Lowering Blood Pressure Reduces Renal Events in Type 2 Diabetes (original) (raw)
Abstract
BP is an important determinant of kidney disease among patients with diabetes. The recommended thresholds to initiate treatment to lower BP are 130/80 and 125/75 mmHg for people with diabetes and nephropathy, respectively. We sought to determine the effects of lowering BP below these currently recommended thresholds on renal outcomes among 11,140 patients who had type 2 diabetes and participated in the Action in Diabetes and Vascular disease: preterAx and diamicroN-MR Controlled Evaluation (ADVANCE) study. Patients were randomly assigned to fixed combination perindopril-indapamide or placebo, regardless of their BP at entry. During a mean follow-up of 4.3 yr, active treatment reduced the risk for renal events by 21% (P < 0.0001), which was driven by reduced risks for developing microalbuminuria and macroalbuminuria (both P < 0.003). Effects of active treatment were consistent across subgroups defined by baseline systolic or diastolic BP. Lower systolic BP levels during follow-up, even to <110 mmHg, was associated with progressively lower rates of renal events. In conclusion, BP-lowering treatment with perindopril-indapamide administered routinely to individuals with type 2 diabetes provides important renoprotection, even among those with initial BP <120/70 mmHg. We could not identify a BP threshold below which renal benefit is lost.
Type 2 diabetes is the leading cause of end-stage kidney disease, accounting for 30 to 50% of new cases in the industrialized world.1 Microalbuminuria is one of the earliest detectable manifestations of kidney disease in diabetes, with a prevalence of 25% after 10 yr of diabetes duration and with an annual rate of progression to overt nephropathy of approximately 3%.2 The risk for the development and progression of microalbuminuria is highly dependent on BP.3 Accumulating evidence suggests that BP lowering reduces the risk for new-onset or progressive nephropathy, particularly when angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) are used.4–9
Current guidelines recommend commencement of BP-lowering treatment for patients with diabetes and a BP of ≥130/80 mmHg to a therapeutic target of <130/80 mmHg.10–13 The threshold for those with diabetes and nephropathy has been set at 125/75 mmHg.13 These cut points are based largely on the strong, positive, and graded association between higher levels of BP and the risk for clinical events, including end-stage renal disease.14 Evidence from major intervention trials to support these thresholds is, limited however, because average BP levels at study entry were often much higher.5–9,15 Conversely, observational analyses reporting continuous associations of renal disease with BP16,17 suggested that individuals with initial BP levels below the currently recommended thresholds may benefit from BP-lowering treatment. Again, evidence from randomized comparisons supporting such a hypothesis is lacking.
The BP arm of the Action in Diabetes and Vascular disease: preterAx and diamicroN-MR Controlled Evaluation (ADVANCE) study recently reported that the routine administration of a fixed combination of the ACEI perindopril and the diuretic indapamide to a broad cross-section of patients with type 2 diabetes reduced the risks for major vascular events and death from all causes.18 The risk for renal events was reduced by 21% overall. In this report, we examine the effects of study treatment on a range of renal outcomes in more detail and assess whether there are benefits of such treatment in patients with BP levels below those currently recommended as thresholds for commencing BP-lowering treatment.
RESULTS
A total of 12,877 potentially eligible participants were registered; 1737 (13.5%) were subsequently withdrawn during the 6-wk active run-in period, and 11,140 (86.5%) were randomly assigned. The characteristics of the ADVANCE study participants are summarized in Table 1. Entry BP levels averaged 145/81 mmHg overall, and 20% of patients had a BP <130/80 mmHg.
Table 1.
Baseline characteristics recorded before active run-ina
| Variable | Randomized Treatment | |
|---|---|---|
| Perindopril-Indapamide (n = 5569) | Placebo (n = 5571) | |
| Age (yr; mean [SD]) | 66 (6) | 66 (7) |
| Female (n [%]) | 2366 (42.5) | 2369 (42.5) |
| Age when diabetes first diagnosed (yr; mean [SD]) | 58 (9) | 58 (9) |
| Duration of diabetes (yr; mean [SD]) | 8 (6) | 8 (6) |
| BP control (mmHg; mean [SD]) | ||
| SBP | 145 (22) | 145 (21) |
| DBP | 81 (11) | 81 (11) |
| history of currently treated hypertension (n [%]) | 3802 (68.3) | 3853 (69.2) |
| Renal parameters | ||
| UACR (μg/mg; median [IQR]) | 15 (7 to 40) | 15 (7 to 40) |
| microalbuminuria (n [%]) | 1441 (25.9) | 1421 (25.5) |
| macroalbuminuria (n [%])b | 197 (3.5) | 204 (3.7) |
| serum creatinine (μmol/L; mean [SD])c | 87 (23) | 87 (26) |
| eGFR (ml/min per 1.73 m2; mean [SD]) | 78 (25) | 78 (25) |
| eGFR <60 ml/min per 1.73 m2 (n [%]) | 1063 (19.1) | 1094 (19.6) |
| Previous vascular disease | ||
| history of major macrovascular disease (n [%]) | 1798 (32.3) | 1792 (32.2) |
| history of myocardial infarction (n [%]) | 678 (12.2) | 656 (11.8) |
| history of stroke (n [%]) | 502 (9.0) | 520 (9.3) |
| history of microvascular eye disease (n [%])d | 389 (7.0) | 404 (7.3) |
| Other major risk factors | ||
| current smoker (n [%]) | 804 (14.4) | 878 (15.8) |
| serum total cholesterol (mmol/L; mean [SD])e | 5.2 (1.2) | 5.2 (1.2) |
| serum HDL cholesterol (mmol/L; mean [SD])e | 1.3 (0.3) | 1.3 (0.4) |
| serum HbA1c concentration (%; mean [SD]) | 7.5 (1.6) | 7.5 (1.6) |
| body weight (kg; mean [SD]) | 78.3 (16.8) | 78.0 (16.8) |
| BMI (kg/m2; mean [SD]) | 28.3 (5.2) | 28.3 (5.1) |
| waist-to-hip ratio (mean [SD]) | 0.93 (0.08) | 0.93 (0.08) |
| Region of origin (n [%]) | ||
| Europe | 2539 (45.6) | 2544 (45.7) |
| Asia | 2070 (37.2) | 2066 (37.1) |
| Treatment (n [%]) | ||
| ACEIs | 2402 (43.1) | 2388 (42.9) |
| ARBs | 289 (5.2) | 320 (5.7) |
| calcium channel blockers | 1669 (30.0) | 1758 (31.6) |
| β blockers | 1344 (24.1) | 1385 (24.9) |
| diuretics | 1260 (22.6) | 1247 (22.4) |
| antiplatelets | 2597 (46.6) | 2601 (46.7) |
| lipid-modifying drugs | 1938 (34.8) | 1996 (35.8) |
Vital status was known at the end of follow-up for all but 15 patients.18 The mean systolic BP (SBP) during follow-up was 134.7 mmHg in patients assigned active treatment and 140.3 mmHg in patients assigned placebo (P < 0.0001); mean diastolic BP (DBP) levels were 74.8 and 77.0 mmHg in the two groups, respectively (P < 0.0001). Mean body weight decreased by 0.3 kg in patients assigned active and increased by 0.2 kg in patients assigned placebo treatment (P < 0.0001), but there were no differences with respect to glycosylated hemoglobin (6.9%) or total cholesterol levels (5.0 mmol/L) at study completion. At the end of follow-up, 4081 (73%) patients assigned active treatment and 4143 (74%) patients assigned placebo were adherent to study medication, whereas concomitant BP-lowering drugs were used more by patients on placebo than by those on active treatment (Table 2).
Table 2.
Concomitant BP-lowering treatment at the end of follow-upa
| Variable | End of Follow-up (n [%]) | |
|---|---|---|
| Perindopril-Indapamide | Placebo | |
| Open-label perindopril | 2128 (44.5) | 2591 (54.9) |
| Other ACEIs | 232 (4.9) | 213 (4.5) |
| ARBs | 453 (9.5) | 618 (13.1) |
| β blockers | 1492 (31.2) | 1671 (35.4) |
| Calcium antagonists | 1531 (32.0) | 2040 (43.2) |
| Thiazide diuretics | 158 (3.3) | 217 (4.6) |
| Other diuretics | 673 (14.1) | 749 (15.9) |
| Other BP-lowering drugs | 463 (9.7) | 638 (13.5) |
| Any BP-lowering drugs | 3634 (74.0) | 4024 (82.7) |
Values for serum creatinine and urinary albumin-creatinine ratio (UACR) were available at baseline for 10,996 (99%) and 10,640 (95.5%) patients, respectively. Nineteen percent of participants had an estimated GFR (eGFR) <60 ml/min per 1.73 m2. Microalbuminuria was present in 26% and macroalbuminuria in 4% of the participants. The presence of microalbuminuria or macroalbuminuria was independently associated with higher risks for developing end-stage kidney disease, cardiovascular events, cardiovascular death, and death from any cause (all P < 0.03; Table 3).
Table 3.
Adjusted risks of end-stage kidney disease, macrovascular events, cardiovascular death, and all-cause death by baseline albuminuria levela
| End Point | Normoalbuminuria (n = 7877) | Micro- or Macroalbuminuria (n = 3263) | HR (95% CI) |
|---|---|---|---|
| End-stage kidney diseaseb | 19 (0.2%) | 27 (0.8%) | 1.99 (1.08 to 3.70) |
| Macrovascular eventsc | 585 (7.4%) | 415 (12.7%) | 1.61 (1.42 to 1.84) |
| Cardiovascular death | 239 (3.0%) | 229 (7.0%) | 2.07 (1.72 to 2.50) |
| All-cause death | 496 (6.3%) | 383 (11.7%) | 1.70 (1.48 to 1.96) |
Effect of Randomized Treatment on Renal Outcomes
A total of 1243 (22.3%) patients assigned active treatment and 1500 (26.9%) patients assigned placebo developed the composite renal outcome of new-onset microalbuminuria, new-onset nephropathy, doubling of serum creatinine above 200 μmol/L, or end-stage kidney disease (hazard ratio [HR] 0.79; 95% confidence interval [CI] 0.73 to 0.85; P < 0.0001; Table 4). On this basis, one such event would be prevented among every 20 (95% CI 15 to 30) patients assigned active treatment for a 5-yr period.
Table 4.
Incidence of renal end pointsa
| End Point | Perindopril- Indapamide (No. of Events/Patient [%]) | Placebo | HR (95% CI) | P | NNT |
|---|---|---|---|---|---|
| Progression of nephropathy | |||||
| all renal events | 1243/5569 (22.3) | 1500/5571 (26.9) | 0.79 (0.73 to 0.85) | <0.0001 | 20 |
| progression of ≥1 albuminuria stage | 1179/5436 (21.7) | 1442/5412 (26.6) | 0.78 (0.72 to 0.84) | <0.0001 | 18 |
| new-onset microalbuminuria | 1094/3995 (27.4) | 1317/3991 (33.0) | 0.79 (0.73 to 0.86) | <0.0001 | 16 |
| new-onset macroalbuminuria | 114/5436 (2.1) | 163/5412 (3.0) | 0.69 (0.54 to 0.88) | 0.0027 | 97 |
| patients with normoalbuminuria | 25/3995 (0.6) | 35/3991 (0.9) | 0.71 (0.42 to 1.18) | 0.1841 | NA |
| patients with microalbuminuria | 89/1441 (6.2) | 128/1421 (9.0) | 0.69 (0.52 to 0.91) | 0.0074 | 32 |
| doubling of serum creatinine >200 μmol/L | 55/5569 (1.0) | 45/5571 (0.8) | 1.21 (0.81 to 1.79) | 0.3483 | NA |
| end-stage kidney diseaseb | 25/5569 (0.4) | 21/5571 (0.4) | 1.18 (0.66 to 2.11) | 0.5736 | NA |
| Regression of nephropathy | |||||
| regression of ≥1 albuminuria stage | 908/1638 (55.4) | 816/1625 (50.2) | 1.16 (1.06 to 1.28) | 0.0017 | 19 |
| regression to normoalbuminuria | 848/1638 (51.8) | 745/1625 (45.8) | 1.15 (1.04 to 1.27) | 0.0059 | 16 |
| patients with microalbuminuria | 797/1441 (55.3) | 698/1421 (49.1) | 1.15 (1.04 to 1.27) | 0.0067 | 16 |
| patients with macroalbuminuria | 51/197 (25.9) | 47/204 (23.0) | 1.08 (0.72 to 1.60) | 0.7146 | NA |
Active treatment also reduced the risk for progression of albuminuria among patients who had either normo- or microalbuminuria at baseline (HR 0.78; 95% CI 0.72 to 0.84; P < 0.0001) and the risk for new-onset microalbuminuria in patients with normoalbuminuria at baseline (HR 0.79; 95% CI 0.73 to 0.86; P < 0.0001; Table 4).
A total of 286 patients developed overt nephropathy, as defined by macroalbuminuria, during follow-up: 114 (2.1%) patients assigned active treatment and 163 (3.0%) patients assigned placebo (HR 0.69; 95% CI 0.54 to 0.88; P = 0.003). The relative risk reductions were of comparable magnitude in patients with normoalbuminuria or microalbuminuria at baseline (P = 0.3 for heterogeneity).
Regression of albuminuria was observed in more than half of all patients with micro- or macroalbuminuria at baseline, most of whom regressed to normoalbuminuria (Table 4). Proportionally more patients assigned active than assigned placebo treatment achieved restoration of normoalbuminuria (P = 0.006), with approximately similar absolute UACR declines according to treatment assignment (patients with microalbuminuria: from 55.0 to 10.6 μg/mg [active treatment] versus from 54.2 to 11.0 μg/mg [placebo]; patients with macroalbuminuria: from 490.6 to 9.0 μg/mg [active treatment] versus from 465.9 to 13.4 μg/mg [placebo]).
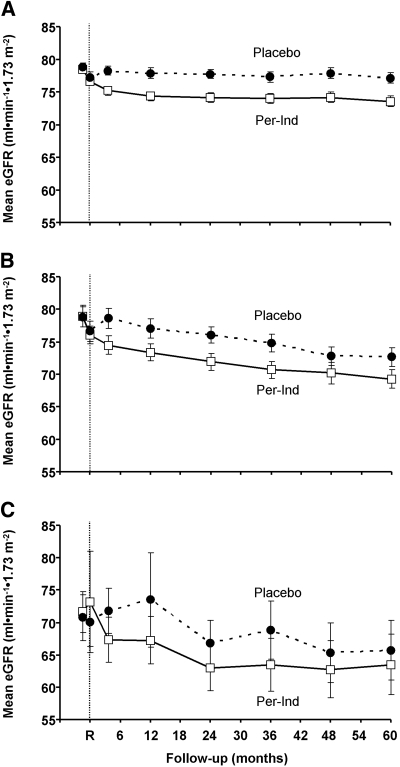
Active treatment reduced eGFR to a greater extent than placebo (P < 0.0001) in the first 4 mo (Figure 1), but annual declines in eGFR were similar in the active and placebo treatment groups thereafter (0.5 _versus_ 0.6 ml/min per 1.73 m2). This was true for patients with normoalbuminuria (0.1 _versus_ 0.0 ml/min per 1.73 m2; _P_ = 0.69), patients with microalbuminuria (1.1 _versus_ 1.4 ml/min per 1.73 m2; _P_ = 0.45), and patients with macroalbuminuria (1.5 _versus_ 2.7 ml/min per 1.73 m2; _P_ = 0.34). Both end-stage kidney disease and doubling of serum creatinine to a level >200 μmol/L were infrequently observed in ADVANCE, and the occurrence of these two outcomes was not significantly different between randomly assigned groups (Table 4).
Figure 1.
(A through C) Mean eGFR during follow-up according to treatment assignment in patients with normoalbuminuria (A), microalbuminuria (B), and macroalbuminuria (C) at baseline. R, randomization; Per-Ind, perindopril-indapamide treatment group.
Effects of Randomized Treatment in Patient Subgroups
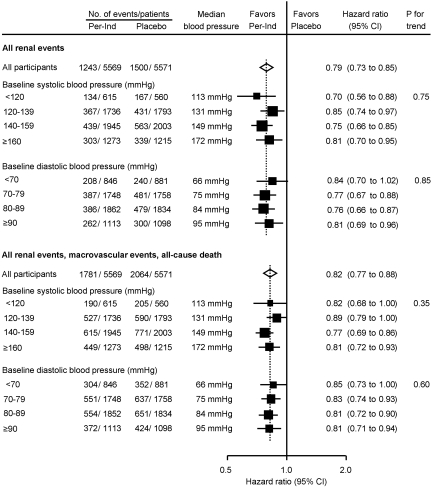
Median initial values of SBP for the four groups defined by baseline SBP ranged from 113 to 172 mmHg, and those of DBP for DBP subgroups ranged from 66 to 95 mmHg. Separately significant reductions in the occurrence of renal events and the composite of macrovascular events, renal events, and all-cause mortality were observed for almost every BP subgroup with no evidence of differences in the size of effect at different levels of baseline SBP or DBP (Figure 2). Even in the subgroup with lowest baseline SBP (<120 mmHg; median 113 mmHg), active treatment reduced the risk for the composite renal outcome in patients with normoalbuminuria (HR 0.78; 95% CI 0.61 to 0.99; P = 0.04) and in patients with higher levels of albuminuria (HR 0.37; 95% CI 0.16 to 0.83; P = 0.02) at baseline.
Figure 2.
Effect of randomized treatment on all renal events (top) and the composite of all renal events, macrovascular events, or all-cause mortality (bottom) according to baseline SBP and DBP. The center of the diamond represents the estimate and its width the 95% CI for overall treatment effect. Solid boxes represent estimates of treatment effect in subgroups; the centers of the boxes are placed at the estimates of effect, the areas of boxes are proportional to the number of events, and horizontal lines represent the corresponding 95% CIs. The vertical dotted line represents the point estimate for overall effect. The “P trend” tested the consistency of treatment effect in subgroups.
The same pattern was observed with regard to renal benefits for subgroups defined by commonly used BP thresholds. As such, comparable proportional benefits were achieved in patients with starting BP levels above (HR 0.79; 95% CI 0.73 to 0.85; P < 0.0001) and below 125/75 mmHg (HR 0.78; 95% CI 0.63 to 0.96; _P_ = 0.02; _P_ = 0.88 for heterogeneity) and similarly for patients with BP levels above and below 130/80 or 140/90 mmHg (both _P_ > 0.1 for heterogeneity).
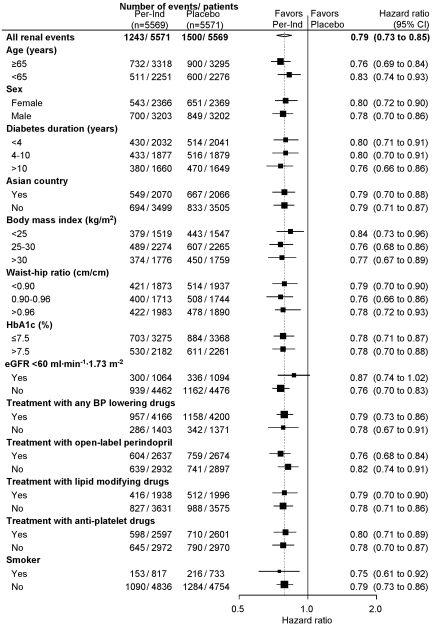
The benefits of active treatment with respect to renal events were also consistent across a broad range of participant subgroups defined by background use of a variety of concomitant therapies, age, gender, kidney function, diabetes duration, and glycemic control (all P > 0.1 for trend; Figure 3). Specifically, there was no evidence that background ACEI treatment modified the effects of study treatment.
Figure 3.
Effect of randomized treatment on all renal events in subgroups of participants defined by characteristics at baseline. For other conventions, see Figure 1. P > 0.1 for trend for all subgroup comparisons.
Very few serious adverse events were observed in ADVANCE. Overall, there were slightly higher rates of hypotension (0.5 versus 0.3%) and electrolyte disorders (0.7 versus 0.3%) in patients assigned active than in those assigned placebo treatment. These findings were similar for all baseline BP subgroups (data not shown).
Risk for Renal Events According to Achieved BP during Follow-up
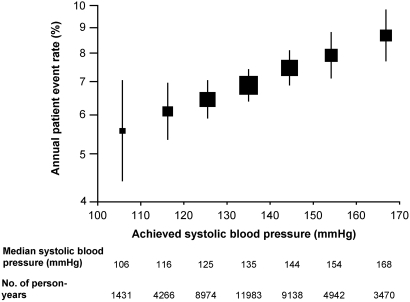
In age- and gender-adjusted analyses, there was an approximately log-linear relationship between the rate of any renal event and achieved SBP during follow-up. This association was unchanged after adjustment for a wide range of other risk factors (P < 0.0001 trend; Figure 4). The lowest risk for renal events was observed among those with a median achieved SBP of 106 mmHg. The same association was observed for achieved follow-up DBP levels within the range 62 to 93 mmHg (P = 0.008).
Figure 4.
Incidence of all renal events according to achieved BP levels, adjusted for age, gender, duration of diabetes, glycosylated hemoglobin, currently treated hypertension, history of macrovascular disease, electrocardiogram abnormalities (ventricular hypertrophy, Q waves, or atrial fibrillation), triglycerides, LDL cholesterol, HDL cholesterol, body mass index, current smoking, current alcohol use, and study drug. Solid boxes represent estimates of event rates, centers of which are placed at the intersection of the point estimate and median SBP value. Areas of the boxes are proportional to the number of events, and vertical bars represent 95% CI. The rate of all renal events was significantly associated with achieved SBP levels (P < 0.0001 for trend).
DISCUSSION
The main report from the BP-lowering part of ADVANCE showed that routine BP-lowering treatment with perindopril-indapamide produced a one-fifth reduction in the risk for developing a renal outcome among patients with type 2 diabetes.18 These analyses expand on that first report demonstrating both reductions in the risks for albuminuria progression and an increased likelihood of regression of albuminuria. We also found that widely used BP thresholds for treatment initiation do not differentiate between patient groups that will and will not derive benefit from BP lowering. The effects of active treatment on renal events in ADVANCE seem independent of the initial BP level with subgroup analyses showing clear benefits among individuals with baseline BP levels <120/70 mmHg. Separate observational analyses support these findings with the lowest risk for renal events observed among participants with achieved BP levels <110 mmHg systolic or 65 mmHg diastolic.
Most guidelines have progressively lowered the threshold BP level at which treatment is recommended for patients with diabetes, although evidence from randomized, controlled trials to support these recommendations is limited.10–13 The data presented here provide new evidence that goes toward justifying the lower BP thresholds used in this patient group with respect to renal disease. The consistency of benefits across the broad range of initial BP levels for both all renal events and the composite end point that included macrovascular events and all-cause mortality studied here suggests that the decision to use BP-lowering treatment in patients with diabetes should not be based on the BP level. Indeed, applying current recommendations to the ADVANCE trial population would have denied renoprotective treatment to one fifth of the ADVANCE study population, one quarter of whom developed a renal event during follow-up. This conclusion is directly compatible with that of the main study result: Treatment was shown to be safe and to reduce the risks for major vascular events and death to the same extent in patients with BP levels below or above 140/90 mmHg.18 Other data showing that BP lowering reduced the risks for macrovascular outcomes in patients with normotension and type 2 diabetes19 and in patients with normotension and coronary artery disease20 also support the more liberal use of BP-lowering treatment in high-risk individuals.
The benefits of active treatment were observed against a background of standard care that caused regression to normoalbuminuria in almost half of the patients who had micro- or macroalbuminuria and were assigned placebo. Treatments based on blockade of the renin-angiotensin system have generally been associated with remission rates of approximately 30% among patients with microalbuminuria,6,21 although rates up to 64% have been reported with combination drug therapy.22 Both optimal levels of glycemic control and lipid modification and a protocol that permitted open-label perindopril treatment to all patients may have contributed to the high rate of albuminuria regression in ADVANCE.
In ADVANCE, levels of GFR declined acutely after the institution of BP-lowering therapy before stabilizing. This pattern is the typical response to treatment with agents that block the renin-angiotensin system.23 For the remainder of the study, GFR declined at lower than anticipated rates24 in patients with normoalbuminuria and at slightly higher rates in those with micro- or microalbuminuria with no effect of treatment assignment detectable. Similar acute reductions in GFR, without seeming benefit during 3 to 4 yr of follow-up, have been reported with the use of ACE inhibition in other populations with more severe forms of kidney disease.25,26 In those studies, however, the group assigned to the lower BP target did have a reduction in the risk for kidney failure after prolonged follow-up.27 Given that the risk for end-stage kidney disease is directly and strongly related to the level of albuminuria,28,29 that reductions in albuminuria are associated with reductions in the risk for end-stage kidney disease,30 and that remissions to normoalbuminuria predict an attenuated decline in kidney function,21 beneficial effects of the ADVANCE regimen on such outcomes would be anticipated in the longer term. Moreover, levels of albuminuria are strongly associated with risks for cardiovascular events and death (Table 3), which would be positively affected by treatment that reduces albuminuria.31
At the end of follow-up, patients who were randomly assigned to active treatment had lost 0.5 kg of body weight relative to patients who were randomly assigned to placebo. This difference is suggestive of modest volume depletion possibly as a result of the diuretic component of the fixed combination drug used in ADVANCE and the more limited use of diuretics by patients who were randomly assigned to placebo treatment. However, because instructions to reduce body weight could be provided in relation to the intensive glucose control arm of the study32 and no data were collected on intake or excretion of sodium, this remains speculative. Although the diuretic component is likely to have contributed to the BP-lowering and antiproteinuric effect of the fixed combination perindopril-indapamide,33 ADVANCE was not designed and is therefore not able to determine the separate effects of its components, the results of which can reliably be attributed only to the combination as a whole. Moreover, BP lowering per se is probably more important than the means by which such lowering is achieved.10–13,34 The strong and continuous association we and others16,17 observed between achieved BP and renal events supports this assertion. The absence of a lower limit of BP below which the risks for renal events did not continue to decline reemphasizes the broad range of individuals with diabetes who could derive benefit from BP-lowering therapy.
These analyses show that routine treatment with a fixed combination of perindopril and indapamide significantly reduced the risk for renal disease progression in a large representative population of patients with type 2 diabetes. In absolute terms, this simple, well-tolerated treatment strategy prevented one renal event among every 20 patients who were treated for 5 yr, with no selection of patients on the basis of BP or BP-lowering treatment. These data therefore suggest that BP lowering may be considered routinely for the prevention of renal complications in all patients with diabetes, regardless of the baseline BP level.
CONCISE METHODS
ADVANCE is a randomized factorial trial of BP and glucose-lowering regimens. The ADVANCE study methods have been previously published35 and are described here in brief. The findings reported here relate to the double-blind BP-lowering component of the trial, which ended in June 2007. The results of the glucose-lowering arm have been reported separately.32
Participants
A total of 11,140 individuals who had type 2 diabetes and were aged ≥55 yr were enrolled from 215 centers in 20 countries. Patients with diabetes were potentially eligible when they had evidence of a substantially elevated risk for vascular disease, as described in detail elsewhere.18 Of note, there were no inclusion or exclusion criteria with respect to BP, albuminuria rate, renal function, or use of BP-lowering agents. Approval for the trial was obtained from each center's institutional review board, and all participants provided written informed consent.
Study Treatment
Eligible participants were randomly assigned to either fixed-combination perindopril-indapamide (2 mg/0.625 mg) or matching placebo after a 6-wk run-in period during which all patients received active treatment. Study treatment dosages were doubled after 3 mo so that participants were receiving either perindopril-indapamide 4 mg/1.25 mg or matching placebo. Other treatments were continued at the discretion of the responsible physician, except that ACEIs other than perindopril were substituted with open-label perindopril at a dosage of 2 or 4 mg/d, which could be prescribed at any time through to the end of follow-up, thereby ensuring that the maximum recommended dosage of 8 mg was not exceeded. Use of thiazide or thiazide-like diuretics was not permitted18; however, if at any time a definite indication arose for a thiazide or an ACEI other than perindopril 4 mg/d, study treatment could be withdrawn and open-label therapy provided. There were neither limitations nor instructions formulated with respect to the use of concomitant treatments, diets, or other lifestyle interventions during follow-up related to the BP-lowering comparison, which all remained at the discretion of the responsible physician.
Follow-up and Assessments
Participants were seen at 3, 4, and 6 mo after randomization and subsequently every 6 mo. Mean participant follow-up was 4.3 yr. At each study visit, BP was recorded as the mean of two measurements made in the seated position using an automated sphygmomanometer (Omron HEM-705 CP; Omron, Kyoto, Japan). Measurement of UACR was performed on spot urine samples at 24, 48, and 60 mo after randomization and at the end of follow-up. Serum creatinine was measured 4 and 12 mo after randomization and subsequently at yearly intervals. Both UACR and serum creatinine levels were measured at local laboratories. The Modification of Diet in Renal Disease (MDRD) equation was used to calculate eGFR.36
Outcomes
The main outcome for this analysis was a composite of renal events defined by new-onset microalbuminuria (UACR 30 to 300 μg/mg), new-onset nephropathy (new-onset macroalbuminuria defined as UACR >300 μg/mg, which required confirmation by a second sample), doubling of serum creatinine to >200 μmol/L, or end-stage kidney disease (defined as requirement for renal replacement therapy or renal death). Other outcomes included separate components of the main outcome; progression of albuminuria, defined as the worsening of at least one albuminuria stage (from normoalbuminuria to either micro- or macroalbuminuria or from micro- to macroalbuminuria); regression of albuminuria, defined as improvement of at least one albuminuria stage; and the composite of renal events, nonfatal macrovascular events (nonfatal stroke or nonfatal myocardial infarction), or all-cause mortality.
Statistical Analysis
The effects of randomized treatment on primary and secondary end points were estimated from unadjusted Cox proportional hazard models, based on the intention-to-treat principle. For participants with more than one outcome event during follow-up, survival time to the first relevant end point was used in each analysis. Participants were censored at their date of death or, for those still alive at the end of follow-up, the date of their last clinic visit before the termination of this study arm. Patients with unknown vital status were censored when they were last known to be alive. Relative risk reductions are described in the text and figures as percentage reductions ([1 − hazard ratio] × 100). Differences between randomized groups in BP during follow-up were estimated from linear mixed models.37 Numbers needed to treat were calculated as reciprocals of the absolute risk differences with their normally approximated 95% CIs.38
Separate estimates for treatment effects were calculated for subgroups of participants defined by demographic factors, ancillary treatments, and standard microvascular and macrovascular risk factors at study entry. For BP, participants were grouped into two sets of the following four ordinal categories: SBP <120, 120 to 139, 140 to 159, and ≥160 mmHg and DBP <70, 70 to 79, 80 to 89, and ≥90 mmHg. Trends for treatment effects assessed as both categorical and continuous variables were tested by adding interaction terms to the relevant Cox models.
Associations between achieved BP levels and renal outcomes were assessed using the pooling of repeated observations method.39,40 Briefly, each participant's follow-up period was divided into a series of intervals defined by the 2-yr visits. The presence or absence of the relevant outcome was documented during each interval and coupled to the time-weighted average of BP levels recorded during each interval. Missing follow-up BP values at any one visit (8.2% missing for all visits) were imputed by using the BP values recorded during the previous visit. The participants generated 28,969 intervals with follow-up SBP and DBP for the intervals ranging from 78 to 241 mmHg and from 41 to 121 mmHg, respectively. These intervals were then divided into seven ordinal achieved follow-up BP categories, separated by 10 mmHg for SBP and by 5 mmHg for DBP. Incidence rates of the relevant outcome were estimated using Poisson log-linear regression model that included risk factors for the development of chronic kidney disease.41 Trends in relationships between achieved BP levels and the risk for outcomes were tested using each median value of BP for BP categories. The SAS 9.1 for Windows (SAS Institute, Cary, NC) was used to perform all statistical analyses. All P values were calculated from two-tailed tests of statistical significance with a type I error rate of 5%.
DISCLOSURES
S.M. and J.C. hold research grants from Servier as principal investigators for ADVANCE. B.E.d.G., V.P., A.P., A.C., B.N., N.P., S.H., M.C., M.M., B.W., P.H., G.M., P.G., D.E.G., S.M., and J.C. have received lecturing fees from Servier.
Acknowledgments
This work was presented at the scientific meeting of the American Diabetes Association; June 6 through 10, 2008; San Francisco, CA; and published in abstract form (Diabetes 57[Suppl 1]: A218–A29, 2008).
ADVANCE was funded by grants from Servier and the National Health and Medical Research Council of Australia. The sponsors had no role in the design of the study, data collection, data analysis, data interpretation, and the writing of the manuscript. Study data were not made available to the sponsors. The Management Committee, whose membership did not include any sponsor representatives, had final responsibility for the decision to submit for publication. The first four authors had full access to the data of the study and take responsibility for the accuracy of the analysis.
The following are members of the writing committee of the ADVANCE Collaborating Group: Bastiaan E. de Galan, Vlado Perkovic, Toshiharu Ninomiya, Avinesh Pillai, Anushka Patel, Alan Cass, Bruce Neal, Neil Poulter, Stephen Harrap, Carl-Erik Mogensen, Mark Cooper, Michel Marre, Bryan Williams, Pavel Hamet, Giuseppe Mancia, Mark Woodward, Diederick Grobbee, Stephen MacMahon, and John Chalmers. All members of the ADVANCE Collaborating Group have been listed in full previously.18
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.US Renal Data System: USRDS Annual Data Report, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2007
- 2.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR: Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 63: 225–232, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Ritz E, Orth SR: Nephropathy in patients with type 2 diabetes mellitus. N Engl J Med 341: 1127–1133, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Ravid M, Brosh D, Levi Z, Bar-Dayan Y, Ravid D, Rachmani R: Use of enalapril to attenuate decline in renal function in normotensive, normoalbuminuric patients with type 2 diabetes mellitus: A randomized, controlled trial. Ann Intern Med 128: 982–988, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Ruggenenti P, Fassi A, Ilieva AP, Bruno S, Iliev IP, Brusegan V, Rubis N, Gherardi G, Arnoldi F, Ganeva M, Ene-Iordache B, Gaspari F, Perna A, Bossi A, Trevisan R, Dodesini AR, Remuzzi G: Preventing microalbuminuria in type 2 diabetes. N Engl J Med 351: 1941–1951, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P: The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 345: 870–878, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: Results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 355: 253–259, 2000 [PubMed] [Google Scholar]
- 8.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I: Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A: 2007 ESH-ESC practice guidelines for the management of arterial hypertension: ESH-ESC Task Force on the Management of Arterial Hypertension. J Hypertens 25: 1751–1762, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Standards of medical care in diabetes: 2008. Diabetes Care 31[Suppl 1]: S12–S54, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ: Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42: 1206–1252, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Bakris GL, Williams M, Dworkin L, Elliott WJ, Epstein M, Toto R, Tuttle K, Douglas J, Hsueh W, Sowers J: Preserving renal function in adults with hypertension and diabetes: A consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis 36: 646–661, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, Shulman NB, Stamler J: Blood pressure and end-stage renal disease in men. N Engl J Med 334: 13–18, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 317: 703–713, 1998 [PMC free article] [PubMed] [Google Scholar]
- 16.Bakris GL, Weir MR, Shanifar S, Zhang Z, Douglas J, van Dijk DJ, Brenner BM: Effects of blood pressure level on progression of diabetic nephropathy: Results from the RENAAL study. Arch Intern Med 163: 1555–1565, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Pohl MA, Blumenthal S, Cordonnier DJ, De Alvaro F, Deferrari G, Eisner G, Esmatjes E, Gilbert RE, Hunsicker LG, de Faria JB, Mangili R, Moore J Jr, Reisin E, Ritz E, Schernthaner G, Spitalewitz S, Tindall H, Rodby RA, Lewis EJ: Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: Clinical implications and limitations. J Am Soc Nephrol 16: 3027–3037, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Patel A, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, Harrap S, Poulter N, Marre M, Cooper M, Glasziou P, Grobbee DE, Hamet P, Heller S, Liu LS, Mancia G, Mogensen CE, Pan CY, Rodgers A, Williams B: Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): A randomised controlled trial. Lancet 370: 829–840, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Schrier RW, Estacio RO, Esler A, Mehler P: Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int 61: 1086–1097, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Nissen SE, Tuzcu EM, Libby P, Thompson PD, Ghali M, Garza D, Berman L, Shi H, Buebendorf E, Topol EJ: Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: The CAMELOT study—A randomized controlled trial. JAMA 292: 2217–2225, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Gaede P, Tarnow L, Vedel P, Parving HH, Pedersen O: Remission to normoalbuminuria during multifactorial treatment preserves kidney function in patients with type 2 diabetes and microalbuminuria. Nephrol Dial Transplant 19: 2784–2788, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Fogari R, Corradi L, Zoppi A, Lazzari P, Mugellini A, Preti P, Rinaldi A: Addition of manidipine improves the antiproteinuric effect of candesartan in hypertensive patients with type II diabetes and microalbuminuria. Am J Hypertens 20: 1092–1096, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Hollenberg NK: Renal response to angiotensin-converting enzyme inhibition. Am J Cardiol 49: 1425–1429, 1982 [DOI] [PubMed] [Google Scholar]
- 24.Lindeman RD, Tobin J, Shock NW: Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 33: 278–285, 1985 [DOI] [PubMed] [Google Scholar]
- 25.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G: The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med 330: 877–884, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Wright JT Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG: Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the AASK trial. JAMA 288: 2421–2431, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Sarnak MJ, Greene T, Wang X, Beck G, Kusek JW, Collins AJ, Levey AS: The effect of a lower target blood pressure on the progression of kidney disease: Long-term follow-up of the modification of diet in renal disease study. Ann Intern Med 142: 342–351, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Rossing K, Christensen PK, Hovind P, Tarnow L, Rossing P, Parving HH: Progression of nephropathy in type 2 diabetic patients. Kidney Int 66: 1596–1605, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Keane WF, Brenner BM, de Zeeuw D, Grunfeld JP, McGill J, Mitch WE, Ribeiro AB, Shahinfar S, Simpson RL, Snapinn SM, Toto R: The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: The RENAAL study. Kidney Int 63: 1499–1507, 2003 [DOI] [PubMed] [Google Scholar]
- 30.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM: Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: Lessons from RENAAL. Kidney Int 65: 2309–2320, 2004 [DOI] [PubMed] [Google Scholar]
- 31.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM: Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 110: 921–927, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R Travert F: Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358: 2560–2572, 2008. 18539916 [Google Scholar]
- 33.Mogensen CE, Viberti G, Halimi S, Ritz E, Ruilope L, Jermendy G, Widimsky J, Sareli P, Taton J, Rull J, Erdogan G, De Leeuw PW, Ribeiro A, Sanchez R, Mechmeche R, Nolan J, Sirotiakova J, Hamani A, Scheen A, Hess B, Luger A, Thomas SM: Effect of low-dose perindopril/indapamide on albuminuria in diabetes: Preterax in albuminuria regression: PREMIER. Hypertension 41: 1063–1071, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Turnbull F, Neal B, Algert C, Chalmers J, Chapman N, Cutler J, Woodward M, MacMahon S: Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: Results of prospectively designed overviews of randomized trials. Arch Intern Med 165: 1410–1419, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Rationale and design of the ADVANCE study: A randomised trial of blood pressure lowering and intensive glucose control in high-risk individuals with type 2 diabetes mellitus. Action in Diabetes and Vascular Disease: PreterAx and DiamicroN Modified-Release Controlled Evaluation. J Hypertens Suppl 19: S21–S28, 2001 [PubMed] [Google Scholar]
- 36.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW: Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Verbeke G, Molenberghs G: Linear Mixed Models for Longitudinal Data, New York, Springer, 2000
- 38.Woodward M: Epidemiology: Study Design and Data Analysis, Boca Raton, Chapman and Hall/CRC Press, 2005
- 39.Cupples LA, D'Agostino RB, Anderson K, Kannel WB: Comparison of baseline and repeated measure covariate techniques in the Framingham Heart Study. Stat Med 7: 205–222, 1988 [DOI] [PubMed] [Google Scholar]
- 40.Arima H, Chalmers J, Woodward M, Anderson C, Rodgers A, Davis S, Macmahon S, Neal B: Lower target blood pressures are safe and effective for the prevention of recurrent stroke: The PROGRESS trial. J Hypertens 24: 1201–1208, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Taal MW, Brenner BM: Predicting initiation and progression of chronic kidney disease: Developing renal risk scores. Kidney Int 70: 1694–1705, 2006 [DOI] [PubMed] [Google Scholar]