Parkinson’s disease and mitochondrial complex I: a perspective on the Ndi1 therapy (original) (raw)
. Author manuscript; available in PMC: 2010 Dec 1.
Published in final edited form as: J Bioenerg Biomembr. 2009 Dec;41(6):493–497. doi: 10.1007/s10863-009-9249-z
Abstract
Mitochondrial impairment has been collecting more and more attention as a contributing factor to the etiology of Parkinson’s disease. Above all, the NADH-quinone oxidoreductase, complex I, of the respiratory chain seems to be most culpable. Complex I dysfunction is translated to an increased production of reactive oxygen species and a decreased energy supply. In the brain, the dopaminergic neurons are one of the most susceptible cells. Their death is directly linked to the disease apparition. Developing an effective gene therapy is challenged by harmful actions of reactive oxygen species. To overcome this problem a therapeutic candidate must be able to restore the NADH-quinone oxidoreductase activity regardless of how complex I is impaired. Here we discuss the potency of the yeast alternative NADH dehydrogenase, the Ndi1 protein, to reinstate the mitochondrial respiratory chain compensating for disabled complex I and the benefit Ndi1 brings toward retardation of Parkinson’s disease.
Keywords: Gene therapy, Complex I, NDI1, Parkinson’s disease, Neuroprotection
Introduction
Parkinson’s disease (PD) is one of the most common neurodegenerative disorders affecting people over 60 years old. The clinical hallmarks of this disease are tremor, bradykinesia, rigidity and postural instability (Bulpitt et al. 1985; Olanow and Tatton 1999). Those symptoms are the result of the alteration of the nigro-striatal pathway in the brain. Indeed, during Parkinson’s disease the dopaminergic neurons of the substantia nigra pars compacta undergo a massive death. The clinical presentation of the disease starts when about 50% to 70% of the dopaminergic neurons degenerate. The primary cause of PD remains elusive. A multiplicity of factors including genetic predispositions and/or environmental expositions to toxins might be part of the equation triggering the onset of the disease. Among a variety of causes implicated in the pathogenesis of PD, mitochondrial defects have gained a tremendous support from diverse studies. Mutations of the genes Parkin, Pink1 and DJ-1 have been identified as causes for parkinsonism (Canet-Aviles et al. 2004; Palacino et al. 2004; Valente et al. 2004) and all those genes encode proteins directly related to mitochondrial functions. In the mid 80’s, drugs abusers developed Parkinson disease-like symptoms. They were injecting themselves with a neurotoxic by-product called MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) that target specifically the mitochondria of the dopaminergic neurons. The active metabolite of MPTP, MPP+ (N-methyl-phenylpyridinium ion) is a specific inhibitor of the proton-pumping NADH-quinone oxidoreductase (complex I) of the mitochondrial respiratory chain (Mizuno et al. 1987; Nicklas et al. 1985). Inhibition of complex I leads to decreased production of ATP and generation of reactive oxygen species (ROS) (Langston and Ballard 1983; Ramsay and Singer 1986). Complex I is a major entry point of the respiratory chain and its deficiencies can be translated into a dramatic loss of bioenergetic functions and a great instability of the mitochondria. Whether or not complex I is the triggering factor of all cases of sporadic PD may be debatable. However, it is clear that defects of complex I constitute an important step in the cascade of events leading to the death of the dopaminergic cells. Lowered complex I activity has been observed in mitochondria from platelets as well as substantia nigra and frontal cortex of the brain of PD patients (Schapira et al. 1990; Haas et al. 1995; Keeney et al. 2006). Moreover, in the cybrid system consisting of the mitochondria from PD patients transferred into mitochondria-less normal cells, complex I activity was found to be lower than average (Gu et al. 1998). Specific inhibitors of complex I have been shown to bring about biochemical, anatomical and behavioral characteristics of PD in animal models (Betarbet et al. 2000; Thiruchelvam et al. 2000; Vila and Przedborski 2003). These and other findings suggest that complex I impairments may be central to the pathogenesis of dopamine neuronal death in sporadic PD. It was recently argued that complex I inhibition is not required for dopaminergic neuronal death induced by chemicals such as rotenone, MPP+ or paraquat (Choi et al. 2008). However, there is substantial evidence indicating that augmenting the function of complex I with a replacement enzyme is sufficient enough to counteract the effect of MPP+ or rotenone (Marella et al. 2008; Richardson et al. 2007; Seo et al. 2006b) or other complex I inhibitors such as pyridaben and annonacin (Escobar-Khondiker et al. 2007; Sherer et al. 2007). This review summarizes our work on successful use of the alternative NADH dehydrogenase, Ndi1, as a replacement molecule for complex I. The data not only strengthen the link between complex I impairment and dopaminergic neuron death but also demonstrate a great potential of this single-subunit enzyme as a therapeutic agent for PD.
Emergence of a trans-kingdom gene therapy
There is no doubt that the mitochondrial physiology is involved in the development of PD and that, within the mitochondria, alteration of complex I activity has the strongest impact on this disease. Mammalian complex I is by far the largest complex in the respiratory chain with at least 45 subunits. Alternative NADH dehydrogenases found in bacteria, plant and fungal mitochondria but not in mammalian mitochondria are single-subunit enzymes that catalyzes NADH-quinone oxidoreduction similar to complex I. Unlike complex I, they do not have proton pumping function. In mitochondria of Saccharomyces cerevisiae, there are two types of alternative NADH dehydrogenases. One is directed to the matrix and catalyzes NADH oxidation in the matrix (designated the internal NADH dehydrogenase or Ndi), while the other faces the inter-membrane space and oxidizes NADH in the cytoplasmic space (designated the external NADH dehydrogenases or Nde). The Ndi1 protein belongs to the former type and is similar to complex I in terms of NADH oxidation in the matrix (Yagi et al. 2006). It is a single polypeptide of 53 kDa and contains FAD as a cofactor. Our idea was based on a simple thinking; to get the Ndi1 enzyme to work as the alternative dehydrogenase in mammalian mitochondria and to establish a therapeutic method of restoring the NADH oxidation where complex I is impaired.
Benefit of Ndi1 expression in mitochondria
When the NDI1 gene is expressed in the host cells, the protein is directed to the mitochondria via its own signal peptide. Once imported, Ndi1 shows perfect functional integration into the mammalian respiratory chain. The yeast protein delivers electrons from NADH to quinone and thus is able to maintain the ATP production (Seo et al. 2002). Although Ndi1 is not a proton pump, experiments carried out with cultured cells showed little or no influence on the mitochondrial membrane potential by the presence of Ndi1. Both cellular ATP level and membrane potential were resistant to complex I inhibitors (Marella et al. 2007). This may illustrate that the two other proton pumping sites of the mitochondrial respiratory chain (i.e. complex III and complex IV) are enough to maintain the electrochemical gradient even when coupling site 1 was lost.
When complex I is impaired either structurally or by specific inhibitors, NADH is accumulated and redox potential of the matrix becomes significantly low. These conditions can trigger a burst of reactive oxygen species (ROS) from complex I. ROS has colossal negative repercussions on the viability of cells as they damage DNA, proteins and lipids. Keeney et al. demonstrated that PD patients carry oxidatively damaged complex I resulting in functional impairment (Keeney et al. 2006). The mammalian complex I is trapped in a vicious cycle as its impairment induces the ROS production and ROS in turn harms the complex I subunits reinforcing its defects. The benefit of having the Ndi1 protein in mitochondria is not limited to the role as a replacement dehydrogenase. Generation of ROS from complex I caused by complex I inhibitors is greatly suppressed when Ndi1 is present in the mitochondria (Seo et al. 2006a). This apparent “antioxidant” effect was most likely because Ndi1 keeps the NADH/NAD ratio in the matrix low (the redox potential high). In other words, because ROS formation from complex I requires the redox potential to be low (approximately −350 mV) (Kushnareva et al. 2002), the redox environment created by the action of Ndi1 prevents such ROS production even when complex I is inhibited by rotenone. It was in fact confirmed that the NADH/NAD ratio of the matrix remains low when Ndi1 exists even in the presence of a complex I inhibitor (Marella et al. 2007).
Relevance of the Ndi1 gene therapy in PD
As described above, expression of the yeast Ndi1 in mammalian mitochondria demonstrated its ability not only to maintain the energetic balance in cells but also to fight against ROS production caused by complex I malfunction. In order to test the therapeutic potential of Ndi1 during the development of PD, we carried out a series of in vivo experiments in animal models of PD.
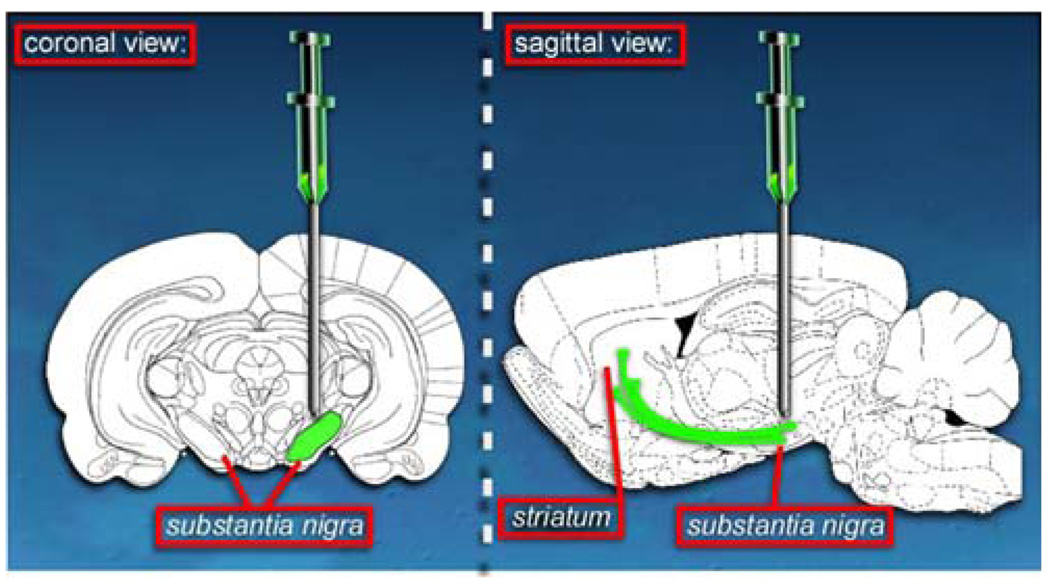
The first attempt of Ndi1 expression was done in a mouse model of PD involving MPTP administration. In those mice, MPTP targets specifically the dopaminergic cells and inhibits the complex I activity. This inhibition results in a dramatic loss of the cells in the nigro-striatal pathway which can be monitored by following the quantity of the dopaminergic cells specific tyrosine hydroxylase enzyme and the quantity of glial fibrillary acidic protein which is specific for astrocytes. Astrocytes are the cells that will fill the empty space left by the dead neurons. In order to deliver the NDI1 gene into the substantia nigra of animals, we used a recombinant adeno-associated virus system (rAAV). The rAAV carrying the NDI1 gene was injected in the vicinity of the substantia nigra, which resulted in a good expression of the transgene in all cells of this brain region (Fig. 1). The mice expressing Ndi1 but also treated with MPTP showed a preserved nigro-striatal pathway and intact dopaminergic cells (Seo et al. 2006b). This study confirmed in vivo integration of the yeast protein in the host mitochondrial respiratory chain and demonstrated that the yeast Ndi1 can protect the animal from the MPTP insult.
Fig. 1.
Delivery of the yeast Ndi1 into rodent brain. In order to express Ndi1 in the cell mitochondria recombinant adeno-associated virus particles carrying the yeast gene is injected near the substantia nigra. The dopaminergic neurons in the substantia nigra project their terminals into the striatum. This whole nigro-striatal pathway undergoes neurodegeneration in Parkinson’s disease
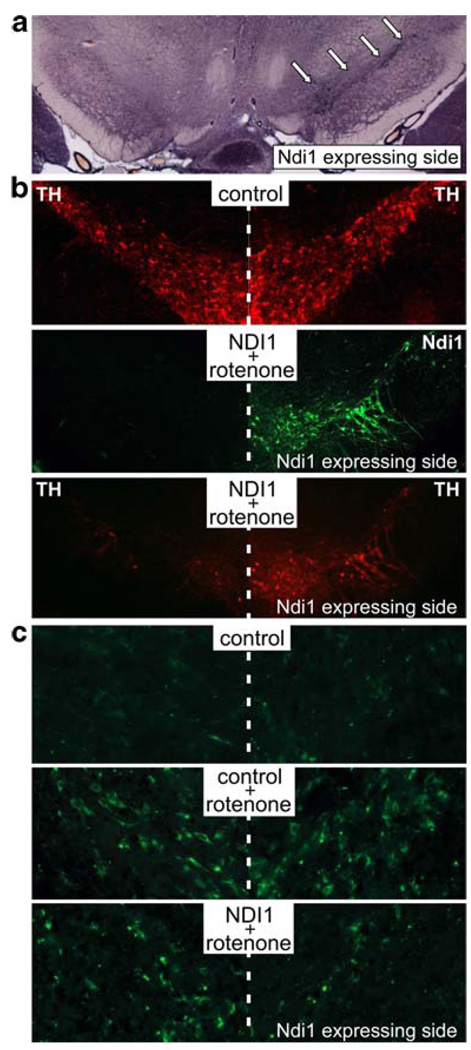
Next, we corroborated the protective effect of Ndi1 expression in a chronic rotenone animal model of PD (Marella et al. 2008). The experiments performed also demonstrated that the Ndi1 expression was able to preserve the nigro-striatal function in spite of the constant diffusion of rotenone into the animal. This animal model was obtained with the subcutaneous injection of rotenone trap into biopolymer microspheres. The beads are designed to release the chemical slowly and persistently throughout the course of the experiments. The actual measurement of rotenone concentration in plasma showed that it was maintained above 1 µM for at least 2 months. Even under these harsh conditions, the Ndi1 expression diminished the mitochondrial ROS production and influenced positively the behavior of the animals (Fig. 2). The behavioral experiments consisted of monitoring the animals lateralization under a dopaminergic agonist, apomorphine hydrochloride. Because Ndi1 was unilaterally expressed, the resulting difference in the integrity between the right and the left side of the dopaminergic pathway lead to an asymmetric behavior and the animal expressing the yeast protein and treated with rotenone displayed a strong lateralization.
Fig. 2.
Protection by the Ndi1 protein against the rotenone insult. Coronal brain sections through the substantial nigra were analyzed histochemically. a NADH dehydrogenase activity staining. The substantia nigra that received the NDI1 gene clearly exhibits functional expression of Ndi1 (arrows). b Immunohistochemical staining for the tyrosine hydroxylase (TH) and the Ndi1 protein. Rotenone exposure caused substantial loss of the dopaminergic cells in the substantia nigra, which is prevented by the expression of Ndi1. c Generation of reactive oxygen species as assessed by oxidative DNA damage. Oxidatively modified DNA of the dopaminergic cells was visualized by immunohistochemical staining for 8-oxo-deoxyguanine. Rotenone causes a high degree of oxidative damage. The presence of Ndi1 exerts protective effect against ROS-driven injuries (from Marella et al. 2008)
More recently, we demonstrated that the protective action of Ndi1 against parkinsonian symptoms was a long term event. Eight months after receiving injection of rAAV-NDI1 into the brain, mice were challenged with MPTP. In this model, the protection provided by the presence of Ndi1 into the mitochondria of dopaminergic cells was still fully effective (Barber-Singh et al. 2009).
Besides its substantial capability the Ndi1 gene therapy showed a great versatility because it is efficacious on different animal models of PD. The extreme structural differences between the big and very intricate complex I and the much simpler Ndi1 turned out to be of considerable advantage. The Ndi1 enzyme is totally insensitive to the chemicals that lead to complex I damages. Therefore, the yeast protein may provide a sustainable protection from a wide variety of environmental mitochondrial toxins.
A yeast protein in my brain, are you crazy?
One of the questions raised when considering the substitution of an endogenous function is whether the elected replacement is incorporated harmoniously into the cells. The gene therapy presented above relies on the expression of the yeast protein Ndi1 into the respiratory chain of mammalian mitochondria. To express this protein into the brain of rodents we used the rAAV delivery system. The rAAV vectors are widely used as they ensure a safe gene transfer with minimum deleterious effects. Moreover, the immune response to this vector is almost null and it has been considered safe enough to use in human gene therapy trials (Bessis et al. 2004; Kaplitt et al. 2007). Nevertheless, once the transgene is expressed it could display a high intrinsic toxicity resulting in the failure of the therapy or even worse, increase in the damage due to the ongoing inflammation process linked to the immune response of the individual.
We have monitored directly on brain sections containing Ndi1 the recruitment of immunological cells such as microglia and T-cells. Immune cells were only found in the area immediately surrounding the mechanical damages resulting from the needle injection (unpublished data). The humoral immune response was also recorded after Ndi1 expression into the brain. None of the serums of injected animal were positive for the presence of antibodies directly raised against the yeast protein.
The apparent absence of immune response to the Ndi1 was not because this yeast protein was not immunogenic in rodents. In fact, injection of purified Ndi1 protein into the blood stream gave rise to a high amount of Ndi1-specific antibodies in the serum. This paradigm could be explained by the fact that our protocol involves the injection of the gene and not the protein itself. The protein once expressed in the cell is, in fine, confined in the mitochondria. This implies a multiple layer of membranes segregating Ndi1 from the immune system. The fact that the yeast Ndi1 could be detected more than a year (1 year 8 months) after a single injection of rAAV-NDI1 indicates that the yeast protein is stably integrated into the mammalian mitochondrial physiology and it does not elicit any immune response which would have degraded the expressing cells. Moreover on those tissues, in situ hybridization experiments clearly showed a high content of Ndi1 mRNA indicating a stable transgene DNA sequence (unpublished data).
The mammalian mitochondria proved to be a good receptacle for the foreign protein Ndi1. The apparent peaceful and functional integration of the yeast protein within the respiratory chain brings a satisfactory alternative to diminish or to stop the development of the symptoms linked to PD and raises hopes for patients affected by this terrible disease.
Acknowledgments
We thank Dr. Jennifer Barber-Singh for her work described in the review. We are also grateful to all current and past colleagues involved in our project. This work was supported by NIH R01DK053244 and NIH R01NS048441 (to T.Y. and A.M.-Y.).
Contributor Information
Mathieu Marella, Email: marellam@scripps.edu, Department of Molecular and Experimental Medicine, The Scripps Research Institute, La Jolla, CA 92037, USA.
Byoung Boo Seo, Department of Molecular and Experimental Medicine, The Scripps Research Institute, La Jolla, CA 92037, USA.
Takao Yagi, Department of Molecular and Experimental Medicine, The Scripps Research Institute, La Jolla, CA 92037, USA.
Akemi Matsuno-Yagi, Email: ayagi@scripps.edu, Department of Molecular and Experimental Medicine, The Scripps Research Institute, La Jolla, CA 92037, USA.
References
- Barber-Singh J, Seo BB, Nakamaru-Ogiso E, Lau YS, Matsuno-Yagi A, Yagi T. [(August 5, 2009)];Rejuvenation Res. 2009 doi: 10.1089/rej.2009.0854. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessis N, GarciaCozar FJ, Boissier MC. Gene Ther. 2004;11 Suppl 1:S10–S17. doi: 10.1038/sj.gt.3302364. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Bulpitt CJ, Shaw K, Clifton P, Stern G, Davies JB, Reid JL. Clin Neuropharmacol. 1985;8:175–183. doi: 10.1097/00002826-198506000-00007. [DOI] [PubMed] [Google Scholar]
- Canet-Aviles RM, Wilson MA, Miller DW, Ahmad R, McLendon C, Bandyopadhyay S, Baptista MJ, Ringe D, Petsko GA, Cookson MR. Proc Natl Acad Sci USA. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WS, Kruse SE, Palmiter RD, Xia Z. Proc Natl Acad Sci USA. 2008;105:15136–15141. doi: 10.1073/pnas.0807581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Khondiker M, Hollerhage M, Muriel MP, Champy P, Bach A, Depienne C, Respondek G, Yamada ES, Lannuzel A, Yagi T, Hirsch EC, Oertel WH, Jacob R, Michel PP, Ruberg M, Hoglinger GU. J Neurosci. 2007;27:7827–7837. doi: 10.1523/JNEUROSCI.1644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu M, Cooper JM, Taanman JW, Schapira AH. Ann Neurol. 1998;44:177–186. doi: 10.1002/ana.410440207. [DOI] [PubMed] [Google Scholar]
- Haas RH, Nasirian F, Nakano K, Ward D, Pay M, Hill R, Shults CW. Ann Neurol. 1995;37:714–722. doi: 10.1002/ana.410370604. [DOI] [PubMed] [Google Scholar]
- Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, During MJ. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- Keeney PM, Xie J, Capaldi RA, Bennett JP., Jr J Neurosci. 2006;26:5256–5264. doi: 10.1523/JNEUROSCI.0984-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnareva YE, Murphy AN, Andreyev AY. Biochem J. 2002;368:545–553. doi: 10.1042/BJ20021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston JW, Ballard PA., Jr N Engl J Med. 1983;309:310. doi: 10.1056/nejm198308043090511. [DOI] [PubMed] [Google Scholar]
- Marella M, Seo BB, Matsuno-Yagi A, Yagi T. J Biol Chem. 2007;282:24146–24156. doi: 10.1074/jbc.M701819200. [DOI] [PubMed] [Google Scholar]
- Marella M, Seo BB, Nakamaru-Ogiso E, Greenamyre JT, Matsuno-Yagi A, Yagi T. PLoS ONE. 2008;3:e1433. doi: 10.1371/journal.pone.0001433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno Y, Saitoh T, Sone N. Biochem Biophys Res Commun. 1987;143:294–299. doi: 10.1016/0006-291x(87)90664-4. [DOI] [PubMed] [Google Scholar]
- Nicklas WJ, Vyas I, Heikkila RE. Life Sci. 1985;36:2503–2508. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Tatton WG. Annu Rev Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- Palacino JJ, Sagi D, Goldberg MS, Krauss S, Motz C, Wacker M, Klose J, Shen J. J Biol Chem. 2004;279:18614–18622. doi: 10.1074/jbc.M401135200. [DOI] [PubMed] [Google Scholar]
- Ramsay RR, Singer TP. J Biol Chem. 1986;261:7585–7587. [PubMed] [Google Scholar]
- Richardson JR, Claudle WM, Guillot TS, Watson JL, Nakamaru-Ogiso E, Seo BB, Sherer TB, Greenamyre JT, Yagi T, Matsuno-Yagi A, Miller GW. Toxicol Sci. 2007;95:196–204. doi: 10.1093/toxsci/kfl133. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Mann VM, Cooper JM, Dexter D, Daniel SE, Jenner P, Clark JB, Marsden CD. J Neurochem. 1990;55:2142–2145. doi: 10.1111/j.1471-4159.1990.tb05809.x. [DOI] [PubMed] [Google Scholar]
- Seo BB, Nakamaru-Ogiso E, Flotte TR, Yagi T, Matsuno-Yagi A. Mol Ther. 2002;6:336–341. doi: 10.1006/mthe.2002.0674. [DOI] [PubMed] [Google Scholar]
- Seo BB, Marella M, Yagi T, Matsuno-Yagi A. FEBS Lett. 2006a;580:6105–6108. doi: 10.1016/j.febslet.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Seo BB, Nakamaru-Ogiso E, Flotte TR, Matsuno-Yagi A, Yagi T. J Biol Chem. 2006b;281:14250–14255. doi: 10.1074/jbc.M600922200. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Richardson JR, Testa CM, Seo BB, Panov AV, Yagi T, Matsuno-Yagi A, Miller GW, Greenamyre JT. J Neurochem. 2007;100:1469–1479. doi: 10.1111/j.1471-4159.2006.04333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiruchelvam M, Richfield EK, Baggs RB, Tank AW, Cory-Slechta DA. J Neurosci. 2000;20:9207–9214. doi: 10.1523/JNEUROSCI.20-24-09207.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- Vila M, Przedborski S. Nat Rev Neurosci. 2003;4:365–375. doi: 10.1038/nrn1100. [DOI] [PubMed] [Google Scholar]
- Yagi T, Seo BB, Nakamaru-Ogiso E, Marella M, Barber-Singh J, Yamashita T, Kao MC, Matsuno-Yagi A. Rejuvenation Res. 2006;9:191–197. doi: 10.1089/rej.2006.9.191. [DOI] [PubMed] [Google Scholar]