An innately dangerous balancing act: intestinal homeostasis, inflammation, and colitis-associated cancer (original) (raw)
Abstract
Inflammatory bowel disease (IBD) is characterized by dysregulated immune responses to the intestinal microbiota, and by chronic intestinal inflammation. Several recent studies demonstrate the importance of innate microbial recognition by immune and nonimmune cells in the gut. Paradoxically, either diminished or exacerbated innate immune signaling may trigger the breakdown of intestinal homeostasis, leading to IBD and colitis-associated cancer (CAC). This dichotomy may reflect divergent functional roles for immune sensing in intestinal epithelial cells and leukocytes, which may vary with distinct disease mechanisms.
Much evidence supports the central contribution of the microbiota to intestinal health and disease. Although it plays beneficial roles in host development, metabolism, and defense, the relationship between the microbiota and host can break down, culminating in IBD. With few exceptions, murine models of intestinal inflammation are attenuated in germ-free animals (Strober et al., 2002). In humans, both antibiotic treatment and diversion of the fecal stream can similarly attenuate disease in some Crohn’s disease (CD) patients (Rutgeerts et al., 1991; Perencevich and Burakoff, 2006). CD clinical symptoms also tend to develop in the terminal ileum and colon, the sites of the intestine with the highest bacterial load (Sartor, 2008).
Genetic association studies have linked pattern recognition receptors (PRRs) that mediate microbial sensing with the development of IBD. These include NOD2 and NLRP3 (Hugot et al., 2001; Ogura et al., 2001; Villani et al., 2009) as well as multiple TLR genes (De Jager et al., 2007; Pierik et al., 2006; Török et al., 2004). However, the functional characterization of PRRs in IBD is just starting to be unraveled. Recent studies published in this journal (Salcedo et al. [this issue]; Allen et al., 2010) and elsewhere (Dupaul-Chicoine et al., 2010; Zaki et al., 2010) provide fresh insights into the regulation of intestinal inflammation and carcinogenesis by the signaling pathways triggered by these innate sensors. These studies also highlight the diverse functions of microbial sensing in intestinal homeostasis.
Epithelial protection
One hallmark of IBD is disruption of the intestinal epithelium. Normally, maintenance of the intestinal epithelium involves a dynamic process in which intestinal stem cells (ISCs) located at the crypt base undergo differentiation, proliferation, and migration along the crypt–villus axis. At the end of this journey, intestinal epithelial cells (IECs) undergo apoptosis. These processes appear to be dysregulated in the inflamed mucosa of IBD patients, which exhibit hyperplasia, goblet cell depletion, and enhanced apoptosis. More severe damage may manifest as either total epithelial destruction and ulceration, or dysplasia and progressive tumorigenesis. Because IECs and intestinal leukocytes both express PRRs, downstream signaling has the potential to affect epithelial homeostasis at several levels.
A landmark study using a mouse colitis model in which dextran sodium sulfate (DSS) acutely damages the intestinal epithelium demonstrated that _Myd88_−/− mice (deficient in Toll-like receptor [TLR], interleukin 1 receptor [IL-1R], and IL-18R signaling) develop significantly enhanced disease compared with wild-type mice (Rakoff-Nahoum et al., 2004). In this context, innate signals are clearly protective and promote epithelial integrity via several mechanisms. These include the induction of antiapoptotic and cytoprotective factors and the promotion of tight junction formation (Rakoff-Nahoum et al., 2004; Cario et al., 2007; Kim et al., 2009). Moreover, microbial sensing by PRRs drives the recruitment of stromal and myeloid cells to the ISC niche, thereby facilitating regeneration of the epithelium after injury (Pull et al., 2005; Brown et al., 2007). In addition to facilitating epithelial renewal, PRR activation may limit bacterial translocation by inducing the production of antimicrobial peptides, such as defensins and RegIIIγ, by IECs (Kobayashi et al., 2005; Brandl et al., 2007; Nenci et al., 2007). These functions are partially mediated by activation of NF-κB– and PI3-Akt–dependent pathways in IECs (for review see Cario, 2008). For example, mice whose IECs lack NEMO (NEMOIEC-KO, a component of the IκB kinase required for NF-κB activation, develop spontaneous intestinal inflammation (Nenci et al., 2007).
Recent studies also support a role for NOD-like receptor (NLR)–mediated inflammasome activation in maintaining epithelial integrity (Allen et al., 2010; Dupaul-Chicoine et al., 2010; Zaki et al., 2010). _Nlrp3_−/−, _Pycard_−/−, and _Casp-1_−/− mice, which lack the inflammasome signaling components Nlrp3, Asc, and caspase-1, respectively, all exhibit enhanced DSS-induced colitis compared with wild-type controls (Allen et al., 2010; Dupaul-Chicoine et al., 2010; Zaki et al., 2010). These findings are consistent with a protective role for NLR signaling in this model, analogous to that of MyD88 signaling. Indeed, because microbial stimuli and host danger signals, such as uric acid, extracellular ATP, and reactive oxygen species, synergize to activate the inflammasome (Franchi et al., 2009), acute epithelial damage in the microbe-rich gut may create a potent niche for such regulation. However, the question of whether inflammasome activation in epithelial or hematopoietic cells confers this protection has produced mixed findings (Allen et al., 2010; Dupaul-Chicoine et al., 2010; Zaki et al., 2010).
A major effector function of inflammasome-mediated activation of caspase-1 is the processing of pro–IL-1β and pro–IL-18 into their active forms. Tellingly, Il1r−/−, Il18r−/−, and _Il18_−/− mice exhibit enhanced DSS-induced colitis compared with wild-type controls (Takagi et al., 2003; Lebeis et al., 2009; Salcedo et al., 2010), and recombinant IL-18 can attenuate disease (Dupaul-Chicoine et al., 2010; Zaki et al., 2010), supporting a role for inflammasome-derived mediators in epithelial repair. Crucially, because MyD88 is a signaling adaptor shared by IL-1R, IL-18R, and TLRs, its indirect activation by NLRs and/or direct activation by TLRs may be a key event in the restoration of epithelial function. Nonetheless, the role of IL-1 and IL-18 in intestinal inflammation remains contentious, given that earlier studies support a pathogenic role of these cytokines (for review see Siegmund, 2002).
A comparable and perhaps more physiological model where TLR or IL-1R family signals limit colitis is that induced by the attaching and effacing bacterium Citrobacter rodentium. Indeed, increased numbers of mucosally associated adherent-invasive Escherichia coli have been reported in CD and UC patients (Rolhion and Darfeuille-Michaud, 2007). Infected Myd88−/− mice exhibit delayed neutrophil recruitment and enhanced bacterial outgrowth in the intestinal mucosa with pronounced ulceration, bleeding, and mortality (Lebeis et al., 2007; Gibson et al., 2008a). In this context, dysregulated epithelial restitution and repair is compounded by pathogen outgrowth and further immune activation. However, some PRRs may contribute more to immune pathology than to protective immunity. For example, Tlr4−/− mice develop protective immunity to C. rodentium, but develop less severe intestinal pathology than infected wild-type mice (Khan et al., 2006). This pathogenic role of TLR4 contrasts with that of TLR2 and IL-1R, which both limit _C. rodentium_–induced inflammation (Gibson et al., 2008b; Lebeis et al., 2009).
Chronic inflammation
These broadly protective roles for gut TLR and inflammasome signals in acute intestinal inflammation contrast starkly with pathogenic roles played by these signals in the _Il10_−/−or _Helicobacter hepaticus–_induced chronic colitis models. MyD88 signaling promotes spontaneous colitis in both _Il10−/−_mice and LysMStat3KO mice; the latter mice are rendered hyporesponsive to IL-10 by a myeloid cell–specific deletion of Stat3 (Kobayashi et al., 2003; Rakoff-Nahoum et al., 2006). Moreover, we have recently shown that colitis induced by H. hepaticus colonization of RAG−/− mice requires MyD88 expression by hematopoietic cells (Asquith et al., 2010). Together, these findings suggest that a failure to regulate TLR/IL-1R family–dependent activation of myeloid cells in the presence of a triggering microbiota can precipitate chronic intestinal inflammation.
A key difference in these chronic colitis models is that damage to the epithelium is likely secondary to the microbiota-driven inflammatory response mediated by tissue-resident leukocytes. This notion is supported by the spontaneous colitis that occurs in NemoIEC-KO mice, in which tumor necrosis factor produced by MyD88-activated myeloid cells is thought to drive epithelial apoptosis (Nenci et al., 2007). In contrast, acute epithelial disruption by DSS or C. rodentium infection may be a primary pathophysiological mechanism, with gross disruption of the epithelium allowing MyD88-independent activation by the intestinal microbiota. In this acute disease setting, the epithelial repair functions of MyD88 play a protective role, promoting restoration of epithelial barrier function and a return to homoeostasis. It will be of interest to extend studies of inflammasome function to chronic colitis models with distinct pathophysiological mechanisms.
Tumorigenesis
Intestinal tumorigenesis is intimately linked to intestinal inflammation, and by extension to microbial sensing by PRRs. Intestinal tumors may originate from dysplastic epithelial or intestinal stem cells, and are infiltrated by several cell types, including lymphoid and myeloid cells (Terzic et al., 2010). Evidently, as in models of colitis, in models of CAC, PRR signals may modulate the neoplastic and infiltrating leukocyte populations distinctly, complicating the dissection of their role in CAC pathophysiology. Several previous studies have indicated that signals mediated by the TLR/IL-1R family promote intestinal tumorigenesis. For example, TLR4 is up-regulated in both human and mouse neoplastic lesions of the inflamed intestine and promotes CAC in mice treated with the chemical procarcinogen azoxymethane (AOM) followed by DSS (Fukata et al., 2007). In addition, the size and frequency of adenocarcinomas in ApcMin+/− mice, which express a mutant version of the tumor suppressor gene APC, are greater than in ApcMin+/−MyD88−/− mice (Rakoff-Nahoum and Medzhitov, 2007). Moreover, Il10−/− mice treated with AOM develop MyD88-dependent CAC (Uronis et al., 2009).
In contrast to the tumorigenic role of TLR4 signals in the DSS + AOM model, the Nlrp3 inflammasome and MyD88 signals prevent CAC in this setting. This paradox remains unresolved. However, whereas epithelial TLR4 signals promote tumorigenesis (Fukata et al., 2009), hematopoietic Nlrp3 activation may be required for protection (Allen et al., 2010). Thus, distinct compartmentalized roles may underscore these divergent functions. Alternatively, MyD88-independent TLR4 signaling through the adaptor TRIF may play a role, and merits further investigation.
When treated with DSS, MyD88−/− (Fukata et al., 2005; Rakoff-Nahoum et al., 2004), Nlrp3−/−, and Casp-1−/− mice exhibit enhanced epithelial apoptosis and diminished or comparable epithelial proliferation relative to DSS-treated wild-type mice (Zaki et al., 2010). Rather counterintuitively, MyD88−/−, Nlrp3−/−, and Casp-1−/− mice show enhanced tumorigenesis compared with wild-type mice if also treated with AOM (Allen et al., 2010; Salcedo et al., 2010). A possible explanation provided by Salcedo et al. (2010) is that MyD88−/− mice treated with AOM + DSS exhibit down-regulation of multiple DNA repair genes and have a higher frequency of mutations in the oncoprotein β-catenin, specifically in the domain required to target the protein for degradation. This indicates that failed repair of DNA damage in MyD88−/− mice may render epithelial cells prone to deregulation of the cell cycle and tumor progression, despite their increased propensity for cell death. Nonetheless, untreated Myd88−/− mice exhibit “normal” expression of DNA repair genes (Salcedo et al., 2010), suggesting that this is a downstream effect of enhanced inflammation in these mice.
Excessive TLR/IL-1R–driven signaling can itself drive proinflammatory responses that fuel tumorigenesis, as shown by the MyD88-dependent development of CAC in _Il10_−/− mice treated with AOM (Uronis et al., 2009). Moreover, the removal of negative regulators of TLR/IL-1R family and inflammasome signaling, such as Tir8 or caspase-12, respectively, also leads to increased colitis and CAC in the DSS + AOM model (Dupaul-Chicoine et al., 2010; Garlanda et al., 2007). In addition to excessive production of inflammatory cytokines in these mice, hyperproliferative epithelial repair responses may also drive tumorigenesis.
PRR signals may also modulate tumorigenesis independently of their pathogenic and protective roles in inflammation. This is exemplified by _ApcMin+/−_mice, which model familial adenomatous polyposis patients with mutations in the APC gene who develop intestinal polyps and colorectal cancer early in life. TLR-MyD88 signals promote tumorigenesis in these mice, partially through stabilization of oncoprotein c-Myc, which induces epithelial hyperproliferation and inhibits apoptosis (Lee et al., 2010). In this case, the presence of a genetic lesion such as APC mutations may divert the homeostatic function of PRR signaling toward tumorigenesis.
Concluding remarks
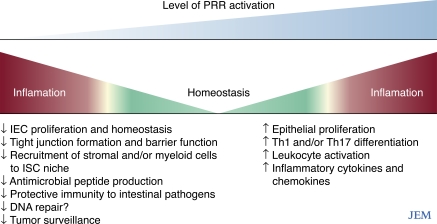
Functional analyses of PRR pathways in intestinal homeostasis have revealed a complex picture with evidence for both protective and pathogenic roles. Optimally, PRR-signaling should be maintained at a homeostatic level at which tissue repair and host defense are preserved (Fig. 1). Beyond this middle ground, immune pathology can result from either impaired or exuberant PRR responses. A key challenge now is to translate these diverse functions of PRR in different animal models to determine which pathophysiological mechanisms underpin distinct forms of IBD and CAC. For example, ablation of PRR-driven leukocyte activation may limit pathogenesis in some settings, but may prove futile, and even exacerbate symptoms, if barrier dysfunction is the primary disease mechanism. Careful dissection of PRR-signaling networks in different cellular compartments and in acute and chronic models of intestinal inflammation is an important first step in designing rational therapeutic strategies designed to restore PRR-mediated intestinal homeostasis in IBD.
Figure 1.
Diminished or enhanced intestinal PRR signals may promote intestinal inflammation and tumorigenesis. PRR signals are maintained at a critical threshold to maintain intestinal homeostasis. PRR signals may be required to restore barrier function after epithelial insult and for protective immunity against pathogens; impairment of these processes caused by insufficient PRR signaling may result in pathogen outgrowth and, indirectly, excessive subsequent inflammation (left). Excessive PRR-driven repair or inflammatory responses (right) may also threaten homeostasis, e.g., through dysregulated epithelial proliferation leading to tumorigenesis and overexuberant pathogenic inflammatory responses to the intestinal microbiota.
Acknowledgments
We thank Michael Barnes for critical reading of the manuscript.
F. Powrie and M. Asquith are funded by the Wellcome Trust.
References
- Allen I.C., TeKippe E.M., Woodford R.M., Uronis J.M., Holl E.K., Rogers A.B., Herfarth H.H., Jobin C., Ting J.P. 2010. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J. Exp. Med. 207:1045–1056 10.1084/jem.20100050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asquith M.J., Boulard O., Powrie F., Maloy K.J. 2010. Pathogenic and protective roles of MyD88 in leukocytes and epithelial cells in mouse models of inflammatory bowel disease. Gastroenterology. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl K., Plitas G., Schnabl B., DeMatteo R.P., Pamer E.G. 2007. MyD88-mediated signals induce the bactericidal lectin RegIII gamma and protect mice against intestinal Listeria monocytogenes infection. J. Exp. Med. 204:1891–1900 10.1084/jem.20070563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S.L., Riehl T.E., Walker M.R., Geske M.J., Doherty J.M., Stenson W.F., Stappenbeck T.S. 2007. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J. Clin. Invest. 117:258–269 10.1172/JCI29159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cario E. 2008. Innate immune signalling at intestinal mucosal surfaces: a fine line between host protection and destruction. Curr. Opin. Gastroenterol. 24:725–732 10.1097/MOG.0b013e32830c4341 [DOI] [PubMed] [Google Scholar]
- Cario E., Gerken G., Podolsky D.K. 2007. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 132:1359–1374 10.1053/j.gastro.2007.02.056 [DOI] [PubMed] [Google Scholar]
- De Jager P.L., Franchimont D., Waliszewska A., Bitton A., Cohen A., Langelier D., Belaiche J., Vermeire S., Farwell L., Goris A., et al. ; Quebec IBD Genetics Consortium; NIDDK IBD Genetics Consortium 2007. The role of the Toll receptor pathway in susceptibility to inflammatory bowel diseases. Genes Immun. 8:387–397 10.1038/sj.gene.6364398 [DOI] [PubMed] [Google Scholar]
- Dupaul-Chicoine J., Yeretssian G., Doiron K., Bergstrom K.S., McIntire C.R., LeBlanc P.M., Meunier C., Turbide C., Gros P., Beauchemin N., et al. 2010. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 32:367–378 10.1016/j.immuni.2010.02.012 [DOI] [PubMed] [Google Scholar]
- Franchi L., Warner N., Viani K., Nuñez G. 2009. Function of Nod-like receptors in microbial recognition and host defense. Immunol. Rev. 227:106–128 10.1111/j.1600-065X.2008.00734.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M., Michelsen K.S., Eri R., Thomas L.S., Hu B., Lukasek K., Nast C.C., Lechago J., Xu R., Naiki Y., et al. 2005. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 288:G1055–G1065 10.1152/ajpgi.00328.2004 [DOI] [PubMed] [Google Scholar]
- Fukata M., Chen A., Vamadevan A.S., Cohen J., Breglio K., Krishnareddy S., Hsu D., Xu R., Harpaz N., Dannenberg A.J., et al. 2007. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 133:1869–1881 10.1053/j.gastro.2007.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M., Hernandez Y., Conduah D., Cohen J., Chen A., Breglio K., Goo T., Hsu D., Xu R., Abreu M.T. 2009. Innate immune signaling by Toll-like receptor-4 (TLR4) shapes the inflammatory microenvironment in colitis-associated tumors. Inflamm. Bowel Dis. 15:997–1006 10.1002/ibd.20880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C., Riva F., Veliz T., Polentarutti N., Pasqualini F., Radaelli E., Sironi M., Nebuloni M., Zorini E.O., Scanziani E., Mantovani A. 2007. Increased susceptibility to colitis-associated cancer of mice lacking TIR8, an inhibitory member of the interleukin-1 receptor family. Cancer Res. 67:6017–6021 10.1158/0008-5472.CAN-07-0560 [DOI] [PubMed] [Google Scholar]
- Gibson D.L., Ma C., Bergstrom K.S., Huang J.T., Man C., Vallance B.A. 2008a. MyD88 signalling plays a critical role in host defence by controlling pathogen burden and promoting epithelial cell homeostasis during Citrobacter rodentium-induced colitis. Cell. Microbiol. 10:618–631 10.1111/j.1462-5822.2007.01071.x [DOI] [PubMed] [Google Scholar]
- Gibson D.L., Ma C., Rosenberger C.M., Bergstrom K.S., Valdez Y., Huang J.T., Khan M.A., Vallance B.A. 2008b. Toll-like receptor 2 plays a critical role in maintaining mucosal integrity during Citrobacter rodentium-induced colitis. Cell. Microbiol. 10:388–403 10.1111/j.1462-5822.2007.01071.x [DOI] [PubMed] [Google Scholar]
- Hugot J.P., Chamaillard M., Zouali H., Lesage S., Cézard J.P., Belaiche J., Almer S., Tysk C., O’Morain C.A., Gassull M., et al. 2001. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 411:599–603 10.1038/35079107 [DOI] [PubMed] [Google Scholar]
- Khan M.A., Ma C., Knodler L.A., Valdez Y., Rosenberger C.M., Deng W., Finlay B.B., Vallance B.A. 2006. Toll-like receptor 4 contributes to colitis development but not to host defense during Citrobacter rodentium infection in mice. Infect. Immun. 74:2522–2536 10.1128/IAI.74.5.2522-2536.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.Y., Kajino-Sakamoto R., Omori E., Jobin C., Ninomiya-Tsuji J. 2009. Intestinal epithelial-derived TAK1 signaling is essential for cytoprotection against chemical-induced colitis. PLoS One. 4:e4561 10.1371/journal.pone.0004561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Kweon M.N., Kuwata H., Schreiber R.D., Kiyono H., Takeda K., Akira S. 2003. Toll-like receptor-dependent production of IL-12p40 causes chronic enterocolitis in myeloid cell-specific Stat3-deficient mice. J. Clin. Invest. 111:1297–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K.S., Chamaillard M., Ogura Y., Henegariu O., Inohara N., Nuñez G., Flavell R.A. 2005. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 307:731–734 10.1126/science.1104911 [DOI] [PubMed] [Google Scholar]
- Lebeis S.L., Bommarius B., Parkos C.A., Sherman M.A., Kalman D. 2007. TLR signaling mediated by MyD88 is required for a protective innate immune response by neutrophils to Citrobacter rodentium. J. Immunol. 179:566–577 [DOI] [PubMed] [Google Scholar]
- Lebeis S.L., Powell K.R., Merlin D., Sherman M.A., Kalman D. 2009. Interleukin-1 receptor signaling protects mice from lethal intestinal damage caused by the attaching and effacing pathogen Citrobacter rodentium. Infect. Immun. 77:604–614 10.1128/IAI.00907-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Hu L.L., Gonzalez-Navajas J., Seo G.S., Shen C., Brick J., Herdman S., Varki N., Corr M., Lee J., Raz E. 2010. ERK activation drives intestinal tumorigenesis in Apc(min/+) mice. Nat. Med. 16:665–670 10.1038/nm.2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenci A., Becker C., Wullaert A., Gareus R., van Loo G., Danese S., Huth M., Nikolaev A., Neufert C., Madison B., et al. 2007. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 446:557–561 10.1038/nature05698 [DOI] [PubMed] [Google Scholar]
- Ogura Y., Bonen D.K., Inohara N., Nicolae D.L., Chen F.F., Ramos R., Britton H., Moran T., Karaliuskas R., Duerr R.H., et al. 2001. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 411:603–606 10.1038/35079114 [DOI] [PubMed] [Google Scholar]
- Perencevich M., Burakoff R. 2006. Use of antibiotics in the treatment of inflammatory bowel disease. Inflamm. Bowel Dis. 12:651–664 10.1097/01.MIB.0000225330.38119.c7 [DOI] [PubMed] [Google Scholar]
- Pierik M., Joossens S., Van Steen K., Van Schuerbeek N., Vlietinck R., Rutgeerts P., Vermeire S. 2006. Toll-like receptor-1, -2, and -6 polymorphisms influence disease extension in inflammatory bowel diseases. Inflamm. Bowel Dis. 12:1–8 10.1097/01.MIB.0000195389.11645.ab [DOI] [PubMed] [Google Scholar]
- Pull S.L., Doherty J.M., Mills J.C., Gordon J.I., Stappenbeck T.S. 2005. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc. Natl. Acad. Sci. USA. 102:99–104 10.1073/pnas.0405979102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S., Medzhitov R. 2007. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 317:124–127 10.1126/science.1140488 [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S., Paglino J., Eslami-Varzaneh F., Edberg S., Medzhitov R. 2004. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 118:229–241 10.1016/j.cell.2004.07.002 [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S., Hao L., Medzhitov R. 2006. Role of toll-like receptors in spontaneous commensal-dependent colitis. Immunity. 25:319–329 10.1016/j.immuni.2006.06.010 [DOI] [PubMed] [Google Scholar]
- Rolhion N., Darfeuille-Michaud A. 2007. Adherent-invasive Escherichia coli in inflammatory bowel disease. Inflamm. Bowel Dis. 13:1277–1283 10.1002/ibd.20176 [DOI] [PubMed] [Google Scholar]
- Rutgeerts P., Goboes K., Peeters M., Hiele M., Penninckx F., Aerts R., Kerremans R., Vantrappen G. 1991. Effect of faecal stream diversion on recurrence of Crohn’s disease in the neoterminal ileum. Lancet. 338:771–774 10.1016/0140-6736(91)90663-A [DOI] [PubMed] [Google Scholar]
- Salcedo R., Worschech A., Cardone M., Jones Y., Gyulai Z., Dai R.-M., Wang E., Ma W., Haines D., O’hUigin C., Marincola F.M., Trinchieri G. 2010. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J. Exp. Med. 207:1625–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor R.B. 2008. Microbial influences in inflammatory bowel diseases. Gastroenterology. 134:577–594 10.1053/j.gastro.2007.11.059 [DOI] [PubMed] [Google Scholar]
- Siegmund B. 2002. Interleukin-1beta converting enzyme (caspase-1) in intestinal inflammation. Biochem. Pharmacol. 64:1–8 10.1016/S0006-2952(02)01064-X [DOI] [PubMed] [Google Scholar]
- Strober W., Fuss I.J., Blumberg R.S. 2002. The immunology of mucosal models of inflammation. Annu. Rev. Immunol. 20:495–549 10.1146/annurev.immunol.20.100301.064816 [DOI] [PubMed] [Google Scholar]
- Takagi H., Kanai T., Okazawa A., Kishi Y., Sato T., Takaishi H., Inoue N., Ogata H., Iwao Y., Hoshino K., et al. 2003. Contrasting action of IL-12 and IL-18 in the development of dextran sodium sulphate colitis in mice. Scand. J. Gastroenterol. 38:837–844 10.1080/00365520310004047 [DOI] [PubMed] [Google Scholar]
- Terzic J., Grivennikov S., Karin E., Karin M. 2010. Inflammation and colon cancer. Gastroenterology. 138:2101–2114. [DOI] [PubMed] [Google Scholar]
- Török H.P., Glas J., Tonenchi L., Bruennler G., Folwaczny M., Folwaczny C. 2004. Crohn’s disease is associated with a toll-like receptor-9 polymorphism. Gastroenterology. 127:365–366 10.1053/j.gastro.2004.05.051 [DOI] [PubMed] [Google Scholar]
- Uronis J.M., Mühlbauer M., Herfarth H.H., Rubinas T.C., Jones G.S., Jobin C. 2009. Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS One. 4:e6026 10.1371/journal.pone.0006026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani A.C., Lemire M., Fortin G., Louis E., Silverberg M.S., Collette C., Baba N., Libioulle C., Belaiche J., Bitton A., et al. 2009. Common variants in the NLRP3 region contribute to Crohn’s disease susceptibility. Nat. Genet. 41:71–76 10.1038/ng.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki M.H., Boyd K.L., Vogel P., Kastan M.B., Lamkanfi M., Kanneganti T.D. 2010. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 32:379–391 10.1016/j.immuni.2010.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]