Safety and tolerability of putaminal AADC gene therapy for Parkinson disease (original) (raw)
Abstract
Background:
In Parkinson disease (PD), the benefit of levodopa therapy becomes less marked over time, perhaps because degeneration of nigrostrial neurons causes progressive loss of aromatic l-amino acid decarboxylase (AADC), the enzyme that converts levodopa into dopamine. In a primate model of PD, intrastriatal infusion of an adeno-associated viral type 2 vector containing the human AADC gene (AAV-hAADC) results in robust response to low-dose levodopa without the side effects associated with higher doses. These data prompted a clinical trial.
Methods:
Patients with moderately advanced PD received bilateral intraputaminal infusion of AAV-hAADC vector. Low-dose and high-dose cohorts (5 patients in each) were studied using standardized clinical rating scales at baseline and 6 months. PET scans using the AADC tracer [18F]fluoro-l-m-tyrosine (FMT) were performed as a measure of gene expression.
Results:
The gene therapy was well tolerated, but 1 symptomatic and 2 asymptomatic intracranial hemorrhages followed the operative procedure. Total and motor rating scales improved in both cohorts. Motor diaries also showed increased on-time and reduced off-time without increased “on” time dyskinesia. At 6 months, FMT PET showed a 30% increase of putaminal uptake in the low-dose cohort and a 75% increase in the high-dose cohort.
Conclusion:
This study provides class IV evidence that bilateral intrastriatal infusion of adeno-associated viral type 2 vector containing the human AADC gene improves mean scores on the Unified Parkinson’s Disease Rating Scale by approximately 30% in the on and off states, but the surgical procedure may be associated with an increased risk of intracranial hemorrhage and self-limited headache.
GLOSSARY
AADC
= l-amino acid decarboxylase;
AAV-hAADC
= adeno-associated viral type 2 vector containing the human AADC gene;
DA
= dopamine;
FMT
= [18F]fluoro-l-m-tyrosine;
MPTP
= 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine;
MSN
= medium spiny neurons;
NS
= not significant;
PD
= Parkinson disease;
UPDRS
= Unified Parkinson’s Disease Rating Scale.
After initial improvement with levodopa, many patients with Parkinson disease (PD) eventually require higher doses for benefit, and their condition is then often complicated by side effects such as motor fluctuations, dyskinesias, hallucinations, and autonomic symptoms. The reason for the waning benefit of levodopa is unknown.
Peripherally administered levodopa is decarboxylated to dopamine (DA) by l-amino acid decarboxylase (AADC), presumably within dopaminergic terminals in the striatum. One reason for the waning benefit of levodopa treatment may be that the conversion to DA is reduced due to declining levels of AADC.1 We chose a strategy to restore dopaminergic function by delivering the AADC gene directly into nondegenerating striatal neurons (the site of DA action) by means of an adeno-associated viral vector (AAV-hAADC), thereby eliminating the need for the nigral neurons in DA synthesis. Potential advantages of this approach are that it targets the striatal regions affected by the disease and the treatment effect can be adjusted simply by changing the levodopa dose.
Preclinical studies have shown that this treatment results in robust gene expression for more than 7 years, with restoration of the response to levodopa therapy and widening of the therapeutic window.2,3 These data prompted initiation of a phase 1 (safety) study of intrastriatal infusion of AAV-hAADC in subjects with moderately advanced PD who had response fluctuations. Here we report the complete results for 2 cohorts of patients studied at 6 months, the study’s primary endpoint. A preliminary report of the first cohort was recently published.4
METHODS
Standard protocol approvals, registrations, and patient consents.
The protocol and consent forms were approved by the institutional review board at the University of California, San Francisco, and US Food and Drug Administration. The protocol was also reviewed and approved by the Recombinant DNA Advisory Committee of the National Institutes of Health. A data safety monitoring board was established to review ongoing data from the study. All subjects reviewed the consent form and provided written consent. The clinical trial identifier is NCT00229736.
Study procedure.
Ten patients (5 men and 5 women) with a mean age of 64 years (range, 57–71 years) were entered into the study. Two dose cohorts (5 patients each) were evaluated. The low-dose cohort underwent 2 infusions into each putamen consisting of 50 μL of vehicle per infusion site, receiving a total dose of 9 × 1010 vector genomes. The high-dose cohort underwent similar infusions and received 3 × 1011 vector genomes.
Entry criteria included PD with intractable motor fluctuations despite optimized medical treatment, Hoehn and Yahr stage III to IV off medication, history and screening examinations showing improvement with dopaminergic therapy, and optimized and stable anti-PD medication for at least 2 months before screening. Exclusion criteria included atypical parkinsonism, violent dyskinesias in the previous 6 months, prior stereotactic neurosurgery for PD, Mini-Mental State Examination5 score <26, hallucinations or delusions in the 6 months before screening, major psychiatric disorder, Geriatric Depression Scale score6 of greater than 10 (or greater than 5 if on antidepressants), history of malignancy within the past 5 years (excluding basal cell carcinoma), or neutralizing antibody titer to AAV-2 ≥1:1,200.
Subjects were evaluated clinically at baseline and then monthly for 6 months postoperatively. Standardized evaluations using the Unified Parkinson’s Disease Rating Scale (UPDRS)7 and the stand-walk-sit test8 were performed at baseline and 6 months in the off- and on-medication states. The UPDRS is a widely used rating scale that evaluates cognitive, functional, and motor deficits (UPDRS part III) and medication-related complications. It was administered in the morning in the off-state, 12 hours after the last dose of dopaminergic medication, and in the fully on-state, as judged by the patient and clinician. In most cases, this occurred 1 hour after taking the usual morning medications, but if the subject was not fully “on,” an additional one-half or whole tablet of carbidopa/levodopa 25/100 was administered, and the patient was evaluated when fully “on.” Motor state diaries were obtained at baseline, at 3 months, and 6 months postoperatively.9 Subjects were trained on how to complete motor diaries by scoring their motor status at half-hour intervals; the possible choices were “asleep,” “off,” “on,” “on with nontroublesome dyskinesias,” and “on with troublesome dyskinesias.”9 Subjects completed motor diaries for 3 consecutive days before the baseline, 3-month, and 6-month evaluations. For analysis, the average time per day spent in each motor state was calculated. The total on-time was calculated as the time spent in any on-state: “on without dyskinesias” plus “on with nontroublesome dyskinesias” plus “on with troublesome dyskinesias.” To compare the changes in antiparkinsonian medications, we calculated levodopa equivalents in the following manner: 100 mg of levodopa was defined as equivalent to 133 mg of controlled-release levodopa, 75 mg of levodopa plus entacapone, 1 mg of pramipexole, and 5 mg of ropinirole.10 Although no adjustment was made for treatment with rasagiline, no patient initiated or discontinued this medication during the 6-month observation period. The Mini-Mental State Examination and Geriatric Depression Scale were performed at the screening visit and at the 6-month visit.
AAV vector.
An expression cassette containing the human AADC complementary DNA was cloned into an AAV-2 shuttle plasmid, and a recombinant AAV-2 containing AADC under the control of the cytomegalovirus promoter was generated by a triple transfection of human embryonic kidney 293 cells.11,12 AAV-hAADC was purified from clarified cell lysates by differential precipitation, double cesium chloride ultracentrifugation, and buffer exchange. A detailed description of AAV vector production was published previously.3
AAV-hAADC infusion.
On the morning of the operation, subjects were admitted to the neurosurgical service, and antiparkinsonian medications were withheld. The subjects were fitted with a Leksell stereotactic head frame (Elekta, Norcross, GA) using local anesthesia at the pin sites and IV sedation with midazolam. The target in the postcommissural putamen was localized using gadolinium-enhanced volumetric T1 and either T2 or inversion recovery slab magnetic resonance (MR) acquisitions of the brain. A surgical planning workstation (StealthStation; Medtronic, Minneapolis, MN) was used to perform targeting and trajectory planning. Two parallel cannulae were placed 6 mm apart in the center of the postcommissural putamen bilaterally, one anterior and one posterior. Trajectories to these targets were planned so as to avoid traversing the lateral ventricle, sulci, and cortical veins. Subjects were infused with a total volume of 200 μL over the 4 injection sites (50 μL per site) using convection-enhanced delivery at a flow rate of 1 μL per minute.3 Customized infusion cannulae were designed to minimize potential reflux up the injection tract, and a “waiting period” of 10 minutes was observed after infusion before the cannulae were removed. Infusion was performed simultaneously through the anterior and posterior cannulae in each putamen to minimize operative time. Brain MRIs were performed postoperatively to identify any surgical complications. A subsequent MR scan was also obtained 6 months to 3 years postoperatively.
PET scanning.
Subjects underwent PET scans using the AADC tracer [18F]fluoro-l-m-tyrosine (FMT) 1 to 10 days before surgery and at 1 and 6 months after surgery. PET studies were performed on a Siemens (Munich, Germany) ECAT EXACT HR+ PET scanner in 3-dimensional acquisition mode. All subjects were studied approximately 60 to 90 minutes after an oral dose of 2.5 mg/kg of carbidopa. Before the emission scan, a 5-minute transmission scan was obtained for attenuation correction. Subsequently, approximately 1 to 2 mCi of FMT was injected as a bolus in an antecubital vein, and a 90-minute dynamic acquisition sequence was obtained. Data were quantified as previously described,4 yielding the term Kic to describe tracer uptake.
Statistical analysis.
Paired t tests were performed to evaluate differences in baseline and post–gene transfer clinical outcome measures and Kic values. For the clinical outcome measurements, a Bonferroni adjustment for multiple comparisons was used.
RESULTS
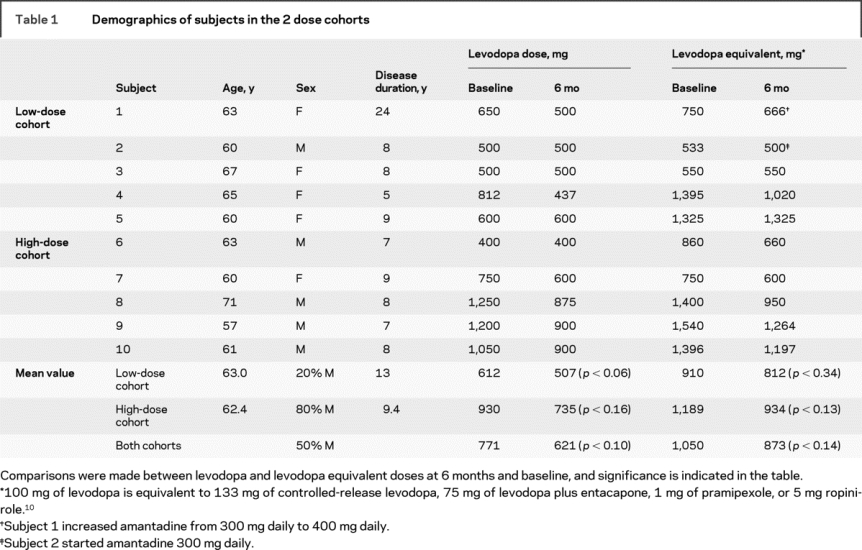
Of 12 patients who underwent screening, 2 were excluded because of elevated antibody titers to AAV. All 10 enrolled subjects had received long-term levodopa therapy (average 8.4 years, range 5–25 years) and were potential candidates for surgical therapy for PD because of intractable motor fluctuations that were not responsive to optimal medical therapy. Table 1 shows baseline demographic information.
Table 1 Demographics of subjects in the 2 dose cohorts
Adverse effects occurring in the study are shown in table e-1 on the _Neurology_® Web site at www.neurology.org. Asymptomatic hemorrhage, identified by postoperative MRI, occurred in 2 subjects; 1 (subject 4) had a small subdural/subarachnoid hemorrhage, and the other (subject 5) had an intracerebral hemorrhage associated with venous infarction. Subject 9 had a symptomatic hemorrhagic infarct (attributed to an arterial rupture) causing transient hemiplegia and aphasia from which he made an almost complete recovery. In all 3 instances, the hemorrhages occurred along the trajectory of the catheter but far from the infusion site, and were considered secondary to the surgical procedure. The most common adverse events were self-limited headache and discomfort at the surgical site, which was short-lived. No significant adverse events were related to the AAV-hAADC investigational product itself. Although subject 1 obtained clinical benefit after treatment, she elected to have deep brain stimulation of the subthalamic nucleus 18 months after gene transfer therapy because of incomplete resolution of long-standing wearing-off symptoms.
MRIs of the brain performed immediately postoperatively and then 6 or more months thereafter showed T2 and fluid-attenuated inversion recovery signal changes along the trajectory path of the infusion cannulae that did not enhance after administration of gadolinium. These signal changes were most notable just beneath the location of brain penetration and were attributed to the surgical procedure. No increase in baseline FMT signal was observed in these areas.
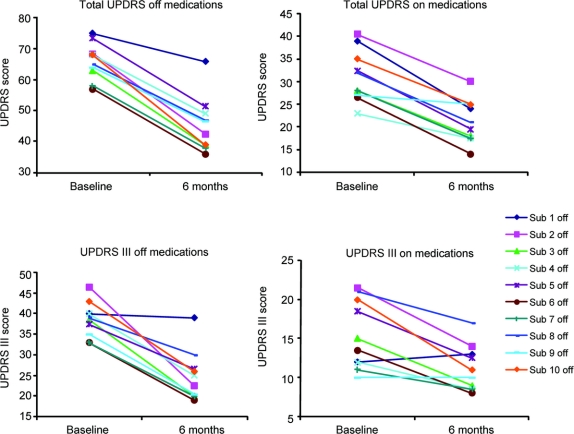
Results from the UPDRS are shown in figure 1. At 6 months, all subjects showed improvement in the total UPDRS in both the off- and on-states. The mean improvements in the total UPDRS were 31% in the off-state and 32% in the on-state (table 2). There were similar improvements in the UPDRS III (motor score) except that 1 patient did not show improvement in the on-state (figure 1, lower right panel). The mean improvement in the UPDRS III was 36% in the off-state and 28% in the on-state. Although off- and on-state evaluations are not available for all subjects at later dates beyond the primary (6-month) endpoint, results at 1 and 2 years postoperatively showed continuing reductions in total mean UPDRS scores for the combined cohorts: off-state 37% (n = 5) at 1 year and 38% (n = 7) at 2 years, and on-state 32% (n = 5) at 1 year and 22% (n = 7) at 2 years. Similar reductions in the UPDRS motor scores were present at 1 and 2 years.
Figure 1 Total and motor UPDRS scores for individual patients at baseline and 6 months after treatment in both the on- and off-states
UPDRS = Unified Parkinson’s Disease Rating Scale.
Table 2 Summary of outcomes
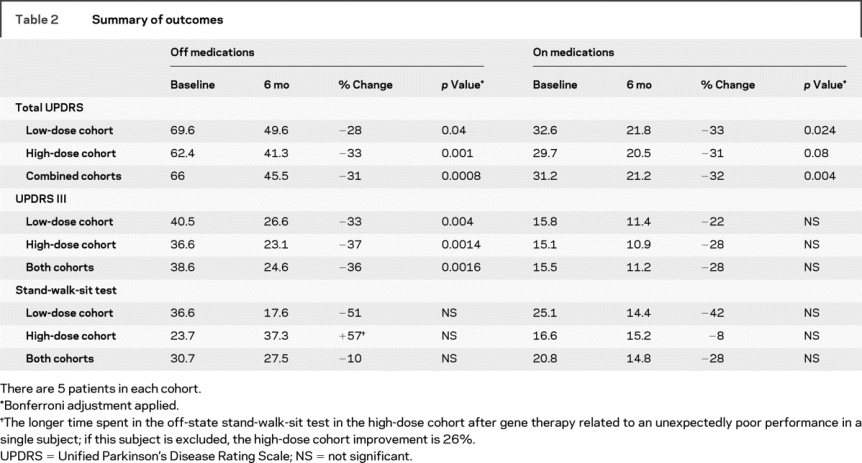
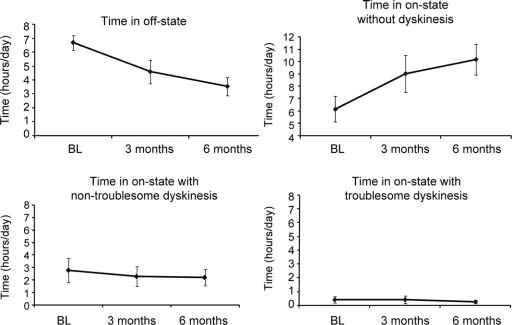
Improvements were also observed in the motor state diaries, as demonstrated by a reduction in off-time and an increase in on-time (figure 2). The mean off-time was reduced by 3.1 hours (p < 0.014), whereas total mean “on” time increased 3.3 hours (p < 0.14). As shown in the patient diaries and as reported by subjects, there was a gradual improvement in on-time and in mobility over the 6-month postoperative period. These improvements were not associated with an increase in severe dyskinesia; troublesome dyskinesias were reduced at 6 months (figure 2). Two patients had a transient increase in mild dyskinesias, but nontroublesome dyskinesias were reduced for the group as a whole (figure 2). Reductions in the time required to perform the stand-walk-sit test occurred in most patients, but this result did not reach statistical significance (table 2).
Figure 2 Time spent in different motor states at baseline and at 3 and 6 months postoperatively
BL = baseline.
Levels of dopaminergic medications were reduced in 8 patients (all of the 5 in the high-dose cohort and 3 of those in the low-dose group), although the absolute difference in dosage was not significant (table 1). These dose reductions occurred at the request of the subjects, who often noted a prolongation of on-time or reduced wearing-off. This is precisely what would be expected if gene therapy increased AADC activity and thus conversion of levodopa to DA. No patient required an increase in dose. Amantadine was started in 1 subject, and the dose was increased in another subject because of a transient increase in preexisting dyskinesias.
The success of gene transfer was monitored objectively using FMT PET imaging. Figure 3 shows the median increase in the FMT uptake (Kic) values in the putamen (averaged over right and left hemispheres) at 1 month and 6 months after gene transfer according to the dose received. As shown, the average increase was higher in the high-dose cohort (75% vs 30%), consistent with higher levels of AADC expression in the high-dose cohort.
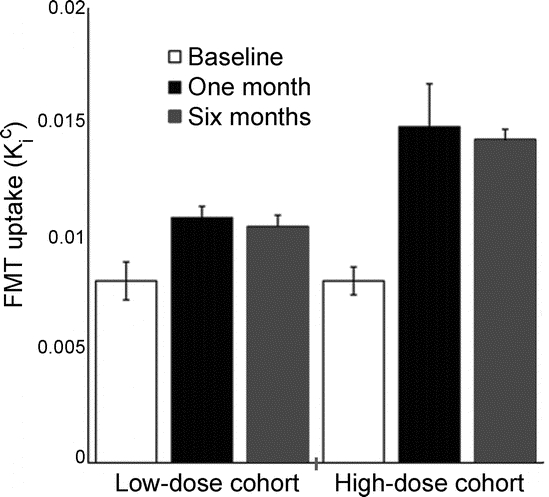
Figure 3 Plot showing change in FMT uptake of both putamens (mean ± SEM) over time for each cohort
These improvements were significant for both the low-dose (1 month, p = 0.02; 6 months, p = 0.009) and high-dose (1 month, p = 0.007; 6 months, p = 0.004) cohorts. FMT = [18F]fluoro-l-m-tyrosine.
Cognitive and behavioral screens were stable over the course of the study. The mean Mini-Mental State Examination score was 29.6 at baseline and 29.1 at 6 months (not significant [NS]). The mean Geriatric Depression Scale was 2.3 at baseline and 1.5 at 6 months (NS).
Patient titers to AAV were measured at the screening visit and at 2 weeks, 6 months, and 12 months postoperatively. Complete data were available for 6 subjects. Modest increases in titers (2- to 8-fold) were seen in 4 subjects, which peaked at the 2-week visit in 3 subjects and at the 6-month visit in 1 subject. One subject had no increase in antibody titers, and another had a prominent increase at 6 months. The baseline and postoperative titers did not correlate with clinical outcome or with other changes in clinical state.
DISCUSSION
We found that AAV-hAADC therapy was safe and well tolerated in 2 dose cohorts, although adverse effects did occur and may have been related to the method of vector administration. Although symptomatic or asymptomatic intracranial hemorrhages occur with deep brain stimulation, they seemed to occur more commonly with our infusion procedure. Intracranial hemorrhage is a well-described risk of stereotactic craniotomy. In a series of 481 lead implantations, there was a 3.3% risk of asymptomatic hemorrhage in microelectrode-guided deep brain stimulation surgery and a 0.6% risk of permanent neurologic injury from hematoma.13 In our study, factors that might have increased this risk include surgical technique, catheter design, infusion time, or other aspects of the infusion protocol itself; it is also possible that the incidence of hemorrhage was increased by chance. The independent data safety monitoring board did not identify any specific factors related to the surgical procedure or the infusion catheter design that might have been associated with the hemorrhage events. Given that hemorrhages occurred at a distance from the site of the infusion, we doubt that the infusion itself was directly responsible.
Our results regarding efficacy (improvement in both the off- and on-state UPDRS scores and in motor diaries) must be regarded as preliminary given the open-label design and small number of subjects. Similar degrees of improvement, eventually attributed to a placebo effect, were described in an open-label study of infused glial cell–derived neurotrophic factor in 5 subjects with advanced PD.14
There have been 2 other phase 1 studies using gene therapy for PD. In one, glutamic acid decarboxylase delivered by AAV-2 was injected unilaterally into the subthalamic nucleus in 12 subjects. At 6 months, the authors reported a 28% reduction in the total UPDRS in the off-state and 26% reduction in the on-state.15 In the AAV-Neurturin study, 12 subjects underwent bilateral intraputaminal injections.16 In this study, the mean off-state motor score at 6 months was reduced by 31.8% (p < 0.001), but there was no significant improvement in on-state motor score (W.J. Marks, personal communication, 2008). This contrasts with our findings in which, at 6 months, total UPDRS was reduced by 31% (p < 0.01) in the off-state and 32% (p < 0.01) in the on-state, and UPDRS III was reduced by 36% (p < 0.001) and 28% (NS).
One interpretation of the improvement in the off-medication state is that AADC treatment allows for improved conversion of endogenous levodopa in the remaining dopaminergic nigrostriatal axons. This interpretation is consistent with findings in nonhuman primates.17 An additional mechanism that might explain improvement in the off-state is increased endogenous levodopa production secondary to sprouting of surviving dopaminergic neurons. This phenomenon has been observed in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)–treated monkeys undergoing neural implant surgery and may be due to increased glial reaction at the site of the implant18 or, as in the present instance, to gene transfer.19 One additional explanation might be of a prolongation of the long-duration effect of levodopa. The improvement in the on-medication state may relate to more reliable conversion of peripherally administered levodopa by AADC, which after gene therapy is present at increased levels in the putamen. The lesser improvement in the on-state than in the off-state presumably reflects the lesser room for improvement when patients are already showing a good—but nevertheless incomplete—response to pharmacotherapy.
Phase 1 studies are often limited by the placebo effect. A particular feature of our study was the use of PET imaging to monitor the success and stability of gene transfer over time.4 Unlike other gene therapy studies for PD, our imaging technique provided direct evidence of gene expression in the target area by using a specific tracer for the gene product (AADC) consistent with results from preclinical studies.2,17 In addition, we observed higher signal in the high-dose group than in the low-dose group. Such a dose-response relationship in the imaging findings makes a placebo-associated clinical effect less likely, but does not exclude it.
Animal studies support the rationale for this approach. They show that the neurotropic vector (AAV-2), when infused into the striatum, mainly targets the striatal medium spiny neurons (MSN), which in turn express AADC.2,3,20 After systemic administration of levodopa, the neutral amino acid transporter is likely responsible for its entry into MSN.20 Although the mechanism of release has not been fully characterized, MSN then release DA as shown in microdialysis experiments in parkinsonian rodents treated with levodopa.20
Our approach, using intraputaminal gene transfer of the enzyme that converts levodopa to DA, differs from those previously reported because it replaces a principal enzyme that becomes deficient as a result of degeneration of nigrostrial neurons and because it allows the magnitude of the effect of gene therapy to be adjusted by altering the dose of exogenous levodopa. In our study, improvement occurred in several clinical measures. Further studies using a placebo group will be important to establish the safety of this treatment and to determine whether any benefit is due to AAV-hAADC. In addition, higher-dose therapy may also be considered, because a marked reduction in levodopa dose requirements was observed in parkinsonian nonhuman primates2 but not in our patients.
AUTHOR CONTRIBUTIONS
Statistical analysis was performed by Chadwick Christine at UCSF.
ACKNOWLEDGMENT
The authors thank Dr. James P. O’Neil of the Lawrence Berkeley National Laboratory for providing the [18F]FMT tracer used in this study.
DISCLOSURE
Financial support for this study and trial materials were provided by Avigen, Inc. and Genzyme Corporation. The cost of neurosurgical intervention was paid and Dr. Aminoff received salary and travel support from the sponsors through a grant to the University of California. Dr. Christine receives research support from Genzyme Corporation, Kyowa Pharmaceutical, Inc., Eisai Inc., and the NIH [NINDS 5U10NS044460 (Co-I) and RO1NS046487 (Site Investigator)]; and has received salary and travel support from Avigen, Inc. and Genzyme Corporation though a grant to the University of California. Dr. Starr serves on a scientific advisory board for Protein Therapeutics, Inc.; serves on the editorial board of Stereotactic and Functional Neurosurgery; has received honoraria from Medtronic, Inc.; and receives research support from SurgiVision, and the Dystonia Medical Research Foundation. Dr. Larson is an Associate Editor for Neurosurgery; has received honoraria and/or funding for travel to meetings from Ceregene, Inc. and NuVasive, Inc.; and receives research support from SurgiVision, Inc., Ceregene, Inc., and Genzyme Corporation. Dr. Eberling may accrue revenue on US Patent 6309634 (Issued: 1999) [Methods of Treating Parkinson’s Disease Using Recombinant Adeno-associated Vector (rAAV)] and received partial salary support from Genzyme Corporation to the Lawrence Berkeley National Laboratory. Dr. Jagust has served as a consultant to Synarc, Elan Corporation, Genentech, Inc., Ceregene, Schering-Plough Corp., and Merck Serono; and receives research support from the NIH [AG027859 (PI), AG027984 (PI), AG 024904 (Co-I)] and from the Alzheimer’s Association. Dr. Hawkins receives honoraria from the UCSF Department of Radiology (CME Lecture Series); and serves as a consulting radiologist to New Approaches to Neuroblastoma Therapy (NANT). Dr. VanBrocklin serves as an editor of Nuclear Medicine and Biology, Letters in Drug Design & Discovery, Cancer Research, and Reports in Medical Imaging; has filed US Patent Application US-2009-0136424-A1 (filed: November 18, 2008); has received honoraria from the US Department of Energy and the American Chemical Society; receives research support from Avid Radiopharmaceuticals, Inc. (NIH R41 AG030241 subcontract Co-I), Bayer Schering Pharma, and Varian BioSynergy; and receives research support from the Department of Energy [DE-FG02-08ER64699 (PI)] and the NIH [R01 CA119414 (Co-PI), R01 CA135626 (Co-I), R01 CA135358 (Co-I), S10 RR023051 (PI), R01 CA94253-01 (PI), R01 EB000482-01 (PI), and U54 CA90788 (Co-PI)], and the Alliance for Lupus Research; and holds stock options in Molecular Insight Pharmaceuticals, Inc. Dr. Wright has received funding for travel to invited talks from Genzyme Corporation and honoraria for invited talks not funded by industry; holds a financial interest in patent application PCT/US08/60654, 2008: Humanized viral vectors and methods of use thereof; and has served as a consultant to Tacere Therapeutics, Inc. and Genzyme Corporation. Dr. Bankiewicz has served as consultant for Genzyme Corporation; accrues revenue and receives license fee payments from patent US 7,534,613 B2 (issued: 2009): Methods of treating Parkinson’s disease using viral vectors; and may accrue revenue on US Patent 6309634 (Issued: 1999) [Methods of Treating Parkinson’s Disease Using Recombinant Adeno-associated Vector (rAAV)]. Dr. Aminoff served as Editor-in-Chief of Muscle & Nerve (1998-2007); receives royalties from publishing Neurology & General Medicine (Elsevier, 2008), Electrodiagnosis in Clinical Neurology (Elsevier, 2005), Clinical Neurology (McGraw-Hill, 2009), chapters in Cecil Textbook of Medicine (W.B. Saunders; 2004 and 2008), Harrison’s Principles of Internal Medicine (McGraw-Hill, 1994-2008), Handbook of Clinical Neurology (Elsevier; 2003-2009), and Current Medical Diagnosis & Treatment (McGraw-Hill, 1985-2009); has received honoraria for lectures or educational activities not funded by industry; serves as Editor-in-chief, Neurology section, Up-to-Date, for which he receives royalties; and receives research support from Genzyme Corporation, the NIH [NINDS 5 U10 NS044460 (Site PI) and R01 NS37167 (Site PI)] and the University of Rochester.
Supplementary Material
[Data Supplement]
Address correspondence and reprint requests to Dr. Michael J. Aminoff, UCSF Department of Neurology, 505 Parnassus Ave., Room 795-M, San Francisco, CA 94143-0114 aminoffm@neurology.ucsf.edu
Supplemental data at www.neurology.org
e-Pub ahead of print on October 14, 2009, at www.neurology.org.
Disclosure: Author disclosures are provided at the end of the article.
Received May 1, 2009. Accepted in final form August 14, 2009.
REFERENCES
- 1.Nagatsu T, Sawada M. Biochemistry of postmortem brains in Parkinson’s disease: historical overview and future prospects. J Neural Transm Suppl 2007;113–120. [DOI] [PubMed] [Google Scholar]
- 2.Bankiewicz KS, Forsayeth J, Eberling JL, et al. Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol Ther 2006;14:564–570. [DOI] [PubMed] [Google Scholar]
- 3.Bankiewicz KS, Eberling JL, Kohutnicka M, et al. Convection-enhanced delivery of AAV vector in parkinsonian monkeys: in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp Neurol 2000;164:2–14. [DOI] [PubMed] [Google Scholar]
- 4.Eberling JL, Jagust WJ, Christine CW, et al. Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology 2008;70:1980–1983. [DOI] [PubMed] [Google Scholar]
- 5.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 6.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982;17:37–49. [DOI] [PubMed] [Google Scholar]
- 7.van Hilten JJ, van der Zwan AD, Zwinderman AH, Roos RA. Rating impairment and disability in Parkinson’s disease: evaluation of the Unified Parkinson’s Disease Rating Scale. Mov Disord 1994;9:84–88. [DOI] [PubMed] [Google Scholar]
- 8.O’Sullivan JD, Said CM, Dillon LC, Hoffman M, Hughes AJ. Gait analysis in patients with Parkinson’s disease and motor fluctuations: influence of levodopa and comparison with other measures of motor function. Mov Disord 1998;13:900–906. [DOI] [PubMed] [Google Scholar]
- 9.Hauser RA, Friedlander J, Zesiewicz TA, et al. A home diary to assess functional status in patients with Parkinson’s disease with motor fluctuations and dyskinesia. Clin Neuropharmacol 2000;23:75–81. [DOI] [PubMed] [Google Scholar]
- 10.Deuschl G, Schade-Brittinger C, Krack P, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med 2006;355:896–908. [DOI] [PubMed] [Google Scholar]
- 11.Matsushita T, Elliger S, Elliger C, et al. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther 1998;5:938–945. [DOI] [PubMed] [Google Scholar]
- 12.Wright JF, Qu G, Tang C, Sommer JM. Recombinant adeno-associated virus: formulation challenges and strategies for a gene therapy vector. Curr Opin Drug Discov Devel 2003;6:174–178. [PubMed] [Google Scholar]
- 13.Binder DK, Rau GM, Starr PA. Risk factors for hemorrhage during microelectrode-guided deep brain stimulator implantation for movement disorders. Neurosurgery 2005;56:722–732. [DOI] [PubMed] [Google Scholar]
- 14.Gill SS, Patel NK, Hotton GR, et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med 2003;9:589–595. [DOI] [PubMed] [Google Scholar]
- 15.Kaplitt MG, Feigin A, Tang C, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet 2007;369:2097–2105. [DOI] [PubMed] [Google Scholar]
- 16.Marks WJ Jr, Ostrem JL, Verhagen L, et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson’s disease: an open-label, phase I trial. Lancet Neurol 2008;7:400–408. [DOI] [PubMed] [Google Scholar]
- 17.Forsayeth JR, Eberling JL, Sanftner LM, et al. A dose-ranging study of AAV-hAADC therapy in parkinsonian monkeys. Mol Ther 2006;14:571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bankiewicz KS, Plunkett RJ, Jacobowitz DM, Kopin IJ, Oldfield EH. Fetal nondopaminergic neural implants in parkinsonian primates: histochemical and behavioral studies. J Neurosurg 1991;74:97–104. [DOI] [PubMed] [Google Scholar]
- 19.Sanftner LM, Sommer JM, Suzuki BM, et al. AAV2-mediated gene delivery to monkey putamen: evaluation of an infusion device and delivery parameters. Exp Neurol 2005;194:476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez-Pernaute R, Harvey-White J, Cunningham J, Bankiewicz KS. Functional effect of adeno-associated virus mediated gene transfer of aromatic L-amino acid decarboxylase into the striatum of 6-OHDA-lesioned rats. Mol Ther 2001;4:324–330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Data Supplement]