Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by co-targeting MEK and IGF-1R/PI3K (original) (raw)
. Author manuscript; available in PMC: 2011 Dec 15.
Published in final edited form as: Cancer Cell. 2010 Dec 14;18(6):683–695. doi: 10.1016/j.ccr.2010.11.023
Summary
BRAF is an attractive target for melanoma drug development. However, resistance to BRAF inhibitors is a significant clinical challenge. We describe a model of resistance to BRAF inhibitors developed by chronic treatment of BRAFV600E melanoma cells with the BRAF inhibitor SB-590885; these cells are cross resistant to other BRAF-selective inhibitors. Resistance involves flexible switching among the three RAF isoforms, underscoring the ability of melanoma cells to adapt to pharmacological challenges. IGF-1R/PI3K signaling was enhanced in resistant melanomas, and combined treatment with IGF-1R/PI3K and MEK inhibitors induced death of BRAF inhibitor-resistant cells. Increased IGFR-1R and pAKT levels in a post-relapse human tumor sample are consistent with a role for IGF-1R/PI3K-dependent survival in the development of resistance to BRAF inhibitors.
Keywords: melanoma, BRAF, MEK, IGF-1R, targeted therapy, drug resistance
Introduction
Melanoma, a malignancy originating in pigment-producing melanocytes, is the most aggressive form of skin cancer. Although surgical treatment of early melanoma leads to 90% cure rates, unresectable advanced melanoma is notorious for its intrinsic resistance to chemotherapy, aggressive clinical behavior, and tendency to rapidly metastasize. Ten-year survival rates for patients with metastatic disease remain below 14% (Cockburn Myles). Additionally, the incidence of melanoma continues to rise worldwide (WHO, 2001). This dismal clinical and epidemiological picture underscores the need for effective therapeutic strategies to target this aggressive neoplasia. Over 50% of melanomas harbor activating V600E mutations in BRAF (BRAFV600E) (Davies et al., 2002), an oncogene known to be critical for the proliferation and survival of melanoma cells through activation of the RAF/MEK/ERK mitogen activated protein kinase pathway (MAPK) (Fecher et al., 2008; Garnett and Marais, 2004), making BRAF an attractive target for anti-melanoma therapy. Thus, there is an ongoing effort to develop small molecule inhibitors to target the BRAF/MAPK pathway. Several BRAF and MEK inhibitors are currently being tested; for example, the BRAF inhibitors RAF-265 (Novartis), XL281 (Exelixis), PLX4032 (Plexxikon/Roche), and GSK2118436 (GSK) are in advanced stages of clinical trials (ClinicalTrials.gov). Encouraging results from a recent trial with the BRAF inhibitor PLX4032 were recently reported (Flaherty, 2010). Data from this study indicate that chronic treatment with PLX4032 leads to tumor shrinkage and progression-free survival of ~7 months in patients with BRAFV600E mutant melanomas. However, most patients who initially responded to treatment with PLX4032 relapsed, suggesting that chronic treatment with BRAF inhibitors is associated with development of drug resistance.
Drug resistance is a common problem associated with chronic treatment with anti-cancer drugs (Engelman and Janne, 2008; Engelman et al., 2007; Kobayashi et al., 2005; Pao et al., 2005). Clinical experience with other neoplasms, as well as early data with PLX4032, suggest that resistance to BRAF inhibitors will likely be a significant clinical challenge. Therefore, it is critical to proactively direct research efforts to: 1) develop good models of resistance to BRAF inhibitors; 2) investigate the mechanisms underlying resistance; and 3) design alternative therapeutic strategies to overcome drug resistance. Models of acquired resistance should mimic chronic treatment conditions used in the clinical setting. The evaluation of mechanisms of resistance should address the well documented adaptability of melanoma cells (Lipkin, 2008; Hendrix et al., 2003) and consider the possibility that resistance to a drug can be linked to multiple mechanisms. Understanding the mechanisms underlying acquired resistance to anti-cancer agents will be instrumental in developing alternative therapeutic strategies.
Here we examine mechanisms underlying acquired resistance to BRAF inhibitors in melanomas with BRAFV600E mutations and evaluate therapeutic strategies to overcome it.
Results
Chronic BRAF inhibition leads to acquired drug resistance
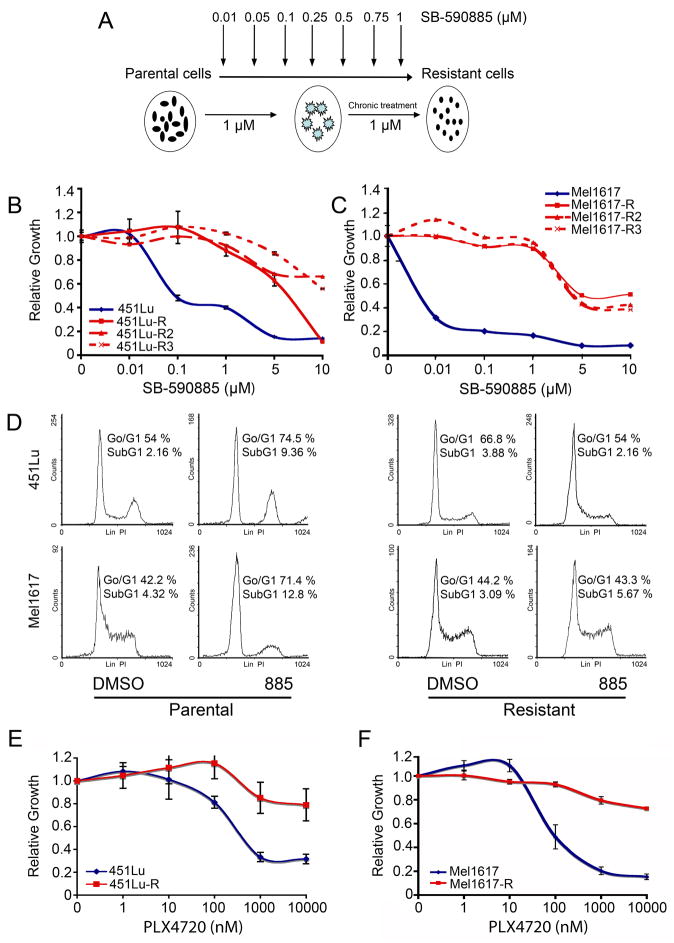
To investigate if chronic BRAF inhibition could lead to acquired drug resistance, a panel of BRAF inhibitor sensitive melanoma cell lines harboring the V600E mutation in the BRAF gene and expressing PTEN (Table S1) were chronically treated with increasing concentrations of the specific BRAF inhibitor SB-590885 (885; Figure 1A) (King et al., 2006). We focused on PTEN-expressing cells because we have found that cells that lack PTEN are often substantially less sensitive to BRAF inhibitors than PTEN expressing cells (our unpublished data). MTT assays showed that while parental cells (451Lu and Mel1617) were highly sensitive to BRAF inhibition by 885 (IC50 ~ 0.01–0.1 μM), melanoma cells which had been chronically treated with 885 (451Lu-R and Mel1617-R) required higher doses of the drug for partial growth inhibition (IC50 ~ 5–10 μM) (Figure 1B–C). Chronic treatment of additional BRAFV600E melanoma cell lines with 885 led to the emergence of drug resistance (Figure S1A–C and Table S1). Cell cycle analysis showed that while treatment with 1 μM of 885 led to a G0/G1 cell cycle arrest after 24h (p<0.05) and an increase in the percentage of cells in the SubG1 fraction after 72h (p<0.05) in 451Lu and Mel1617 parental cells, it had no significant effect on 451Lu-R and Mel1617-R cells (p>0.05) (Figures 1D and S1D–E).
Figure 1. BRAFV600E mutant melanomas chronically treated with BRAF inhibitors develop drug resistance.
(A) Schematic representation of generation of SB-590885 (885) resistant cells. The resistant cells are indicated by the name of the parental cell line followed by R. (B–C) Sensitivity to BRAF inhibition of parental (blue) and 885 chronically treated melanoma cells (red) was assessed by MTT assays. Relative growth (RG) was calculated as the ratio of treated to untreated cells at each dose for each replicate. Data are represented as mean ± SEM (n=7). (B) At all doses less than 10 μM, RG was significantly lower for 451Lu cells (p<0.05). (C) At all doses RG was significantly lower for Mel1617 cells (_p_<0.05). (D) Cells were treated with DMSO or 1 μM 885 for 24h, stained with propidium iodide and analyzed for cell cycle progression by flow cytometry. Response to treatment between the two cell lines was not significantly different (_p_>0.05). The percentage of cells in G0/G1 and SubG1 are shown. Representative cell cycle plots from one experiment are shown. (E–F) Sensitivity of 451Lu (E) and Mel1617 (F) parental and the corresponding 885-resistant cells to PLX4720 was assessed by MTT assays as in (B). Cells were treated with the indicated concentrations of PLX4720 (nM). Data represent mean of three independent experiments ± SEM. Parental and resistant cells were significantly different (p<0.05) at doses > 1 μM. See also Figure S1.
Cells chronically treated with the BRAF inhibitor 885 exhibited cross-resistance to other specific BRAF inhibitors, including PLX4720 (PLX) (Tsai et al., 2008) as well as two other BRAF inhibitors currently in clinical trials (not shown). Treatment of parental cells with PLX notably reduced viability (IC50 ~100–500 nM) of BRAFV600E mutant melanomas. However, PLX had no major effect on 885-resistant cells (IC50>5 μM; Figure 1E–F). These data demonstrate that chronic treatment with a specific BRAF inhibitor can lead to development of drug resistance to multiple selective BRAF inhibitors in melanomas harboring BRAFV600E mutations which were initially highly sensitive to these compounds.
SB-590885-resistant cells proliferate, form colonies in soft agar, and grow in 3D-collagen–based matrices despite BRAF inhibition
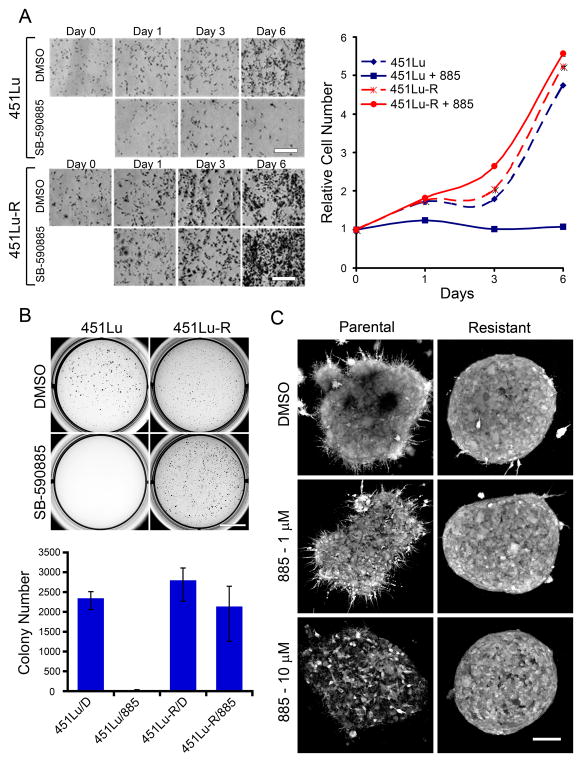
To further characterize the growth properties of melanoma cells with acquired resistance to BRAF inhibitors, we investigated the effects of BRAF inhibition on proliferation, anchorage independent growth, and growth in a 3D-tumor-like microenvironment of the parental metastatic melanoma and 885-resistant cell lines (Figure 2). While treatment of 451Lu parental cells with 885 led to inhibition of proliferation (Figure 2A; p<0.05), it did not affect the growth of 451Lu-R cells (_p_>0.05). 451Lu-R cells exhibited similar growth rates as untreated 451Lu cells, even when grown in the presence of 885 (_p_>0.05). Anchorage-independent growth assays demonstrated that while BRAF inhibition precluded the ability of parental cells to form colonies in soft agar (Figure 2B; p<0.05), it did not affect the colony-forming ability of cells resistant to BRAF inhibitors (_p_>0.05). Previous studies have shown that growth of melanoma cells as 3D collagen-implanted spheroids more closely mimics the in vivo behavior of melanoma tumors and considerably increases their drug resistance (Horning et al., 2008; Smalley et al., 2006). We examined the effect of BRAF inhibition by 885 in parental and resistant cells grown as multicellular spheroids in 3D collagen-based matrices (Figure 2C). Consistent with our previous studies (King et al., 2006), treatment of the BRAFV600E mutant cells with 885 for 72 h led to a dose-dependent loss of cell viability. In contrast, BRAF-inhibitor resistant spheroids remained viable. The growth properties of these cells both in 2D and 3D, and their ability to form colonies in soft agar, demonstrate that treatment with BRAF inhibitors leads to acquired drug resistance and the emergence of cells able to grow and proliferate even under anchorage-independent conditions.
Figure 2. Growth properties of SB-590885-resistant melanoma cells.
(A) Cells were treated with 1 μM 885 for 6 days, fixed at the indicated days, stained with crystal violet and photographed. Cell number was determined relative to day 0. Data represents mean ± SEM (n=3). P<0.05 when comparing 451Lu + 885 with 451Lu + DMSO or 451Lu-R +/− 885 at day 6; _p_>0.05 when comparing 451Lu-R +/− 885 with 451Lu. Scale bar, 800 μm (B) 451Lu parental and resistant cells were grown in soft agar for 12 days +/− 1 μM 885. Anchorage-independent growth was assessed by counting individual colonies using ImagePro-Plus software in triplicate and normalizing to vehicle controls for each condition. Data represent mean ± SEM (n=3), p<0.05 DMSO-treated vs. 885-treated parental cells, and _p_>0.05 for parental DMSO-treated vs. resistant cells. Scale bar, 10 mm. (C) 451Lu (left panels) and 451Lu-R (right panels) collagen-embedded spheroids were treated with the indicated concentrations of 885. Spheroids were stained with calcein-AM and imaged with a confocal microscope. Relative viability was assessed based on the % of cells remaining after treatment and morphological appearance. Scale bar, 150 μm.
BRAF-inhibitor-resistant melanomas switch among RAF isoforms to activate the MAPK pathway and induce proliferation
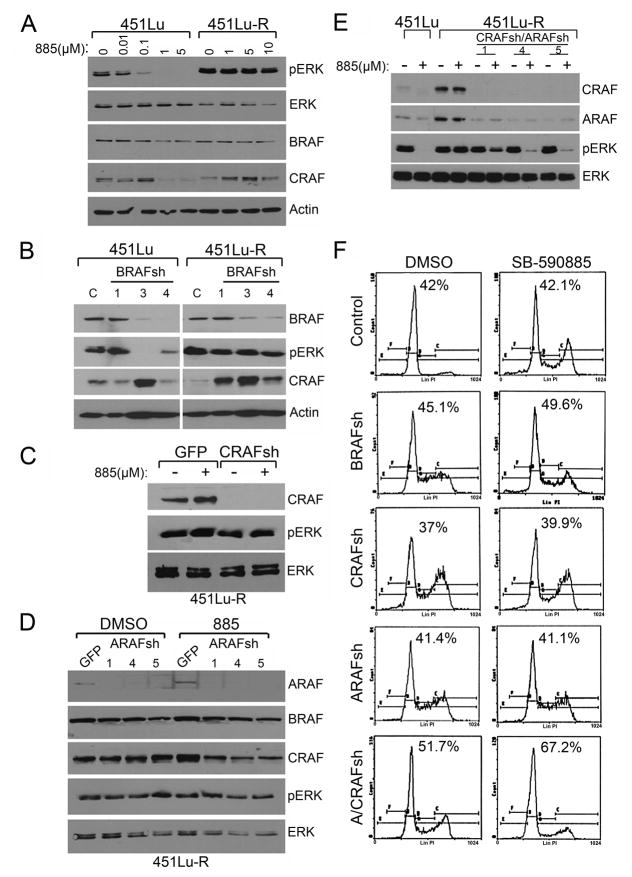
To investigate the molecular basis underlying acquired resistance to BRAF inhibitors, we analyzed the effect of 885 on downstream ERK activation in both parental and resistant cells. Treatment of 451Lu cells with 885 caused a dose-dependent inhibition of ERK activation (Figure 3A). In contrast, ERK remained phosphorylated in the resistant cells despite treatment with high doses of the BRAF inhibitor up to 10 μM, raising the possibility that ERK activation could be mediated by a kinase other than BRAF (Figures 3A and S2). To confirm the results obtained with 885, as well as to determine if ERK activation was dependent on BRAF, we knocked-down BRAF using shRNA (Figure 3B). Short hairpin RNA-mediated BRAF knockdown led to inhibition of ERK phosphorylation in 451Lu parental cells, but had no effect on 451Lu-R cells, suggesting that ERK activation is BRAF-independent in these cells.
Figure 3. Abrogation of ERK activity in SB-590885-resistant melanomas requires inhibition of all three RAF isoforms and leads to cell cycle arrest.
(A) 451Lu and 451Lu-R cells were treated with increasing concentrations of 885. Cells lysates were analyzed by immunoblotting. (B) 451Lu and 451Lu-R cells were infected with either a control [C] lentiviral shRNA or 3 different clones targeting BRAF (1, 3 or 4). Cell lysates were analyzed by immunoblotting. (C) 451Lu-R cells were infected with lentiviral shRNA directed against CRAF or GFP. Infected cells were treated with 1 μM 885 (+) or left untreated (−) for 24h. Cell lysates were analyzed by immunoblotting with antibodies against CRAF, pERK, and total ERK (loading control). (D) 451Lu-R cells were infected with lentiviral shRNA against ARAF or GFP. Infected cells were treated with 1 μM 885 or DMSO for 24h. Cells were harvested, lysed and analyzed by immunoblotting with antibodies against ARAF, BRAF, CRAF, pERK, and total ERK (loading control). (E) 451Lu–R cells were sequentially infected with lentiviral shRNA directed against CRAF followed by ARAF. Infected and control cells were then treated with 1 μM 885 for 24h (+) or left untreated (−). Cell lysates were analyzed by immunoblotting. (F) After lentiviral infection, cells were treated with DMSO or 1 μM 885 for 72h. Cells were harvested, fixed, stained with propidium iodide and analyzed by flow cytometry. The percentage of cells in G0/G1 is indicated for each condition. See also Figure S2.
We also examined if secondary mutations in BRAF could be associated with development of resistance to BRAF inhibitors. Mutational analysis of exons 6 and 11–17 in the BRAF gene was performed in all parental and resistant cell lines. These exons represent those in which mutations in melanoma and genetic syndromes have been described. We did not identify any mutations beyond V600E (Table S1). Moreover, we sequenced other genes commonly mutated in melanoma, including, NRAS (exons 2 and 3), CKIT (exons 9, 11, 13, 14, 17 and 18) and PTEN (exons 5–9) and did not find de novo mutations in these genes. We also found that resistance to BRAF inhibitors was not associated with changes in copy number of BRAF, NRAS, c-KIT, or PTEN (not shown).
We noted that short-term treatment with 885 at 1–5 μM led to a decrease in CRAF protein levels in 451Lu cells, while CRAF levels remained steady or in some instances even increased in the resistant cells (Figure 3A). Similarly, knockdown of BRAF using shRNA, led to an increase in CRAF protein levels in both the parental and resistant cells (Figure 3B; sh-3). We next examined the possibility that CRAF could be mediating ERK activation in response to BRAF inhibition (Montagut et al., 2008). Lentiviral-mediated infection of 451Lu-R cells with CRAF shRNA inhibited CRAF expression, but had no effect on ERK activation (Figure 3C). Treatment of CRAF shRNA-infected cells with 885 had no effect on phospho-ERK levels, indicating that 885-resistant cells can activate the MAPK pathway independently of BRAF and CRAF. Similarly, infection of 451Lu-R cells with 3 different ARAF shRNAs led to knockdown of this gene, but had no effect on phospho-ERK (Figure 3D). Inhibition of BRAF activity by 885 in conjunction with ARAF-knockdown did not preclude phosphorylation of ERK in 451Lu-R cells (Figure 3D, lanes 6–8). Given that 885-resistant cells are able to activate ERK despite inhibition of either one or two RAF isoforms, we hypothesized that these cells only require one active RAF isoform to activate the MAPK pathway. To test this hypothesis, we sequentially infected 451Lu-R cells with lentivirus carrying shRNAs against CRAF followed by infection with shRNAs against ARAF (Figure 3E). Simultaneous shRNA-mediated inhibition of CRAF and ARAF did not have a significant effect on phospho-ERK levels; however, treatment of these cells with 1 μM 885 resulted in downregulation of ERK phosphorylation (Figure 3E). We conclude that inhibition of ERK activity in BRAF-inhibitor-resistant cells requires concomitant abrogation of all three RAF isoforms. Together these data argue that cells with acquired resistance to BRAF inhibitors can rewire their signaling properties and indistinctly use any of the three active RAF isoforms to trigger ERK activation. While inhibition of one or two RAF isoforms did not considerably affect cell cycle progression in 451Lu-R cells, simultaneous inhibition of all three RAF isoforms led to G0/G1 cell cycle arrest; no major increase in the number of cells accumulating in the SubG1 fraction of the cell cycle was observed (Figure 3F). We conclude that any RAF isoform can activate ERK and regulate proliferation of melanoma cells resistant to BRAF inhibitors.
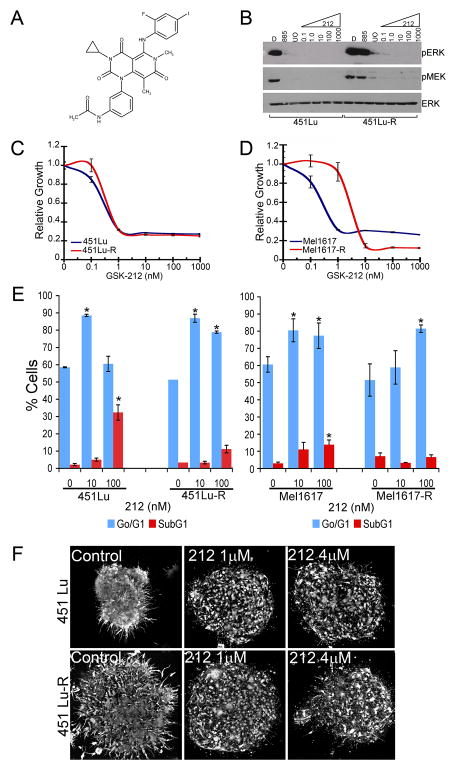
To confirm that 885 resistant cells remain dependent on MAPK activation for proliferation, we examined the effect of MEK inhibition in parental and resistant cells using the MEK inhibitors GSK1120212 (212), AZD6244 (AZD) and U0126 (UO) (Figures 4 and S3). 212 is a potent and selective allosteric MEK1/2 inhibitor currently in phase I/II clinical trials for solid tumors and lymphoma (Clinicaltrials.gov; Figure 4A). In biochemical assays, 212 inhibits MEK1 activation by RAF (IC50 = 0.7 ± 0.1 nM) and phospho-MEK1 kinase activity (IC50 = 10 ± 2 nM) (not shown). 212 blocks full activation of MEK1/2 by inhibiting phosphorylation of S217 and shows no significant activity against ~200 unique kinases when tested at 10 μM. Treatment with 212 inhibited ERK phosphorylation and decreased viability in both parental and resistant cell lines (Figures 4B–D and S3A). Consistent with these data, MEK inhibition by 212 resulted in G0/G1 cell cycle arrest in parental and resistant melanomas (Figure 4E; p<0.05). However, a 10-fold higher dose of 212 was required to inhibit ERK phosphorylation, cell viability, and G0/G1 cell cycle arrest in Mel1617-R cells. Interestingly, while treatment with 212 significantly increased the number of cells in SubG1 in the parental cells (_p_<0.05), it did not have a considerable effect on the resistant cells (_p_>0.05). To confirm our findings with 212, we used two additional MEK inhibitors (AZD6244 and UO126) displaying different mechanisms of action. Treatment of parental and resistant cells with AZD6244 or UO126 led to inhibition of ERK phosphorylation (Figures 4B and S3A–C), G0/G1 cell cycle arrest (Table S2) and decreased cell viability (Figure S3D). Similar to the results with 212, a 10-fold higher dose of AZD6244 was required to inhibit phosphorylation of ERK and viability of Mel1617R cells compared to their parental counterparts. Treatment of 885-sensitive and -resistant melanomas in a 3D context with 212, AZD6244, or U0126 over 72h showed that both parental and 885-resistant cells were partially sensitive to MEK inhibition when maintained in a 3D tumor-like microenvironment (Figures 4F and S3E–F). These results suggest that although ERK activity remains sensitive to MEK inhibition in BRAF-inhibitor resistant cells, abrogating MAPK signaling has primarily cytostatic effects and raises the possibility that additional pathways may promote survival of these cells.
Figure 4. The MEK inhibitor GSK1120212 prevents ERK activation and proliferation in both SB-590885 sensitive and resistant cell lines.
(A) Chemical structure of GSK1120212 (212). (B) 451Lu and 451Lu-R cells were treated with 1 μM 885, 10 μM UO126 (UO) or increasing concentrations of 212 (nM) for 24h. Cell lysates were analyzed by immunoblotting. (C–D) Sensitivity to the MEK inhibitor 212 was assessed by MTT assays as in Figure 1. Data represent means ± SEM (n=7). (E) Parental and 885-resistant cells were treated with 212 for 72 h, stained with propidium iodide, and analyzed by flow cytometry. Data represent mean ± SEM (n=3); (*) p<0.05 when compared to DMSO-treated cells. (F) 451Lu and 451Lu-R collagen-embedded spheroids were treated with the indicated concentrations of 212 for 72 hours. Cells were imaged with a confocal microscope. Scale bar, 150 μm. See also Figure S3 and Table S2.
IGF-1R leads to induction of pro-survival signals in BRAF inhibitor resistant cells
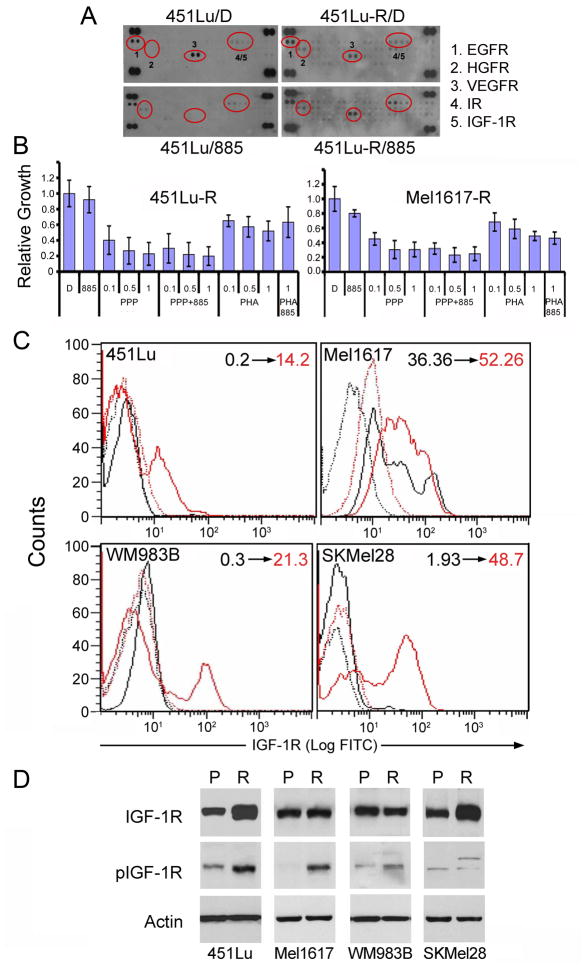
To investigate if additional pathways were stimulated in response to chronic BRAF inhibition, we examined the activation of several tyrosine kinase receptors (RTKs). Analysis of RTK phosphorylation using an antibody array suggested that some RTKs were differentially phosphorylated in the resistant cells compared to their parental counterparts (Figure 5A). Using pharmacological inhibitors of these receptors, we found that only treatment with the IGF-1R inhibitors cyclolignan picropodophyllin (PPP; Girnita et al., 2004) or tyrphostin AG1024 (Parrizas et al., 1997) (Figures 5B, S4A–B, and data not shown) led to decreased viability of melanomas resistant to BRAF inhibitors. Consistent with an established role of IGF-1 mediating proliferation and survival in melanoma (Satyamoorthy et al., 2001; Hilmi et al., 2008), PPP had a partial effect decreasing viability in both parental and resistant melanoma spheroids (Figure S4D–E). We next evaluated both the surface expression of IGF-1R and phosphorylation of IGF-1R at Tyr1131, which is indicative of kinase activation. Analysis of IGF-1R surface expression by flow cytometry revealed that BRAF-inhibitor resistant cells upregulate IGF-1R (Figure 5C). Moreover, IGF-1R remained phosphorylated in the resistant cells after treatment with 885 compared with parental cells (Figure 5D, S4C). We did not find mutations in IGF-1R, nor did we observe changes in copy number, suggesting that the regulation of IGF-1R is mediated at least in part by increased surface expression of the receptor in the BRAF-inhibitor resistant cells. Analysis of IGF-1 and IGF-1R mRNA by qRT-PCR indicated that even short-term treatment of parental cells with 885 led to an increase in both growth factor and receptor mRNA (not shown); however, this increase does not seem to be sufficient to persistently activate the IGF-1 system, as it does not correlate with increased IGF-1R protein expression or activation in parental cells treated with 885. Similarly, analysis of IGF-1 and IGF-1R mRNA by qRT-PCR in resistant cells showed a modest increase in mRNA levels for both growth factor and receptor that did not correlate with protein expression. These results suggest that the persistent IGF-1R activity in cells resistant to BRAF inhibitors is most likely regulated at the post-transcriptional level and that additional factors, such as IGFBP expression, may be required to fully engage the system. Indeed, qRT-PCR analysis showed that IGFBP-3 mRNA was increased after acute treatment of parental cells with 885, whereas it was downmodulated in the resistant cells (Figure S4F). IGFBP3 negatively regulates the activation of IGF-1R by sequestering IGF-1 and preventing ligand binding to the receptor (Karas et al., 1997); thus, the regulation of IGFBP3 may be one of several factors modulating IGF-1-mediated signaling in response to BRAF inhibition.
Figure 5. Enhanced IGF-1R in cells chronically treated with BRAF inhibitors.
(A) 451Lu and 451Lu-R cells were treated with 1 μM 885 or DMSO (D) for 24h. Whole-cell lysates were incubated on RTK antibody arrays. Each RTK antibody is spotted in duplicate. Positive RTK dots are circled in red and indicated by a number; the corresponding RTKs are listed next to the arrays. (B) Sensitivity to the IGF-1R inhibitor PPP (μM) or the c-Met inhibitor PHA (μM) was assessed in 451Lu-R and Mel1617-R by MTT assays as in Figure 1. Data represent means ± SEM (n=7) (C) IGF-1R surface expression was assed by indirect immunoflourescence in parental (black) and resistant (red) melanomas treated with 885 (1 μM) for 20h. Dotted lines denote control rabbit antibody for the corresponding parental or resistant cells. Numbers on the top right indicate percent positive surface expression of IGF-1R in parental (black) and resistant (red) cells. (D) Expression and phosphorylation of IGF-1R was assessed in parental (P) and resistant (R) melanomas treated with 885 (1 μM) for 20h. Cell lysates were analyzed by immunoblotting with the indicated antibodies. See also Figure S4.
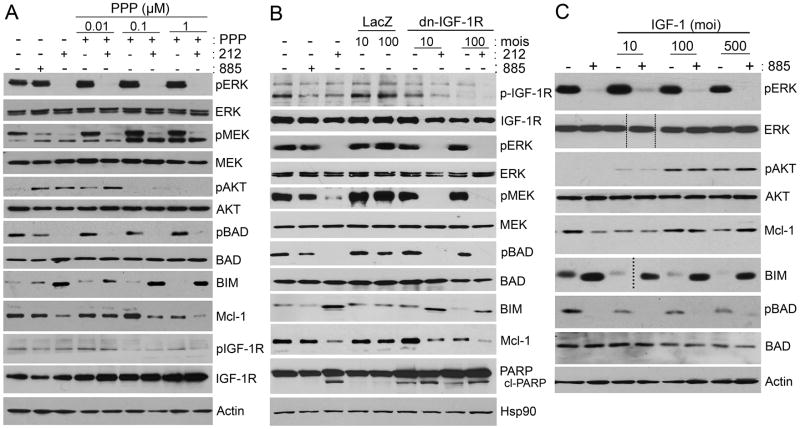
IGF-1R plays an important role in tumorigenesis, resistance to apoptosis and resistance to anti-cancer agents (Casa et al., 2008; Pollak, 2008; Tao et al., 2007). IGF-1R has gained increasing attention as a promising target in cancer therapy, but its role as a therapeutic target in melanoma has not been systematically explored. IGF-1R can activate both the MAPK and PI3K pathways, both of which play critical roles in melanomagenesis. We examined the effect of IGF-1R inhibition on MAPK- and PI3K-mediated signaling. Treatment with PPP or AG1024 had no effect on ERK activation in 885-resistant cells (Figures 6A, S4C, and S5A–B). However, phosphorylation of AKT was inhibited by treatment with PPP (Figure 6A). Consistent with our results using IGF-1R small molecule inhibitors, expression of dominant negative (dn) IGF-1R (Min et al., 2003) in 885-resistant cells did not inhibit MEK and ERK phosphorylation (Figure 6B), but had an inhibitory effect on AKT phosphorylation (Figure S5C). Overexpression of the IGF-1R ligand, IGF-1, in Mel1617 parental cells led to increased phosphorylation of AKT, but had no significant effect on ERK phosphorylation (Figures 6C and S5C–D). Together these data suggest that persistent IGF-1R signaling induces PI3K/AKT activation in V600E mutant melanomas-resistant to BRAF inhibitors. However, our data do not preclude the possibility that additional factors could also affect inter-regulation of IGF-1R and PI3K in BRAF inhibitor resistant cells.
Figure 6. IGF-R mediates PI3K signaling in BRAF-inhibitor resistant cells.
(A) Mel1617-R cells were treated with increasing concentrations of PPP (μM) as single agent or in combination with 0.1 μM 212. The effect of IGF-1R inhibition on MAPK, AKT, and Bcl-2 family proteins was assessed by immunoblotting. (B) Mel1617-R cells were infected with adenoviruses encoding dominant negative (dn) IGF-1R at 10 or 100 MOI, or LacZ as a negative control. Infected cells were treated 48h post infection with 0.1 μM 212 or left untreated. Cells lysates were analyzed by immunoblotting; cl-PARP, cleaved PARP. (C) Parental Mel1617 cells were infected in serum-free medium with adenoviruses encoding IGF-1 at 10, 100 or 500 MOI. Infected cells were serum starved for 48 h and then treated with 1 μM 885 or left untreated. Cells lysates were analyzed by immunoblotting. Doted lines indicate where blot was cut to remove an empty lane. See also Figure S5.
Considering that IGF-1R and PI3K/AKT play important roles mediating cell survival, we examined the effect of MEK and IGF-1R inhibition on the expression of some Bcl2-family members known to be important for melanoma survival, including Mcl-1, BAD and BIM (Boisvert-Adamo et al., 2009). Mel1617-R cells expressed high levels of phospho-BAD and Mcl-1, neither of which were completely inhibited by treatment with 885 (Figures 6A and S5A–B). Unphosphorylated BAD binds and inactivates the pro-survival factors Bcl-2 and Bcl-xl promoting apoptosis; phosphorylated BAD associates with 14-3-3 allowing unbound Bcl-2/Bcl-xl to promote survival. While inactivation of MEK/ERK by 212 or AZD6244 was sufficient to inhibit BAD phosphorylation and to induce BIM, inhibition of IGF-1R signaling did not have any considerable effect on these pro-apoptotic factors (Figures 6A–B and S5A–B). Inhibition of either MEK or IGF-1R led to a partial downregulation of the pro-survival factor Mcl-1 (Figures 6A–B and S5A–B). Moreover, concomitant inhibition of MEK and IGF-1R/AKT-mediated signaling had an additive effect on downregulating Mcl-1 in Mel1617-R cells (Figures 6A–B and S5A–B). MEK and IGF-1R appear to cooperate and promote survival of melanomas resistant to BRAF inhibitors; while MEK alone regulates BIM and BAD, both pathways jointly regulate Mcl-1 expression. Overexpression of IGF-1 decreased BIM expression, but it did not preclude the ability of 885 to induce BIM (Figure 6C). Although treatment of Mel1617 cells with 885 resulted in partial downregulation of Mcl-1, overexpression of IGF-1 led to increased Mcl-1 levels, which could not be downregulated by 885 alone. These results suggest that MEK and IGF-1R cooperate to promote cell survival in part through the coordinated regulation of Mcl-1. Our data suggest that co-inhibition of MEK and IGF-1R shifts the balance of apoptotic BH3-family member activity towards cell death, although other survival factors in addition to BAD, BIM, and Mcl-1 could also be regulating survival of BRAF-inhibitor resistant melanomas.
Simultaneous MEK and IGF-1R/PI3K inhibition leads to cytotoxicity in melanomas resistant to BRAF inhibitors
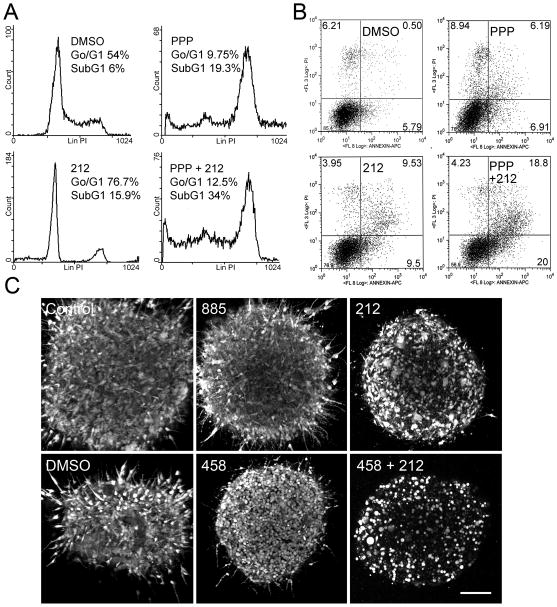
To investigate if combined MEK and IGF-1R inhibition could induce cytotoxic effects on 885-resistant cells, 451Lu-R and Mel1617-R cells were treated with MEK inhibitors (212 or AZD6244), an IGF-1R inhibitor (PPP), or the potent pan-PI3K inhibitor GSK2126458 (458) (Knight et al., 2010), as single agents or in combination. Treated cells were analyzed for cell cycle progression (Figure 7A and Tables S3–4) and Annexin-V expression (Figures 7B and S6A). Cell-cycle analyses established that while BRAF inhibition did not have a significant effect on proliferation or induction of apoptosis in 885-resistant cells (p> 0.05; Figures 1D and S1D–E), MEK inhibition in BRAF inhibitor-resistant cells was sufficient to induce cell-cycle arrest after 24h of treatment (Figure 4C, Table S2). Prolonged exposure to 212 (72h) led to minor increases in cell death as determined by the number of cells accumulating in the SubG1 fraction of the cell cycle as well as an increase in Annexin V-positive cells (Figure 7A–B) in resistant cells. Treatment of BRAF inhibitor-resistant melanomas with PPP increased the number of cells in the G2/M phase of the cell cycle, the number of cells in the SubG1 phase (Figure 7A and Table S3–4), and Annexin V-positive cells (Figures 7B and S6A). Concomitant MEK and IGF-1R inhibition by 212 and PPP led to an increase in the fraction of cells in the SubG1 phase of the cell cycle, as well as an increase in the number of Annexin V-positive cells, indicating that co-inhibition of MEK and IGF1-R leads to increased melanoma cell death. Similar results were observed when inhibiting MEK with AZD6244 in combination with PPP (Figure S6A) or by combined treatment with 212 and 458 (Table S4). We confirmed the results from our 2D-platforms by using 3D-spheroid assays to determine if combined MEK and IGF-1R or MEK and PI3K inhibition could induce cytotoxicity in melanoma cells resistant to BRAF inhibitors in the context of a 3D-collagen matrix. Simultaneous treatment with 212 and 458 confirmed that BRAFV600E cells resistant to BRAF inhibitors undergo apoptosis (assessed by the percentage of viable cells remaining after treatment and morphological appearance) in response to combination treatment to a much greater extent than when treated with each individual compound (Figures 7C and S6B). Treatment with PPP in combination with 212 or AZD6244 resulted in decreased cell viability in 885-resistant melanoma spheroids (Figure S6C–D). The collective data suggest that co-targeting MEK and IGF-1R/PI3K can result in striking anti-melanoma activity in melanomas resistant to BRAF inhibitors.
Figure 7. Co-inhibition of IGF-1R/PI3K and MEK induces cytotoxicity in BRAF-inhibitor resistant cells.
(A) Cell cycle profiles of 451Lu-R cells treated with DMSO, 1 μM 212, 1 μM PPP, or a combination of both inhibitors for 72h. Percentage of cells in G0/G1 and SubG1 are shown. (B) 451Lu-R cells were treated with DMSO, 212 (1 μM), PPP (1 μM) or both inhibitors at the same concentrations for 72h. Cells were collected and apoptosis was assessed by Annexin-V staining. Numbers in each quadrant indicate percentage of cells. Representative results of 2 independent experiments are shown. (C) Collagen-embedded Mel1617-R spheroids were treated with DMSO, 10 μM 885, 1 μM 212, 1 μM 458 or 1 μM 212 + 1 μM 458 for 72 hours. Cells were imaged with a confocal microscope. Scale bar, 150 μm. See also Figure S6 and Tables S3–4.
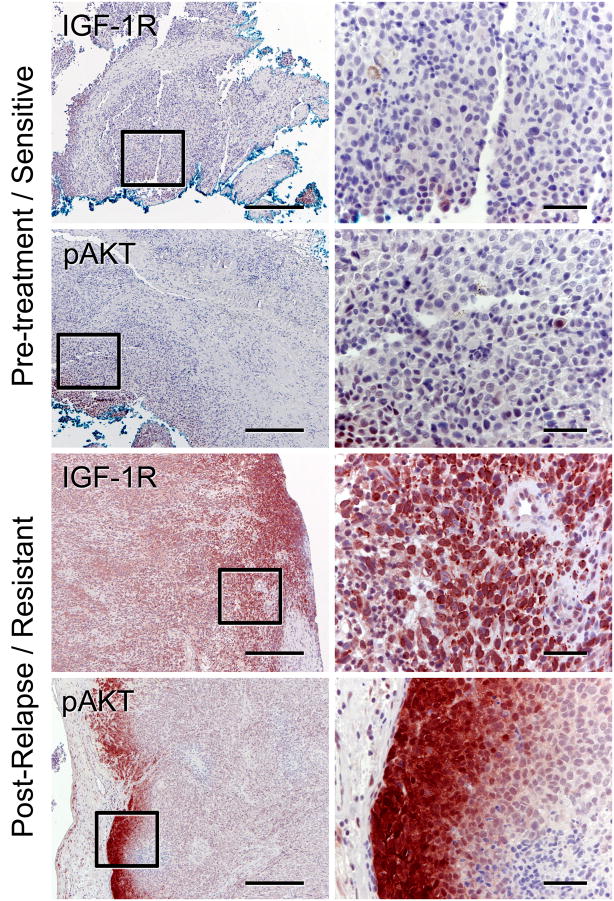
Increased IGF-1R expression and phoshorylation of AKT correlate with resistance to BRAF inhibitors in one out of five paired tissue samples from relapsed patients
To evaluate the potential clinical implications of our in vitro findings, we examined by immunohistochemistry (IHC) tumor biopsies from five patients with metastatic melanoma treated with the BRAF inhibitor PLX4032. All five patients’ tumors were BRAFV600E+ and initially responded to treatment with PLX4032 (Table S5) but relapsed after 4–15 months, suggesting that they developed resistance to the BRAF inhibitor. Five sets of paired tumor samples (pre-treatment and post-relapse) were stained and analyzed for IGF-1R and pAKT blindly by a pathologist. We found increased levels of IGF-1R and pAKT in post-relapse tumor biopsies of one patient (Figure 8; pt 1 in Table S5). This patient did not have secondary BRAF mutations, NRAS mutations, or changes in PTEN status. Patient 1 had brain and subcutaneous metastases but no other organ involvement before enrolling in the study. The patient was dose escalated from 160 to 720mg BID of PLX4032, had a good response to the BRAF inhibitor as judged by CT scans (Figure S7A–B), and had a progression-free survival (PFS) of 466 days, but relapsed on PLX4032. A progressing intra-abdominal lesion was not seen at presentation (Figure S7C), but was then observed at progression using PET/CT scan fusion (Figure S7D). These findings are consistent with our in vitro data, where increased IGF-1R expression and phosphorylation of AKT, in the absence of changes in BRAF, NRAS, or PTEN mutation status, is associated with resistance to BRAF inhibitors. Additionally, we also found increased IGF-1R levels in post-relapse samples of patient 5 (Figure S7E–F); however, pAKT levels were not increased. The absence of pAKT in the post-relapse biopsy of patient 5 could be due to the rapid loss of phospho-proteins in FFPE human tissue samples that often occurs during the processing of the sample (Jones et al., 2008).
Figure 8. Increased IGF-1R expression and phosphorylation of AKT in relapsed patient samples.
Paired tumor samples (pt 1, Table S5) taken before treatment (pre-treatment, sub-cutaneous back lesion) and drug resistant (post-relapse, small bowel) were analyzed for IGF-1R expression or phospho-AKT by immunohistochemistry. Low magnification representative images are shown on the left (scale bar, 500 μm) and higher magnification images are on shown on the right (scale bar, 50 μm). See also Figure S7 and Table S5.
Partial information on PTEN status was available for patients 2, 4 and 5. (Table S5). The post-relapse sample of patient 2, which did not have secondary mutations in BRAF or mutations in NRAS, had a homozygous loss of PTEN that was not present in the pre-treatment sample. Interestingly, there was an increase in pAKT in the post-relapse sample of this patient without a concomitant IGF-1R increase (not shown). Although the number of specimens examined was small, due to limited access to human samples, our findings suggest that increased expression of IGF-1R and activation of the IGF-1R/PI3K/AKT pathway could occur in association with development of resistance to BRAF inhibitors in the clinical setting.
Discussion
We report that BRAFV600E melanomas chronically treated with a specific BRAF inhibitor acquire cross-resistance to several selective BRAF inhibitors through a RAF kinase switch. Chronic BRAF inhibition is associated with enhanced IGF-1R and PI3K/AKT activity in melanoma cells resistant to BRAF inhibitors. We propose that drug combinations co-targeting MEK and IGF-1R/PI3K may offer valid therapeutic approaches to overcome resistance to BRAF inhibitors.
Acquired resistance to anticancer agents is frequently encountered in clinical practice. Resistance to kinase inhibitors is often associated with secondary mutations in the target gene, which render the kinase insensitive to the inhibitor (Engelman and Settleman, 2008). However, in our in vitro system, we did not find secondary mutations in BRAF that could explain resistance to BRAF inhibitors. We also did not identify de novo mutations or changes in copy number in NRAS, KIT, or PTEN, three oncogenes commonly associated with melanoma. BRAFV600E promotes persistent MAPK activity, leading to increased proliferation and survival. Acute BRAFV600E inhibition by genetic depletion or kinase inhibitors can lead to cell cycle arrest and, in some instances, apoptosis in melanomas addicted to this oncogene (Bollag et al., 2010; Hingorani et al., 2003; Lee et al., 2010; King et al., 2006; Tsai et al., 2008). Our studies demonstrate that upon chronic BRAF inhibition, melanomas rewire their signaling circuitries in order to utilize one of the other two RAF isoforms, ARAF or CRAF, to overcome the effect of BRAF inhibition.
Our data are consistent with a model whereby melanomas are initially addicted to the BRAF/MAPK pathway. If BRAF is repressed, melanomas trigger an alternative signaling program, involving a kinase switch, which allows the addicted tumor to continue to rely on MAPK for maintenance of the neoplastic phenotype. Our findings have important therapeutic implications as they highlight the relevance of MAPK signaling in melanoma and argue that targeting the MAPK pathway constitutes a valid therapeutic strategy.
Recent studies demonstrated that in the context of mutant RAS, acute inhibition of BRAF kinase activity promotes altered scaffolding and activation of CRAF, phosphorylation of ERK, and oncogenesis (Hatzivassiliou et al., 2010; Heidorn S et al., 2010; Poulikakos, 2010). While Hatzivassiliou et al. and Heidorn et al. suggested that BRAF inhibition does not activate CRAF in V600E-mutant cells, our studies indicate that BRAFV600E melanomas can flexibly switch among the three different RAF isoforms by a yet unidentified mechanism to overcome the effect of chronic BRAF inhibition and activate the MAPK pathway.
Montagut and colleagues described a model of resistance to the RAF inhibitor AZ628 through increased levels of CRAF protein (Montagut et al., 2008). We also observed increased CRAF levels in cells chronically treated with the BRAF inhibitor 885. However, in our system, shRNA-mediated inhibition of CRAF did not affect ERK activation or proliferation, as resistant cells can also switch to ARAF. The differences between the two studies may be due to the distinct molecular and genetic profiles of the cell lines used, the mechanism of action of the drug used to target the tumor cells, and/or the duration of treatment among other factors.
Our data demonstrate that under conditions of chronic BRAF inhibition, melanomas rely on IR/IGF-1R -mediated survival pathways in order to circumvent adverse conditions favoring cell death. IGF-1R, which is expressed in all cells of melanocytic origin, has been implicated in resistance to therapy in other neoplasia, including lung and breast cancer (Casa et al., 2008). Recently, Sharma et al. have reported the existence of a subpopulation of drug-tolerant cells that survive acute drug treatment via engagement of IGF-1R signaling (Sharma et al., 2010). The enhanced activity of PI3K/AKT associated with chronic BRAF inhibition suggests the possible existence of a negative crosstalk between the two pathways. Crosstalk between MAPK and PI3K has been reported in several cancer systems (Carracedo et al., 2008; Cheung et al., 2008; Mirzoeva et al., 2009), but not much is known in melanoma; this issue deserves further exploration.
BRAFV600E/PTEN+ melanomas, which are sensitive to BRAF inhibitors, have low levels of pAKT (our unpublished data). In contrast, melanoma cells that acquire resistance to BRAF inhibitors have enhanced levels of pAKT associated with increased IGF-1R signaling. These observations raise the possibility that IGF-1R/PI3K-mediated signaling in the context of chronic BRAF inhibition promotes survival of BRAF inhibitor-resistant melanomas, and cooperates with the MAPK pathway to support drug resistance. Consistent with this notion, inhibitors of MEK and IGF-1R or PI3K in combination were more effective inducing cell death of BRAF-inhibitor resistant cells than when used as single agents.
Although results from recent clinical trials with PLX4032 are encouraging, responding tumors eventually develop resistance. Increased expression of IGF-1R in post-relapse tumor biopsies of two patients who developed resistance to PLX4032, one of whom also had increased levels of phospho-AKT, constitute proof-of-principle that IGF-1R/PI3K/AKT-mediated signaling may be associated with resistance to BRAF inhibitors, and provide insight into future therapies for the treatment of patients who become refractory to these drugs. The absence of changes in BRAF, NRAS, and PTEN mutation status in patient 1 supports the idea that a non-genetic mechanism can be underlying resistance to BRAF inhibitors in some patients. Our findings suggest that melanomas can respond to chronic BRAF inhibition through dynamic changes by re-wiring their signaling circuitries, allowing the tumor cells to adapt to pharmacological challenges. Given the high degree of heterogeneity and plasticity of melanoma, it is likely that several mechanisms of resistance will arise in response to chronic BRAF inhibition, raising challenges to our quest in search of effective therapies for this malignancy. Of note, homozygous loss of PTEN and increased phospho-AKT were identified in post-relapse samples in one patient, suggesting that alternative mechanisms leading to PI3K/AKT activation may also be associated with acquired resistance to BRAF inhibitors.
Our studies and others’ demonstrate that targeting solely one pathway is not sufficient to eradicate melanoma (Lasithiotakis et al., 2008; Smalley et al., 2006). This study provides further evidence that combination strategies targeting key oncogenic pathways are required for successful therapy. Furthermore, our findings provide a molecular rationale for combining MEK and IGF-1R/PI3K inhibitors as we demonstrate that: 1) melanomas are addicted to the MAPK pathway - thus, shutting off this pathway results in oncogenic shock, rendering cells susceptible to apoptosis; 2) chronic BRAF inhibition is associated with enhanced IGF-1R/PI3K-dependent survival pathways as a protective cellular mechanism, and 3) concomitant MEK and IGF-1R/PI3K inhibition shifts the balance towards induction/activation of pro-apoptotic molecules and inhibition of pro-survival factors in melanomas resistant to BRAF inhibitors.
Combining MEK and IGF-1R/PI3K inhibitors constitutes a promising approach, as these two signaling pathways cooperate to drive tumor growth, survival, and resistance to therapy. Thus, combination strategies targeting these two pathways merit further evaluation as a potential approach to treat melanomas refractory to BRAF inhibitors.
Experimental Procedures
Reagents
SB-590885, GSK1120212, and GSK2126458 were provided by GlaxoSmithKline. PLX4720 was provided by Plexxikon. AZD6244 was synthesized by Chemietek (Indianapolis, IN). U0126 was purchased from Promega (Madison, WI); cyclolignan picropodophyllin (PPP), AG1024 and PHA-665752 were purchased from Calbiochem (San Diego, CA).
Cell Culture
Human melanoma cell lines have previously been described (Satyamoorthy et al., 2003; Iliopoulos et al., 1989). Melanoma cells were cultured in DMEM supplemented with 5% fetal bovine serum. 451Lu and 451Lu-R clones were isolated from single cells. Resistant cell lines were generated by treating parental cells with increasing concentrations of 885. Cells with the ability to grow in 1 μM of 885 were obtained ~6 months after the initial drug exposure. Resistant lines were maintained in the continuous presence of 1 μM 885, supplemented every 72h. The consistency of cellular genotypes and identities was confirmed by DNA fingerprinting using Coriell’s microsatellite kit.
Cell growth/viability, colony formation and apoptosis assays
Cell viability was measured by MTT assays as previously described (Smalley et al., 2009). For cell cycle and apoptosis analysis, melanoma cells were treated with small molecule inhibitors for 24–72h as previously described (Tsai et al., 2008). For Annexin V analysis, cells were stained with annexin-APC (Molecular probes) and propidium iodide. Samples were subsequently analyzed with an EPICS XL (Beckman-Coulter) apparatus.
Immunoblotting and antibody arrays
All antibodies used were from Cell Signaling Technology (Beverly, MA, USA), except β-Actin, which was purchased from Sigma (St. Louis, MO), and Mcl-1 from Santa Cruz Biotechnology (Santa Cruz, CA). To identify the relative levels of phosphorylation of RTKs, we used a human phospho-RTK array kit (ARRY-001; R&D Systems Minneapolis, MN), according to manufacturer instructions.
Three-dimensional spheroid growth/survival assays
Melanoma spheroids were prepared as previously described (Tsai, et al, 2008). Collagen-embedded spheroids were treated with inhibitors for 72–96 hours. Spheroids were imaged using a Leica TCS SP2 confocal microscope.
Lentivirus and adenovirus infection
Lentiviral shRNA constructs were obtained from Sigma. Recombinant adenovirus encoding IGF-1 has previously been described (Satyamoorthy et al., 2001). Dominant-negative mutant IGF-1R adenoviral vector (DN-IGF-1R) was a generous gift from Dr. Y. Adachi and described elsewhere (Lee et al., 2003).
Patients’ samples
Tumor specimens collected to evaluate the pathology of melanoma and pharmacodynamics of PLX4032, as well as clinical information from patients treated with PLX4032 were obtained under institutional review board-approved studies at Vanderbilt University Medical Center (Nashville, TN) and Peter MacCallum Cancer Centre (Victoria, Australia). All patients provided informed written consent. Mutational and immunohistochemical analysis is described in the supplemental experimental procedures.
Statistical analysis
The analysis of variance (ANOVA) was used to identify significant experimental factors including cell line, dose, day and/or experiment that influenced the primary experimental outcomes. When the ANOVA model was significant, pair-wise differences in experimental group means were evaluated using Tukey’s procedure controlling for multiple hypothesis tests. Statistical analyses were done in SAS (version 9.2) using Proc ANOVA and Proc GLM.
Significance.
Effective strategies to overcome anti-cancer drug resistance are sorely needed and can only be developed by understanding the molecular mechanisms of resistance in models that mimic the chronic administration of anti-cancer drugs used in the clinic. BRAFV600E mutant melanomas developed resistance to BRAF inhibitors by switching among BRAF, CRAF and ARAF isoforms to activate the MAPK pathway. Our study not only establishes the mechanism of resistance to BRAF inhibition but also proposes a strategy to overcome it. We find that enhanced IGF-1R/PI3K signaling promotes survival of BRAF inhibitor resistant cells, thus the requirement to co-target both pathways. Our findings suggest that co-inhibition of MEK and IGF-1R/PI3K warrants further investigation as a strategy to treat melanomas refractory to BRAF inhibitors.
Supplementary Material
01
Acknowledgments
We thank T. Nguyen and F. Keeney for assistance with graphics, T. Wang for help with Annexin-V analysis, J. Kong, Y, Chen, S. Huang, M. Neri, and S. Lee for technical assistance. We thank the Microscopy and Flow cytometry facilities at the Wistar Institute and G. Bollag for providing PLX4720. This work was supported by grants from the National Cancer Institute (P01 CA114046, P01 CA025874, P30 CA010815, RO1 CA117881), the Adelson Medical Research Foundation, and GSK. Sylvie Laquerre is an employee and shareholder of GSK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boisvert-Adamo K, Longmate W, Abel EV, Aplin AE. Mcl-1 is required for melanoma cell resistance to anoikis. Mol Cancer Res. 2009;7:549–556. doi: 10.1158/1541-7786.MCR-08-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, Spevak W, Zhang C, Zhang Y, Habets G, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. The Journal of clinical investigation. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casa AJ, Dearth RK, Litzenburger BC, Lee AV, Cui X. The type I insulin-like growth factor receptor pathway: a key player in cancer therapeutic resistance. Front Biosci. 2008;13:3273–3287. doi: 10.2741/2925. [DOI] [PubMed] [Google Scholar]
- Cheung M, Arati Sharma, MV, Robertson GP. Akt3 and Mutant V600EB-Raf Cooperate to Promote Early Melanoma Development. Cancer research. 2008;68:3429–3439. doi: 10.1158/0008-5472.CAN-07-5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn Myles PD. Key Charles Melanoma SEER Survival Monograph. National Cancer Institute; pp. 93–100. [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Janne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2008;14:2895–2899. doi: 10.1158/1078-0432.CCR-07-2248. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Settleman J. Acquired resistance to tyrosine kinase inhibitors during cancer therapy. Current opinion in genetics & development. 2008;18:73–79. doi: 10.1016/j.gde.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science (New York, NY) 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- Fecher LA, Amaravadi RK, Flaherty KT. The MAPK pathway in melanoma. Current opinion in oncology. 2008;20:183–189. doi: 10.1097/CCO.0b013e3282f5271c. [DOI] [PubMed] [Google Scholar]
- Flaherty KT, Puzanov Igor, MD, Kim Kevin B, MD, Ribas Antoni, MD, McArthur Grant A, MB, BS, PhD, Sosman Jeffrey A, MD, O'Dwyer Peter J, MD, Lee Richard J, MD, PhD, Grippo Joseph F, PhD, Nolop Keith, MD, Chapman Paul B., MD Inhibition of Mutated, Activated BRAF in Metastatic Melanoma. The New England journal of medicine. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett MJ, Marais R. Guilty as charged: B-RAF is a human oncogene. Cancer cell. 2004;6:313–319. doi: 10.1016/j.ccr.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Girnita A, Girnita L, del Prete F, Bartolazzi A, Larsson O, Axelson M. Cyclolignans as inhibitors of the insulin-like growth factor-1 receptor and malignant cell growth. Cancer research. 2004;64:236–242. doi: 10.1158/0008-5472.can-03-2522. [DOI] [PubMed] [Google Scholar]
- Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, Ludlam MJ, Stokoe D, Gloor SL, Vigers G, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–435. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, Hussain J, Reis-Filho JS, Springer CJ, Pritchard C, Marais R. Kinase-Dead BRAF and Oncogenic RAS Cooperate to Drive Tumor Progression through CRAF. Cell. 2010;114:209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Molecular plasticity of human melanoma cells. Oncogene. 2003;22:3070–3075. doi: 10.1038/sj.onc.1206447. [DOI] [PubMed] [Google Scholar]
- Hilmi C, Larribere L, Giuliano S, Bille K, Ortonne JP, Ballotti R, Bertolotto C. IGF1 promotes resistance to apoptosis in melanoma cells through an increased expression of BCL2, BCL-X(L), and survivin. The Journal of investigative dermatology. 2008;128:1499–1505. doi: 10.1038/sj.jid.5701185. [DOI] [PubMed] [Google Scholar]
- Hingorani SR, Jacobetz MA, Robertson GP, Herlyn M, Tuveson DA. Suppression of BRAF(V599E) in human melanoma abrogates transformation. Cancer research. 2003;63:5198–5202. [PubMed] [Google Scholar]
- Horning JL, Sahoo SK, Vijayaraghavalu S, Dimitrijevic S, Vasir JK, Jain TK, Panda AK, Labhasetwar V. 3-D tumor model for in vitro evaluation of anticancer drugs. Molecular pharmaceutics. 2008;5:849–862. doi: 10.1021/mp800047v. [DOI] [PubMed] [Google Scholar]
- Iliopoulos D, Ernst C, Steplewski Z, Jambrosic JA, Rodeck U, Herlyn M, Clark WH, Jr, Koprowski H, Herlyn D. Inhibition of metastases of a human melanoma xenograft by monoclonal antibody to the GD2/GD3 gangliosides. Journal of the National Cancer Institute. 1989;81:440–444. doi: 10.1093/jnci/81.6.440. [DOI] [PubMed] [Google Scholar]
- Jones RJ, Boyce T, Fennell M, Jacobs V, Pinto F, Duffield E, Clack G, Green T, Kelly J, Robertson J. The impact of delay in cryo-fixation on biomarkers of Src tyrosine kinase activity in human breast and bladder cancers. Cancer chemotherapy and pharmacology. 2008;61:23–32. doi: 10.1007/s00280-007-0440-9. [DOI] [PubMed] [Google Scholar]
- Karas M, Danilenko M, Fishman D, LeRoith D, Levy J, Sharoni Y. Membrane-associated insulin-like growth factor-binding protein-3 inhibits insulin-like growth factor-I-induced insulin-like growth factor-I receptor signaling in ishikawa endometrial cancer cells. The Journal of biological chemistry. 1997;272:16514–16520. doi: 10.1074/jbc.272.26.16514. [DOI] [PubMed] [Google Scholar]
- King AJ, Patrick DR, Batorsky RS, Ho ML, Do HT, Zhang SY, Kumar R, Rusnak DW, Takle AK, Wilson DM, et al. Demonstration of a genetic therapeutic index for tumors expressing oncogenic BRAF by the kinase inhibitor SB-590885. Cancer research. 2006;66:11100–11105. doi: 10.1158/0008-5472.CAN-06-2554. [DOI] [PubMed] [Google Scholar]
- Knight S, Adams N, Burgess J, Chaudhari A, Darcy M, Donatelli C, Luengo J, Newlander K, Parrish C, Ridgers L, et al. Discovery of GSK2126458, a Highly Potent Inhibitor of PI3K and the Mammalian Target of Rapamycin. ACS Med Chem Lett. 2010;1:39–43. doi: 10.1021/ml900028r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG, Halmos B. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. The New England journal of medicine. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- Lasithiotakis KG, Sinnberg TW, Schittek B, Flaherty KT, Kulms D, Maczey E, Garbe C, Meier FE. Combined inhibition of MAPK and mTOR signaling inhibits growth, induces cell death, and abrogates invasive growth of melanoma cells. The Journal of investigative dermatology. 2008;128:2013–2023. doi: 10.1038/jid.2008.44. [DOI] [PubMed] [Google Scholar]
- Lee CT, Park KH, Adachi Y, Seol JY, Yoo CG, Kim YW, Han SK, Shim YS, Coffee K, Dikov MM, Carbone DP. Recombinant adenoviruses expressing dominant negative insulin-like growth factor-I receptor demonstrate antitumor effects on lung cancer. Cancer gene therapy. 2003;10:57–63. doi: 10.1038/sj.cgt.7700524. [DOI] [PubMed] [Google Scholar]
- Lee JT, Li L, Brafford PA, Van Den Eijnden M, Halloran MB, Sproesser K, Haass N, Smalley KSM, Tsai J, Bollag G, Herlyn M. PLX4032, a potent inhibitor of the B-Raf V600E oncogene, selectively inhibits V600E-positive melanomas. Pigment Cell Melanoma Res. 2010;23:820–827. doi: 10.1111/j.1755-148X.2010.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkin G. Plasticity of the cancer cell: implications for epigenetic control of melanoma and other malignancies. The Journal of investigative dermatology. 2008;128:2152–2155. doi: 10.1038/jid.2008.69. [DOI] [PubMed] [Google Scholar]
- Min Y, Adachi Y, Yamamoto H, Ito H, Itoh F, Lee CT, Nadaf S, Carbone DP, Imai K. Genetic blockade of the insulin-like growth factor-I receptor: a promising strategy for human pancreatic cancer. Cancer research. 2003;63:6432–6441. [PubMed] [Google Scholar]
- Mirzoeva OK, Das D, Heiser LM, Bhattacharya S, Siwak D, Gendelman R, Bayani N, Wang NJ, Neve RM, Guan Y, et al. Basal subtype and MAPK/ERK kinase (MEK)-phosphoinositide 3-kinase feedback signaling determine susceptibility of breast cancer cells to MEK inhibition. Cancer research. 2009;69:565–572. doi: 10.1158/0008-5472.CAN-08-3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagut C, Sharma SV, Shioda T, McDermott U, Ulman M, Ulkus LE, Dias-Santagata D, Stubbs H, Lee DY, Singh A, et al. Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma. Cancer research. 2008;68:4853–4861. doi: 10.1158/0008-5472.CAN-07-6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, Kris MG, Varmus H. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS medicine. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrizas M, Gazit A, Levitzki A, Wertheimer E, LeRoith D. Specific inhibition of insulin-like growth factor-1 and insulin receptor tyrosine kinase activity and biological function by tyrphostins. Endocrinology. 1997;138:1427–1433. doi: 10.1210/endo.138.4.5092. [DOI] [PubMed] [Google Scholar]
- Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–30. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyamoorthy K, Li G, Gerrero MR, Brose MS, Volpe P, Weber BL, Van Belle P, Elder DE, Herlyn M. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer research. 2003;63:756–759. [PubMed] [Google Scholar]
- Satyamoorthy K, Li G, Vaidya B, Patel D, Herlyn M. Insulin-like growth factor-1 induces survival and growth of biologically early melanoma cells through both the mitogen-activated protein kinase and beta-catenin pathways. Cancer research. 2001;61:7318–7324. [PubMed] [Google Scholar]
- Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, McDermott U, Azizian N, Zou L, Fischbach MA, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley KS, Haass NK, Brafford PA, Lioni M, Flaherty KT, Herlyn M. Multiple signaling pathways must be targeted to overcome drug resistance in cell lines derived from melanoma metastases. Molecular cancer therapeutics. 2006;5:1136–1144. doi: 10.1158/1535-7163.MCT-06-0084. [DOI] [PubMed] [Google Scholar]
- Smalley KS, Xiao M, Villanueva J, Nguyen TK, Flaherty KT, Letrero R, Van Belle P, Elder DE, Wang Y, Nathanson KL, Herlyn M. CRAF inhibition induces apoptosis in melanoma cells with non-V600E BRAF mutations. Oncogene. 2009;28:85–94. doi: 10.1038/onc.2008.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Pinzi V, Bourhis J, Deutsch E. Mechanisms of disease: signaling of the insulin-like growth factor 1 receptor pathway--therapeutic perspectives in cancer. Nature clinical practice. 2007;4:591–602. doi: 10.1038/ncponc0934. [DOI] [PubMed] [Google Scholar]
- Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, Bremer R, Gillette S, Kong J, Haass NK, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3041–3046. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. GLOBOCAN: Cancer Incidence, Mortality and Prevalence Worldwide. IARC Cancerbase. 2001;(5) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01