Impaired dendritic cell maturation and cytokine production in patients with chronic mucocutanous candidiasis with or without APECED (original) (raw)
Abstract
Patients with chronic mucocutaneous candidiasis (CMC) suffer persistent infections with the yeast Candida. CMC includes patients with autoimmune regulator (AIRE) gene mutations who have autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED), and patients without known mutations. CMC patients have dysregulated cytokine production, and dendritic cells (DCs), as central orchestrators, may underlie pathogenic disease mechanisms. In 29 patients with CMC (13 with APECED) and controls, we generated monocyte-derived DCs, stimulated them with Candida albicans, Toll-like receptor-2/6 ligand and lipopolysaccharide to assess cytokine production [interleukin (IL)-12p70, IL-23, interferon (IFN)-γ, IL-2, tumour necrosis factor (TNF)-α, IL-6, transforming growth factor-β, IL-10, IL-5, IL-13] and cell-surface maturation marker expression (CD83, CD86, human leucocyte antigen D-related). In both APECED and non-APECED CMC patients, we demonstrate impairment of DC function as evidenced by altered cytokine expression profiles and DC maturation/activation: (1) both groups over-produce IL-2, IFN-γ, TNF-α and IL-13 and demonstrate impaired DC maturation. (2) Only non-APECED patients showed markedly decreased _Candida_-stimulated production of IL-23 and markedly increased production of IL-6, suggesting impairment of the IL-6/IL-23/T helper type 17 axis. (3) In contrast, only APECED patients showed DC hyperactivation, which may underlie altered T cell responsiveness, autoimmunity and impaired response to Candida. We demonstrate different pathogenic mechanisms on the same immune response pathway underlying increased susceptibility to Candida infection in these patients.
Keywords: AIRE, APECED, chronic mucocutaneous candidiasis, dendritic cells, human
Introduction
Candida is an opportunistic yeast, colonizing the skin and mucosa of most healthy humans without causing tissue damage [1], but which quickly establishes disease in a variety of permissive circumstances, often on a background of impaired immune function. Protective immunity to Candida involves both the innate and adaptive immune systems [2]. Defects in cell-mediated immunity predispose to mucocutaneous candidiasis, as well as to a wide range of other infectious agents [3]. As opposed to this, there are very rare patients with a selective susceptibility to mucocutaneous infections with Candida who suffer from recurring or persistent, often severe, debilitating infections with this yeast [4]. The diagnosis of this disease, coined chronic mucocutaneous candidiasis (CMC), is clinical and encompasses a heterogeneous group of conditions [5]. The autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED) syndrome, also known as autoimmune polyendocrinopathy type 1 identifies patients with CMC who have associated organ-specific autoimmune involvement of endocrine glands and other organs, and an underlying mutation of the autoimmune regulator (AIRE) gene (online Mendelian inheritance in man – OMIM 240300) (reviewed in [6,7]. There are surprisingly few data addressing the link between AIRE mutations, the associated autoimmunity and the immune defect seen in APECED patients which underlies susceptibility to Candida infections [8,9]. Other subgroups of CMC have also been defined clinically and include patients with associated thyroid disease (OMIM 606415), isolated CMC with various modes of inheritance (OMIM 11458, OMIM 212050) and sporadic CMC [4]. In these CMC patients the diagnosis remains clinical, given that a genetic or biochemical marker is not yet available.
In recent years, a wealth of new knowledge has emerged elucidating the mechanisms involved in protective responses against Candida in both mouse and human models [2,10]. Cytokines produced by the innate immune system, in particular interleukin (IL)-12 secreted by dendritic cells (DCs), are crucial for generating a protective T helper type 1 (Th1) response in mice. However, in striking contrast, patients with inborn errors of the IL-12/interferon (IFN)-γ pathway do not show increased susceptibility to Candida or other fungal infections [11], suggesting strongly that our current understanding of immune mechanisms involved in protection against fungi may need reassessment. A newly identified Th17 pathway, involving IL-6 in the initiation phase and IL-23 in the perpetuation of IL-17-secreting T cells [12], was shown recently to be involved crucially in both human [13,14] and murine [15] immune responses to Candida, and was not identical in mice and man [16].
Very little is known about the immune defect underlying increased susceptibility to Candida infections in CMC patients. These patients have different clinical diseases and (known and unknown) genetic defects, but they all demonstrate the same selective susceptibility to mucocutaneous Candida infections, which suggests that they either harbour the same underlying immune defect or, more likely, have different defects on the same immune response pathway necessary for protection against Candida. Earlier studies both in vivo and in vitro demonstrated defects in cell-mediated immunity, interpreted generally as disorders of effector T cell function [4,17]. More recently, we [18,19] and others [20] demonstrated dysregulated cytokine production in response to Candida, suggesting that the immune defect might be at the level of orchestrating appropriate Th1 (or other?) cytokine responses, rather than the effector T cell level itself.
In the current study we investigated DC function in response to Candida and non-Candida stimuli, to assess if impairment of these central orchestrators of cytokine production could underlie pathogenic disease mechanisms in CMC. Our results demonstrate that DCs from both APECED and non-APECED patients show hyperresponsive cytokine expression profiles following stimulation with lipopolysaccharide (LPS), with over-production of IFN-γ, IL-2, tumour necrosis factor (TNF)-α, IL-5 and IL-13, as well as impaired DC maturation. Only non-APECED patients showed markedly decreased production of IL-23 and markedly increased production of IL-6 specifically in response to Candida, suggesting that impairment of the IL-6/IL-23/Th17 axis may underlie defective clearance and susceptibility to Candida infections. Thus, both APECED and non-APECED CMC patients have impaired/altered DC function, albeit with different defects, suggesting different pathogenic mechanisms on the same immune response pathway underlying increased susceptibility to Candida infection in these patients.
Materials and methods
Our study included 29 CMC patients, 13 APECED patients with the AIRE gene mutation, 16 non-APECED patients without a detectable AIRE gene mutation and 25 age- and sex-matched healthy controls (Table 1). In unstimulated (immature) and stimulated (mature) monocyte-derived DC (moDC) cultures, we assessed supernatants for secreted cytokines [IL-12p70, IL-23, IFN-γ, IL-2, TNF-α, IL-6, transforming growth factor (TGF)-β, IL-10, IL-5 and IL-13], as well as moDCs cell-surface maturation and activation markers [CD83, CD86 and human leucocyte antigen D-related (HLA-DR)]. Toll-like receptor (TLR)-1–10 and other receptor expression was also studied (data not shown, manuscript in preparation). Monocyte-derived DCs were used as representatives of skin and mucosal myeloid-DCs involved in Candida recognition, because obtaining skin biopsies from CMC patients for purely research purposes was unacceptable for ethical reasons.
Table 1.
Patients and controls.
| Total | Adults | Children | Autoantibodies | Endocrinopathy | ||
|---|---|---|---|---|---|---|
| Organ-specific | Systemic | |||||
| Patients | 13 | 5 (1M 4F) | 8 (6M 2F) | 7 | 4 | 10 |
| APECED | 16 | 7 (2M 5F) | 9 (5M 4F) | 2 | 5 | 3 |
| Non-APECED | 29 | 12 | 17 | 9 | 9 | 13 |
| Total controls | 25 | 14 (7M 7F) | 11 (5M 6F) |
We stimulated moDCs with C. albicans hyphae (CH) rather than yeasts, as several studies suggest that hyphae are the invasive morphotype of Candida in clinical infections [21]. With the aim of investigating putative impaired Candida binding to DCs, we assessed moDC stimulation with a TLR-2/6 ligand (MALP2) that selectively engages the same TLRs that are known to bind Candida and other yeasts [22]. LPS was used as a ‘positive’ non-Candida control, in order to assess moDC functionality in response to other potent stimuli. Assessment of additional stimuli was limited by the quantity of blood we could draw from each patient, particularly children.
Generation of moDC from patient blood
moDC were generated from peripheral blood CD14-positive cells in the presence of IL-4 and granulocyte–macrophage colony-stimulating factor (GM-CSF). Peripheral blood mononuclear cells were isolated by density centrifugation (LymphoPrep, Axis-Shield, Oslo, Norway) and CD14-positive cells purified by magnetic separation on an LS column following labelling with anti-CD14-coated magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany). Monocytes were seeded into 24-well plates at 0·75 × 106 per well in 1 ml total volume of RF10 culture media (RPMI-1640 media (BioWhittaker, Lonza, Wokingham, UK), supplemented with 10% fetal calf serum (PAA Laboratories, Pasching, Austria), 2 mM l-glutamine (Sigma Aldrich, St Louis, MO, USA) and 1% penicillin–streptomycin (Gibco, Carlsbad, CA, USA). Fifty ng/ml IL-4 and GM-CSF (Immunotools, Friesoythe, Germany) were added to each well on days 0 and 3. Cells were incubated at 37°C with 5% CO2.
Dendritic cell maturation
On day 6 of the DC culture, immature DCs were activated as follows: no treatment (unstimulated); addition of 1:10 000 final dilution (10 mg/l protein content or 25 × 106 cells) of heat-killed CH (American Type Culture collection no. 18804, Manassas, VA, USA); 10 ng/ml of the purified TLR-2/6 ligand, MALP-2 (Apotech, Epalinges, Switzerland); and 1 µg/ml of LPS (Invivogen, San Diego, CA, USA). Cells or cytokines were harvested after 24 h on day 7.
Cytokine analysis
Culture supernatants were harvested 24 h after activation and stored at −20°C. Cytokine levels were assessed either by sandwich enzyme-linked immunosorbent assay or the electrochemiluminescence-based meso scale discovery (MSD) (Meso Scale Discovery, Gaithersburg, MD, USA) immunoassay. IL-12p70, IFN-γ, IL-2, IL-5, IL-10 and IL-13 were part of an MSD multiplex Th1/Th2 plate (detection limits for IL-12p70, IFN-γ and IL-10 were 4 pg/ml; for IL-2, IL-5 and IL-13, 2 pg/ml); IL-6 and TNF-α were MSD duplex custom-made plates (extra-sensitive, detection limit 3 pg/ml for both cytokines); the TGF-β duoset kit was purchased from R&D Systems (Minneapolis, MN, USA; detection limit 20 pg/ml) and IL-23 Ready-Set-Go kit from eBioscience (San Diego, CA, USA; detection limit 15 pg/ml). Cytokine levels were calculated using the manufacturer's software, given in pg/ml and presented as medians with interquartile ranges (IQR).
Flow cytometry
To assess DC maturation, DCs were harvested 24 h after activation and stained for 20 min on ice with the following antibodies: CD86-fluorescein isothiocyanate, CD83-phycoerythrin (PE) and HLA-DR-peridinin chlorophyll and appropriate isotype controls (BD Biosciences, San Jose, CA, USA). Stained cells were washed in fluorescence activated cell sorter (FACS) wash (1 × phosphate-buffered saline + 0·1% bovine serum albumin) and fixed in 1% paraformaldehyde (Sigma Aldrich). All stained cells were acquired using a FACScan (BD Biosciences) equipped with a 488 nm laser and a 633 nm laser upgrade. Acquired events were analysed using FlowJo software (Tree Star, Inc., Ashland, OR, USA). Monocyte transformation into DCs was confirmed by absence of CD14 staining in cultures. Results of DC activation marker analysis are presented as percentage of positive cells and median fluorescence intensity (MFI).
Candida albicans
Freeze-dried C. albicans was purchased from American Type Culture Collection (no. 18804) and rehydrated according to the supplier's instructions. To culture the hyphal form, C. albicans was grown in autoclaved 1× broth (67 g/l yeast nitrogen base and 10% d-glucose; Becton-Dickinson, Sparks, MD, USA) at 30°C, heat-killed (pressure cooker for 30 min at 120°C), pelleted at 400 g for 10 min and used in cell cultures at a final concentration of 1:10 000, defined as optimal in previous titrations.
Subjects
We investigated 29 CMC patients, of whom 13 were APECED patients with the AIRE gene mutation, 16 were non-APECED patients without a detectable AIRE gene mutation and 25 were age- and sex-matched healthy controls (Table 1).
Patients
We studied 29 patients with CMC, who were all screened for the two most common AIRE gene mutations: p.R257X (non-sense mutation in exon 6) and c·964del13 [13 base pairs (bp) deletion in exon 8] in either Huch-Laboratory Diagnostics (Helsinki University Hospital, Finland) or Northern Molecular Genetics Service (Institute of Human Genetics, Newcastle Upon Tyne, UK). Thirteen patients (children 3–15 years of age, adults 17–38) had an AIRE gene mutation and the APECED syndrome, of whom nine had the c·964del13 deletion. In the remaining 16 non-APECED patients (children 2–15 years of age, adults 19–47) an AIRE mutation was not detected. All patients were also screened for auto-antibodies to Type 1 IFNs, shown to be highly specific for APECED patients [23]; these autoantibodies were present in all APECED patients and none of the non-APECED patients and controls.
Ten patients in the APECED group and nine in the non-APECED group had affected siblings, who are all included in this study (maximum three patients from any one family). Three non-APECED patients had hypothyroidism, two with thyroid peroxidase antibodies. At the time of sampling, patients did not have other serious infections, were not on systemic antibiotic treatment or receiving steroids. All patients suffered with recurrent mucocutaneous Candida infection (mouth, nails, skin, oesophagus and perineum). Patients were screened for systemic autoantibodies including anti-nuclear factor, smooth muscle, liver–kidney microsomal, mitochondrial and gastric parietal cell antibodies. Organ-specific autoantibodies and/or endocrinopathy affected parathyroid, thyroid, adrenal cortex, gonads and pancreas. Autoantibodies were evaluated in patients' sera using indirect immunofluorescence on commercial rodent tissue (Euroimmune, Lubeck, Germany) for systemic autoantibodies and monkey organ tissue (The Binding Site, Birmingham, UK) for organ-specific autoantibodes. Endocrinopathy was diagnosed if/when there was clinical and laboratory evidence of glandular hypofunction.
Controls
Twenty-five age- and sex-matched controls were recruited for the study. Adults (19–55 years of age) were healthy laboratory volunteers, while control children (2–16 years of age) were undergoing general anaesthesia for surgery to treat non-infectious causes (eye squints, circumcision, hernia, etc.).
The number of patients and controls in each experiment may vary, due to the limitation of blood available. Both patients and healthy controls – parents on behalf of children – received verbal and written explanations of the study and signed informed consent forms. Ethical approval was obtained from the Newcastle and North Tyneside Local Research Ethics Committee 1.
Statistical analysis
Statistical analysis and graphic presentations were performed using the GraphPad prism software package. Average values are presented as medians with IQRs. _P_-values were calculated using the two-tailed, 95% confidence intervals Mann–Whitney rank sum test for independent, non-parametric data. The level of significance was set at P < 0·05.
Results
Cytokine production by moDCs
The IL-6/IL-23/Th17 axis cytokine production
For IL-6, one of the most important findings in this study was the selectively increased IL-6 production in non-APECED CMC patients, who demonstrated significantly higher unstimulated IL-6 production compared with both APECED patients and controls, where IL-6 levels were mainly undetectable or at very low levels (Fig. 1a). Importantly, non-APECED patients produced significantly more IL-6 in response to CH and TLR-2/6 ligand stimulation compared with both APECED patients and controls (Fig. 1a). LPS stimulation resulted in high levels of IL-6 produced in both patient groups which were higher, on average, albeit not significantly different compared with controls (Fig. 1a). There was no major difference between levels produced by adults and children in any of the groups (data not shown).
Fig. 1.
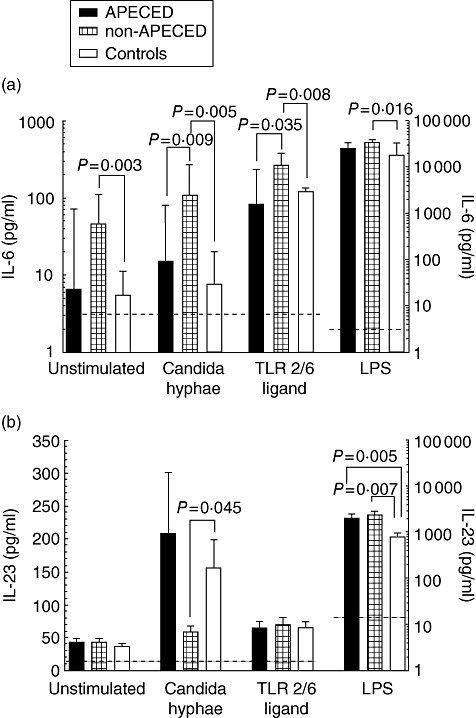
Non-autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED) patients produce very high interleukin (IL)-6 but low IL-23 levels in response to _Candida_-specific stimuli. Immature monocyte-derived dendritic cells (moDCs) from patients and controls were either left unstimulated or treated with C. albicans hyphae, a specific Toll-like receptor (TLR)-2/6 ligand or lipopolysaccharide (LPS). Culture supernatants were collected at 24 h and measured by meso scale discovery (MSD) multiplex assay for detection of IL-6 or enzyme-linked immunosorbent assay for detection of IL-23. Note log scale for LPS. The level of significance was set at P < 0·05. Average values are presented as medians with interquartile ranges (IQR). (a) Increased production of IL-6. Detection limit (----) was 3 pg/ml. (b) Decreased production of IL-23. Detection limit (----) was 15 pg/ml.
For IL-23, impaired production of IL-23 in non-APECED patients was another important finding in our study (Fig. 1b). In response to Candida stimulation, non-APECED patients produced significantly less IL-23 than controls, whereas this was not the case with APECED patients. The difference was most marked in non-APECED adults (P = 0·035 compared with controls, data not shown). TLR-2/6 stimulation resulted in modest and comparable IL-23 increases in all groups (Fig. 1b). Interestingly, the non-Candida stimulant LPS resulted in huge increases of IL-23 levels in all groups, with both APECED and non-APECED patients producing significantly more IL-23 compared with controls.
The Th1 cytokine production
Production of IL-12p70 in unstimulated cultures was barely detectable in all groups. Stimulation with Candida and TLR-2/6 ligand resulted in a low but clearly detectable and similar response in all groups (data not shown). The response to LPS was impressively and significantly higher in non-APECED patients (particularly children, data not shown) compared with levels produced by APECED patients and controls, which were almost identical (Table 2).
Table 2.
LPS-stimulated cytokine hyperproduction in autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED) and non-APECED patients.
| pg/ml | APECED | Non-APECED | CTRLS† | APECED | Non-APECED | CTRLS |
|---|---|---|---|---|---|---|
| Unstimulated | LPS | |||||
| IL-12p70 | 7† (6–8) | 7 (4–8) | 4 (4–5) | 291 (73–1583) | 1112** (570–2623) | 292 (13–861) |
| IFN-γ | 93 (84–114) | 97 (98–115) | 87 (82–101) | 130* (101–146) | 141* (115–165) | 102 (83–118) |
| IL-2 | 27 (25–31) | 26 (25–29) | 25 (23–28) | 32* (29–43) | 37** (33–44) | 29 (23–33) |
| TNF-α | 47* (14–89) | 56** (32–84) | 1 (1–22) | 6555* (5233–9692) | 5456* (4019–9596) | 3568 (928–6618) |
| IL-10 | 38 (35–41) | 39 (35–42) | 37 (32–44) | 73 (49–98) | 78 (61–148) | 65 (50–100) |
| IL-5 | 11 (9–12) | 11 (10–12) | 9 (8–13) | 15 (12–20) | 17** (13–21) | 12 (11–15) |
| IL-13 | 3 (3–4) | 3 (3–4) | 2 (2–3) | 5 (4–7) | 6 (3–7) | 3 (2–4) |
Background levels of IFN-γ in unstimulated cultures were detectable in all groups and neither CH nor TLR-2/6 increased IFN-γ production above unstimulated levels. However, LPS induced IFN-γ production in all groups, although significantly more in CMC patients than in controls (Table 2). Healthy children produced less IFN-γ than healthy adults, although this was not statistically significant (data not shown).
The IL-2 cytokine was produced only with LPS stimulation and non-APECED patients, interestingly produced significantly more IL-2 than controls (Table 2).
Inflammatory cytokine production
For TNF-α, high unstimulated production was seen in both APECED and non-APECED patients compared with controls, where it was mainly undetectable or low. Following CH and TLR-2/6 ligand stimulation all groups responded to a similar degree, but in response to LPS, APECED and non-APECED patients produced significantly more TNF-α compared with controls (Table 2).
Anti-inflammatory cytokine production
For TGF-β, production of this cytokine was not enhanced and remained close to baseline in both patients and controls (data not shown). Assessment of mRNA levels yielded similar results (unpublished data).
The Th2 and Th2-inducing cytokine production
With regard to IL-10, IL-5 and IL-13, CH and TLR-2/6 did not stimulate production of these cytokines by moDCs. LPS increased production of IL-10 to low and comparable levels in all groups, while LPS-stimulated IL-5 and IL-13 production was markedly higher in APECED and non-APECED patients than in controls (Table 2).
Cytokine plasma levels
With regard to IL-6, TNF-α and TGF-β plasma levels, levels of IL-6 and TNF-α in plasma for all groups studied were mainly low or undetectable, while TGF-β levels were detectable and significantly higher in APECED and non-APECED patients than in controls (data not shown).
The DC activation markers
CD83
For CD83, we present only findings in children, where the differences were most marked.
Percentages of CD83+ cells in unstimulated cultures were comparable between patients and controls (Fig. 2a). In response to CH and TLR-2/6 ligand stimulation, APECED and non-APECED children had markedly lower percentages of CD83+ cells than controls (Fig. 2a). Following LPS stimulation, the trend was the same but did not reach statistical significance (Fig. 2a). In adults, the percentages of CD83+ cells were not significantly different between the groups for any of the stimuli used (data not shown).
Fig. 2.
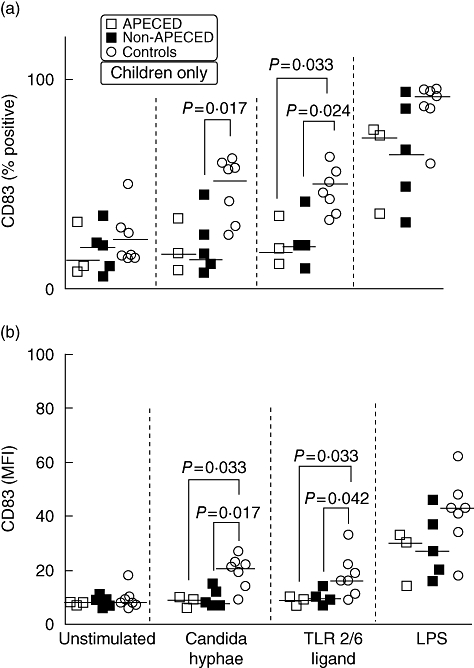
Impaired upregulation of CD83 in both autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED) and non-APECED children in response to Candida albicans. Immature monocyte-derived dendritic cells (moDCs) were cultured overnight with either the hyphal form of C. albicans, a specific Toll-like receptor (TLR)-2/6 ligand, lipopolysaccharide (LPS) or were left untreated. After 24 h, moDCs were harvested, stained with anti-CD83-phycoerythrin (PE) and analysed by flow cytometry for CD83 expression. The level of significance was set at P < 0·05. Average values are presented as medians with interquartile ranges (IQR). (a) Percentage of cells. (b) Median fluorescence intensity (MFI).
The MFI levels of CD83 in unstimulated DC cultures were low and comparable in all groups (Fig. 2b). In response to stimulation with CH and TLR-2/6 ligand, APECED and non-APECED children up-regulated CD83 to a significantly lesser degree than controls. LPS increased CD83 expression in all groups; this was again to a lesser degree in APECED and non-APECED children than in controls, albeit not statistically significant (Fig. 2b). CD83 expression in adults did not differ between groups (data not shown).
CD86
Percentages of CD86+ cells in unstimulated cultures were significantly higher in APECED patients compared with controls (Fig. 3a). Following stimulation with CH and TLR-2/6 ligand, the percentage of CD86+ cells in APECED patients increased significantly compared with both controls and non-APECED patients. In contrast, percentages of CD86+ cells in non-APECED patients remained similar to control levels throughout. LPS stimulation resulted in almost all cells expressing CD86 in all individuals (Fig. 3a).
Fig. 3.
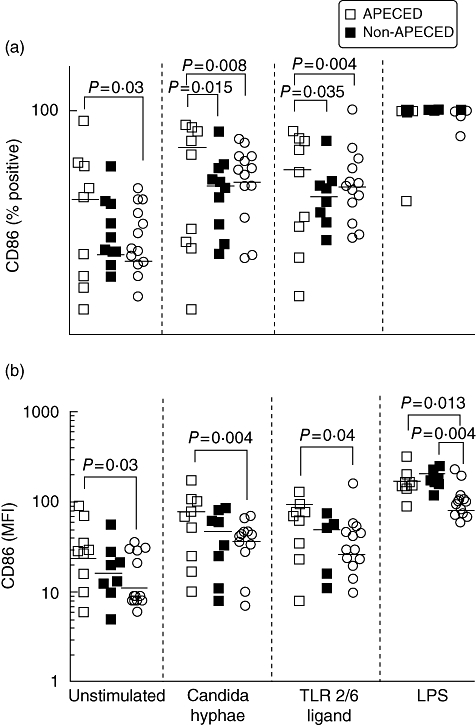
Monocyte-derived dendritic cells (moDCs) from autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED) patients express higher CD86 levels than controls. Immature monocyte-derived dendritic cells (DCs) were cultured overnight with either the hyphal form of Candida albicans, a specific Toll-like receptor (TLR)-2/6 ligand, lipopolysaccharide or were left untreated. After 24 h, DCs were harvested, stained with anti-CD86-fluorescein isothiocyanate (FITC) and analysed by flow cytometry for CD86 expression. The level of significance was set at P < 0·05. Average values are presented as medians with interquartile ranges (IQR). (a) Percentage of cells. (b) Median fluorescence intensity (MFI). Note log scale.
The MFI levels of CD86 in unstimulated cultures were significantly higher in APECED patients compared with controls. Following stimulation with CH and TLR-2/6 ligand, CD86 MFI levels in APECED patients were significantly higher compared with controls, whereas MFI levels in non-APECED patients also increased, but not significantly (Fig. 3b). Stimulation with LPS resulted in higher CD86 MFI in both APECED and non-APECED compared with controls (Fig. 3b).
The HLA-DR
Percentages and MFI of HLA-DR+ cells after stimulation by CH, TLR-2/6 and LPS were not statistically different between groups (data not shown).
Discussion
We demonstrate for the first time that both APECED and non-APECED CMC patients have impaired DC function, implying that this may be the pathogenic mechanism underlying increased susceptibility to Candida infection in these patients. Importantly, although some DC defects were common for both patient groups, other defects were unique, suggesting different pathogenic mechanisms on the same immune response pathway, resulting in a similar phenotype of increased susceptibility to Candida infection.
Our most important finding was the _Candida-_specific decreased IL-23 and increased IL-6 production seen in only non-APECED patients, implicating impairment of the IL-6/IL-23/IL-17 axis as the mechanism underlying defective clearance of Candida in these patients. In contrast, only APECED patients showed DC hyperactivation, which may underlie altered T cell responsiveness leading to autoimmunity as well as impaired cell-mediated responses to Candida. Both APECED and non-APECED patients demonstrated LPS-induced cytokine hyperproduction (IFN-γ, IL-2, TNF-α, IL-5, IL-13) and impaired DC maturation.
It is believed currently that protective immunity to Candida in both mice and humans is highly dependent upon the IL-12 initiation of a protective Th1 response, and there is ample evidence supporting the role of DCs as master orchestrators of this scenario [10]. DCs phagocytose Candida yeasts leading to production of Th1 cytokines (IL-12), while the β-glucan receptor Dectin-1 and TLR-2 collaborate to trigger phagocytosis and secretion of IL-12 and TNF-α. In contrast, Candida hyphae stimulate production of Th2 cytokines (IL-4 and IL-10) and are poor stimulators of IL-12 production. In our study, the low IL-12 produced in both patients and controls was due probably to stimulation with the hyphal rather than the yeast Candida morphotype. This is consistent with our previous work [19], where we also found low/undetectable IL-12 production in CMC patients. A recent study demonstrated that rather than IL-12, hyphae induce IL-23, which plays a key role in the differentiation of Th17 cells [13]. Fungal triggering of TLRs on DCs is now known to be of paramount importance, with TLR-4 implicated in triggering Th1-inducing cytokines (IL-12) and TLR-2 in initiating the Th2 cytokine cascade (IL-4, IL-5) [2]. Our own results (in preparation) demonstrate that stimulated mRNA expression of TLR-1–10 and Dectin-1 in the same moDCs as used in this study differs compared with healthy controls. The high IL-12 levels produced by non-APECED patients in response to LPS in this study suggest poor control, i.e. dysregulation of IL-12 production. Notably, under the same circumstances APECED patients did not show any abnormalities of IL-12 production.
The current belief that the IL-12/IFN-γ axis is central for generating protective immunity to fungi is questioned crucially by the fact that patients with inborn errors of this pathway do not demonstrate increased susceptibility to Candida or other fungal infections [11], suggesting that other cytokine pathways may be the main players (see below). Indeed, the role of IFN-γ in murine models of candidiasis has been controversial [10], while in CMC patients the effects of IFN-γ treatment have been disappointing [24].
A new and significant finding in our study was that non-APECED CMC patients produced markedly less IL-23 in response to Candida stimulation compared with controls, which was not seen in APECED patients. The importance of IL-23 in the generation of IL- 17-producing T helper (Th17) cells, initiated by the effect of IL-6 and IL-1 on newly primed CD4 T cells in humans (IL-6 and TGF-β in mice), with IL-23 required for further Th17 expansion, was recognized recently [12]. IL-23 receptor–ligand interaction activates the human signal transducer and activator of the transcription 3 (STAT3) gene, resulting in binding of IL-17A and IL-17F promoters [12]. Th17 cells were reported to be induced preferentially by fungal binding and signalling through Dectin-1 [15]. It was also demonstrated that in humans _C. albicans_-specific T memory cells have a Th17 phenotype and chemokine receptor expression pattern indicative of mucosal homing [13,14]. In light of these findings, the low levels of IL-23 produced by non-APECED CMC patients in response to Candida stimulation could translate into an inability to mount and sustain an anti-fungal Th17 response resulting in impaired clearance of Candida in vivo, which is currently under investigation by our group. Interestingly, stimulation of IL-23 production by TLR-2/6 ligand and LPS was comparable in all groups, suggesting that the defect in IL-23 production in non-APECED CMC patients is _Candida_-specific and may be at the level of Candida recognition, which is known to involve simultaneously multiple receptors and signalling pathways activated by Candida, but not engaged by other stimuli (e.g. TLRs, Dectin-1 and 2, complement receptors, mannose receptor, etc.) [13].
A crucial finding in our study is the markedly increased production of IL-6 by non-APECED CMC patients, specifically in response to Candida and _Candida_-like ligands, but not to LPS. It has been reported that mice deficient in IL-6 demonstrate increased susceptibility to systemic candidiasis [25], while newer studies demonstrate a crucial role for IL-6, together with TGF-β or IL-1 in the initiation of Th17 cells [12]. Recently [26], in a model of experimental autoimmune encephalomyelitis (EAE), it was demonstrated that stimulation of myelin-reactive Th17 cells by IL-6 and TGF-β alone leads to IL-17 production but abrogates their pathogenic role, due to co-expression of IL-10. In contrast, stimulation of Th17 by IL-23 leads to IL-17 production in the absence of IL-10, leading to inflammation. In our CMC patients, there is clearly a defect in IL-23 production together with elevated IL-6. It seems this could lead similarly to IL-17 and IL-10 production by T cells, which might suppress the ability to clear the pathogen. Indeed, although moDCs in this study did not produce high IL-10 levels, our previous studies demonstrated very high IL-10 production by _Candida_-stimulated whole-blood cultures in CMC patients [18].
An alternative way in which IL-6 could influence susceptibility to Candida infections in CMC patients would be through its effect on T regulatory cells. It was reported that TLR activation of DCs abrogated the suppressive effects of CD4+CD25+ T regulatory cells due partially to the effect of DC-produced IL-6 on responder T cells [27]. Recently, a direct blocking effect of IL-6 on the de novo induction of adaptive T regulatory cells in mice has also been reported [28]. High IL-6 levels seen in non-APECED patients could abrogate the suppressive effects of T regulatory cells, which could explain the cytokine hyperproduction in response to LPS (IL-12, IL-23, IFN-γ, IL-2, TNF-α, IL-5, IL-13). As opposed to this, in APECED patients, the hyperproduction in response to LPS (IFN-γ, IL-2, TNF-α, IL-13) could also be due to impaired T regulatory cell numbers and/or function, albeit not involving IL-6, as was indeed reported by us [29] and confirmed by others [30]. The resulting dysregulation of cytokine production could undermine an efficient immune response needed to clear the Candida infection in CMC patients.
An important and novel finding in this study was impaired moDC maturation in young APECED and non-APECED patients, as evidenced by low CD83 percentages and MFI expression on moDCs. CD83 is a well-recognized marker of DC maturation, and is upregulated on moDCs together with co-stimulatory molecules CD80 and CD86 [31], implying that expression of CD83 is crucial in regulating the development of cellular immunity. It is tempting to hypothesize that this may be of major importance in understanding impaired immunity to Candida in CMC patients, and is the first evidence of a DC maturational defect in CMC patients. Our data on defective TLR mRNA down-regulation in the same moDCs also suggests impaired DC maturation (in preparation). It is intriguing that this defect is seen only in children and not adults with CMC, suggesting that the immune system gradually overcomes or compensates this defect. Interestingly, APECED (but not non-APECED) patients demonstrated a higher percentage and MFI of CD86+ moDCs after stimulation with CH and TLR-2/6, suggesting more of their DCs become activated when exposed to Candida antigens. This may be linked to the ongoing Candida infections in vivo, as Candida hyphae have generally been reported to increase expression of co-stimulatory molecules and major histocompatibility complex (MHC) class II on DCs [10,21]. Activation of DCs without maturation may render these cells efficient in phagocytosing Candida antigens, but less inefficient in antigen presentation and activation of T cell responses. Altered DC activation in APECED patients may underlie aberrant T cell responsiveness, leading both to autoimmunity as well as to impaired cell-mediated responses, resulting in increased susceptibility to Candida infections.
Taken together, our findings suggest strongly that both APECED and non-APECED CMC patients have impaired/altered DC function, albeit with different defects on the same immune response pathway necessary for protection against Candida, and that this impairment may be the pathogenic mechanism underlying increased susceptibility to Candida infection in these patients. In APECED patients, we demonstrate impaired DC maturation and hyperactivation which may underlie increased T cell responsiveness (also demonstrated previously by others [9]), leading to autoimmunity and impaired handling of Candida. As opposed to this, in non-APECED patients, the DC defect is probably at the level of DC cytokine secretion, with inadequate production of IL-23 and an overproduction of IL-6, which together leads to an inefficient IL-17 response. In non-APECED patients the gene defect is not known, but the degree of cytokine dysregulation is reminiscent of findings in the ‘classical’ hyperimmunoglobulin (Ig)E syndrome, where it was reported recently that the underlying defect is a mutation in the human STAT3 gene, which is activated in response to IL-23 (see above) as well as to a wide variety of cytokines and growth factors, resulting in dysregulation of multiple cytokines [32].
Elucidating the immune defect(s) in APECED and non-APECED CMC patients is of paramount significance not only because of the obvious implications for patient management, but because these conditions are prime examples of recently defined ‘non-conventional’ primary immune deficiencies [33], which are characterized by a narrow spectrum of specific infections limited usually to one microbe (in this case Candida) as a consequence of inborn errors of immunity. Dissection of underlying mechanisms in diseases such as CMC contributes to the understanding of fundamental pathways in immunity.
Acknowledgments
This work was supported by the Primary Immunodeficiency Association (PIA), UK, Registered Charity no. 803217 and the UK/China Scholarships for Excellence Programme. We are very grateful to patients, healthy volunteers and their families for supporting and participating in this research. We thank Dr Idse Harrema, Dr Mike Clarke and the medical and nursing staff in the departments of paediatric surgery and paediatric ophtalmology, Royal Victoria Infirmary, NUTH NHS Foundation Trust for enabling and supporting collection of blood samples from healthy children. We thank Anne Curtis, Head of the Molecular Genetic Diagnostics Laboratory, NUTH NHS Foundation Trust for screening for AIRE gene mutations. We are grateful to Daniel Swan, Bioinformatics Department, Newcastle University, for help with data analysis. The authors do not have any conflicts of interests to disclose.
References
- 1.Odds FC. Candida and candidosis. 2nd. London: Baillière-Tindall; 1988. [Google Scholar]
- 2.Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008;6:67–78. doi: 10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
- 3.Notarangelo L, Casanova JL, Conley ME, et al. Primary immunodeficiency diseases: an update from the International Union of Immunological Societies Primary Immunodeficiency Diseases Classification Committee Meeting in Budapest, 2005. J Allergy Clin Immunol. 2006;117:883–96. doi: 10.1016/j.jaci.2005.12.1347. [DOI] [PubMed] [Google Scholar]
- 4.Kirkpatrick CH, Rich RR, Bennett JE. Chronic mucocutaneous candidiasis: model-building in cellular immunity. Ann Intern Med. 1971;74:955–78. doi: 10.7326/0003-4819-74-6-955. [DOI] [PubMed] [Google Scholar]
- 5.Lilic D, Haynes K. Candida. In: Brown GD, Netea MG, editors. Immunology of fungal infections. Dordrecht, the Netherlands: Springer; 2007. pp. 361–82. [Google Scholar]
- 6.Perheentupa J. Autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy. J Clin Endocrinol Metab. 2006;91:2843–50. doi: 10.1210/jc.2005-2611. [DOI] [PubMed] [Google Scholar]
- 7.Mathis D, Benoist C. A decade of AIRE. Nat Rev Immunol. 2007;7:645–50. doi: 10.1038/nri2136. [DOI] [PubMed] [Google Scholar]
- 8.Pontynen N, Miettinen A, Arstila TP, et al. Aire deficient mice do not develop the same profile of tissue-specific autoantibodies as APECED patients. J Autoimmun. 2006;27:96–104. doi: 10.1016/j.jaut.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Ramsey C, Winqvist O, Puhakka L, et al. Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum Mol Genet. 2002;11:397–409. doi: 10.1093/hmg/11.4.397. [DOI] [PubMed] [Google Scholar]
- 10.Romani L. Immunity to fungal infections. Nat Rev Immunol. 2004;4:1–23. doi: 10.1038/nri1255. [DOI] [PubMed] [Google Scholar]
- 11.Ottenhoff TH, Kumararatne D, Casanova JL. Novel human immunodeficiencies reveal the essential role of type-I cytokines in immunity to intracellular bacteria. Immunol Today. 1998;19:491–4. doi: 10.1016/s0167-5699(98)01321-8. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt-Weber CB, Akdis M, Akdis CA. TH17 cells in the big picture of immunology. J Allergy Clin Immunol. 2007;120:247–54. doi: 10.1016/j.jaci.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 13.Acosta-Rodriguez EV, Rivino L, Geginat J, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–46. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 14.Zhou M, Yang B, Ma R, Wu C. Memory Th-17 cells specific for C. albicans are persistent in human peripheral blood. Immunol Lett. 2008;118:72–81. doi: 10.1016/j.imlet.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 15.LeibundGut-Landmann S, Gross O, Robinson MJ, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–8. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 16.Laurence A, O'Shea JJ. T(H)-17 differentiation: of mice and men. Nat Immunol. 2007;8:903–5. doi: 10.1038/ni0907-903. [DOI] [PubMed] [Google Scholar]
- 17.Valdimarsson H, Higgs JM, Wells RS, Yamamura M, Hobbs JR, Holt PJ. Immune abnormalities associated with chronic mucocutaneous candidiasis. Cell Immunol. 1973;6:348–61. doi: 10.1016/0008-8749(73)90035-x. [DOI] [PubMed] [Google Scholar]
- 18.Lilic D, Cant AJ, Abinun M, Calvert JE, Spickett GP. Chronic mucocutaneous candidiasis. I. Altered antigen-stimulated IL-2, IL-4, IL-6 and interferon-gamma (IFN-gamma) production. Clin Exp Immunol. 1996;105:205–12. doi: 10.1046/j.1365-2249.1996.d01-764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lilic D, Gravenor I, Robson N, et al. Deregulated production of protective cytokines in response to Candida albicans infection in patients with chronic mucocutaneous candidiasis. Infect Immun. 2003;71:5690–9. doi: 10.1128/IAI.71.10.5690-5699.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobrynski LJ, Tanimune L, Kilpatrick L, Campbell DE, Douglas SD. Production of T-helper cell subsets and cytokines by lymphocytes from patients with chronic mucocutaneous candidiasis. Clin Diagn Lab Immunol. 1996;3:740–5. doi: 10.1128/cdli.3.6.740-745.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.d'Ostiani CF, Del Sero G, Bacci A, et al. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J Exp Med. 2000;191:1661–74. doi: 10.1084/jem.191.10.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozinsky A, Underhill DM, Fontenot JD, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766–71. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meloni A, Furcas M, Cetani F, et al. Autoantibodies against type I interferons as an additional diagnostic criteria for APSI. J Clin Endocrinol Metab. 2008 doi: 10.1210/jc.2008-0935. E-pub doi 10.1210jc/2008-0935. [DOI] [PubMed] [Google Scholar]
- 24.Abinun M, Lilic D, Cant AJ, Calvert JE. Clinical and immunological response to IFNγ treatment in a patient with chronic mucocutaneous candidiasis. In: Caragol I, Espanol T, Fontan G, Matamoros N, editors. Progress in immune deficiency. Iberica: Springer-Verlag; 1995. pp. 255–6. [Google Scholar]
- 25.van Enckevort FH, Netea MG, Hermus AR, et al. Increased susceptibility to systemic candidiasis in interleukin-6 deficient mice. Med Mycol. 1999;37:419–26. doi: 10.1046/j.1365-280x.1999.00247.x. [DOI] [PubMed] [Google Scholar]
- 26.McGeachy MJ, Bak-Jensen KS, Chen Y, et al. F-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–7. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 27.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4 + CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–6. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 28.Dominitzki S, Fantini MC, Neufert C, et al. Cutting edge: trans-signaling via the soluble IL-6R abrogates the induction of FoxP3 in naive CD4 + CD25 T cells. J Immunol. 2007;179:2041–5. doi: 10.4049/jimmunol.179.4.2041. [DOI] [PubMed] [Google Scholar]
- 29.Ryan KR, Lawson CA, Lorenzi AR, Arkwright PD, Isaacs JD, Lilic D. CD4 + CD25+ T-regulatory cells are decreased in patients with autoimmune polyendocrinopathy candidiasis ectodermal dystrophy. J Allergy Clin Immunol. 2005;116:1158–9. doi: 10.1016/j.jaci.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 30.Kekalainen E, Tuovinen H, Joensuu J, et al. A defect of regulatory T cells in patients with autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy. J Immunol. 2007;178:1208–15. doi: 10.4049/jimmunol.178.2.1208. [DOI] [PubMed] [Google Scholar]
- 31.Lechmann M, Berchtold S, Hauber J, Steinkasserer A. CD83 on dendritic cells: more than just a marker for maturation. Trends Immunol. 2002;23:273–5. doi: 10.1016/s1471-4906(02)02214-7. [DOI] [PubMed] [Google Scholar]
- 32.Minegishi Y, Saito M, Tsuchiya S, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–62. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 33.Casanova JL, Abel L. Primary immunodeficiencies: a field in its infancy. Science. 2007;317:617–9. doi: 10.1126/science.1142963. [DOI] [PubMed] [Google Scholar]