The biology of ovarian cancer: new opportunities for translation (original) (raw)
. Author manuscript; available in PMC: 2010 Feb 1.
Published in final edited form as: Nat Rev Cancer. 2009 Jun;9(6):415. doi: 10.1038/nrc2644
Abstract
Over the past two decades, the 5-year survival for ovarian cancer patients has substantially improved owing to more effective surgery and treatment with empirically optimized combinations of cytotoxic drugs, but the overall cure rate remains approximately 30%. Many investigators think that further empirical trials using combinations of conventional agents are likely to produce only modest incremental improvements in outcome. Given the heterogeneity of this disease, increases in long-term survival might be achieved by translating recent insights at the molecular and cellular levels to personalize individual strategies for treatment and to optimize early detection.
- Several factors make ovarian cancer a difficult disease to treat effectively. Although many patients experience symptoms, these often overlap with other ailments, and many patients are diagnosed after the cancer has metastasized. Ovarian cancer is also heterogeneous — multiple genetic and epigenetic changes are evident in patients with ovarian cancer; however, how such changes are selected for during tumorigenesis is not yet clear.
- Mutation and loss of TP53 function is one of the most frequent genetic abnormalities in ovarian cancer and is observed in 60–80% of both sporadic and familial cases. Of the 16 candidate tumour suppressor genes identified to date in ovarian cancer, 3 are imprinted genes. Several growth inhibitory genes are also silenced by methylation or imprinting.
- Inheritance of DNA repair defects contributes to as many as 10–15% of ovarian cancers. The lifetime risk of developing ovarian cancer in mutation carriers varies with the genetic defect (for BRCA1 30–60%, for BRCA2 15–30% and for hereditary non-polyposis colon cancer 7%).
- At least 15 oncogenes have been implicated in ovarian cancers, and DNA copy number abnormalities have also been found in loci that are known to contain non-coding microRNAs. At least seven signalling pathways are activated in >50% of ovarian cancers, and mutations that affect cell proliferation, apoptosis and autophagy are also evident.
- Ovarian cancer can be split into two groups on the basis of genetic changes: low-grade tumours with mutations in KRAS, BRAF and PIK3CA, loss of heterozygosity (LOH) on chromosome Xq, microsatellite instability and expression of amphiregulin; and high-grade tumours with aberrations in TP53 and potential aberrations in BRCA1 and BRCA2, as well as LOH on chromosomes 7q and 9p.
- Changes in cell adhesion and motility also contribute to disease development and metastasis. Adhesion of ovarian cancer cells to the mesothelial cells and to the underlying stroma is mediated by CD44, CA125 and b1 intergrin on the surface of ovarian cancer cells that bind to mesothelin and hyaluronic acid on mesothelial cells, or to fibronectin, laminin and type IV collagen in the underlying matrix.
- A crucial goal is to identify patients who would benefit from particular targeted therapies. Given the complexity of crosstalk between protein signalling pathways, predicting the impact and efficacy of any one signalling inhibitor is difficult. Inhibition of multiple pathways will almost certainly be required to substantially affect ovarian cancer growth.
- Effective methods for early detection are needed. Given the prevalence of ovarian cancer, strategies for early detection must have a high sensitivity for early-stage disease (>75%), but an extremely high specificity (99.6%) to attain a positive predictive value of at least 10% (ten operations for each case of ovarian cancer). Using rising values of serum biomarkers such as CA125 to trigger transvaginal sonography is a promising approach.
Ovarian cancer has a distinctive biology and behaviour at the clinical, cellular and molecular levels. Clinically, ovarian cancers often present as a complex cystic mass in the pelvis. Although ovarian cancer has been termed the 'silent killer', more than 80% of patients have symptoms, even when the disease is still limited to the ovaries1. These symptoms are, however, shared with many more common gastrointestinal, genitourinary and gynaecological conditions and have not yet proved useful for early diagnosis. Metastases can occur through lymphatics to nodes at the renal hilus or through blood vessels to the parenchyma of the liver or lung. Most frequently, small clusters of cancer cells are shed by the ovary and implant on the peritoneal surface, forming numerous nodules. For cancer in the ovary, unlike cancers at many other sites, no anatomical barrier exists to widespread metastasis throughout the peritoneal cavity. Tumour implants block lymphatic vessels that pass through the diaphragm, preventing the outflow of ascites fluid that leaks from disordered tumour vessels in the presence of high levels of tumour-derived vascular endothelial growth factor A (VEGFA), which is a vascular permeability factor2. Antibodies that neutralize VEGFA have decreased the accumulation of ascites in animal models and in clinical studies3, 4
Despite ongoing efforts to develop an effective screening strategy (Box 1), only 20% of ovarian cancers are diagnosed while they are still limited to the ovaries (stage 1). At this stage, up to 90% of patients can be cured using currently available therapy5. After the disease has metastasized to the pelvic organs (stage 2), the abdomen (stage 3) or beyond the peritoneal cavity (stage 4), the cure rate decreases substantially. Ovarian cancer is one of the few malignancies in which cytoreductive surgery is carried out to remove the bulk of the tumour, even when complete resection is impossible. At least 70% of ovarian cancers will respond to a combination of platinum- and taxane-based chemotherapy administered after surgery. In at least one-half of these patients, residual cancer cannot be detected using imaging studies and serum markers after 5 months of treatment.
Box 1|Ovarian cancer screening.
Despite advances in therapy, ovarian cancer remains the most lethal of the gynaecological cancers. This is largely related to late diagnosis122. Although up to 90% of stage I patients with ovarian cancer can be cured with conventional surgery and chemotherapy, only 20% of ovarian cancers are currently detected in stage I owing to the absence of an effective screening strategy. Given its prevalence, strategies for early ovarian cancer detection must have a high sensitivity for early-stage disease (>75%), but an extremely high specificity (99.6%) to attain a positive predictive value of at least 10% (10 operations for each case of ovarian cancer). Transvaginal sonography (TVS), serum markers and a combination of the two modalities have been evaluated for their ability to detect ovarian cancer at early stages. Among the serum markers, cancer antigen 125 (CA125) has received the most attention, but lacks the sensitivity or specificity to be used alone as a screening test when measured on a single occasion133. Greater specificity can be achieved by combining CA125 and TVS or by monitoring increases in CA125 over time. Two-stage screening strategies promise to be cost effective; in these strategies, abnormal serum assay results are used to prompt the application of TVS to detect lesions that require laparotomy. Accrual has been completed for a trial with 200,000 subjects in the United Kingdom that will test the ability of increased CA125 levels to prompt TVS and subsequent exploratory surgery. Data from the first 2 years of the trial suggest that this strategy could increase the percentage of disease detected at early stages (48%) with adequate sensitivity (89.4%) and specificity (99.8%)121.
As only 80% of ovarian cancers express CA125, additional markers will be required to detect all early-stage disease. New biomarkers have been identified using the empirical generation of monoclonal antibodies, gene expression array analysis, proteomics, lipomics and by cloning related proteases. One of the most promising biomarkers is whey-associated protein four-disulphide core domain protein 2 (WFDC2; also known as HE4), which might be transcriptionally upregulated by nuclear factor-κB123. At a tissue level, >95% of ovarian cancers can be identified with panels of four or five biomarkers124. Two recent abstracts have reported that, in serum, panels of four conventional or proteomic markers have detected 87–90% of stage I cancers125, 126. The development of multiplex technologies that simultaneously measure multiple serum markers and the creation of statistical methods that increase sensitivity without sacrificing specificity are promising.
Strategies for the early detection of ovarian cancer depend on several biological assumptions: first, that most ovarian cancers must be clonal and arise from the ovary or fallopian tube in which TVS can detect early tumour masses; second, that the interval before metastasis must be sufficiently long to allow cost-effective annual screening; and third, that most advanced-stage disease must develop from clinically detectable stage I or II lesions. In the case of sporadic disease, 90% of tumours are clonal, most arise from the ovary or fallopian tube, and estimates from preclinical increases in CA125 and from comparison of prevalent and incident cases in the UKCTOCS (UK Collaborative Trial of Ovarian Cancer Screening) are consistent with a lead time of 1.9–2 years121. Moreover, a similar profile of gene expression is observed in early- and late-stage high-grade serous cancers127. However, these assumptions might not apply to familial ovarian cancer, which is often multifocal, can show TP53 mutations and metastatic potential in cysts of <1 cm and, at least anecdotally, can present within 3 months of a normal TVS and CA125 result122. Although patients with BRCA1 or BRCA2 mutations are arbitrarily screened at 3–6 month intervals, the prophylactic removal of both ovaries and fallopian tubes (salpingo-oophorectomy) is generally recommended as soon as women at risk have completed their families.
Small numbers of drug-resistant cells can, however, persist for many months and remain dormant in the peritoneal cavity, only to grow progressively, leading to the death of the patient despite aggressive treatment of recurrent disease. Metastatic nodules form fibrous adhesions between loops of the bowel, causing intestinal obstruction that prevents normal alimentation, leading to malnutrition and eventual death from factors that includeintercurrent infection. Given the importance of disease on the peritoneal surface, intraperitoneal delivery of chemotherapy to achieve high local concentrations of a drug has substantially improved the survival of patients who have minimal gross disease remaining after surgery and who can tolerate the side effects of treatment6. Thus, the clinical biology of ovarian cancer suggests that late diagnosis and the persistence of dormant, drug-resistant cancer cells limit our ability to cure this disease.
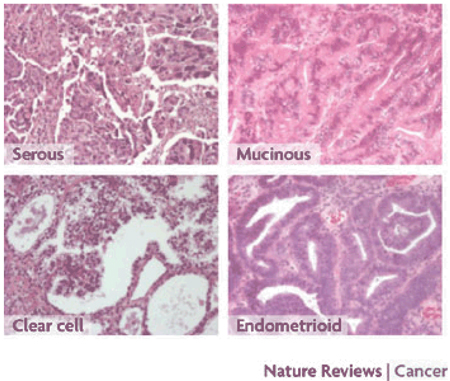
At the cellular and molecular levels, ovarian cancers are remarkably heterogeneous. The normal ovary is a complex tissue with several distinct components. Although ovarian cancers can develop from germ cells or granulosa–theca cells, more than 90% of ovarian cancers have an epithelial histology and are thought to arise from cells that cover the ovarian surface or that line subsurface inclusion cysts7 Cancers that have a similar histology can also arise from the lining of the fallopian tube, deposits of endometriosis or the surface of the peritoneal cavity. Substantial heterogeneity has been observed in the cellular grade, proliferative index and histotype of ovarian cancers (Box 2).
Box 2|Histotypes of epithelial ovarian cancer.
Unlike most cancers, which become less differentiated during transformation, epithelial ovarian cancers develop from simple flattened epithelial cells into four distinct main histotypes (see the figure) that resemble the well-differentiated normal cells that line the fallopian tube (serous), endometrium (endometrioid) and endocervix (mucinous), or that form nests within the vagina (clear cell). At a molecular level, altered patterns of gene expression in different histotypes have correlated with distinctive patterns of gene expression in the normal fallopian tube, endometrial and intestinal mucosa128. Histotypes have also correlated with the abnormal re-expression of homeobox (Hox) genes that are normally only expressed during gynaecological organogenesis129. HOXA9, HOXA10, HOXA11 and HOXA13 are associated with the developing fallopian tube, uterus, lower uterine segment and cervix, and upper vagina, respectively. Although these genes are not expressed in the normal ovarian surface epithelium, HOXA9 is expressed in serous, HOXA10 in endometrioid and HOXA11 in mucinous ovarian cancers. Moreover, forced expression of each gene in spontaneously transformed undifferentiated murine ovarian surface epithelium cells can induce the formation of histotype-specific cancers. The distinct ovarian cancer histotypes differ with regard to their epidemiology, genetic changes, gene expression, tumour markers and responsiveness to therapy. Although each histotype shows a distinctive pattern of gene expression, similarities have been observed between serous and endometrioid subtypes in some studies130, 131 and between endometrioid and clear-cell histology in others126. Clear-cell and mucinous cancers generally do not respond as well as serous and endometrioid cancers to platinum- and taxane-based chemotherapeutic regimens.
In addition to invasive low-grade and high-grade ovarian cancers, borderline tumours of low malignant potential (LMP) show the epithelial proliferation associated with malignancy without obvious invasion of the underlying stroma. In approximately 10% of cases, borderline cancers can metastasize, recur only after many years and eventually prove lethal. In contrast to high-grade disease, low-grade ovarian cancers and LMP tumours generally resist conventional cytotoxic chemotherapy.
The phenotype of the ovarian cancer stem cell has not been completely defined. Side populations of murine ovarian cancer cells that exclude Hoechst dyes during cell sorting show increased tumour-initiating activity in vivo78. When human ovarian cancer cells are dissociated from surgical specimens, as few as 100 CD44+CD117+ cells can establish growth as xenografts, whereas 105 CD44−CD117− cells cannot establish growth, suggesting that CD44+CD117+ cells are cancer stem cells8. Tumour-initiating cells show increased chemoresistance to cisplatin or paclitaxel and upregulation of stem cell markers (BMI1, stem cell factor (SCF), NOTCH1, NANOG, nestin, ATP-binding cassette G2 (ABCG2) andOCT4) compared with parental tumour cells grown under similar conditions. In other studies, CD44+MYD88+ ovarian cancer cells showed increased tumour-initiating activity, constitutive nuclear factor- κB (NF-κB) expression, cytokine and chemokine production, high capacity for repair of DNA damage, chemoresistance to conventional chemotherapies and resistance to tumour necrosis factor-α (TNF α)-mediated apoptosis9 The relevance of these studies to the heterogeneity of ovarian cancer has yet to be established.
As in other solid tumours, 90% of epithelial ovarian cancers are clonal10. They develop from the progeny of single cells that have accumulated a series of 5 or more genetic alterations selected from a repertoire of more than 30 oncogenes and tumour suppressor genes that have been implicated in ovarian oncogenesis. Genetic changes drive altered signalling that induces proliferation, inhibits apoptosis, blocks anoikis, increases motility, adhesion and invasion, and attracts stromal components, including mesenchymal stem cells and new blood vessels. Although many of these changes are observed in cancers at different sites, only those that are distinctive or characteristic of ovarian cancer will be considered in this Review.
Pathogenesis of ovarian cancer
Multiple genetic and epigenetic abnormalities have been detected in ovarian cancers from different individuals11 (Table 1). Activation of genes occurs through amplification, mutation and hypomethylation, whereas genetic inactivation results from deletion of large chromosomal regions, more limited loss of heterozygosity (LOH) at particular loci and promoter methylation. Evidence for genetic instability is provided by the observation of multiple changes using comparative genomic hybridization, particularly in cancers that have a high histological grade and are of a late stage12 Only 10–15% of ovarian cancers are associated with germline mutations of BRCA1, BRCA2 or the heritable non-polyposis colorectal cancer mismatch repair genes, loss of which predisposes to transformation and genetic instability13.
TABLE 1.
Genetic abnormalities in epithelial ovarian cancer
| Event | Effect | Chromosome | Gene |
|---|---|---|---|
| Geneamplification‡ | Activation | 1q223q265q318q2419q20p20q13.2 | RAB25 PRKCI, EVI1 and PIK3CA FGF1 MYC PIK3R1 and AKT2_ND_AURKA |
| Gene deletion‡ | Inactivation | 4q, 5q, 16q, 17p, 17q, Xp and Xq | ND |
| Mutation§ | Activation | NA | KRAS (15%), BRAF (12%), CTNNB1 (12%), CDKN2A (10%),APC (9%), PIK3CA (8%), KIT (7%) and SMAD4 (7%) |
| Hypomethylation | Activation | NA | IGF2 and SAT2 |
| Loss ofheterozygosity | Inactivation | 17p13 and 17q21 (in 50% of cases or more)1p, 3p, 5q, 5q, 6q, 7q and 8q (in fewer than 30% of cases) | ARHI, PEG3, PLAGL1, RPS6KA2, TP53, BRCA1, BRCA2, PTEN, OPCML and WWOX |
| Mutation | Inactivation | NA | TP53 (62%), BRCA1 (5%), BRCA2 (<5%) and PTEN (3–8%) |
| Promotermethylation# | Inactivation | NA | ARHI, DAPK1, CDH13, MLH1, ICAM1, PLAGL1, DNAJC15, MUC2, OPCML, PCSK6, PEF3, CDKN2A, CDKN1A, RASSF1, SOCS1, SOCS2, PYCARD and SFN |
The source of much genetic damage remains uncertain. Chemical carcinogens with mutagenic potential have generally not been linked to ovarian cancer risk, although exposure to the chronic inflammation produced by talc has been cited as a risk factor in some studies14, 15. Consistent with an absence of strong exogenous chemical carcinogens, the pattern of TP53 mutations in sporadic ovarian cancers is most consistent with spontaneous mutations that occur during cell proliferation16. Epithelial cells covering the ovary are generally quiescent and have a low proliferative index. Following the rupture of mature follicles to release oocytes, epithelial cells proliferate to repair damage to the ovarian surface. Ovulation is also crucial to the development of epithelial cell-lined subsurface inclusion cysts from which a substantial fraction of ovarian cancers develop. Epidemiological factors that increase the number of ovulatory cycles — early onset of menses, late menopause and infertility — increase the risk of ovarian cancer, whereas factors that decrease ovulation — multiple pregnancies, prolonged lactation and use of oral contraceptives — decrease the risk of ovarian cancer in later life. After menopause, proliferation of ovarian epithelial cells could be driven by increased levels of follicle-stimulating hormone, luteinizing hormone, oestrone and androgen. Given the low rates of spontaneous mutation, it is not surprising that few oncogenes are activated by mutation and several of the most frequently affected tumour suppressor genes are either haploinsufficient or are imprinted genes and are therefore heterozygous and subject to LOH.
Tumour suppressor genes
Many well-established and putative tumour suppressorgenes have been implicated in ovarian oncogenesis (Table 2)11. Although oncogenes can be activated by a single genetic event, both alleles of a tumour suppressor gene generally must be inactivated to lose function. If spontaneous mutation during proliferation is an important mechanism in ovarian oncogenesis, loss of tumour suppressor function could be less frequent than in carcinogen-driven cancers with higher rates of mutation. There are, however, several important exceptions to the generalization that two genetic events must occur to lose tumour suppressor function.
TABLE 2.
Putative tumour suppressor genes of which function is lost in epithelial ovarian cancer
| Gene | Chromosome | Percentage of cancers in which downregulated or inactivated | Mechanisms of downregulation | Function |
|---|---|---|---|---|
| ARHI (DIRAS3) | 1p31 | 60% | Imprinting; LOH; promoter methylation; transcription downregulated by E2F1 and E2F4 | 26 kDa GTPase; inhibits proliferation and motility; induces autophagy and dormancy; upregulates P21; inhibits cyclin D1, PI3K, Ras–Mapk signalling and STAT3 |
| RASSF1A | 3p21 | 60% | Hypermethylation | Inhibits proliferation and tumorigenicity in many different cancers; interacts with Ras inhibiting and downregulating cyclin D and signalling through JNK; stabilizes microtubules; regulates spindle checkpoint; regulates CD95- and TNFα-induced apoptosis |
| DLEC1 | 3p22.3 | 73% | Promoter hypermethylation and histone hypoacetylation | 166 kDa cytoplasmic protein that inhibits anchorage-dependent growth |
| SPARC | 5q31 | 70–90% decreased expression; 9% lost expression | Transcription | 32 kDa Ca2*-binding protein; prevents adhesion |
| DAB2 (DOC2) | 5q13 | 58–85% lost expression | Transcription | 105 kDa protein binds GRB2, preventing Ras and Mapk activation; prevents FOS induction and decreases ILK activity; contributes to anoikis; inhibits proliferation; inhibits anchorage-independent growth and tumorigenicity |
| PLACL1 (LOT1) | 6q25 | 39% | Imprinting; LOH; transcription downregulated by EGF end TPA | 55 kDa nuclear zinc-finger protein; inhibits proliferation and tumorigenicity |
| RPS6KAZ | 6q27 | 64% | Monoallelic expression in ovary; LOH | 90 kDa ribosomal S6 serine threonine kinase; inhibits growth; induces apoptosis; decreases Erk phosphorylation and cyclin D1; increases p21 and p27 |
| PTEN | 10q23 | 3–8% mutated; expression lost in 27%, particularly in endometrioid and clear-cell histotypes | Promoter methylation; LOH; mutation | PI3 phosphatase; decreases proliferation, migration and survival; decreases cyclin D; increases p27 |
| OPCML | 11q25 | 56–83% | Promoter methylation; LOH; mutation | GPI-anchored IgLON family member induces aggregation; inhibits proliferation and tumorigenicity |
| BRCA2 | 13q12–13 | 3–6% | Mutation; LOH | Binds RAD51 during repair of DNA DSBs |
| ARL11 | 13q14 | 62% | Promoter methylation | ADP ribosylation factor; induces apoptosis |
| WWOX | 16q23 | 30–49%, particularly in mucinous end clear–cell histotypes | LOH; mutation | Decreases anchorage-independent growth and tumorigenicity; mouse homologue required for apoptosis |
| TP53 | 17p13.1 | 50–70% | Mutation | 53 kDa nuclear protein; induces p21 leading to cell cycle arrest and promotion of DNA stability; induces apoptosis |
| DPH1 | 17p13.3 | 37% | LOH | 50 kDa protein; decreases proliferation and clonogenicity; decreases cyclin Dl |
| BRCA1 | 17q21 | 6–8% | Mutation; LOH | E3 ubiquitin ligase that participates directly in repair of DNA DSBs through homologous recombination; regulates ABL1; induces p53, androgen receptor, oestrogen receptor and MYC |
| PEG3 | 19q13 | 75% | Imprinting; LOH; promoter methylation; transcription | Induces p53-dependent apoptosis |
TP53
Owing to the dominant negative activity of mutant p53 proteins, TP53 function can be lost with a single genetic event. Mutation and loss of TP53 function is one of the most frequent genetic abnormalities in ovarian cancer and is observed in 60–80% of both sporadic and familial cases. TP53 mutation and consequent overexpression is seen in approximately 4% of pre-invasive borderline tumours17, 10–20% of early cancers and 40–60% of advanced cancers, and correlates with metastatic potential18, 19. Mutated TP53 has been found in microscopic ovarian cancers in oophorectomy specimens resected prophylactically from women who carry BRCA1 or BRCA2 mutations, which is consistent with an early role in familial disease. TP53 mutation has been associated with resistance to platinum-based therapy in some studies. Although 43% of 215 published analyses from 64 papers reported a correlation between TP53 mutation and a clinical end point that was relevant to chemoresistance, none of the 6 most crucial studies showed a statistically significant correlation20. Interestingly, TP53 mutations have been associated with a short-term survival benefit19.
Clinically, restoration of p53 function has been attempted using a replication-deficient adenoviral vector containing recombinant wild-type TP53 that was delivered into the peritoneal cavities of heavily pretreated patients with or without additional chemotherapy. Despite a decrease in the levels of the cancer antigen 125 (CA125) serum biomarker in half of the evaluable patients, this approach was abandoned owing to the failure of p53 therapy to provide a more dramatic improvement in the response to retreatment with carboplatin and paclitaxel21. The selective replication of a genetically modified E1B-deficient adenovirus (ONYX-015) has been achieved, allowing the lysis of ovarian cancer cells with_TP53_ mutations in culture and the inhibition of xenograft growth while sparing normal cells. Intraperitoneal administration of ONYX-015 in the clinic failed, however, to inhibit ovarian cancer growth22. MDM2 is an E3 ubiquitin ligase that binds p53 and targets it for proteasomal degradation. In leukaemias with wild-type p53, disruption of the p53–MDM2 association with small molecular mass compounds such as nutlins increased p53 stability and induced apoptosis23. Approximately one-third of ovarian cancers overexpress MDM2 and a similar strategy might be pursued in those cancers with high levels of MDM2 and low levels of wild-type p53.
PTEN
Aside from TP53, inactivating somatic mutations of other growth suppressive genes are uncommon in sporadic epithelial ovarian cancer. Inactivating mutations of PTEN, for example, are found in only 3–8% of sporadic cancers, which are largely of the endometrioid histotype and are usually of a low grade.
Imprinted tumour suppressor genes
The function of imprinted tumour suppressor genes can also be lost in a single step. Approximately 70 human genes are known to be imprinted: only one allele is expressed in each cell of the embryo and adult and silencing of the maternal or paternal allele is inherited despite a normal nucleotide sequence. Of the 16 candidate tumour suppressor genes identified to date in ovarian cancer, 3 are known to be imprinted: ARHI (also known as DIRAS3), pleiomorphic adenoma gene-like 1 (PLAGL1, also known as LOT1) and paternally expressed 3 (PEG3). All three genes are downregulated in a substantial fraction of ovarian cancers.
ARHI encodes a 26 kDa GTPase that shares homologywith Ras, but has a distinctive 34 amino acid amino-terminal extension that mediates its tumour-suppressive effect. The functional allele of ARHI is downregulated in >60% of ovarian cancers owing to LOH (30%), promoter hypermethylation (10%), transcriptional regulation (>20%) or decreased mRNA half-life24. Expression of ARHI is associated with prolonged progression-free survival. Re-expression of ARHI inhibits proliferation, motility and angiogenesis, and induces autophagy (see below). PLAGL1 encodes a 55 kDa zinc-finger protein that is downregulated in 39% of ovarian cancers through LOH and transcriptional regulation25. Re-expression of _PLAGL1_inhibits proliferation in culture and tumour formation in xenografts. PEG3 encodes a 140 kDa Krüppel-type zinc-finger protein that is downregulated in 75% of ovarian cancers by LOH (20%), promoter methylation (26%) and, in all probability, transcriptional regulation26. Re-expression of PEG3 inhibits growth and induces apoptosis through translocation of BAX downstream of p53. However, the persistence of PEG3 expression does not improve prognosis. Treatment with demethylating agents and histone deacetylase inhibitors has induced expression of ARHI and PEG3 in ovarian cancer cell lines after treatment, correlating with growth inhibition27. Consequently, increased expression of imprinted and silenced genes might prove useful as a biomarker for monitoring epigenetic therapy.
Other epigenetically silenced genes
Several growth inhibitory genes are silenced by methylation or imprinting in ovarian cancer including RASSF1, deleted in lung and esophageal cancer 1 (DLEC1) and opioid binding protein/cell adhesion molecule-like (OPCML) (Table 2). Additional growth-inhibitory genes such as DLEC1 are directly or indirectly downregulated by histone deacetylation. A recently published abstract showed that the histone deacetylase inhibitor belinostat has clinical activity against borderline ovarian cancers that are generally refractory to chemotherapy28. Platinum resistance has been associated with CpG methylation of the MLH1 mismatch repair gene in relapsed invasive ovarian cancers and can be reversed with demethylating agents in preclinical models29. An initial report on a Phase II trial at M. D. Anderson Cancer Center showed that the combination of the demethylating agent azacytidine and carboplatin had clinical activity in 22% of 18 patients with platinum-resistant ovarian cancer30.
BRCA1 and BRCA2
Inheritance of DNA repair defects contributes to as many as 10–15% of ovarian cancers31,32. The lifetime risk of developing ovarian cancer in mutation carriers varies with the genetic defect (for BRCA1 30–60%, for BRCA2 15–30% and for hereditary non-polyposis colon cancer 7%). In contrast to sporadic disease, BRCA-related familial ovarian cancers are more frequently multifocal, with genetically distinct clones involving multiple sites33, and progress faster, but are more sensitive to platinum-based chemotherapy34. BRCA1 and BRCA2 are required for reliable DNA double strand break repair by homologous recombination35, 36. As in familial breast cancers, an inactivated allele is inherited by all cells, and loss of BRCA1 or BRCA2 function occurs in ovarian cancer cells through LOH of the normal allele. Cells lacking BRCA1 or BRCA2 repair DNA by error-prone mechanisms such as non-homologous end joining, leading to chromosomal rearrangements and genomic instability. Ovarian cancer cells with truncating mutations of BRCA1 or BRCA2 or with lower expression of these genes are less able to repair DNA damage induced by platinum compounds. Indeed, platinum chemoresistance can arise from mutations that restore the_BRCA2_ open reading frame and thus the cellular capacity for homologous recombination37,38. Similar to platinum drugs, poly(ADP-ribose) polymerase (PARP) inhibitors are particularly effective in the presence of defects in homologous recombination repair, and clinical trials with these agents are underway in ovarian cancer patients with inherited _BRCA1_and BRCA2 mutations39, 40.
Although the BRCA1 and BRCA2 genes have been thought to be mutated only rarely in sporadic ovarian cancers, somatic mutations have recently been documented in approximately 10% of non-familial cases41, and expression of BRCA1 and BRCA2 can also be silenced by methylation in additional cancers42. Remarkably, the gene expression profiles in sporadic cases have been reported to fall into two categories that resemble the expression in cancers from patients carrying either BRCA1 or BRCA2 mutations, although this observation requires confirmation43. High-grade sporadic serous epithelial ovarian cancer might show dysfunction of the BRCA1 or BRCA2 pathway, contributing to sensitivity to platinum-containingtherapies. PARP inhibitors might also find broader clinical application in sporadic cancers in which these pathways are also defective.
Oncogenes
At least 15 oncogenes have been implicated in ovarian cancers (Table 3)11, and 11 of these oncogenes show genomic amplification11, 44. DNA copy number abnormalities have also been found in 37% of 283 loci that are known to contain non-coding microRNAs45. In contrast to copy number anomalies, activating mutations in oncogenes are uncommon in the most frequently occurringhistotypes.
TABLE 3.
Oncogenes associated with epithelial ovarian cancer
| Gene | Chromosome | Percentage of cancers in which amplified | Percentage of cancers in which overexpressed | Percentage of cancers in which mutated | Function |
|---|---|---|---|---|---|
| RAB25 | 1q22 | 54% | 80–89% | ND | Cytoplasmic GTPase and apical vessel trafficking |
| EVI1 | 3q26 | ND | ND | ND | Transcription factor |
| EIF5A2 | 3q26 | ND | ND | ND | Elongation factor |
| PRKCI | 3q26 | 44% | 78% | ND | Cytoplasmic serine-threonine protein kinase |
| PIK3CA | 3q26 | 9–11% | 32% | 8–12% | Cytoplasmic lipid kinase |
| FGF1 | 5q31 | ND | 51% | ND | Growth factor for cancer and angiogenesis |
| MYC | 8q24 | 20% | 41–66% | ND | Transcription factor |
| ECFR | 7q12 | 11–20% | 9–28% | <1% | Protein tyrosine kinase growth factor receptor |
| NOTCH3 | 9p13 | 20–21% | 62% | ND | Cell surface growth factor receptor |
| KRAS | 12P11–12 | 5% | 30–52% | 2–24% | Cytoplasmic GTPase |
| ERBB2 | 17q12–21 | 6–11% | 4–12% | ND | Protein tyrosine kinase growth factor receptor |
| PIK3R1 | 19q | ND | ND | ND | Cytoplasmic lipid kinase |
| CCNE1 | 19q12 | 12–36% | 42–63% | ND | Cyclin |
| AKT2 | 19q13.2 | 12–27% | 12% | ND | Cytoplasmic serine–threonine protein kinase |
| AURKA | 20q13 | 10–15% | 48% | ND | Nuclear serine–threonine protein kinase |
KRAS is mutated in >20% of the less common low-grade (type I) cancers, but _KRAS_mutations are rare in the more numerous high-grade (type II) lesions132 (Box 3). PI3K catalytic subunit-α (PIK3CA) mutations occur commonly only in the small subset of endometrioid and clear-cell ovarian cancers. Thus, ovarian cancer can be split into two groups: low-grade tumours with mutations in KRAS, BRAF and PIK3CA, LOH on Xq, microsatellite instability46 and expression of amphiregulin; and high-grade tumours with aberrations in TP53 and potential aberrations in BRCA1 and BRCA2, as well as LOH on 7q and 9p47, 48.
Box 3|Molecular classification of epithelial ovarian cancers.
Type I ovarian cancers constitute a minority of epithelial lesions and include low-grade and borderline serous cancers, endometrioid, mucinous and clear-cell cancers132. This group has more frequent PTEN, PI3K catalytic subunit-α (PIK3CA), KRAS, BRAF and b-catenin (CTNNB1) mutations, along with genomic stability and _TP53_mutations in only a small fraction of cases. Type II ovarian cancers constitute most epithelial ovarian cancers and include high-grade serous cancers, mixed malignant mesodermal tumours, carcinosarcomas and undifferentiated cancers132. These tumours have TP53 mutations in up to 80% of cases, along with marked genomic instability. Type II cancers arise more frequently from the fallopian tubes and the peritoneum. Ovarian cancers that arise in women with inherited BRCA1 and _BRCA2_mutations are usually type II tumours.
| Type | Histology | Precursor | Molecular features |
|---|---|---|---|
| I | Low-grade serous carcinoma | Cystadenoma–borderline tumour–carcinoma sequence | Mutations in KRAS and/or BRAF (≥ 60%) |
| I | Low-grade endometrioid carcinoma | Endometriosis and endometrial cell-like hyperplasia* | Mutations in CTNNB1, PTEN and PIK3CA with microsatellite instability |
| I | Mucinous carcinoma | Cystadenoma–borderline tumour–carcinoma sequence; metastases from bowel | Mutations in KRAS; TP53 mutation associated with transition from borderline tumour to carcinoma |
| I | Clear cell carcinoma | Endometriosis | PTEN mutation or loss of heterozygosity; PIK3CA mutation‡ |
| II | High-grade serous carcinoma | De novo in epithelial inclusion cysts; fallopian tube | TP53 mutation (up to 80%) and BRCA1 dysfunction |
| II | High-gradeendometrioid carcinoma | Epithelial inclusion glands or cysts | TP53 mutation and BRCA1 dysfunction; PIK3CA mutation |
Well-differentiated tumours with a low histological grade are associated with a better prognosis than are high-grade neoplasms, despite the resistance of low histological grade tumours to chemotherapy. At least a fraction of low-grade serous cancers seem to arise from LMP tumours. Karyotypically, LMP tumours are generally diploid49 and have few DNA copy number changes. Expression of the oestrogen receptor is found more frequently in LMP and low-grade ovarian cancers, suggesting that hormonal manipulation might be effective for controlling the growth of these neoplasms50. Ovarian surface epithelial cells immortalized with human telomerase reverse transcriptase and SV40 t antigen have been transformed with mutant HRAS or KRAS, and lead to cells that grow slowly but progressively as xenografts with serous papillary histology in the peritoneal cavity of nude mice51. Gene expression profile analysis of the transformed cells showed the increased expression of several cytokines, including interleukin 1 (IL-1), IL-6 and IL-8, which are upregulated by the NF-κB pathway51. Each of these molecules might provide targets for therapeutic intervention in type I cancers with Ras mutations.
The small G-protein RAB25 is amplified and upregulated in most ovarian cancers and regulates motility, aggressiveness, apoptosis and autophagy52. RAB25 also mediates survival in response to stress, such as that induced by chemotherapy, ultraviolet radiation, serum depletion and glucose starvation. Delivery of specific small-interfering RNAs (siRNAs) could allow targeting of RAB25.
Several other amplified oncogenes are potential targets for ovarian cancer therapy. Small molecule inhibitors of Aurora kinases are currently being evaluated in several cancers53. Antibodies that inhibit NOTCH3 or its ligands are being developed54. New gold compounds have been shown to target the N-terminal PHOX-BEM1 domain of PKC ι that is required for PKC ι-driven transformation.
Signalling
At least seven signalling pathways are activated in >50% of ovarian cancers (Box 4).
Box 4|Development of targeted therapies.
New screening small-interfering (siRNA) libraries have been used to identify targets such as Src and Akt that increase the cytotoxicity of paclitaxel in ovarian cancer cells. Phase II clinical trials of the Src inhibitor dasatinib have been initiated.
Although small molecule inhibitors can be developed for intracellular targets and antibodies can be developed for the inhibition of cell surface receptors, growth factors and cytokines, the therapeutic use of siRNA promises to provide more rapid translation of this approach to the clinical setting. Recent studies have shown that the intravenous injection of siRNA in neutral liposomes can deliver adequate amounts of siRNA to inhibit the growth of intraperitoneal xenografts104.
New technologies are being introduced that allow the simultaneous measurement of multiple signalling pathways. Gene expression analysis has identified ovarian cancer signatures that predict optimal cytoreductive surgery, poor overall survival and chemoresistance, although the predictive values of these signatures have not been sufficient to affect patient management. Recently, gene expression profiles have also been reported that identify the activation of particular signalling pathways (see the table) and responsiveness to targeted therapies. New reverse-phase protein lysate arrays can measure the simultaneous activation of multiple signalling pathways, thus allowing a more accurate definition of signalling heterogeneity and prediction of responsiveness to targeted therapies.
| Signalling pathway | Percentage of cancers in which activation is observed |
|---|---|
| PI3K | 70% |
| Src | >50% |
| IL-6–IL-6R; Jak–STAT3 | 70% |
| LPA | 90% |
| MEKK3–IKK–NK-κB | >50% |
| Mullerian inhibitory substance receptor | >50% |
| PKCι | 78% |
| Ras–Mek–Mapk* | <50% (activated in most low-grade type I cancers) |
Epidermal growth factor receptor (EGFR) mutations are rare in ovarian cancer, and the receptor is not markedly overexpressed. Gefitinib and erlotinib, which are inhibitors of EGFR, stabilized disease in 11–44% of patients with ovarian cancer but produced objective regression in only 4–6% of cases55, 56. As shown in colorectal cancers, activation of the Ras–Mapk signalling pathway might also minimize the effect of EGFR inhibitors57. ERBB2 (also known as HER2) is not frequently amplified and is overexpressed in only 11% of ovarian cancers58. Objective responses were observed in only 7% of 41 patients with ovarian cancer that overexpressed ERBB2 who were treated with trastuzumab, and only 1 complete response and 2 partial responses were observed58.
The PI3K pathway is activated in approximately 70% of ovarian cancers, and activation of this pathway is associated with resistance to cytotoxic chemotherapy. Activation of the PI3K pathway can be driven by gene amplification (PIK3CA and AKT2), activating mutations (PIK3CA) or inactivating mutations (PTEN), but in many cancers the pathway is activated by autocrine or paracrine signalling through protein tyrosine kinase growth factor receptors. Inhibitors of PI3K and Akt prevent the growth of ovarian cancer xenografts and potentiate the cytotoxic effects of paclitaxel and cisplatin59. Perifosine inhibits Akt, and an ovarian cancer clinical trial is currently underway at M. D. Anderson Cancer Center combining perifosine with docetaxel. More specific Akt inhibitors are being developed and PI3K inhibitors are entering Phase I–II trials60.
IL-6 is overexpressed in most ovarian cancers, providing autocrine stimulation of the IL-6 receptor, which activates Janus kinase 2 (JAK2), facilitating phosphorylation and nuclear translocation of the signal transducer and activator of transcription 3 (STAT3), which upregulates genes that stimulate proliferation, inhibit apoptosis and induce angiogenesis. Nuclear localization of activated phosphorylated STAT3 occurs in more than 70% of ovarian cancers and is associated with decreased overall survival61. Activated STAT3 also translocates to focal adhesion complexes and stimulates motility in combination with SRC. In addition to antibodies against IL-6 and inhibitors of JAK2 (Refs 62, 63), new inhibitors of STAT3 are being developed that might be used to target most ovarian cancers64.
Lysophosphatidic acid (LPA) is produced by the phosphodiesterase autotoxin (ATX; also known as lysophospholipase D). The G protein-linked LPA receptors LPAR2 and LPAR3 are upregulated during the malignant transformation of ovarian surface epithelial cells. LPAR3 responds to LPAs with unsaturated fatty acyl chains that are produced by ovarian cancers. Interestingly, cyclic phosphatidic acid blocks ATX, reducing LPA levels and metastasis, but not the growth of primary cancers65. LPA-neutralizing antibodies have been developed, and LPA receptor inhibitors are being sought that might block the proliferation of ovarian cancer cells66.
The NF-κB transcription factor is constitutively activated in more than half of ovarian cancers67, 68 through signalling initiated by several cytokines (IL-1 and TNFα) and growth factors (EGF). Inactive NF-κB is complexed with inhibitor of NF κB (IκB) and is generally activated by the IκB kinase (IKK) complex, which contains two kinase subunits (IKKα and IKKβ) and a regulatory subunit (IKKγ)69. MEKK3 is one of several kinases that can activate the IKK complex70, 71 and is overexpressed in >50% of ovarian cancers. The resultant activation of NF-κB upregulates anti-apoptotic genes (for example, CFLAR), antioxidant proteins (superoxide dismutase or ferritin heavy chain), growth regulatory cytokines (IL-6 or growth regulated-α (GRO1)) and angiogenic factors (IL-8)71. Selective inhibition of NF-κB has been difficult to achieve, but gene therapy with adenoviral E1A reduces NF-κB signalling and increases sensitivity to paclitaxel in xenograft models, which has prompted a clinical trial at the University of Texas M. D. Anderson Cancer Center of liposomal E1A in combination with paclitaxel in patients with intra-abdominal recurrence of ovarian cancer.
Tumour biology
Proliferation
The fraction of cycling cells in different ovarian cancers varies over a wide range from 1% to 79% (Ref. 72). Upregulation of cyclin D1 or E1, E2F1 or cyclin-dependent kinases (CDK2), and downregulation of CDK inhibitors (p16, p21 and p27) have been observed in a fraction of ovarian cancers, and the levels of cyclins and CDKs correlate with prognosis73, 74. In addition to intrinsic deregulation of checkpoints in the cell cycle, a range of autocrine and paracrine growth factors stimulate ovarian cancer proliferation, including EGF, transforming growth factor-α(TGFα), amphiregulin, heregulin, VEGFA, insulin-like growth factor 1 (IGF1), IL-6 and LPA.
In normal ovarian surface epithelial cells, autocrine growth inhibition is maintained by TGFβ75. In approximately 40% of ovarian cancers, expression of or responsiveness to TGFβ is lost. Although mutation of SMAD4 is sometimes observed, TGF βRI and TGF βRII receptors are generally intact, as is Smad signalling downstream of the receptors. Loss of growth inhibition and increased invasiveness might relate to ecotropic viral integration site 1 (EVI1) overexpression in 43% of ovarian cancers76. EVI1 inhibits transcription of TGFβ-responsive genes. Ski-like protein (SKIL) and runt-related transcription factor 1 (RUNX1), which bind to and regulate Smads, are also deregulated in ovarian cancer.
Mullerian inhibition substance (MIS) is a homologue of TGFβ and binds to a TGFβ-like receptor, leading to regression of gynaecological precursors during the development of normal male embryos77. MIS also inhibits the growth of epithelial ovarian cancer cells by binding to MISII G protein-coupled receptors and upregulating p16, leading to cell cycle arrest in G1. MISII receptors have been detected in 56% of human ovarian cancers, and clonogenic growth can be inhibited with MIS in more than 80% of the cancers that express MISII receptors78. Recombinant MIS also increases the activity of cisplatin against ovarian cancer cell lines79. As sufficient quantities of MIS become available, clinical trials could be undertaken.
Apoptosis
Decreased sensitivity to apoptotic stimuli occurs in epithelial ovarian cancer. The pro-apoptotic receptor CD95 was detected in 85% of inclusion cysts, 94% of cystadenomas, 35% of borderline tumours and 4% of invasive cancers80. Thus, invasive ovarian cancers are likely to resist the apoptosis that is induced by exposure to CD95 ligand or agonistic anti-CD95 antibodies. When compared with normal ovaries, overexpression (4–10-fold) of the anti-apoptotic proteins BCL-2, BCL-XL(also known as BCL2L1) and survivin (also known as BIRC5) was observed in >70% of invasiveepithelial ovarian cancers81. The altered expression of apoptosis-regulating proteins has also been associated with poor outcomes (decreased BAX), platinum resistance (XIAP upregulation) and taxane resistance (BCL-2 and survivin overexpression)82, 83, 84.
Autophagy
During autophagy, cellular organelles are enclosed and digested in double-walled autophagosomes, recycling amino acids and fatty acids and providing substrates for the generation of ATP. Autophagy can serve as a short-term survival mechanism that sustains cancer cells in an environment that is poor in nutrients, but prolonged autophagy can induce nonapoptotic cancer cell death. The development of breast cancers in beclin+/− mice with impaired autophagy supports the notion that autophagy can suppress cancer progression85. However, recent studies suggest that autophagy might be crucial for the maintenance of tumour dormancy.
Expression of the imprinted tumour suppressor gene ARHI is decreased or lost in 60% of ovarian cancers. Re-expression of ARHI at physiological levels blocks growth, motility and angiogenesis, and also induces autophagy by inhibiting PI3K and downregulating mTOR activity86. In addition to triggering autophagy, ARHI participates directly in autophagosome formation by upregulating the ATG4 enzyme that processes the microtubule-associated protein LC3I to LC3II. Although ARHI re-expression caused autophagic death of ovarian cancer cells in culture within days, induction of ARHI in xenografts does not kill ovarian cancer cells, but instead induces tumour dormancy for a period of weeks86. Ovarian cancer xenografts rapidly resume growth when ARHI is downregulated. Treatment with chloroquine, an inhibitor of autophagy, substantially delays the outgrowth of xenografts, which is consistent with the possibility that autophagy is required for tumour cell survival. Cytokines (IGF1, IL-8 and VEGFA) and matrix proteins (fibronectin and collagen) that are found in the microenvironment of xenografts can rescue ovarian cancer cells from autophagic death in culture and reverse ARHI-induced inhibition of PI3K signalling. If autophagy is required for tumour dormancy and if this model is relevant to human ovarian cancer, strategies could be devised using siRNAs (to target beclin and ATG5) and small molecules (class III PI3K inhibitors) to prevent autophagy in dormant cancer cells. Alternatively, crucial growth factors (IL-8 and VEGFA) that sustain autophagic cancer cells or their receptors (IGF1R and VEGFR) could be targeted using antibodies or protein tyrosine kinase inhibitors.
Motility and invasion
To metastasize, ovarian cancer cells must not only detach from neighbouring epithelial cells and adopt a mesenchymal phenotype, but they must also acquire the ability to move and invade stroma, vessels and the walls of the cysts in which they arise. Signals regulating motility and invasion are transduced through the Ras–Mapk, PI3K–Akt–p70S6K and STAT3 signalling pathways. A range of ligands activate these pathways, including VEGFA, EGF, heregulin, hepatocyte growth factor, macrophage colony-stimulating factor, TGFβ, bone morphogenetic protein 4, TNFα and LPA87, 88. LPA is frequently detectable in serum and ascites, and also induces the secretion of VEGFA, IL-6, IL-8 and GRO1 by ovarian cancer cells89. Interestingly, stress hormones, including adrenaline, noradrenaline and cortisol, have also recently been shown to upregulate matrix metalloproteinase 2 (MMP2) and MMP9 in ovarian cancer cells, thereby increasing invasion90. These experiments have provided one of the first physiological mechanisms by which psychological stress might influence tumour progression.
Directed motion of cancer cells and invasion can also be stimulated by the binding of cancer-associated integrins to the extracellular matrix. Clustering of collagen-binding integrins during the adhesion of ovarian cancer cells to collagen activates the Src family kinases to induce expression of early growth response 1 (EGR1), resulting in transcriptional activation of the MMP14 promoter and subsequent collagen invasion catalysed by MMP14 (Ref. 91). Laminin, collagen and fibronectin can all increase chemotactic activity, whereas increased invasion is observed only with laminin and collagen. α3, α6 and β1 integrin-mediated signalling through Ras–Mapk, Erk and Akt regulate both processes.
Effector proteases, which are crucial for invasion, have been detected in ovarian cancer cells and in stromal components. MMP2, MMP7, MMP9, urokinase plasminogen activator and the kallikreins are found in ovarian cancer cells in culture and in surgical specimens92. Twelve of the fifteen human kallikreins are transcriptionally upregulated in ovarian cancer. Kallikreins degrade matrix components, including fibronectin, vitronectin, laminin and collagens I, II, III and IV, thereby increasing ovarian cancer cell invasiveness93. They are therefore being evaluated as biomarkers for early detection or prognosis in ovarian cancer94.
Adhesion
Metastasis to the peritoneal surface requires the adhesion of ovarian cancer cells to the mesothelial cells that line the cavity and to the underlying stroma. Adhesion is mediated by β integrins, CD44 and CA125 (Ref. 95) on the surface of ovarian cancer cells that bind to mesothelin and hyaluronic acid on mesothelial cells, or to fibronectin, laminin and type IV collagen in the underlying matrix. CA125 is a high molecular mass (1 MDa) glycosylated transmembrane mucin that is expressed by 80% of ovarian cancers95. The N-glycans of CA125 bind to mesothelin and might be the initial point of contact between ovarian cancer cells and the peritoneal surface96. Mesothelin-specific antibodies block the adhesion of ovarian cancer cells to mesothelial cells97. In addition, knockdown of CA125 decreases the invasion of ovarian cancers, consistent with active signalling by the intracellular domain of the mucin. Both CA125 and mesothelin are shed into body fluids and are being evaluated as serum biomarkers for the monitoring and early diagnosis of ovarian cancer (Box 1). CD44–hyaluronic acid and β1 integrin–fibronectin, laminin and type IV collagen interactions might also contribute to peritoneal metastases. More than 70% of ovarian cancers show a diverse mixture of CD44 splice variants that can bind to hyaluronic acid98. CD44-specific antibodies can partially block adhesion of ovarian cancer cells to peritoneal mesothelial cells99. Thus, motility, adhesion and invasion might be inhibited using antibodies (LPA-, CA125-, CD44- and mesothelin-specific) and small molecule inhibitors (adrenergic receptors and proteases). Even after diagnosis and primary treatment, continued metastasis in the peritoneal cavity occurs during the progression of drug-resistant disease, ultimately producing intestinal obstruction. However, given dramatic differences in the rate of intra-abdominal progression from patient to patient, as well as the availability of multiple conventional drugs to treat recurrent disease, the design of appropriate clinical trials to test these strategies is problematic.
Angiogenesis
As is the case in other cancers, tumour vessels must be recruited if ovarian cancers and their metastases are to grow to more than 2 mm3. Microvessel density correlates with the propensity of ovarian cancers to metastasize and with disease-free survival100. VEGFA, LPA, IL-6, IL-8, fibroblast growth factor 2 (FGF2) and FGF1 all contribute to angiogenesis in ovarian cancer100, 101. Treatment with bevacizumab, a VEGFA-specific antibody, induced an objective response rate in 16% of patients with recurrent ovarian cancer and stabilized disease for 5.5 months in 50% of patients102. In general, anti-angiogenic therapy has been well tolerated, although hypertension has been observed in approximately one-third of the women treated and bowel perforation has occurred in approximately 5% of cases. In combination with cytotoxic chemotherapy, improved response rates have been reported in platinum-resistant disease. Daily metronomic chemotherapy with agents such as cyclophosphamide can take advantage of the differences in the proliferative rates of tumour-associated and normal endothelial cells103. New methods for inhibiting VEGFA activity are also being explored; for example, VEGF Trap104.
Overexpression of the ephrin type A receptor 2 (EPHA2) in tumour cells or endothelial cells has been observed in >75% of ovarian cancers and is associated with increased microvascular density and increased expression of MMP2, MMP9 and MMP14 (Ref. 105). Xenograft growth can be inhibited by agonistic antibodies against EPHA2 (Ref. 106) or by the delivery of EPHA2 siRNA in neutral liposomes107.
Pericytes contribute to the maintenance of endothelial cells108 and secrete VEGFA to promote endothelial survival. Their survival, in turn, depends on platelet-derived growth factor (PDGF) produced by endothelial cells. Both endothelial cells and pericytes could thus be targeted by the simultaneous inhibition of VEGFA and PDGF101. Trials are planned at the M. D. Anderson Cancer Center to exploit this mechanism for the treatment of ovarian cancer.
Other stromal components
In addition to tumour vessels, ovarian cancers recruit a stroma that contains fibroblasts, lymphocytes and inflammatory cells. Haematopoietic mesenchymal stem cells have been shown to home to ovarian cancer xenografts and differentiate into fibroblasts, contributing to microvascularization, stromal networks and production of tumour-stimulating cytokines (for example, IL-6)134. Once recruited, fibroblasts can also be affected by the tumour microenvironment. Ovarian surface epithelial cells that have been immortalized by Ras produce GRO1, which induces the senescence of stromal fibroblasts that produce factors that increase ovarian cancer growth109.
Lymphocytes and macrophages infiltrate ovarian tumours and are found in ascites in addition to a wide range of cytokines and chemokines110. T cells are most prevalent in solid tumour nodules, and their presence is associated with a 5-year survival rate of 38% compared with 4.5% for patients who have tumours that lack T cell infiltrates111, 112. A high ratio of CD8+ to regulatory T cells is also associated with an improved prognosis112. Interferons produced by activated T cells can inhibit tumour growth and IL-8 secretion, block angiogenesis and upregulate major histocompatibility complex expression, which increases immune recognition. Macrophage colony-stimulating factor and macrophage chemotactic protein 1 secreted by ovarian cancer cells and by T cells are potent chemoattractants for macrophages. Activated macrophages can produce proteins that stimulate (IL-1, IL-6 and TNFα) or inhibit (nitric oxide and TNFα) tumour growth. However, tumour-associated macrophages have impaired phagocytic activity and decreased effector function for antibody-dependent cell-mediated cytotoxicity. The C–X–C chemokine receptor CXCR4 is expressed in 59% of ovarian cancers and is associated with decreased disease-free and overall survival duration; its ligand CXCL12 is found in 91% of ovarian cancers113, 114,115. Inhibition of CXCR4 inhibits intraperitoneal dissemination of ovarian cancer xenografts116. Clinical trials should become feasible with the development of potent orally available CXCR4 inhibitors.
Approximately 80% of ovarian cancers express TNFα (which is regulated both translationally and transcriptionally through NF-κB), in addition to TNFRI and TNFRII receptors that allow both autocrine and paracrine stimulation117. TNFα can stimulate clonal growth, and TNFα knockdown inhibits growth and dissemination in ovarian cancer xenografts118. Clinical trials have therefore been undertaken with a TNFα-specific antibody for ovarian cancer119.
Conclusion
Given the large number of potential targets in ovarian cancer, an immediate challenge is to identify promising candidates for which inhibition would provide synthetic lethality using conventional drugs or other targeted therapies (Box 4). A crucial goal is to identify patients who would benefit from particular targeted therapies. Given the complexity of crosstalk between protein signalling pathways, predicting the impact and efficacy of any one signalling inhibitor is difficult. Individual inhibitors produce only modest xenograft growth inhibition. Thus, inhibition of multiple pathways will almost certainly be required to substantially affect ovarian cancer growth.
With many new targets and more than 400 potential drugs in the pharmaceutical pipeline, the clinical evaluation of new agents individually, and particularly in combination, is likely to limit the rate of progress. Participation of more ovarian cancer patients, enrichment of Phase I–II trials with cancers that express relevant targets, more efficient Bayesian trial design, use of surrogate biomarkers such as CA125 for response120, and studying tissues and imaging to determine whether drugs are 'hitting the target' will contribute to accelerating progress in early clinical trials.
As new agents become available, future trials should focus on inhibitors of the molecules (Src, MIS, RAB25 and PKCι) and signalling pathways (PI3K, IL-6–JAK2–STAT3, LPA and NF-κB) that are most frequently activated or overexpressed in ovarian cancers. Targeted therapy should be accelerated by the delivery of siRNAs, which have been shown to be effective in xenograft models of ovarian cancer and are moving towards clinical trials. Greater emphasis should be placed on targeting the interactions of cancer cells with stromal components. In addition to VEGFA, anti-angiogenic therapy could be directed towards other relevant factors (IL-8, PDGF, FGF1, FGF2 and LPA), receptors (EPHA2, PDGFR and FGFR) and cells (pericytes) in combination with metronomic dosing of cytotoxic drugs. If animal models prove to be relevant to the clinic, dormant ovarian cancer cells might be eliminated by targeting autophagy (chloroquine and siRNA) or survival factors (VEGFA, IGF and IL-8) that are required to prevent autophagic death in cancer cells that still express ARHI.
Ultimately, we must improve our ability to predict response in the clinic and to identify patients who are most likely to benefit from particular combinations of drugs. Given the heterogeneity of ovarian cancer at a molecular level, if we are to match the abnormalities present in an ovarian cancer to appropriate therapy, extensive characterization of every cancer specimen must be carried out using DNA sequencing of all relevant genes, high-resolution comparative genomic hybridization, single nucleotide polymorphism analysis, expression arrays, reverse-phase protein arrays and immunohistochemistry of selected biomarkers. As a greater rate of response to targeted therapy might be seen when signalling pathways are activated by genetic events, integrating mutation data from the Cancer Genome Atlas Project and DNA copy number with other 'omics' data should be particularly relevant for defining subsets of patients for therapy. Biopsy samples of fresh ovarian cancer tissue will be required from all patients before Phase I and II trials. Logistically, this is a particular challenge in recurrent ovarian cancer in which much of the disease is often localized in the abdominal cavity. The reward of more effective, potentially less toxic, individualized targeted therapy provides a substantial incentive to design and execute such trials.
Currently available biomarkers (p53 and KRAS) support the treatment of low-grade serous and non-serous type I and high-grade serous and endometrioid type II ovarian cancers with different agents. Reverse-phase protein array analysis suggests that distinct subsets of patients might benefit from either a combination of PI3K and MAPK inhibitors, a combination of KIT and cyclin E2 inhibitors, hormonal therapy or antiangiogenic approaches. A multi-arm trial is under development to determine the value of predictive tests for assigning patients to each of these treatment groups. As clinical trials mature with PI3K pathway inhibitors, the response in ovarian cancers with gene amplifications (_PIK3CA_and AKT2) or mutation (PIK3CA and PTEN) of pathway components can be compared with cancers in which PI3K activation is driven by autocrine or paracrine stimulation. If mutations are important drivers for cancer cell proliferation and survival, we anticipate that type I ovarian cancers will show a higher response rate than type II cancers to inhibitors of the PI3K–Akt and Ras–Mapk pathways.
In the long term, earlier detection of ovarian cancer is likely to have an important effect on the management of this disease. In the next 3 years, the results of a trial involving 200,000 subjects in the United Kingdom will establish whether measuring increasing CA125 levels followed by transvaginal ultrasound will detect sufficient numbers of patients with early-stage disease to affect survival121. Regardless of the outcome, it is likely that multiple marker panels will be required to detect >90% of patients with preclinical or early-stage disease. Understanding the heterogeneity of different ovarian cancers at a molecular level has facilitated the development of candidate panels of markers.
Acknowledgments
This work was supported by grants from the M. D. Anderson Ovarian SPORE National Cancer Institutes (P50CA83639), the National Foundation for Cancer Research, the Ovarian Cancer Research Fund, the Mossy Foundation and the Zarrow Foundation.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- 1.Goff BA, et al. Ovarian carcinoma diagnosis. Cancer. 2000;89:2068. doi: 10.1002/1097-0142(20001115)89:10<2068::aid-cncr6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Zebrowski BK, et al. Markedly elevated levels of vascular endothelial growth factor in malignant ascites. Ann. Surg. Oncol. 1999;6:373. doi: 10.1007/s10434-999-0373-0. [DOI] [PubMed] [Google Scholar]
- 3.Mesiano S, Ferrara N, Jaffe R. Role of vascular endothelial growth factor in ovarian cancer: inhibition of ascites formation by immunoneutralization. Am. J. Path. 1998;153:1249. doi: 10.1016/S0002-9440(10)65669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Numnum TM, et al. The use of bevacizumab to palliate symptomatic ascites in patients with refractory ovarian cancer. Gynecol. Oncol. 2006;102:425. doi: 10.1016/j.ygyno.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 5.Berek JS. In: Practical Gynecologic Oncology 4th edn Ch. 11 Ovarian Cancer. Berek JS, Hacker NF, editors. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 443–511. [Google Scholar]
- 6.Armstrong DK, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 7.Feeley KM, Wells M. Precursor lesions of ovarian epithelial malignancy. Histopathology. 2001;38:87. doi: 10.1046/j.1365-2559.2001.01042.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhang S, et al. Identification and characterization of ovarian cancer initiating cells from primary human tumors. Cancer Res. 2008;68:4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. This report established the phenotype of tumour-initiating ovarian cancer cells.
- 9.Alvero AB, et al. Molecular phenotyping of human ovarian cancer stem cells unravel the mechanisms for repair and chemoresistance. Cell Cycle. 2009;8:188–169. doi: 10.4161/cc.8.1.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs IJ, et al. Clonal origin of epithelial ovarian cancer: analysis by loss of heterozygosity, p53 mutation and X chromosome inactivation. J. Natl Cancer Inst. 1992;84:1793–1798. doi: 10.1093/jnci/84.23.1793. [DOI] [PubMed] [Google Scholar]
- 11.Bast RC, Jr, Mills GB. In: The Molecular Basis of Cancer. 3rd edn. Mendelsohn J, Howley P, Israel M, Gray J, Thompson C, editors. Philadelphia: W. B. Saunders Co.; 2008. pp. 441–455. [Google Scholar]
- 12.Iwabuchi H, et al. Genetic analysis of benign, low-grade and high-grade ovarian tumors. Cancer Res. 1995;55:6172–6180. [PubMed] [Google Scholar]
- 13.Risch HA, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J. Natl Cancer Inst. 2006;98:1675–1677. doi: 10.1093/jnci/djj465. [DOI] [PubMed] [Google Scholar]
- 14.Cramer DW, et al. Genital talc exposure and risk of ovarian cancer. Int. J. Cancer. 1999;81:351–356. doi: 10.1002/(sici)1097-0215(19990505)81:3<351::aid-ijc7>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 15.Muscat JE, Huncharek MS. Perineal talc use and ovarian cancer: a critical review. Eur. J. Cancer Prev. 2006;62:358–360. doi: 10.1097/CEJ.0b013e32811080ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohler MF, et al. Spectrum of mutation and frequency of allelic deletion of the p53 gene in ovarian cancer. J. Natl Cancer Inst. 1993;85:1513–1519. doi: 10.1093/jnci/85.18.1513. This paper indicated that ovarian cancers undergo spontaneous mutation.
- 17.Berchuck A, et al. Overexpression of p53 is not a feature of benign and early-stage borderline epithelial ovarian tumors. Gynecol. Oncol. 1994;52:232–236. doi: 10.1006/gyno.1994.1037. [DOI] [PubMed] [Google Scholar]
- 18.Berchuck A, et al. The p53 tumor suppressor gene frequently is altered in gynecologic cancers. Am. J. Obstet. Gynecol. 1994;170:246–252. doi: 10.1016/s0002-9378(94)70414-7. [DOI] [PubMed] [Google Scholar]
- 19.Havrilesky L, et al. Prognostic significance of p53 mutation and p53 overexpression in advanced epithelial ovarian cancer: a Gynecologic Oncology Group study. J. Clin. Oncol. 2003;21:3814–3825. doi: 10.1200/JCO.2003.11.052. [DOI] [PubMed] [Google Scholar]
- 20.Hall J, et al. Critical evaluation of p53 as a prognostic marker in ovarian cancer. Exp. Rev. Mol. Med. 2004;12:1–20. doi: 10.1017/S1462399404007781. A thoughtful and thorough review of the prognostic significance of p53 in ovarian cancer.
- 21.Buller RE, et al. A phase I/II trial of rAd/p53 ( SCH58500) gene replacement in recurrent ovarian cancer. Cancer Gene Ther. 2002;9:553–566. doi: 10.1038/sj.cgt.7700472. [DOI] [PubMed] [Google Scholar]
- 22.Vasey PA, et al. Phase I trial of intraperitoneal injection of the E1B-55-kd-gene-deleted adenovirus ONYX-015 (dl1520) given on days 1 through 5 every 3 weeks in patients with recurrent/refractory epithelial ovarian cancer. J. Clin. Oncol. 2002;15:1562–1569. doi: 10.1200/JCO.2002.20.6.1562. [DOI] [PubMed] [Google Scholar]
- 23.Kojima K, et al. MDM2 antagonists induce p53-dependent apoptosis in AML: implications for leukemia therapy. Blood. 2005;106:3150–3159. doi: 10.1182/blood-2005-02-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu Y, et al. Methods in Enzymology: Regulators and Effectors of Small GTPases. In: Balch WE, Der C, Hall A, editors. Ras Proteins. Vol. 407. New York: Academic; 2006. pp. 455–467. A comprehensive review of the role of DIRAS3 (ARHI) in ovarian cancer.
- 25.Cvetkovic D, et al. Altered expression and loss of heterozygosity of the LOT1 gene in ovarian cancer. Gynecol. Oncol. 2004;95:449–455. doi: 10.1016/j.ygyno.2004.08.051. [DOI] [PubMed] [Google Scholar]
- 26.Feng W, et al. Imprinted tumor suppressor genes ARHI and PEG3 are the most frequently down-regulated in human ovarian cancers by loss of heterozygosity and promoter methylation. Cancer. 2008;112:1489–1502. doi: 10.1002/cncr.23323. [DOI] [PubMed] [Google Scholar]
- 27.Chen MY, et al. Synergistic inhibition of ovarian cancer cell growth with demethylating agents and histone deacetylase inhibitors. Proc. Amer. Assoc. Cancer Res. 2007;681 [Google Scholar]
- 28.Mackay H, et al. A phase II trial of the histone deacetylase inhibitor belinostat (PSC101) in patients with platinum resistant epithelial ovarian tumors and micropapillary/borderline (LMP) ovarian tumors. A PMH phase II consortium trial. J. Clin. Oncol. 2008;26 Suppl.:5518. [Google Scholar]
- 29.Balch C, et al. The epigenetics of ovarian cancer drug resistance and resensitization. Am. J. Obstet. Gynecol. 2004;191:1552–1572. doi: 10.1016/j.ajog.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 30.Bast RC, et al. A phase IIa study of a sequential regimen using azacitidine to reverse platinum resistance to carboplatin in patients with platinum resistant or refractory epithelial ovarian cancer. J. Clin. Oncol. 2008;26 Suppl.:3500. [Google Scholar]
- 31.Rubin SC, et al. BRCA1, BRCA2, and hereditary nonpolyposis colorectal cancer gene mutations in an unselected ovarian cancer population: relationship to family history and implications for genetic testing. Am. J. Obstet. Gynecol. 1998;178:670–677. doi: 10.1016/s0002-9378(98)70476-4. [DOI] [PubMed] [Google Scholar]
- 32.Lancaster JM, et al. BRCA2 mutations in primary breast and ovarian cancers. Nature Genet. 1996;13:238–240. doi: 10.1038/ng0696-238. [DOI] [PubMed] [Google Scholar]
- 33.Boyd J. In: Ovarian Cancer 5. Sharp F, Blackett T, Berek J, Bast R, editors. Oxford: Isis Medical Media; 1998. pp. 3–16. [Google Scholar]
- 34.Chetrit A, Hirsh-Yechezkel G, Ben-David Y, Lubin F, Friedman E. Effect of BRCA 1/2 mutations on long-term survival of patients with ovarian cancer: the national Israeli study of ovarian cancer. J. Clin. Oncol. 2008;26:20–25. doi: 10.1200/JCO.2007.11.6905. [DOI] [PubMed] [Google Scholar]
- 35.Moynahan ME, et al. Homology directed DNA repair, mitomycin-c resistance, and chromosome stability is restored with correction of a Brca1 mutation. Cancer Res. 2001;61:4842–4850. [PubMed] [Google Scholar]
- 36.Narod SA, Foulkes WD. BRCA1 and BRCA2, 1994 and beyond. Nature Rev. Cancer. 2004;4:665–676. doi: 10.1038/nrc1431. [DOI] [PubMed] [Google Scholar]
- 37.Edwards SL, et al. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451:1111–1115. doi: 10.1038/nature06548. [DOI] [PubMed] [Google Scholar]
- 38.Sakai W, et al. Secondary mutations as a mechanism of resistance to cisplatin in BRCA2-mutated cancers. Nature. 2008;451:1116–1120. doi: 10.1038/nature06633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drew Y, Calvert H. The potential of PARP inhibitors in genetic breast and ovarian cancers. Ann. NY Acad. Sci. 2008;1138:126–145. doi: 10.1196/annals.1414.020. [DOI] [PubMed] [Google Scholar]
- 40.Yap TA, Carden CT, Kaye SB. Beyond chemotherapy: targeted therapies in ovarian cancer. Nature Rev. Cancer. 2009;9:167–181. doi: 10.1038/nrc2583. A thorough and up-to-date review of molecular therapeutics for ovarian cancer.
- 41.Hennessey B, et al. BRCA status in ovarian cancer. Proc. Amer. Soc. Clin. Oncol. (in the press) [Google Scholar]
- 42.Umayahara K, et al. In: Ovarian Cancer 5. Sharp F, Blackett T, Berek J, Bast R, editors. Oxford: Isis Medical Media; 1998. pp. 17–23. [Google Scholar]
- 43.Jazaeri AA, et al. Gene expression profiles of BRCA1 -linked, BRCA2 -linked, and sporadic ovarian cancers. J. Natl Cancer Inst. 2002;13:990–1000. doi: 10.1093/jnci/94.13.990. This provocative paper suggests that sporadic ovarian cancers are either BRCA1 or BRCA2-like.
- 44.Eder AM, et al. Atypical PKC ι contributes to poor prognosis through loss of apical–basal polarity and cyclin E overexpression in ovarian cancer. Proc. Natl Acad. USA. 2005;102:12519–12524. doi: 10.1073/pnas.0505641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang L, et al. MicroRNAs exhibit high frequency genomic alterations in human cancer. Proc. Natl Acad. Sci. USA. 2003;103:9136–9141. doi: 10.1073/pnas.0508889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tangir J, et al. Frequent microsatellite instability in epithelial borderline ovarian tumors. Cancer Res. 1996;56:2501–2505. [PubMed] [Google Scholar]
- 47.Rodabaugh KJ, et al. Detailed deletion mapping of chromosome 9p and p16 gene alterations in human borderline and invasive epithelial ovarian tumors. Oncogene. 1995;11:1249–1254. [PubMed] [Google Scholar]
- 48.Berchuck A, et al. Overexpression of p53 is not a feature of benign and early- stage borderline epithelial ovarian tumors. Gynecol. Oncol. 1994;52:232–236. doi: 10.1006/gyno.1994.1037. [DOI] [PubMed] [Google Scholar]
- 49.Iwabuchi H, et al. Genetic analysis of benign, low-grade, and high-grade ovarian tumors. Cancer Res. 1995;55:6172–6180. [PubMed] [Google Scholar]
- 50.Abu-Jawdeh GM, et al. Estrogen receptor expression is a common feature of ovarian borderline tumors. Gynecol. Oncol. 1996;60:301–307. doi: 10.1006/gyno.1996.0043. [DOI] [PubMed] [Google Scholar]
- 51.Liu J, et al. A genetically defined model for human ovarian cancer. Cancer Res. 2004;64:1655–1663. doi: 10.1158/0008-5472.can-03-3380. This paper showed that an ovarian phenotype can be induced in xenografts by transfecting normal human ovarian surface epithelial cells with SV40 T antigen, telomerase and mutant Ras.
- 52.Cheng KW, et al. Emerging role of Rab GTPases in cancer and human disease. Cancer Res. 2005;65:2516–2519. doi: 10.1158/0008-5472.CAN-05-0573. [DOI] [PubMed] [Google Scholar]
- 53.Gautschi O, et al. Aurora kinases as cancer drug targets. Clin. Cancer Res. 2008;14:1639–1648. doi: 10.1158/1078-0432.CCR-07-2179. [DOI] [PubMed] [Google Scholar]
- 54.Li K, et al. Modulation of Notch signaling by antibodies specific for the extracellular regulatory region of Notch3. J. Biol. Chem. 2008;283:8046–8054. doi: 10.1074/jbc.M800170200. [DOI] [PubMed] [Google Scholar]
- 55.Schilder RJ, et al. Phase II study of gefitinib in patients with relapsed or persistent ovarian or primary peritoneal carcinoma and evaluation of epidermal growth factor receptor mutations and immunohistochemical expression: a Gynecologic Oncology Group Study. Clin. Cancer Res. 2005;11:5539–5548. doi: 10.1158/1078-0432.CCR-05-0462. [DOI] [PubMed] [Google Scholar]
- 56.Gordon AN, et al. Efficacy and safety of erlotinib HCI, an epidermal growth factor receptor (HER1/EGFR) tyrosine kinase inhibitor, in patients with advanced ovarian carcinoma: results from a phase II multicenter study. Int. J. Gynecol. Cancer. 2005;15:785–792. doi: 10.1111/j.1525-1438.2005.00137.x. [DOI] [PubMed] [Google Scholar]
- 57.Heinemann V, Stintzing S, Kirchner T, Boeck S, Jung A. Clinical relevance of EGFR- and KRAS-status in colorectal cancer patients treated with monoclonal antibodies directed against the EGFR. Cancer Treat. Rev. 2009;35:262–271. doi: 10.1016/j.ctrv.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 58.Bookman MA, et al. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the Gynecologic Oncology Group. J. Clin. Oncol. 2003;21:283–290. doi: 10.1200/JCO.2003.10.104. [DOI] [PubMed] [Google Scholar]
- 59.Hu L, Hofmann J, Lu Y, Mills GB, Jaffe RB. Inhibition of phophatidylinositol 3' kinase increases efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Cancer Res. 2002;62:1087–1092. [PubMed] [Google Scholar]
- 60.Raynaud FL, et al. Pharmacologic characterization of a potent inhibitor of class I phophatidylinositide 3-kinases. Cancer Res. 2007;67:5840–5850. doi: 10.1158/0008-5472.CAN-06-4615. [DOI] [PubMed] [Google Scholar]
- 61.Rosen DG, et al. The role of constitutively active signal transducer and activator of transcription 3 in ovarian tumorigenesis and prognosis. Cancer. 2006;107:2730–2740. doi: 10.1002/cncr.22293. [DOI] [PubMed] [Google Scholar]
- 62.Burke WM, et al. Inhibition of constitutively active Stat3 suppresses growth of human ovarian and breast cancer cells. Oncogene. 2001;20:7925–7934. doi: 10.1038/sj.onc.1204990. [DOI] [PubMed] [Google Scholar]
- 63.Duan Z, et al. 8-benzyl-4-oxo-8-azabicyclo[3.2.1]oct-2-ene-6, 7-dicarboxylic acid (SD-1008), a novel janus kinase 2 inhibitor, increases chemotherapy sensitivity in human ovarian cancer cells. Mol. Pharmacol. 2007;72:1137–1145. doi: 10.1124/mol.107.038117. [DOI] [PubMed] [Google Scholar]
- 64.McMurray JS. A new small molecule Stat 3 inhibitor. Chem. Biol. 2006;13:1123–1124. doi: 10.1016/j.chembiol.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 65.Murph M, et al. Of spiders and crabs: the emergence of lysophospholipids and their metabolic pathways as targets for therapy in cancer. Clin. Cancer Res. 2006;12:6598–6602. doi: 10.1158/1078-0432.CCR-06-1721. [DOI] [PubMed] [Google Scholar]
- 66.Beck HP, et al. Discovery of potent LPA2 (EDG4) antagonists as potential anticancer agents. Bioorg. Med. Chem. Lett. 2008;18:1037–1041. doi: 10.1016/j.bmcl.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 67.Lin YG, et al. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-κB pathway. Clin. Cancer Res. 2007;13:3423–3430. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- 68.Samanta AK, Huang HJ, Bast RC, Jr, Liao W. Overexpression of MEKK3 confers resistance to apoptosis through activation of NF κB. J. Biol. Chem. 2004;279:7576–7583. doi: 10.1074/jbc.M311659200. [DOI] [PubMed] [Google Scholar]
- 69.Häcker H, Karin M. Regulation and function of IKK and IKK-related kinases. Science STKE. 2006;357:re 13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- 70.Yang J, et al. The essential role of MEKK3 in TNF-induced NF-κB activation. Nature Immunol. 2001;2:620–624. doi: 10.1038/89769. [DOI] [PubMed] [Google Scholar]
- 71.Karin M. Nuclear factor κB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 72.See HT, Kavanagh JJ, Hu W, Bast RC., Jr Targeted therapy for epithelial ovarian cancer: current status and future prospects. Int. J. Gynecol. Cancer. 2004;13:701–734. doi: 10.1111/j.1525-1438.2003.13601.x. [DOI] [PubMed] [Google Scholar]
- 73.Suh DS, Yoon MS, Choi KU, Kim JY. Significance of E2F-1 overexpression in ovarian cancer. Int. J. Gynecol. Cancer. 2008;18:492–498. doi: 10.1111/j.1525-1438.2007.01044.x. [DOI] [PubMed] [Google Scholar]
- 74.Reimer D, et al. Expression of the E2 family of transcription factors and its clinical relevance in ovarian cancer. Ann. NY Acad. Sci. 2006;1091:270–286. doi: 10.1196/annals.1378.073. [DOI] [PubMed] [Google Scholar]
- 75.Berchuck A, et al. Regulation of growth of normal ovarian epithelial cells and ovarian cancer cell lines by transforming growth factor-β. Am. J. Obstet. Gynecol. 1992;166:676–684. doi: 10.1016/0002-9378(92)91697-9. [DOI] [PubMed] [Google Scholar]
- 76.Sunde JS, et al. Expression profiling identifies altered expression of genes that contribute to the inhibition of transforming growth factor-β signaling in ovarian cancer. Cancer Res. 2006;66:8404–8412. doi: 10.1158/0008-5472.CAN-06-0683. [DOI] [PubMed] [Google Scholar]
- 77.Fuller AF, Jr, Guy S, Budzik GP, Donahoe PK. Mullerian inhibiting substance inhibits colony growth of a human ovarian carcinoma cell line. J. Clin. Endocrinol. Metabol. 1982;54:1051–1055. doi: 10.1210/jcem-54-5-1051. [DOI] [PubMed] [Google Scholar]
- 78.Szotek PP, et al. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian inhibiting substance responsiveness. Proc. Natl Acad. Sci. USA. 2006;103:11154–11159. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pieretti-Vanmarcke R, et al. Mullerian inhibiting substance enhances subclinical doses of chemotherapeutic agents to inhibit human and mouse ovarian cancer. Proc. Natl Acad. Sci. USA. 2006;103:17426–17431. doi: 10.1073/pnas.0607959103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reed J, et al. Significance of Fas receptor protein expression in epithelial ovarian cancer. Hum. Pathol. 2005;36:971–976. doi: 10.1016/j.humpath.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 81.Kar R, et al. Role of apoptotic regulators in human epithelial ovarian cancer. Cancer Biol. Ther. 2007;6:1101–1105. doi: 10.4161/cbt.6.7.4329. [DOI] [PubMed] [Google Scholar]
- 82.Schuyer M, et al. Reduced expression of BAX is associated with poor prognosis in patients with epithelial ovarian cancer: a multifactorial analysis of TP53, p21, BAX and BCL-2. Br. J. Cancer. 2001;85:1359–1367. doi: 10.1054/bjoc.2001.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lancaster JM, et al. High expression of tumor necrosis factor apoptosis- inducing ligand is associated with favorable ovarian cancer survival. Clin. Cancer Res. 2003;9:762–766. [PubMed] [Google Scholar]
- 84.De la Torre FJ, et al. Apoptosis in epithelial ovarian tumours: prognostic significance of clinical and histopathologic factors and its association with the immunohistochemical expression of apoptotic regulatory proteins (p53, bcl-2 and bax) Eur. J. Obstet. Gynecol. Reprod. Biol. 2007;130:121–128. doi: 10.1016/j.ejogrb.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 85.Liang XH, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 86.Lu Z, et al. A novel tumor suppressor gene ARHI induces autophagy and tumor dormancy in ovarian cancer xenografts. J. Clin. Invest. 2008;118:3917–3929. doi: 10.1172/JCI35512. The initial report that linked autophagy and tumour dormancy.
- 87.Ren J, et al. Lysophosphatidic acid is constitutively produced by human peritoneal mesothelial cells and enhances adhesion, migration, and invasion of ovarian cancer cells. Cancer Res. 2006;15:3006–3014. doi: 10.1158/0008-5472.CAN-05-1292. [DOI] [PubMed] [Google Scholar]
- 88.Fishman DA, et al. Lysophosphatidic acid promotes matrix metalloproteinase (MMP) activation and MMP-dependent invasion in ovarian cancer cells. Cancer Res. 2001;1:3194–3199. [PubMed] [Google Scholar]
- 89.Fang X, et al. Mechanisms for lysophosphatidic acid-induced cytokine production in ovarian cancer cells. J. Biol. Chem. 2004;279:9653–9661. doi: 10.1074/jbc.M306662200. [DOI] [PubMed] [Google Scholar]
- 90.Sood AK, et al. Stress hormone-mediated invasion of ovarian cancer cells. Clin. Cancer Res. 2006;15:369–375. doi: 10.1158/1078-0432.CCR-05-1698. This report links stress to ovarian cancer growth through physiological mechanisms.
- 91.Barbolina MV, et al. Microenvironmental regulation of membrane type 1 matrix metalloproteinase activity in ovarian carcinoma cells via collagen-induced EGR1 expression. J. Biol. Chem. 2007;16:4924–4931. doi: 10.1074/jbc.M608428200. [DOI] [PubMed] [Google Scholar]
- 92.Cai KQ, et al. Prominent expression of metalloproteinases in early stages of ovarian tumorigenesis. Mol. Carcinog. 2007;46:130–143. doi: 10.1002/mc.20273. [DOI] [PubMed] [Google Scholar]
- 93.Prezas P, et al. Overexpression of the human tissue kallikrein genes KLK4, 5, 6, and 7increases the malignant phenotype of ovarian cancer cells. Biol. Chem. 2006;387:807–811. doi: 10.1515/BC.2006.102. [DOI] [PubMed] [Google Scholar]
- 94.Paliouras M, et al. Human tissue kallikreins: the cancer biomarker family. Cancer Lett. 2007;28:61–79. doi: 10.1016/j.canlet.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 95.Yin BWT, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J. Biol. Chem. 2001;276:27371–27375. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- 96.Gubbels JA, et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol. Cancer. 2006;5:50. doi: 10.1186/1476-4598-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rump A, Morikawa Y, Tanaka M. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J. Biol. Chem. 2004;279:9190–9198. doi: 10.1074/jbc.M312372200. [DOI] [PubMed] [Google Scholar]
- 98.Cannistra SA, et al. CD44 variant expression is a common feature of epithelial ovarian cancer: lack of association with standard prognostic factors. J. Clin. Oncol. 1995;13:1912–1921. doi: 10.1200/JCO.1995.13.8.1912. [DOI] [PubMed] [Google Scholar]
- 99.Strobel T, Swanson L, Cannistra SA. In vivo inhibition of CD44 limits intra-abdominal spread of a human ovarian cancer xenograft in nude mice: a novel role for CD44 in the process of peritoneal implantation. Cancer Res. 1997;57:1228–1232. [PubMed] [Google Scholar]
- 100.Yoneda J, et al. Expression of angiogenesis-related genes and progression of human ovarian carcinomas in nude mice. J. Natl Cancer Inst. 1998;90:447–454. doi: 10.1093/jnci/90.6.447. [DOI] [PubMed] [Google Scholar]
- 101.Birrer MJ, et al. Whole genome oligonucleotide-based array comparative genomic hybridization analysis identified fibroblast growth factor 1 as a prognostic marker for advanced-stage serous ovarian adenocarcinomas. J. Clin Oncol. 2007;1:2281–2287. doi: 10.1200/JCO.2006.09.0795. A recent comparative genomic hybridization analysis that indicates the importance of FGF1 in the pathogenesis of ovarian cancer.
- 102.Monk BJ, et al. Salvage bevacizumab (rhuMAB VEGF)-based therapy after multiple prior cytoxic regimens in advanced refractory epithelial ovarian cancer. Gynecol. Oncol. 2006;102:140–144. doi: 10.1016/j.ygyno.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 103.Kamat AA, et al. Metronomic chemotherapy enhances the efficacy of antivascular therapy in ovarian cancer. Cancer Res. 2007;67:281–288. doi: 10.1158/0008-5472.CAN-06-3282. [DOI] [PubMed] [Google Scholar]
- 104.Lu C, et al. Impact of vessel maturation on antiangiogenic therapy in ovarian cancer. Am. J. Obstet. Gynecol. 2008;198:477.e1–477.e9. doi: 10.1016/j.ajog.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lin YG, et al. EphA2 overexpression in associated with angiogenesis in ovarian cancer. Cancer. 2007;109:332–340. doi: 10.1002/cncr.22415. [DOI] [PubMed] [Google Scholar]
- 106.Landen CN, et al. Efficacy and antivascular effects of EphA2 reduction with an agonistic antibody in ovarian cancer. J. Natl Cancer Inst. 2006;98:1558–1570. doi: 10.1093/jnci/djj414. [DOI] [PubMed] [Google Scholar]
- 107.Landen CN, et al. Intraperitoneal delivery of liposomal siRNA for therapy of advanced ovarian cancer. Cancer Biol. Ther. 2006;5:1708–1713. doi: 10.4161/cbt.5.12.3468. This paper showed that neutral liposomes allow the efficient delivery of siRNA to human ovarian cancer xenografts.
- 108.Mancuso MR, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J. Clin. Invest. 2006;116:2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang G, et al. The chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis. Proc. Natl Acad. Sci. USA. 2006;31:16472–16477. doi: 10.1073/pnas.0605752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Milliken D, et al. Analysis of chemokines and chemokine receptor expression in ovarian cancer ascites. Clin. Cancer Res. 2002;8:1108–1114. [PubMed] [Google Scholar]
- 111.Zhang L, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003;16:203–213. doi: 10.1056/NEJMoa020177. A thorough study that documents the prognostic significance of T cell infiltration in ovarian cancer.
- 112.Sato E, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl Acad. Sci. USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jiang YP, et al. Expression of chemokine CXCL12 and its receptor CXCR4 in human epithelial ovarian cancer: an independent prognostic factor for tumor progression. Gynecol. Oncol. 2006;103:226–233. doi: 10.1016/j.ygyno.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 114.Kryczek I, et al. CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Res. 2005;65:465–472. [PubMed] [Google Scholar]
- 115.Curiel TJ, et al. Dendritic cell subsets differentially regulate angiogenesis in human ovarian cancer. Cancer Res. 2004;64:5535–5538. doi: 10.1158/0008-5472.CAN-04-1272. [DOI] [PubMed] [Google Scholar]
- 116.Kajiyana H, et al. Involvement of SDF-1α/CXCR4 axis in the enhanced peritoneal metastasis of epithelial ovarian cancer. Int. J. Cancer. 2008;122:91–99. doi: 10.1002/ijc.23083. [DOI] [PubMed] [Google Scholar]
- 117.Szosarek PW, et al. Expression and regulation of tumor necrosis factor α in normal and malignant ovarian epithelium. Mol. Cancer Ther. 2006;5:382–390. doi: 10.1158/1535-7163.MCT-05-0303. [DOI] [PubMed] [Google Scholar]
- 118.Kulbe H, et al. The inflammatory cytokine tumor necrosis factor-α generates an autocrine tumor-promoting network in epithelial ovarian cancer cells. Cancer Res. 2007;67:585–592. doi: 10.1158/0008-5472.CAN-06-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Madhusdan S, et al. Study of etanercept, a tumor necrosis α inhibitor, in recurrent ovarian cancer. J. Clin. Oncol. 2005;23:5950–5959. doi: 10.1200/JCO.2005.04.127. [DOI] [PubMed] [Google Scholar]
- 120.Rustin GJS, et al. Use of CA-125 in clinical trial evaluation of new therapeutic drugs for ovarian cancer. Clin. Cancer Res. 2004;10:3919–3926. doi: 10.1158/1078-0432.CCR-03-0787. A recent review regarding the application of CA125 to clinical trials.
- 121.Menon U, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) Lancet Oncol. 2009;10:327–340. doi: 10.1016/S1470-2045(09)70026-9. Initial data from this trial suggest that combining CA125 and transvaginal ultrasound will be an effective strategy for the early detection of ovarian cancer.
- 122.Das PM, Bast RC., Jr Early detection of ovarian cancer. Biomarkers Med. 2008;2:291–303. doi: 10.2217/17520363.2.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bouchard D, et al. Proteins with whey-acidic protein motifs and cancer. Lancet Oncol. 2006;7:167–174. doi: 10.1016/S1470-2045(06)70579-4. [DOI] [PubMed] [Google Scholar]
- 124.Lu KH, et al. Selection of potential markers for epithelial ovarian cancer with gene expression arrays and recursive descent partition analysis. Clin. Cancer Res. 2004;10:3291–3300. doi: 10.1158/1078-0432.CCR-03-0409. [DOI] [PubMed] [Google Scholar]
- 125.Clarke CH, et al. A panel of proteomic markers improves the sensitivity of CA125 for detecting stage I epithelial ovarian cancer. J. Clin. Oncol. 2008;26 Suppl.:5542. [Google Scholar]
- 126.Bast RC, et al. Optimizing a two-stage strategy for early detection of ovarian cancer. NCI Translational Science Meeting 300, #292. National Cancer Institute. 2008 [Google Scholar]
- 127.Shridhar V, et al. Genetic analysis of early- versus late-stage ovarian tumors. Cancer Res. 2001;61:5895–5904. [PubMed] [Google Scholar]
- 128.Marquez RT, et al. Patterns of gene expression in different histotypes of epithelial ovarian cancer correlate with those in normal fallopian tube, endometrium and colon. Clin. Cancer Res. 2005;11:6116. doi: 10.1158/1078-0432.CCR-04-2509. This study showed that the gene expression profiles of different ovarian cancer histotypes correlate with their morphological counterparts in normal tissues.
- 129.Cheng W, et al. Lineage infidelity of epithelial ovarian cancers is controlled by HOX genes that specify regional identity in the reproductive tract. Nature Med. 2005;11:531. doi: 10.1038/nm1230. The authors make a convincing argument that the HOX genes have a role in determining ovarian cancer histotypes.
- 130.Tothill RW, et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin. Cancer Res. 2008;14:5198–5208. doi: 10.1158/1078-0432.CCR-08-0196. [DOI] [PubMed] [Google Scholar]
- 131.Schwartz DR, et al. Gene expression in ovarian cancer reflects both morphology and biological behavior, distinguishing clear cell from other poor-prognosis ovarian carcinomas. Cancer Res. 2002;63:4722–4729. [PubMed] [Google Scholar]
- 132.Kurman RJ, Shih LEM. Pathogenesis of ovarian cancer: lessons from morphology and biology and their clinical implications. Int. J. Gynecol. Pathol. 2008;27:151–160. doi: 10.1097/PGP.0b013e318161e4f5. This review summarizes the evidence for type I and type II ovarian cancer.
- 133.Bast RC, Jr, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. New Engl. J. Med. 1983;309:883–887. doi: 10.1056/NEJM198310133091503. This is the original report of the CA125 assay.
- 134.Spaeth EL, et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contribures to fibrovascular network expansion and tumor progression. PLoS ONE. 2009;4:e4992. doi: 10.1371/journal.pone.0004992. [DOI] [PMC free article] [PubMed] [Google Scholar]