Bright Ideas for Chemical Biology (original) (raw)
. Author manuscript; available in PMC: 2010 Jan 6.
Published in final edited form as: ACS Chem Biol. 2008 Mar 20;3(3):142–155. doi: 10.1021/cb700248m
Abstract
Small-molecule fluorescent probes embody an essential facet of chemical biology. Although numerous compounds are known, the ensemble of fluorescent probes is based on a modest collection of modular “core” dyes. The elaboration of these dyes with diverse chemical moieties is enabling the precise interrogation of biochemical and biological systems. The importance of fluorescence-based technologies in chemical biology elicits a necessity to understand the major classes of small-molecule fluorophores. Here, we examine the chemical and photophysical properties of oft-used fluorophores, and highlight classic and contemporary examples in which utility has been built upon these scaffolds.
Introduction
Small fluorescent molecules are indispensable tools for chemical biology, being ubiquitous as biomolecular labels, enzyme substrates, environmental indicators, and cellular stains (1–12). Choosing a suitable fluorophore to visualize a biochemical or biological process can be daunting, given the countless molecules available either commercially (13) or through de novo design and synthesis. Fortunately, the plethora of fluorescent probes has an intrinsic modularity. Attachment of various reactive groups, substrate moieties, chelating components, and other chemical entities to a small number of “core” fluorophores gives rise to the ensemble of extant probes. Overall, these core fluorophores are well-established (9, 14), consisting of molecules with excellent spectral characteristics, high chemical stabilities, and facile syntheses. Probe selection and design can, therefore, be simplified by understanding the properties of these foundational fluorescent compounds.
In this review, we trek along the electromagnetic spectrum and discuss the properties of the main classes of fluorescent molecules used in bioresearch. We also give examples of tools constructed from these fluorophores. We believe that comprehension of the strengths, weaknesses, and common uses of each dye class will equip the chemical biologist for expeditions to reveal new biochemical and biological phenomena.
A Brief History
The first well-defined small-molecule fluorophore was the natural product quinine (1), an important compound for both medicinal and organic chemistry (15). The visible emission from an aqueous quinine solution was reported by Herschel in 1845 (16). Stokes showed that this phenomenon was due to the absorption and then emission of light by quinine, and coined the term “fluorescence” to describe this process (17). The importance of quinine as an antimalarial would later lead to an attempted synthesis by Perkin, starting from aniline derivatives. Of course, the total synthesis of quinine would tarry for many decades, realized in essence by Woodward and Doering, and in practice by Stork (18). Instead, Perkin's fated synthetic route produced the first synthetic textile dye, mauvine, in 1865. Perkin's success in the commercialization of mauvine and other “aniline dyes” is often considered to be the birth of the modern chemical industry (15, 19). This achievement foreshadowed the discovery of many useful dye molecules, fluorescent and otherwise (20). These colored synthetic molecules were fodder for new biological experiments, and many found diagnostic or even clinical utility (21).
The intrinsic fluorescence of quinine also motivated the development of the fluorometer, which was needed to evaluate antimalarial drug cocktails during World War II (10). The commercialization of such instrumentation in the 1950's allowed increased use of fluorescence-based bioanalytical techniques (22). In the 1960's, the advent of the dye laser spurred much interest in the synthesis of novel or improved fluorescent molecules with desirable photophysical properties (23). Indeed, some structural permutations developed to enhance laser dyes persist in modern fluorescent bioprobes.
More recently, additional classes of fluorophores have joined the foray, including inorganic “quantum dots” and fluorescent proteins (9, 14). Although beyond the scope of this review, green fluorescent protein (GFP) and its variants deserve special mention. These genetically-encoded fluorophores are, in essence, small-molecule imidazolinone dyes embedded within a protein having a β-barrel tertiary structure (24, 25):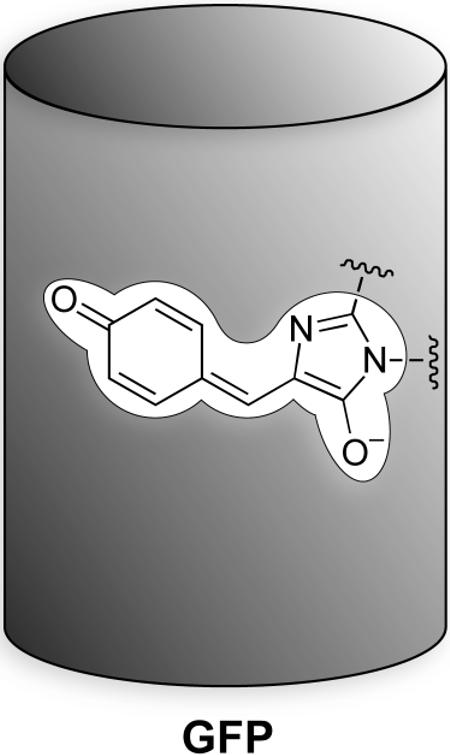 The dye is produced in an autocatalytic manner from native amino-acid residues, its full maturation requiring molecular oxygen and producing an equivalent of hydrogen peroxide (26), a reactive oxygen species. The protein casing is essential, as the naked imidazolinone dye exhibits only meager fluorescence (27). Mutagenesis has produced an assortment of fluorescent proteins with disparate chemical and spectral properties (28, 29) that enable, for example, impressive in vivo imaging experiments (30). Going forward, we expect small-molecule, inorganic, and proteineous fluorophores—each with particular benefits and drawbacks—to continue to facilitate both basic and applied research in chemical biology.
The dye is produced in an autocatalytic manner from native amino-acid residues, its full maturation requiring molecular oxygen and producing an equivalent of hydrogen peroxide (26), a reactive oxygen species. The protein casing is essential, as the naked imidazolinone dye exhibits only meager fluorescence (27). Mutagenesis has produced an assortment of fluorescent proteins with disparate chemical and spectral properties (28, 29) that enable, for example, impressive in vivo imaging experiments (30). Going forward, we expect small-molecule, inorganic, and proteineous fluorophores—each with particular benefits and drawbacks—to continue to facilitate both basic and applied research in chemical biology.
Fluorescence
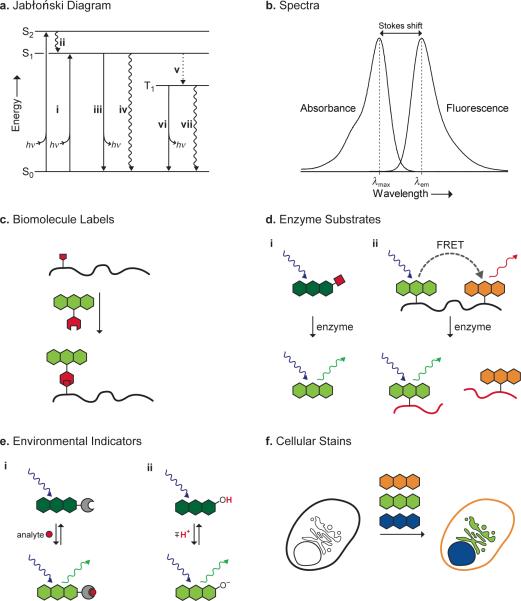
The process of fluorescence is illustrated in the Jabłoński diagram shown in Figure 1a (10). Although this review is focused on single-photon excitation processes, multiphoton excitation is also an important and vibrant field (31). The fluorescence process begins when a molecule in a singlet electronic ground state (S0) absorbs a photon of suitable energy. This promotes an electron to higher energy orbitals, which relax quickly to the first singlet excited state (S1). The decay of the excited state can occur with photon emission (i.e., fluorescence) or in a non-radiative (NR) fashion. This non-radiative “quenching” of the fluorophore excited state can occur through one of a variety of processes, including bond rotation or vibration, molecular collision (32), and photoinduced electron transfer (PeT) (33). The excited state can also undergo forbidden intersystem crossing (ITC) to the triplet excited state (T1) and subsequent relaxation either by photon emission (i.e., phosphorescence) or NR decay. ITC efficiency is increased by substitution with, or proximity to, atoms with high atomic number due to spin-orbit coupling—a phenomenon commonly termed the “heavy atom effect” (34). Another important pathway for decay of the singlet excited state involves Förster resonance energy transfer (FRET) to an acceptor molecule. This process is distance dependent, and can be used as a “spectroscopic ruler” to measure the proximity of labeled entities (35).
Figure 1.
Photophysical concepts (a, b) and biological applications (c–f) of small-molecule fluorophores. a) Jabłoński diagram. i) Absorption of a photon gives an excited state. ii) Internal conversion to S1. iii) Fluorescence. iv) Non-radiative decay. v) Intersystem crossing to T1. vi) Phosphorescence. vii) Non-radiative decay. b) Generic absorption and emission spectra. c) Site-specific labeling of a biomolecule by an orthogonal reaction between two functional groups (red). d) Enzyme substrates. i) Enzyme-catalyzed removal of a blocking group (red) elicits a change in fluorescence. ii) Enzyme catalyzes the cleavage of a labeled biomolecule (red) and concomitant decrease in FRET. e) Environmental indicators. i) Binding of an analyte (red) elicits a change in fluorescence. ii) Protonation of a fluorophore elicits a change in fluorescence. f) Staining of subcellular domains by distinct fluorophores.
A generic absorption/emission spectrum is shown in Figure 1b. The maximal absorption (λmax) is related to the energy between the S0 and the higher energy levels. The absorptivity of a molecule at λmax is given by the extinction coefficient (ε), defined by the Beer–Lambert–Bouguer law. The maximal emission wavelength (λem) is longer (i.e., lower in energy) than λmax due to energy losses by solvent reorganization or other processes (6). Stokes demonstrated this phenomenon by using a rudimentary filter set consisting of a stained glass window and a goblet of wine (17). The difference between λmax and λem is therefore termed the “Stokes shift”. Fluorophores with small Stokes shifts are susceptible to self-quenching via energy transfer, therefore limiting the number of labels that can be attached to a biomolecule (36). The lifetime of the excited state (τ) can range from 0.1 to >100 ns, and is an important parameter for time-resolved measurements (37) and fluorescence polarization applications (38). Another critical property of a fluorophore is the quantum yield or quantum efficiency (Φ)—essentially the ratio of photons fluoresced to those absorbed.
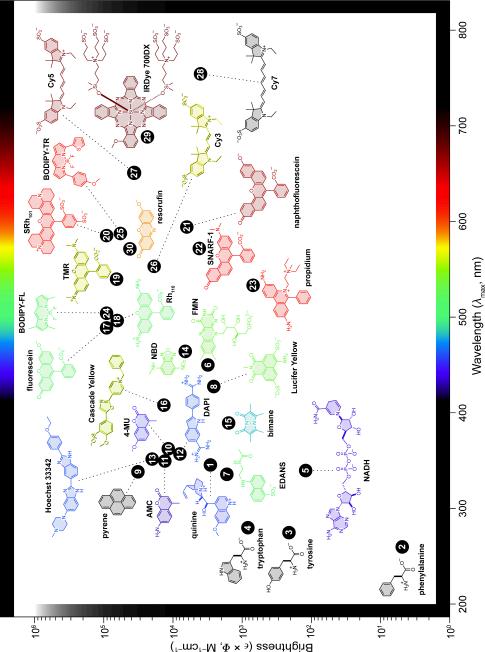
Fluorophores are utilized in many ways, including as labels for biomolecules (Figure 1c), enzyme substrates (Figure 1d), environmental indicators (Figure 1e), and cellular stains (Figure 1f). The utility of a particular fluorophore is dictated by its specific chemical properties (e.g., reactivity, lipophilicity, p_K_a, stability) and photophysical properties (e.g., λmax, λem, ε, Φ, τ). A simple parameter for making meaningful comparisons between different fluorescent molecules is the product of the extinction coefficient and the quantum yield (ε × Φ). This term is directly proportional to the brightness of the dye, accounting for both the amount of light absorbed and the quantum efficiency of the fluorophore. Accurate comparisons between dye molecules must include both of these parameters. A plot of ε × Φ versus λmax for the major classes of biologically significant fluorescent dyes is shown in Figure 2. A more detailed table of the properties of these fluorophores can be found in Table S1 (see: Supporting Information).
Figure 2.
Plot of fluorophore brightness (ε × Φ) versus the wavelength of maximum absorption (λmax) for the major classes of fluorophores. The color of the structure indicates its wavelength of maximum emission (λem). For clarity, only the fluorophoric moiety of some molecules is shown.
Classes of Fluorescent Dyes
Endogenous Fluorophores
Like quinine (1), many naturally occurring compounds exhibit measurable fluorescence (39). These include the aromatic amino acids, whose fluorescence properties were first described by Weber (40). Phenylalanine (2) and tyrosine (3) exhibit weak fluorescence under UV excitation wavelengths. Tryptophan (4) is the most fluorescent natural amino acid, with a λmax of 280 nm, λem of 348 nm, extinction coefficient of 6.3 × 103 M−1cm−1, and a quantum yield of 0.13 (39). Tryptophan fluorescence is environmentally-sensitive and has been used as an index for a variety of processes, including protein folding and ligand binding (41). Tryptophan can also be used in FRET applications (35), or serve as a quencher for a variety of fluorophores by PeT (42).
Other naturally-occurring fluorophores include reduced nicotinamide cofactors (e.g., NADH; 5) that show measurable fluorescence with a λmax/λem of 340/435 nm (43). Flavins are also very important intrinsic fluorophores, with flavin mononucleotide (FMN; 6) showing significant fluorescence with λmax = 450 nm, λem = 530 nm, ε = 1.22 × 104 M−1cm−1, and Φ = 0.25 (39, 44). Other native moieties are fluorescent, including porphyrins and pyridoxal derivatives (39). Collectively, endogenous fluorophores can give rise to “autofluorescence”, which can obfuscate desired signals from labeled entities in imaging and other in cellulo or in vivo experiments (45). Red-shifted dyes can circumvent this background problem, while allowing deeper tissue penetration (46). Long-wavelength excitation is also gentle to DNA, as nucleosides absorb at λmax ≈ 260 nm with ε ≈ 7–15 × 103 M−1cm−1 (47).
Polycyclic Aromatics
Polycyclic aromatic compounds are a widely-used subset of fluorescent dyes. In general, spectral properties correlate to size, and substitution on the abundant open valencies affords a variety of useful probes. A classic category of synthetic biomolecule labels are naphthalene derivatives These include the amine-reactive 5-dimethylaminonaphthalene-1-sulfonyl (dansyl) chloride (48), and other associated fluorophores (49, 50). Another related naphthalene derivative is 5-((2-aminoethyl)amino)naphthalene-1-sulfonic acid (EDANS). Derivatives of this fluorophore, such as compound 7, exhibit a λmax of 336 nm, λem of 520 nm, extinction coefficient of 6.1 × 103 M−1cm−1, and a quantum yield of 0.27 in water (51). EDANS remains in wide use, particularly in FRET-based experiments (52, 53). Naphthalene can be further elaborated to give 4-amino-3,6-disulfonylnaphthalimides (e.g., compound 8) that absorb at 428 nm (54). These fluorophores bear the moniker “Lucifer Yellow”, and are useful polar tracers (13).
Pyrene-derived molecules also find use as probes. Derivatives of pyrene (9) shows λmax/λem of 340/376 nm, ε = 4.3 × 104 M−1cm−1, and Φ = 0.75 (13, 55). The environmental sensitivity of this fluorophore can be used to report on RNA folding (56). Pyrene also exhibits a long-lived excited state (τ >100 ns). This long lifetime allows an excited pyrene molecule to associate with a pyrene in the ground state. The resulting eximer exhibits a bathochromic (i.e., red) shift in fluorescence intensity (λem ≈ 490 nm). This process can be used to measure important biomolecular processes, such as protein conformation (57). Sulfonation of pyrene elicits a bathochromic shift, affording useful compounds that are excited at >390 nm. These compounds include the pH probe 8-hydroxy-1,3,6-pyrenetrisulfonate (HPTS or pyranine) (58), and valuable sulfonated pyrene labels with high water solubility (13, 59).
Other polycyclic aromatic molecules are also sometimes used to construct useful fluorescent tools. Anthracene has been elaborated to prepare sensors for anions such as pyrophosphate (60). Perylene derivatives constitute another intriguing class of fluorophores that exhibit very high quantum yields in organic solvents (61), but require significant structural elaboration to become useful in water (62). Still another functional scaffold is coronene, which exhibits a long lifetime (τ ≈ 200 ns) that is useful in some time-resolved experiments (63).
Coumarins
Coumarins represent a broad class of natural products, pharmaceuticals, and fluorophores. Heteroatom substitution at position 7 of coumarin gives fluorescent molecules with UV or near-UV excitation wavelengths. A common example is 7-hydroxy-4-methylcoumarin (i.e., 4-methylumbelliferone; 4-MU; 10). Under basic conditions, the phenolate form of 4-MU (p_K_a = 7.8) exhibits λmax = 360 nm, λem = 450 nm, ε = 1.7 × 103 M−1cm−1, and Φ = 0.63 (64). The related 7-amino-4-methylcoumarin (AMC; 11) displays similar spectral properties, which are constant above pH 5 (13). The large Stokes shift of coumarins is due in part to the significant change in dipole upon excitation and subsequent loss in energy by solvent reorganization (6).
Molecular probes built on the coumarin scaffold include useful biomolecular labels. Different reactive groups are compatible with this fluorophore, and are typically attached at the 3 or 4 position of coumarin (13). The spectral characteristics of AMC can be tuned through different nitrogen substitution patterns (65). Still other substitutions (e.g., fluoronation or sulfonation) can yield coumarin dyes with desirable chemical properties, such as higher solubility in aqueous solution and lower sensitivity to pH (64, 66).
Coumarins are also useful for assembling enzyme substrates. Various derivatives of 7-hydroxycoumarin can be used to assay an assortment of hydrolases (67, 68) and dealkylases (69). Peptidyl derivatives of AMC are widely used to measure protease activity (70). Microarrays of coumarin substrates have been built to examine protease specificities (71). AMC has also been elaborated to prepare substrates for other enzymes including deacetylases (72) and esterases (73).
Quinolines
The archetypal fluorophore quinine (1), is still employed as a fluorescence standard (74, 75). The 6-methoxyquinoline moiety can be alkylated and the resulting quinolinium species is quenched collisionally by halide ions in solution. Several quinolinium compounds find use as indicators for chloride ion (76). The chelating properties of hydroxyquinoline derivatives have been exploited to create useful fluorescence-based kinase substrates (77) and fluorescent ion indicators (78).
Indoles and Imidizoles
The indole fluorophore has been elaborated beyond tryptophan to construct useful tools such as the calcium indicator “Indo-1” (79). Another notable indole-based probe is 4′,6-diamidino-2-phenylindole (DAPI; 12), which binds in the minor groove of DNA (80). As this binding is accompanied by a large increase in fluorescence, this molecule can be used to stain DNA for cellular imaging or other experiments (81).
The dibenzimidizole dyes originally developed by Hoechst AG are useful DNA-binding probes. Like DAPI, the Hoechst dyes bind in the minor groove of DNA and can be used for fluorescence microscopy and flow cytometry (1). Hoechst 33342 (13) is sufficiently cell-permeable for use in live cells (81). Unlike DAPI, the Hoechst dyes are quenched upon binding to DNA containing 5-bromo-2-deoxyuridine due to the heavy atom effect, thereby allowing cell-cycle analyses (82).
NBD
Another notable example of a small heterocyclic fluorophore is 4-nitrobenz-2-oxa-1,3-diazole (NBD) and other related benzoxadiazole compounds. Examples include the amine- or thiol-reactive NBD-Cl (83) and the thiol-reactive 7-chlorobenz-2-oxa-1,3-diazole-4-sulfonate (SBD-Cl) (84). Primary amine adducts of NBD-Cl (e.g., compound 14) exhibit photophysical properties that belie the size of the molecule. Such derivatives emit in the green portion of the spectrum, with a λmax = 465 nm, λem = 535 nm, ε = 2.2 × 104 M−1cm−1, and Φ = 0.3 in MeOH (13). This lightweight fluorophore allows conjugates with small molecules, such as sugars, to retain biological activity (85). The environmentally-sensitive fluorescence of NBD derivatives (86) can be exploited in a variety of ways, including the preparation of lipid probes (87) and novel kinase substrates (88).
Other UV-Excited Fluorophores
There are numerous examples of other small heterocyclic molecules as useful fluorescent probes. These include the 1,5-diazabicyclo[3.3.0]octa-3,6-diene-2,8-dione (i.e., bimane) structure (15) that exhibits moderate fluorescence with a λmax = 390 nm, λem = 482 nm, and Φ = 0.3 in aqueous solution (89). Halogenated versions of these fluorophores are useful thiol-reactive labels, and can be used as fluorescent cross-linkers (90). Additional significant core dyes involve diaryloxazole structures, which can exhibit large Stokes shifts (91). This structure can be elaborated to yield useful organelle stains (92) and fluorescent labels (93). An example is the “Cascade Yellow” fluorophore (16), which shows λmax = 409 nm, λem = 558 nm, ε = 2.4 × 104 M−1cm−1, and Φ = 0.56 (13, 93).
Fluorescein
The well known xanthene dye fluorescein (17) was first synthesized by Baeyer in 1871 (94). Despite its antiquity, fluorescein remains one of the most widely utilized fluorophores in modern biochemical, biological, and medicinal research. Fluorescein exhibits several interesting (and underappreciated) properties in aqueous solution. For example, fluorescein can exist in seven prototropic forms, with the most biologically-relevant molecular forms being the monoanion and the dianion that interchange with a p_K_a ≈ 6.4 (95). The dianion is the most fluorescent form with a λmax of 490 nm, λem of 514 nm, extinction coefficient of 9.3 × 104 M−1cm−1, and a quantum yield of 0.95 (10, 13).
Fluorescein is an extremely versatile core dye. Fluorescein can be appended with reactive groups to yield important biomolecule labels (96). The structure of fluorescein can be modified further to tune properties such as p_K_a or wavelength. For example, 2′,7′-difluorofluorescein (i.e., Oregon Green) is less basic (p_K_a = 4.6) than fluorescein, maintains fluorescein-like wavelengths, and exhibits increased photostability relative to fluorescein (97). The addition of other substituents, such as chloro groups, affects not only pH sensitivity (98) but also elicits a bathochromic shift in excitation wavelength. Examples include the traditional automated DNA sequencing dye 2′,4,7,7′-tetrachlorofluorescein (TET), which exhibits a λmax/λem of 521/536 nm (13). Fluoresceins containing bromine or iodine substituents have red-shifted spectra and also exhibit significant intersystem crossing due to the heavy atom effect (99).
Fluorescein also serves as a scaffold for preparing indicator molecules. In particular, the pH sensitivity of fluorescein has been exploited to prepare small-molecule pH sensors (100). Changes in the p_K_a of fluorescein can be used as an index to report on the status of fluorescein-labeled biomolecules (95). Appending fluorescein with various chelating moieties affords sensors for biologically important ions. A most noteworthy example is the calcium indicator “Fluo-3” developed by Tsien and co-workers (101), which can be used to measure calcium ion fluxes in live cells and is employed widely in high-throughput screening (102). Other notable examples of fluorescein-based indicators include compounds for detecting sodium (103), zinc (104), palladium (105), mercury(II) (106), and fluoride (107) ions, as well as clever nitric oxide sensors based on chelates with copper(II) (108).
Fluorescein exists in equilibrium between a “closed” lactone and an “open” quinoid form. Acylation or alkylation of the phenolic groups locks the molecule into the nonfluorescent lactone in an aqueous environment, and serves as the basis for a variety of fluorogenic substrates for esterases, phosphatases, glycosylases, and other enzymes (109–112):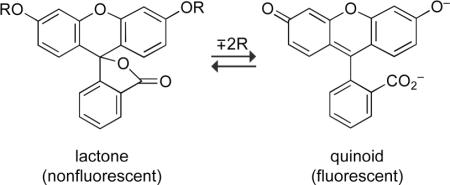 Fluorescein can also be “caged” with photolabile groups and unmasked by distinct wavelengths of light (113). Other substitutions can confer redox sensitivity to the fluorescein molecule (114). Appending fluorescein derivatives with electron-donating substituents on the pendant phenyl ring allows the construction of enzyme substrates with only one substrate moiety. These “Tokyo Green” substrates show improved enzyme kinetics relative to disubstituted fluorescein substrates (115), and can be used for in vivo imaging (116).
Fluorescein can also be “caged” with photolabile groups and unmasked by distinct wavelengths of light (113). Other substitutions can confer redox sensitivity to the fluorescein molecule (114). Appending fluorescein derivatives with electron-donating substituents on the pendant phenyl ring allows the construction of enzyme substrates with only one substrate moiety. These “Tokyo Green” substrates show improved enzyme kinetics relative to disubstituted fluorescein substrates (115), and can be used for in vivo imaging (116).
Rhodamine
Isologues of fluorescein, the rhodamines are used widely as fluorophores. Some key characteristics of this dye class include low pH sensitivity and tunable spectral properties. Different _N_-alkyl substitution patterns on the rhodamine core can modify spectral characteristics. The simplest member of this class, rhodamine 110 (Rh110; 18), exhibits fluorescein-like spectral properties with λmax = 496 nm, λem = 517 nm, ε = 7.4 × 104 M−1cm−1, and Φ = 0.92 in aqueous solution (117). Substitution to tetramethylrhodamine (TMR; 19) gives longer excitation and emission wavelengths (λmax/λem of 540/565 nm) but a lower quantum yield. (Φ = 0.68) (3). This lower quantum yield is likely due to decay of the excited state via rotation around the C–N bond (23). This undesirable decay process can be circumvented by freezing the C–N bond via appropriate substitution. Rhodamines containing rigid julolidine ring systems show higher quantum yields than do the unrestricted dyes (118), and exhibit longer excitation and emission wavelengths (13). Sulforhodamine 101 (SRh101; 20) is a julolidine-based dye that is common in bioresearch. Amine-reactive sulfonyl chloride derivatives of SRh101 are sold under the trademark “Texas Red” (13).
Rhodamine labels are often paired with fluorescein derivatives for FRET-based experiments due to efficient energy transfer between these xanthene compounds (13). Dye constructs containing both fluorescein and rhodamine moieties have proven useful for DNA sequencing. The fluorescein donor of these “BigDye” fluorophores can be excited by a single-wavelength light source, and the emission is dictated by the specific rhodamine derivative that serves as the FRET acceptor (119).
Rhodamines can also be used to assemble enzyme substrates. Acyl substitution of both the amino groups of a rhodamine locks the molecule into a nonfluorescent lactone form. As with fluorescein, this property can be exploited to prepare caged compounds (113), or fluorogenic molecules for enzymatic studies. Substrates based on Rh110 for simple proteases were first described by Mangel in 1983 (120). More recent developments have centered on using Rh110 to build useful caspase substrates to assay apoptosis (121). Rh110-based substrates have also been developed for phophatases (122), esterases (117), and metal-ion catalysis in a cellular context (123).
Rhodamines have been used to build indicators for ions such as sodium (124) and calcium (e.g., Rhod-2) (101). Other rhodamine derivatives have been assembled to detect reactive oxygen species in cells (125). Hybrid structures between fluorescein and rhodamine (i.e., xanthene dyes with one oxygen and one nitrogen substituent) are termed “rhodols” and exhibit interesting spectral properties (126). The unique properties of these rhodol fluorophores can be harnessed to build probes such as ion indicators (127).
Naphthoxanthene Dyes
A notable modification to the fluorescein and rhodamine dyes is the introduction of a fused benzo ring into the xanthene structure. This modification elicits a severe bathochromic shift in excitation and emission wavelengths. A classic example is naphthofluorescein (21), which exhibits much longer wavelengths than does fluorescein (λmax/λem of 595/660 nm) under basic conditions (128). Unfortunately, the advantageous bathochromic shift is countered by an undesirable p_K_a = 8.0—well above the physiological pH—and a lower extinction coefficient (ε = 4.4 × 104 M−1cm−1) and quantum yield (Φ = 0.14) (128). The poor fluorescence properties of naphthofluorescein limits the utility of this scaffold, though some useful derivatives have been reported (128–130).
Xanthene dyes that bear only one fused benzo ring display interesting spectral properties. Unlike the symmetrical fluoresceins and rhodamines, the resonance forms of these seminaphtho dyes are not equivalent, and therefore exhibit dissimilar spectral properties. Thus, the asymmetry of the dye can be yoked to construct ratiometric fluorescent indicators. Probes from the seminaphthofluorescein (SNAFL) core include pH sensors (131) and other ion indicators (132). Rhodol-type seminaphthoxanthenes are also useful pH indicators (131, 133). One example is ratiometric pH sensor 22, which bears the common name “seminaphthorhodafluor-1” (SNARF-1) This compound displays a λmax = 573 nm, λem = 631 nm, ε = 4.4 × 104 M−1cm−1, and Φ = 0.092 at high pH values (131). Derivatives of dye 22 boast useful p_K_a values around 7.5, that can be tuned to lower values by fluorine substitution (133).
Phenanthridines
Phenanthridines derivatives are widely used DNA intercalators that exhibit higher fluorescence intensity upon binding to nucleic acids. Examples include the cationic dyes ethidium and propidium (23). In the presence of DNA, propidium presents λmax = 535 nm, λem = 617 nm, ε = 5.4 × 103 M−1cm−1, and Φ = 0.13 (13, 134). These values constitute a 20–30-fold increase in fluorescence relative to the free dye. The fixed ionic character of compound 23 limits passive diffusion through the intact membrane of living cells. Thus, propidium can be used to identify dead cells with compromised membranes (135).
BODIPY
The boron difluoride dipyrromethene (BODIPY) dye structure has been used to build a variety of useful fluorescent labels and other probes (136). Key features of this dye class are the insensitivity of the spectral properties to environment, the small Stokes shift, and the overall lipophilicity of the dye (13, 137). The core structure of BODIPY is somewhat base sensitive, limiting its use in applications such as solid-phase peptide synthesis (138). The simplest BODIPY 24 shows fluorescein-like parameters with λmax = 505 nm, λem = 511 nm, ε = 9.1 × 104 M−1cm−1, and Φ = 0.94 and bears the common name “BODIPY-FL” (3, 13). Another important property of this class of dyes is the tunability of wavelength through appropriate substitution. BODIPY dyes can thus serve as surrogates for traditional dyes such as fluorescein, tetramethylrhodamine, and many others. One example is the “BODIPY-TR” fluorophore (25) which exhibits spectral properties similar to those of Texas Red (i.e., SRh101; 20; refs (3, 13)).
The ensemble of probes built on the BODIPY scaffold are centered largely on fluorescent labels, but some indicators for ions and other molecules have been reported (103, 139). These fluorophores are particularly useful labels for fluorescence polarization techniques (140). The nonpolar character of BODIPY allows incorporation into lipophilic probes (137). Moreover, the small Stokes shift of BODIPY dyes causes efficient self-quenching of overlabeled biomolecules. This phenomenon can be utilized to create useful protease substrates, as proteolysis of densely labeled proteins leads to an increase in fluorescence intensity (141).
Cyanines
The term “cyanine dye” denotes a dye system with a polymethine chain between two nitrogens (i.e., R2N–(CH=CH)n–CH=N+R2). This dye system, which resembles the retinaldimine visual pigment of rhodopsin (142), has been the subject of many seminal studies on the molecular basis of color (143). Numerous cyanines and associated polymethine structures are useful as labels (144), DNA stains (134), and membrane potential sensors (145–147). Perhaps the most well-known cyanine dyes in modern bioresearch are the “CyDye” fluorophores, which are based on a sulfoindocyanine structure (148). These compounds are given common names according to the number of carbon atoms between the dihydroindole units. Cy3 (26) shows spectral characteristics that are comparable to TMR with λmax = 554, λem = 568 nm, ε = 1.3 × 105 M−1cm−1, and Φ = 0.14 in water. Cy5 (27) exhibits longer wavelengths with λmax = 652 nm, λem = 672 nm, ε = 2.0 × 105 M−1cm−1, and Φ = 0.18. Longer cyanine constructs, such as Cy7 (28), exhibit a λmax/λem of 755/788 nm, albeit with a lower quantum yield (Φ = 0.02) (2). Further elaboration of the cyanine core can provide control over wavelength. For example, introduction of a fused benzo ring in the dihydroindole moieties elicits a bathochromic shift of ~20–30 nm (149). This structural modification is designated with a “.5” suffix (e.g., “Cy5.5”).
The CyDyes are useful biomolecular labels and are now the standard fluorophores for microarrays and many other analyses (14). CyDye pairs are also often used for FRET experiments (150) and can be utilized as photo-switchable probes for ultrahigh-resolution imaging (151). A significant drawback to cyanine labels is the severe dependence of the fluorescence of their bioconjugates on the number of fluorophores per biomolecule. This phenomenon likely has several causes, and can limit the utility of CyDye conjugates in some applications (152). Newer (albeit structurally mysterious) sulfonated cyanine dyes reportedly overcome this problem (153).
Phthalocyanines
The phthalocyanine structure serves as a scaffold for a variety of interesting compounds, from pigments to photosensitizers. Wavelength absorption and other properties can be tuned by structural modification or through substitution of metal centers (20). To prevent dye aggregation and facilitate water solubility, inclusion of numerous ionic substituents is necessary (154). A successful example of a phthalocyanine fluorescent label is IRDye 700DX (29), which shows λmax = 689 nm, λem = 700 nm, ε = 1.7 × 105 M−1cm−1, Φ = 0.14, and excellent photostability (155).
Oxazines
Substituted oxazine compounds are useful fluorophores. Of particular importance is resorufin (30), whose anion exhibits λmax = 572 nm, λem = 585 nm, ε = 5.6 × 104 M−1cm−1, and quantum yield = 0.74. These attributes have some sensitivity to pH, as resorufin has a p_K_a of 5.8 (156). Use of the resorufin scaffold to prepare fluorescent labels has been limited (157), though this dye has been used to construct fluorogenic molecules that are unmasked by various hydrolases (158–160) and cytochrome P450 enzymes (161).
Resorufin exhibits interesting redox properties. Oxidation to the _N_-oxide yields resazurin, which is only weakly fluorescent. Resazurin can be reduced to resorufin by biological reducing equivalents, and thus has been used to assay cell viability (162). In addition, reduced versions of resorufin are nonfluorescent, but can be oxidized to resorufin by hydrogen peroxide in the presence of horseradish peroxidase. These compounds are useful for the ELISA and other assays (163).
Other important oxazine dyes include cresyl violet, which can be elaborated to give substrates for proteases (164) and esterases (73). A key property of several oxazine fluorophores is their environmental sensitivity. These compounds can be used to prepare useful compounds, such as labels to report on protein conformation (165).
Conclusions
Known small-molecule fluorophores have a wide range of spectral and chemical properties. Elaboration of these core structures has provided numerous probes for assaying biological systems. Nonetheless, extraordinary opportunities remain, as delving deeper into biochemical and biological phenomena will require ever more sophisticated and tailored probes. Scientists who straddle the fields of chemistry and biology are best equipped to fashion these tools, and then wield them to illuminate otherwise inscrutable life processes.
Supplementary Material
Supporting Information
Acknowledgment
We are grateful to Z.J. Diwu. and T.J. Rutkoski for contributive discussions, and H.A. Steinberg for artistic assistance with Figure 2 and the TOC graphic. L.D.L was supported by Biotechnology Training Grant 08349 (NIH) and an ACS Division of Organic Chemistry Graduate Fellowship sponsored by The Genentech Foundation. Related work in our laboratory was supported by grant CA73808 (NIH).
Footnotes
Supporting Information Available: A table detailing the photophysical properties of fluorophores 1–30 (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Petit J-M, Denis-Gay M, Ratinaud M-H. Assessment of fluorochromes for cellular structure and function studies by flow cytometry. Biol. Cell. 1993;78:1–13. doi: 10.1016/0248-4900(93)90109-r. [DOI] [PubMed] [Google Scholar]
- 2.Waggoner A, Kenneth S. Covalent labeling of proteins and nucleic acids with fluorophores. Methods Enzymol. 1995;246:362–373. doi: 10.1016/0076-6879(95)46017-9. [DOI] [PubMed] [Google Scholar]
- 3.Johnson I. Fluorescent probes for living cells. Histochem. J. 1998;30:123–140. doi: 10.1023/a:1003287101868. [DOI] [PubMed] [Google Scholar]
- 4.Boonacker E, Van Noorden CJF. Enzyme cytochemical techniques for metabolic mapping in living cells, with special reference to proteolysis. J. Histochem. Cytochem. 2001;49:1473–1486. doi: 10.1177/002215540104901201. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Campbell RE, Ting AY, Tsien RY. Creating new fluorescent probes for cell biology. Nat. Rev. Mol. Cell Biol. 2002;3:906–918. doi: 10.1038/nrm976. [DOI] [PubMed] [Google Scholar]
- 6.Valeur B. Molecular Fluorescence: Principles and Applications. Wiley–VCH; Weinheim: 2002. [Google Scholar]
- 7.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 2003;7:626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Goddard J-P, Reymond J-L. Enzyme assays for high-throughput screening. Curr. Opin. Biotechnol. 2004;15:314. doi: 10.1016/j.copbio.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Giepmans BNG, Adams SR, Ellisman MH, Tsien RY. The fluorescent toolbox for assessing protein location and function. Science. 2006;312:217–224. doi: 10.1126/science.1124618. [DOI] [PubMed] [Google Scholar]
- 10.Lakowicz JR. Principles of Fluorescence Spectroscopy. 3rd ed. Springer; New York: 2006. [Google Scholar]
- 11.Sadaghiani AM, Verhelst SHL, Bogyo M. Tagging and detection strategies for activity-based proteomics. Curr. Opin. Chem. Biol. 2007;11:20–28. doi: 10.1016/j.cbpa.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 12.Johnsson N, Johnsson K. Chemical tools for biomolecular imaging. ACS Chem. Biol. 2007;2:31–38. doi: 10.1021/cb6003977. [DOI] [PubMed] [Google Scholar]
- 13.Haugland RP, Spence MTZ, Johnson ID, Basey A. The Handbook: A Guide to Fluorescent Probes and Labeling Technologies. 10th ed. Molecular Probes; Eugene, OR: 2005. [Google Scholar]
- 14.Waggoner A. Fluorescent labels for proteomics and genomics. Curr. Opin. Chem. Biol. 2006;10:62–66. doi: 10.1016/j.cbpa.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Kaufman TS, Rúveda EA. The quest for quinine: Those who won the battles and those who won the war. Angew. Chem., Int. Ed. 2005;44:854–885. doi: 10.1002/anie.200400663. [DOI] [PubMed] [Google Scholar]
- 16.Herschel JFW. On a case of superficial colour presented by a homogeneous liquid internally colourless. Phil. Trans. R. Soc. London. 1845;135:143–145. [Google Scholar]
- 17.Stokes GG. On the change of refrangibility of light. Phil. Trans. R. Soc. London. 1852;142:463–562. [Google Scholar]
- 18.Seeman JI. The Woodward–Doering/Rabe–Kindler total synthesis of quinine: Setting the record straight. Angew. Chem., Int. Ed. 2007;46:1378–1413. doi: 10.1002/anie.200601551. [DOI] [PubMed] [Google Scholar]
- 19.Garfield S. Mauve: How One Man Invented a Color that Changed the World. W.W. Norton & Co.; New York: 2001. [Google Scholar]
- 20.Christie RM. Colour Chemistry. Royal Society of Chemistry; Cambridge, UK: 2001. [Google Scholar]
- 21.Wainwright M. The use of dyes in modern biomedicine. Biotech. Histochem. 2003;78:147–155. doi: 10.1080/10520290310001602404. [DOI] [PubMed] [Google Scholar]
- 22.Udenfriend S. Development of the spectrophotofluorometer and its commercialization. Protein Sci. 1995;4:542–551. doi: 10.1002/pro.5560040321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drexhage KH. Structure and Properites of Laser Dyes. In: Schäfer FP, editor. Dye Lasers. 2nd ed. Springer–Verlag; Berlin: 1977. pp. 144–193. [Google Scholar]
- 24.Yang F, Moss LG, Phillips GN., Jr. The molecular structure of green fluorescent protein. Nat. Biotechnol. 1996;14:1246–1251. doi: 10.1038/nbt1096-1246. [DOI] [PubMed] [Google Scholar]
- 25.Ormö M, Cubitt AB, Kallio K, Gross LA, Tsien RY, Remington SJ. Crystal structure of the Aequorea victoria green fluorescent protein. Science. 1996;273:1392–1395. doi: 10.1126/science.273.5280.1392. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Patel HN, Lappe JW, Wachter RM. Reaction progress of chromophore biogenesis in green fluorescent protein. J. Am. Chem. Soc. 2006;128:4766–4772. doi: 10.1021/ja0580439. [DOI] [PubMed] [Google Scholar]
- 27.Niwa H, Inouye S, Hirano T, Matsuno T, Kojima S, Kubota M, Ohashi M, Tsuji Frederick I. Chemical nature of the light emitter of the Aequorea green fluorescent protein. Proc. Natl. Acad. Sci. U.S.A. 1996;93:13617–13622. doi: 10.1073/pnas.93.24.13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Remington SJ. Fluorescent proteins: Maturation, photochemistry and photophysics. Curr. Opin. Struct. Biol. 2006;16:714–721. doi: 10.1016/j.sbi.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Shaner NC, Patterson GH, Davidson MW. Advances in fluorescent protein technology. J. Cell Sci. 2007;120:4247–4260. doi: 10.1242/jcs.005801. [DOI] [PubMed] [Google Scholar]
- 30.Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, Sanes JR, Lichtman JW. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007;450:56–62. doi: 10.1038/nature06293. [DOI] [PubMed] [Google Scholar]
- 31.Svoboda K, Yasuda R. Principles of two-photon excitation microscopy and its applications to neuroscience. Neuron. 2006;50:823–839. doi: 10.1016/j.neuron.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 32.Zelent B, Kusba J, Gryczynski I, Johnson ML, Lakowicz JR. Time-resolved and steady-state fluorescence quenching of N-acetyl-tryptophanamide by acrylamide and iodide. Biophys. Chem. 1998;73:53. doi: 10.1016/s0301-4622(98)00137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Silva AP, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, Rice TE. Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 1997;97:1515–1566. doi: 10.1021/cr960386p. [DOI] [PubMed] [Google Scholar]
- 34.McGlynn SP, Daigre J, Smith FJ. External heavy-atom spin–orbital coupling effect. IV. Intersystem crossing. J. Chem. Phys. 1963;39:675–679. [Google Scholar]
- 35.Sapsford KE, Berti L, Medintz IL. Materials for fluorescence resonance energy transfer analysis: Beyond traditional donor–acceptor combinations. Angew. Chem., Int. Ed. 2006;45:4562–4588. doi: 10.1002/anie.200503873. [DOI] [PubMed] [Google Scholar]
- 36.Hemmilä IA. Applications of Fluorescence in Immunoassays. Wiley; New York: 1991. [Google Scholar]
- 37.Bright FV, Munson CA. Time-resolved fluorescence spectroscopy for illuminating complex systems. Anal. Chim. Acta. 2003;500:71–104. [Google Scholar]
- 38.Owicki JC. Fluorescence polarization and anisotropy in high throughput screening: Perspectives and primer. J. Biomolecular Screen. 2000;5:297–306. doi: 10.1177/108705710000500501. [DOI] [PubMed] [Google Scholar]
- 39.Wolfbeis OS. The fluorescence of organic natural products. In: Schulman SG, editor. Molecular Luminescence Spectroscopy: Methods and Applications—Part 1. Wiley; New York: 1985. pp. 167–317. [Google Scholar]
- 40.Teale FW, Weber G. Ultraviolet fluorescence of the aromatic amino acids. Biochem. J. 1957;65:476–482. doi: 10.1042/bj0650476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beechem JM, Brand L. Time-resolved fluorescence of proteins. Annu. Rev. Biochem. 1985;54:43–71. doi: 10.1146/annurev.bi.54.070185.000355. [DOI] [PubMed] [Google Scholar]
- 42.Marmé N, Knemeyer J-P, Wolfrum J, Sauer M. Highly sensitive protease assay using fluorescence quenching of peptide probes based on photoinduced electron transfer. Angew. Chem., Int. Ed. 2004;43:3798–3801. doi: 10.1002/anie.200453835. [DOI] [PubMed] [Google Scholar]
- 43.Weber G. Intramolecular transfer of electronic energy in dihydrodiphosphopyridine nucleotide. Nature. 1957;180:1409. [Google Scholar]
- 44.Whitby LG. A new method for preparing flavin-adenine dinucleotide. Biochem. J. 1953;54:437–442. doi: 10.1042/bj0540437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aubin JE. Autofluorescence of viable cultured mammalian cells. J. Histochem. Cytochem. 1979;27:36–43. doi: 10.1177/27.1.220325. [DOI] [PubMed] [Google Scholar]
- 46.Ballou B, Ernst LA, Waggoner AS. Fluorescence imaging of tumors in vivo. Curr. Med. Chem. 2005;12:795–805. doi: 10.2174/0929867053507324. [DOI] [PubMed] [Google Scholar]
- 47.Cavaluzzi MJ, Borer PN. Revised UV extinction coefficients for nucleoside-5'-monophosphates and unpaired DNA and RNA. Nucleic Acids Res. 2004;32:e13. doi: 10.1093/nar/gnh015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weber G. Polarization of the fluorescence of macromolecules II. Fluorescent conjugates of ovalbumin and bovine serum albumin. Biochem. J. 1952;51:155–167. doi: 10.1042/bj0510155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daniel E, Weber G. Cooperative effects in binding by bovine serum albumin. I. The binding of 1-anilino-8-naphthalenesulfonate. Fluorimetric titrations. Biochemistry. 1966;5:1893–1900. doi: 10.1021/bi00870a016. [DOI] [PubMed] [Google Scholar]
- 50.Weber G, Farris FJ. Synthesis and spectral properties of a hydrophobic fluorescent probe: 6-Propionyl-2-(dimethylamino)naphthalene. Biochemistry. 1979;18:3075–3078. doi: 10.1021/bi00581a025. [DOI] [PubMed] [Google Scholar]
- 51.Hudson EN, Weber G. Synthesis and characterization of two fluorescent sulfhydryl reagents. Biochemistry. 1973;12:4154–4161. doi: 10.1021/bi00745a019. [DOI] [PubMed] [Google Scholar]
- 52.Maggiora LL, Smith CW, Zhang ZY. A general method for the preparation of internally quenched fluorogenic protease substrates using solid-phase peptide synthesis. J. Med. Chem. 1992;35:3727–3730. doi: 10.1021/jm00099a001. [DOI] [PubMed] [Google Scholar]
- 53.Tyagi S, Kramer FR. Molecular beacons: Probes that fluoresce upon hybridization. Nat. Biotechnol. 1996;14:303–308. doi: 10.1038/nbt0396-303. [DOI] [PubMed] [Google Scholar]
- 54.Stewart WW. Synthesis of 3,6-disulfonated 4-aminonaphthalimides. J. Am. Chem. Soc. 1981;103:7615–7620. [Google Scholar]
- 55.Karpovich DS, Blanchard GJ. Relating the polarity-dependent fluorescence response of pyrene to vibronic coupling. Achieving a fundamental understanding of the py polarity scale. J. Phys. Chem. 1995;99:3951–3958. [Google Scholar]
- 56.Smalley MK, Silverman SK. Fluorescence of covalently attached pyrene as a general RNA folding probe. Nucleic Acids Res. 2006;34:152–166. doi: 10.1093/nar/gkj420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sahoo D, Narayanaswami V, Kay CM, Ryan RO. Pyrene excimer fluorescence: A spatially sensitive probe to monitor lipid-induced helical rearrangement of apolipophorin III. Biochemistry. 2000;39:6594–6601. doi: 10.1021/bi992609m. [DOI] [PubMed] [Google Scholar]
- 58.Kano K, Fendler JH. Pyranine as a sensitive pH probe for liposome interiors and surfaces. pH gradients across phospholipid vesicles. Biochim. Biophys. Acta. 1978;509:289–299. doi: 10.1016/0005-2736(78)90048-2. [DOI] [PubMed] [Google Scholar]
- 59.Whitaker JE, Haugland RP, Moore PL, Hewitt PC, Reese M, Haugland RP. Cascade blue derivatives: Water soluble, reactive, blue emission dyes evaluated as fluorescent labels and tracers. Anal. Biochem. 1991;198:119–130. doi: 10.1016/0003-2697(91)90515-u. [DOI] [PubMed] [Google Scholar]
- 60.Gunnlaugsson T, Glynn M, Tocci GM, Kruger PE, Pfeffer FM. Anion recognition and sensing in organic and aqueous media using luminescent and colorimetric sensors. Coord. Chem. Rev. 2006;250:3094. [Google Scholar]
- 61.Süßmeier F, Langhals H. Novel fluorescence labels: The synthesis of perylene-3,4,9-tricarboxylic imides. Eur. J. Org. Chem. 2001;2001:607–610. [Google Scholar]
- 62.Kohl C, Weil T, Qu J, Müllen K. Towards highly fluorescent and water-soluble perylene dyes. Chem.—Eur. J. 2004;10:5297–5310. doi: 10.1002/chem.200400291. [DOI] [PubMed] [Google Scholar]
- 63.Davenport L, Shen B, Joseph TW, Straher MP. A novel fluorescent coronenyl-phospholipid analogue for investigations of submicrosecond lipid fluctuations. Chem. Phys. Lipids. 2001;109:145–156. doi: 10.1016/s0009-3084(00)00214-0. [DOI] [PubMed] [Google Scholar]
- 64.Sun W-C, Gee KR, Haugland RP. Synthesis of novel fluorinated coumarins: Excellent UV-light excitable fluorescent dyes. Bioorg. Med. Chem. Lett. 1998;8:3107–3110. doi: 10.1016/s0960-894x(98)00578-2. [DOI] [PubMed] [Google Scholar]
- 65.Grandberg II, Denisov LK, Popova OA. 7-Aminocoumarins. Chem. Heterocycl. Compd. (N.Y.) 1987;23:117–142. [Google Scholar]
- 66.Panchuk-Voloshina N, Haugland RP, Bishop-Stewart J, Bhalgat MK, Millard PJ, Mao F, Leung W-Y, Haugland RP. Alexa Dyes, a series of new fluorescent dyes that yield exceptionally bright, photostable conjugates. J. Histochem. Cytochem. 1999;47:1179–1188. doi: 10.1177/002215549904700910. [DOI] [PubMed] [Google Scholar]
- 67.Gee KR, Sun W-C, Bhalgat MK, Upson RH, Klaubert DH, Latham KA, Haugland RP. Fluorogenic substrates based on fluorinated umbelliferones for continuous assays of phosphatases and β-galactosidases. Anal. Biochem. 1999;273:41–48. doi: 10.1006/abio.1999.4202. [DOI] [PubMed] [Google Scholar]
- 68.Babiak P, Reymond JL. A high-throughput, low-volume enzyme assay on solid support. Anal. Chem. 2005;77:373–377. doi: 10.1021/ac048611n. [DOI] [PubMed] [Google Scholar]
- 69.Yamazaki H, Inoue K, Mimura M, Oda Y, Guengerich FP, Shimada T. 7-Ethoxycoumarin O-deethylation catalyzed by cytochromes P450 1A2 and 2E1 in human liver microsomes. Biochem. Pharmacol. 1996;51:313. doi: 10.1016/0006-2952(95)02178-7. [DOI] [PubMed] [Google Scholar]
- 70.Zimmerman M, Ashe B, Yurewicz EC, Patel G. Sensitive assays for trypsin, elastase, and chymotrypsin using new fluorogenic substrates. Anal. Biochem. 1977;78:47–51. doi: 10.1016/0003-2697(77)90006-9. [DOI] [PubMed] [Google Scholar]
- 71.Salisbury CM, Maly DJ, Ellman JA. Peptide microarrays for the determination of protease substrate specificity. J. Am. Chem. Soc. 2002;124:14868–14870. doi: 10.1021/ja027477q. [DOI] [PubMed] [Google Scholar]
- 72.Wegener D, Wirsching F, Riester D, Schwienhorst A. A fluorogenic histone deacetylase assay well suited for high-throughput activity screening. Chem. Biol. 2003;10:61–68. doi: 10.1016/s1074-5521(02)00305-8. [DOI] [PubMed] [Google Scholar]
- 73.Lavis LD, Chao TY, Raines RT. Latent blue and red fluorophores based on the trimethyl lock. ChemBioChem. 2006;7:1151–1154. doi: 10.1002/cbic.200500559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schulman SG, Threatte RM, Capomacchia AC, Paul WL. Fluorescence of 6-methoxyquinoline, quinine, and quinidine in aqueous media. J. Pharm. Sci. 1974;63:876–880. doi: 10.1002/jps.2600630615. [DOI] [PubMed] [Google Scholar]
- 75.Eaton DF. Reference materials for fluorescence measurement. Pure Appl. Chem. 1988;60:1107–1114. [Google Scholar]
- 76.Jayaraman S, Verkman AS. Quenching mechanism of quinolinium-type chloride-sensitive fluorescent indicators. Biophys. Chem. 2000;85:49–57. doi: 10.1016/s0301-4622(00)00146-0. [DOI] [PubMed] [Google Scholar]
- 77.Shults MD, Carrico-Moniz D, Imperiali B. Optimal Sox-based fluorescent chemosensor design for serine/threonine protein kinases. Anal. Biochem. 2006;352:198–207. doi: 10.1016/j.ab.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 78.Tsien RY. New calcium indicators and buffers with high selectivity against magnesium and protons: Design, synthesis, and properties of prototype structures. Biochemistry. 1980;19:2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- 79.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 80.Larsen TA, Goodsell DS, Cascio D, Grzeskowiak K, Dickerson RE. The structure of DAPI bound to DNA. J. Biomol. Struct. Dyn. 1989;7:477–491. doi: 10.1080/07391102.1989.10508505. [DOI] [PubMed] [Google Scholar]
- 81.Crissman HA, Hirons GT. Staining of DNA in live and fixed cells. Methods Cell Biol. 1994;41:195–209. doi: 10.1016/s0091-679x(08)61718-5. [DOI] [PubMed] [Google Scholar]
- 82.Mozdziak PE, Pulvermacher PM, Schultz E, Schell K. Hoechst fluorescence intensity can be used to separate viable bromodeoxyuridine-labeled cells from viable non-bromodeoxyuridine-labeled cells. Cytometry. 2000;41:89–95. doi: 10.1002/1097-0320(20001001)41:2<89::aid-cyto2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 83.Ghosh PB, Whitehouse MW. 7-Chloro-4-nitrobenzo-2-oxa-1,3-diazole: A new fluorogenic reagent for amino acids and other amines. Biochem. J. 1968;108:155–156. doi: 10.1042/bj1080155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Andrews JL, Ghosh P, Ternai B, Whitehouse MW. Ammonium 4-chloro-7-sulfobenzofurazan: A new fluorigenic [sic] thiol-specific reagent. Arch. Biochem. Biophys. 1982;214:386–396. doi: 10.1016/0003-9861(82)90043-1. [DOI] [PubMed] [Google Scholar]
- 85.Levi J, Cheng Z, Gheysens O, Patel M, Chan CT, Wang Y, Namavari M, Gambhir SS. Fluorescent fructose derivatives for imaging breast cancer cells. Bioconjugate Chem. 2007;18:628–634. doi: 10.1021/bc060184s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin S, Struve WS. Time-resolved fluorescence of nitrobenzoxadiazole aminohexanoic acid: Effect of intermolecular hydrogen-bonding on nonradiative decay. Photochem. Photobiol. 1991;54:361–365. doi: 10.1111/j.1751-1097.1991.tb02028.x. [DOI] [PubMed] [Google Scholar]
- 87.Chattopadhyay A. Chemistry and biology of N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-labeled lipids: Fluorescent probes of biological and model membranes. Chem. Phys. Lipids. 1990;53:1–15. doi: 10.1016/0009-3084(90)90128-e. [DOI] [PubMed] [Google Scholar]
- 88.Dai Z, Dulyaninova NG, Kumar S, Bresnick AR, Lawrence DS. Visual snapshots of intracellular kinase activity at the onset of mitosis. Chem. Biol. 2007;14:1254–1260. doi: 10.1016/j.chembiol.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kosower NS, Kosower EM, Newton GL, Ranney HM. Bimane fluorescent labels: Labeling of normal human red cells under physiological conditions. Proc. Natl. Acad. Sci. U.S.A. 1979;76:3382–3386. doi: 10.1073/pnas.76.7.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim JS, Raines RT. Dibromobimane as a fluorescent crosslinking reagent. Anal. Biochem. 1995;225:174–176. doi: 10.1006/abio.1995.1131. [DOI] [PubMed] [Google Scholar]
- 91.Diwu Z, Zhang C, Klaubert DH, Haugland RP. Fluorescent molecular probes VI: The spectral properties and potential biological applications of water-soluble Dapoxyl™ sulfonic acid. J. Photochem. Photobiol., A. 2000;131:95–100. [Google Scholar]
- 92.Diwu Z, Chen CS, Zhang C, Klaubert DH, Haugland RP. A novel acidotropic pH indicator and its potential application in labeling acidic organelles of live cells. Chem. Biol. 1999;6:411–418. doi: 10.1016/s1074-5521(99)80059-3. [DOI] [PubMed] [Google Scholar]
- 93.Anderson MT, Baumgarth N, Haugland RP, Gerstein RM, Tjioe T, Herzenberg LA, Herzenberg LA. Pairs of violet-light-excited fluorochromes for flow cytometric analysis. Cytometry. 1998;33:435–444. doi: 10.1002/(sici)1097-0320(19981201)33:4<435::aid-cyto7>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 94.Baeyer A. Ueber eine neue Klasse von Farbstoffen. Ber. Dtsch. Chem. Ges. 1871;4:555–558. [Google Scholar]
- 95.Lavis LD, Rutkoski TJ, Raines RT. Tuning the pKa of fluorescein to optimize binding assays. Anal. Chem. 2007;79:6775–6782. doi: 10.1021/ac070907g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brinkley M. A brief survey of methods for preparing protein conjugates with dyes, haptens, and cross-linking reagents. Bioconjugate Chem. 1992;3:2–13. doi: 10.1021/bc00013a001. [DOI] [PubMed] [Google Scholar]
- 97.Sun W-C, Gee KR, Klaubert DH, Haugland RP. Synthesis of fluorinated fluoresceins. J. Org. Chem. 1997;62:6469–6475. [Google Scholar]
- 98.Mchedlov-Petrossyan NO, Rubtsov MI, Lukatskaya LL. Ionization and tautomerism of chloro-derivatives of fluorescein in water and aqueous acetone. Dyes Pigm. 1992;18:179–198. [Google Scholar]
- 99.Fleming GR, Knight AWE, Morris JM, Morrison RJS, Robinson GW. Picosecond fluorescence studies of xanthene dyes. J. Am. Chem. Soc. 1977;99:4306–4311. [Google Scholar]
- 100.Graber ML, Dilillo DC, Friedman BL, Pastorizamunoz E. Characteristics of fluoroprobes for measuring intracellular pH. Anal. Biochem. 1986;156:202–212. doi: 10.1016/0003-2697(86)90174-0. [DOI] [PubMed] [Google Scholar]
- 101.Minta A, Kao JP, Tsien RY. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J. Biol. Chem. 1989;264:8171–8178. [PubMed] [Google Scholar]
- 102.Inglese J, Johnson RL, Simeonov A, Xia M, Zheng W, Austin CP, Auld DS. High-throughput screening assays for the identification of chemical probes. Nat. Chem. Biol. 2007;3:466–479. doi: 10.1038/nchembio.2007.17. [DOI] [PubMed] [Google Scholar]
- 103.Martin VV, Rothe A, Gee KR. Fluorescent metal ion indicators based on benzoannelated crown systems: A green fluorescent indicator for intracellular sodium ions. Bioorg. Med. Chem. Lett. 2005;15:1851–1855. doi: 10.1016/j.bmcl.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 104.Kikuchi K, Komatsu K, Nagano T. Zinc sensing for cellular application. Curr. Opin. Chem. Biol. 2004;8:182–191. doi: 10.1016/j.cbpa.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 105.Song F, Garner AL, Koide K. A highly sensitive fluorescent sensor for palladium based on the allylic oxidative insertion mechanism. J. Am. Chem. Soc. 2007;129:12354–12355. doi: 10.1021/ja073910q. [DOI] [PubMed] [Google Scholar]
- 106.Yoon S, Miller Evan W., He Q, Do Patrick H., Chang Christopher J. A bright and specific fluorescent sensor for mercury in water, cells, and tissue. Angew. Chem., Int. Ed. 2007;46:6658–6661. doi: 10.1002/anie.200701785. [DOI] [PubMed] [Google Scholar]
- 107.Yang X-F, Ye S-J, Bai Q, Wang X-Q. A fluorescein-based fluorogenic probe for fluoride ion based on the fluoride-induced cleavage of tert-butyldimethylsilyl ether. J. Fluoresc. 2007;17:81–87. doi: 10.1007/s10895-006-0140-6. [DOI] [PubMed] [Google Scholar]
- 108.Lim MH, Lippard SJ. Metal-based turn-on fluorescent probes for sensing nitric oxide. Acc. Chem. Res. 2007;40:41–51. doi: 10.1021/ar950149t. [DOI] [PubMed] [Google Scholar]
- 109.Rotman B, Zderic JA, Edelstein M. Fluorogenic substrates for β-D-galactosidases and phosphatases derived from fluorescein (3,6-dihydroxyfluoran) and its monomethylether. Proc. Natl. Acad. Sci. U.S.A. 1963;50:1–6. doi: 10.1073/pnas.50.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rotman B, Papermaster BW. Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. Proc. Natl. Acad. Sci U.S.A. 1966;55:134–141. doi: 10.1073/pnas.55.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huang Z, Wang QP, Ly HD, Gorvindarajan A, Scheigetz J, Zamboni R, Desmarais S, Ramachandran C. 3,4-Fluorescein diphosphate: A sensitive fluorogenic and chromogenic substrate for protein tyrosine phosphatases. J. Biomol. Screen. 1999;4:327–334. doi: 10.1177/108705719900400608. [DOI] [PubMed] [Google Scholar]
- 112.Zaikova TO, Rukavishnikov AV, Birrell GB, Griffith OH, Keana JFW. Synthesis of fluorogenic substrates for continuous assay of phosphatidylinositol-specific phospholipase C. Bioconjugate Chem. 2001;12:307–313. doi: 10.1021/bc0001138. [DOI] [PubMed] [Google Scholar]
- 113.Mitchison TJ, Sawin KE, Theriot JA, Gee K, Mallavarapu A, Gerard M. Caged fluorescent probes. Methods Enzymol. 1998;291:63–78. doi: 10.1016/s0076-6879(98)91007-2. [DOI] [PubMed] [Google Scholar]
- 114.Miller EW, Bian SX, Chang CJ. A fluorescent sensor for imaging reversible redox cycles in living cells. J. Am. Chem. Soc. 2007;129:3458–3459. doi: 10.1021/ja0668973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Urano Y, Kamiya M, Kanda K, Ueno T, Hirose K, Nagano T. Evolution of fluorescein as a platform for finely tunable fluorescence probes. J. Am. Chem. Soc. 2005;127:4888–4894. doi: 10.1021/ja043919h. [DOI] [PubMed] [Google Scholar]
- 116.Kamiya M, Kobayashi H, Hama Y, Koyama Y, Bernardo M, Nagano T, Choyke PL, Urano Y. An enzymatically activated fluorescence probe for targeted tumor imaging. J. Am. Chem. Soc. 2007;129:3918–3929. doi: 10.1021/ja067710a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lavis LD, Chao T-Y, Raines RT. Fluorogenic label for biomolecular imaging. ACS Chem. Biol. 2006;1:252–260. doi: 10.1021/cb600132m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Karstens T, Kobs K. Rhodamine B and rhodamine 101 as reference substances for fluorescence quantum yield measurements. J. Phys. Chem. 1980;84:1871–1872. [Google Scholar]
- 119.Lee LG, Spurgeon SL, Heiner CR, Benson SC, Rosenblum BB, Menchen SM, Graham RJ, Constantinescu A, Upadhya KG, Cassel JM. New energy transfer dyes for DNA sequencing. Nucl. Acids Res. 1997;25:2816–2822. doi: 10.1093/nar/25.14.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Leytus SP, Patterson WL, Mangel WF. New class of sensitive and selective fluorogenic substrates for serine proteinases: Amino acid and dipeptide derivatives of rhodamine. Biochem. J. 1983;215:253–260. doi: 10.1042/bj2150253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu J, Bhalgat M, Zhang C, Diwu Z, Hoyland B, Klaubert DH. Fluorescent molecular probes V: A sensitive caspase-3 substrate for fluorometric assays. Bioorg. Med. Chem. Lett. 1999;9:3231–3236. doi: 10.1016/s0960-894x(99)00566-1. [DOI] [PubMed] [Google Scholar]
- 122.Kupcho K, Hsiao K, Bulleit B, Goueli SA. A homogeneous, nonradioactive high-throughput fluorogenic protein phosphatase assay. J. Biomol. Screen. 2004;9:223–231. doi: 10.1177/1087057103262840. [DOI] [PubMed] [Google Scholar]
- 123.Streu C, Meggers E. Ruthenium-induced allylcarbamate cleavage in living cells. Angew. Chem., Int. Ed. 2006;45:5645–5648. doi: 10.1002/anie.200601752. [DOI] [PubMed] [Google Scholar]
- 124.Martin VV, Rothe A, Diwu Z, Gee KR. Fluorescent sodium ion indicators based on the 1,7-diaza-15-crown-5 system. Bioorg. Med. Chem. Lett. 2004;14:5313–5316. doi: 10.1016/j.bmcl.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 125.Koide Y, Urano Y, Kenmoku S, Kojima H, Nagano T. Design and synthesis of fluorescent probes for selective detection of highly reactive oxygen species in mitochondria of living cells. J. Am. Chem. Soc. 2007;129:10324–10325. doi: 10.1021/ja073220m. [DOI] [PubMed] [Google Scholar]
- 126.Whitaker JE, Haugland RP, Ryan D, Hewitt PC, Haugland RP, Prendergast FG. Fluorescent rhodol derivatives: Versatile, photostable labels and tracers. Anal. Biochem. 1992;207:267–279. doi: 10.1016/0003-2697(92)90011-u. [DOI] [PubMed] [Google Scholar]
- 127.Burdette SC, Lippard SJ. The rhodafluor family. An initial study of potential ratiometric fluorescent sensors for Zn2+ Inorg. Chem. 2002;41:6816–6823. doi: 10.1021/ic026048q. [DOI] [PubMed] [Google Scholar]
- 128.Lee LG, Berry GM, Chen CH. Vita Blue: A new 633-nm excitable fluorescent dye for cell analysis. Cytometry. 1989;10:151–164. doi: 10.1002/cyto.990100206. [DOI] [PubMed] [Google Scholar]
- 129.Sarpara GH, Hu SJ, Palmer DA, French MT, Evans M, Miller JN. A new long-wavelength fluorigenic substrate for alkaline phosphatase: Synthesis and characterisation. Anal. Commun. 1999;36:19–20. [Google Scholar]
- 130.Xu K, Tang B, Huang H, Yang G, Chen Z, Li P, An L. Strong red fluorescent probes suitable for detecting hydrogen peroxide generated by mice peritoneal macrophages. Chem. Commun. 2005:5974–5976. doi: 10.1039/b512440a. [DOI] [PubMed] [Google Scholar]
- 131.Whitaker JE, Haugland RP, Prendergast FG. Spectral and photophysical studies of benzo[c]xanthene dyes: Dual emission pH sensors. Anal. Biochem. 1991;194:330–344. doi: 10.1016/0003-2697(91)90237-n. [DOI] [PubMed] [Google Scholar]
- 132.Chang CJ, Jaworski J, Nolan EM, Sheng M, Lippard SJ. A tautomeric zinc sensor for ratiometric fluorescence imaging: Application to nitric oxide-induced release of intracellular zinc. Proc. Natl. Acad. Sci. U.S.A. 2004;101:1129–1134. doi: 10.1073/pnas.0308079100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu J, Diwu Z, Leung W-Y. Synthesis and photophysical properties of new fluorinated benzo[c]xanthene dyes as intracellular pH indicators. Bioorg. Med. Chem. Lett. 2001;11:2903–2905. doi: 10.1016/s0960-894x(01)00595-9. [DOI] [PubMed] [Google Scholar]
- 134.Cosa G, Focsaneanu KS, McLean JRN, McNamee JP, Scaiano JC. Photophysical properties of fluorescent DNA-dyes bound to single- and double-stranded DNA in aqueous buffered solution. Photochem. Photobiol. 2001;73:585–599. doi: 10.1562/0031-8655(2001)073<0585:PPOFDD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 135.Darzynkiewicz Z, Bruno S, Del Bino G, Gorczyca W, Hotz MA, Lassota P, Traganos F. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13:795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- 136.Loudet A, Burgess K. BODIPY dyes and their derivatives: Syntheses and spectroscopic properties. Chem. Rev. 2007;107:4891–4932. doi: 10.1021/cr078381n. [DOI] [PubMed] [Google Scholar]
- 137.Karolin J, Johansson LBA, Strandberg L, Ny T. Fluorescence and absorption spectroscopic properties of dipyrrometheneboron difluoride (BODIPY) derivatives in liquids, lipid membranes, and proteins. J. Am. Chem. Soc. 1994;116:7801–7806. [Google Scholar]
- 138.Lumbierres M, Palomo JM, Kragol G, Roehrs S, Müller O, Waldmann H. Solid-phase synthesis of lipidated peptides. Chem.—Eur. J. 2005;11:7405–7415. doi: 10.1002/chem.200500476. [DOI] [PubMed] [Google Scholar]
- 139.Gabe Y, Urano Y, Kikuchi K, Kojima H, Nagano T. Highly sensitive fluorescence probes for nitric oxide based on boron dipyrromethene chromophore—rational design of potentially useful bioimaging fluorescence probe. J. Am. Chem. Soc. 2004;126:3357–3367. doi: 10.1021/ja037944j. [DOI] [PubMed] [Google Scholar]
- 140.Banks P, Gosselin M, Prystay L. Impact of a red-shifted dye label for high throughput fluorescence polarization assays of G protein-coupled receptors. J. Biomol. Screen. 2000;5:329–334. doi: 10.1177/108705710000500504. [DOI] [PubMed] [Google Scholar]
- 141.Thompson VF, Saldaña S, Cong J, Goll DE. A BODIPY fluorescent microplate assay for measuring activity of calpains and other proteases. Anal. Biochem. 2000;279:170–178. doi: 10.1006/abio.1999.4475. [DOI] [PubMed] [Google Scholar]
- 142.Nathans J. Molecular biology of visual pigments. Annu. Rev. Neurosci. 1987;10:163–194. doi: 10.1146/annurev.ne.10.030187.001115. [DOI] [PubMed] [Google Scholar]
- 143.Lewis GN, Calvin M. The color of organic substances. Chem. Rev. 1939;25:273–328. [Google Scholar]
- 144.Buschmann V, Weston KD, Sauer M. Spectroscopic study and evaluation of red-absorbing fluorescent dyes. Bioconjugate Chem. 2003;14:195–204. doi: 10.1021/bc025600x. [DOI] [PubMed] [Google Scholar]
- 145.Smith JC. Potential-sensitive molecular probes in membranes of bioenergetic relevance. Biochim. Biophys. Acta. 1990;1016:1–28. doi: 10.1016/0005-2728(90)90002-l. [DOI] [PubMed] [Google Scholar]
- 146.Plasek J, Sigler K. Slow fluorescent indicators of membrane potential: A survey of different approaches to probe response analysis. J. Photochem. Photobiol., B. 33:101–124. doi: 10.1016/1011-1344(96)07283-1. [DOI] [PubMed] [Google Scholar]
- 147.Zhou WL, Yan P, Wuskell JP, Loew LM, Antic SD. Intracellular long-wavelength voltage-sensitive dyes for studying the dynamics of action potentials in axons and thin dendrites. J. Neurosci. Methods. 2007;164:225–239. doi: 10.1016/j.jneumeth.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mujumdar RB, Ernst LA, Mujumdar SR, Lewis CJ, Waggoner AS. Cyanine dye labeling reagents: Sulfoindocyanine succinimidyl esters. Bioconjugate Chem. 1993;4:105–111. doi: 10.1021/bc00020a001. [DOI] [PubMed] [Google Scholar]
- 149.Mujumdar SR, Mujumdar RB, Grant CM, Waggoner AS. Cyanine-labeling reagents: Sulfobenzindocyanine succinimidyl esters. Bioconjugate Chem. 1996;7:356–362. doi: 10.1021/bc960021b. [DOI] [PubMed] [Google Scholar]
- 150.Schobel U, Egelhaaf HJ, Brecht A, Oelkrug D, Gauglitz G. New donor-acceptor pair for fluorescent immunoassays by energy transfer. Bioconjugate Chem. 1999;10:1107–1114. doi: 10.1021/bc990073b. [DOI] [PubMed] [Google Scholar]
- 151.Bates M, Huang B, Dempsey GT, Zhuang X. Multicolor super-resolution imaging with photo-switchable fluorescent probes. Science. 2007;317:1749–1753. doi: 10.1126/science.1146598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gruber HJ, Hahn CD, Kada G, Riener CK, Harms GS, Ahrer W, Dax TG, Knaus HG. Anomalous fluorescence enhancement of Cy3 and Cy3.5 versus anomalous fluorescence loss of Cy5 and Cy7 upon covalent linking to IgG and noncovalent binding to avidin. Bioconjugate Chem. 2000;11:696–704. doi: 10.1021/bc000015m. [DOI] [PubMed] [Google Scholar]
- 153.Berlier JE, Rothe A, Buller G, Bradford J, Gray DR, Filanoski BJ, Telford WG, Yue S, Liu J, Cheung C-Y, Chang W, Hirsch JD, Beechem JM, Haugland RP, Haugland RP. Quantitative comparison of long-wavelength Alexa Fluor dyes to CyDyes: Fluorescence of the dyes and their bioconjugates. J. Histochem. Cytochem. 2003;51:1699–1712. doi: 10.1177/002215540305101214. [DOI] [PubMed] [Google Scholar]
- 154.Liu W, Jensen TJ, Fronczek FR, Hammer RP, Smith KM, Vicente GH. Synthesis and cellular studies of nonaggregated water-soluble phthalocyanines. J. Med. Chem. 2005;48:1033–1041. doi: 10.1021/jm049375b. [DOI] [PubMed] [Google Scholar]
- 155.Peng X, Draney DR, Volcheck WM, Bashford GR, Lamb DT, Grone DL, Zhang Y, Johnson CM. Phthalocyanine dye as an extremely photostable and highly fluorescent near-infrared labeling reagent. Proc. SPIE-Int. Soc. Opt. Eng. 2006;6097E:1–12. [Google Scholar]
- 156.Bueno C, Villegas ML, Bertolotti SG, Previtali CM, Neumann MG, Encinas MV. The excited-state interaction of resazurin and resorufin with amines in aqueous solutions. Photophysics and photochemical reaction. Photochem. Photobiol. 2002;76:385–390. doi: 10.1562/0031-8655(2002)076<0385:tesior>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 157.Christoph S, Meyer-Almes FJ. Novel fluorescence based receptor binding assay method for receptors lacking ligand conjugates with preserved affinity: Study on estrogen receptor α. Biopolymers. 2003;72:256–263. doi: 10.1002/bip.10402. [DOI] [PubMed] [Google Scholar]
- 158.Hofmann J, Sernetz M. Immobilized enzyme kinetics analyzed by flow-through microfluorimetry: Resorufin-b-galactopyranoside as a new fluorogenic substrate for b-galactosidase. Anal. Chim. Acta. 1984;163:67–72. [Google Scholar]
- 159.Kitson TM. Comparison of resorufin acetate and p-nitrophenyl acetate as substrates for chymotrypsin. Bioorg. Chem. 1996;24:331–339. [Google Scholar]
- 160.Gao W, Xing B, Tsien RY, Rao J. Novel fluorogenic substrates for imaging β-lactamase gene expression. J. Am. Chem. Soc. 2003;125:11146–11147. doi: 10.1021/ja036126o. [DOI] [PubMed] [Google Scholar]
- 161.Burke MD, Thompson S, Weaver RJ, Wolf CR, Mayer RT. Cytochrome P450 specificities of alkoxyresorufin O-dealkylation in human and rat liver. Biochem. Pharmacol. 1994;48:923–936. doi: 10.1016/0006-2952(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 162.O'Brien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000;267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 163.Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: Applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal. Biochem. 1997;253:162–168. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
- 164.Boonacker E, Elferink S, Bardai A, Fleischer B, Van Noorden CJF. Fluorogenic substrate [Ala-Pro]2-cresyl violet but not Ala-Pro-rhodamine 110 is cleaved specifically by DPPIV activity: A study in living Jurkat cells and CD26/DPPIV-transfected Jurkat cells. J. Histochem. Cytochem. 2003;51:959–968. doi: 10.1177/002215540305100711. [DOI] [PubMed] [Google Scholar]
- 165.Cohen BE, Pralle A, Yao X, Swaminath G, Gandhi CS, Jan YN, Kobilka BK, Isacoff EY, Jan LY. A fluorescent probe designed for studying protein conformational change. Proc. Natl. Acad. Sci. USA. 2005;102:965–970. doi: 10.1073/pnas.0409469102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information