E3 ubiquitin ligase APC/C-Cdh1 accounts for the Warburg effect by linking glycolysis to cell proliferation (original) (raw)
Abstract
Cell proliferation is known to be accompanied by activation of glycolysis. We have recently discovered that the glycolysis-promoting enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase, isoform 3 (PFKFB3), is degraded by the E3 ubiquitin ligase APC/C-Cdh1, which also degrades cell-cycle proteins. We now show in two different cell types (neoplastic and nonneoplastic) that both proliferation and aerobic glycolysis are prevented by overexpression of Cdh1 and enhanced by its silencing. Furthermore, we have coexpressed Cdh1 with PFKFB3—either wild-type or a mutant form resistant to ubiquitylation by APC/C-Cdh1—or with the glycolytic enzyme 6-phosphofructo-1-kinase and demonstrated that whereas glycolysis is essential for cell proliferation, its initiation in the presence of active Cdh1 does not result in proliferation. Our experiments indicate that the proliferative response, regardless of whether it occurs in normal or neoplastic cells, is dependent on a decrease in the activity of APC/C-Cdh1, which activates both proliferation and glycolysis. These observations have implications for cell proliferation, neoplastic transformation, and the prevention and treatment of cancer.
Keywords: aerobic glycolysis, cell cycle, PFKFB3, cancer
The anaphase-promoting complex/cyclosome (APC/C) is an E3 ubiquitin ligase that plays an essential role in G1 phase and mitosis through the degradation of cell-cycle proteins. To initiate degradation it utilizes two activator proteins, Cdc20 and Cdh1, which associate with the enzyme in a cell-cycle-dependent manner and target different substrates for destruction (1). Substrates bind specifically to the APC/C-activator complex through degradation motifs, the best known of which are the D box (2–4) and the KEN box (5). Although both activator proteins recognize the D box, the KEN box is a targeting signal for APC/C-Cdh1 (5). Whereas APC/C-Cdc20 regulates the proteins responsible for metaphase-to-anaphase transition, inactivation of APC/C-Cdh1 at the restriction point in G1 is necessary for initiation of the S phase of the cell cycle, in which DNA is replicated and chromosomes are duplicated.
Proliferation in cancer cells is accompanied by activation of glycolysis, which occurs even in the presence of a normal oxygen concentration. The purpose and mechanism of this aerobic glycolysis, known as the Warburg effect (6), are still unclear. The two main reasons for this are, first, that the presence in cancer of a mitochondrial defect, originally thought to be responsible for aerobic glycolysis (6), remains controversial and, second, that aerobic glycolysis also occurs in proliferating nonneoplastic cells (7, 8). This has led to the suggestion that aerobic glycolysis may be required for new biomass formation (9).
We have recently discovered, using cells from rat brains, that up-regulation of glycolysis in astrocytes is dependent on the activity of the enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase, isoform 3 (PFKFB3) (10). PFKFB3, an isoform of 6-phosphofructo-2-kinase (PFK2), is responsible for the generation of fructose-2,6-bisphosphate (F2,6P2), the allosteric activator of 6-phosphofructo-1-kinase (PFK1) and therefore of glycolysis. We further found that this enzyme contains a KEN box and that it is constantly degraded in neurons by APC/C-Cdh1, explaining the inability of these cells to increase their glycolysis (10).
In view of the above, we tested the hypothesis that APC/C-Cdh1 is responsible for the linking of glycolysis to cell proliferation using two cell lines, neoplastic human neuroblastoma SH-SY5Y cells in which proliferation can be inhibited by incubation with retinoic acid (RA) (11), and embryonically derived human kidney cells (HEK 293), which proliferate in the presence of serum.
Results
Cell Proliferation Correlates with Glycolytic Capacity.
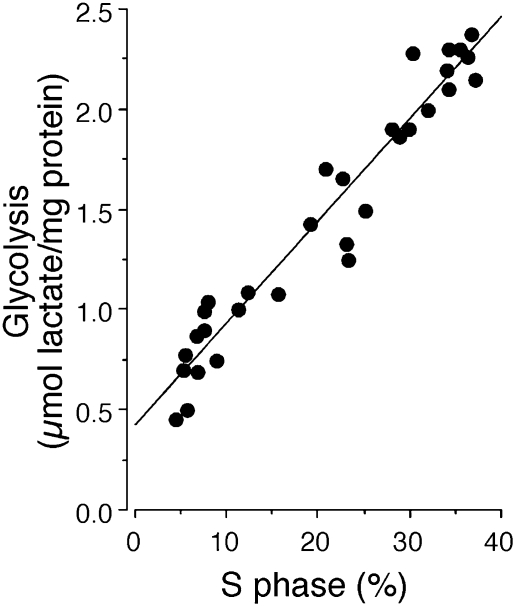
SH-SY5Y human neuroblastoma cells were treated with RA (10 μM) to induce cell-cycle arrest. Their rate of proliferation during 1-h incubation periods at 0, 1, 2, and 3 days after addition of RA was determined by measurement of BrdU incorporation into DNA to show the proportion of cells in S phase. The glycolytic capacity of the cells at these times was assessed as lactate produced during inhibition of ATP synthase by oligomycin. As shown in Fig. 1, the proportion of cells in S phase correlated positively with the glycolytic capacity.
Fig. 1.
Glycolysis and proliferation in human neuroblastoma SH-SY5Y cells. S phase (BrdU-staining analysis by flow cytometry) and glycolysis correlated positively in SH-SY5Y cells at different stages of proliferation.
Effect of Overexpression of Cdh1 on Cell Proliferation, Glycolysis, and Amount of PFKFB3 in Different Cell Types.
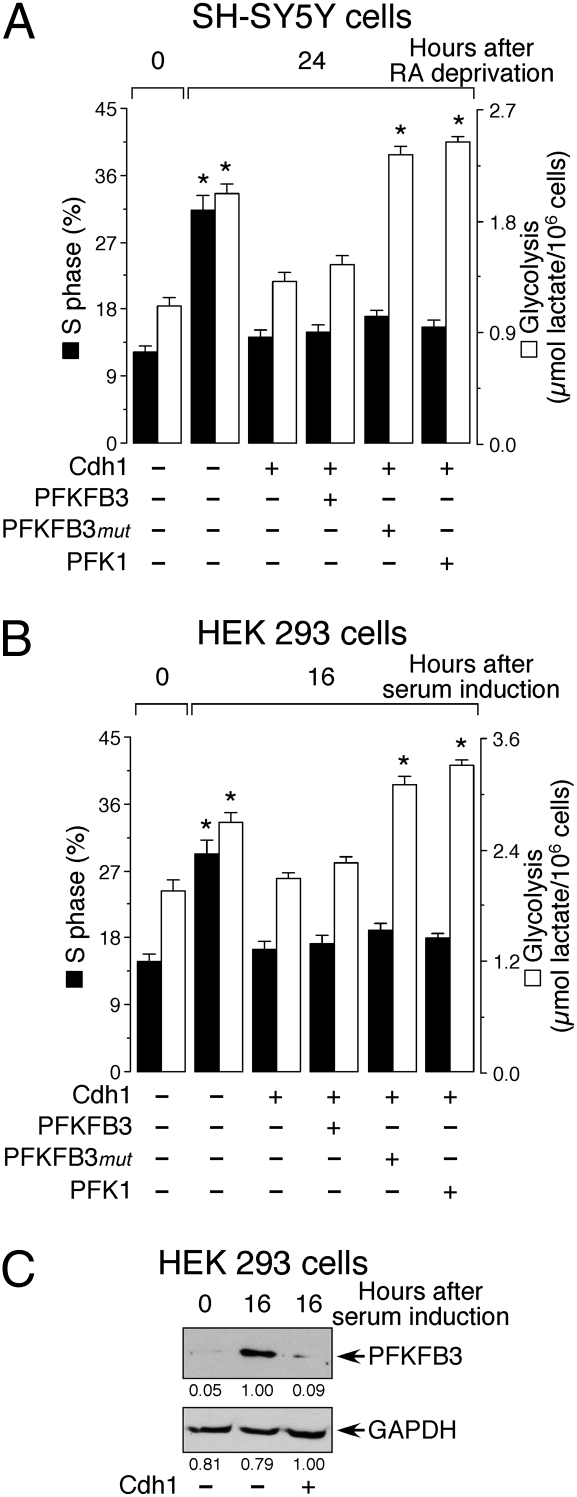
SH-SY5Y human neuroblastoma cells were treated with RA (10 μM) for 3 days to inhibit proliferation. Twenty-four hours after they were allowed to reenter the cell cycle by removal of RA, the proportion of cells in S phase was significantly increased, as was the glycolytic rate (Fig. 2_A_). A similar effect was observed in HEK 293 cells which had previously been deprived of serum to induce quiescence and in which proliferation was reestablished by addition of serum for 16 h (Fig. 2_B_). Proliferation in HEK 293 cells, demonstrated by an increase in the proportion in S phase, was associated with an increase in glycolytic activity (Fig. 2_B_) and with an increase in PFKFB3 protein abundance, as assessed by western blotting (Fig. 2_C_). Overexpression of Cdh1 largely prevented the increase in the proportion of cells in S phase and the increase in glycolysis induced in SH-SY5Y and HEK 293 cells by RA deprivation and addition of serum, respectively (Fig. 2 A and B). These effects of Cdh1, which were due to the degradation of PFKFB3 protein (Fig. 2_C_), could not be restored by coexpression of rat PFKFB3 (RB2K6 splice variant) (Fig. 2 A and B) because this enzyme (which is homologous to the human RBK5 splice variant) was actively degraded (Fig. 3_A_). However, the increase in glycolytic capacity, but not the proportion of cells in S phase, could be completely restored in these cells by coexpressing a PFKFB3 form in which the KEN box was mutated to AAA (PFKFB3_mut_; Fig. 2 A and B), and was therefore not targeted by APC/C-Cdh1 for destruction (Fig. 3_A_). Glycolysis, but not the proportion of cells in S phase, could also be restored by overexpression of the glycolytic enzyme PFK1 (Fig. 2 A and B). These results show that in both cell types the increase in glycolysis, promoted either by PFKFB3 or PFK1, is not sufficient to trigger cell proliferation in the presence of elevated amounts of Cdh1.
Fig. 2.
Effect of overexpression of Cdh1 on glycolysis, proliferation, and amount of PFKFB3 in different cell types. (A) The increase in S phase and glycolysis in SH-SY5Y cells, following exit from quiescence by removal of RA, were both prevented by Cdh1 overexpression. Glycolysis, but not proliferation, was restored by expression of PFKFB3_mut_, which is not recognized by Cdh1, or by overexpression of 6-phosphofructo-1-kinase, human muscle isoform (PFK1). (B) S phase and glycolysis were both increased following exit from quiescence by addition of serum to HEK 293 cells. Both responses were prevented by Cdh1 overexpression. Glycolysis, but not proliferation, was restored by expression of PFKFB3_mut_ or by overexpression of PFK1. (C) Serum addition to HEK 293 cells increased their PFKFB3 protein abundance, an effect that was abolished by Cdh1 overexpression. The detected protein was quantified by densitometry from three independent experiments. *P < 0.05 versus the corresponding 0-h points.
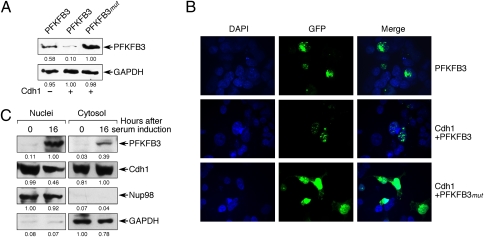
Fig. 3.
Cdh1 promotes PFKFB3 degradation in the nucleus. (A) Cdh1 overexpression promoted the degradation of coexpressed rat PFKFB3 cDNA (RB2K6 splice variant), but did not affect its KEN-box-mutant form (PFKFB3_mut_). (B) In Cdh1-overexpressing cells, coexpressed rat PFKFB3 (RB2K6 splice variant) was present only in the nucleus, whereas its KEN-box-mutant form (PFKFB3_mut_) accumulated in the nucleus and was spread throughout the cytosol. (C) Serum-induced exit from quiescence promoted PFKFB3 accumulation, both in the nucleus and in the cytosol, as well as a decrease in Cdh1 in the nucleus. The detected protein in A and C was quantified by densitometry from three independent experiments.
Localization and Degradation of PFKFB3.
HEK 293 cells transfected with either GFP-PFKFB3 or GFP-PFKFB3_mut_ were studied by confocal microscopy 16 h after the initiation of proliferation. Cotransfection of Cdh1 greatly reduced the amount of PFKFB3 protein (Fig. 3 A and B). Cdh1-induced degradation appeared to take place in the nucleus, where APC/C-Cdh1 is known to be active, as shown by the punctuate appearance of GFP-PFKFB3 fusion protein in this organelle (Fig. 3_B_, center row). Coexpression of Cdh1 and PFKFB3_mut_ resulted in a subcellular distribution of the enzyme in the nucleus and surrounding cytosol (Fig. 3_B_, lower row), which correlated with an increase in total PFKFB3 protein abundance (Fig. 3_A_). We also performed cell fractionation to confirm the cellular localization of endogenous PFKFB3. As shown in Fig. 3_C_, exit from quiescence induced PFKFB3 accumulation in the nucleus and in the cytosol, an effect that was concomitant with a slight decrease in nuclear Cdh1.
Proliferation and Glycolysis in Cells Lacking Cdh1.
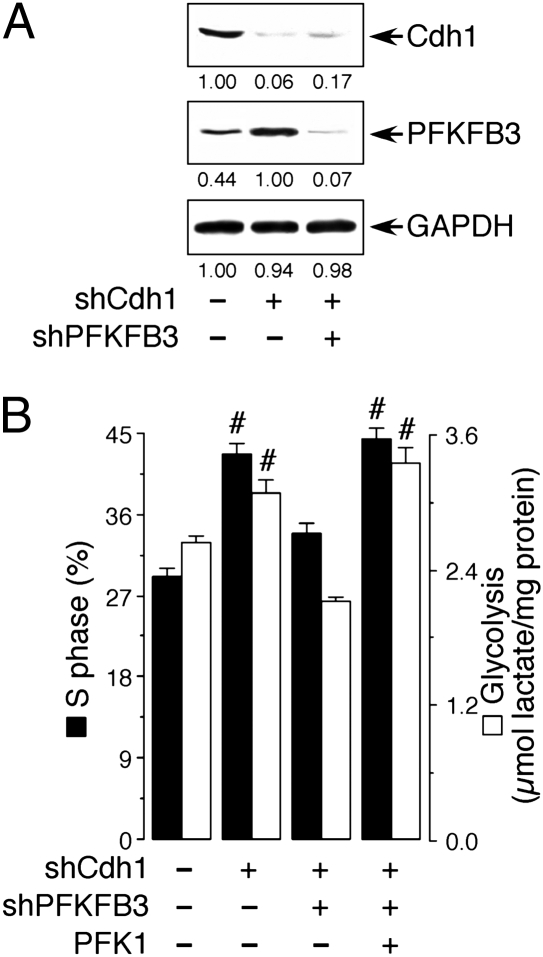
Cdh1 was knocked down in proliferating serum-induced HEK 293 cells (Fig. 4_A_). As shown in Fig. 4_B_, Cdh1 silencing significantly increased the proportion of cells in S phase, an effect that was accompanied by an increase in glycolytic activity. When PFKFB3 was also knocked down in Cdh1-silenced cells, both the increase in proliferation and in glycolysis caused by the knockdown of Cdh1 were prevented (Fig. 4_B_). Furthermore, reestablishment of glycolysis, in the absence of PFKFB3, by overexpressing PFK1, was sufficient to increase the proportion of cells in S phase (Fig. 4_B_). These results indicate that, in the absence of Cdh1 activity, an up-regulation of glycolysis by any means is sufficient to promote cell proliferation.
Fig. 4.
Cdh1 coordinates glycolysis with S-phase entry. (A) Treatment of HEK 293 cells with an shRNA against Cdh1 efficiently promoted a decrease in Cdh1 protein abundance and a concomitant increase in PFKFB3; the increase in PFKFB3 protein resulting from Cdh1 silencing was prevented by cotreatment with an shRNA against PFKFB3. The detected protein was quantified by densitometry from three independent experiments. (B) S phase and glycolysis in serum-treated HEK 293 cells were increased by Cdh1 silencing. This was counteracted by cosilencing PFKFB3 (shPFKFB3), but not when PFK1 was overexpressed. #P < 0.05 versus the corresponding control (no plasmids added).
Discussion
Changes in the activity of Cdh1 are known to play a central role in cell quiescence and proliferation. APC/C-Cdh1 is responsible for the ubiquitylation and destruction of mitotic cyclins, among other substrates, in late mitosis. Inactivation of APC/C-Cdh1, resulting from phosphorylation of the Cdh1 protein, occurs in late G1 phase of the cell cycle and is required for the renewed accumulation of the regulatory cell-cycle proteins necessary for the cells to enter S phase, toward a new round of cell division (1, 12). This point in late G1 phase, known as the restriction point (13), has long been recognized to be sensitive to lack of nutrients (12, 14). Although early studies demonstrated that glucose is essential for the synthesis of macromolecules in proliferating cells (7, 8, 13), the way in which this increased requirement for glucose is met during the cell cycle, or in general during cell proliferation, has remained unresolved. Our recent finding that PFKFB3 is degraded in neurons through an APC/C-Cdh1-dependent mechanism (10) provided the clue for the explanation of this phenomenon.
PFKFB3 activity is known to be increased in cancer and in highly proliferative cells (15). It is the only PFK2 isoform amenable to destruction by APC/C-Cdh1 (10). Furthermore, it is the one that has the highest kinase-to-bisphosphatase ratio (740:1), and therefore its activity is mostly dedicated to the generation of F2,6P2 and hence to the promotion of glycolysis (15). Thus, if both regulatory cell-cycle proteins and PFKFB3 are degraded via APC/C-Cdh1, the decrease in activity of APC/C-Cdh1 at the restriction point would synchronize an increase in mitotic cyclins to initiate transition toward S phase with an increase in glycolysis, to supply the metabolic intermediates necessary for the biosynthesis of macromolecules.
The present results, from experiments using two types of cell lines, of which only one is neoplastic, support our hypothesis. Indeed, overexpression of Cdh1 in either neuroblastoma or HEK 293 cells resulted in a decrease in both glycolysis and cell proliferation which could not be reversed by transfection of PFKFB3 alone. Transfection of a PFKFB3 with a mutated KEN box, rendering it resistant to ubiquitylation by APC/C-Cdh1, increased glycolysis without a concomitant increase in cell proliferation. In a similar manner, transfection of PFK1 increased glycolysis without increasing cell proliferation. These results show that, although Cdh1 controls both cell proliferation and glycolysis, an increase in glycolysis alone in the presence of increased Cdh1 activity is not sufficient to initiate proliferation.
Our experiments have also confirmed the nuclear localization of PFKFB3 (16). The fact that Cdh1 is also localized in the nucleus (17) indicates that this is the site where both cyclins and PFKFB3 are targeted for degradation by APC/C-Cdh1. Evidence for the nucleus being the site of PFKFB3 ubiquitylation, and probably degradation, comes from our experiments using confocal microscopy showing that PFKFB3_mut_ accumulates in the nucleus and spreads out into the cytosol. Moreover, exit from quiescence induced accumulation of endogenous PFKFB3 in the nucleus and in the cytosol, consistent with the enzyme overflowing from the nucleus following inhibition of its degradation. A nonglycolytic role for PFKFB3 in the nucleus, related to the activation of a cyclin-dependent kinase(s), has been suggested (16). Our results, however, indicate that PFKFB3 is degraded in the nucleus by APC/C-Cdh1 and that increased glycolysis occurs when inhibition of its destruction allows it to overflow into the cytoplasm.
In a separate set of experiments using HEK 293 cells, we have shown that, even when these cells were proliferating following addition of serum, silencing Cdh1 led to a further increase in cell proliferation and glycolysis. Both of these effects could be prevented by cosilencing PFKFB3 and reversed by concomitant expression of PFK1, demonstrating that, as long as the activity of APC/C-Cdh1 is low, enhancing glycolysis is sufficient to activate cell proliferation.
Thus, our experiments show that reduction in the activity of APC/C-Cdh1 is the step that coordinates glycolysis to cell proliferation. The linking of increased glycolysis to cell proliferation was originally described in neoplastic cells (6). However, aerobic glycolysis also occurs in nonneoplastic, highly proliferative cells such as lymphocytes (7, 8, 18). Our experiments indicate that the proliferative response, whether it occurs in normal or neoplastic cells, is dependent on a decrease in the activity of APC/C-Cdh1 which, through a single mechanism (i.e., stopping the destruction of crucial KEN-box-containing proteins), activates both proliferation and glycolysis.
In relation to neoplastic transformation, Cdh1 therefore lies at the crossroads of two crucial pathways that define cancer. Cdh1 has been shown to be down-regulated during malignant progression in a murine B-lymphoma cell line, and its reexpression reduced tumor development in these cells (19). It has recently been demonstrated that, whereas the absence of Cdh1 is lethal at the embryonic stage, Cdh1−/+ heterozygous mice are more susceptible to spontaneous tumors (20). Furthermore, mutations of APC subunits have been described in colon and breast cancer (21, 22). These data and our observations suggest that suppression of activity of APC/C-Cdh1 is likely to be a component of neoplastic transformation or an enabling mechanism without which cancer cannot proceed.
Materials and Methods
Cell Culture.
SH-SY5Y human neuroblastoma cells and human embryonic kidney (HEK) 293 cells were seeded at 104 cells/cm2 and 105 cells/cm2, respectively, in Dulbecco’s modified Earls medium (DMEM; Sigma-Aldrich) supplemented with 10% FCS. Eighteen hours after seeding, quiescence was induced in SH-SY5Y cells by incubation with retinoic acid (RA; 10 μM; Sigma) for 3 days (11) and in HEK 293 cells by serum deprivation (0.5% FCS) for 2 days. Quiescence was terminated by incubating cells with DMEM supplemented with 10% FCS for a further 24 h (SH-SY5Y cells) or 16 h (HEK 293 cells).
Cell Transfection.
Transfections were performed 24 h before termination of quiescence, using Lipofectamine 2000 (Invitrogen) with the following plasmid constructs: (i) pIRES-EGFP from Invitrogen, either empty or containing the full-length cDNA of rat PFKFB3 (RB2K6 splice variant) (10), PFKFB3_mut_ (KEN box mutated to AAA, starting at amino acid 142; QuikChange XL site-directed mutagenesis kit from Stratagene) (10), or human muscle PFK1 (NCBI accession number NM_000289.1); (ii) pcDNA3.0 (Invitrogen), either empty or including human Cdh1 (23); (iii) pd2EGFP-C1 (Clontech) including rat PFKFB3 (GFP-PFKFB3, RB2K6 splice variant) or its corresponding mutant PFKFB3_mut_ form (GFP-PFKFB3_mut_); and (iv) pSuper-neo.gfp (Oligoengine), including the small hairpin sequences for luciferase (control) (10), Cdh1 (24), or PFKFB3 (10). In each experiment, the total cDNA transfected was 1.6 μg/mL. Control cells were treated with 1.4 μg/mL of empty pcDNA3.0 plus 0.2 μg/mL of pIRES-EGFP, whereas all other groups were treated with 1.4 μg/mL of Cdh1 plus 0.2 μg/mL of the appropriate other plasmid. Cdh1 was administered at 1.4 μg/mL and GFP-PFKFB3 or GFP-PFKFB3_mut_ at 0.2 μg/mL. For RNA interference, each plasmid was administered at 0.53 μg/mL except in the control cells (luciferase shRNA), which were treated with 1.6 μg/mL.
Cell Fractionation.
Cells were washed with ice-cold PBS containing 1 mM MgCl2, and then were harvested with a rubber policeman and taken up in ice-cold hypotonic buffer (10 mM Hepes, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 300 mM sucrose, 1 mM EDTA, 1 mM DTT, 0.1 mM Na3VO4, and 0.1% Nonidet P-40) supplemented with protease inhibitors (100 μM phenylmethylsulfonyl fluoride, 50 μg/mL pepstatin, 50 μg/mL amastatin, 50 μg/mL leupeptin, 50 μg/mL bestatin, and 50 μg/mL soybean trypsin inhibitor). After swelling on ice for 15 min, plasma membranes were disrupted by repeated pipetting through a Gilson microtip. The samples were centrifuged at 500 × g for 5 min at 4°C to recover a cytoplasmic fraction (supernatant). Nuclei (pellet) were lysed in ice-cold hypertonic buffer containing the protease inhibitors for 2 h on ice. Both cytosolic and nuclear fractions were boiled for 5 min and identical amounts of protein were loaded on the gel. Nup98 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as control markers for nuclei and cytosol, respectively.
Western Blotting.
Aliquots (50 μg of protein) of cell lysate (in 2% sodium dodecylsulfate, 2 mM EDTA, 2 mM EGTA, 5 mM Tris, 100 μM phenylmethylsulfonyl fluoride, 50 μg/mL pepstatin, 50 μg/mL amastatin, 50 μg/mL leupeptin, 50 μg/mL bestatin, and 50 μg/mL soybean trypsin inhibitor) were centrifuged (14,000 × g, 10 min) and electrophoresed in an 8% SDS acrylamide gel. Proteins were transferred electrophoretically to nitrocellulose membranes, which were blocked in 5% (wt/vol) low-fat milk in 20 mM Tris, 500 mM sodium chloride, and 0.1% (wt/vol) Tween 20 (at pH 7.5) for 1 h, and further incubated with either anti-human PFKFB3 monoclonal antibody (H00005209-M08; Novus Biologicals), specific anti-rat PFKFB3 (RB2K3–RB2K6 splice variants) polyclonal antibody (10), anti-Cdh1 (AR38; J. Gannon, Clare Hall Laboratories, Cancer Research U.K., London), or anti-GAPDH (Sigma) overnight at 4°C. Signal detection was performed with an enhanced chemiluminescence kit (Pierce).
Measurement of Proportion of Cells in S Phase of the Cell Cycle.
The proportion of cells in S phase was assessed in nonconfluent cells by flow cytometric analysis of bromodeoxyuridine (BrdU) incorporation into DNA. This was achieved after 1 h of incubation with 10 μg/mL BrdU using the APC-BrdU flow kit (Becton Dickinson Biosciences), following the manufacturer’s instructions. APC-BrdU-stained cells were analyzed on the FL4 channel of a FACSCalibur flow cytometer (15 mW argon ion laser tuned at 488 nm; CellQuest software, Becton-Dickinson Biosciences).
Determination of Aerobic Glycolytic Capacity.
The glycolytic capacity of the cells was assessed by determining the rate of lactate released into the medium after inhibition of ATP synthase with oligomycin (6 μM; Sigma) for 1 h (25). Lactate concentrations were measured spectrophotometrically (Uvikon XL; Microbeam), as previously described (26).
Confocal Microscopy.
Cells were grown on glass coverslips and transfected with 0.2 μg/mL GFP-PFKFB3 or GFP-PFKFB3_mut_, either in the absence or presence of 1.4 μg/mL of Cdh1, as described above. Coverslips were mounted in glycerol/PBS (90/10, vol/vol) containing DAPI (30 μM) on glass slides. Microphotographs were taken by confocal microscopy 24 and 48 h after transfection.
Statistical Analysis.
Measurements from individual cultures were always performed in triplicate, and the results are expressed as mean ± SEM values across three different cell-culture preparations (n = 3). Statistical analysis of the results was performed by one-way analysis of variance, followed by the least significant difference multiple range test. In all cases, P < 0.05 was considered significant.
Acknowledgments
We thank Annie Higgs for help in the preparation of this manuscript. J.P.B. is supported by the Ministerio de Ciencia e Innovación (SAF2007-61492 and Consolider-Ingenio CSD2007-00020, Spain) and by the Junta de Castilla y León (SA066A07 and Red de Terapia Celular y Medicina Regenerativa). A.A. is supported by the Instituto de Salud Carlos III (FIS06/0794 and Renevas) and by the Junta de Castilla y León. J.P.B and A.A are recipients of "Salvador de Madariaga" grants from the "Programa Nacional de Movilidad de Recursos Humanos 2008-2011" from the Spanish Ministry of Science and Innovation.
Footnotes
The authors declare no conflict of interest.
References
- 1.Li M, Zhang P. The function of APC/CCdh1 in cell cycle and beyond. Cell Div. 2009;4(2) doi: 10.1186/1747-1028-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glotzer M, Murray AW, Kirschner MW. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- 3.Yamano H, Tsurumi C, Gannon J, Hunt T. The role of the destruction box and its neighbouring lysine residues in cyclin B for anaphase ubiquitin-dependent proteolysis in fission yeast: Defining the D-box receptor. EMBO J. 1998;17:5670–5678. doi: 10.1093/emboj/17.19.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters J-M. The anaphase promoting complex/cyclosome: A machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- 5.Pfleger CM, Kirschner MW. The KEN box: An APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 2000;14:655–665. [PMC free article] [PubMed] [Google Scholar]
- 6.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 7.Kay JE. The importance of changes in membrane transport in lymphocyte activation by phytohaemagglutinin. Biochem Soc Trans. 1976;4:1120–1122. doi: 10.1042/bst0041120. [DOI] [PubMed] [Google Scholar]
- 8.Hume DA, Radik JL, Ferber E, Weidemann MJ. Aerobic glycolysis and lymphocyte transformation. Biochem J. 1978;174:703–709. doi: 10.1042/bj1740703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrero-Mendez A, et al. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- 11.Cuende J, Moreno S, Bolaños JP, Almeida A. Retinoic acid downregulates Rae1 leading to APC(Cdh1) activation and neuroblastoma SH-SY5Y differentiation. Oncogene. 2008;27:3339–3344. doi: 10.1038/sj.onc.1210987. [DOI] [PubMed] [Google Scholar]
- 12.Morgan DO. In: The Cell Cycle. Lawrence E, editor. London: New Science; 2007. [Google Scholar]
- 13.Pardee AB. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci USA. 1974;71:1286–1290. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zetterberg A, Larsson O. Kinetic analysis of regulatory events in G1 leading to proliferation or quiescence of Swiss 3T3 cells. Proc Natl Acad Sci USA. 1985;82:5365–5369. doi: 10.1073/pnas.82.16.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yalcin A, Telang S, Clem B, Chesney J. Regulation of glucose metabolism by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases in cancer. Exp Mol Pathol. 2009;86:174–179. doi: 10.1016/j.yexmp.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Yalcin A, et al. Nuclear targeting of 6-phosphofructo-2-kinase (PFKFB3) increases proliferation via cyclin-dependent kinases. J Biol Chem. 2009;284:24223–24232. doi: 10.1074/jbc.M109.016816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaquenoud M, van Drogen F, Peter M. Cell cycle-dependent nuclear export of Cdh1p may contribute to the inactivation of APC/C(Cdh1) EMBO J. 2002;21:6515–6526. doi: 10.1093/emboj/cdf634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang T, Marquardt C, Foker J. Aerobic glycolysis during lymphocyte proliferation. Nature. 1976;261:702–705. doi: 10.1038/261702a0. [DOI] [PubMed] [Google Scholar]
- 19.Wang CX, Fisk BC, Wadehra M, Su H, Braun J. Overexpression of murine fizzy-related (fzr) increases natural killer cell-mediated cell death and suppresses tumor growth. Blood. 2000;96:259–263. [PubMed] [Google Scholar]
- 20.García-Higuera I, et al. Genomic stability and tumour suppression by the APC/C cofactor Cdh1. Nat Cell Biol. 2008;10:802–811. doi: 10.1038/ncb1742. [DOI] [PubMed] [Google Scholar]
- 21.Wang Q, et al. Alterations of anaphase-promoting complex genes in human colon cancer cells. Oncogene. 2003;22:1486–1490. doi: 10.1038/sj.onc.1206224. [DOI] [PubMed] [Google Scholar]
- 22.Park KH, Choi SE, Eom M, Kang Y. Downregulation of the anaphase-promoting complex (APC)7 in invasive ductal carcinomas of the breast and its clinicopathologic relationships. Breast Cancer Res. 2005;7:R238–R247. doi: 10.1186/bcr978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almeida A, Bolaños JP, Moreno S. Cdh1/Hct1-APC is essential for the survival of postmitotic neurons. J Neurosci. 2005;25:8115–8121. doi: 10.1523/JNEUROSCI.1143-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 25.Almeida A, Moncada S, Bolaños JP. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat Cell Biol. 2004;6:45–51. doi: 10.1038/ncb1080. [DOI] [PubMed] [Google Scholar]
- 26.Gutmann I, Wahlefeld AW. L-(+)-Lactate. Determination with lactate dehydrogenase and NAD. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Weinheim, Germany: Verlag Chemie; 1974. pp. 1464–1468. [Google Scholar]