COGNITIVE BEHAVIORAL STRESS MANAGEMENT EFFECTS ON PSYCHOSOCIAL AND PHYSIOLOGICAL ADAPTATION IN WOMEN UNDERGOING TREATMENT FOR BREAST CANCER (original) (raw)
. Author manuscript; available in PMC: 2010 Jul 1.
Published in final edited form as: Brain Behav Immun. 2008 Sep 20;23(5):580–591. doi: 10.1016/j.bbi.2008.09.005
Abstract
BACKGROUND
A diagnosis of breast cancer and treatment are psychologically stressful events, particularly over the first year after diagnosis. Women undergo many demanding and anxiety-arousing treatments such as surgery, radiation and chemotherapy. Psychosocial interventions that promote psychosocial adaptation to these challenges may modulate physiological processes (neuroendocrine and immune) that are relevant for health outcomes in breast cancer patients.
METHODS
Women with Stage 1 – 3 breast cancer recruited 4 – 8 weeks after surgery were randomized to either a 10-week group-based cognitive behavioral stress management (CBSM) intervention or a 1-day psychoeducational control group and completed questionnaires and late afternoon blood samples at study entry and 6 and 12 months after assignment to experimental condition.
RESULTS
Of 128 women initially providing psychosocial questionnaire and blood samples at study entry, 97 provided complete data for anxiety measures and cortisol analysis at all time points, and immune assays were run on a subset of 85 of these women. Those assigned to a 10-week group-based CBSM intervention evidenced better psychosocial adaptation (lower reported cancer-specific anxiety and interviewer-rated general anxiety symptoms) and physiological adaptation (lower cortisol, greater Th1 cytokine [interleukin-2 and interferon-γ production and IL-2:IL-4 ratio) after their adjuvant treatment compared to those in the control group. Effects on psychosocial adaptation indicators and cortisol appeared to hold across the entire 12-month observation period. Th1 cytokine regulation changes held only over the initial 6-month period.
CONCLUSIONS
This intervention may have facilitated a “recovery or maintenance” of Th1 cytokine regulation during or after the adjuvant therapy period. Behavioral interventions that address dysregulated neuroendocrine function could play a clinically significant role in optimizing host immunologic resistance during a vulnerable period.
INTRODUCTION
Psychosocial Adaptation to Breast Cancer
The American Cancer Society estimated 178,480 new cases and over 40,000 deaths from invasive breast cancer (BCa) last year (American Cancer Society 2007). Localized BCa can be treated by surgery, radiation, and/or chemotherapy, and survival rates for localized and regional disease are 97% and 78%, respectively. Diagnosis of BCa and subsequent treatment are clearly stressful. Over the first year after diagnosis women undergo many demanding and anxiety-arousing treatments such as surgery, radiation and chemotherapy. Dealing with these events requires a significant amount of adaptational energies and individuals differ widely in their ability to manage this period of time (Bloom et al., 1987) (Penman et al., 1977) (Irvine et al., 1991) (van't Spijker et al., 1997) (Cella and Tross, 1986).
Intrusive thoughts about the diagnosis and its treatment have been documented as a common experience in women with BCa, prompting some to claim that many patients experience a post-traumatic stress disorder (Jacobsen et al., 1998). Prior to receiving adjuvant therapy these women deal with anticipatory anxiety over how treatment will affect their bodies, while during and after treatment they can experience physical complications of these treatments, residual psychological strain of cancer diagnosis, shifts in social support and fear of recurrence and death (Weisman & Worden, 1976) Thomas, Madden, & Jehu, 1987) (van der Pompe, Antoni, & Heijen, 1998), (Andersen, Karlsson, Anderson, & Tewfik, 1984), (Morrow, Roscoe, Hickok, Andrews, & Matteson, 2002), (Spencer et al., 1999b). Persisting cognitive and affective symptoms of anxiety during cancer treatment can compromise many emotional and physical aspects of quaility of life. Thus anxiety symptoms are an important indicator of psychosocial adaptation in women with BCa and were a central focus of this study.
Physiological Adaptation to Breast Cancer
While there is a long history of studies tying distress and other psychosocial factors to quality of life in breast cancer (Holland, 1998) there is controversy regarding the influence of such factors on disease progression and survival after BCa diagnosis and treatment (Antoni & Lutgendorf, 2007). One mechanism proposed to explain the association between psychosocial factors and disease outcomes in BCa involves neuroendocrine and immunologic regulation, and more recently tumor growth processes related to angiogenesis and tissue invasion (Antoni, Lutgendorf, Cole et al., 2006). Since neuroendocrine and immune regulation may be negatively affected by distress and anxiety (Segerstom, 2004) (Taylor, Repetti, & Seeman, 1997) (Levy et al., 1990) it is plausible that distress-related neuroendocrine changes may account for the effects of psychosocial adaptation on health outcomes in women undergoing treatment for BCa (Antoni, Lutgendorf, Cole et al., 2006).
Periods of chronic or continuing levels of high stress and anxiety are associated with decrements in cellular immune functions that help prevent infectious disease and metastasis (Andersen, Kiecolt-Glaser, & Glaser, 1994) (Andersen et al., 1998) (Herbert & Cohen, 1993a; Herbert & Cohen, 1993b). Andersen et al.’s (Andersen et al., 1994) biobehavioral model relates stress to disease course via biologic, psychological, and behavioral paths relevant to cancer patients. Accordingly, cancer diagnosis and treatment induce acute and chronic stress, which may be accompanied by negative health behaviors as well as dysregulations in neuroendocrine and immune functioning. Animal work demonstrates that stress-induced neuroendocrine and immunologic changes may contribute (directly and synergistically) to local and metastatic disease progression (Thaker et al., 2006; Ben-Eliyahu et al., 1999). After surgical treatment of tumors, optimal cellular and natural immunity may be important in eliminating residual disease and micrometastases. Some have posited that among patients receiving surgical treatments for cancer, intact perioperative immune function is involved in tumor control (Ben-Eliyahu, 2003). This suggests the value of identifying stressed surgical cancer patients during the vulnerable peri-surgical period who might benefit from stress reduction interventions to improve health outcomes (Shakhar & Ben-Eliyahu, 2003).
Effects of Psychosocial Interventions on Physiological Processes in Cancer Patients
If psychosocial and physiological adaptation processes run in parallel among women treated for BCa it follows that psychosocial interventions designed to promote psychosocial adaptation may also facilitate physiological adaptation with possible implications for health outcomes. However, establishing reliable associations between psychosocial and physiological indicators can be challenging in the midst of active treatment for cancer. A major methodological challenge here is the potentially confounding effects of cancer treatments on physiological variables such as cortisol and cellular immune function indicators (van der Pompe et al., 1998). The effects of adjuvant therapy vary significantly over short periods and there is inter-individual variation in neuroimmunological responses that may be modified by exposure to surgery and treatments such as chemotherapy, radiation, hormonal, immunomodulator treatments, and anti-emetic corticosteroid treatments (van der Pompe et al., 1998). Importantly, not all BCa patients receive standardized adjuvant regimens with clear beginning or ending points (van der Pompe et al., 1998). Some ways to deal with this group of methodological issues are: (1) to time baseline data collection to be at a circumscribed point after surgery yet before the onset of adjuvant therapy; (2) to delay follow-up measurements until after the completion of adjuvant therapies; and (3) to carefully assess and statistically control for adjuvant treatment including chemotherapy, radiation, endocrine therapy and immunotherapy received.
Physiological measures in psychosocial intervention research with BCa patients have included immunologic outcome measures that lacked specificity and it is unclear what role the variables may play in patient disease outcomes. For instance, some studies have focused on the effects on relaxation on non-specific immune indicators such as lymphocyte counts (Schedlowski et al., 1994). More recent studies have tested the effects of psychosocial interventions on functional measures such as lymphocyte proliferation to phytohemagglutinin (PHA) (van der Pompe, Antoni, & Heijen, 1997) (Andersen et al., 2004), (McGregor et al., 2004) though the mechanisms underlying these effects remain unclear. One way to examine how these forms of intervention affect immune cell functioning is to examine their effects on the regulation of cell-signaling cytokines that can direct the functioning of T-lymphocytes and other important effector cells, such as natural killer (NK) cells, known to be relevant in cancer (Whiteside and Herberman, 1989).
Cytokine Regulation and Breast Cancer
The past decade has seen significant work in immunotherapy with response modifiers such as the cytokines interleukin 2 (IL-2) and interferon-gamma (IFN-γ) in the treatment of different cancers (DeVita, Hellman, & & Rosenberg, 1997). Cytokine regulation may play a role in disease outcomes in BCa patients through their role in tumor surveillance. These include what are referred to as T-lymphocyte Helper-Type 1 and 2 (Th1 and Th2) cytokines, which are known to help regulate lymphocyte proliferation and cytotoxicity. Th1 cytokines IFN-γ and IL-2 play a major role in upregulating cytotoxicity. Lymphokine-activated killer (LAK) cells—T cells that have been stimulated with substances such as IL-2 or IFN-γ—may be more cytotoxic than resting NK cells against a wider variety of tumor cells, including breast cancer (Whiteside et al., 1989) (Baxevanis et al., 1993). These two cytokines are largely produced in-vivo by T-helper (CD4+) type -1 lymphocytes.
In-vitro treatment of peripheral blood leukocytes with IL-2 in Stage I-III BCa patients provided a dose-dependent enhancement of their NK activity, to levels higher than those of healthy controls (Konjevic & Spuzic, 1993). Low-dose subcutaneous IL-2 based immunotherapy significantly increased NK (CD56+CD3−) cells, and NK/LAK lysis of MCF-7 antigen in BCa patients following autologous transplantation. This suggests that IL-2 stimulation may be an important in-vivo phenomenon for conferring immunologic surveillance in BCa patients and may do so by selectively increasing specific subpopulations of NK cells (Miller et al., 1997). IFN-γ directly inhibits the growth of certain cancer cells (Woodworth, Lichti, Simpson, Evans, & DiPaolo, 1992) and poorer prognosis and tumor recurrence in some cancers is associated with a lower number of IFN-γ mRNA copies in tumor biopsy specimens (Tartour et al., 1998). In sum, IL-2 and IFN-γ may work together to promote surveillance of tumor cells and may play a role in disease recurrence after treatment.
Other work shows that Th2 cytokines (IL-4, IL-5 and IL-10) may antagonize these Th1 effects. Th2 cytokines such as IL-4 can antagonize the actions of IL-2 and IFN-γ on cell-mediated immune functions such as lymphocyte proliferation and cytotoxicity (Saito et al., 1996). (Roussel, Gingras, Grimm, Bruner, & & Moser, 1996). Several studies have identified a predominance of Th2 cytokines over Th1 cytokines at tumor sites (Elsasser-Beile, von Kleist, & Martin, 1992) and have shown suppressed production of IL-2 and IFN-γ across various cancer populations (Elsasser-Beile et al., 1992) (Elsasser-Beile et al., 1993) (Fischer et al., 1997). Since longer-term health sequelae of breast cancer after surgery may depend to a considerable extent on the ability of the immune system to recognize and kill residual tumor cells, the regulation of Th1 and Th2 cytokines may be critical.
Importantly, stressful events have been linked to decreases in Th1 cytokines such as IFN-γ and IL-2, reduced IL-2 receptor expression, and increases in some Th2 cytokines such as IL-4 and IL-5 in students undergoing examination stress (Kang & Fox, 2001). Animal studies show that psychosocial stress can compromise the ability of tumor-specific CD4 cells to produce IFN-γ and IL-2 thus possibly facilitating tumor progression via suppressed cytotoxic abilities (Ben Eliyahu, Page, Yirmiya, & Shakhar, 1999). Recent work has also indicated that emotional stress is associated with decreases in NK activity and related TH1 cytokines IL-2 and IFN-γ in PHA-stimulated PBMC supernatants among daughters of women with BCa. Decreased NK activity and IL-2 production were associated with increased cortisol and norepinephrine suggesting neuroendocrine mediation (Cohen et al., 2002).
Lymphocytes express receptors for beta2 adrenergic ligands, glucocorticoids, ACTH and corticotrophin-releasing hormone. The immunosuppressive effects of these hormones are well established and hypothesized to be mediated by alterations in cytokine production and communication (Smith, Hobish, Lin, Culig, & & Keller, 2001) (Reichlin, 1993) (Savino & Dardenne, 1995). Exposure to chronic stress leads to chronically elevated cortisol release, which in turn may promote a shift from a Th1 to a Th2 cytokine response (DeRijk et al., 1997) (Elenkov et al., 1999b). Alterations in cortisol regulation relates to disruption in immune parameters in BCa that may promote cancer disease progression (Touitou et al., 1995), though the extent to which these associations are mediated by Th1 and Th2 cytokine regulation remain unclear.
Present Study
The body of work just reviewed establishes associations among psychological states, cortisol and cell-mediated immune function, and the value of considering the roles of Th1 and Th2 cytokines in stress-immune relations in women with BCa. Our prior work established that among women recruited in the weeks after surgery for BCa, a 10-week group-based cognitive behavioral stress management (CBSM) intervention was associated with improvements in psychosocial adaptation (Antoni, Wimberly, Lechner et al., 2006; Antoni, Lechner, Kazi et al., 2006) as well as reductions in serum cortisol (Cruess et al., 2000b) and one indicator of T-lymphocyte functioning—lymphocyte proliferation to T-cell receptor stimulation (McGregor et al., 2004)—immune changes that have been observed using similar interventions in breast cancer patients under treatment (e.g., Andersen et al., 2004). Are the immune effects of psychosocial interventions explained in part by alterations in Th1 and Th2 cytokines? The present study tested the effects of a 10-week CBSM intervention on indicators of psychosocial adaptation (cancer-specific anxiety and general anxiety symptoms), serum cortisol, and in-vitro Th1 and Th2 cytokine production in women undergoing treatment for non-metastatic breast cancer. We hypothesized that women assigned to the CBSM condition would report improvements in psychosocial adaptation (reduced anxiety), and would show parallel changes in physiological adaptation (reduced afternoon serum cortisol levels, and greater Th1 and less Th2 cytokine production) as they moved through treatment for breast cancer.
METHOD
Participants
Recruitment focused on women diagnosed with non-metastatic breast cancer (BCa) who were approached in the weeks after surgery but before adjuvant therapy had begun. Potentially eligible participants received a letter and study brochure from their physician or the local chapter of the American Cancer Society and were asked to contact the study team if they were interested in participating. The study was described as an opportunity for women under treatment for breast cancer to learn stress management. Interested women contacted the study coordinator and spoke with a female assistant who screened for eligibility. Participants were eligible if they had surgery for histologically-confirmed primary breast cancer 4 – 8 weeks prior to initial assessment. Women with Stage IV breast cancer were ineligible. Additional exclusion criteria included a previous diagnosis of cancer (except minor skin cancer), age over 70, panic disorder, suicidality, non-fluent in English, and unwillingness to be randomized. Participants were also excluded if they had a co-morbid major medical condition, were taking medications with known effects on endocrine or immune functioning, or if they began adjuvant treatment before study entry. These criteria were established to create a reasonably homogeneous sample of individuals with similar disease status and medical treatments, and individuals who would be able to comprehend assessment materials, and regularly attend intervention sessions.
All women were part of a larger trial of CBSM (Antoni, Kazi, Lechner et al., 2006). Only women who had complete cortisol and psychological data were included in the present study. For this substudy, 97 women provided blood samples for cortisol assays at all three time points, and for subsequent analyses involving cytokines, the sample sizes differed slightly. Women from the parent study (Antoni et al., 2006c) who did not provide blood samples (and were thus not included in this substudy) were more likely to have received chemotherapy during their treatment course than those in the present study (n = 77 and 60, respectively), χ2 (1) 11.67, p = 0.001; to have received radiation therapy (n = 80 and 68, respectively), χ2 (1) 8.47, p = .004; and to have presented with a greater stage of disease (those without blood samples: Stage: 0 = 15, I = 35, II = 47, III = 15 vs those with blood samples: Stage: 0 = 23, I = 56, II = 45, III = 4, χ2 (3) 11.93, p < .001). Women who did not provide blood samples had a greater number of positive lymph nodes (M = 2.37, SD = 4.00) than women in the present study (M = 0.87, SD = 2.40), t(237) = 3.57, p < 0.01. Finally, women from the parent study who did not provide blood samples were more likely to have been unemployed than those in the present study, χ2 (1) = 6.65, p = 0.01. Thus women composing the sample used in the present study represented a group with less advanced disease than those women from the parent sample who did not provide blood samples. This difference may be due to the fact that women with more advanced disease were likely to have received neo-adjuvant (pre-surgery) treatments that excluded them from the present substudy of biological outcomes. These advanced cases are also more likely to have received adjuvant therapes after surgery and to have been unemployed due to health reasons. These factors singly or in combination may have made them less willing or able to provide blood samples for the study. Thus caution is in order in generalizing the present results to all women being treated for non-metastic breast cancer. Despite these differences in disease status, there were no differences between women in the parent study who did not provide samples vs those who participated in the present study for any other clinicopathological (e.g., ER/PR status), demographic (e.g., age, education, marital status) or outcome variable assessed at Time 1. Demographic and disease-related information is presented in Table 1.
Table 1.
Demographic and Medical Characteristics of the Sample at Study Entry
| Control | Intervention | Statistic | p | |
|---|---|---|---|---|
| Age at diagnosis | 49.31 (8.33) | 50.08 (7.48) | F(1,126)=0.30 | .58 |
| Years education | 15.31 (2.42) | 15.97 (2.77) | F(1,126)=2.07 | .15 |
| Ethnicity | χ_2_(2,N=128)=0.11 | .95 | ||
| Non-Hispanic | 45 (69.2%) | 45 (71.4%) | ||
| White | ||||
| Hispanic | 15 (23.1%) | 13 (20.6%) | ||
| African American | 5 (7.7%) | 5 (7.9%) | ||
| Married/partnered | 42 (64.6%) | 40 (63.5%) | χ_2_(1,N=128)=0.02 | .90 |
| Employed | 54 (83.1%) | 50 (79.4%) | χ_2_(1,N=128)=0.29 | .59 |
| Stage | χ_2_(3,N=128)=2.36 | .50 | ||
| 0 | 11 (16.9%) | 12 (19.0%) | ||
| I | 32 (49.2%) | 24 (38.1%) | ||
| II | 21 (32.3%) | 24 (38.1%) | ||
| III | 1 (1.5%) | 3 (4.8%) | ||
| Positive Lymph Nodes | 0.37 (0.86) | 1.38 (3.24) | F(1,126)=5.89 | .02 |
| Time since surgery (days) | 42.59 (22.84) | 37.33 (19.71) | F(1,125)= 1.93 | .17 |
| Type of surgery | χ_2_(1,N=128)=0.76 | .38 | ||
| Lumpectomy | 37 (56.9%) | 31 (49.2%) | ||
| Mastectomy | 28 (43.1%) | 32 (50.8%) | ||
| Radiation | 32 (49.2%) | 36 (57.1%) | χ_2_(1,N=128)=0.80 | .37 |
| Chemotherapy | 25 (38.5%) | 35 (55.6%) | χ_2_(1,N=128)=3.75 | .05 |
| Hormonal | 5 (7.9%) | 2 (3.2%) | χ_2_(1,N=125)=1.31 | .25 |
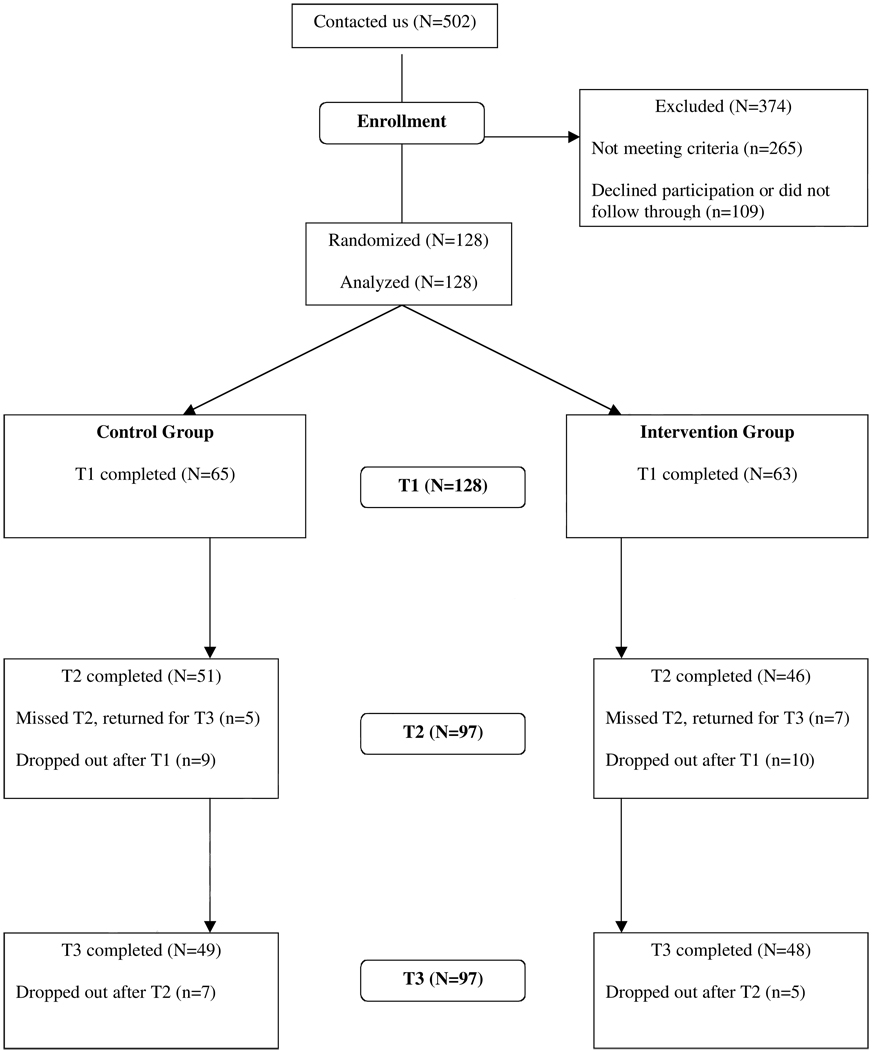
Outcome variables were collected at study entry (Time 1), and 6 and 12 months after entry (Times 2 and 3). Attrition is described in the CONSORT flow diagram in Figure 1. (for CONSORT criteria, Altman, Schulz, Moher, Egger, Davidoff , Elbourne et al., 2001). Attrition did not differ significantly by condition at Time 2, χ2 (1) = .40, p > .54, or Time 3, χ2 (1) = 1.21, p > .38. Those stopping between Times 2 and 3 did not differ from completers on any psychosocial or physiological outcome variable or on any medical or demographic variable, all _F_s < 1, _p_s > .3,.
Figure 1.
Experimental design and flow diagram of participation
Procedure
Participants completed a first assessment (described below) upon recruitment, corresponding to 4 – 8 weeks post-surgery, and were randomly assigned to intervention or control condition on a 1:1 basis within cohorts of an average of 14 women. The intervention (described below) occurred over a 10-week period. Women in the control group were invited to attend a 1-day seminar at the midpoint of this 10-week period. A second assessment (Time 2) occurred 3 months after the intervention ended (6 months after the initial assessment). A third assessment (Time 3) occurred 6 months later. Thus the period of follow-up spanned approximately one year after randomization.
Participants in both conditions met in groups of up to eight in a room equipped with flat couches for muscle relaxation exercises, and a table and chairs for group discussions. Both the intervention and the 1-day seminar (control condition) were co-led by female post-doctoral fellows and advanced pre-doctoral trainees in clinical psychology. Leaders rotated between intervention and control cohorts. Assessments were administered by persons not conducting the intervention with that cohort.
Intervention
The CBSM intervention was delivered in a closed, structured group format in weekly sessions for a period of 10 weeks (Antoni, 2003). CBSM techniques were presented using didactic explanations, in-session experiential exercises (role-playing) and out-of-session assignments (e.g., practicing relaxation and completing homework assignments examining responses to stressors encountered during the week). Women received recordings of one of their group leaders reciting relaxation and guided imagery exercises, which they were strongly encouraged to use on a daily basis. The intervention was focused on teaching stress reduction techniques such as rational thought replacement with specific modules to assist women in learning to reframe their appraisals of stressful situations, improve their coping strategies and better match their coping choices to the nature of these situations, and learn interpersonal skills to optimize their communication skills and use of social resources. The intervention facilitators encouraged emotional expression; taught methods to replace doubt appraisals with more confident appraisals (Beck & Emery, 1975); honed skills in anxiety reduction (by muscle relaxation and relaxing imagery, Bernstein & Borkovec, 1973) and taught skills in conflict resolution and emotional expression (via assertion training, Fensterheim & Baer, 1975 and anger management). Participants averaged 7.08 sessions attendance (SD = 2.58, median = 8, range 1–10). All interventionists participated in a 10-week long training protocol. All sessions were videotaped and treatment fidelity was insured by two clinical psychologists who monitored the videotapes at multiple points during each cohort. Any deviations in intervention protocol were communicated to interventionists at the point that they were detected during weekly supervision meetings.
Control seminar
Control participants received a condensed, educational version of the information from the intervention, lasting 5–6 hours, at approximately the midpoint of the 10-week period of the intervention group within their cohort. It lacked the therapeutic group environment and emotional support, opportunities to role-play the techniques and receive group feedback, the opportunity to observe other members model new appraisals, relaxation techniques, and coping strategies, and lacked the weekly home practice experience. This procedure has at least two benefits over a no-treatment (usual care) control. First, by providing information relevant to breast cancer experiences, it provides something perceived as useful to participants and thereby diminishes differential attrition in the control condition, a major pitfall of no-treatment control. Second, since this condition provided information related to adjustment in an group-based educational seminar it created a stronger test of the intervention’s impact than would a usual care control. In fact this control condition provides a dose of most ingredients of the intervention, thus creating a rather stringent test of the intervention’s effects. Unfortunately, this procedure did not control for attention time, with less than one-third the contact hours of the CBSM groups (20 vs. 6 hrs).
Physiological Adaptation Indicators
Sample collection
Blood samples (30–40 ml) were taken 4 – 8 weeks after surgery, before adjuvant therapy started. All participants were requested to refrain from alcohol use, recreational drug use and caffeinated beverages on the day of the blood draw. Any women reporting use of antibiotics or allergy medications were rescheduled for their assessments until one week after the cessation of these products. Blood was drawn in heparinized Vacutainer tubes (Becton-Dickinson, USA) between 4:00 pm and 6:30 pm, in order to avoid circadian variations, kept overnight at room temperature and then processed the following morning. Our previous studies showed that the immune assays measured here did not vary between samples immediately processed and those processed after holding overnight at room temperature (Blomberg et al., under review).
Isolation of peripheral blood mononuclear cells (PBMC)
Blood was separated on Ficoll density gradient (Lymphocyte Separation Medium, ICN Biochemicals, USA). PBMC were collected from the gradient interface, washed twice with Phosphate Buffered Saline (PBS) (Gibco-BRL, USA) and resuspended in RPMI-1640 (Gibco-BRL, USA), supplemented with 10% fetal bovine serum, 100 U/ml penicillin (Gibco-BRL, USA), 100 ug/ml streptomycin (Gibco-BRL, USA), 1mM Sodium Pyruvate (Gibco-BRL, USA), 1mM non-essential amino acids (Gibco-BRL, USA) and 5 × 10−5 mM 2-mercaptoethanol, referred to as complete tissue culture media (cRPMI). Cell counts were performed by 0.4% Trypan blue dye exclusion and viability was always higher than 90%. All assays were performed with fresh (not frozen) samples (within 20 hours after the blood was drawn).
Cytokine production assay
PBMCs at a concentration of 2 × 106 cells/ml were stimulated with plate-bound anti-CD3 antibody (OKT-3, 1 µg/ml) routinely or plate bound anti-CD3 plus soluble anti-CD28 (Pharmingen, 2 µg/ml) in initial conditions and supernatants were collected at different times of stimulation, then frozen at −20°C until analysis. IL-2, IFN-γ, and IL-4 were measured using ELISA kits following the manufacturer recommendations; optimal conditions for stimulation and supernatant collection were established for each cytokine. Kinetic studies revealed that optimal stimulation times were 48 hours for IFN-γ and 24 hours for IL-2 and IL-4. All kits were from Biosource International except for IL-2 (R and D Systems).
Cortisol
Serum cortisol was used as a measure of physiological stress. We collected 10 ml of peripheral venous blood via venipuncture in red-topped vacutainer tubes containing no anticoagulants. Blood tubes were incubated in a water bath at 37°C for 30 minutes, following which they were placed on ice for approximately 1 hour until centrifuged for 5 minutes at 2000 rpm. The serum was collected and frozen at –20°C until analysis. Cortisol levels were measured by competitive ELISA (Diagnostic Systems Laboratories, Webster, TX). All assays were run neat, unless the results were outside of the standard curve, in which case dilutions were assayed.
Psychosocial Adaptation Indicators
Intrusive thoughts about breast cancer
Impact of Event Scale (IES; Horowitz, Wilner, & Alvarez, 1979). This measure is a 15-item self-report instrument assessing degree of thought intrusion and avoidance about particular life situations. Response options coded 0, 1, 3, and 5. The Impact of Event Scale has two subscales, of which the Intrusion scale was used in the present study. The intrusion scale measures the extent of unwanted thoughts and images related to the stressor (e.g., “I had trouble falling asleep or staying awake because pictures or thoughts about it came into my mind”) over the past two weeks. Our average alpha reliability across time points and conditions was 0.86.
Clinician rating of anxiety
(Hamilton Rating Scale for Anxiety ; Schwab, Bialow, Clemmons, & Holzer, 1967). Anxiety symptoms experienced over the past week were measured by the Hamilton Rating Scale for Anxiety (HAM-Anxiety). Procedures for scoring these clinical ratings followed the format of the structured interview guide. High interrater reliability, internal consistency, and discriminant validity have been reported to be adequate for this scale. Assessors who coded the ratings were trained by a clinical psychologist with extensive training in the use of this measure.
Negative mood
We measured negative mood with the Affects Balance Scale (ABS; Derogatis, 1996). This measure incorporates scales to assess negative affect (i.e., depression, hostility, guilt, and anxiety) along with scales to measure positive emotions. Items are self-descriptive adjectives, and respondents indicate the extent to which they have been feeling the emotional quality the item portrays during the past week. For the present study a negative affect score was computed by summing the depression, hostility, guilt and anxiety scales. The reliability of ABS subscales is α > .85 for all scales.
Statistical Analysis
All variables were analyzed for outliers and normality. Logarithmic transformation was applied to all physiological variables to achieve normal distributions. The transformed variables were then used in all calculations. Statistics were conducted with SPSS version 15 (Chicago, IL). Tests of intervention effects included Repeated Measures Analysis of Co-variance (RANCOVA) using carefully selected covariates. The key analysis was the test for a group X time interaction. Significant group X time interactions were followed up with simple effects tests to examine the effects of condition across different sets of time points. Several variables were examined as potential covariates/control variables based on the extant literature and available social, demographic, health behavior, medical, and treatment related variables (Kiecolt-Glaser & Glaser, 1988; Andersen, Kiecolt-Glaser & Glaser, 1994; van der Pompe et al., 1998). Potential social and demographic covariates included age, ethnicity/race, educational attainment, income, insurance status, marital status, employment, and religion. Health behaviors were physical activity, caffeine intake, cigarette use, alcohol use, and illegal drug use. Potential covariates in the medical/treatment category were assessed at study entry (and at additional time points as indicated) and included type of surgical procedure, chemotherapy (every time point), radiation therapy (every time point), stage (American Joint Committee on Cancer [AJCC] criteria), number of positive lymph nodes, tamoxifen (every time point), estrogen receptor (ER) status and progesterone receptor (PR) status. Covariates were determined using correlations for continuous data and t-test or ANOVA for categorical data and were examined for a statistically significant relationship to each dependent variable. Covariates were considered significantly correlated with neuroendocrine and immune variables at p < .05. Covariates are specified in each of the repeated measures ANOVA analyses described below.
RESULTS
Characteristics of the sample, by condition, are in Table 1. Comparisons revealed no significant difference between conditions with the exception of a greater number of positive lymph nodes collected during surgery in women assigned to the intervention condition. However, the number of positive nodes was not associated with any outcome variable at any time point nor with changes in outcome measures between time points, all p’s > .25.
Intervention Effects on Psychosocial Adaptation
Intrusive thoughts about breast cancer
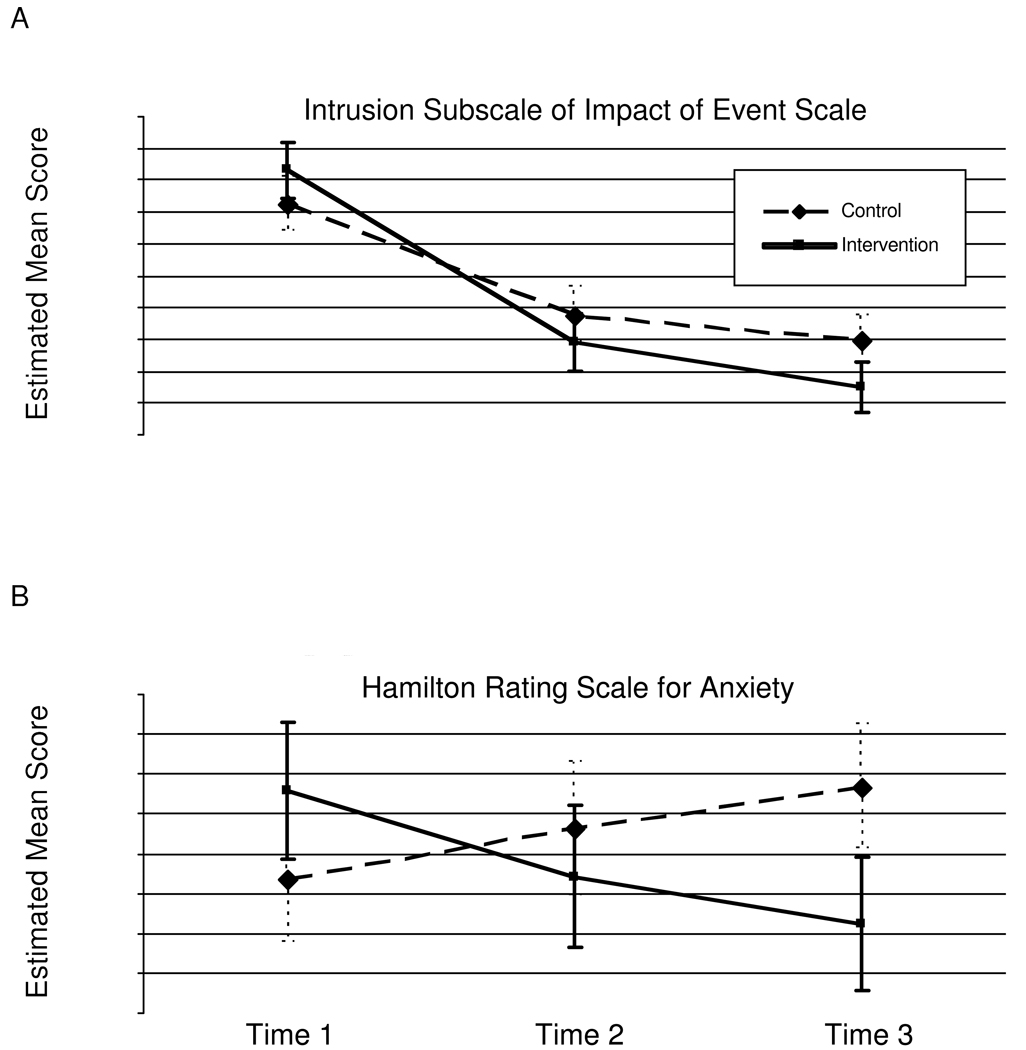
We examined whether psychological variables changed as a function of condition over the course of the study using RANCOVA (2: condition x 3: time). The omnibus group X time interaction test for IES-Intrusive thoughts was statistically significant, F (2,83) = 3.24, p <.05. When simple effects were examined, results showed a significant change from T1–T2 (p<.05), marginally significant change from T2–T3 (p<.08), and significant change from T1–T3 (p<.05). Control and intervention conditions did not differ at T1 or T2, however, the participants in the CBSM condition showed significantly lower IES-intrusive thoughts scores at T3. (See Figure 2a).
Figure 2.
Means (and SEM) for Impact of Event Scale Intrusive Thoughts scores (Panel A) and Hamilton Rating Scale-Anxiety scores (Panel B) in breast cancer patients assigned to the intervention or control condition at T1 (study entry),T2 (6-month follow-up), and T3 (12-month follow-up).
Rated anxiety symptoms
The repeated measures group by time interaction for HAM-Anx was statistically significant F(2,81) = 3.86, p<.05. Simple effects analyses revealed no discernable change from T1–T2, T2–T3 or T1–T3. The groups differed marginally at T1, whereby the control group had lower anxiety scores at baseline relative to CBSM participants. There were no group differences at T2. At T3, the CBSM group showed markedly lower anxiety scores compared to controls. See Figure 2b.
Negative affect
The group X time interaction for ABS-NEG was not statistically significant for change from T1–T3, F(2,83) < 1. Therefore, simple effects tests were not conducted.
Intervention Effects on Physiological Adaptation
Serum cortisol
The pattern of change in serum cortisol was analyzed using RANCOVA (2: condition x 3: time). The group by time effect was significant F(2,82)= 6.87, p<.01. The pattern of these results have been described in detail elsewhere (Phillips et al., in press). Briefly, results suggested that CBSM and control participants differed at T1 (Control mean = 1.92 (SE= .076) vs CBSM mean 2.16 (SE=.079)) and T3 (Control mean = 2.10 (SE= .074) vs CBSM mean 1.91 (SE=.076). The groups did not differ at T2 ((Control mean = 2.02 (SE= .073) vs CBSM mean 2.10 (SE=.075)).
IL-2
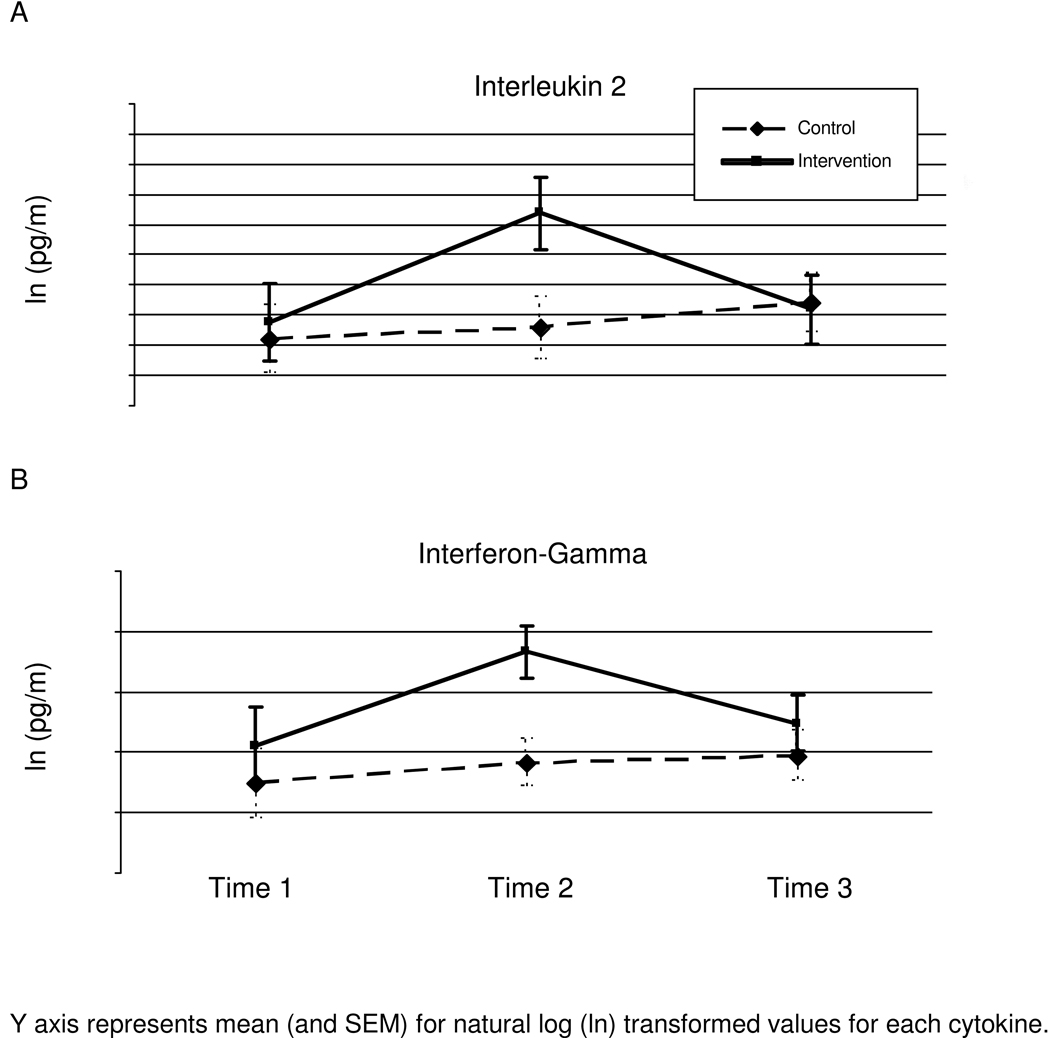
The group X time interaction (2: condition X 3: time (T1, T2, T3)) for IL-2 production was significant, F(2,44)= 3.323, _p_=.045, with baseline radiation therapy covaried. Pairwise comparison analyses revealed significant change over time between T1–T2 (mean difference=−.397, p <.05), however, mean differences did not differ between T2–T3 (mean difference= .236, ns) or T1–T3 (mean difference=−.161, ns). CBSM and control participants did not differ at T1 or T3, however, the groups diverged significantly at T2 t(49)=− 2.61, p<.05 (see Figure 3a).
Figure 3.
Natural-log (ln) transformed means (and SEMs) for production of IL-2 (Panel A) and IFN-γ (Panel B) in breast cancer patients assigned to the intervention or control condition at T1 (study entry), T2 (6-month follow-up), and T3 (12-month follow-up).
IFN-γ
The group X time interaction (2: condition X 3: time (T1, T2, T3)) for IFN-γ production was significant F(2,72)= 3.76, _p_=.028 with baseline radiation therapy covaried. Pairwise comparison analyses revealed significant change over time between T1–T2 (mean difference= −479, p<.05), however, mean differences did not differ for T2–T3 (mean difference= 0.27, ns) or T1–T3 (mean difference= −0.209, ns). CBSM and control participants did not differ at T1 or T3, however, the groups diverged significantly at T2 t(78)=− 2.67, p<.01 (see Figure 3b).
IL-4
The group X time interaction (2: condition (CBSM, control) X 3: time (T1, T2, T3)) for IL-4 production was not statistically significant F (2,49) < 1 (not pictured). Therefore no simple effects test were conducted.
IL-2:IL-4 ratio
The group X time interaction (2: condition X 3: time (T1, T2, T3)) for the ratio of IL-2 to IL-4 production was significant F(2,40)= 4.367, _p_=.019 with baseline radiation therapy covaried. Pairwise comparison analyses revealed no significant change over time between T1–T2 (mean difference=.06, ns), T2–T3 (mean difference=.04, ns), or T1–T3 (mean difference=.10, ns). CBSM and control participants did not differ at T1 or T3, however, the groups diverged at T2 (Control Mean = 2.47 (SE = .25) vs CBSM mean = 3.46 (SE=.33), t(44)=− 2.56, p<.05. (not pictured)
IFN-γ: IL-4 ratio
The group X time interaction (2: condition (CBSM, control) X 3: time (T1, T2, T3)) for the ratio of IFN-γ to IL-4 production was not statistically significant F(2,44) < 1. (not pictured). Therefore no simple effects were tested for this variable.
Lymphocyte subsets
There were no group X time effects for CD4, CD8, CD56, CD56+CD3+, or CD19 cell counts, all p’s > .10. Therefore changes in cytokine production were not likely due to alterations in cells involved in the stimulation assays.
Associations Among Psychosocial and Physiological Adaptation Indicators
We first examined relations among individual cytokine values collected at baseline, and 6- and 12-month follows-up. We observed significant positive correlations among IL-2, and γ-IFN at study entry, all p’s < .01. A similar pattern emerged at 6 and 12 month follow-up, all p’s < .05 - .01. Cytokine production was significantly associated over time as well. In lagged correlations IL-2 and IFN-γ values were all positively associated with their respective values at baseline, 6 and 12 months, p’s < .05 - .01.
To examine associations among changes in cytokines, and between changes in cortisol and changes in cytokines over time, we computed correlations between residualized changes scores in cortisol and cytokines between baseline and 6 months (T1 – T2), baseline and 12 months (T1 –T3) and 6 and 12 months (T2 – T3). (see Table 2). As can be seen, associations in changes among cytokines were significant and positive across each time frame. In terms of associations between cortisol changes and cytokine changes we found that T1 – T2 decreases in cortisol were not associated with cytokines changes in the T1–T2 period. This may be due to the fact that cortisol did not change significantly over the 6-month follow-up period. Increases in IL-2 from T1 to T2, were however associated with changes in cortisol over the final follow-up frame (T2 – T3) and over the entire study period (T1 – T3). These correlations are most in line with the intervention findings of this study wherein CBSM showed increases in IL-2 production that held only to the 6-month follow-up while decreases in cortisol were evident out to the 12-month follow-up period, The pattern for cortisol-IFN-γ changes was somewhat different. Cortisol decreases from T2 – T3 were associated with increases in IFN-γ from T1 to T3. Lower cortisol values at 12-month follow-up were also associated with greater IFN-γ production at 12 months, r =−.21, p < .05.
Table 2.
Pearson correlations between unstandardized residual change scores for physiological adaptation indicators between baseline and 6 months (t1–t2), 6 months and 1 year (t2–t3), and baseline and 1 year (t1–t3).
| IL2t1–t2 | IL2t2–t3 | IL2t1–t3 | IL4t1–t2 | IL4t2–t3 | IL4t1–t3 | IFNγt1–t2 | IFNγt2–t3 | IFNγt1–t3 | |
|---|---|---|---|---|---|---|---|---|---|
| IL2 t1–t2 | -- | ||||||||
| IL2 t2–t3 | −.13 | -- | |||||||
| IL2 t1-t3 | .28* | .91** | -- | ||||||
| IL4 t1–t2 | .48** | −.29* | −.11 | -- | |||||
| IL4 t2–t3 | −.09 | .24 | .18 | −.05 | -- | ||||
| IL4 t1–t3 | .20 | −.04 | .19 | .56** | .76** | -- | |||
| IFNγ t1–t2 | .53** | −.10 | .16 | .40** | .14 | .32* | -- | ||
| IFNγ t2–t3 | .04 | .31* | .29* | −.10 | .24 | .15 | −.01 | -- | |
| IFNγ t1–t3 | .34* | .24 | .39** | .12 | .28 | .27* | .54** | .84** | -- |
| cortisol t1–t2 | −.19 | −.12 | .10 | −.16 | .30* | .14 | .10 | .03 | .07 |
| cortisol t2–t3 | −.29* | −.04 | −.04 | −.02 | .04 | .06 | −.12 | −.19 | −.25* |
| cortisol t1–t3 | −.31* | −.08 | .04 | −.09 | .18 | .15 | −.02 | −.14 | −.17 |
Finally, we examined associations between individual changes in those physiological and psychosocial adaptation indicators that were affected by CBSM over the course of the study. Specifically, we sought to determine if the changes in IES-intrusive thoughts and Hamilton anxiety symptoms were associated with changes in IL-2, IFN-γ, IL-2/IL-4 ratio or cortisol . There were no significant associations between changes in these psychosocial and physiological parameters across similar time frames or in lagged (psychosocial → physiological) analyses. The lack of associations between hypothesized changes in psychosocial and immune/endocrine variables precluded our ability to test for mediation effects (Baron & Kenny, 1986).
DISCUSSION
Women confront much adversity in adapting to diagnosis and treatment for breast cancer. Breast cancer patients with poor cognitive coping skills and a negative outlook (Carver et al., 1993; Stanton et al., 1993) and fewer social resources (Alferi et al., 2001) experience greater anxiety and distress during the stressful period of treatment. How such adaptations can be modulated by psychosocial interventions that improve individuals’ outlook, coping skills and social support has been of interest to clinical researchers for several years (Antoni, 2003). Reviews based on intervention studies conducted in the past 10 years have revealed that psychosocial and relaxation-based interventions are helpful in terms of improving emotional adjustment and quality of life in cancer patients undergoing medical treatments (e..g., Luebbert, Dahme & Hasenbring, 2001; Meyer & Mark, 1995). In one meta-analysis, relaxation-based interventions (including progressive muscle relaxation, imagery, autogenics and other techniques) were found to be especially effective in reducing anxiety, tension, depression, and overall negative mood (Luebbert et al., 2001). Cognitive behavioral interventions have been shown to be effective in improving depression, anxiety, and quality of life in cancer patients, with anxiety reductions showing the largest effect sizes (Kissane et al; 2003; Edmonds, Lockwood & Cunningham, 1999; Larson et al., 2000; Trask, Paterson, Griffith, Riba, & Schwartz, 2003). Less is known about whether such experiences relate to physiological changes, which in turn could affect post-adjuvant physical status and possibly even reduce the risk of disease recurrence over the years after surgery and adjuvant therapy (Antoni & Lutgendorf, 2007). To address this knowledge gap, the present study sought to document whether a well-established psychosocial intervention modulates psychosocial adaptation in parallel with physiological adaptation in women undergoing treatment for BCa.
Previously we showed in two separate trials that a 10-week Cognitive Behavioral Stress Management (CBSM) intervention facilitated psychosocial adaptation in women being treated for BCa. These effects included decreased prevalence of clinically-elevated depression, less thought intrusion, anxiety symptoms, negative affect, and social disruption, and more benefit finding, positive affect, and positive states of mind (Antoni et al., 2001; Antoni, Wimberly, Lechner et al., 2006; Antoni, Lechner, Kazi et al., 2006). All of these findings appeared to hold up to 12 months. This intervention was also found to decrease afternoon levels of serum cortisol immediately after the completion of the intervention in a prior trial (Cruess et al., 2000), and up to 12 month follow-up in the women participating in the present study—results that were published previously (Phillips et al., in press). Women assigned to CBSM also showed greater cellular immune function (lymphocyte proliferative responses to anti-CD3 stimulation) at 3-month follow-up (McGregor et al., 2004). Other work similarly suggested that psychosocial interventions of this theoretical orientation, format and length were capable of decreasing self-reported stress and cortisol, and modulating immune parameters such as lymphocyte counts and lymphocyte proliferation (Schedlowski et al., 1994; Andersen et al., 2004). In one study the size of the immune effect (McGregor et al., 2004a) was greatest in women with the largest psychological improvements.
It is plausible that some of the immunological changes observed in these prior psychosocial intervention studies may have been explained by changes in Th1 and Th2 cytokine regulation, though few prior studies have shown that psychosocial interventions can affect cytokine regulation in cancer patients. A recently published study showed that women with early stage BCa who received a 8-week mindfulness-based stress reduction (MBSR) group intervention starting 10 days after surgery showed a better recovery of IFN-γ production, decreased late afternoon (4 – 6pm) plasma cortisol, and improved quality of life for up to one-month post-intervention compared to a non-MBSR (usual care) control group (Witek-Janusek, Albuquerque, Chroniak, Chroniak, Durazo-Arvizu & Mathews, in press). In another study, breast and prostate cancer survivors, who had completed treatment 3 months to 20 years prior, who were assigned to MBSR showed increases in Th1 cytokines IFN-γ and tumor necrosis factor (TNF) that were apparent at 6 – 12 month follow-up (Carlson, Speca, Patel & Faris, 2007). However, since patients in each of these studies were self-selected into treatment conditions one cannot assume that MBSR caused the changes observed over time. The present study sought to add to these findings by testing the effects of a 10-week group-based CBSM intervention on psychological, neuroendocrine and cytokine production indicators using a randomized controlled trial design.
First we found that women with BCa assigned to the CBSM intervention showed improvements in psychosocial adaptation evidenced by reductions in cancer-specific intrusive thoughts and general anxiety symptoms. Our confidence in these anxiety reduction effects is reinforced by the fact that they were evident in both a self-report measure and in an interviewer rating scale. Physiological adaptation effects associated with participation in the intervention included decreases in afternoon serum cortisol levels, which we have previously described in detail elsewhere (Phillips et al., in press). Our rationale for collecting blood samples in the late afternoon is detailed in that paper as well as the limitation in using this protocol. Briefly, we decided to use the 4 – 6 pm sampling window in order to (a) replicate the time frame used in a prior study of CBSM in breast cancer patients (Cruess et al., 2000) that we sought to expand upon; (b) because elevated PM cortisol levels may underlie the association between flat di-urnal cortisol ouput pattern and poorer health outcomes in breast cancer (Sephton, Sapolsky, Kraemer & Spiegel, 2000); and (c) because this was a time frame that best accommodated participants’ schedules and would likely lead to optimal compliance. Caution is in order in interpreting these single afternoon serum samples as being reflective of HPA activity as they represent total (bound and unbound) levels of cortisol rather than free cortisol levels, and fail to capture momentary fluctuations that vary with stressor exposure or across the diurnal cycle (Yehuda, 2003). Future work should investigate the effects of stress management on serial salivary samples (which reflect free cortisol levels) collected across the di-urnal cycle, which allow for the calculation of multiple indicators of cortisol output and regulation (Fekedulegn, Andrew, Burchfiel et al., 2007).
We also observed increases in the production of Th1 cytokines IL-2 and IFN-γ from stimulated PBMCs during the initial 6-month follow-up in the CBSM condition in contrast to apparent IL-2 and INF-γ decreases in those assigned to a psychoeducational control condition. Since most women underwent some form of adjuvant therapy during the time of the intervention these findings suggested that being in a CBSM group seemed to “buffer” women from the ongoing effects of adjuvant therapy. These effects were no longer evident and had receded to baseline levels at 12-month follow-up, a point well after adjuvant therapy had been completed. When we created a ratio of IL-2 to IL-4 production to reflect the Th1:Th2 balance, as hypothesized we found that women assigned to CBSM showed significant increases in the ratio of IL-2 to IL-4 production compared to controls who showed declines. These effects were also only evident between T1 and T2. Thus all of the effects of CBSM on immune effects were concentrated in the initial 6 month follow-up. During this period women received no further therapeutic contact once their 10 week groups had ended. It is plausible that had we offered a longer intervention or periodic maintenance sessions, effects on cytokine production may have held up longer.
This pattern of results—intervention-related decreases in psychological distress (anxiety) and cortisol levels over the entire 12-month period and intervention effects on Th1 cytokines out to 6 months only—are hard to explain. Explaining these disparities is challenging as the kinetics of immunologic indicators during and after adjuvant therapy for cancer is poorly understood. As a preliminary step it will be important to carefully map out the changes that occur in Th1 and Th2 cytokine production (and other immune parameters) during the typical course of adjuvant therapies for breast cancer patients and then catalog the time course for each cytokine change during each form of treatment. The present study is not equipped to do so.
Despite the fact that women who received the CBSM intervention showed decreases in anxiety, decreases in serum cortisol, and increases in Th1 cytokines there was no evidence that individual differences in psychosocial adaptation changes tracked in synchrony with physiological adaptation changes. It is likely that anxiety reduction alone may not be affecting the cytokine changes, but rather there may have been effects more proximal to intervention participation that are linked to cytokine changes. Specifically, it may be some combination of skills training (e.g., increased perceived efficacy in using stress management skills) or being in a supportive group (e.g., increased perceived social support and bonding with other cancer patients) that are tied more directly to Th1 cytokine production increases. Future work should examine how changes in CBSM specific skills and non-specific group processes track with anxiety, cortisol and immune changes over time in this population.
Our prior work testing CBSM in other populations (men with Human Immunodeficiency Virus [HIV] infection) has shown that cortisol reductions during CBSM parallel reductions in depressed affect but not decreases in anxiety, while reductions in anxiety during CBSM parallel reductions in norepinephrine (Antoni, 2003b). Although we measured changes in the negative affect in the present study, the measure used (ABS) did not focus on depressed mood exclusively and was not affected by CBSM. Since we did not measure norepinephrine in these breast cancer patients we are unable to test whether changes in anxiety were associated with changes in this neurohormone in the present study. This precludes our ability to test whether parallel CBSM-associated changes in psychosocial and endocrine variables established in prior work are also evident in breast cancer patients. We did however note that greater decreases in cortisol over the course of the study (from baseline to 12 month, and from 6 to 12 month follow-up) were associated with greater increases in Th1 cytokine production, but only up to the 6-month follow-up for IL-2 and up to the 12-month follow-up for IFN-γ It is possible that stress management effects on IL-2 production may have been greatest when patients were acutely recovering from their treatments (up to the 6 month follow-up), and that thereafter other phenomena (e.g., additional medical treatments) acted to decrease cytokine production in the direction of values evident at study entry. On the other hand, decreases in cortisol during the 6 – 12 month follow-up period were associated with increases in IFN-γ production from study entry to 12 month follow-up. The reasons for the discrepant findings for these two Th1 cytokines are unclear. As mentioned previously it will be helpful in future work to do fine-grained analyses of cytokine fluctuations that occur during and after the period of adjuvant therapy in cancer patients in order to better understand the clinical significance of the findings observed in the present study.
The most consistent immunologic effect of psychosocial interventions conducted to date with BCa patients has been increases in lymphocyte proliferation, suggesting that cell-mediated immune indices may be most sensitive to the stress-reducing effects of these interventions (van der Pompe et al., 1997; Andersen et al., 2004; McGregor et al., 2004 In related work one group demonstrated that an 8-week cognitive behavioral therapy intervention focused on improving sleep in BCa patients was associated with better subjective sleep, decreases in quality of life, and decreases in anxiety and depression (Savard, Simard, Ivers, & Morin, 2005a), and increases in IFN-γ production (Savard, Simard, Ivers, & Morin, 2005b). The present effects of CBSM on increases in Th1 cytokine production in women treated for Stage I – III BCa mirror these effects and may be relevant for health outcomes given prior associations between Th1-directed immune functions and disease progression in mice and BCa patients (Baxevanis, 1993; Dighe, Richards, Old, & Schreiber, 1994; Kaplan, Shankaran, Dighe, Stockert, Aguet, Old & Schreiber, 1998; Street, Cretney & Smyth, 2001; Whiteside & Herberman, 1989).
We also found that women assigned to CBSM showed reductions in serum cortisol, which are in line with prior studies of CBSM in BCa patients (Cruess et al., 2000). In other work, reductions in plasma cortisol paralleled a “normalization” of Th1 and Th2 cytokine production in breast cancer patients receiving MBSR intervention during treatment (Witek-Janusek et al., in press). Some work has suggested that alterations in cortisol regulation may predict survival in women with metastatic BCa (Sephton et al., 2000) though it is unclear what health consequences may be associated with cortisol changes in the present sample of non-metastatic patients. Glucocorticoids regulate a wide variety of cellular processes through glucocorticoid receptor-mediated activation or repression of target genes. Glucocorticoids such as cortisol may act in a synergistic fashion with catecholamines to facilitate cancer growth (for review see Antoni, Lutgendorf, Cole et al., 2006). It is thus plausible that stressful situations characterized by both elevated catecholamine and cortisol levels (e.g., uncontrollable stress) may have the greatest impact on cancer-related processes. It is reasonable then to suggest that interventions such as CBSM, which blends relaxation training with cognitive behavioral techniques, to diminish anxiety and perceived stress and restore a sense of control over life are helpful for women dealing with BCa and its treatment. These psychosocial changes may be able to modulate the production of these neuroendocrine substances and possibly their effects on not only cytokine regulation but also tumor growth processes. The present data support the provocative notion that interventions such as CBSM might be used in conjunction with adjuvant therapy to optimize immunologic recovery during critical periods of medical treatment that may be relevant for optimal disease surveillance (Shakhar & Ben-Eliyahu, 2003).
Caution should be exercised in interpreting these findings as evidence that CBSM can improve cancer outcomes, though recent work suggests that similar psychosocial interventions may improve health outcomes in BCa patients by decreasing emotional distress (Andersen et al., 2007). This study used in vitro cytokine production as an indicator of immune status. It is important to keep in mind that in-vitro production assays may not provide adequate indicators of the processes that occur in-vivo. Although recent work (e.g., Carlson, Speca, Patel & Faris, 2007; Witek-Janusek et al., in press) has also shown that psychosocial interventions are associated with increases in in-vitro Th1 cytokine production assays, it is unclear how well these assays represent in-vivo cytokine production and signaling. It is therefore unclear whether the changes in cytokine production observed in these studies are relevant to biological processes or clinical outcomes in women with breast cancer. In fact, the question of the relevance of peripheral indicators of the immune system as they relate to changes in the tumor environment remains controversial. While tumor-specific immune responses to immunotherapy have been demonstrated in-vivo, contemporary research focuses on subverting immune tolerizing mechanisms in tumors, supplementing immune functions, and suppressing tumor angiogenesis and growth (Zou, 2005). Future work should examine whether psychosocial interventions are capable of modulating some of these tumor-relevant processes and then tie the changes to neuroendocrine changes, and longer-term health outcomes by following cohorts of women with BCa over time.
Caution is also in order in interpreting the results presented herein as they are based upon a middle-class well-educated sample that was likely motivated to participate in health research. There was also evidence that this sample of women may have been more healthy than prior samples used to demonstrate the effects of CBSM on psychosocial adaptation in breast cancer patients (Antoni et al., 2006). Finally the use of repeated measure ANCOVA models in the present study eliminated all subjects who had missing data for the outcome studies under investigation. This resulted in analyses based on different sample sizes, and reflect “completer” analyses rather than intent to treat analyses. These factors could have biased our results. We considered using other analytic techniques that we and others have used elsewhere (e.g., Latent Growth Modeling; Muthen, 1997), which use Full Information Maximum Likelihood (FIML) for estimating parameters in the presence of missing data (Enders, 2006). However, we decided against using LGM due to the small sample size. Results should be considered with these limitations in mind.
Conclusion
The pattern of effects observed in the present study suggested that women with non-metastatic BCa offered a 10-week group-based CBSM intervention evidenced better psychosocial adaptation and physiological adaptation during and after their adjuvant treatment. Effects on psychosocial adaptation indicators (reduced cancer-specific and general anxiety) appeared to hold across the entire 12-month observation period. Th1 cytokine regulation changes were most pronounced in the initial 6-month period then returned to pre-intervention levels. Findings suggest that CBSM intervention may have facilitated a “recovery or maintenance” of Th1 cytokine regulation through the adjuvant therapy period. Psychosocial interventions that address dysregulated neuroendocrine function could play a clinically significant role in optimizing host resistance to cancer during this vulnerable period.
Acknowledgements
This study was supported by the National Cancer Institute R01-CA-064710 to MHA. We thank all the women who agreed to participate in this study at a time when they were dealing with so much else in their lives. We also acknowledge Janny Rodriquez for recruiting and scheduling women for this study, Alain Diaz, Maria Romero, and Juan Alvarez for conducting many of the immunologic assays, and Lynne Hudgins, B.S. for managing the fiscal aspects of the project. We also acknowledge the contribution of to Robert P. Derhagopian, MD, Sharlene Weiss, Ph.D., Alan S. Livingstone, MD, Frederick L. Moffat, Jr., MD, Jodeen E. Powell, MD, Eli Avisar, MD, Stefan Gluck, MD, PhD, Joyce Slingerland, MD, PhD, and the Dade County American Cancer Society for their help in recruiting participants, and to Maria Llabre, Ph.D. for statistical consultation. Finally we thank the Psychology graduate students and post-doctoral fellows Patricia Arena, Amy Boyers, Jennifer Culver, Sophie Guellati, Aisha Kazi, Jessica Lehman, Roselyn Smith, Vida Petronis, Tammy Sifre, Kenya urcuyo, Kurrie Wells, and Sara Wimberly who participated in screening, assessment and intervention activities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altman D, Schulz K, Moher D, Egger M, Davidoff F, Elbourne D, et al. The Revised CONSORT statement for reporting randomized trials: Explanation and Elaboration. Annals of Internal Medicine. 2001;134:663–694. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- Alferi SM, Carver CS, Antoni MH, Weiss S, Duran RE. An exploratory study of social support, distress, and life disruption among low-income Hispanic women under treatment for early stage breast cancer. Health Psychology. 2001;20:41–46. doi: 10.1037//0278-6133.20.1.41. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Cancer Statistics. 2007 www.cancer.org [on-line]
- Andersen BL, Farrar WB, Golden-Kreutz D, Kutz LA, MacCallum R, Courtney ME, et al. Stress and immune responses after surgical treatment for regional breast cancer. Journal of the National Cancer Institute. 1998;90:30–36. doi: 10.1093/jnci/90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL, Farrar WB, Golden-Kreutz DM, Glaser R, Emery CF, Crespin TR, et al. Psychological, behavioral, and immune changes after a psychological intervention: a clinical trial. Journal of Clinical Oncology. 2004;22:3570–3580. doi: 10.1200/JCO.2004.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL, Kiecolt-Glaser JK, Glaser R. A biobehavioral model of cancer stress and disease course. American Journal of Psychology. 1994;49:389–404. doi: 10.1037//0003-066x.49.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL, Farrar WB, Golden-Kreutz DM, Emery CF, Glaser R, Crespin T, Carson WE., III Distress reduction from a psychological intervention contributes to improved health for cancer patients. Brain, Behavior, and Immunity. 2007;21:953–961. doi: 10.1016/j.bbi.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL, Karlsson JA, Anderson B, Tewfik HH. Anxiety and cancer treatment: response to stressful radiotherapy. Health Psychology. 1984;3:535–551. doi: 10.1037//0278-6133.3.6.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH. Stress Management Intervention for Women with Breast Cancer. Washington D.C: American Psychological Association Press; 2003. (a) [Google Scholar]
- Antoni MH. Stress management effects on psychological, endocrinological and immune function in men with HIV: Empirical support for a psychoneuroimmunological model. Stress. 2003;6:173–188. doi: 10.1080/1025389031000156727. (b) [DOI] [PubMed] [Google Scholar]
- Antoni MH, Lehman JM, Kilbourn KM, Boyers AE, Culver JL, Alferi SM, et al. Cognitive-behavioral stress management intervention decreases the prevalence of depression and enhances benefit finding among women under treatment for early-stage breast cancer. Health Psychology. 2001;20:20–32. doi: 10.1037//0278-6133.20.1.20. [DOI] [PubMed] [Google Scholar]
- Antoni MH, Lutgendorf S. Psychosocial Factors and Disease Progression in Cancer. Current Directions in Psychological Science. 2007;16:42–46. [Google Scholar]
- Antoni MH, Lutgendorf S, Cole S, Dhabhar F, Sephton S, McDonald P, Stefanek M, Sood A. The influence of biobehavioral factors on tumor biology, pathways and mechanisms. Nature Reviews Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Wimberly S, Lechner S, Kazi A, Sifre T, Urcuyo K, Phillips K, Smith R, Petronis V, Guellati S, Wells K, Blomberg B, Carver CS. Stress Management Intervention Reduces Cancer-Specific Thought Intrusions and Anxiety Symptoms among Women Undergoing Treatment for Breast Cancer. American Journal of Psychiatry. 2006;163:1791–1797. doi: 10.1176/ajp.2006.163.10.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Kazi A, Lechner S, Wimberly S, Gluck S, Carver CS. How Stress Management Improves Quality of Life After Treatment for Breast Cancer. J. Consulting and Clinical Psychology. 2006;74:1143–1152. doi: 10.1037/0022-006X.74.6.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychology research: Conceptual, strategic and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Baxevanis CN, Reclos GJ, Gritzapis AD, Dedousis GV, Missitzis I, Papamichail M. Elevated prostaglandin E2 production by monocytes is responsible for the depressed levels of natural killer and lymphokine-activated killer cell function in patients with breast cancer. Cancer. 1993;72:491–501. doi: 10.1002/1097-0142(19930715)72:2<491::aid-cncr2820720227>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Beck AT, Emery G. Anxiety disorders and phobias: A cognitive perspective. New York: Basic Books; 1985. [Google Scholar]
- Bernstein B, Borkovec T. Progressive muscle relaxation training: A manual for the helping professions. Champaign, Ill: Research Press; 1973. [Google Scholar]
- Ben-Eliyahu S, Page G, Yimura R, et al. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int J. Cancer. 1999;80:880–888. doi: 10.1002/(sici)1097-0215(19990315)80:6<880::aid-ijc14>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Ben-Eliyahu S. The promotion of tumor metastasis by surgery and stress: Immunologial basis and implications for psychoneuroimmunology. Brain, Behavior and Immunity. 2003;17 suppl 1:S27–S36. doi: 10.1016/s0889-1591(02)00063-6. [DOI] [PubMed] [Google Scholar]
- Bloom JR, et al. Psychological response to mastectomy: A prospective comparison study. Cancer. 1987;59:189–196. doi: 10.1002/1097-0142(19870101)59:1<189::aid-cncr2820590136>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Carlson LE, Speca M, Patel KD, Faris P. One year pre-post intervention follow-up of psychological, immune, endocrine and blood prerssure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain, Behavior and Immunity. 2007;21:1038–1049. doi: 10.1016/j.bbi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Carver CS, Pozo C, Harris SD, Noriega V, Scheier MF, Robinson DS, et al. How coping mediates the effect of optimism on distress: a study of women with early stage breast cancer. Journal of Personality and Social Psychology. 1993;65:375–390. doi: 10.1037//0022-3514.65.2.375. [DOI] [PubMed] [Google Scholar]
- Cella DF, Tross S. Psychological adjustment to survival from Hodgkin’s disease. Journal of Consulting and Clinical Psychology. 1986;54:616–622. doi: 10.1037//0022-006x.54.5.616. [DOI] [PubMed] [Google Scholar]
- Cohen M, Klein E, Kuten A, Fried G, Zinder O, Pollack S. Increased emotional distress in daughters of breast cancer patients is associated with decreased natural cytotoxic activity, elevated levels of stress hormones and decreased secretion of Th1 cytokines. International Journal of Cancer. 2002;100:347–354. doi: 10.1002/ijc.10488. [DOI] [PubMed] [Google Scholar]
- Cruess DG, Antoni MH, McGregor BA, Kilbourn KM, Boyers AE, Alferi SM, et al. Cognitive-behavioral stress management reduces serum cortisol by enhancing benefit finding among women being treated for early stage breast cancer. Psychosomatic Medicine. 2000a;62:304–308. doi: 10.1097/00006842-200005000-00002. [DOI] [PubMed] [Google Scholar]
- DeRijk R, Michelson D, Karp B, Petrides J, Galliven E, Deuster P, et al. Exercise and circadian rhythm-induced variations in plasma cortisol differentially regulate interleukin-1 beta (IL-1 beta), IL-6, and tumor necrosis factor-alpha (TNF alpha) production in humans: high sensitivity of TNF alpha and resistance of IL-6. J.Clin Endocrinol.Metab. 1997;82:2182–2191. doi: 10.1210/jcem.82.7.4041. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. The Affects Balance Scale. Baltimore, MD: Clinical Psychometric Research; 1996. [Google Scholar]
- Fensterheim H, Baer J. Don’t say yes when you want to say no. New York: David McKay; 1975. [Google Scholar]
- DeVita V, Hellman S, Rosenberg S. Cancer: Principles and Practice of Oncology. 5th Edition. Philadelphia, PA: Lippincott-Raven; 1997. [Google Scholar]
- Edmonds CV, Lockwood GA, Cunningham AJ. Psychological responses to long term group therapy: A randomized trial with metastatic breast cancer patients. Psycho-Oncology. 1999;8:74–91. doi: 10.1002/(SICI)1099-1611(199901/02)8:1<74::AID-PON339>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Chrousos GP. Stress Hormones, Th1/Th2 patterns, Pro/Anti-inflammatory Cytokines and Susceptibility to Disease. Trends Endocrinol.Metab. 1999a;10:359–368. doi: 10.1016/s1043-2760(99)00188-5. [DOI] [PubMed] [Google Scholar]
- Elsasser-Beile U, von Kleist S, Martin M. Comparison of mitogen- and virus-induced interferon production in whole blood cell cultures of patients with various solid carcinomas and controls. Tumour.Biol. 1992;13:358–363. doi: 10.1159/000217787. [DOI] [PubMed] [Google Scholar]
- Elsasser-Beile U, von Kleist S, Stahle W, Schurhammer-Fuhrmann C, Monting JS, Gallati H. Cytokine levels in whole blood cell cultures as parameters of the cellular immunologic activity in patients with malignant melanoma and basal cell carcinoma. Cancer. 1993;71:231–236. doi: 10.1002/1097-0142(19930101)71:1<231::aid-cncr2820710136>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Enders CK. A primer on the use of modern missing-data methods in psychosomatic medicine research. Psychosom Med. 2006;68:427–436. doi: 10.1097/01.psy.0000221275.75056.d8. [DOI] [PubMed] [Google Scholar]
- Fekedulegn D, Andrew M, Burchfiel O, Violanti J, Hartley T, Charles L, Miller D. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosomatic Medicine. 2007;69:651–659. doi: 10.1097/PSY.0b013e31814c405c. [DOI] [PubMed] [Google Scholar]
- Fischer JR, Schindel M, Bulzebruck H, Lahm H, Krammer PH, Drings P. Decrease of interleukin-2 secretion is a new independent prognostic factor associated with poor survival in patients with small-cell lung cancer. Ann.Oncol. 1997;8:457–461. doi: 10.1023/a:1008242000431. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Brit J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Herbert TB, Cohen S. Stress and immunity in humans: a meta-analytic review. Psychosomatic Medicine. 1993b;55:364–379. doi: 10.1097/00006842-199307000-00004. [DOI] [PubMed] [Google Scholar]
- Herbert TB, Cohen S. Depression and immunity: a meta-analytic review. Psychological Bulletin. 1993a;113:472–486. doi: 10.1037/0033-2909.113.3.472. [DOI] [PubMed] [Google Scholar]
- Holland J. Textbook of Psycho-Oncology. New York: Oxford University Press; 1998. The role of psychosocial factors in the development of cancer. [Google Scholar]
- Horowitz M, Wilner N, Alvarez W. Impact of event scale. Psychosomatic Medicine. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- Irvine D, Brown B, Crooks D, Roberts J, Browne G. Psychosocial Adjustment in Women with Breast-Cancer. Cancer. 1991;67:1097–1117. doi: 10.1002/1097-0142(19910215)67:4<1097::aid-cncr2820670438>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Jacobsen PB, Widows MR, Hann DM, Andrykowski MA, Kronish LE, Fields KK. Posttraumatic stress disorder symptoms after bone marrow transplantation for breast cancer. Psychosomatic Medicine. 1998;60:366–371. doi: 10.1097/00006842-199805000-00026. [DOI] [PubMed] [Google Scholar]
- Kang D, Fox C. Th1 and Th2 cytokine responses to academic stress. Research in Nursing and Health. 2001;24:245–257. doi: 10.1002/nur.1027. [DOI] [PubMed] [Google Scholar]
- Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc. Natl. Acad. Science. USA. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser J, Glaser R. Methodological issues in behavioral immunology research with humans. Brain, Behavior and Immunity. 1988;2:67–78. doi: 10.1016/0889-1591(88)90007-4. [DOI] [PubMed] [Google Scholar]
- Kissane DW, Boloch S, Smith GC, et al. Cognitive-existential group psychotherapy for women with primary breast cancer: A randomised controlled trial. Psycho-Oncology. 2003;12:532–546. doi: 10.1002/pon.683. [DOI] [PubMed] [Google Scholar]
- Konjevic G, Spuzic I. Stage dependence of NK cell activity and its modulation by interleukin 2 in patients with breast cancer. Neoplasma. 1993;40:81–85. [PubMed] [Google Scholar]
- Larson M, Duberstein P, Talbot N, Caldwell C, Moynihan J. A pre-surgical psychosocial intervention for breast cancer patients: Psychological distress and the immune response. Journal of Psychosomatic Research. 2000;48:187–194. doi: 10.1016/s0022-3999(99)00110-5. [DOI] [PubMed] [Google Scholar]
- Levy S, Herberman R, Whiteside T. Perceived social support and tumor estrogen/progesterone receptor status as predictors of natural killer cell activity in breast cancer patients. Psychosomatic Medicine. 1990;52:73–85. doi: 10.1097/00006842-199001000-00006. [DOI] [PubMed] [Google Scholar]
- Luebbert K, Dahme B, Hasenbring M. The effectiveness of relaxation training in reducing treatment-related symptoms and improving emotional adjustment in acute non-surgical cancer treatment: a meta-analytical review. Psychooncology. 2001;10:490–502. doi: 10.1002/pon.537. [DOI] [PubMed] [Google Scholar]
- McGregor BA, Antoni MH, Boyers A, Alferi SM, Blomberg BB, Carver CS. Cognitive-behavioral stress management increases benefit finding and immune function among women with early-stage breast cancer. Journal of Psychosomatic Research. 2004b;56:1–8. doi: 10.1016/S0022-3999(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Mark MM. Effects of psychosocial interventions with adult cancer patients: A meta-analysis of randomized experiments. Health Psychology. 1995;14:101–108. doi: 10.1037//0278-6133.14.2.101. [DOI] [PubMed] [Google Scholar]
- Miller JS, Tessmer-Tuck J, Pierson BA, Weisdorf D, McGlave P, Blazar BR, et al. Low dose subcutaneous interleukin-2 after autologous transplantation generates sustained in vivo natural killer cell activity. Biol .Blood Marrow Transplant. 1997;3:34–44. [PubMed] [Google Scholar]
- Morrow GR, Roscoe JA, Hickok JT, Andrews PR, Matteson S. Nausea and emesis: evidence for a biobehavioral perspective. Supportive Care in Cancer. 2002;10:96–105. doi: 10.1007/s005200100294. [DOI] [PubMed] [Google Scholar]
- Muthén B. Latent variable modeling with longitudinal and multilevel data. In: Raferty A, editor. Sociological Methodology. Boston: Blackwell; 1997. pp. 453–480. [Google Scholar]
- Penman DT, Bloom JR, Fotpoulos S, Cook MR, Holland JC, Gates C, Flamer D, Murawski B, Ross R, Brandt U, Muenz LR, Pee D. The impact of mastectomy on self-concept and social function: A combined cross-sectional and longitudinal study with comparison groups. Women and Health. 1987;11:101–130. doi: 10.1300/j013v11n03_08. [DOI] [PubMed] [Google Scholar]
- Phillips K, Antoni MH, Lechner S, Blomberg B, Llabre M, Avisar E, Gluck S, DehHagopian R, Carver C. Psychosomatic Medicine. Stress Management Intervention Reduces Serum Cortisol and Increases Relaxation During Treatment for Non-Metastatic Breast Cancer. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichlin S. Neuroendocrine-immune interactions. New England Journal of Medicine. 1993;329:1246–1253. doi: 10.1056/NEJM199310213291708. [DOI] [PubMed] [Google Scholar]
- Roussel E, Gingras MC, Grimm EA, Bruner JM, Moser RP. Predominance of a type 2 intratumoral immune response in fresh tumour-infiltrating lymphocytes from human gliomas. Clinical Experimental Immunology. 1996;105:344. doi: 10.1046/j.1365-2249.1996.d01-753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Umekage H, Nishikawa K, Morii T, Narita N, Enomoto M, et al. Interleukin 4 (IL-4) blocks the IL-2-induced increased in natural killer activity and DNA synthesis of decidual CD16-CD56bright NK cells by inhibiting expression of the IL-2 receptor alpha, beta, and gamma. Cell Immunol. 1996;170:71–77. doi: 10.1006/cimm.1996.0135. [DOI] [PubMed] [Google Scholar]
- Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part i: Sleep and psychological effects. J Clin Oncol. 2005a;23(25):6083–6096. doi: 10.1200/JCO.2005.09.548. [DOI] [PubMed] [Google Scholar]
- Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part ii: Immunologic effects. J Clin Oncol. 2005b;23(25):6097–6106. doi: 10.1200/JCO.2005.12.513. [DOI] [PubMed] [Google Scholar]
- Savino W, Dardenne M. Immune-neuroendocrine interactions. Immunololgy Today. 1995;16:318–322. doi: 10.1016/0167-5699(95)80144-8. [DOI] [PubMed] [Google Scholar]
- Schedlowski M, Jungk C, Schimanski G, Tewes U, Schmoll HJ. Effects of behavioral intervention on plasma cortisol and lymphocytes in breast cancer patients: An exploratory study. Psycho-Oncology. 1994;3:181–187. [Google Scholar]
- Schwab JJ, Bialow MR, Clemmons RS, Holzer CE. Hamilton rating scale for depression with medical in-patients. British Journal of Psychiatry. 1967;113:83–88. doi: 10.1192/bjp.113.494.83. [DOI] [PubMed] [Google Scholar]
- Segerstom S. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. Journal of the National Cancer Institute. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- Shakhar G, Ben-Eliyahu S. Potential prophylactic measures against postoperative immunosuppression: Could they reduce recurrence rates in oncological patients? Ann Surg Oncol. 2003;16:972–992. doi: 10.1245/aso.2003.02.007. [DOI] [PubMed] [Google Scholar]
- Smith PC, Hobish A, Lin DL, Culig Z, Keller ET. Interleukin-6 and prostate cancer progression. Cytokine and Growth Factor Reviews. 2001;12:33–40. doi: 10.1016/s1359-6101(00)00021-6. [DOI] [PubMed] [Google Scholar]
- Spencer SM, Lehman JM, Wynings C, Arena P, Carver CS, Antoni MH, et al. Concerns about breast cancer and relations to psychosocial well-being in a multiethnic sample of early-stage patients. Health Psychology. 1999;18:159–168. doi: 10.1037//0278-6133.18.2.159. [DOI] [PubMed] [Google Scholar]
- Stanton AL, Snider PR. Coping with a breast cancer diagnosis: A prospective study. Health Psychology. 1993;12:16–23. doi: 10.1037//0278-6133.12.1.16. [DOI] [PubMed] [Google Scholar]
- Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood. 2001;97:192–197. doi: 10.1182/blood.v97.1.192. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Repetti RL, Seeman T. Health psychology: what is an unhealthy environment and how does it get under the skin? Annual Review of Psychology. 1997;48:411–447. doi: 10.1146/annurev.psych.48.1.411. [DOI] [PubMed] [Google Scholar]
- Tartour E, Gey A, Sastre-Garau X, Lombard SI, Mosseri V, Fridman WH. Prognostic value of intratumoral interferon gamma messenger RNA expression in invasive cervical carcinomas. Journal of the National Cancer Institute. 1998;90:287–294. doi: 10.1093/jnci/90.4.287. [DOI] [PubMed] [Google Scholar]
- Thaker P, Han L, Kamat A, Arevalo J, Takahashi R, Chunhua L, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma [Electronic version] Nature Medicine. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- Thomas C, Madden F, Jehu D. Psychological effects of stomas--I. Psychosocial morbidity one year after surgery. Journal of Psychosomatic Research. 1987;31:311–316. doi: 10.1016/0022-3999(87)90050-x. [DOI] [PubMed] [Google Scholar]
- Touitou Y, Levi F, Bogdan A, Benavides M, Bailleul F, Misset JL. Rhythm alteration in patients with metastatic breast cancer and poor prognostic factors. J Cancer Res.Clin.Oncol. 1995;121:181–188. doi: 10.1007/BF01198101. [DOI] [PubMed] [Google Scholar]
- Trask PC, Paterson AG, Griffith KA, Riba MB, Schwartz JL. Cognitive-behavioral intervention for distress in patients with melanoma. Cancer. 2003;98:854–864. doi: 10.1002/cncr.11579. [DOI] [PubMed] [Google Scholar]
- van der Pompe G, Antoni MH, Heijnen C. The relations of plasma ACTH and cortisol levels with the distribution and function of peripheral blood cells in response to a behavioral challenge in breast cancer: An empirical exploration by means of statistical modeling. International Journal of Behavioral Medicine. 1997;4:145–167. doi: 10.1207/s15327558ijbm0402_4. [DOI] [PubMed] [Google Scholar]
- van der Pompe G, Antoni MH, Heijnen C. The effects of surgical stress and psychological stress on the immune function of operative cancer patients. Psychology and Health. 1998;13:1015–1026. [Google Scholar]
- van't Spijker A, Trijsburg RW, Duivenvoorden HJ. Psychological sequelae of cancer diagnosis: A meta-analytic review of 58 studies after 1980. Psychosomatic Medicine. 1997;59:280–293. doi: 10.1097/00006842-199705000-00011. [DOI] [PubMed] [Google Scholar]
- Weisman AD, Worden JW. The existential plight in cancer: significance of the first 100 days. International Journal of Psychiatry in Medicine. 1976;7:1–15. doi: 10.2190/uq2g-ugv1-3ppc-6387. [DOI] [PubMed] [Google Scholar]
- Whiteside TL, Herberman RB. The role of natural killer cells in human disease. Clinical Immunology and Immunopathology. 1989;53:1–23. doi: 10.1016/0090-1229(89)90096-2. [DOI] [PubMed] [Google Scholar]
- Woodworth CD, Lichti U, Simpson S, Evans CH, DiPaolo JA. Leukoregulin and gamma-interferon inhibit human papillomavirus type 16 gene transcription in human papillomavirus-immortalized human cervical cells. Cancer Research. 1992;52:456–463. [PubMed] [Google Scholar]
- Witek-Janusek L, Albuquerque K, Chroniak KR, Chroniak C, Durazo-Arvizu R, Mathews H. Effect of mindfulness-based stress reduction on immune function, quality of life and coping in women newly diagnosed with early stage breast cancer. Brain, Behavior and Immunity. doi: 10.1016/j.bbi.2008.01.012. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R. Hypothalamic-pituitary-adrenal alterations in PTSD: are they relevant to understanding cortisol alterations in cancer? Brain, Behavior and Immunity. 2003;17:S73–S83. doi: 10.1016/s0889-1591(02)00070-3. [DOI] [PubMed] [Google Scholar]
- Zou W. Immunosuppressive networks in the tumor environment and their therapeutic relevance. Nature Reviews Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]