The effects of a soluble activin type IIB receptor on obesity and insulin sensitivity (original) (raw)
. Author manuscript; available in PMC: 2010 May 1.
Published in final edited form as: Int J Obes (Lond). 2009 Aug 11;33(11):1265–1273. doi: 10.1038/ijo.2009.162
Abstract
Myostatin, also known as Growth and Differentiation Factor 8, is a secreted protein that inhibits muscle growth. Disruption of myostatin signaling increases muscle mass and decreases glucose, but it is unclear whether these changes are related. We treated mice on chow and high-fat diets with a soluble activin receptor type IIB (ActRIIB.Fc) which is a putative endogenous signaling receptor for myostatin and other ligands of the TGF-β superfamily. After 4 weeks, RAP-031 increased lean and muscle mass, grip strength, and contractile force. RAP-031 enhanced the ability of insulin to suppress glucose production under clamp conditions in high-fat fed mice, but did not significantly change insulin-mediated glucose disposal. The hepatic insulin sensitizing effect of RAP-031 treatment was associated with increased adiponectin levels. RAP-031 treatment for 10 weeks further increased muscle mass and drastically reduced fat content in mice on either chow or high-fat diet. RAP-031 suppressed hepatic glucose production and increased peripheral glucose uptake in chow fed mice. In contrast, RAP-031 suppressed glucose production with no apparent change in glucose disposal in high-fat diet mice. Our findings demonstrate that disruption of ActRIIB signaling is a viable pharmacological approach for treating obesity and diabetes.
Keywords: activin receptor, myostatin, muscle, insulin, glucose, mice
Introduction
Myostatin is a member of the TGF-β superfamily that is predominantly expressed and secreted by skeletal muscle (1). Loss-of-function mutations in the myostatin gene results in massive increase in muscle mass in mice, cattle, dog and human (1-4). The biology of myostatin has been explored pharmacologically through injection of neutralizing antibodies or the myostatin propeptide which both increase muscle mass in mice (5-7). Proteins that bind to myostatin, e.g. follistatin, follistatin-related gene (FLRG), and growth and differentiation factor-associated serum protein-1 (GASP-1), result in inhibition of myostatin activity and increased muscle growth (8). In addition to its effects on muscle, myostatin has been reported to promote or inhibit adipogenesis (9-11).
The activin type IIB receptor (ActRIIB) is a signaling receptor for multiple TGF-β superfamily members, including activin A, nodal, BMP2, BMP6, BMP7, GDF5, GDF8 (myostatin) and GDF11, that are involved in the negative regulation of muscle. ActRIIB is widely distributed in skeletal muscle, adipose tissue and various organs (12). Myostatin and ActRIIB levels are increased in obese mice (13). Interestingly, myotubes from extremely obese individuals secrete high levels of myostatin, and this is related to the severity of insulin resistance (14). Obesity is characterized by increased fat content and relative decrease in lean tissue; thus, it was postulated that inhibition of myostatin would increase lean and muscle mass and ameliorate insulin resistance and diabetes (15, 16). In agreement, crossing myostatin knockout mice with _Lep_ob/ob and agouti lethal yellow (Ay/a) mice attenuated body fat and glucose levels (17). Myostatin deficient mice generated from ENU mutagenesis manifested reduced fat content and improved hepatic insulin sensitivity on high-fat diet (16). Disruption of myostatin signaling through transgenic myostatin propeptide expression also prevented diet-induced obesity and insulin resistance (18). However, these genetic models do not clarify whether the effect of myostatin deficiency on glucose homeostasis is directly attributable to increased muscle mass.
A soluble ActRIIB:Fc fusion protein (RAP-031) has been shown to increase muscle mass and strength in a mouse model of amyotrophic lateral sclerosis (19). In the current study we treated mice on chow or high-fat diets with this compound, and studied the temporal relationships between body composition and insulin sensitivity.
Materials and Methods
Animals and treatment
Experiments were performed according to protocols reviewed and approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania School of Medicine. Eight-week-old wild-type male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were housed (n=5 per cage) under a 12: 12-h light-dark cycle (light on at 0700) and an ambient temperature of 22°C and allowed free access to water and food. The mice were fed a high-fat diet (Research Diets, New Brunswick, NJ; #D12451, containing 45% fat, 35% carbohydrate, 20% protein of dry weight, and 4.7 kcal/g of dry weight (20, 21). ActRIIB:Fc fusion protein (RAP-031 10 mg/kg; provided by Acceleron Pharmaceuticals, Cambridge, MA) was injected intraperitoneally twice weekly (19). Preliminary studies showed that RAP-031 treatment increased lean and muscle mass in a dose dependent manner. The vehicle was phosphate-buffered saline. Control mice on regular chow diet (LabDiet, Richmond, IN, #5001, containing 4.5% fat, 49.9% carbohydrate, 23.4% protein of dry weight; 4 kcal/g of dry weight) received RAP-031 (10 mg/kg IP twice weekly) or vehicle. Food intake was measured weekly, and body composition was assessed prior to treatment, and 4 and 10 weeks later with nuclear magnetic resonance (NMR) (Echo Medical Systems, Houston, TX) (21, 22).
Treadmill and muscle studies
RAP-031- or vehicle-treated mice were acclimatized to a modular treadmill connected to an open circuit indirect calorimeter (CLAMS, Columbus Instruments, Columbus, OH). After 4 weeks of treatment, the mice were deprived of food for 5 hours, and indirect calorimetry was performed at rest for 1 hour followed by forced exercise on the treadmill (angle 10°, 15 min at 10 meters/min and then 2 hours at 15 meters/min).
The muscle strength in the forelimbs was measured with a grip meter (TSE; Bad Hamburg, Germany). The mice were trained to grasp a horizontal metal bar while being pulled by their tail, and the force was detected by a sensor. Ten measurements were determined for each mouse and averaged. The mice were euthanized with carbon dioxide, muscles were dissected and weighed, and contraction of the extensor digitorum longus (EDL) muscle was analyzed ex vivo. Twitch and tetanic responses were evaluated as previously described (5, 23, 24). At the end of the physiological studies, muscles were flash-frozen in isopentane cooled in liquid nitrogen and stored at -80°C prior to sectioning. Serial frozen sections (10 μm) were cut at mid-belly of the EDL muscle, fixed using 100% ice-cold methanol (5 min), and stored in airtight containers at -80°C. Sections were examined after staining with hematoxylin and eosin (H&E) or ATPase. Pictures were taken using an Olympus BX51 microscope equipped with a Magnafire camera. Morphometric measurements were performed on digitized images using the Scion Image 4.02 software as previously described (5).
Hyperinsulinemic-Euglycemic Clamp
RAP-031 or vehicle-treated mice on normal chow or high-fat diets for 4 or 10 weeks respectively underwent hyperinsulinemic-euglycemic clamp to assess glucose kinetics (21, 22). An indwelling catheter was inserted in the right internal jugular vein under sodium pentobarbital anesthesia and extended to the right atrium. Four days after recovery, the mice were fasted for 6 hours (0800-1300), placed in restrainers and administered a bolus injection of 5 μCi of [3-3H] glucose, followed by continuous intravenous infusion at 0.05 μCi/min. Baseline glucose kinetics were measured for 60 min, followed by hyperinsulinemic clamp for 120 min. A priming dose of regular insulin (16 mU/kg, Humulin; Eli Lilly, Indianapolis, IN) was given intravenously, followed by continuous infusion at 2.5 mU•kg-1•min-1. A variable intravenous infusion of 20% glucose was administered to attain blood glucose levels of 120-140 mg/dL. The target glucose concentration was maintained for 90 min. The mice were euthanized, and liver, perigonadal white adipose tissue (WAT), and soleus/gastrocnemius muscles were excised, frozen immediately in liquid nitrogen and stored at -80°C for analysis of glucose uptake. Rates of whole body glucose uptake and basal glucose turnover are measured as the ratio of the [3H] glucose infusion rate (dpm) to the specific activity of plasma glucose. Hepatic glucose production (HGP) during clamp is measured by subtracting the glucose infusion rate (GIR) from the whole body glucose uptake (Rd).
Chemistry
RAP-031 or vehicle-treated mice on normal chow or high-fat diets for 4 or 10 weeks respectively, were deprived of food from 0800-1300h, and then euthanized with carbon dioxide. Cardiac blood was drawn and serum was stored at -20°C. Serum glucose and lipids were measured using colorimetric enzymatic assays (Stanbio, Boerne, TX). Insulin, leptin, and adiponectin were measured with enzyme immunoassays (Crystal Chem, Evanston, IL; Linco, St. Charles, MO).
Statistical Analysis
The effect of RAP-031 treatment on various parameters within each diet group was assessed using unpaired t-test. P<0.05 is significant.
Results
Short-term RAP-031 treatment increases muscle mass, strength and contraction
RAP-031 (10 mg/kg IP twice weekly) significantly increased body weight in mice on normal chow diet (31± 0.89 vs 27.1±1.25 g) or high-fat diet (35.5±1.34 vs 29.8±1.42 g) after 4 weeks (Fig. 1A). There was a significant increase in food intake in RAP-031-treated mice on chow diet (12.8±0.46 in vs 11.7±0.39 kcal/mouse/day, _P_= 0.036), and a slight increase on high-fat diet (14.6±0.69 vs 13±0.57 kcal/mouse/day; _P_=0.11). Lean mass was increased by RAP-031 but fat content was not significantly different (Figs. 1B, C). RAP-031 treatment increased the weights of gastrocnemius, quadriceps, and extensor digitorum longus (EDL) muscles (Figs. 1D-F). Morphometric analysis showed that RAP-031 did not significantly increase the number of EDL muscle fibers compared to vehicle treatment (chow diet: 807.5±76.1 vs. 848.6±99.7, _P_=0.75; high-fat diet: 1044±60.2 vs. 982.7±27.4, _P_=0.38). Rather, the cross-sectional area of EDL fibers was shifted to the right and the mean fiber area increased in response to RAP-031 treatment, consistent with muscle hypertrophy (Figs. 2A-D).
Figure 1.
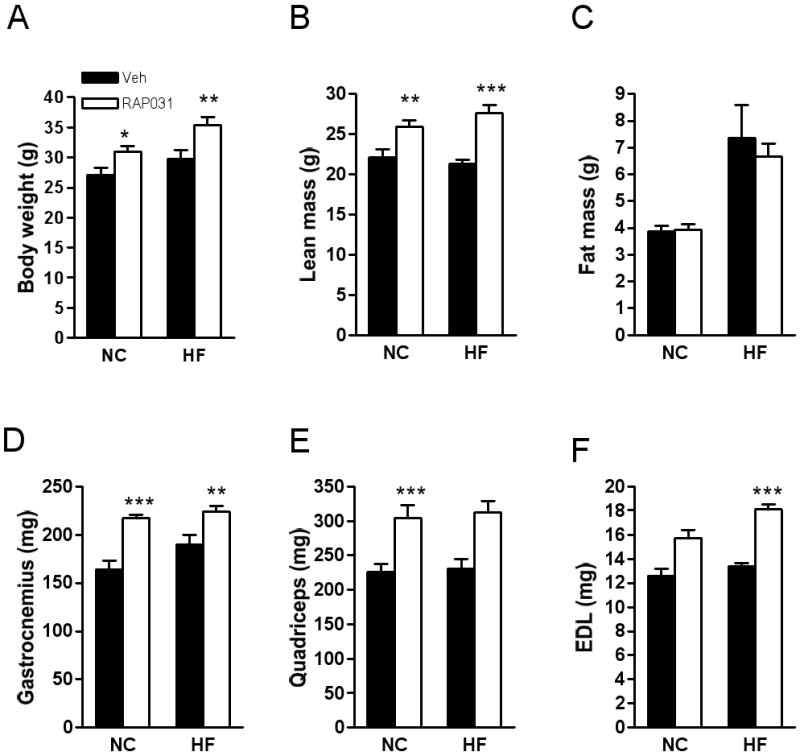
Effects of 4 weeks RAP-031 treatment (white bar) or vehicle (black bar) on (A) body weight, (B) lean, and (C) fat mass. (D-F) skeletal muscle weights. Data are mean ± SEM, N=10; *P<0.05 vs vehicle; **P<0.01 vs vehicle; ***P<0.001 vs vehicle. NC, normal chow diet; HF, high-fat diet.
Figure 2.
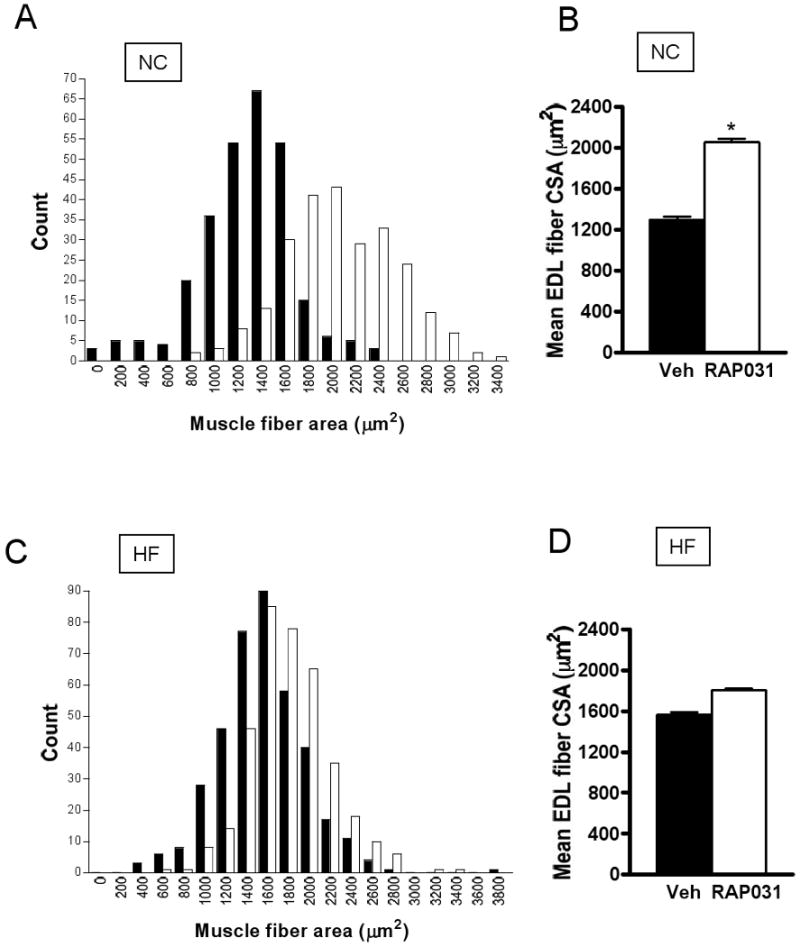
Effects of 4 weeks RAP-031 treatment (white bar) or vehicle (black bar) on EDL muscle fiber cross-sectional areas in mice on normal chow diet (A, B) or high-fat diet (C, D). Data are mean ± SEM. N=5; *P<0.01 vs vehicle. NC, normal chow diet; HF, high-fat diet.
RAP-031 treatment did not significantly alter the basal or peak VO2 levels during treadmill exercise (Figs. 3A, B), or the respiratory quotient (RQ) under basal or peak exercise conditions (data not shown). On the other hand, the forelimb grip strength was increased by RAP-031 on both chow and high-fat diets (Fig. 3C). EDL twitch force was increased by RAP-031 treatment (Fig. 3D). Moreover, EDL contraction under tetanic stimulation was enhanced by RAP-031 in high-fat fed mice (Fig. 3E).
Figure 3.
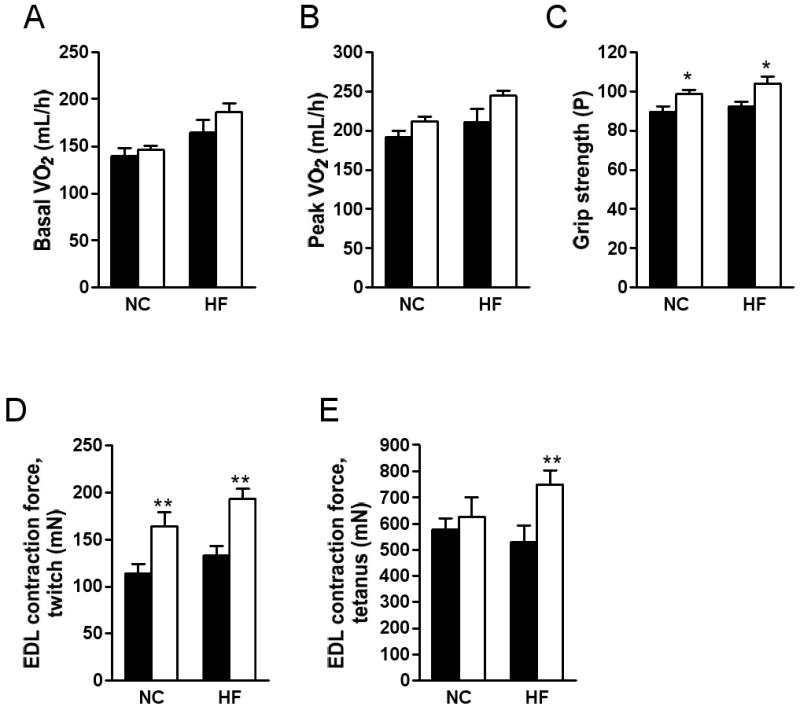
Effects of 4 weeks RAP-031 treatment (white bar) or vehicle (black bar) on oxygen consumption (VO2) under (A) basal and (B) peak treadmill exercise conditions, (C) grip strength, and (D, E) ex vivo EDL contraction. Data are mean ± SEM. N=5; *P<0.05 vs vehicle, **P<0.01 vs vehicle, ***P<0.001 vs vehicle. NC, normal chow diet; HF, high-fat diet.
RAP-031 treatment for 4 weeks resulted in elevation of serum adiponectin in high-fat fed mice, but glucose, lipids, insulin and leptin were not significantly different (Table 1). We performed hyperinsulinemic-euglycemic clamp with radioisotopic tracers to examine the effects of 4 weeks RAP-031 treatment on glucose homeostasis. The basal glucose production was not significantly affected by RAP-031 treatment in chow-fed mice (Fig. 4A). Moreover, we did not detect a statistically significant difference in the glucose infusion rate (GIR) needed to maintain euglycemia in vehicle and RAP-031-treated mice under insulin clamp (Fig. 4A). Hepatic glucose production (HGP), glucose disposal rate (Rd), and glucose uptake by white adipose tissue (WAT) and muscle under insulin clamp were not different between chow-fed mice treated with RAP-031 or vehicle (Figs. 4A, B). In contrast, RAP-031 treatment increased GIR (43.9±4.33 vs 27±6 mL/kg/min, _P_=0.03) in mice on a high-fat diet, consistent with improvement of insulin sensitivity (Fig. 4C). The ability of insulin to suppress HGP (25.3±2.9 vs 33.7±2.1 mL/kg/min, _P_=0.047) was enhanced by RAP-031, but we did not detect a statistically significant effect of RAP-031 on Rd (69.2±2.35 vs 60.7±4.48 mL/kg/min, _P_=0.12) (Fig. 4C), or glucose uptake by WAT or muscle (Fig. 4D).
Table 1. Effects of 4 weeks RAP-031 treatment on serum chemistry.
| Normal chow diet | High fat diet | |||
|---|---|---|---|---|
| Vehicle | RAP-031 | Vehicle | RAP-031 | |
| Glucose (mg/dL) | 144 ± 8.87 | 144 ± 6.1 | 188 ± 12 | 164 ± 6.35 |
| Insulin (ng/mL) | 0.55 ± 0.16 | 0.62 ± 0.13 | 1.36 ± 0.57 | 0.8 ± 0.08 |
| Leptin (ng/mL) | 2.6 ± 0.8 | 3.5 ± 0.8 | 5.0 ± 0.6 | 4.2 ± 0.6 |
| Adiponectin (μg/mL) | 11.9 ± 0.72 | 13.9 ± 2.14 | 7.55 ± 0.9 | 17.1 ± 0.87* |
| Triglycerides (mg/dL) | 81 ± 12 | 91.4 ± 10.1 | 78.7 ± 9.4 | 92 ± 10.7 |
| NEFA (mEq/L) | 0.7 ± 0.1 | 0.69 ± 0.09 | 0.75 ± 0.09 | 0.82 ± 0.09 |
| Cholesterol (mg/dL) | 85.9 ± 2.36 | 103 ± 7.3 | 151 ± 8.3 | 149 ± 8.01 |
Figure 4.
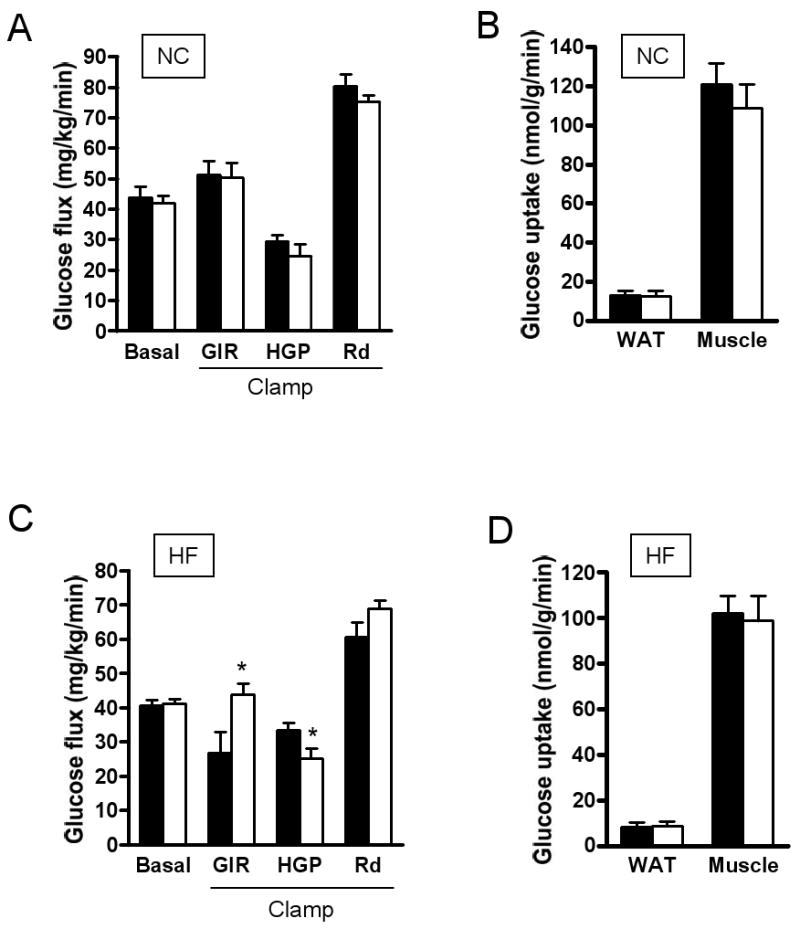
Basal glucose production and hyperinsulinemic-euglycemic clamp in chow (NC) or high-fat (HF) fed mice treated with RAP031 (white bar) or vehicle (black bar) for 4 weeks. (A) Glucose kinetics under basal and clamp conditions on NC diet; (B) WAT and muscle glucose uptake on NC diet; (C) Glucose kinetics under basal and clamp conditions on HF diet; (D) WAT and muscle glucose uptake on HF diet. Data are mean ± SEM. N=5. *P<0.05 vs vehicle.
Long-term RAP-031 treatment increases muscle mass, decreases fat and enhances insulin sensitivity
RAP-031 treatment did not significantly alter body weight at 10 weeks (Fig. 5A), but increased lean mass (Fig. 5B). Body fat was drastically reduced by RAP-031 after 10 weeks (Figs. 5C-I). Serum levels of leptin, glucose and cholesterol levels fell in parallel with reduced adiposity in high-fat diet mice after RAP-031 treatment (Table 2). Adiponectin concentration in the serum was highly variable and did not change significantly after 10 weeks RAP031 treatment (Table 2). However, on the high-fat diet, the ratio of serum adiponectin-to-body fat was higher in mice treated with RAP-031 than vehicle (1.42 vs. 0.76). The basal glucose production rate was not significantly different between vehicle or RAP-031-treated mice on chow diet (Fig. 6A). Under insulin clamp, RAP-031 treatment resulted in significant increase of GIR (113.2±25 vs 45.7±6.1 mL/kg/min, _P_=0.03) and Rd (118±15.3 vs 62.6±5.5 mL/kg/min, _P_=0.009), while HGP was suppressed (5.67±0.63 vs 16.9±3.17 mL/kg/min, _P_=0.008) (Fig. 6A). Insulin-stimulated glucose uptake in WAT and muscle was increased by RAP-031 (Fig. 6B). On the high-fat diet, RAP-031 increased GIR (43.3±3.9 vs 27.8±2.7 mL/kg/min, _P_=0.03) and suppressed HGP (6.78±0.5 vs 22.3±4.8 mL/kg/min, _P_=0.008) (Fig. 6C), but did not change Rd (50.2±3.5 vs 51.1±3.3 mL/kg/min, _P_=0.12) or glucose uptake by WAT or muscle (Fig. 6D).
Figure 5.
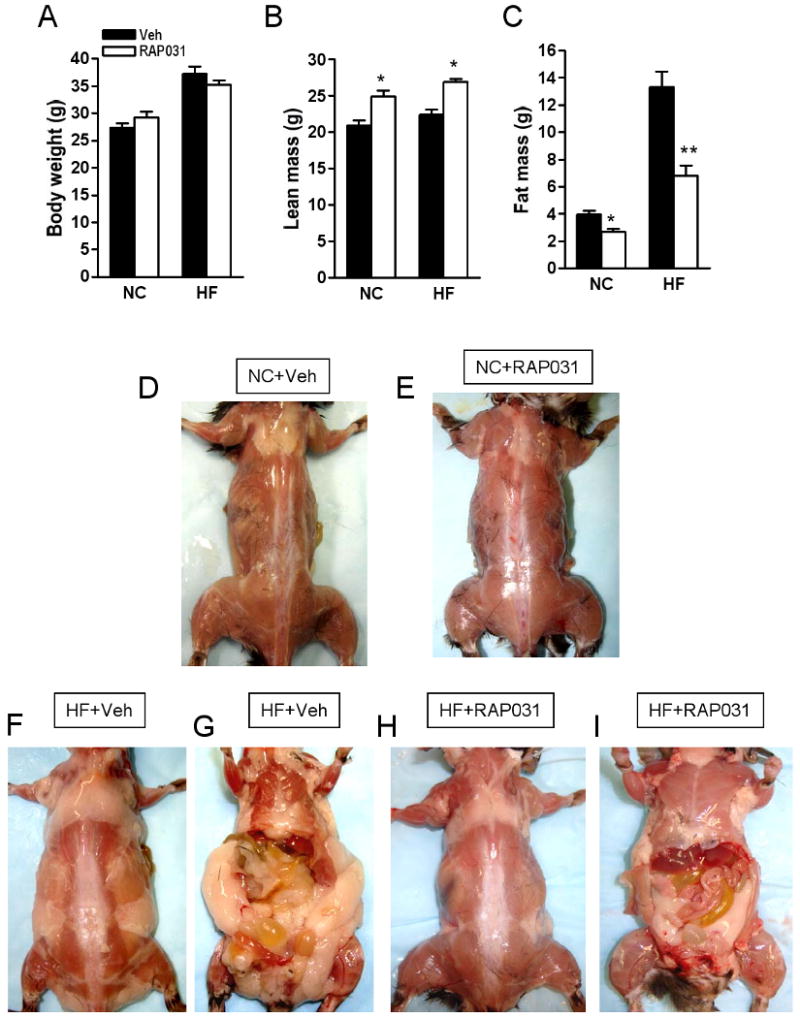
Effects of 10 weeks RAP-031 treatment (white bar) or vehicle (black bar) on (A) body weight, (B) lean mass, (C) fat mass. Data are mean ± SEM. N=5; *P<0.01 vs vehicle; **P<0.001 vs vehicle. NC, normal chow diet; HF, high-fat diet. Photographs showing the effects of (D) vehicle and (E) RAP-031 treatment on muscle and fat in the dorsal region of NC mice. Effects of (F, G) vehicle or (H, I) RAP-031 treatment on fat and muscle in HF mice.
Table 2. Effects of 10 weeks RAP-031 treatment on serum chemistry.
| Normal chow diet | High fat diet | |||
|---|---|---|---|---|
| Vehicle | RAP-031 | Vehicle | RAP-031 | |
| Glucose (mg/dL) | 131 ± 5.47 | 95.3 ± 11.7* | 166 ± 9.1 | 136 ± 6.81* |
| Insulin (ng/mL) | 1.96 ± 0.27 | 5.35 ± 2.63 | 4.75 ± 1.45 | 2.04 ± 0.8 |
| Leptin (ng/mL) | 3.35 ± 0.8 | 2.37 ± 0.24 | 36.2 ± 5.37 | 19.1 ± 3.1* |
| Adiponectin (μg/mL) | 6.76 ± 0.65 | 19.9 ± 10.1 | 10.2 ± 1.15 | 9.64 ± 0.32 |
| Triglycerides (mg/dL) | 57.8 ± 10.4 | 83.8 ± 17.4 | 58.8 ± 3.9 | 68.7 ± 16.8 |
| NEFA (mEq/L) | 0.35 ± 0.05 | 0.54 ± 0.27 | 0.57 ± 0.08 | 0.36 ± 0.08 |
| Cholesterol (mg/dL) | 82.8 ± 7.68 | 111 ± 16.7 | 150 ± 5.4 | 106 ± 5.63** |
Figure 6.
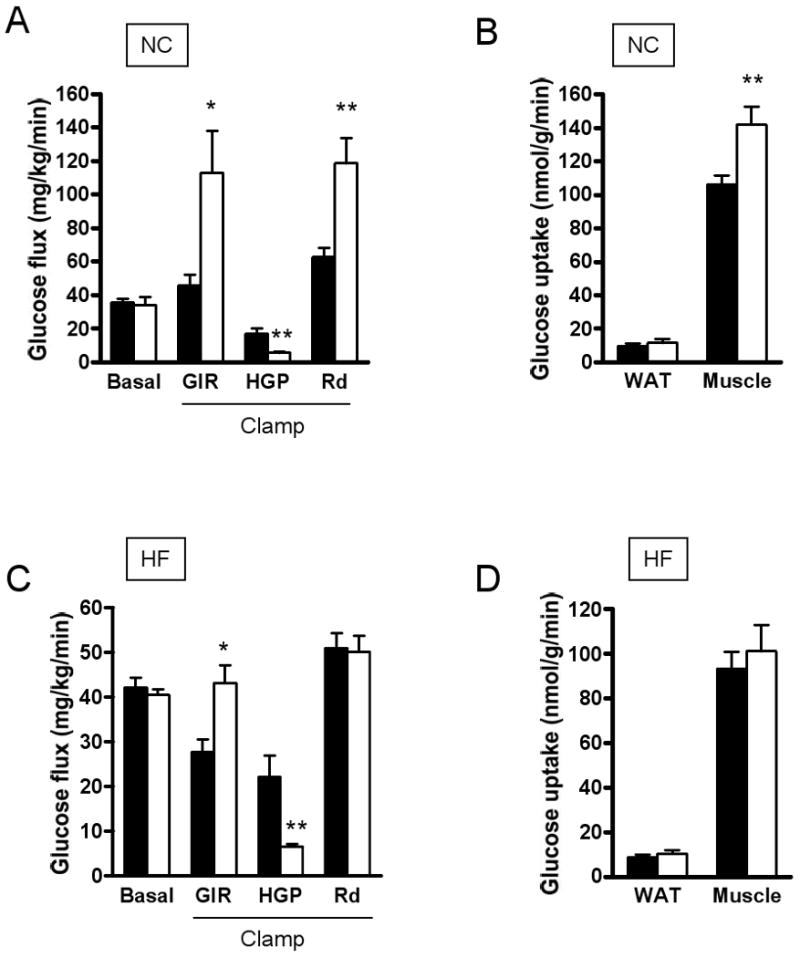
Basal glucose production and hyperinsulinemic-euglycemic clamp in mice chow (NC) or high-fat (HF) fed mice, treated with RAP-031 (white bar) or vehicle (black bar) for 10 weeks. (A) Glucose kinetics under basal or clamp conditions on NC diet; (B) WAT and muscle glucose uptake on NC diet; (C) Glucose kinetics under basal and clamp conditions on HF diet; (D) WAT and muscle glucose uptake on HF diet. Data are mean ± SEM. N=5. *P<0.01, **P<0.001 vs vehicle.
Discussion
There is evidence to suggest that multiple ligands including myostatin control muscle growth by signaling through ActRIIB (15). We confirm here with the use of RAP-031 that disrupting ActRIIB signaling leads to increased muscle mass and improved functional parameters such as grip strength and ex vivo muscle contraction (19). This increase in functional muscle mass occurs rapidly within 4 weeks of treatment, and is in agreement with published reports on myostatin knock-out (Mstn-/-)mice (2, 16), transgenic myostatin propeptide overexpression (18), chronic administration of anti-myostatin antibodies (5, 19) and myostatin propeptide antibodies (6, 7), and viral overexpression of myostatin inhibitors (8). The change in EDL mass and contraction force after 4 weeks of RAP-031 treatment is similar to that of 10-12 month old Mstn heterozygous and null mice (25). However, unlike Mstn-/- mice, our results showed that RAP-031 treatment resulted in muscle hypertrophy rather than hyperplasia (23).
Obesity is associated with increased accumulation of fat and a relative decrease in lean and muscle mass (26). Adipose tissue has been linked to insulin resistance and type 2 diabetes through the secretion of fatty acids, adipokines and proinflammatory cytokines (27). Skeletal muscle is normally responsible for more than 75% of insulin-mediated glucose disposal, thus the reduction in muscle mass associated with obesity is thought to contribute to the development of insulin resistance and type 2 diabetes (27, 28). Other factors implicated in skeletal muscle insulin resistance include the accumulation of intramyocellular lipids and metabolites, such as diacylglycerol and ceramides (29, 30). Low-grade chronic inflammation and oxidative stress also contribute to skeletal muscle insulin resistance (28). In contrast to the secretion of proinflammatory cytokines by adipose tissue, studies indicate that IL-6 is secreted by skeletal muscle during exercise and stimulates the release of other anti-inflammatory cytokines, e.g. IL-1ra and IL-10, which inhibit the production of TNFα. In addition, IL-6 stimulates lipolysis and fat oxidation, thereby preventing fat accumulation in muscle and liver (31). Thus, it appears IL-6 is a muscle-secreted peptide (myokine) that protects against diabetes and cardiovascular disease (31). In contrast, myostatin secretion is increased in extremely obese women and associated with insulin resistance (14). Myostatin treatment in mice induces insulin resistance (16).
Several lines of evidence led us to believe that postnatal inhibition of ActRIIB signaling would increase muscle, reduce fat and lead to improved glucose homeostasis. Myostatin and ActRIIB expression is altered in adipose tissue and skeletal muscle of obese mice (13). Mstn-/- mice have increased muscle, decreased fat and improved insulin sensitivity (16, 17). Likewise, transgenic mice overexpressing the myostatin propeptide that blocks myostatin signaling develop improved glucose tolerance and insulin sensitivity on a high-fat diet (18). However, in vivo myostatin treatment has produced inconsistent effects on muscle and fat. Zimmers et al. reported that subcutaneous injection of 2 μg myostatin for 7 days decreased body fat in female mice (32). These results could not be reproduced in another study (33). Instead, 120 μg myostatin administered for 21 days decreased muscle mass without changing fat in wild type or genetically obese mice (33).
Our study revealed an interesting dynamic between body composition and glucose homeostasis following RAP-031 treatment. As predicted, RAP-031 rapidly increased lean and muscle mass. However, this did not lead to a significant enhancement of insulin-mediated glucose disposal in muscle or fat of mice on chow or high-fat diet. Instead, 4 weeks of RAP-031 treatment in high-fat diet mice resulted in a significant increase in insulin's effect to suppress glucose production. Interestingly, the hepatic insulin sensitizing action of RAP-031 was associated with elevation of adiponectin levels. Adiponectin is expressed in adipose tissue, circulates as low and high molecular weight complexes, and has been shown enhance insulin sensitivity (34). Similar to our results, transgenic overexpression of myostatin propeptide in mice fed a high-fat diet resulted in elevation of adiponectin levels (7). The expression of adiponectin mRNA in epidydimal fat of transgenic myostatin propeptide mice was 12 times higher than in wild type littermates, suggesting that enhanced muscle growth somehow stimulates the expression of adiponectin (7).
We observed that longer RAP-031 treatment for 10 weeks increased muscle mass, decreased body fat content significantly increased hepatic and peripheral insulin sensitivity, particularly in chow-fed mice. Adiponectin concentration in the serum was not significantly increased by RAP-031, however, the ratio of adiponectin to body fat was greater, suggesting an increase in adiponectin secretion. The insulin sensitizing action of adiponectin has been associated with fatty acid oxidation, which may be a potential mechanism for RAP-031 (34). The ability of prolonged RAP-031 treatment to improve sensitivity in the liver and peripheral organs is related to muscle hypertrophy and loss of body fat respectively. A similar phenotype resulted from chronic activation of Akt signaling in skeletal muscle (35). As with RAP-031 treatment, constitutive Akt expression in skeletal muscle caused skeletal muscle hypertrophy, increased strength, diminished fat deposition and improvement in whole body metabolism (35). Interestingly, the anti-obesity effect of Akt activation was attributed to increased fatty acid oxidation in muscle (35). Based on these findings, it was proposed that muscle-secreted proteins (known as myokines), serve as critical signals linking muscle to liver, adipose tissue and various organs. Indeed, follistatin-like 1 has recently been identified as a myokine that promotes limb vascularization (36).
We believe ours is the first study to show that pharmacological disruption of ActRIIB signaling results in reduced fat content, enhanced insulin sensitivity, as well as increased skeletal muscle mass. These results demonstrate that inhibition of ActRIIB signaling is a viable strategy for the prevention or treatment of obesity, insulin resistance and diabetes. The signaling mechanisms by which RAP-031 regulates muscle mass and insulin sensitivity are yet to be determined. A recent study showed that myostatin induced muscle atrophy through the transcription factors, Smad 2 and Smad3 (37). Conversely, Smad2/3 inhibition promoted muscle hypertrophy partially mTOR and Akt signaling (37). These results were corroborated by another study which showed that myostatin inhibited myoblast differentiation by suppressing Akt/TORC1/p70S6 kinase activity (38). Further work is needed to determine whether these pathways mediate the effects of ActRIIB on insulin sensitivity, adiposity and other metabolic processes.
Acknowledgments
This work was supported by National Institutes of Health grants RO1-DK062348 and PO1-DK049210 to R.S.A, University of Pennsylvania Diabetes Endocrinology Research Center (DERC) Mouse Metabolic Phenotyping Core (P30-DK19525), and World Anti-Doping Agency (WADA, Institution No: 801619) to T.S.K, and Acceleron Pharmaceuticals.
Footnotes
Conflict of interest: Jeffrey Ucran and Jennifer Lachey are employees of Acceleron Pharma which provided RAP-031 for the studies.
References
- 1.McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci U S A. 1997;94:12457–12461. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 3.Mosher DS, Quignon P, Bustamante CD, Sutter NB, Mellersh CS, Parker HG, et al. A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet. 2007;3:e79. doi: 10.1371/journal.pgen.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuelke M, Wagner KR, Stolz LE, Hubner C, Riebel T, Komen W, et al. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med. 2004;350:2682–2688. doi: 10.1056/NEJMoa040933. [DOI] [PubMed] [Google Scholar]
- 5.Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS, et al. Functional improvement of dystrophic muscle by myostatin blockade. Nature. 2002;420:418–421. doi: 10.1038/nature01154. [DOI] [PubMed] [Google Scholar]
- 6.Bogdanovich S, Perkins KJ, Krag TO, Whittemore LA, Khurana TS. Myostatin propeptide-mediated amelioration of dystrophic pathophysiology. FASEB J. 2005;19:543–549. doi: 10.1096/fj.04-2796com. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki ST, Zhao B, Yang J. Enhanced muscle by myostatin propeptide increases adipose tissue adiponectin, PPAR-alpha, and PPAR-gamma expressions. Biochem Biophys Res Commun. 2008;369:767–773. doi: 10.1016/j.bbrc.2008.02.092. [DOI] [PubMed] [Google Scholar]
- 8.Haidet AM, Rizo L, Handy C, Umapathi P, Eagle A, Shilling C, et al. Long-term enhancement of skeletal muscle mass and strength by single gene administration of myostatin inhibitors. Proc Natl Acad Sci U S A. 2008;105:4318–4322. doi: 10.1073/pnas.0709144105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Artaza JN, Bhasin S, Magee TR, Reisz-Porszasz S, Shen R, Groome NP, et al. Myostatin inhibits myogenesis and promotes adipogenesis in C3H 10T(1/2) mesenchymal multipotent cells. Endocrinology. 2005;146:3547–3557. doi: 10.1210/en.2005-0362. [DOI] [PubMed] [Google Scholar]
- 10.Feldman BJ, Streeper RS, Farese RV, Jr, Yamamoto KR. Myostatin modulates adipogenesis to generate adipocytes with favorable metabolic effects. Proc Natl Acad Sci U S A. 2006;103:15675–15680. doi: 10.1073/pnas.0607501103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HS, Liang L, Dean RG, Hausman DB, Hartzell DL, Baile CA. Inhibition of preadipocyte differentiation by myostatin treatment in 3T3-L1 cultures. Biochem Biophys Res Commun. 2001;281:902–906. doi: 10.1006/bbrc.2001.4435. [DOI] [PubMed] [Google Scholar]
- 12.Rebbapragada A, Benchabane H, Wrana JL, Celeste AJ, Attisano L. Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis. Mol Cell Biol. 2003;23:7230–7242. doi: 10.1128/MCB.23.20.7230-7242.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen DL, Cleary AS, Speaker KJ, Lindsay SF, Uyenishi J, Reed JM, et al. Myostatin, activin receptor IIb, and follistatin-like-3 gene expression are altered in adipose tissue and skeletal muscle of obese mice. Am J Physiol Endocrinol Metab. 2008;294:E918–927. doi: 10.1152/ajpendo.00798.2007. [DOI] [PubMed] [Google Scholar]
- 14.Hittel DS, Berggren JR, Shearer J, Boyle K, Houmard JA. Increased secretion and expression of myostatin in skeletal muscle from extremely obese women. Diabetes. 2009;58:30–38. doi: 10.2337/db08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SJ, Reed LA, Davies MV, Girgenrath S, Goad ME, Tomkinson KN, et al. Regulation of muscle growth by multiple ligands signaling through activin type II receptors. Proc Natl Acad Sci U S A. 2005;102:18117–18122. doi: 10.1073/pnas.0505996102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilkes JJ, Lloyd DJ, Gekakis N. A loss-of-function mutation in myostatin reduces TNF{alpha} production and protects liver against obesity-induced insulin resistance. Diabetes. 2009;58:1133–1143. doi: 10.2337/db08-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McPherron AC, Lee SJ. Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest. 2002;109:595–601. doi: 10.1172/JCI13562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao B, Wall RJ, Yang J. Transgenic expression of myostatin propeptide prevents diet-induced obesity and insulin resistance. Biochem Biophys Res Commun. 2005;337:248–255. doi: 10.1016/j.bbrc.2005.09.044. [DOI] [PubMed] [Google Scholar]
- 19.Morrison BM, Lachey JL, Warsing LC, Ting BL, Pullen AE, Underwood KW, et al. A soluble activin type IIB receptor improves function in a mouse model of amyotrophic lateral sclerosis. Exp Neurol. 2009;217:258–268. doi: 10.1016/j.expneurol.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi N, Patel HR, Qi Y, Dushay J, Ahima RS. Divergent effects of leptin in mice susceptible or resistant to obesity. Horm Metab Res. 2002;34:691–697. doi: 10.1055/s-2002-38251. [DOI] [PubMed] [Google Scholar]
- 21.Varela GM, Antwi DA, Dhir R, Yin X, Singhal NS, Graham MJ, et al. Inhibition of ADRP prevents diet-induced insulin resistance. Am J Physiol Gastrointest Liver Physiol. 2008;295:G621–628. doi: 10.1152/ajpgi.90204.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singhal NS, Lazar MA, Ahima RS. Central resistin induces hepatic insulin resistance via neuropeptide Y. J Neurosci. 2007;27:12924–12932. doi: 10.1523/JNEUROSCI.2443-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moens P, Baatsen PH, Marechal G. Increased susceptibility of EDL muscles from mdx mice to damage induced by contractions with stretch. J Muscle Res Cell Motil. 1993;14:446–451. doi: 10.1007/BF00121296. [DOI] [PubMed] [Google Scholar]
- 24.Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci U S A. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendias CL, Marcin JE, Calerdon DR, Faulkner JA. Contractile properties of EDL and soleus muscles of myostatin-deficient mice. J Appl Physiol. 2006;101:898–905. doi: 10.1152/japplphysiol.00126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leibel RL, Hirsch J. Metabolic characterization of obesity. Ann Intern Med. 1985;103:1000–1002. doi: 10.7326/0003-4819-103-6-1000. [DOI] [PubMed] [Google Scholar]
- 27.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 29.Galgani JE, Moro C, Ravussin E. Metabolic flexibility and insulin resistance. Am J Physiol Endocrinol Metab. 2008;295:E1009–1017. doi: 10.1152/ajpendo.90558.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moro C, Bajpeyi S, Smith SR. Determinants of intramyocellular triglyceride turnover: implications for insulin sensitivity. Am J Physiol Endocrinol Metab. 2008;294:E203–213. doi: 10.1152/ajpendo.00624.2007. [DOI] [PubMed] [Google Scholar]
- 31.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 32.Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, et al. Induction of cachexia in mice by systemically administered myostatin. Science. 2002;296:1486–1488. doi: 10.1126/science.1069525. [DOI] [PubMed] [Google Scholar]
- 33.Stolz LE, Li D, Qadri A, Jalenak M, Klaman LD, Tobin JF. Administration of myostatin does not alter fat mass in adult mice. Diabetes Obes Metab. 2008;10:135–142. doi: 10.1111/j.1463-1326.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 34.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 35.Izumiya Y, Hopkins T, Morris C, Sato K, Zeng L, Viereck J, et al. Fast/Glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab. 2008;7:159–172. doi: 10.1016/j.cmet.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ouchi N, Oshima Y, Ohashi K, Higuchi A, Ikegami C, Izumiya Y, et al. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J Biol Chem. 2008;283:32802–32811. doi: 10.1074/jbc.M803440200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sartori R, Milan G, Patron M, Mammucari C, Blaauw B, Abraham R, et al. Smad2 and 3 Transcription Factors Control Muscle Mass in Adulthood. Am J Physiol Cell Physiol. 2009;296:C1248–1257. doi: 10.1152/ajpcell.00104.2009. [DOI] [PubMed] [Google Scholar]
- 38.Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol. 2009;296:C1258–1270. doi: 10.1152/ajpcell.00105.2009. [DOI] [PubMed] [Google Scholar]