An autoimmune response to OBP1a is associated with dry eye in the Aire-deficient mouse (original) (raw)
. Author manuscript; available in PMC: 2011 Apr 15.
Published in final edited form as: J Immunol. 2010 Mar 17;184(8):4236–4246. doi: 10.4049/jimmunol.0902434
Abstract
Sjögren’s Syndrome is a human autoimmune disease characterized by immune-mediated destruction of the lacrimal and salivary glands. Here, we show that the _Aire_-deficient mouse represents a new tool to investigate autoimmune dacryoadenitis and keratoconjunctivitis sicca, features of Sjögren’s Syndrome. Previous work in the _Aire_-deficient mouse suggested a role for alpha-fodrin, a ubiquitous antigen, in the disease process. Using an unbiased biochemical approach, however, we have identified a novel lacrimal gland autoantigen, odorant binding protein 1a, targeted by the autoimmune response. This novel autoantigen is expressed in the thymus in an _Aire-_dependent manner. The results from our study suggest that defects in central tolerance may contribute to Sjögren’s Syndrome and provide a new and clinically relevant model to investigate the pathogenic mechanisms in lacrimal gland autoimmunity and associated ocular surface sequelae.
Keywords: T cells, Autoimmunity, Autoantibodies, Tolerance, Thymus
INTRODUCTION
Primary Sjögren’s Syndrome (SS) is an autoimmune disease characterized by a lymphocytic infiltrate in, and subsequent destruction of, the lacrimal and salivary glands (1). Immune-mediated impairment of glandular function results in keratoconjunctivitis sicca and xerostomia (dryness of the eyes and mouth, respectively). However, these symptoms represent late-stage events and little is known about the pathologic mechanisms that result in a failure of immune tolerance.
Traditionally, animal models have been employed to dissect the cellular and genetic mechanisms underlying many human diseases. A major barrier in understanding autoimmune disease, however, is the lack of knowledge of the inciting autoantigens. Multiple animal models of Sjögren’s Syndrome exist including the non-obese diabetic mouse, MRL/lpr, and more recently the C57BL/6.NOD-Aec1Aec2 (reviewed in (2–4)), however, few autoantigens have been identified that are implicated in the disease process. Alpha-fodrin was identified as a potential autoantigen in mouse models (5–7) and some human patients (8), although the relevance of this putative autoantigen to disease remains controversial (9). Anti-alpha-fodrin autoantibodies have been demonstrated in systemic lupus erythematosus, multiple sclerosis, Moyamoya, and glaucoma patients (10–13) suggesting that reactivity to alpha-fodrin may represent a generalized feature of autoimmunity or disease. Thus, the identification of additional autoantigens remains a priority for understanding Sjögren’s Syndrome.
Here, we investigated autoimmune dacryoadenitis and associated dry eye complications in _Aire_-deficient mice, a mouse model of the human disease autoimmune polyglandular syndrome type 1 (APS1). The AIRE gene was identified as the defective gene in APS1 by positional cloning (14, 15). APS1 is an autosomal recessive, monogenic autoimmune disorder that results in immune-mediated destruction of a number of organs, predominantly of endocrine origin (16). Importantly, Sjögren’s Syndrome has also been described in a subset of patients with APS1 (16). In addition, keratoconjunctivitis sicca (KCS), a clinical hallmark of Sjögren’s Syndrome, has been described in a recent study of APS1 patients (17).
The animal model for APS1 has been critical in unraveling the cellular and molecular mechanisms of central tolerance that result in autoimmunity. Like their human counterparts, _Aire_-deficient mice develop spontaneous autoimmunity against multiple organs including the retina, the salivary and lacrimal glands, the exocrine pancreas, the lungs, the liver, the stomach and the reproductive organs (18). Here, we describe a spontaneous dacryoadenitis that consumes the lacrimal tissue and results in defects in tear production, culminating in keratoconjunctivitis sicca and damage to the ocular surface. Using an unbiased biochemical approach, we identified a novel autoantigen, odorant binding protein 1a, as a target of the immune response. This ~18kDa secreted protein is present in the lacrimal gland and in the tear fluid. The identification of this novel autoantigen and characterization of the spontaneous dacryoadenitis in _Aire_-deficient mice should be a useful new tool to study Sjögren’s Syndrome and allow the opportunity for more critical analysis of the pathogenesis of autoimmune mediated ocular complications.
MATERIALS AND METHODS
Mice
_Aire_-deficient mice were generated as previously described (18). _Aire_-deficient mice used here were backcrossed into the BALB/c and NOD Lt/J backgrounds greater than 10 generations. NOD.scid mice were purchased from Jackson labs. BALB/c Nude mice were purchased from Taconic. All mice were housed in a pathogen-free barrier facility at UCSF. Experiments complied with the Animal Welfare Act and NIH guidelines for the ethical care and use of animals in biomedical research and were approved by the UCSF Animal Care and Use Committee.
Ocular surface studies
Animals were anesthetized with 100mg/kg of ketamine and 5 mg/kg of xylazine prior to the procedure. The ocular surface was illuminated and imaged with a Nikon SMZ800 stereozoom microscope and Nikon D100 digital camera. Lissamine green (Leiter’s Pharmacy, San Jose, CA) was applied to the ocular surface of mice at the six week time point.
Histology & Scoring
Organs from mice were harvested and fixed overnight in 10% formalin, embedded in paraffin, sectioned, and stained for hematoxylin and eosin. Tissues were scored on a 4 point scale where: 0 – no histological infiltrate, 1 – 1–25% of the lacrimal gland is infiltrated, 2 – 26–50% of the lacrimal gland is infiltrated, 3 – 51–75% of the lacrimal gland is infiltrated, few ducts are present and fibrotic tissue is present, 4 – 76–100% of the lacrimal gland is infiltrated and no ducts are present.
Tear fluid collection
6–8 week old _Aire_-deficient and _Aire_-sufficient animals were anesthetized with 100 mg/kg of ketamine and 5 mg/kg of xylazine prior to the procedure. To procure tear fluid, anesthetized animals were treated with 4.5 mg/kg pilocarpine (Sigma-Aldrich, St. Louis, MT) via intraperitoneal injection. Animals were rested comfortably for 5 minutes then tear fluid was collected via the application of a Zone-Quik thread (FCI Opthalmics, Marshfield Hills, MA) to the intercanthus of the eye for 15 seconds.
Immunoblotting
Sera was screened for the presence of autoantibodies by western blotting as previously described (19). Briefly, lacrimal glands from 6 week old NOD.scid mice were homogenized on ice in Laemmli sample buffer (2% SDS, 10% glycerol, 5% 2-mercaptoethanol, 60mM Tris-Cl and 0.01% bromophenol blue). For tear fluid, 6 week old NOD.scid mice were anesthetized as described above and stimulated with 4.5mg/kg of pilocarpine. Tear fluid was collected using a P10 pipette tip applied to the intercanthus of the eye and mixed with an equal volume of Laemmli sample buffer. Samples were incubated at 95°C for 10 minutes, then centrifuged at 13,000 rpm for 10 minutes. The extracts were separated by 10% Bis-Tris gel in MES buffer (Invitrogen, Carlsbad, CA) and transferred to polyvinylidene fluoride membrane. The membranes were blocked with TBS-T (25 Mm Tris-HCl, pH 7.6,150 mM NaCl, and 0.1% Tween 20) containing 5% nonfat milk overnight, assembled in a Multiscreen apparatus(Bio-Rad Laboratories, Hercules, CA) and incubated with a 1:500 dilution of sera for 1 hour. After washing five times with TBS-T, the bound antibodies were reacted with horseradish peroxidase–conjugated donkey anti–mouse IgG (1:12,000; Bio-Rad Laboratories) for 1 h and revealed with an enhanced chemiluminescence reagent(Thermo Scientific, Waltham, MA) and autoradiography.
Immunoaffinity Purification
Immunoaffinity purification of autoantigens was performed using protein G agarose coupled to _Aire_-sufficient or _Aire_-deficient sera as described previously (20). Briefly, tissue extracts were prepared from immunodeficient mouse eyes (to reduce endogenous immunoglobulin contaminants) were homogenized in 0.15M NaCl, 0.05M Tris pH8.0, and 0.1% CHAPS (Sigma-Aldrich). Protein agarose G coupled columns were washed in 30mL phosphate buffered saline (PBS), then immunodeficient eye extracts prepared in CHAPS buffer were passed through the matrix. Columns were washed with 30mL PBS, then washed again with 30mL 10mM phosphate pH6.8. Eluates were collected by passing 0.5ml of 100mM glycine pH2.5 over the column and collecting the flow-through. Eluates from multiple runs were pooled and concentrated in a Vivaspin centrifugal protein concentrator (Sartorius, UK).
In-gel Digestion and Peptide Mass Fingerprinting (PMF)
OBP1a was identified by provisional peptide mass fingerprint as previously described (21–24). Briefly, gel bands were excised, destained (stain-stripped) three times in 50% acetonitrile and 25 mM ammonium bicarbonate, pH 8.0, dehydrated with 100% acetonitrile, and dried in a Speed-Vac. Gel pieces were rehydrated with a solution of sequencing grade trypsin (10 μg trypsin [Promega, Madison, WI]/ml in 25 mM ammonium bicarbonate) and the digestion was carried out for 16 h at 37°C. Peptides were extracted three times by the addition of two volumes of an aqueous solution of 50% acetonitrile and 5% trifluoroacetic acid. The extracts were combined and reduced to a final volume of 5–10 μl. PMF was used for preliminary protein identification. Portions (typically 5%) of the unseparated tryptic digest was co-crystallized in a matrix of alpha-cyano-4-hydroxycinnamic acid (5 mg/ml) and analyzed on a Voyager DE-STR MALDI-TOF mass spectrometer (Applied Biosystems, Foster City, CA) operating in reflector mode. Mass spectra were produced representing protonated molecular ions (MH+) of tryptic peptides from the protein(s) present in each gel spot. The mass spectra were internally mass calibrated using two trypsin autolysis products present in the digest mixture (842.5100 Da and 2211.1046 Da). The mass measurement accuracy for all peptides was ± 25 ppm, and the mass measurement precision, defined as the standard deviation of differences between the experimental and theoretical peptide masses, was typically ≤25 ppm. Preliminary protein identities were established by matching the experimentally determined peptide masses to those produced by an in silico tryptic digestion of the NCBInr.2005.01.06 database within the window of experimental mass measurement accuracy. The PMF data-searching algorithm MS-Fit (http://prospector.ucsf.edu/cgi-bin/msform.cgi?form=msfitstandard) was used to perform the database searches.
Preparation of cDNA from organs
RNA from various organs was prepared according to manufacturers instructions using a mini RNA isolation kit (Stratagene, La Jolla, CA). RNA was reverse transcribed into cDNA using Superscript III (Invitrogen) and poly-dT primers.
Amplification of OBP1a and cyclophilin from organs
Polymerase chain reaction was used to amplify OBP1a or cyclophilin from various organs. Primers used for OBP1a: forward 5′ ATGGCAAAATTTCTGCTGC reverse 5′ TCATTCAGGACAGTAATCTG. Cyclophilin primers were purchased from SuperArray Biosciences (Frederick, MD).
Fusion protein vectors
Recombinant proteins were produced by cloning full length cDNA sequences into pGEX-3X (GE Healthcare, Piscataway, NJ) or pMAL-C2X (New England Biolabs, Ipswich, MA). Briefly, RNA was prepared from lacrimal tissue using a RNA spin column (Stratagene) and converted into cDNA using Superscript III (Invitrogen) and oligo-dT primers. Primers specific for OBP1a were used to generate amplicons containing the full length sequence with restriction sites for subcloning. For pGEX-3X, primers used were: forward primer 5′ CGGGATCCGCATGGCAAAATTTCTGC and reverse primer 5′ ATAGTTTAGCGGCCGCTCATTCAGGACAGTAAT. These primers incorporated a BamHI site on the 5′ end and a NotI site on the 3′ end of the amplicon for subcloning. For pMAL-C2X, primers used were: forward primer 5′ TAGGATCCATGGCAAAATTTCTGCTGC and reverse primer 5′ TACTGCAGTCATTCAGGACAGTAATCTG. These primers incorporated a BamHI site on the 5′ end and a PstI site on the 3′ end of the amplicon for subcloning. Constructs were transformed into BL-21 bacteria for protein production.
Fusion protein production
BL-21 bacteria containing the recombinant protein vectors were grown overnight at 37°C in 3ml LB with antibiotics. The following day, this culture was used to seed a 1 liter culture without antibiotics. When the culture reached an O.D. of 0.6, 0.01mM IPTG was added and the culture was incubated at 30°C for an additional 3 hours.
GST fusion protein purification
For GST constructs, fusion protein was harvested using a GST renaturation kit (Cell Biosystems, San Diego, CA). Briefly, cultures of BL21 bacteria transformed with OBP1a-GST were pelleted and resuspended in 1x STE buffer. Cells were lysed by sonication, then incubated in detergent solubilization buffer for 1 hour on ice. The resulting supernatant fraction was collected by centrifugation at 15,000g for 10 minutes, then incubated for 1 hour with detergent neutralization solution. Glutathion sepharose 4B (GE Healthcare, Waukesha, WI) was incubated with the supernatant containing fusion protein for 2 hours, washed three times with PBS with 1% Triton X-100, then eluted in Laemmli sample buffer (2% SDS, 0.1M dithiothreitol, 10% glycerol, 60mM Tris). Recombinant protein was precipitated using chloroform and methanol then resuspended in 0.5% CHAPS buffer.
Maltose fusion protein purification
For MBP constructs, fusion protein was prepared by lysing the bacteria by sonication followed by centrifugation at 8,000g for 30 minutes. Supernatants were collected and used for protein purification. Fusion protein was bound to amylose resin on a column support and washed with 20mM Tris-HCl, 200mM NaCl, and 1mM EDTA (column buffer). Fusion proteins were eluted in column buffer supplemented with 10mM maltose.
Removal of LPS from fusion proteins
LPS was removed from the fusion protein samples using Detoxigel (Pierce Endogen, Rockford, IL) according to the manufacturers instructions.
Immunostaining
Immune cell subtypes were visualized by immunohistochemistry using antibodies specific for CD4 (clone RM4-5), CD8 (clone 53-6.7), and IgD (clone 11-26x.2a; BD Pharmingen), a donkey anti-rat secondary antibody conjugated to horseradish peroxidase (Jackson Labs) and a DAB staining kit (Vector Labs, Burlingame, CA). Briefly, lacrimal glands from 6–8 week old NOD _Aire_-deficient or _Aire_-sufficient animals were embedded in OCT Tissue Tek freezing media (Sakura Finetek, Torrance, CA). Ten micron thick sections were prepared from these tissues using a cryostat (Leica, Germany) and mounted on SuperFrost Plus slides (Fisher Scientific, Pittsburgh, PA). Sections were fixed for 10 minutes in acetone at −20°C, washed in PBS for 5 minutes, then blocked with 0.1M Tris, 0.05M NaPhos (PBS), 0.3% Tween 20, 3% normal donkey serum for 1 hour at room temperature. After blocking, slides were incubated in 3% hydrogen peroxide to inactivate endogenous peroxidases, then incubated with primary appropriate antibody diluted 1:50 in block for 30 minutes at room temperature, washed three times with PBS for five minutes each, and incubated with secondary antibody diluted 1:200 in block for 30 minutes at room temperature. After three additional washes in PBS for 5 minutes each, slides were developed with substrate and counterstained with hematoxylin.
Preparation and flow cytometry of lymphocytes from lacrimal gland
Whole lacrimal glands were digested in 1mg/ml collagenase P (Roche Biosciences, Palo Alto, CA) in DMEM for 30 minutes at 37°C. Digested tissues were passed through a 70 micron cell strainer. Lymphocytes were prepared by centrifugation in Lympholyte M (Cedarlane Labs, Burlington, NC) according to manufacturers instructions. Lymphocytes were stained with antibodies specific for mouse CD45 (clone 30-F11; BD Biosciences) CD4 (clone RM4-4; BD Biosciences), CD8 (clone 53-6.7; BD Biosciences) and CD19 (clone 6D5; Southern Biotech) and analyzed on a FACS Calibur (Becton Dickinson, San Diego, CA).
Adoptive transfer
Cervical lymph node cells and splenocytes were harvested and CD4+ or CD8+ T cells or CD19+ B cells were depleted using rabbit complement. Briefly, cells were incubated with CD4 (clone GK1.5), CD8 (clone YTS-169) or CD19 (clone 1D3; BD Biosciences) followed by rabbit complement (Sigma-Aldrich) for 1 hour at 37°. Cells were analyzed by flow cytometry to assess removal of the desired cell population. Cell populations (5 × 106 CD4+ depleted, CD8+ depleted, or B cell depleted) were injected I.V. into NOD.scid mice. Animals were aged 40 days post-transfer then sacrificed and analyzed as described.
EliSpots
25,000 CD4+ T cells from the cervical lymph nodes or lacrimal gland infiltrating cells of 10–15 week old BALB/c _Aire_-deficient or _Aire_-wild type animals were isolated by flow cytometry using a FACSAria (Becton Dickinson). Purity was greater than 98% for all samples. For lacrimal gland infiltrating cells, the lacrimal glands were prepared as described above and purified by flow cytometry using a FACSAria. 250,000 APCs from Aire wild-type BALB/c mice were used as target cells and co-cultured with 5 micrograms per ml OBP1a-MBP or MBP as the control protein for 24 hours at 37°C in a CO2 incubator. The release of IFN-γ by CD4 T cells was measured by Elispot assay. In brief, BD Immunospot M200 plates (Becton Dickinson) were coated with anti-mouse IFN-γ mAb (2ug/ml, #551216, BD Pharmingen) and incubated overnight at 4 C. The plates were washed with PBS and blocked with medium containing 10% FCS for 2h at 37 C. The effector CD4+ T cells and irradiated APC (3000 rad) and antigen were added to each well and incubated for 24h in RPMI complete medium. The plates were then washed thoroughly with PBS before adding biotin-labeled IFN-γ mAb (2ug/ml, #554410, BD Pharmingen) and incubating overnight at 4 °C. After further incubation with Avidin-HRP (1:100 dilutions, #551950, BD Pharmingen) for 1h at room temperature, the plates were developed using BD Pharmingen’s AEC substrate Solution. Positive spots displayed in the plate membranes were examined using the Transtec ELISPOT reader system (Cell Technology, Columbia, MD). The number of spot-forming cells was the average number of spots in duplicate wells.
Adoptive transfer of stimulated cells
Cervical lymph node cells were harvested from 6 week old NOD._Aire_-deficient mice. 20 million cells were placed in culture with 50ug/ml of either OBP1a-MBP fusion protein or MBP protein alone. After 4 days in culture, lymphocytes were purified by Ficoll centrifugation. 1.5 million cells were adoptively transferred into NOD.scid recipients (Jackson) via intravenous injection. The mice were sacrificed 4 weeks after transfer and analyzed by histology as described.
Immunostaining of adoptive transfer sections
Paraffin embedded tissues were cut into 5 micron sections on a cryotome and placed on glass microscope slides. Sections were deparaffinized and rehydrated (xylene, 100% ethanol, 95% ethanol; two changes each for five minutes per change). Antigen retrieval was performed in 10 mM Sodium Citrate Buffer pH 6 at 95 degrees for 30 minutes. Endogenous peroxidases were quenched with 3% hydrogen peroxide for 15 minutes. Slides were blocked with 0.1M Tris, 0.05M NaPhos (PBS), 0.3% Tween 20, 3% normal donkey serum for 1 hour at room temperature. After blocking, slides were washed with TBS-T (3 times, 5 minutes each) and incubated with isotype control or polyclonal rabbit anti-human CD3 (A0452, Dako) diluted 1:500 in block for 1 h at room temperature. Slides were washed again in TBS-T and a mouse anti-rabbit IgG biotinylated secondary was added for 1 hour at room temperature. Slides were washed again in TBS-T and then sections were incubated with Elite ABC reagent for 15 minutes (VectorLabs). Sections were washed a final time in TBS-T and the reaction was developed with DAB. Sections were hematoxylin counterstained and mounted.
Thymic stroma preparation
Thymic epithelial cells (TECs) were prepared according to previously established protocol (25). Briefly, thymi from 5-wk-old BALB/c _Aire_-deficient or _Aire_-sufficient mice were removed and trimmed of fat. Small cuts into the capsule were made, and the thymi were gently agitated in 50 ml RPMI 1640 for 30 min at 4°C. Thymic fragments were manually dispersed via pipetting, recovered by settling, and digested with 0.125% collagenase D (Roche) with 0.1% DNase (Promega) in RPMI 1640 at 37°C. This digestion was repeated for a total of two times, and supernatants were retained. All supernatant fractions were pooled, and cells were collected by centrifugation at 400 g for 10 min. CD45+ cells were removed by negative selection using CD45 microbeads (Miltenyi Biotec) and an AutoMACS instrument (Miltenyi Biotec). RNA was prepared from the CD45 – fraction.
Real time PCR
Real time PCR was carried out on cDNA prepared from DNase treated RNA. GAD67, and cyclophilin primers were used as previously described (18, 26). For OBP1a, the following primers were used: Forward-5′ GAATGCAAGGAAATGAAAGTCACAT Reverse-5′ ATCTTCTTGCAAATACCCAGTGATT Probe-FAM-TCAATGAAAATGGACAGTGCTCATTGACCA -TAMRA. For Spt1, the following primers were used: Forward-5′ GCTTGGTGTTTCCACTATCCTAGTCT Reverse-5′ AATCAGCAGTTCCAGAAGTTTCAGT Probe 5′ FAM-TTGCCAGGACCCGGAGACAAACA -TAMRA. Reactions were run on an Applied Biosystems HT7900 Sequence Detection System machine. For analysis of target gene expression from organ derived cDNA, the standard curve method was used as previously described (20).
Thymic transplantation
Thymi were removed from 1–2 day old BALB/c _Aire_-deficient or _Aire_-sufficient animals and placed into culture in DMEM media supplemented with 100 units per ml penicillin, 100 mcg per ml streptomycin, 2mM glutamine, 10% fetal calf serum and 1.35mM 2-deoxyguanosine (Sigma-Aldrich)for 7d to deplete bone marrow–derived cells. The thymi were washed in DMEM media without 2-deoxyguanosine for 2 h and transplanted under the kidney capsule of 6–8 week old adult nude mice on the BALB/c background (Taconic, Hudson, NY). 4 wk after transplantation, animals were harvested and analyzed for T cell reconstitution by FACS and immune infiltrates by histology.
Statistics
Data was analyzed using Prism 4.0 (GraphPad, San Diego, CA) and a Mann-Whitney non-parametric test.
RESULTS
Dry eye and ocular surface changes in _Aire_-deficient mice mimic Sjögren’s Syndrome
Since a small proportion of patients with APS1 also have the clinical symptoms of Sjögren’s Syndrome (16), we assessed the consequences of autoimmune mediated lacrimal gland destruction and dysfunction in _Aire_-deficient mice. Initial studies focused on evaluation of the ocular surface in _Aire_-deficient mice using established ophthalmic techniques. In contrast with _Aire_-sufficient mice on the non-obese diabetic (NOD) background (Fig 1A), _Aire_-deficient NOD mice at 5 weeks of age demonstrated clinical signs of aqueous-deficient keratoconjunctivitis sicca consistent with human Sjögren’s Syndrome, including significant irregularity of the corneal epithelium and filamentary keratopathy (Fig 1B; arrowheads) signaling inflammation and stress on the corneal surface. At 6 weeks of age, _Aire_-deficient NOD mice showed marked pathologic changes on the ocular surface including extensive and confluent corneal epithelial defects (Fig 1B). Ocular surface changes were also observed in _Aire_-deficient mice on the BALB/c background; however, the onset, severity, and penetrance of the pathologic signs were diminished (data not shown). No obvious changes in the cornea were observed in _Aire_-deficient mice on the C57BL/6 background (data not shown), although recent published data suggests that dessicating stress in Aire-deficient C57BL/6 mice results in keratoconjunctivitis (27). To demonstrate a possible correlation of the ocular surface findings in _Aire_-deficient NOD mice with lacrimal gland dysfunction, next we analyzed tear fluid production. Tear fluid production of 6–8 week old NOD _Aire_-deficient and _Aire_-sufficient mice was stimulated using pilocarpine and total volume was measured using Zone-Quik threads (Fig 1C). Our results revealed that _Aire_-deficient NOD mice had markedly diminished (p = 0.0002) tear fluid production as compared to _Aire_-sufficient animals with many _Aire_-deficient mice producing only trace amounts of tear fluid.
Figure 1. _Aire_-deficient mice develop spontaneous ocular surface abnormalities and diminished tear fluid production.
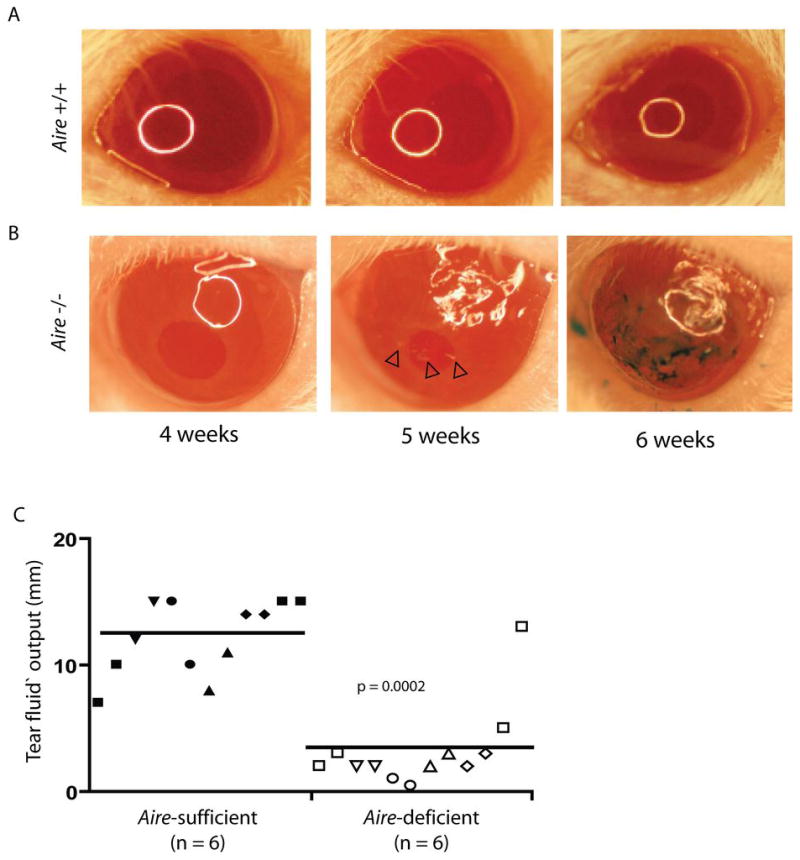
Kinetic analysis of the ocular surface in _Aire_-sufficient (A) and _Aire_-deficient (B) mice on the NOD background. Images of the ocular surface were taken using a stereozoom microscope and digital camera. Pathologic changes to the ocular surface appeared at approximately five weeks of age in _Aire_-deficient mice including disruption of the corneal epithelium (noted by the irregular light source reflection) and filamentary keratopathy (arrowheads). At six weeks of age, lissamine green staining applied to the corneal surface detected profound corneal surface irregularity and extensive epithelial defects. C. Tear fluid volume was measured from 6–8 week old NOD _Aire_-sufficient and _Aire_-deficient animals that were anesthetized with ketamine and xylazine and stimulated with 4.5mg/kg pilocarpine. Tears were collected for 5 minutes. Shown are tear fluid volumes measured in millimeters from each eye using Zone-Quik threads (n = 6 for _Aire_-sufficient, closed symbols; n = 6 for _Aire_-deficient, open symbols, each symbol represents an individual mouse with each eye analyzed individually per animal).
Autoimmune-mediated destruction of the lacrimal gland characterized by autoantibodies and immune infiltrates
To confirm that the observed change in the ocular surface and decreased tear production in _Aire_-deficient mice results from destruction of the lacrimal gland, we performed histological analysis of the lacrimal gland in 6 to 8 week old _Aire_-deficient and _Aire_-sufficient animals on the NOD background (Fig 2A). Mononuclear infiltrates were present in the lacrimal glands of _Aire_-deficient mice, often consuming the entire organ. In contrast, only sparse infiltrates were present in _Aire_-sufficient NOD mice consistent with published observations (28). Furthermore, similar infiltrates were also present in BALB/c Aire-deficient mice (Figure S1). In both strains, there was no gender-dependence and autoimmunity was observed equally in both male and female mice, consistent with clinical observations in APS1. The infiltrates observed in _Aire_-deficient mice appear to surround ductal tissue. To assess whether or not autoantibodies targeted the lacrimal gland, we performed indirect immunohistochemistry using sera from wild-type and _Aire_-deficient mice. Autoantibodies present in the sera of _Aire_-deficient mice recognize and react with proteins present in or around the ducts, as shown in Figure 2B. Importantly, no autoreactivity was observed with wild-type sera reflecting the specificity of the _Aire_-deficient immune response. A previous study suggested that alpha-fodrin may be an autoantigen in this model system (7), although alpha-fodrin is not a thymically _Aire_-regulated antigen and its expression is not restricted to the lacrimal gland. To further characterize the autoantigen or autoantigens targeted by the autoantibody response, we performed immunoblots of lacrimal gland protein extracts with _Aire_-deficient or _Aire_-sufficient sera on the NOD (Fig 2C) and BALB/c (Fig 2D) backgrounds. Immunoblotting revealed an 18 kilodalton autoantigen present in the lacrimal gland extract, a pattern of reactivity not compatible with any described form of alpha-fodrin. Given that the indirect immunohistochemistry suggested that the protein was peri-ductal, we hypothesized that it may be a secreted protein and performed similar immunoblots comparing lacrimal gland extract and tear fluid samples (Fig 2E). Again, reactivity to an 18 kilodalton autoantigen was observed in sera from _Aire_-deficient animals. Animals sacrificed at various time points showed that autoantibodies were present at 4 weeks of age in NOD _Aire_-deficient mice and their frequency increased with age (Table 1). NOD _Aire_-sufficient mice as old as 1 year were screened and this autoantibody reactivity was not observed (data not shown). The 18 kilodalton antigen observed in NOD _Aire_-deficient mice was not dependent on the NOD background, as similar autoantibody reactivity and histological infiltrates are observed in _Aire_-deficient mice on the BALB/c background (Figure S1).
Figure 2. The lacrimal gland is a target of autoimmunity and expresses an 18 kilodalton autoantigen.
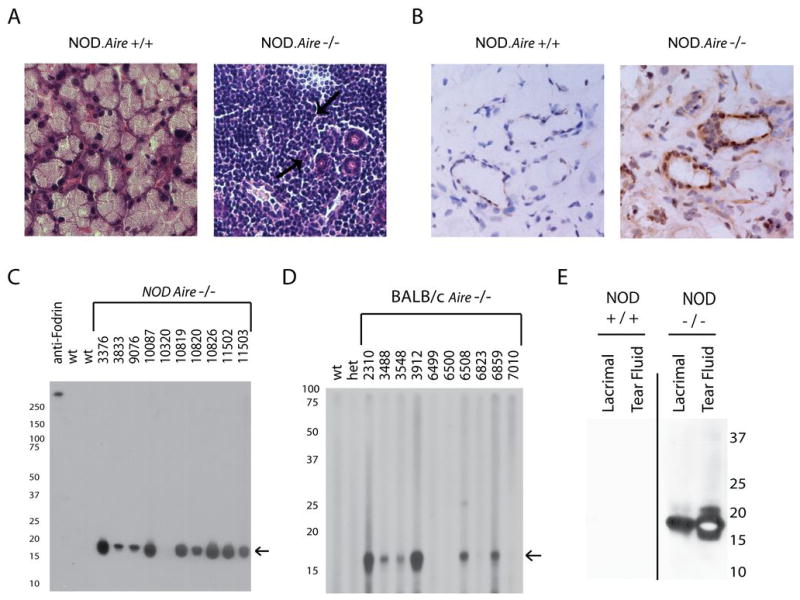
A. Histological analysis of 6 week old NOD _Aire_-sufficient (+/+) and _Aire_-deficient (−/−) animals demonstrated a profound immune infiltrate in the lacrimal glands of _Aire_-deficient animals absent from _Aire-_sufficient animals. B. NOD.scid lacrimal gland sections were embedded in OCT, sectioned, and used to perform indirect immunohistochemistry with sera derived from _Aire_-deficient mice and _Aire_-sufficient animals. C. NOD.scid lacrimal gland extracts prepared in Laemmli sample buffer and immunoblotted with sera derived from 6–8 week old NOD _Aire_-deficient (individual animals listed by number) and _Aire_-sufficient (wt) animals target an approximately 18 kilodalton protein. D. Reactivity to the 18 kilodalton antigen is not limited to the NOD background and Aire-deficient BALB/c mice (individual animals listed by number) also show reactivity to this protein whereas age matched Aire-sufficient (both wild-type [wt] and heterozygous [het]) do not. E. Tear fluid and lacrimal gland extracts prepared from NOD.scid animals were immunoblotted using pooled sera from three 6–8 week old NOD _Aire_-deficient animals or three 10 week old NOD _Aire_-sufficient controls.
Table 1.
frequency of autoantibodies against OBP1a and histology in NOD _Aire_-deficient mice at various ages
| Age at sacrifice | Autoantibody positive | Autoantibody Negative | Positive (%) | Infiltrates |
|---|---|---|---|---|
| 4–6 weeks | 6 | 4 | 60 | 100% |
| 6–8 weeks | 5 | 3 | 62.5 | 100% |
| 8–10 weeks | 13 | 1 | 93 | 100% |
Identification of the 18 kDa autoantigen as odorant binding protein 1a
Using an unbiased biochemical approach, we immunoaffinity purified an 18 kilodalton protein identified by peptide mass fingerprinting as odorant binding protein 1a (OBP1a; Fig 3A) from whole eye extracts. The original screen was undertaken to identify the retina-specific protein Interphotoreceptor retinoid binding protein (IRBP), however, OBP1a was also identified. Odorant binding protein 1a is a putative pheromone transporter that is part of the lipocalin family (Fig 3B). It is a secreted protein and contains an N terminal signal sequence. At the genetic level, it is encoded on the X chromosome of mice and is composed of 7 exons that result in a transcript of 757 base pairs. This transcript is translated into a 163 amino acid protein, part of which is presumably cleaved off (the signal sequence) during maturation and export. It has a predicted N-linked glycosylation site at position 104, which may explain the multiple bands observed by immunoblotting and identified by mass spectrometry as OBP1a (Fig 3A). Interrogating the available databases determined that this protein was not expressed in the eye, a result that was confirmed by screening cDNA libraries prepared from various organs. Rather, the only organs from which detectable levels of transcript were identified were the lacrimal gland and the vomeronasal organ (Fig 3C). We therefore reasoned that the immunoaffinity purification of OBP1a from whole eye extracts represented a contamination of the eye extract preparation with tear fluid, which was present on the surface of the eyes at the time of removal (see figure 2E).
Figure 3. The 18 kilodalton autoantigen is identified as odorant binding protein 1a by immunoaffinity purification.
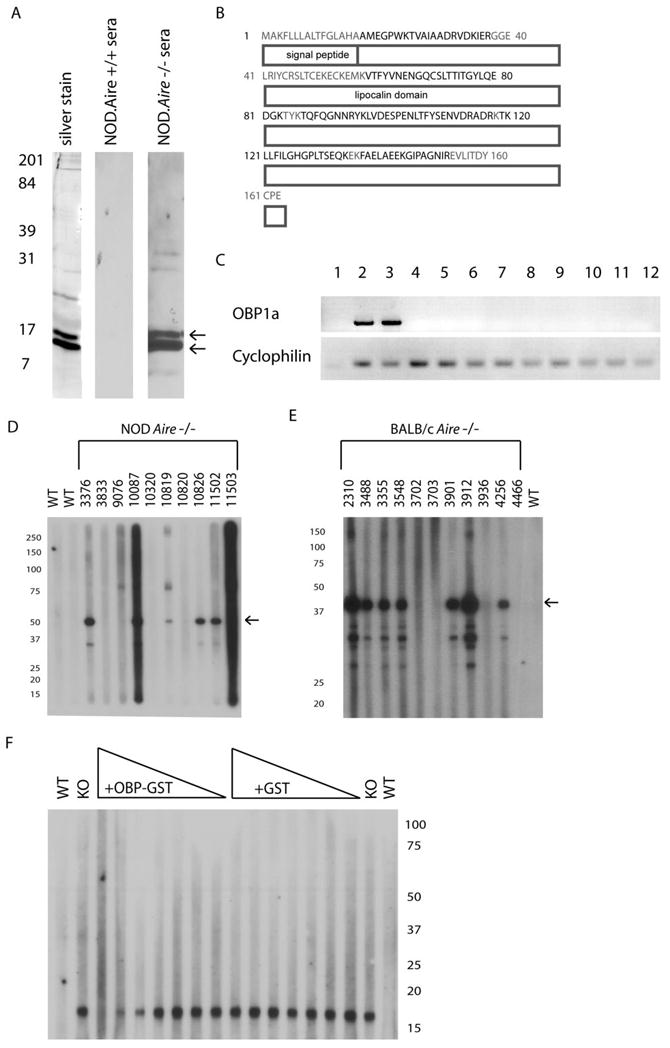
NOD.scid eye extracts were prepared in non-denaturing conditions (CHAPS detergent) and the 18 kilodalton protein was purified by immunoaffinity purification using _Aire_-deficient animal sera. The immunopurified protein was given the provisional identification of odorant binding protein 1a (OBP1a) by peptide mass fingerprinting. Arrows in (A) indicate the bands that were immunoaffinity purified and excised from an acrylamide gel (3A, left column) and identified as odorant binding protein 1a (OBP1a). The immunoprecipitated protein reacted strongly with _Aire_-deficient (−/−) sera pooled from 4 individual animals (3A, right column) but not with _Aire_-sufficient (+/+) sera pooled from 4 individual animals (3A, middle column). B. Peptide mass fingerprinting of the eluted proteins identified many epitopes (identified residues are in black) and resulted in 74% coverage of the OBP1a protein. C. cDNA was prepared from RNA derived from various tissues and using a PCR reaction with OBP1a specific primers. OBP1a expression is limited to the vomeronasal organ (lane 2) and the lacrimal gland (lane 3), but not expressed in the salivary gland, lung, pancreas, liver, prostate, ovary, eye, stomach, or thyroid (lanes 4–12; lane 1 is a water control). D. Full length OBP1a was cloned into a pGEX-3X fusion protein vector and used to generate OBP1a-GST fusion protein. Immunoblotting OBP1a-GST fusion protein (indicated by arrow) with NOD._Aire_-deficient sera (individual animals listed by number, WT denotes wild-type) confirmed autoantibody reactivity. E. Reactivity to OBP1a-GST was also observed in BALB/c _Aire_-deficient sera (individual animals listed by number, WT denotes wild-type). F. To ensure that the 18 kilodalton band observed in the lacrimal gland was OBP1a, 6 week old NOD.Aire deficient sera was pre-incubated with increasing concentrations of recombinant OBP1a-GST fusion protein, or GST tag alone, and used to immunoblot whole lacrimal gland extract prepared from NOD.scid animals (KO denotes Aire-deficient NOD sera, WT denotes wild-type sera, triangle represents increasing concentrations of recombinant fusion protein).
To confirm that OBP1a was a target of autoantibodies present in _Aire_-deficient mice, we constructed an OBP1a-glutathione-S-transferase (GST) fusion protein that was produced in E. coli. This fusion protein, consisting of the 18 kilodalton OBP1a and a 25 kilodalton glutathione-S-transferase (GST) tag, migrated at approximately 43 kilodaltons. Immunoblotting of recombinant OBP1a-GST fusion protein with sera from _Aire_-deficient mice confirmed the presence of autoantibodies against OBP1a in both the NOD background (Fig 3D) and the BALB.c background (Fig 3E). Not all NOD _Aire_-deficient mice that responded to OBP1a from lacrimal gland extracts were reactive to the OBP1a-GST fusion protein, presumably reflecting antibody specificities to native folded protein or glycosylated moieties. When recombinant OBP1a-GST was incubated with _Aire_-deficient sera before immunoblotting, reactivity against the 18-kD band in whole mouse lacrimal extract was reduced (Fig 3F). In contrast, incubation with an equal concentration of purified GST tag alone failed to reduce immunoreactivity. Similarly, recombinant OBP1a-GST, but not GST tag alone, was able to reduce immunoreactivity against mouse tear fluid extract (data not shown). From these experiments, we conclude that OBP1a is the 18 kilodalton autoantigen present in the tear fluid and lacrimal glands and targeted by _Aire_-deficient mice.
Lacrimal infiltrate in _Aire_-deficient mice includes B and T cells
To determine the nature of the infiltrate present in the lacrimal glands of _Aire_-deficient mice, we performed immunohistochemistry with antibodies specific for the T cell markers CD4 and CD8 and the B cell marker IgD. In control tissues from age and gender matched _Aire_-sufficient animals, few immunolabeled cells are present. In contrast, the lacrimal glands of NOD _Aire_-deficient mice are infiltrated with CD4+ and CD8+ T cells, as well as IgD+ B cells (Fig 4A). To determine the relative amount of each cell type present in the lesions, we performed flow cytometry with cell-specific markers. Lymphocytes were prepared from infiltrated lacrimal glands and interrogated for cell-surface expression of CD4, CD8, and CD19. The majority of the cells present in the lesions, as suggested by immunohistochemistry, are CD45+ CD19+ B cells. This finding was also confirmed in BALB/c _Aire-_deficient mice (Figure S2).
Figure 4. CD4+ and CD8+ T cells and B cells are present in the immune infiltrates.
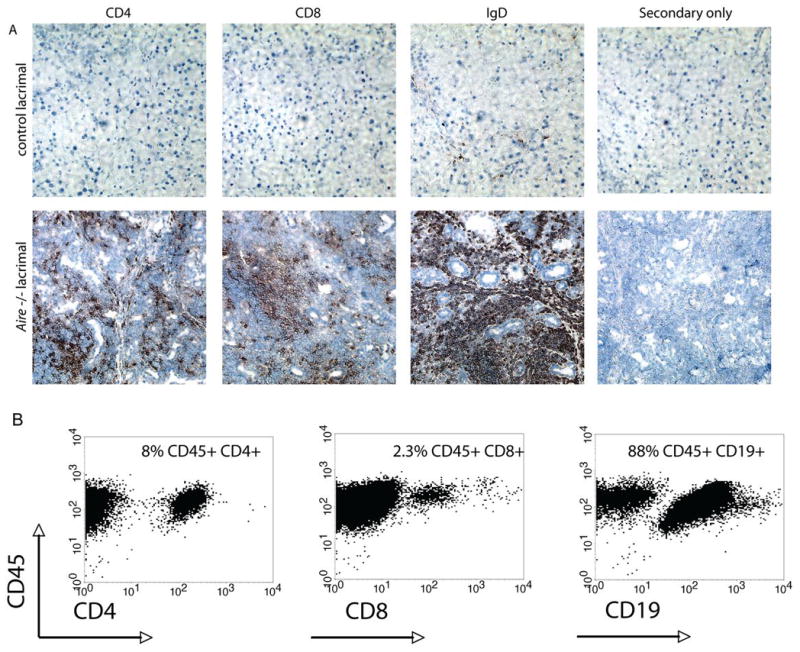
A. The lacrimal glands of 6 week old _Aire_-sufficient and _Aire_-deficient NOD animals were analyzed by immunostaining of frozen sections with antibodies specific for CD4 and CD8 (T cells) or IgD (B cells). B. Flow cytometry was also used to determine the relative composition of immune cells in the infiltrate. Numbers represent the percentage of cells within the lymphocyte gate that are surface marker positive. Data are representative of at least 5 independent experiments.
The presence of a large proportion of B cells in the immune infiltrates, contradicting data as to the relevance of B cells in the _Aire_-deficient mouse model (29, 30), and the demonstration that autoantibodies are important in the pathogenesis of other mouse models of Sjogren’s syndrome (31) led us to further investigate whether or not B cells were playing a role in lacrimal autoimmunity in the _Aire_-deficient mouse model. In an attempt to resolve whether one cell population was more important than the others in the disease process, we adoptively transferred lymphocytes depleted of individual cell populations into immunodeficient hosts. The purity of the transferred fractions was confirmed by FACS analysis prior to transfer (CD4 depleted: 1.2% CD4+, 8.7% CD8+, 57.7% CD19+; CD8 depleted fraction: 64.1% CD4+, 0.9% CD8+, 22.1% CD19+; B cell depleted: 57.4% CD4+, 30.4% CD8+, 3.7% CD19+). Consistent with previous experiments (20, 29), the transfer of CD4 depleted populations has a significantly lower average histological score, indicating an important role for CD4+ T cells in the disease process. In contrast, CD8 or B cell depleted lymphocytes were equally capable of mediating lacrimal disease in immunodeficient recipients (Fig 5). Coupled with our previous data showing that lacrimal autoimmunity is not reduced in Aire-deficient, B cell deficient animals (29), these data suggest that B cells play a limited role in lacrimal gland autoimmunity in this model.
Figure 5. CD4+ T cells are important in the disease process.
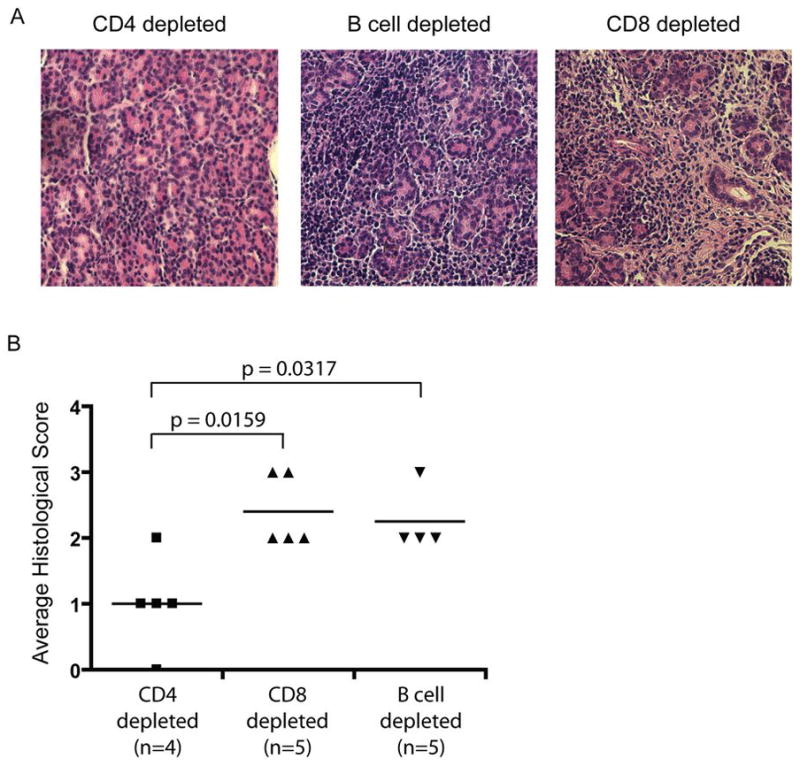
To determine what cells were capable of transferring disease, cell populations derived from 6–8 week old NOD._Aire_-deficient animals and depleted of either CD4, CD8, or B cells using rabbit complement were injected into immunodeficient NOD.scid hosts. Recipient mice were analyzed for the presence or absence of immune infiltrates 40 days post transfer. A. Hematoxylin and eosin stained sections were used to assess disease. B. Disease scores are shown for individual animals in each group. Data are representative of 2 independent experiments.
OBP1a is expressed in the thymus in an _Aire_-dependent fashion
The current model of Aire function postulates that Aire upregulates self-antigen expression in the thymus. This model, however, was called into question with the identification of alpha-fodrin as a candidate autoantigen in lacrimal gland autoimmunity (7) and led many groups to postulate that Aire functions to shape T cell tolerance through mechanisms in addition to TSA upregulation. To determine whether or not OBP1a was expressed in the thymus under the control of Aire, we purified thymic stroma from _Aire_-sufficient and _Aire_-deficient mice. cDNA generated from these cells was used to interrogate known _Aire_-regulated and _Aire_-independent tissue specific antigens (TSAs), as well as OBP1a, using quantitative real-time PCR. OBP1a was expressed in the thymus in an _Aire_-regulated manner (Fig 6). To confirm the accuracy of this analysis, SPT1, a known _Aire_-regulated antigen, was also shown to be _Aire_-dependent. In contrast, glutamic acid decarboxylase 67 (GAD67), which is known to be _Aire_-independent, was not _Aire_-regulated. Thus, the expression of OBP1a in the thymus appears to be _Aire_-dependent.
Figure 6. OBP1a is expressed in the thymus in an _Aire_-regulated fashion.
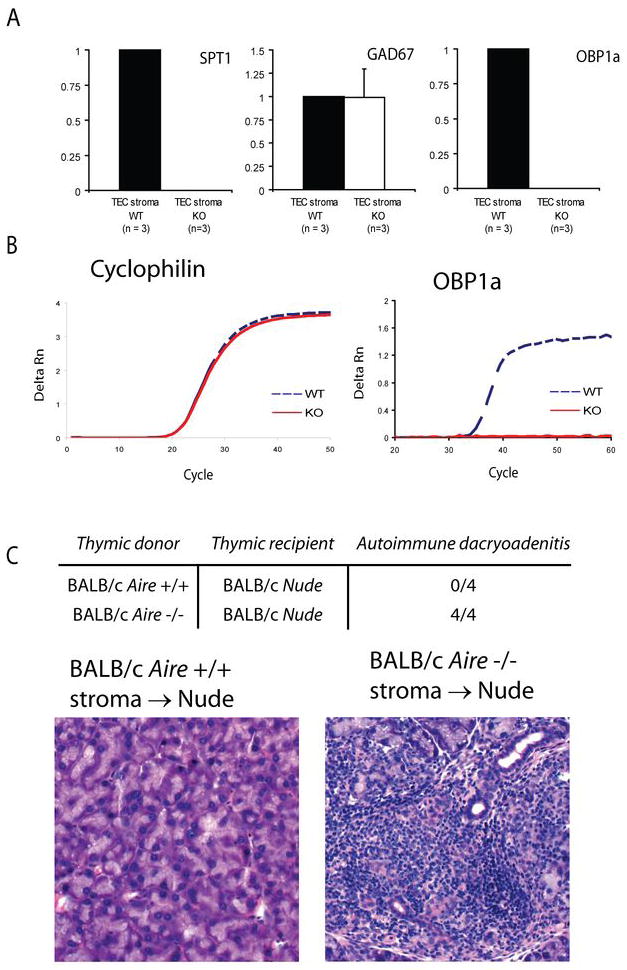
cDNA derived from thymic stroma of BALB/c _Aire_-deficient (n = 3) and _Aire_-sufficient (n = 3) animals was prepared. A. Quantitative real-time PCR was used to determine the expression of known _Aire_-dependent (Spt1) and _Aire_-independent antigens (Gad67) along with OBP1a. Values are normalized to cyclophilin and relative to expression in the WT thymic stroma, data are representative of three independent experiments. B. Representative amplification plots of cyclophilin and OBP1a are shown. C. A lobe of thymus from newborn (1–2 days post birth) BALB/c _Aire_-deficient or _Aire_-sufficient mice were treated for 1 week with 2-deoxyguanosine and then implanted under the kidney capsule of 8 week old BALB/c Nude animals. Recipient animals were harvested 1 month post transplant and analyzed for immune infiltrates in the lacrimal gland by histology.
An Aire-deficient thymus is sufficient for disease
The transplantation of _Aire_-deficient stroma into Nude recipients was shown to result in retinal, stomach, and salivary autoimmunity (18, 32), supporting a critical role for the thymus in autoimmunity in _Aire_-deficient mice,. To ascertain whether or not a defect in thymic TSA expression would result in lacrimal autoimmunity, we transplanted thymi from BALB/c _Aire_-sufficient and _Aire_-deficient newborn mice (1–2 days post birth) into 6–8 week old BALB/c Nude recipients. Nude mice have a mutation in the gene FoxN1, preventing the formation of thymic architecture and resulting in a complete lack of mature T cells. Transplantation of _Aire_-sufficient or _Aire_-deficient thymi generates a mouse deficient in Aire only in the stromal compartment of the thymus. Consistent with a model in which autoimmunity is dependent on an absence of Aire in the medullary stroma, BALB/c Nude recipients of _Aire_-deficient thymic implants developed lacrimal disease (Figure 6B & C). As expected, disease was not restricted to the lacrimal gland and infiltrates were also observed in the liver, lung, pancreas, prostate, salivary glands, and stomach (data not shown).
T cells from _Aire_-deficient mice recognize OBP1a
To demonstrate that a lack of OBP1a in the thymic compartment results in a defect in central tolerance, we performed ELISPOT analysis to determine the frequency of autoreactive cells in the draining cervical lymph node of wild-type and _Aire_-deficient mice. CD4+ T cells were isolated from 10–15 week old BALB/c _Aire_-sufficient or _Aire_-deficient animals and cultured with irradiated antigen presenting cells loaded with recombinant OBP1a-maltose binding protein (OBP1a-MBP) or maltose binding protein tag (MBP) alone. The number of cells that produced interferon-gamma when stimulated with OBP1a was significantly increased in _Aire_-deficient mice (Fig 7A), suggesting the release of autoreactive cells from the _Aire_-deficient thymus and their expansion in the periphery. To further demonstrate that OBP1a-reactive cells were present in the immune cells infiltrating the target organ, CD4+ T cells were purified from the lacrimal glands by flow cytometry and stimulated as described above (Fig 7B). Due to the heterogeneity in the number of OBP1a specific cells present in Aire-deficient mice, this result did not achieve statistical significance (p = 0.2). However, 3 of the 4 Aire-deficient mice had OBP1a-specific cells present in their lacrimal glands whereas none of the Aire wild-type mice had OBP1a-specific cells present.
Figure 7. OBP1a is an important autoantigen in lacrimal gland autoimmunity.
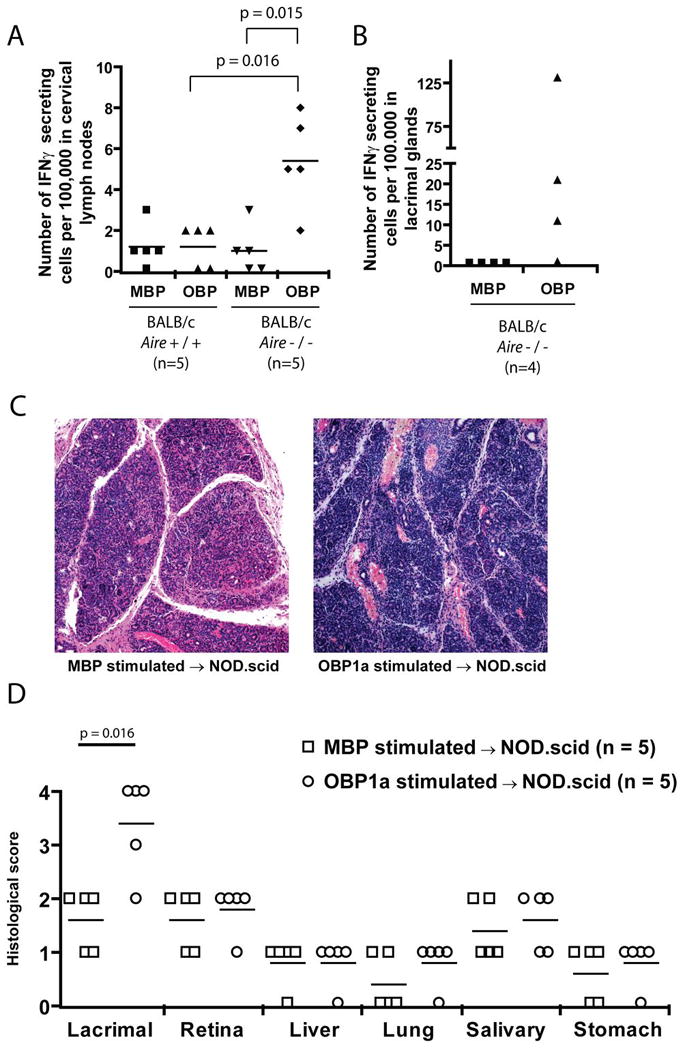
An EliSpot analysis was performed on CD4+ T cells purified from the cervical lymph nodes of 10–15 week old BALB/c _Aire_-sufficient (n = 5) and _Aire_-deficient (n = 5) mice using 5 mcg/ml OBP1a-MBP or MBP tag alone. B. An EliSpot analysis was performed on CD4+ T cells purified from infiltrating lacrimal gland cells of 10–15 week old BALB/c _Aire_-deficient mice (n = 4) stimulated with 5 mcg/ml OBP1a-MBP or MBP. Data are representative of two independent experiments. C. Lymphocytes from the cervical lymph node of 6 week old NOD _Aire_-deficient mice were stimulated in vitro in the presence of 50mcg/ml OBP1a-MBP fusion protein or MBP tag alone for 4 days. After stimulation and Ficoll purification, 1.5 million cells were transferred into NOD.scid recipients and the animals were aged for 4 weeks. Hematoxylin and eosin stained sections of the affected organs were scored for disease severity. D. Disease scores are shown for individual animals in each group.
To further demonstrate that OBP1a-specific cells can generate lacrimal autoimmunity, we stimulated cervical lymph node cells from 6 week old NOD _Aire_-deficient mice in vitro with either OBP1a-MBP fusion protein or MBP fusion protein alone. After 4 days of stimulation, cells were purified by ficoll centrifugation and 1.5 million cells per mouse were transferred into 6–8 week old NOD.scid immunodeficient recipients. Upon transfer into immunodeficient hosts, lacrimal disease in mice that received OBP1a stimulated cells was significantly more severe than in MBP stimulated cells (Figure 7C). As a control, disease severity in the remaining organs was equivalent in the two groups (Figure 7D), indicating that stimulation of the cells with OBP1a specifically exacerbated disease only in the lacrimal gland. Paraffin embedded sections were also stained with anti-CD3 antibodies to confirm the presence of T cells in the lesions (Figure S3).
DISCUSSION
In this study we have demonstrated that _Aire_-deficient mice represent a model for autoimmune dry eye and have further characterized the autoimmune response. Of note, previous work in the model has suggested that the _Aire_-deficient autoimmune response to the salivary and lacrimal glands is directed against the known autoantigen alpha-fodrin (7). To carefully assess the antigens potentially targeted in the model we performed immunoblot analysis with sera from multiple NOD and BALB/c _Aire_-deficient mice on lacrimal gland extracts and identified a novel molecular target of 18 kilodaltons that proved to be OBP1a. Interestingly, OBP1a is expressed in the thymus in an _Aire_-dependent fashion, suggesting that defective thymic expression of OBP1a may play a role in the spontaneous dacryoadenitis that develops in the _Aire_-deficient mouse model.
Previous work on the _Aire_-deficient model has suggested that alpha-fodrin is a likely prominent autoantigen associated with Sjögren’s syndrome; however, our data provides evidence for other potential autoantigen targets. In our analysis we screened for autoantibodies using immunoblots of whole lacrimal gland extracts. These extracts had detectable alpha-fodrin present on lacrimal immunoblots. However, no reactivity to alpha-fodrin was observed when these extracts were probed with _Aire_-deficient sera. Our inability to detect alpha-fodrin could be for several reasons. One possibility is that our _Aire_-deficient mice harbor low titer alpha-fodrin autoantibodies that are not detectable in our immunoblot assay. The previous study that had identified alpha-fodrin as an autoantigen in the _Aire_-deficient model used recombinant protein on an immunoblot and an ELISA to detect reactivity. In our broad autoantibody screen of lacrimal extract immunoblots, reactivity to an 18 kDa antigen was noted in sera of multiple _Aire_-deficient mice and this antigen was identified as OBP1a. OBP1a is highly expressed in lacrimal gland tissue and has an expression pattern consistent with that of a tissue-restricted antigen. In addition, we also demonstrated that OBP1a is thymically expressed in an _Aire_-dependent manner. Consistent with a defect in the selection of the T cell repertoire against this antigen, we also have demonstrated that _Aire_-deficient mice have a higher frequency of OBP1a-specific T cells than wild-type control mice of similar age. Finally, cells derived from _Aire_-deficient mice and stimulated in vitro with OBP1a are capable of transferring lacrimal disease.
These results have potential implications for our understanding of the _Aire_-deficient mouse model. There have now been several studies showing structural and cellular changes in the thymus of _Aire_-deficient mice and there is the suggestion that the immune response in _Aire_-deficient mice could also target self-antigens whose expression is not thymically regulated by Aire (7, 26, 33). In fact, alpha-fodrin has been cited as a potential self-antigen that would fit this model as its thymic expression is not _Aire_-dependent and _Aire_-deficient mice develop autoantibodies to it (7). In contrast, previous work by our group and the Mathis group has demonstrated that _Aire_-deficient mice develop autoantibody responses to self-antigens that are thymically expressed in an _Aire_-dependent manner (20, 32). Further, our group has determined that increased T cell precursor frequencies are present for a retinal autoantigen, IRBP, in the model. Likewise, we demonstrate here that in addition to autoantibodies to OBP1a, there is also a detectable increase in the T cell response to OBP1a consistent again with a possible thymic selection defect for this self-antigen. Further study will be needed to determine the relative contribution of the alpha-fodrin and OBP1a autoimmune responses to the dacryoadenitis phenotype in the _Aire_-deficient model, but our data suggest that other antigens besides alpha-fodrin may contribute to the pathological response to the lacrimal gland.
Despite the prevalence of several rodent models for SS, only one lacrimal gland specific autoantigen was identified prior to this study (34–38, 39). Autoantibodies to Ro/SSA and La/SSB as well as other ubiquitous nuclear autoantigens have been identified and/or are used in the diagnostic criteria for SS, but reactivity is not exclusive to SS. As outlined above, autoreactivity to another ubiquitous antigen, alpha-fodrin, has been demonstrated in SS patients (8), as well as numerous mouse models for SS (5) including _Aire_-deficient mice. It is important to note that autoantibodies to alpha-fodrin have been demonstrated in a number of diseases including juvenile rheumatoid arthritis, lupus erythramatosus without secondary SS, multiple sclerosis, Moyamoya, and glaucoma (10–13, 40). In contrast to these ubiquitously expressed autoantigens, OBP1a expression appears to be restricted mainly to the lacrimal gland providing some evidence for tissue specificity of the autoimmune response.
In addition to a lack of identified autoantigens in these mouse models, the exact mechanisms involved in the pathogenesis of disease are unclear. For example, in the NOD mouse model of SS the organs targeted are gender-dependent, the disease is not observed until 12–15 weeks of age and is of mild severity compared to what we observe in _Aire_-deficient mice (34), and autoimmunity may be complicated by the presence of insulin-dependent diabetes. MLR/lpr mice develop lacrimal and salivary gland infiltrates and autoantibodies to the ubiquitous Ro and La antigens but also have generalized lymphoproliferation not observed in SS patients (35–37). More recently, inhibitor of differentiation 3 (Id3)-deficient mice have been shown to have decreased glandular function and lymphocytic infiltrates (41, 42), however Id3 is also expressed in the target tissues and the relevance of this is not clear. In addition, human studies on SS patients have yet to identify a role for Id3 in disease (38). Other models, like the NFS/sld, require the removal of the thymus 3 days post-birth (39). In contrast, the _Aire_-deficient mouse model has been clearly demonstrated to have a mechanistic link to thymic T cell selection, is a spontaneous disease process, and has a human correlate in APS1. While no single mouse system is a perfect model for SS, the Aire-deficient mouse will be a useful tool to study the consequences of a breakdown in immune tolerance to the lacrimal gland.
The role of B cells in this disease process is controversial. While autoantibodies to OBP1a helped identify the autoantigen involved in this disease process, the transfer of B cell depleted populations into NOD.scid mice had no appreciable effect on disease induction. In addition, previously published data from our group has shown that passive transfer of sera from Aire-deficient mice is insufficient to cause disease and that genetically deleting B cells from the immune repertoire in Aire-deficient IgH-deficient mice has no effect on the disease process (29). In an intact mouse, it is possible and likely that B cells play a role in antigen presentation in the draining lymph nodes or target organ (30). However, their role in this process is redundant as removing this cell population did not affect the disease outcome.
Does central tolerance play a role in Sjögren’s Syndrome? Some human patients with mutations in AIRE develop keratoconjunctivitis sicca and SS, suggesting that there may be a link between AIRE and SS. Furthermore, the work here identifying a novel lacrimal gland-specific autoantigen suggests a potential link between SS and thymic expression of lacrimal gland-specific antigens. Thus, we suggest that a similar unbiased approach to identifying autoantigens in patients with APS1 and/or Sjögren’s syndrome may identify additional autoantigens with clinical relevance. While OBP1a is a murine gene with no known homolog in humans, it is a member of the lipocalin family which has been implicated in Sjögren’s syndrome (43). Additional work needs to be conducted in human patients to determine if there is a human homolog or if a different gene is being targeted. The identification of autoantigens like OBP1a could provide the framework to induce antigen-specific tolerance. It is also important to note that such a treatment approach could thus one day be feasible for SS patients.
Importantly, both Aire-deficient mice on both the NOD/LtJ and BALB/c backgrounds develop lacrimal gland autoimmunity consisting of immune infiltrates in the target tissue and autoantibodies specific for the lacrimal gland protein OBP1a. Of note, the onset of ocular surface symptoms is stronger in NOD/LtJ mice than BALB/c mice – comparing the two strains there is a difference in onset, severity and penetrance of ocular surface changes. However, the severity and penetrance of lacrimal gland infiltrates is identical in the two strains (onset is delayed in BALB/c mice as compared to NOD, however). This suggests that lacrimal gland infiltration plays an important role in the ocular surface changes seen in Aire-deficient mice, however, other genetic loci play a key role in the ultimate ocular phenotype. Thus, other genes may be involved in relative susceptibility or resistance to corneal damage and dysfunction.
Finally, our data support the notion that the SS-phenotype in _Aire_-deficient mice may be driven by autoantigens other than alpha-fodrin and that a re-assessment of the role of alpha-fodrin in _Aire_-deficient mice may be helpful. Mechanistic studies similar to our work on IRBP using autoantigen-deficient animals (20) will be required to further clarify the relative contribution of OBP1a and alpha-fodrin to autoimmunity against the lacrimal gland in this model. Despite this unresolved issue, a clearer picture of the antigen specificity in the _Aire_-deficient mouse model is beginning to emerge.
Supplementary Material
Figure S1
Figure S2
Figure S3
Supp Data Legends
Acknowledgments
We thank Jeff Bluestone, Abul Abbas, T. Shum for critical review of the manuscript and members of the Anderson Laboratory for helpful discussions and the UCSF Biomolecular Resource Center Mass Spectrometry Facility for mass spectrometry.
Funding
This work was supported by grants from the National Eye Institute (M.S.A. and E.C.S.), the National Institute of Diabetes and Digestive and Kidney Diseases (M.S.A. and J.J.D.), the Pew Scholars Program in Biomedical Sciences (M.S.A.), the Sandler Foundation (M.S.A.), the Burroughs Wellcome Fund (M.S.A.), the RPB James S. Adams Scholar Award (E.C.S.), the Ocular Immunology Fund (E.C.S.), and the Giannini Foundation (J.J.D).
Abbreviations used in this paper
OBP1a
odorant binding protein 1a
SS
Sjögren’s Syndrome
APS
Autoimmune Polyglandular Syndrome
mTEC
medullary thymic epithelial cell
Aire
autoimmune regulator
LNC
lymph node cell
IRBP
interphotoreceptor retinoid binding protein
WT
wild type
KO
knock-out
Footnotes
Disclosures
The authors have no financial conflict of interest.
References
- 1.Fox RI, Howell FV, Bone RC, Michelson P. Primary Sjogren syndrome: clinical and immunopathologic features. Semin Arthritis Rheum. 1984;14:77–105. doi: 10.1016/0049-0172(84)90001-5. [DOI] [PubMed] [Google Scholar]
- 2.van Blokland SC, Versnel MA. Pathogenesis of Sjogren’s syndrome: characteristics of different mouse models for autoimmune exocrinopathy. Clin Immunol. 2002;103:111–124. doi: 10.1006/clim.2002.5189. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen C, Singson E, Kim JY, Cornelius JG, Attia R, Doyle ME, Bulosan M, Cha S, Peck AB. Sjogren’s syndrome-like disease of C57BL/6.NOD-Aec1 Aec2 mice: gender differences in keratoconjunctivitis sicca defined by a cross-over in the chromosome 3 Aec1 locus. Scand J Immunol. 2006;64:295–307. doi: 10.1111/j.1365-3083.2006.01828.x. [DOI] [PubMed] [Google Scholar]
- 4.Cha S, Peck AB, Humphreys-Beher MG. Progress in understanding autoimmune exocrinopathy using the non-obese diabetic mouse: an update. Crit Rev Oral Biol Med. 2002;13:5–16. doi: 10.1177/154411130201300103. [DOI] [PubMed] [Google Scholar]
- 5.Yanagi K, Ishimaru N, Haneji N, Saegusa K, Saito I, Hayashi Y. Anti-120-kDa alpha-fodrin immune response with Th1-cytokine profile in the NOD mouse model of Sjogren’s syndrome. Eur J Immunol. 1998;28:3336–3345. doi: 10.1002/(SICI)1521-4141(199810)28:10<3336::AID-IMMU3336>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 6.Ishimaru N, Yanagi K, Ogawa K, Suda T, Saito I, Hayashi Y. Possible role of organ-specific autoantigen for Fas ligand-mediated activation-induced cell death in murine Sjogren’s syndrome. J Immunol. 2001;167:6031–6037. doi: 10.4049/jimmunol.167.10.6031. [DOI] [PubMed] [Google Scholar]
- 7.Kuroda N, Mitani T, Takeda N, Ishimaru N, Arakaki R, Hayashi Y, Bando Y, Izumi K, Takahashi T, Nomura T, Sakaguchi S, Ueno T, Takahama Y, Uchida D, Sun S, Kajiura F, Mouri Y, Han H, Matsushima A, Yamada G, Matsumoto M. Development of autoimmunity against transcriptionally unrepressed target antigen in the thymus of Aire-deficient mice. J Immunol. 2005;174:1862–1870. doi: 10.4049/jimmunol.174.4.1862. [DOI] [PubMed] [Google Scholar]
- 8.Haneji N, Nakamura T, Takio K, Yanagi K, Higashiyama H, Saito I, Noji S, Sugino H, Hayashi Y. Identification of alpha-fodrin as a candidate autoantigen in primary Sjogren’s syndrome. Science. 1997;276:604–607. doi: 10.1126/science.276.5312.604. [DOI] [PubMed] [Google Scholar]
- 9.Sordet C, Gottenberg JE, Goetz J, Bengoufa D, Humbel RL, Mariette X, Sibilia J. Anti-{alpha}-fodrin autoantibodies are not useful diagnostic markers of primary Sjogren’s syndrome. Ann Rheum Dis. 2005;64:1244–1245. doi: 10.1136/ard.2004.026419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watanabe T, Tsuchida T, Kanda N, Mori K, Hayashi Y, Tamaki K. Anti-alpha-fodrin antibodies in Sjogren syndrome and lupus erythematosus. Arch Dermatol. 1999;135:535–539. doi: 10.1001/archderm.135.5.535. [DOI] [PubMed] [Google Scholar]
- 11.Okura Y, Shiari R, Hattori Y, Matsuzawa T, Miyazaki Y, Hayashi Y, Kobayashi I. Epitope mapping of anti-alpha-fodrin antibody in a case of early-onset multiple sclerosis. Pediatr Int. 2008;50:135–137. doi: 10.1111/j.1442-200X.2007.02521.x. [DOI] [PubMed] [Google Scholar]
- 12.Ogawa K, Nagahiro S, Arakaki R, Ishimaru N, Kobayashi M, Hayashi Y. Anti-alpha-fodrin autoantibodies in Moyamoya disease. Stroke. 2003;34:e244–246. doi: 10.1161/01.STR.0000100479.63243.48. [DOI] [PubMed] [Google Scholar]
- 13.Grus FH, Joachim SC, Bruns K, Lackner KJ, Pfeiffer N, Wax MB. Serum autoantibodies to alpha-fodrin are present in glaucoma patients from Germany and the United States. Invest Ophthalmol Vis Sci. 2006;47:968–976. doi: 10.1167/iovs.05-0685. [DOI] [PubMed] [Google Scholar]
- 14.Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, Krohn KJ, Lalioti MD, Mullis PE, Antonarakis SE, Kawasaki K, Asakawa S, Ito F, Shimizu N. Positional cloning of the APECED gene. Nat Genet. 1997;17:393–398. [Google Scholar]
- 15.An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997;17:399–403. [Google Scholar]
- 16.Perheentupa J. APS-I/APECED: the clinical disease and therapy. Endocrinol Metab Clin North Am. 2002;31:295–320. vi. doi: 10.1016/s0889-8529(01)00013-5. [DOI] [PubMed] [Google Scholar]
- 17.Chang B, Brosnahan D, McCreery K, Dominguez M, Costigan C. Ocular complications of autoimmune polyendocrinopathy syndrome type 1. J AAPOS. 2006;10:515–520. doi: 10.1016/j.jaapos.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 18.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 19.Jiang W, Anderson MS, Bronson R, Mathis D, Benoist C. Modifier loci condition autoimmunity provoked by Aire deficiency. J Exp Med. 2005;202:805–815. doi: 10.1084/jem.20050693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeVoss J, Hou Y, Johannes K, Lu W, Liou GI, Rinn J, Chang H, Caspi RR, Fong L, Anderson MS. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J Exp Med. 2006;203:2727–2735. doi: 10.1084/jem.20061864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henzel WJ, Billeci TM, Stults JT, Wong SC, Grimley C, Watanabe C. Identifying proteins from two-dimensional gels by molecular mass searching of peptide fragments in protein sequence databases. Proc Natl Acad Sci U S A. 1993;90:5011–5015. doi: 10.1073/pnas.90.11.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.James P, Quadroni M, Carafoli E, Gonnet G. Protein identification by mass profile fingerprinting. Biochem Biophys Res Commun. 1993;195:58–64. doi: 10.1006/bbrc.1993.2009. [DOI] [PubMed] [Google Scholar]
- 23.Pappin DJ, Hojrup P, Bleasby AJ. Rapid identification of proteins by peptide-mass fingerprinting. Curr Biol. 1993;3:327–332. doi: 10.1016/0960-9822(93)90195-t. [DOI] [PubMed] [Google Scholar]
- 24.Yates JR, 3rd, Speicher S, Griffin PR, Hunkapiller T. Peptide mass maps: a highly informative approach to protein identification. Anal Biochem. 1993;214:397–408. doi: 10.1006/abio.1993.1514. [DOI] [PubMed] [Google Scholar]
- 25.Gray DH, Chidgey AP, Boyd RL. Analysis of thymic stromal cell populations using flow cytometry. J Immunol Methods. 2002;260:15–28. doi: 10.1016/s0022-1759(01)00493-8. [DOI] [PubMed] [Google Scholar]
- 26.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Yeh S, de Paiva CS, Hwang CS, Trinca K, Lingappan A, Rafati JK, Farley WJ, Li DQ, Pflugfelder SC. Spontaneous T-cell mediated keratoconjunctivitis in Aire-deficient mice. Br J Ophthalmol. 2009 doi: 10.1136/bjo.2008.153700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi M, Ishimaru N, Yanagi K, Haneji N, Saito I, Hayashi Y. High incidence of autoimmune dacryoadenitis in male non-obese diabetic (NOD) mice depending on sex steroid. Clin Exp Immunol. 1997;109:555–561. doi: 10.1046/j.1365-2249.1997.4691368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Devoss JJ, Shum AK, Johannes KP, Lu W, Krawisz AK, Wang P, Yang T, Leclair NP, Austin C, Strauss EC, Anderson MS. Effector mechanisms of the autoimmune syndrome in the murine model of autoimmune polyglandular syndrome type 1. J Immunol. 2008;181:4072–4079. doi: 10.4049/jimmunol.181.6.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gavanescu I, Benoist C, Mathis D. B cells are required for Aire-deficient mice to develop multi-organ autoinflammation: A therapeutic approach for APECED patients. Proc Natl Acad Sci U S A. 2008;105:13009–13014. doi: 10.1073/pnas.0806874105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen CQ, Cha SR, Peck AB. Sjogren’s syndrome (SjS)-like disease of mice: the importance of B lymphocytes and autoantibodies. Front Biosci. 2007;12:1767–1789. doi: 10.2741/2187. [DOI] [PubMed] [Google Scholar]
- 32.Gavanescu I, Kessler B, Ploegh H, Benoist C, Mathis D. Loss of Aire-dependent thymic expression of a peripheral tissue antigen renders it a target of autoimmunity. Proc Natl Acad Sci U S A. 2007;104:4583–4587. doi: 10.1073/pnas.0700259104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niki S, Oshikawa K, Mouri Y, Hirota F, Matsushima A, Yano M, Han H, Bando Y, Izumi K, Matsumoto M, Nakayama KI, Kuroda N. Alteration of intra-pancreatic target-organ specificity by abrogation of Aire in NOD mice. J Clin Invest. 2006;116:1292–1301. doi: 10.1172/JCI26971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doyle ME, Boggs L, Attia R, Cooper LR, Saban DR, Nguyen CQ, Peck AB. Autoimmune dacryoadenitis of NOD/LtJ mice and its subsequent effects on tear protein composition. Am J Pathol. 2007;171:1224–1236. doi: 10.2353/ajpath.2007.070388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrews BS, Eisenberg RA, Theofilopoulos AN, Izui S, Wilson CB, McConahey PJ, Murphy ED, Roths JB, Dixon FJ. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978;148:1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hang L, Theofilopoulos AN, Dixon FJ. A spontaneous rheumatoid arthritis-like disease in MRL/l mice. J Exp Med. 1982;155:1690–1701. doi: 10.1084/jem.155.6.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoffman RW, Alspaugh MA, Waggie KS, Durham JB, Walker SE. Sjogren’s syndrome in MRL/l and MRL/n mice. Arthritis and rheumatism. 1984;27:157–165. doi: 10.1002/art.1780270206. [DOI] [PubMed] [Google Scholar]
- 38.Sellam J, Miceli-Richard C, Gottenberg JE, Proust A, Ittah M, Lavie F, Loiseau P, Mariette X. Is Inhibitor of differentiation 3 involved in human primary Sjogren’s syndrome? Rheumatology (Oxford) 2008;47:437–441. doi: 10.1093/rheumatology/ken013. [DOI] [PubMed] [Google Scholar]
- 39.Haneji N, Hamano H, Yanagi K, Hayashi Y. A new animal model for primary Sjogren’s syndrome in NFS/sld mutant mice. J Immunol. 1994;153:2769–2777. [PubMed] [Google Scholar]
- 40.Takahashi K, Tatsuzawa O, Yanagi K, Hayashi Y, Takahashi H. Alpha-fodrin auto-antibody in Sjogren syndrome and other auto-immune diseases in childhood. Eur J Pediatr. 2001;160:520–521. doi: 10.1007/pl00008454. [DOI] [PubMed] [Google Scholar]
- 41.Li H, Dai M, Zhuang Y. A T cell intrinsic role of Id3 in a mouse model for primary Sjogren’s syndrome. Immunity. 2004;21:551–560. doi: 10.1016/j.immuni.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 42.Ho SB, Takamura K, Anway R, Shekels LL, Toribara NW, Ota H. The adherent gastric mucous layer is composed of alternating layers of MUC5AC and MUC6 mucin proteins. Dig Dis Sci. 2004;49:1598–1606. doi: 10.1023/b:ddas.0000043371.12671.98. [DOI] [PubMed] [Google Scholar]
- 43.Navone R, Lunardi C, Gerli R, Tinazzi E, Peterlana D, Bason C, Corrocher R, Puccetti A. Identification of tear lipocalin as a novel autoantigen target in Sjogren’s syndrome. J Autoimmun. 2005;25:229–234. doi: 10.1016/j.jaut.2005.09.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Figure S3
Supp Data Legends