Molecular Classification of Low-Grade Diffuse Gliomas (original) (raw)
Abstract
The current World Health Organization classification recognizes three histological types of grade II low-grade diffuse glioma (diffuse astrocytoma, oligoastrocytoma, and oligodendroglioma). However, the diagnostic criteria, in particular for oligoastrocytoma, are highly subjective. The aim of our study was to establish genetic profiles for diffuse gliomas and to estimate their predictive impact. In this study, we screened 360 World Health Organization grade II gliomas for mutations in the IDH1, IDH2, and TP53 genes and for 1p/19q loss and correlated these with clinical outcome. Most tumors (86%) were characterized genetically by TP53 mutation plus IDH1/2 mutation (32%), 1p/19q loss plus IDH1/2 mutation (37%), or IDH1/2 mutation only (17%). TP53 mutations only or 1p/19q loss only was rare (2 and 3%, respectively). The median survival of patients with TP53 mutation ± IDH1/2 mutation was significantly shorter than that of patients with 1p/19q loss ± IDH1/2 mutation (51.8 months vs. 58.7 months, respectively; P = 0.0037). Multivariate analysis with adjustment for age and treatment confirmed these results (P = 0.0087) and also revealed that TP53 mutation is a significant prognostic marker for shorter survival (P = 0.0005) and 1p/19q loss for longer survival (P = 0.0002), while IDH1/2 mutations are not prognostic (P = 0.8737). The molecular classification on the basis of IDH1/2 mutation, TP53 mutation, and 1p/19q loss has power similar to histological classification and avoids the ambiguity inherent to the diagnosis of oligoastrocytoma.
According to the 2007 World Health Organization (WHO) classification,1 astrocytic and oligodendroglial gliomas (WHO grade II) are currently divided into three histological types, that is, diffuse astrocytoma, oligoastrocytomas, and oligodendroglioma. Diffuse astrocytoma is a well-differentiated and slowly growing tumor, but shows a consistent tendency to recur after surgical resection, and this is often associated with progression to a higher grade of malignancy, that is, anaplastic astrocytoma (WHO grade III) and eventually secondary glioblastoma (WHO grade IV).1,2 Oligodendrogliomas also diffusely infiltrate the brain parenchyma, but malignant progression to anaplastic oligodendroglioma (WHO grade III) is inconsistent.1 Oligoastrocytoma is composed of a conspicuous mixture of two distinct neoplastic cell types morphologically resembling oligodendroglioma and diffuse astrocytoma.1
Genetically, low-grade diffuse gliomas contain IDH1 mutations in 70–80% of cases.3,4,5 In addition, diffuse astrocytomas frequently carry TP53 mutations (∼60% of cases), whereas oligodendrogliomas typically show loss of 1p/19q (∼70%).6,7,8,9 Oligoastrocytomas carry either TP53 mutations (∼40%) or loss of 1p/19q (∼45%).1,7 These alterations are mutually exclusive,7,8,10,11 indicating that oligoastrocytomas are genetically monoclonal, and carry genetic alterations similar to either diffuse astrocytomas or oligodendrogliomas.
Histological criteria for the diagnosis of low-grade diffuse gliomas are well established1 but somewhat subjective. In particular, the histological typing of oligoastrocytomas is often difficult, with marked interobserver variability.1,8 Because the vast majority of WHO grade II gliomas are genetically characterized by the presence of an IDH1 mutation plus TP53 mutation or of an IDH1 mutation plus 1p/19q loss, a molecular classification may eventually replace the histological classification.
The present study provides a quantitative basis for the molecular classification of low-grade diffuse gliomas by combining a large number of cases from different institutions. The specific aims are to determine the frequency of various combinations of genetic alterations and to assess whether molecular classification using IDH1 mutations, TP53 mutations, and 1p/19q loss provide reliable outcome data.
Materials and Methods
Tumor Samples and Histology Review
Four hundred forty-three samples of low-grade diffuse glioma of WHO grade II from adults (diffuse astrocytomas, oligoastrocytomas, oligodendrogliomas; patients’ age, ≥20 years) from 400 patients were obtained from the Department of Neuropathology, University Hospital Zurich, Switzerland; the Department of Neuropathology, University Hospital Frankfurt, Germany; the Departments of Neuropathology and Neurosurgery, University Hospital Essen, Germany; the Department of Pathology, Gunma University, Japan; the Institute of Neuropathology and Department of Neurosurgery, University Hospital Munster, Germany; the Institute of Neuroscience, Bordeaux, France; and the Department of Neurosurgery, University Hospital Bern, Switzerland. Of these, 39 patients had two or three biopsies for low-grade diffuse gliomas. This study was performed after the approval of the International Agency for Research on Cancer Ethics Committee.
Histology review was carried out by two neuropathologists (P.K., U.De.G.) according to the 2007 WHO Classification of Tumors of the Central Nervous System,1 and 30 tumors were excluded since they were reclassified as anaplastic astrocytomas WHO grade III (10 tumors), anaplastic oligodendrogliomas WHO grade III (four tumors), anaplastic glioma not otherwise specified WHO grade III (three tumors), glioblastoma WHO grade IV (one tumor), dysembryoplastic neuroepithelial tumor (one tumor), infiltrating zone of glioma (three cases), or low-grade diffuse glioma not otherwise specified, that is, unable to reliably assess astrocytic or oligodendroglial differentiation, due to small tumor areas available on the histological sections (eight cases). Thus, after the histology review, a total of 413 tumors were confirmed as low-grade diffuse gliomas WHO grade II (206 diffuse astrocytomas, 73 oligoastrocytomas, and 134 oligodendrogliomas). After the histology review, the diagnosis remained the same for the vast majority (177 of 225; 79%) of tumors originally diagnosed as diffuse astrocytomas and the vast majority (91 of 115; 79%) of tumors originally diagnosed as oligodendrogliomas, while the diagnosis remained unchanged for fewer than half (47 of 104; 45%) of tumors originally diagnosed as oligoastrocytomas.
DNA was extracted from paraffin-embedded sections, as described previously.12 DNA quality was sufficient for genetic analyses for 360 cases (174 diffuse astrocytomas, 64 oligoastrocytomas, and 122 oligodendrogliomas). For 287 (80%) of these patients, clinical data including age and sex, location of tumors, histological diagnosis, date of surgical resection, extent of surgery, other treatment (radiotherapy, chemotherapy), date of last follow-up or last contact, and date of death were available.
Mutations of the IDH1 and IDH2 Genes
Single-strand conformational polymorphism analysis was carried out to prescreen for mutations in exon 4 of the IDH1 gene, as described previously,3 Samples that gave negative results were then investigated for mutations of exon 4 of the IDH2 gene, as reported previously.13
TP53 Mutations
Prescreening for mutations in exons 5–8 of the TP53 gene by single-strand conformational polymorphism analysis was conducted as described previously.14 Samples that gave negative results were then investigated for mutations in exons 4, 9, and 10 using the following primers: 5′-CTGGTC CTCTGACTGCTCTTT-3′ (sense) and 5′-TGGCATTCTGGGAGCTTCAT-3′ (antisense), 5′-GTCCAGATGAAGCTCCCAGA-3′ (sense) and 5′-TTCTGGGAAGGGACAGAAGA-3′ (antisense), 5′-TCTTCTGTCCCTTCCCAGAA-3′ (sense) and 5′-AACTGACCGTGCAAGTCACA-3′ (antisense) for exon 4; 5′-CCTTTCCTTGCCTCTTTCCT-3′ (sense) and 5′-CCACTTGATAAGAGGTCCCAAG-3′ (antisense) for exon 9; and 5′-TCCTCTGTTGCTGCAGATCC-3′ (sense) and 5′-AAGGGGCTGAGGTCACTCAC-3′ (antisense) for exon 10. Samples exhibiting mobility shifts in single-strand conformational polymorphism analyses were subsequently analyzed by direct sequencing on a ABI 3100 PRISM DNA sequencer (Applied Biosystems, Foster City, CA) with the Big Dye Terminator cycle sequencing kit (ABI PRISM; Applied Biosystems).
Loss of 1p/19q
Loss of 1p/19q was assessed using three microsatellite markers (D1S214, D1S468, and D1S2736) on chromosome 1p, and three markers (D19S408, D19S596, and D19S867) on chromosome 19q as described previously.15
Statistical Analyses
The Student’s _t_-test was performed for comparison of the mean age of the patients. The Kaplan-Meier method and the log-rank test were used for survival analysis. Cox regression models were used to assess the effect of different combination of genetic alterations on the survival of patients after adjusting with patients’ age, sex, and treatment (surgery and/or radiotherapy). For survival analyses for patients with multiple biopsies, the date of the first biopsy was used. Patients who received chemotherapy and those who had only stereotactic biopsy were excluded from survival analyses. Patients who died within 4 months after surgery were also excluded from survival analyses to avoid the inclusion of cases in which death may have been attributable to surgical complications.
Results
IDH1 and IDH2 Mutations
IDH1 mutations were observed in 305 of 360 (85%) of all diffuse gliomas analyzed. IDH1 mutations were similarly frequent in all diffuse astrocytomas (85%), oligoastrocytomas (88%), and oligodendrogliomas (83%). The most frequent IDH1 mutations were R132H (95.7%), followed by R132C (2.6%), R132G (1%), R132S (0.3%), and R132V (0.3%).
Tumors that did not contain an IDH1 mutation (55 cases; 15%) were further screened for IDH2 mutations. Ten tumors (three diffuse astrocytomas, one oligoastrocytomas, and six oligodendrogliomas) carried an IDH2 mutation. The most frequent IDH2 mutation was R172K (six mutations); the others detected in one case each were R172M, R172W, R172S, and R172T (not previously reported).
Because the mean age and survival of patients with IDH1 and those with IDH2 mutations were similar (P = 0.2520 and P = 0.6904, respectively), we combined IDH1 and IDH2 mutations for further analyses of combinations of genetic alterations, age distribution, and survival.
TP53 Mutations
One hundred forty-eight miscoding TP53 mutations were detected in 128 of 360 (36%) of all low-grade diffuse gliomas analyzed, and in 53% of diffuse astrocytomas, 39% of oligoastrocytomas and 8% of oligodendrogliomas. Frequent mutations were G:C→A:T at CpG sites, (62 mutations, 42%) followed by G:C→A:T at non-CpG sites (23 mutations; 16%) and A:T→G:C (20 mutations; 14%). There was no significant difference in distribution and type of TP53 mutations between different histological types.
Loss of 1p/19q
Loss of 1p/19q was observed in 152 of 360 (42%) of all low-grade diffuse gliomas analyzed, and in 17% of diffuse astrocytomas, 44% of oligoastrocytomas, and 78% of oligodendrogliomas. In most oligodendrogliomas (67%), 1p and 19q were codeleted, whereas 1p loss only and 19q loss only were rare (7 and 4%). In oligoastrocytomas, 1p and 19q codeletion was detected in 27% of cases; 1p loss only and 19q loss only were infrequent (14 and 3%).
Frequency of Different Combinations of Genetic Alterations
The majority (69%) of low-grade diffuse gliomas were genetically characterized by either 1p/19q loss plus IDH1/2 mutation (133 cases; 37%) or TP53 mutation plus IDH1/2 mutation (115 cases; 32%; Table 1). Sixty-one cases (17%) had IDH1/2 mutations only. No alterations in IDH1/2, TP53, or 1p/19q were observed in 7% of cases. Tumors with a TP53 mutation only (2%) and those with 1p/19q loss only (3%) were rare (Table 1).
Table 1.
Genetic Alterations in Low-Grade Diffuse Gliomas (WHO Grade II)
| Genetic alteration | Diffuse astrocytoma (n = 174) | Oligoastrocytoma (n = 64) | Oligodendroglioma (n = 122) | Total (n = 360) |
|---|---|---|---|---|
| TP53 mutation + IDH1/2 mutation | 85 (49%) | 21 (33%) | 9 (7%) | 115 (32%) |
| TP53 mutation only | 5 (3%) | 1 (2%) | 0 | 6 (2%) |
| 1p/19q loss + IDH1/2 mutation | 23 (13%) | 22 (34%) | 88 (73%) | 133 (37%) |
| 1p/19q loss only | 3 (2%) | 3 (5%) | 6 (4%) | 12 (3%) |
| IDH1/2 mutation only | 40 (23%) | 12 (19%) | 9 (7%) | 61 (17%) |
| Others* | 3 (2%) | 3 (5%) | 1 (1%) | 7 (2%) |
| No alterations | 15 (9%) | 2 (3%) | 9 (7%) | 26 (7%) |
The majority of tumors with TP53 mutation ± IDH1/2 mutation (74%) and those with IDH1/2 mutation only (66%) were histologically diagnosed as diffuse astrocytomas, whereas most tumors with 1p/19q loss ± IDH1/2 mutation (65%) were histologically diagnosed as oligodendrogliomas (Table 2).
Table 2.
Overlap between Genotype and Histologic Types of Low-Grade Diffuse Gliomas (WHO Grade II)
| Genotype | Histologically diagnosed as | ||
|---|---|---|---|
| Diffuse astrocytoma | Oligoastrocytoma | Oligodendroglioma | |
| TP53 mutation ± IDH1/2 mutation (n = 121) | 90 (74%) | 22 (18%) | 9 (7%) |
| 1p/19q loss ± IDH1/2 mutation (n = 145) | 26 (18%) | 25 (17%) | 94 (65%) |
| IDH1/2 mutation only (n = 61) | 40 (66%) | 12 (20%) | 9 (15%) |
| No genetic alteration (n = 26) | 15 (58%) | 2 (8%) | 9 (35%) |
Timing of Genetic Alterations
Of 39 patients with two or three biopsies, most had the same combinations of genetic alterations in first and last biopsies. Exceptions were two patients who had an IDH1 mutation in the first biopsy and TP53 mutation plus IDH1 mutation in the last biopsy, one patient who had an IDH1 mutation in the first biopsy and 1p/19q loss plus IDH1 mutation in the last biopsy, and one patient who had an IDH1 mutation in the first biopsy and 19q loss plus IDH1 mutation in the last biopsy.
Age Distribution
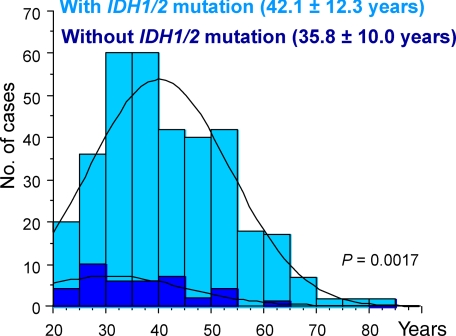
The mean age of patients with IDH1/2 mutations was significantly older than that of patients without IDH1/2 mutations (mean, 42.1 versus 35.8 years; P = 0.0017; Figure 1). This remained true when each histological type was analyzed separately, but the difference was statistically significant only for patients with diffuse astrocytoma.
Figure 1.
Age distribution of patients with and without IDH1/2 mutations in low-grade diffuse gliomas. Note that low-grade diffuse gliomas without IDH1/2 mutations develop in younger patients (mean, 42.1 versus 35.8 years; P = 0.0017).
Clinical Outcome
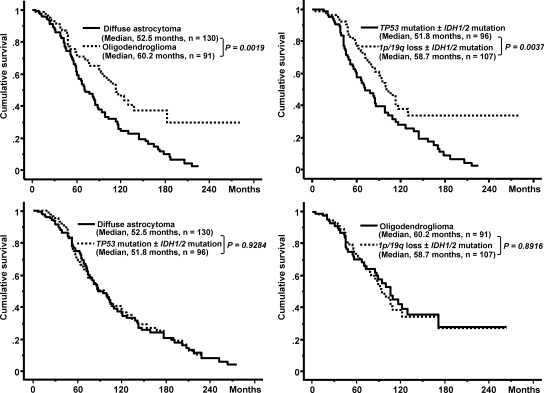
The median survival of patients with tumors histologically classified as oligodendroglioma (60.2 months) was significantly longer than that of patients with diffuse astrocytoma (52.5 months; P = 0.0019; Figure 2).
Figure 2.
Upper panel: The survival of patients with diffuse astrocytoma is significantly shorter than that of patients with oligodendroglioma (left). This difference was also clearly observed when tumors were classified genetically (right). Lower panel: Patients with diffuse astrocytoma and those with low-grade diffuse glioma carrying TP53 mutation + IDH1/2 mutation have very similar survival (left). Patients with oligodendroglioma and those with low-grade diffuse glioma carrying 1p/19q loss + IDH1/2 mutation have very similar survival (right).
The median survival of patients with 1p/19q loss ± IDH1/2 mutation was significantly longer than that of patients with TP53 mutation ± IDH1/2 mutation (58.7 versus 51.8 months; P = 0.0037; Figure 2). The median survival of patients with diffuse astrocytomas was similar to that of patients with low-grade diffuse glioma with TP53 mutation ± IDH1/2 mutation (P = 0.9284; Figure 2); those of oligodendrogliomas were similar to that of patients with low-grade diffuse glioma with 1p/19q loss ± IDH1/2 mutation (P = 0.8916; Figure 2).
Multivariate analysis with adjustment for age, sex, and treatment confirmed these results (P = 0.0087) and also revealed that TP53 mutation is significantly prognostic for shorter survival and 1p/19q loss for longer survival: IDH1/2 mutations were not prognostic for patient outcome (Table 3). With respect to clinical outcome, the molecular classification based on IDH1/2 mutation, TP53 mutation, and 1p/19q loss has a power similar to that of histological classification in low-grade diffuse gliomas (Table 3).
Table 3.
Multivariate Analysis* of Survival of Patients with Low-Grade Diffuse Glioma (WHO Grade II)
| Variable | Number of cases | Hazard ratio (95% confidence interval) | P value |
|---|---|---|---|
| Genetic alterations | |||
| TP53 mutation ± IDH1/2 mutation | 96 | 1 | |
| 1/19q loss ± IDH1/2 mutation | 107 | 0.563 (0.367–0.865) | P = 0.0087 |
| IDH1/2 mutation only | 42 | 0.778 (0.486–1.245) | P = 0.2955 |
| No alteration | 23 | 0.910 (0.474–1.749) | P = 0.7773 |
| TP53 mutation + | 111 | 1 | |
| TP53 mutation − | 157 | 0.518 (0.358–0.751) | P = 0.0005 |
| 1p/19q loss + | 112 | 1 | |
| 1p/19q loss − | 156 | 2.170 (1.454–3.240) | P = 0.0002 |
| IDH1/2 mutation + | 239 | 1 | |
| IDH1/2 mutation − | 29 | 1.047 (0.593–1.850) | P = 0.8737 |
| Histology | |||
| Diffuse astrocytoma | 130 | 1 | |
| Oligodendroglioma | 91 | 0.556 (0.369–0.837) | P = 0.0049 |
| Oligoastrocytoma | 47 | 0.704 (0.442–1.121) | P = 0.1389 |
Discussion
Using a large collection of cases, we show that the vast majority (93%) of low-grade diffuse gliomas carry at least one of the following genetic alterations: IDH1 mutation, IDH2 mutation, TP53 mutation, and 1p/19q loss. Common combinations of genetic alterations were 1p/19q loss plus IDH1/2 mutation (37%), TP53 mutation plus IDH1/2 (32%), and IDH1/2 mutation only (17%), whereas tumors with TP53 mutation only (2%) or 1p/19q loss only (3%) were rare (Table 1). These findings corroborate previous data showing that IDH1 mutations occur at a very early stage3 and are the most frequent genetic alterations in astrocytic and oligodendroglial low-grade diffuse gliomas.3,4,5 Only 26 of 360 cases (7%) of low-grade diffuse gliomas were triple negative, that is, without IDH1/2 mutation, TP53 mutation, or 1p/19q loss. This suggests the existence of alternative genetic pathways involving as yet unidentified genes that play a role in the pathogenesis of a small subset of low-grade diffuse gliomas.
The vast majority of tumors with TP53 mutation ± IDH1/2 mutation (74%) was histologically diagnosed as diffuse astrocytomas, whereas two-thirds of tumors with 1p/19q loss ± IDH1/2 mutation (65%) were oligodendrogliomas (Table 2). It is of interest to note that most tumors (66%) with IDH1/2 mutation only were diagnosed as astrocytomas; only 15% of cases being diagnosed as oligodendrogliomas (Table 2). This suggests that tumor cells with IDH1/2 mutations tend to have an astrocytic phenotype. In oligodendrogliomas, the additional loss of 1p/19q appears to be a driving force toward oligodendroglial differentiation. Tumors with the histological signature of oligodendroglioma (eg, honeycomb appearance of most neoplastic cells) show loss at 1p/19q in the vast majority of cases (>90%).8
IDH1/2 mutations were associated with younger age in patients with glioblastoma,4,13,16 anaplastic astrocytoma,4,5,17 and anaplastic oligoastrocytoma,5,17 but not anaplastic oligodendroglioma.5,17 In most previous studies, the age of patients with and without IDH1/2 mutations appeared to be similar for low-grade diffuse gliomas,5 oligoastrocytomas5 and oligodendrogliomas.3,5 Among the larger number of cases investigated in the present study, IDH1/2 mutations were significantly associated with older age in patients with low-grade diffuse gliomas (mean, 42.1 versus 35.8 years; P = 0.0017; Figure 1). Low-grade diffuse gliomas without IDH1 mutations are clustered in a younger age group (Figure 1).
It is well established that IDH1 mutations are a significant prognostic marker of favorable outcome in patients with glioblastoma4,13,18 and anaplastic glioma.4,19,20 The predictive role of IDH1 mutation in WHO grade II glioma remains to be established. One study in 49 patients with diffuse astrocytoma showed a significant association between the presence of IDH1 mutations and longer overall survival.21 In another study, Sanson et al20 assessed 404 gliomas of grades II–IV (100 being classified as WHO grade II). Univariate analysis showed that IDH1 mutations were prognostic for a more favorable outcome.20 Multivariate analyses of grades II–IV gliomas after adjusting for age, histological grade, treatment, and genetic alterations (including EGFR amplification, MGMT methylation and co-deletion of 1p/19q, but not TP53 mutation) confirmed IDH1 mutations as an independent prognostic marker for longer survival.20 In the present study, using a large number of cases (n = 360), the presence of IDH1/2 mutations was not prognostic for the survival of patients with low-grade glioma in univariate or multivariate analyses.
Loss of 1p/19q is a well-recognized predictive marker in oligodendrogliomas.22,23,24 In contrast, the prognostic value of TP53 mutations in low-grade gliomas has been controversial. In a study of 159 cases of grade II astrocytomas and oligoastrocytomas, Peraud et al25 reported that cumulative progression-free survival was significantly shorter for patients with tumors with TP53 mutation, but that there was no impact on overall survival. Ishii et al26 reported a tendency for shorter survival in patients (34 diffuse astrocytomas/oligoastrocytomas) with TP53 mutations, but the results were not statistically significant. Watanabe et al27 found that TP53 mutations were not significantly prognostic of survival of patients with diffuse astrocytomas (n = 46). We have previously reported in a population-based study that TP53 mutations are predictive of shorter survival of patients with low-grade diffuse gliomas (n = 122).7 The present study clearly indicates that TP53 mutation is a significant prognostic marker for shorter survival in patients with low-grade diffuse glioma.
Table 3 shows that, with respect to clinical outcome, the molecular classification based on IDH1/2 mutation, TP53 mutation, and 1p/19q loss has a power similar to that of histological classification in low-grade diffuse gliomas. Survival of low-grade diffuse glioma patients with 1p/19q loss ± IDH1/2 mutation was significantly longer than that of patients with TP53 mutation ± IDH1/2 mutation, the difference being very similar to that based on histological diagnosis (Figure 2). Despite the observation that IDH1/2 mutations are not prognostic, we recommend that IDH1/2 mutation be assessed, since this is the most reliable marker for a diffuse WHO grade II glioma and excludes other gliomas, including pilocytic astrocytomas, ependymomas, and non-neoplastic brain tissues.3,4,5,28 Screening for TP53 mutations and loss of 1p/19q is important, since these changes are markers of poor and more favorable outcome, respectively (Table 3).
A molecular classification of low-grade diffuse gliomas provides distinct advantages, especially for oligoastrocytomas. The WHO criteria for the histological diagnosis of oligoastrocytoma include the recognition of neoplastic glial cells with convincing astrocytic and oligodendroglial phenotypes,1 but cases with distinct tumor areas exhibiting oligodendroglial and astrocytic differentiation are rare. More commonly, oligoastrocytomas show an intimate mixture of both oligodendroglial and astrocytic tumor cells. There are also tumors composed of cells with phenotypical characteristics intermediate to oligodendroglial and astrocytic differentiation.1 The pronounced phenotypic heterogeneity of the astroglial and oligodendroglial cell lineages and a lack of reliable markers make it difficult to define diagnostic criteria.1 Because poor characterization of these cell lineages causes considerable subjectivity in histological evaluation, interobserver variability for oligoastrocytoma is significantly higher than for astrocytomas and oligodendrogliomas.29,30,31 This was also noted in the present study; only 45% of tumors diagnosed as oligoastrocytomas by the referring pathologist were confirmed by histological review. Despite their histological heterogeneity, oligoastrocytomas are genetically clonal neoplasms.1 One subset appears to be genetically related to diffuse astrocytomas, while another is genetically related to oligodendroglial tumors. The biological basis for the presence of two distinct glial phenotypes may be a common origin from precursor cells with an IDH1 mutation. This is also true for diffuse astrocytomas in which neoplastic cells resembling oligodendrogliomas cells may be encountered in small areas of the tumor, and oligodendrogliomas in which cells with astrocytic features may be present in some tumor areas. Thus, the available evidence suggests that oligoastrocytoma is unlikely to be a distinct entity. It is recommended that the Working Group of the next WHO Classification (fifth edition) re-evaluate this issue.
In conclusion, the molecular profile of low-grade diffuse gliomas based on IDH1/2 mutations, TP53 mutations and 1p/19q loss provides a more objective classification and correlates well with clinical outcome. It should complement and may eventually replace histological typing, particularly in clinical trials including oligoastrocytomas.
Footnotes
Address reprint requests to Hiroko Ohgaki, DVM, Ph.D., Section of Molecular Pathology, International Agency for Research on Cancer, 150 Cours Albert Thomas, 69372 Lyon Cedex 08, France. E-mail: ohgaki@iarc.fr.
Y.-H.K. and S.N. contributed equally to this study.
References
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. Lyon: IARC; WHO Classification of Tumours of the Central Nervous System. 2007:pp 1–306. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64:479–489. doi: 10.1093/jnen/64.6.479. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Nobusawa S, Kleihues P, Ohgaki H. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol. 2009;174:1149–1153. doi: 10.2353/ajpath.2009.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008;116:597–602. doi: 10.1007/s00401-008-0455-2. [DOI] [PubMed] [Google Scholar]
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto Y, Di Patre PL, Burkhard C, Horstmann S, Jourde B, Fahey M, Schuler D, Probst-Hensch NM, Yasargil MG, Yonekawa Y, Lutolf U, Kleihues P, Ohgaki H. Population-based study on incidence, survival rates, and genetic alterations of low-grade astrocytomas and oligodendrogliomas. Acta Neuropathol. 2004;108:49–56. doi: 10.1007/s00401-004-0861-z. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Nakamura M, Kros JM, Burkhard C, Yonekawa Y, Kleihues P, Ohgaki H. Phenotype versus genotype correlation in oligodendrogliomas and low-grade diffuse astrocytomas. Acta Neuropathol. 2002;103:267–275. doi: 10.1007/s004010100464. [DOI] [PubMed] [Google Scholar]
- Reifenberger G, Louis DN. Oligodendroglioma: toward molecular definitions in diagnostic neuro-oncology. J Neuropathol Exp Neurol. 2003;62:111–126. doi: 10.1093/jnen/62.2.111. [DOI] [PubMed] [Google Scholar]
- Maintz D, Fiedler K, Koopmann J, Rollbrocker B, Nechev S, Lenartz D, Stangl AP, Louis DN, Schramm J, Wiestler OD, von Deimling A. Molecular genetic evidence for subtypes of oligoastrocytomas. J Neuropathol Exp Neurol. 1997;56:1098–1104. doi: 10.1097/00005072-199710000-00003. [DOI] [PubMed] [Google Scholar]
- Mueller W, Hartmann C, Hoffmann A, Lanksch W, Kiwit J, Tonn J, Veelken J, Schramm J, Weller M, Wiestler OD, Louis DN, von Deimling A. Genetic signature of oligoastrocytomas correlates with tumor location and denotes distinct molecular subsets. Am J Pathol. 2002;161:313–319. doi: 10.1016/S0002-9440(10)64183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T, Di Patre PL, Burkhard C, Schuler D, Probst-Hensch NM, Maiorka PC, Baeza N, Pisani P, Yonekawa Y, Yasargil MG, Lutolf UM, Kleihues P. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64:6892–6899. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- Nobusawa S, Watanabe T, Kleihues P, Ohgaki H. IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res. 2009;15:6002–6007. doi: 10.1158/1078-0432.CCR-09-0715. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Tachibana O, Sato K, Yonekawa Y, Kleihues P, Ohgaki H. Overexpression of the EGF receptor and p53 mutations are mutually exclusive in the evolution of primary and secondary glioblastomas. Brain Pathol. 1996;6:217–224. doi: 10.1111/j.1750-3639.1996.tb00848.x. [DOI] [PubMed] [Google Scholar]
- Nigro JM, Takahashi MA, Ginzinger DG, Law M, Passe S, Jenkins RB, Aldape K. Detection of 1p and 19q loss in oligodendroglioma by quantitative microsatellite analysis, a real-time quantitative polymerase chain reaction assay. Am J Pathol. 2001;158:1253–1262. doi: 10.1016/S0002-9440(10)64076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura K, Pearson DM, Kocialkowski S, Backlund LM, Chan R, Jones DT, Collins VP. IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neuro Oncol. 2009;11:341–347. doi: 10.1215/15228517-2009-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann C, Meyer J, Balss J, Capper D, Mueller W, Christians A, Felsberg J, Wolter M, Mawrin C, Wick W, Weller M, Herold-Mende C, Unterberg A, Jeuken JW, Wesseling P, Reifenberger G, von DA. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118:469–474. doi: 10.1007/s00401-009-0561-9. [DOI] [PubMed] [Google Scholar]
- Gravendeel LA, Kloosterhof NK, Bralten LB, Van Marion R, Dubbink HJ, Dinjens W, Bleeker FE, Hoogenraad CC, Michiels E, Kros JM, Van den Bent M, Smitt PA, French PJ. Segregation of non-p.R132H mutations in IDH1 in distinct molecular subtypes of glioma. Hum Mutat. 2010;31:E1186–E1199. doi: 10.1002/humu.21201. [DOI] [PubMed] [Google Scholar]
- van den Bent MJ, Dubbink HJ, Marie Y, Brandes AA, Taphoorn MJ, Wesseling P, Frenay M, Tijssen CC, Lacombe D, Idbaih A, Van Marion R, Kros JM, Dinjens WN, Gorlia T, Sanson M. IDH1 and IDH2 mutations are prognostic but not predictive for outcome in anaplastic oligodendroglial tumors: a report of the European Organization for Research and Treatment of Cancer Brain Tumor Group. Clin Cancer Res. 2010;16:1597–1604. doi: 10.1158/1078-0432.CCR-09-2902. [DOI] [PubMed] [Google Scholar]
- Sanson M, Marie Y, Paris S, Idbaih A, Laffaire J, Ducray F, El HS, Boisselier B, Mokhtari K, Hoang-Xuan K, Delattre JY. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009;27:4150–4154. doi: 10.1200/JCO.2009.21.9832. [DOI] [PubMed] [Google Scholar]
- Dubbink HJ, Taal W, Van Marion R, Kros JM, van Heuvel I, Bromberg JE, Zonnenberg BA, Zonnenberg CB, Postma TJ, Gijtenbeek JM, Boogerd W, Groenendijk FH, Smitt PA, Dinjens WN, van den Bent MJ. IDH1 mutations in low-grade astrocytomas predict survival but not response to temozolomide. Neurology. 2009;73:1792–1795. doi: 10.1212/WNL.0b013e3181c34ace. [DOI] [PubMed] [Google Scholar]
- Jenkins RB, Blair H, Ballman KV, Giannini C, Arusell RM, Law M, Flynn H, Passe S, Felten S, Brown PD, Shaw EG, Buckner JC. A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res. 2006;66:9852–9861. doi: 10.1158/0008-5472.CAN-06-1796. [DOI] [PubMed] [Google Scholar]
- Kaloshi G, Benouaich-Amiel A, Diakite F, Taillibert S, Lejeune J, Laigle-Donadey F, Renard MA, Iraqi W, Idbaih A, Paris S, Capelle L, Duffau H, Cornu P, Simon JM, Mokhtari K, Polivka M, Omuro A, Carpentier A, Sanson M, Delattre JY, Hoang-Xuan K. Temozolomide for low-grade gliomas: predictive impact of 1p/19q loss on response and outcome. Neurology. 2007;68:1831–1836. doi: 10.1212/01.wnl.0000262034.26310.a2. [DOI] [PubMed] [Google Scholar]
- Kesari S, Schiff D, Drappatz J, LaFrankie D, Doherty L, Macklin EA, Muzikansky A, Santagata S, Ligon KL, Norden AD, Ciampa A, Bradshaw J, Levy B, Radakovic G, Ramakrishna N, Black PM, Wen PY. Phase II study of protracted daily temozolomide for low-grade gliomas in adults. Clin Cancer Res. 2009;15:330–337. doi: 10.1158/1078-0432.CCR-08-0888. [DOI] [PubMed] [Google Scholar]
- Peraud A, Kreth FW, Wiestler OD, Kleihues P, Reulen HJ. Prognostic impact of TP53 mutations and P53 protein overexpression in supratentorial WHO grade II astrocytomas and oligoastrocytomas. Clin Cancer Res. 2002;8:1117–1124. [PubMed] [Google Scholar]
- Ishii N, Tada M, Hamou MF, Janzer RC, Meagher-Villemure K, Wiestler OD, Tribolet N, Van Meir EG. Cells with TP53 mutations in low grade astrocytic tumors evolve clonally to malignancy and are an unfavorable prognostic factor. Oncogene. 1999;18:5870–5878. doi: 10.1038/sj.onc.1203241. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Katayama Y, Yoshino A, Komine C, Yokoyama T. Deregulation of the TP53/p14ARF tumor suppressor pathway in low-grade diffuse astrocytomas and its influence on clinical course. Clin Cancer Res. 2003;9:4884–4890. [PubMed] [Google Scholar]
- Korshunov A, Meyer J, Capper D, Christians A, Remke M, Witt H, Pfister S, von Deimling A, Hartmann C. Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol. 2009;118:401–405. doi: 10.1007/s00401-009-0550-z. [DOI] [PubMed] [Google Scholar]
- Coons SW, Johnson PC, Scheithauer BW, Yates AJ, Pearl DK. Improving diagnostic accuracy and interobserver concordance in the classification and grading of primary gliomas. Cancer. 1997;79:1381–1393. doi: 10.1002/(sici)1097-0142(19970401)79:7<1381::aid-cncr16>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Kros JM, Gorlia T, Kouwenhoven MC, Zheng PP, Collins VP, Figarella-Branger D, Giangaspero F, Giannini C, Mokhtari K, Mork SJ, Paetau A, Reifenberger G, van den Bent MJ. Panel review of anaplastic oligodendroglioma from European Organization For Research and Treatment of Cancer Trial 26951: assessment of consensus in diagnosis, influence of 1p/19q loss, and correlations with outcome. J Neuropathol Exp Neurol. 2007;66:545–551. doi: 10.1097/01.jnen.0000263869.84188.72. [DOI] [PubMed] [Google Scholar]
- Fuller CE, Schmidt RE, Roth KA, Burger PC, Scheithauer BW, Banerjee R, Trinkaus K, Lytle R, Perry A. Clinical utility of fluorescence in situ hybridization (FISH) in morphologically ambiguous gliomas with hybrid oligodendroglial/astrocytic features. J Neuropathol Exp Neurol. 2003;62:1118–1128. doi: 10.1093/jnen/62.11.1118. [DOI] [PubMed] [Google Scholar]