Effects of intensive glucose lowering in the management of patients with type 2 diabetes mellitus in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial (original) (raw)
. Author manuscript; available in PMC: 2011 Aug 24.
The ACCORD Trial was designed to test whether treatment targeting nearly normal glycemic control reduces the risk of cardiovascular (CV) events in type 2 diabetes. This aim was based on consistent epidemiologic evidence that higher glucose and HbA1c (A1c) levels are associated with greater CV risk, together with inconclusive results from smaller and shorter interventional studies.1 All 10,251 participants in ACCORD were randomized to either a standard treatment strategy which targeted A1c levels between 7.0 and 7.9%, or an intensive strategy which sought to attain A1c <6.0%. With each strategy investigators could prescribe any anti-hyperglycemic agent approved by regulatory authorities. Median A1c with the standard strategy was 7.5%; the intensive strategy achieved median A1c 6.4%.2 However, the intensive strategy was stopped after a median follow-up of 3.4 years, about 60% of that planned, due to 22% higher all-cause mortality.2 Participants previously using the intensive strategy were switched to the standard strategy and continued in the trial. The CV and microvascular effects after the full 5 years of follow-up will soon be reported. Meanwhile, debate continues on why there was higher mortality with intensive treatment, and the clinical implications. Several analyses of the data obtained during randomized treatment have shed light on these issues.
Baseline factors associated with risk during intensive treatment
As expected, various factors such as age and presence of co-morbidities were associated with higher risk of death during randomized treatment.3 After adjustment for these, three baseline characteristics emerged as independent predictors of excess risk with the intensive strategy. Notably, a baseline A1c value higher than 8.5% predicted 64% higher risk. History of taking aspirin (perhaps indicating perceived high CV risk) and self-report of having neuropathy (presumably reflecting microvascular injury) were also independent predictors.
Hypotheses regarding post-randomization factors
Proposed causes of excess mortality during use of the intensive strategy include hypoglycemia, rapid reduction of glucose or maintenance of near-normal levels, effects of drugs or drug-combinations, and weight-gain. Hypoglycemia has attracted the most suspicion. The incidence of first occurrence of hypoglycemic events requiring medical assistance was greater (3.14% yearly) in the intensive group than in the standard treatment group (1.03%).4 However, as an adjudicated cause of death hypoglycemia was uncommon in both groups (8% possible or probable with standard treatment, 11% with intensive), and considered a definite contributor to just a single death in the intensive group.5 In general, the risk of death was higher among individuals who had at least one episode of hypoglycemia requiring medical assistance (hazard ratio 2.87 with standard treatment, 1.28 with intensive treatment).5 However, among those with a prior episode of severe hypoglycemia, the individuals in the intensive group had lower risk of later death than those in the standard group (hazard ratio 0.55, 95% confidence interval 0.31-0.99).5 With both treatment strategies hypoglycemia requiring medical assistance was more frequent when A1c values were high than when they were low.4 Finally, hypoglycemia requiring medical assistance was more likely when participants in the intensive group achieved little reduction of A1c in the first 4 months of randomized treatment, and less likely with larger early reductions.4
The analyses of relationships between A1c and mortality have also produced surprising results. The well-known epidemiologic relationship between glucose levels and greater risk of mortality has been confirmed in the whole ACCORD population.6 With 1% higher average A1c during randomized treatment the risk of death was about 20% greater. This presents a paradox: greater risk with higher A1c, yet higher mortality in the intensive group which was seeking and generally achieved lower A1c. The paradox has been (at least partly) resolved by analyses showing markedly different relationships between A1c and mortality with the two treatment strategies.6 (Figure 1) With the intensive strategy, the lowest risk of death was associated with lower levels of average A1c. As average A1c increased from 6 to 9%, mortality risk increased steadily. The minority subgroup of individuals in the intensive group who had average A1c above 7% accounted for the excess of risk accompanying that treatment regimen. This interpretation was strengthened by another analysis showing higher risk in the intensive group was associated with little reduction of A1c from baseline in the first 4 months or the first 12 months of treatment.
Figure 1.
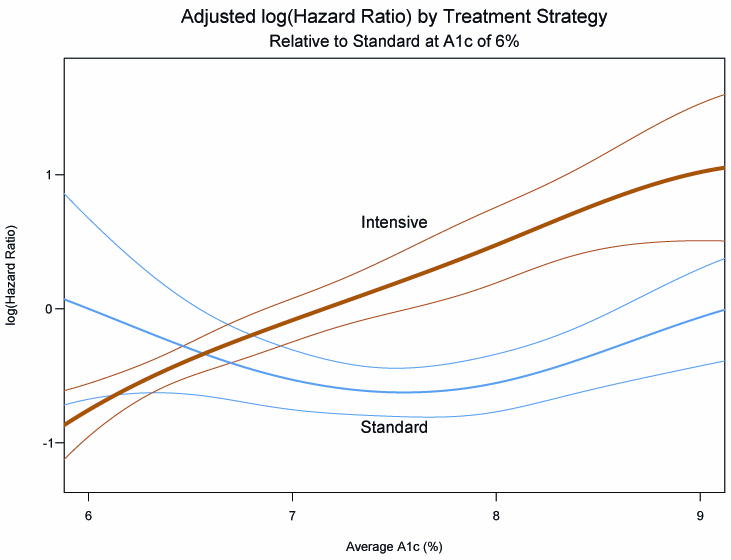
Curves showing the risk of all-cause mortality in the ACCORD population by assigned treatment group, over a wide range of average on-treatment A1c values.6 The fine lines show 95% confidence intervals for each group. Excess risk among participants in the intensively treated group occurs above A1c 7%.
Conclusions from current data and remaining questions
To summarize, analyses from the ACCORD glycemia study confirm that higher levels of A1c generally predict higher risk of mortality in a population of type 2 diabetic persons selected for having high CV risk. They also show that an intensive glucose-lowering strategy, using treatment methods available at the time of the study, caused in the first 3 years a 22% increase of deaths. This adverse outcome was associated with high A1c levels at baseline, and it occurred especially among individuals who attempted the intensive strategy but failed to reduce A1c much from their baseline levels and continued to have A1c levels higher than 7% while using this strategy. A contribution from severe hypoglycemia in the intensively treated group has not been confirmed.
These findings are helpful, but many questions remain. When evidence on the effects of intensive treatment on nephropathy, retinopathy, cognition, and various non-fatal CV endpoints after 5 years of follow-up becomes available, the short-term risk of death can better be weighed against these potential longer-term benefits. Still, it is worrisome that the underlying cause of excess mortality with intensive treatment remains unknown. Effects of specific drugs or of weight-gain certainly might be involved, and require more study. Also, despite lack of clear evidence for a causal role of hypoglycemia, there are reasons not to discard this hypothesis entirely. Hypoglycemia can cause cardiac ischemia or arrhythmia,7 plausibly mediated by secretion of catecholamines. In the intensively treated group in ACCORD, higher risk of severe hypoglycemia and higher risk of death both were associated with average A1c remaining above 7%. An association of greater risk of severe hypoglycemia with higher A1c has been reported in other studies,8,9 and might reflect either physiologic or behavioral characteristics of subgroups of patients. Finally, among participants who had at least one severe hypoglycemic event, minor hypoglycemia (which was more frequent with intensive treatment) was associated with lower risk of subsequent death.5 Repeated minor hypoglycemia has been reported to be protective against brain injury from subsequent severe hypoglycemia.10 Might “hypoglycemic preconditioning”, although associated with increased risk of severe hypoglycemia, protect against CV death resulting from severe hypoglycemia by reducing the catecholamine response?11 If so, were the individuals with A1c above 7% using the intensive strategy at higher risk of death accompanying a severe hypoglycemic event not preceded by milder hypoglycemia? Due to the limitations of post hoc analyses these complex questions may not be resolved from the ACCORD data, but they are worth asking because of their clinical implications.
Practical implications
The ACCORD results pose specific questions for physicians in clinical practice. How can we identify those individuals with type 2 diabetes who may be at risk if they attempt an intensive glycemic treatment strategy? A1c above 8.5% on prior therapy appears to be one predictor of risk. A limited A1c-lowering response in the first 4 to 12 months of treatment may be another indicator, but a more objective way of defining this is needed. Moreover, the idea that a single A1c target is appropriate for all persons with type 2 diabetes is being reexamined. The ACCORD results suggest that in this case one size does not fit all, at least with currently available therapies. Seeking A1c 7% or less for healthy patients with shorter duration of diabetes still seems appropriate, but for patients with long duration of diabetes and established complications, and perhaps other risk factors, a higher target range might be defined. On this point, both further data and systematic review by advisory groups are needed.
Footnotes
References
- 1.Gerstein HC, Riddle MC, Kendall DM, Cohen RM, Goland R, Feinglos MN, Kirk JK, Hamilton BP, Ismail-Beigi F, Feeney P for the ACCORD Study Group. Glycemia treatment strategies in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial. Am J Cardiol. 2007;99(Suppl 12A):34i–43i. doi: 10.1016/j.amjcard.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 2.The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calles-Escandon J, Lovato LC, Simons-Morton DG, Kendall DM, Pop-Busui R, Cohen RM, Bonds DE, Fonseca VA, Ismail-Beigi F, Banerji MA, Failor A, Hamilton B. Effect of intensive compared with standard glycemia treatment strategies on mortality by baseline subgroup characteristics: The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010;33:721–727. doi: 10.2337/dc09-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller ME, Bonds DE, Gerstein HC, Seaquist ER, Bergenstal RM, Calles-Escandon J, Childress RD, Craven TE, Cuddihy RM, Dailey G, Feinglos MN, Ismail-Beigi F, Largay JF, O'Connor PJ, Paul T, Savage PJ, Schubart UK, Sood A, Genuth S for the ACCORD investigators. The effects of baseline characteristics, glycaemia treatment approach, and glycated hemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444. doi: 10.1136/bmj.b5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonds DE, Miller ME, Bergenstal RM, Buse JB, Byington RP, Cutler JA, Dudl RJ, Ismail-Beigi F, Kimel AR, Hoogwerf B, Horowitz KR, Savage PJ, Seaquist ER, Simmons DL, Sivitz WI, Sperl-Hillen JM, Sweeney ME. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiologic analysis of the ACCORD study. BMJ. 2010;430:b4909. doi: 10.1136/bmj.b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riddle MC, Ambrosius WT, Brillon DJ, Buse JB, Byington RP, Cohen RM, Goff DC, Jr, Malozowski S, Margolis KL, Probstfield JL, Schnall A, Seaquist ER for the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Investigators. Diabetes Care. 2010;33:983–990. doi: 10.2337/dc09-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desouza C, Salazar H, Cheong B, Murgo J, Fonseca V. Association of hypoglycemia and cardiac ischemia. A study based on continuous monitoring. Diabetes Care. 2003;26:1485–1489. doi: 10.2337/diacare.26.5.1485. [DOI] [PubMed] [Google Scholar]
- 8.Davis TME, Brown SGA, Jacobs IG, Bulsara M, Bruce DG, Davis WA. Determinants of severe hypoglycemia complicating type 2 diabetes: The Freemantle Study. J Clin Endocrinol Metab. 2010;95:2240–2247. doi: 10.1210/jc.2009-2828. [DOI] [PubMed] [Google Scholar]
- 9.Leese GP, Wang J, Broomhill J, Kelly P, Marsden A, Morrison W, Frier BM, Morris AD. Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: a population-based study of health service resource use. Diabetes Care. 2003;26:1176–1180. doi: 10.2337/diacare.26.4.1176. [DOI] [PubMed] [Google Scholar]
- 10.Puente EC, Silverstein J, Bree AJ, Musikantow DR, Wozniak DF, Maloney S, Daphna-Iken D, Fisher SJ. Recurrent moderate hypoglycemia ameliorates brain damage and cognitive dysfunction induced by severe hypoglycemia. Diabetes. 2010;59:1055–1062. doi: 10.2337/db09-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegel SA, Paramore DS, Cryer PE. Hypoglycemia-associated autonomic failure in advanced type 2 diabetes. Diabetes. 2002;51:724–733. doi: 10.2337/diabetes.51.3.724. [DOI] [PubMed] [Google Scholar]