Circulating Nonphosphorylated Carboxylated Matrix Gla Protein Predicts Survival in ESRD (original) (raw)
Abstract
The mechanisms for vascular calcification and its associated cardiovascular mortality in patients with ESRD are not completely understood. Dialysis patients exhibit profound vitamin K deficiency, which may impair carboxylation of the calcification inhibitor matrix gla protein (MGP). Here, we tested whether distinct circulating inactive vitamin K–dependent proteins associate with all-cause or cardiovascular mortality. We observed higher levels of both desphospho-uncarboxylated MGP (dp-ucMGP) and desphospho-carboxylated MGP (dp-cMGP) among 188 hemodialysis patients compared with 98 age-matched subjects with normal renal function. Levels of dp-ucMGP correlated with those of protein induced by vitamin K absence II (PIVKA-II; r = 0.62, P < 0.0001). We found increased PIVKA-II levels in 121 (64%) dialysis patients, indicating pronounced vitamin K deficiency. Kaplan-Meier analysis showed that patients with low levels of dp-cMGP had an increased risk for all-cause and cardiovascular mortality. Multivariable Cox regression confirmed that low levels of dp-cMGP increase mortality risk (all-cause: HR, 2.2; 95% CI, 1.1 to 4.3; cardiovascular: HR, 2.7; 95% CI, 1.2 to 6.2). Furthermore, patients with higher vascular calcification scores showed lower levels of dp-cMGP. In 17 hemodialysis patients, daily supplementation with vitamin K2 for 6 weeks reduced dp-ucMGP levels by 27% (P = 0.003) but did not affect dp-cMGP levels. In conclusion, the majority of dialysis patients exhibit pronounced vitamin K deficiency. Lower levels of circulating dp-cMGP may serve as a predictor of mortality in dialysis patients. Whether vitamin K supplementation improves outcomes requires further study.
Dialysis patients show an increased total and cardiovascular mortality.1 Cardiovascular calcifications are well-established mortality predictors in ESRD patients.2 Calcification is not only a passive but an actively regulated process dependent on calcification inhibitors.3 Fetuin-A, a liver-derived protein, acts as a systemic calcification inhibitor,4 and low serum levels have been shown to predict mortality in dialysis patients.5 Matrix gla protein (MGP) is produced by vascular smooth muscle cells and acts locally in the vascular wall.6 MGP can be modified by γ-glutamate carboxylation and serine phosphorylation. The function of phosphorylation is not yet known, but recent data suggest that it plays a role in regulating the secretion of proteins into the extracellular environment.7 The role of carboxylation, which depends on vitamin K, is better understood and determines MGP's bioactivity as a calcification inhibitor.8 Impaired carboxylation of MGP is associated with both intimal and medial vascular calcification in human arteries.9 Recently, it was shown that arteries of dialysis patients exhibit a poor MGP carboxylation status, as shown by a high amount of uncarboxylated MGP compared with carboxylated MGP.10 Thus far, only total uncarboxylated MGP (t-ucMGP) and desphospho-uncarboxylated MGP (dp-ucMGP) could be measured in plasma. Here we describe the comparison of conformation-specific ELISAs differentiating between desphospho-carboxylated MGP (dp-cMGP) and desphospho-uncarboxylated MGP (dp-ucMGP) and tested whether dp-cMGP and/or dp-ucMGP predict survival in a cohort of hemodialysis patients. In addition, we tested whether vitamin K2 supplementation can improve the deficient vitamin K status in dialysis patients.
RESULTS
Circulating MGP Levels in Hemodialysis Patients
The characteristics of the dialysis population are given in Table 1. Using MGP species-specific antibodies to distinguish between dp-cMGP and dp-ucMGP, 188 hemodialysis patients exhibited 3.3-fold elevated plasma levels of dp-cMGP (6247 ± 1778 pmol/L) and 6.5-fold elevated plasma levels of dp-ucMGP (2850 ± 1768 pmol/L) compared with 98 age-matched healthy subjects with normal renal function (dp-cMGP 1921 ± 605 pmol/L and dp-ucMGP 442 ± 242 pmol/L; P < 0.0001). dp-cMGP exhibited an inverse correlation with dialysis vintage (r = −0.28, P = 0.0001) and a positive correlation with body mass index (r = 0.24, P = 0.0009), whereas dp-ucMGP did not show such a relationship (data not shown). Age, diabetes, and dialysis efficacy (i.e., Kt/V) were not related to plasma levels of dp-cMGP (Table 1). Patients with lower plasma levels of dp-cMGP had increased C-reactive protein (CRP) levels, whereas other serum parameters were not significantly related to dp-cMGP (Table 1).
Table 1.
Characteristics of patients with high and low levels of desphospho-carboxylated MGP (mean ± SD, range; or number, percent), relative risk (odds ratio), and 95% confidence intervals for low and high levels of dp-cMGP (univariate logistic regression)
| All Patients (n = 188) | dp-cMGP < 6139 pmol/L (n = 94) | dp-cMGP > 6139 pmol/L (n = 94) | Odds Ratio | 95% Confidence Interval | P | |
|---|---|---|---|---|---|---|
| Clinical parameters | ||||||
| age, years | 59 ± 11 | 59 ± 11 | 59 ± 11 | 0.99 | 0.97 to 1.02 | 0.76 |
| male/female | 99/89 | 55 (59%)/39 (41%) | 44 (47%)/50 (53%) | 0.62 | 0.35 to 1.11 | 0.11 |
| diabetes mellitus | 21 (11%) | 11 (12%) | 10 (11%) | 0.90 | 0.36 to 2.23 | 0.82 |
| hypertension | 164 (87%) | 81 (86%) | 83 (88%) | 1.21 | 0.51 to 2.86 | 0.66 |
| smoking | 61 (32%) | 32 (34%) | 29 (31%) | 0.86 | 0.47 to 1.59 | 0.64 |
| body mass index, kg/m2 | 23.5 ± 3.8 | 22.7 ± 3.8 | 24.3 ± 3.6 | 1.12 | 1.03 to 1.22 | 0.007 |
| Dialysis parameters | ||||||
| dialysis vintage, years | 6.8 ± 4.8 | 7.5 ± 5.1 | 6.0 ± 4.3 | 0.93 | 0.88 to 0.99 | 0.03 |
| Kt/V | 1.28 ± 0.20 | 1.28 ± 0.21 | 1.29 ± 0.19 | 0.97 | 0.23 to 4.12 | 0.97 |
| Biochemical serum parameters | ||||||
| protein, g/L | 67.1 ± 4.9 | 67.1 ± 4.5 | 67.0 ± 5.2 | 1.00 | 0.94 to 1.06 | 0.91 |
| calcium, mmol/L | 2.31 ± 0.18 | 2.30 ± 0.17 | 2.31 ± 0.19 | 1.52 | 0.30 to 7.56 | 0.61 |
| phosphate, mmol/L | 1.62 ± 0.42 | 1.61 ± 0.40 | 1.62 ± 0.44 | 1.03 | 0.52 to 2.06 | 0.93 |
| intact PTH, pg/ml | 392 ± 493 (median 192) | 454 ± 513 (median 252) | 331 ± 468 (median 162) | 1.00 | 1.00 to 1.00 | 0.10 |
| cholesterol, mmol/L | 5.13 ± 1.22 | 5.05 ± 1.26 | 5.21 ± 1.18 | 1.12 | 0.88 to 1.42 | 0.36 |
| triglycerides, mmol/L | 2.33 ± 1.34 | 2.16 ± 1.34 | 2.51 ± 1.34 | 1.23 | 0.98 to 1.55 | 0.08 |
| high-sensitivity C-reactive protein, mg/L | 7.90 ± 11.81 (median 3.25) | 9.93 ± 14.78 (median 3.32) | 5.92 ± 7.46 (median 2.93) | 0.97 | 0.94 to 1.00 | 0.03 |
| Calcification, pulse wave velocity, and IMT | ||||||
| iliacal/femoral calcification (n = 183) | 95 (51%) | 50 (53%) | 45 (48%) | 0.79 | 0.44 to 1.40 | 0.41 |
| carotid calcification (n = 183) | 128 (68%) | 64 (68%) | 64 (68%) | 0.90 | 0.48 to 1.69 | 0.74 |
| cardiac valve calcification (n = 181) | 75 (40%) | 42 (45%) | 33 (35%) | 0.63 | 0.35 to 1.14 | 0.12 |
| IMT, mm (n = 184) | 0.81 ± 0.31 | 0.82 ± 0.42 | 0.79 ± 0.10 | 0.70 | 0.22 to 2.21 | 0.54 |
| pulse wave velocity, m/s (n = 177) | 9.7 ± 2.1 | 9.76 ± 2.18 | 9.71 ± 2.13 | 0.99 | 0.86 to 1.14 | 0.88 |
Vitamin K Status in Dialysis Patients
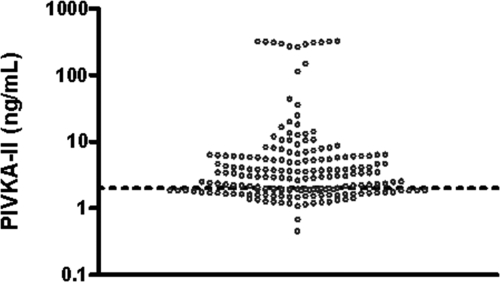
Plasma levels of the liver protein induced by vitamin K absence-II (PIVKA-II) were elevated in 121 (64%) of the patients (median, 2.98; range, 0.45 to 318 ng/ml), indicating hepatic vitamin K deficiency (Figure 1). PIVKA-II levels correlated well with those of dp-ucMGP (r = 0.62; P < 0.0001) and the ratio of dp-ucMGP over dp-cMGP (r = 0.55; P < 0.0001) and slightly with dp-cMGP (r = 0.19; P = 0.01).
Figure 1.
Dialysis patients are deficient in vitamin K. Distribution of PIVKA-II in hemodialysis patients. According to the upper limit of the normal range (dotted line, 2 ng/ml30), 64% of dialysis patients display vitamin K deficiency (as indicated by increased PIVKA-II levels).
MGP and Calcification
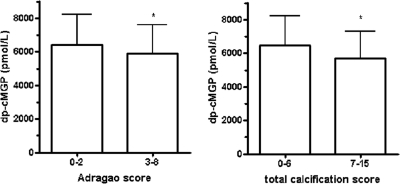
dp-cMGP did not show an association with vascular or valvular calcifications at single sites as determined by ultrasound or x-ray (Table 1). Next we compared MGP levels with two semiquantitative calcification scores, namely the Adragao score11 and the extended composite score (Adragao score plus calcifications of arteriovenous [AV] fistula and neighboring arteries, carotid arteries, and mitral and aortic heart valves).12 These analyses showed 9 and 12% lower levels, respectively, of dp-cMGP in patients with more extensive calcifications compared with patients with fewer calcifications (P = 0.042 and P = 0.011, respectively; Figure 2). dp-ucMGP and PIVKA-II levels did not correlate with the extent of vascular calcifications (data not shown).
Figure 2.
Reduced levels of dp-cMGP in dialysis patients with more calcifications. Serum levels of dp-cMGP in patients with high versus low calcification score (Adragao score and total calcification score). The Adragao score counts vascular calcifications of the pelvis (four quadrants) and hands (four quadrants),11 whereas the extended total calcification score uses the Adragao score plus calcification of both carotid arteries, AV fistula with neighboring arteries, and aortic and mitral heart valves (*P < 0.05).12
dp-ucMGP and Survival in Hemodialysis Patients
We next categorized the hemodialysis patients as being above or below the median of MGP plasma levels to perform a Kaplan-Meier survival analysis over 3 years. Plasma concentrations of dp-ucMGP were not significantly associated with all-cause and cardiovascular mortality (log-rank test: P = 0.08 and P = 0.09, respectively). Using univariate Cox regression analysis, low levels of dp-ucMGP had a hazard ratio (HR) of 1.71 (95% confidence interval [CI], 0.92 to 3.17; P = 0.09) for all-cause mortality and an HR of 1.83 (95% CI, 0.90 to 3.70; P = 0.09) for cardiovascular mortality. High PIVKA-II levels (>2 ng/ml) were not associated with an increased mortality risk (log-rank test: P = 0.67; Cox regression: HR, 1.00; 95% CI, 0.53 to 1.89, P = 1.00).
dp-cMGP and Survival in Hemodialysis Patients
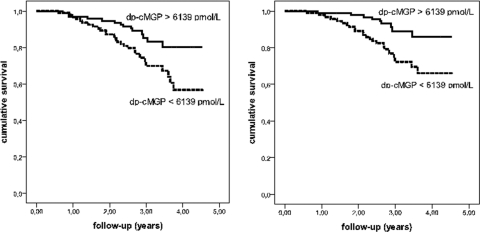
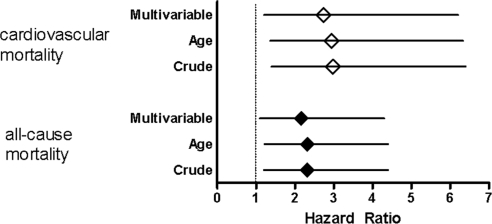
Kaplan-Meier analysis showed that low dp-cMGP levels (<6139 pmol/L) were associated with an increased all-cause and cardiovascular mortality risk (Figure 3; log-rank test: P = 0.008 and P = 0.003, respectively). The univariate Cox regression analysis showed an HR of 2.32 (95% CI, 1.2 to 4.4; P = 0.009) for all-cause mortality and an HR of 2.98 (95% CI, 1.4 to 6.4; P = 0.005) for cardiovascular mortality (Figure 4; Table 2). When adjusting for age, low plasma levels of dp-cMGP remained a significant predictor for all-cause (HR, 2.31; 95% CI, 1.2 to 4.4; P = 0.010) and cardiovascular mortality (HR, 2.94; 95% CI, 1.4 to 6.3; P = 0.006). The multivariable Cox regression analysis with adjustment for age plus adjustment for the serum parameters calcium, phosphate, and high-sensitivity CRP yielded HRs of 2.16 (95% CI, 1.1 to 4.3; P = 0.027) for all-cause and 2.74 (95% CI, 1.2 to 6.2; P = 0.015) for cardiovascular mortality (Figure 4; Table 2).
Figure 3.
Low dp-cMGP levels predict all-cause and cardiovascular mortality in dialysis patients. Kaplan-Meier analysis of all-cause (left) and cardiovascular (right) mortality in patients with low and high levels of dp-cMGP (grouped according to median), log-rank test: P = 0.008 and P = 0.003, respectively.
Figure 4.
With adjustment, low dp-cMGP remains a significant mortality predictor in dialysis patients. Effect of low levels of dp-cMGP on all-cause and cardiovascular mortality determined by Cox regression analysis (crude, adjusted for age, and multivariable adjusted hazard ratios). The multivariable adjusted analysis included age, gender, diabetes mellitus, dialysis vintage, body mass index, presence of vascular disease at the start of the study, and the serum parameters calcium, phosphate, and high-sensitive CRP.
Table 2.
Effect of low levels (below median) of desphospho-carboxylated matrix gla protein (dp-cMGP) on all-cause and cardiovascular mortality determined by Cox regression analysis (crude, adjusted for age, and multivariable adjusted hazard ratios)
| Low dp-cMGP | All-Cause Mortality | Cardiovascular Mortality | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Crude | 2.32 | 1.2 to 4.4 | 0.009 | 2.98 | 1.4 to 6.4 | 0.005 |
| Adjusted for age | 2.31 | 1.2 to 4.4 | 0.010 | 2.94 | 1.4 to 6.3 | 0.006 |
| Multivariable adjusted | 2.16 | 1.1 to 4.3 | 0.027 | 2.74 | 1.2 to 6.2 | 0.015 |
Effect of Vitamin K2 Supplementation
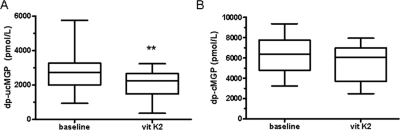
To examine whether vitamin K can affect dp-ucMGP and dp-cMGP levels, we performed a vitamin K2 supplementation pilot study in 17 hemodialysis patients (Figure 5). Daily vitamin K2 supplementation (135 μg of menaquinone-7 orally) over a period of 6 weeks resulted in a significant reduction of dp-ucMGP (baseline: 2750 ± 1120 pmol/L, 6-week K2: 2020 ± 794 pmol/L, P = 0.0027) and of PIVKA-II (baseline: 5.6 ± 3.2 ng/ml, 6-week K2: 3.4 ± 2.2 ng/ml, P = 0.0004), whereas vitamin K2 did not change dp-cMGP levels in a significant way (baseline: 6132 ± 1931 pmol/L, 6-week K2: 5521 ± 1706 pmol/L, P = 0.1).
Figure 5.
Vitamin K2 supplementation reduces dp-ucMGP but not dp-cMGP levels in dialysis patients. Plasma levels of dp-ucMGP and dp-cMGP at baseline and after 6 weeks of treatment with vitamin K2 (135 μg MK-7). (A) Changes in dp-ucMGP levels (**P < 0.01), whereas B depicts dp-cMGP levels (not significant) before (baseline) and after vitamin K2 substitution (vit K2), respectively.
DISCUSSION
In this study, we provided the first data on plasma levels of dp-cMGP in hemodialysis patients as a marker for mortality risk. Moreover, we report that vitamin K2 supplementation can improve the vitamin K status of dialysis patients.
First, we found that dialysis patients exhibited markedly elevated plasma levels of both MGP species (dp-ucMGP and dp-cMGP) compared with subjects with normal renal function. Because both species are based on a capture antibody directed against the nonphosphorylated MGP peptide, impaired MGP phosphorylation in the dialysis population might explain the increased levels of desphospho-MGP. Increased production or release of MGP may also relate to the generally increased vascular damage observed in dialysis patients, and indeed, an accumulation of MGP in and around vascular calcifications has been documented.9 Moreover, decreased MGP renal clearance could be attributed to reduced kidney function. In healthy subjects, MGP, with an apparent molecular mass of 12 kD, is not found in urine, but MGP levels in renal veins are reported to be 13% lower than in renal arteries.13 Thus, as expected with a low-molecular mass protein, the kidneys excrete and metabolize MGP, and some degree of renal retention will occur in dialysis patients.
We previously reported low circulating t-ucMGP levels in hemodialysis patients.14 Although seemingly contradictory to this finding of high dp-ucMGP levels, this may be explained by the fact that low t-ucMGP levels were measured with a mono-antibody assay, irrespective of its phosphorylation status. The concentration of t-ucMGP is in the nanomole range, whereas both dp-cMGP and dp-ucMGP are in the picomole range, with an approximately 2000-fold difference. The t-ucMGP fraction is likely to consist largely of phosphorylated MGP, fragmented MGP, and only a small fraction of dp-ucMGP. The increased dp-ucMGP levels in uremia found in our study are consistent with a recent study showing increasing dp-ucMGP levels with higher chronic kidney disease (CKD) stages.15 In this study, the levels seemed to be even higher than those of our previous study,15 which may be because of the fact that we studied ESRD patients with long dialysis vintage and little to no residual renal function. Additionally, it could also point to a more pronounced vitamin K deficiency in our cohort of patients with long dialysis vintage. Our ESRD patients with long dialysis vintage are likely to represent the patient cohort with the worst vascular dysfunction. The fact that in this population all dp-cMGP levels were extremely high could explain why no other significant correlations of MGP with vascular measures were observed in our study.
The major finding of our study was that dp-cMGP levels below the median predicted all-cause and cardiovascular mortality in dialysis patients. MGP acts locally in the vessel wall, whereas circulating MGP can not prevent vascular calcification.8 Circulating dp-cMGP may thus serve as a biomarker for vascular health. Consistent with this, in this study, dp-cMGP served as an independent predictor of mortality. In two recent studies, dp-ucMGP was identified as a survival marker, with lower levels heralding a better prognosis,15,16 whereas we failed to detect such a relationship. This may be related to the different patient cohort in our study, which consisted of long-term dialysis patients. At present, all data are consistent with the concept that MGP isoforms (dp-cMGP or dp-ucMGP) correlate with better survival and decreased vascular calcification. Current data on the relevance of the different MGP species are summarized in Table 3.
Table 3.
Clinical data on the relevance of different MGP isoforms
| MGP Species | Studies | Interpretation |
|---|---|---|
| Total uc-MGP (uncarboxylated, mainly phosphorylated MGP) | Reduced levels in patients with cardiovascular disease and CKD patients31 | May be partially bioactive |
| Negative association with coronary calcification in dialysis patients32 | Low circulating levels reflect cardiovascular calcifications, possibly because of binding to vascular calcium precipitates | |
| dp-ucMGP (nonphosphorylated, uncarboxylated MGP) | Correlation with PIVKA-II (this study) | Inactive species |
| Increased levels in CKD patients15 and dialysis patients (this study) | Most likely reflects vitamin K status of the vessel wall | |
| Increased levels associated with increased mortality risk in CKD patients but not in dialysis patients15 | ||
| Increased levels associated with increased mortality in aortic stenosis patients16 | ||
| dp-cMGP (nonphosphorylated, carboxylated MGP) | Increased levels in dialysis patients | Partially bioactive MGP |
| Reduced levels in dialysis patients associated with increased all-cause and cardiovascular mortality risk | May reflect vascular health | |
| Reduced levels in dialysis patients with vascular calcification (all this study) |
In our study, dp-cMGP levels were increased in dialysis patients compared with healthy controls. The lack of tests for phosphorylated MGP species makes it technically impossible to decide whether these high levels are caused by impaired phosphorylation, increased synthesis, or slow elimination. Interestingly, within the cohort of dialysis patients, the lower dp-cMGP levels were related to a higher mortality risk. This seemingly paradoxical result cannot easily be explained but may be attributed to the fact that MGP is no longer being produced because of vessel wall dysfunction or reduced vascular smooth muscle cell numbers. In fact, dialysis patients seem to have a reduced number of vascular smooth muscle cells caused by apoptosis.10 Thus, suboptimal production of active MGP (by poor MGP carboxylation, expression, or both) seems to contribute to the elevated mortality in dialysis patients. Obviously, poor vitamin K status is a condition that can be readily addressed by increasing vitamin K intake. Therefore, we first established vitamin K deficiency with a second, independent assay (PIVKA-II) and thereafter studied whether the vascular vitamin K status (as deduced from dp-ucMGP) could be improved by vitamin K supplements.
The majority of dialysis patients in our study exhibited a vitamin K deficiency (some 64%) as indicated by increased PIVKA-II levels. The high correlation of PIVKA-II levels with dp-ucMGP levels implicates that the elevated dp-ucMGP levels observed in dialysis patients largely reflect vascular vitamin K deficiency. This observation suggests that dialysis patients are not only vitamin K deficient in the liver but in the vessel wall as well. Dialysis patients are known to exhibit subclinical vitamin K deficiency.17,18 However, direct assessment of vitamin K levels is difficult to interpret because these levels depend on recent nutritional intake. In two studies, 6 to 29% of the CKD patients had low phylloquinone levels.17,18 More importantly, in these studies, 60 to 93% of the CKD patients had increased levels of uncarboxylated osteocalcin,17,18 and 97% had increased PIVKA-II levels,18 indicating a systemic vitamin K deficiency. However, osteocalcin, as a biomarker of vitamin K deficiency in CKD, can be compromised by the retention of fragments and secondary hyperparathyroidism. In the context of vitamin K deficiency, concerns have been raised about therapeutic vitamin K antagonism (coumarin derivatives) in dialysis patients with atrial fibrillation.19,20 In a retrospective study, it was shown that dialysis patients have an increased risk for both hemorrhagic and ischemic stroke when treated with coumarins compared with no treatment at all.19 In addition, in a randomized trial to prolong graft patency with warfarin, the study had to be stopped because of significantly increased major bleeding events in the treatment group.21 In animal models and humans, warfarin has been shown to induce significantly more cardiovascular calcifications.22,23 Given these data, vitamin K supplementation seems to be an attractive therapeutic option in patients at cardiovascular risk. Indeed, vitamin K2 (menaquinone-7) supplementation in hemodialysis patients significantly reduced the levels of dp-ucMGP in our study. In line with this, supplementing elderly subjects for 3 years with vitamin K1 significantly halted the progression of coronary artery calcification in the group of participants adherent to treatment, supporting our view that improving the vascular vitamin K status positively affects vascular health.24 Moreover, in subjects with normal renal function, the intake of dietary vitamin K2 was inversely correlated with the presence of coronary artery disease, cardiovascular calcification, and cardiovascular death.25,26
Vitamin K2 supplementation in our study showed a significant reduction of dp-ucMGP, whereas dp-cMGP levels were not significantly altered after 6 weeks of daily vitamin K supplementation. There are several explanations for this finding. First, the duration of vitamin K supplementation may have been too short to detect a significant change in dp-cMGP. Second, carboxylation and phosphorylation are linked together so that the gain in cMGP is accompanied by phosphorylation. Third, carboxylated MGP (either in the form of fully maturated p-cMGP or partially maturated dp-cMGP) could remain in the vessel wall and is not released into the circulation.
In our cross-sectional study, we could not detect an association between the vitamin K status of the liver, as reflected by PIVKA-II levels, and vascular calcification and mortality risk. This could be attributed to the rather low number of patients in our study or to the fact that other studies with assessment of vitamin K intake mainly included patients with normal renal function. Therefore, we now initiated intervention studies to test whether vitamin K supplementation can improve vascular health and thereby reduce mortality in dialysis and/or cardiovascular patients.
There are two major limitations to our study. First, the assays we used to analyze dp-cMGP and dp-ucMGP both detect only the nonphosphorylated fraction of MGP. Biochemical tests for the fully maturated circulating MGP (i.e., both carboxylated and phosphorylated) are not available at the moment. Moreover, the function of MGP phosphorylation is not exactly known. Second, because MGP serum levels do not necessarily reflect MGP tissue levels of the vasculature, we can only speculate how MGP as a serum biomarker relates to local MGP as a vascular calcification inhibitor. To improve our understanding of the relationship between locally produced MGP and circulating MGP, studies investigating both MGP in the vascular wall and in the circulation in relation to calcification are in progress.
In conclusion, we report for the first time that dp-cMGP may serve as a potent survival predictor in hemodialysis patients and possibly reflects their vascular health. Given the fact that vitamin K2 supplementation reduces dp-ucMGP levels, our study provides the basis for interventional studies with vitamin K supplementation.
CONCISE METHODS
Patients
We prospectively analyzed 188 prevalent hemodialysis patients with a low prevalence of diabetes (11%) from the Center for Renal Diseases of the Zvezdara University Medical Center, Belgrade (Table 1). All chronic hemodialysis patients were eligible to enter the study if they agreed to participate. Patients on warfarin treatment were excluded. The control group consisted of 98 healthy volunteers with normal renal function (58 ± 15 years). The study protocol was approved by the Ethics Committee of the Zvezdara University Medical Center, Belgrade, and each patient gave informed consent. Patients were enrolled between December 2003 and October 2005 and observed for 221 to 1662 days until the end of June 2008 (mean follow-up, 1104 days). During the observation period, 43 deaths occurred (cardiovascular, 32; malignancy, 7; other, 4). Gender was equally distributed (99 male, 89 female). Etiologies for ESRD were as follows: hypertensive nephrosclerosis, 99 (53%); glomerulonephritis, 24 (13%); autosomal dominant polycystic kidney disease, 19 (10%); pyelonephritis, tubulointerstitial disease, and obstructive nephropathy, 24 (13%); diabetic nephropathy, 10 (5%); systemic lupus erythematosus, 4 (2%); Balkan endemic nephropathy, 8 (4%). The presence of vascular disease at the start of the study was defined as a pre-existing diagnosis of coronary artery disease, cerebrovascular disease, or peripheral artery disease.
Vascular Calcification
Calcifications were assessed by x-ray and ultrasound. X-rays of the pelvis, hands, and AV-fistula arm were performed, and obvious vascular calcifications were counted by two independent observers. In addition, echocardiography of the mitral and aortic heart valve and ultrasound of both carotid arteries were used for the detection of calcifications. Valvular calcifications were determined by one single observer using echocardiography with an Aspen-ACUSON device (Mountain View, CA) equipped with a 2.5-MHz probe and calcified carotid plaques were defined as echogenic structures showing protrusion into the lumen with focal widening that was 50% greater than the intima media thickness (IMT) of adjacent sites.27 In addition to single calcification sites, we calculated two semiquantitative calcification scores.12 First, we determined the Adragao score by analyzing conventional x-rays of the pelvis and hands.11 In brief, x-rays of the pelvis and both hands were divided into four sections by a median vertical line and a horizontal line just above the upper rim of the femoral heads and the metacarpal bones, respectively. The presence of linear vascular calcification in each quadrant was counted as 1 point; thus, a maximum of 8 points could be achieved. Second, we created an extended composite calcification score where not only calcifications of pelvis and hands but also calcifications of the fistula arm plus neighboring arteries, mitral, and aortic heart valves and both carotid arteries were accounted for.12 Each site of calcification assessment was counted as 1 point. Thus, a maximum score of 15 points could be obtained: Adragao (pelvis 4, hands 4), fistula arm, i.e., fistula itself with one (upper arm) or two (lower arm) neighboring arteries (2 or 3 points), mitral plus aortic valves (2), and carotid arteries (2). X-ray images were assessed by two experienced physicians blinded to the patient's condition. To compare patients with a low versus a high Adragao calcification score, patients with a score of 0 to 2 were included in the low calcification group, and patients with a score of 3 to 8 were in the high calcification group.11 For the composite score, patients with a lower score of 0 to 6 were compared with patients with a higher score of 7 to 15. It has been shown for the Adragao score that patients with a score of 0 to 2 have a better prognosis than those with a score of 3 to 8.11,12 Moreover, the presence of calcifications of the heart valves28 and the AV fistula27 have been shown to be a mortality risk factor. Bellasi et al.29 reported that simple measures of cardiovascular calcification had a very good correlation with more sophisticated measurements obtained with CT.
Assessment of Cardiovascular Parameters
To determine cardiovascular parameters we measured systolic and diastolic BP, carotid-femoral pulse wave velocity, and IMT, as described previously.27 Briefly, pulse wave velocity was assessed using one carotid and one femoral sensor simultaneously to determine the velocity of the pulse in relation to the distance between the femoral artery and the suprasternal notch (Complior SP system; Artech Medical, Pantin, France). Two measurements were performed by two trained observers. To determine IMT, B-mode ultrasonography of the carotid arteries was performed using ALOCA SSD 2000 system (Tokyo, Japan) equipment with 7.5-MHz linear transducers. One trained investigator scanned both common carotid arteries, 4 cm from the bulbs, the carotid bulbs, and the first 2 cm of the internal and external carotid arteries. IMT and lumen diameter measurements were performed in a plaque-free area. IMT was measured as the distance between adventitia and the lining of the arterial lumen/intima (mm). This was measured four times on both sides, and the mean of these measurements was recorded.27
Biochemistry
Blood was drawn from the arterial site after a long dialysis interval just before dialysis started (nonfasting) and was collected in citrate and in serum tubes. Levels of circulating dp-ucMGP and dp-cMGP were determined in plasma using sandwich ELISA techniques. Both assays use a monoclonal antibody against the nonphosphorylated sequence 3 to 15 as capture antibody. The dp-ucMGP assay is based on the use of the detection monoclonal antibody directed against the noncarboxylated sequence 35 to 49 in human MGP (VitaK BV, Maastricht, The Netherlands). dp-cMGP levels were measured by a similar sandwich ELISA in which the detection antibody was directed against the carboxylated sequence 35 to 53 in human MGP (VitaK BV). Plasma PIVKA-II levels were assessed by ELISA (Asserachrom PIVKA-II; Stago, Asnières-sur-Seine, France); normal PIVKA-II levels were considered <2 ng/ml.30 Biochemical analysis of serum factors (calcium, phosphate, lipids, protein, cholesterol, triglycerides) was performed by standard laboratory procedures using an automated analyzer. Intact PTH was assessed by a chemiluminescence assay (Diagnostic Product Corporation, Los Angeles, CA). Serum analysis for high-sensitivity CRP was performed by particle-enhanced immunonephelometry using a standard “CardioPhase hsCRP” for “BNII” (Dade Behring Holding GmbH, Liederbach, Germany). Calcium and phosphate measurements were calculated as mean values from four measurements within 4 months before the start of the study. All other parameters were single measurements at the beginning of the study.
Vitamin K2 Supplementation
Seventeen hemodialysis patients (mean age, 62 ± 17 years; 13 male/4 female) were recruited for a 6-week pilot study of daily supplementation with vitamin K2. Inclusion criteria were age >18 years and stable hemodialysis for >3 months. Exclusion criteria were soy allergy, warfarin treatment, history of thrombosis, inflammatory bowel disease, steroid medication, and pregnancy. After obtaining plasma samples for the determination of basal levels of dp-ucMGP and dp-cMGP, patients were orally administered 135 μg of the vitamin K2 form menaquinone-7 (MK-7; NattoPharma, Lysaker, Norway) daily over a period of 6 weeks. The protocol of the pilot study adhered to the Declaration of Helsinki and was approved by the ethics committee of the Rheinisch-Westfälische Technische Hochschule Aachen, and each patient gave written informed consent.
Statistical Analysis
Continuous variables were summarized by means and corresponding SD. Comparisons of the values of continuous variables between two groups were made using an unpaired t test. Paired t test was used for the analysis of dp-ucMGP and dp-cMGP levels before and after 6 weeks of vitamin K2 supplementation. Categorical variables were summarized by relative frequencies. The χ2 test was used for studying associations between various categorical variables. Relative risk (odds ratio) and 95% CIs for numerous variables were calculated by univariate logistic regression. The Kaplan-Meier method was used to estimate the cumulative survival. To identify the prognostic factors of all-cause and cardiovascular death, comparisons between survival curves were made by log-rank test. Cox regression was used (enter method) to determine the effect of MGP levels and PIVKA-II on all-cause and cardiovascular mortality. The multivariable adjusted Cox regression analysis accounted for age, gender, diabetes, dialysis vintage, body mass index, presence of vascular disease at the start of the study, and serum calcium, phosphate, and high-sensitivity CRP as possible confounders. Confounders were selected either on the basis of whether they showed a significant association with dp-cMGP (Table 1) or whether they were known to influence mortality per se. Statistical analysis was performed with SPSS 16.0. P < 0.05 was considered statistically significant.
DISCLOSURES
C.V. is CEO of VitaK, Maastricht University, The Netherlands. L.S. is VitaK's vice president and consultant to NattoPharma (Lysaker, Norway), the supplier of the vitamin K2 form menaquinone-7.
Acknowledgments
We thank Katrin Härthe for excellent technical assistance.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.US Renal Data System: USRDS 1998 annual data report. Am J Kidney Dis 32: 69–80, 1998 [Google Scholar]
- 2.London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H: Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 18: 1731–1740, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Schlieper G, Westenfeld R, Brandenburg V, Ketteler M: Inhibitors of calcification in blood and urine. Semin Dial 20: 113–121, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Schäfer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J, Muller-Esterl W, Schinke T, Jahnen-Dechent W: The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest 112: 357–366, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ketteler M, Bongartz P, Westenfeld R, Wildberger JE, Mahnken AH, Bohm R, Metzger T, Wanner C, Jahnen-Dechent W, Floege J: Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: A cross-sectional study. Lancet 361: 827–833, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G: Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 386: 78–81, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Wajih N, Borras T, Xue W, Hutson SM, Wallin R: Processing and transport of matrix gamma-carboxyglutamic acid protein and bone morphogenetic protein-2 in cultured human vascular smooth muscle cells: Evidence for an uptake mechanism for serum fetuin. J Biol Chem 279: 43052–43060, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Murshed M, Schinke T, McKee MD, Karsenty G: Extracellular matrix mineralization is regulated locally: Different roles of two gla-containing proteins. J Cell Biol 165: 625–630, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schurgers LJ, Teunissen KJ, Knapen MH, Kwaijtaal M, van Diest R, Appels A, Reutelingsperger CP, Cleutjens JP, Vermeer C: Novel conformation-specific antibodies against matrix gamma-carboxyglutamic acid (Gla) protein: Undercarboxylated matrix Gla protein as marker for vascular calcification. Arterioscler Thromb Vasc Biol 25: 1629–1633, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Shroff RC, McNair R, Figg N, Skepper JN, Schurgers L, Gupta A, Hiorns M, Donald AE, Deanfield J, Rees L, Shanahan CM: Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation 118: 1748–1757, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Adragao T, Pires A, Lucas C, Birne R, Magalhaes L, Goncalves M, Negrao AP: A simple vascular calcification score predicts cardiovascular risk in haemodialysis patients. Nephrol Dial Transplant 19: 1480–1488, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Schlieper G, Brandenburg V, Djuric Z, Damjanovic T, Markovic N, Schurgers L, Kruger T, Westenfeld R, Ackermann D, Haselhuhn A, Dimkovic S, Ketteler M, Floege J, Dimkovic N: Risk factors for cardiovascular calcifications in non-diabetic Caucasian haemodialysis patients. Kidney Blood Press Res 32: 161–168, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Rennenberg RJ, Schurgers LJ, Vermeer C, Scholte JB, Houben AJ, de Leeuw PW, Kroon AA: Renal handling of matrix Gla-protein in humans with moderate to severe hypertension. Hypertens Res 31: 1745–1751, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Hermans MM, Vermeer C, Kooman JP, Brandenburg V, Ketteler M, Gladziwa U, Rensma PL, Leunissen KM, Schurgers LJ: Undercarboxylated matrix GLA protein levels are decreased in dialysis patients and related to parameters of calcium-phosphate metabolism and aortic augmentation index. Blood Purif 25: 395–401, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Schurgers LJ, Barreto DV, Barreto FC, Liabeuf S, Renard C, Magdeleyns EJ, Vermeer C, Choukroun G, Massy ZA: The circulating inactive form of matrix Gla protein is a surrogate marker for vascular calcification in chronic kidney disease: A preliminary report. Clin J Am Soc Nephrol 5: 568–575, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ueland T, Gullestad L, Dahl CP, Aukrust P, Aakhus S, Solberg OG, Vermeer C, Schurgers LJ: Undercarboxylated matrix Gla protein is associated with indices of heart failure and mortality in symptomatic aortic stenosis. J Intern Med 268: 483–492, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Pilkey RM, Morton AR, Boffa MB, Noordhof C, Day AG, Su Y, Miller LM, Koschinsky ML, Booth SL: Subclinical vitamin K deficiency in hemodialysis patients. Am J Kidney Dis 49: 432–439, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Holden RM, Morton AR, Garland JS, Pavlov A, Day AG, Booth SL: Vitamins K and D status in stages 3–5 chronic kidney disease. Clin J Am Soc Nephrol 5: 590–597, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan KE, Lazarus JM, Thadhani R, Hakim RM: Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol 20: 2223–2233, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kruger T, Floege J: Coumarin use in dialysis patients with atrial fibrillation: More harm than benefit? Nephrol Dial Transplant 24: 3284–3285, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Crowther MA, Clase CM, Margetts PJ, Julian J, Lambert K, Sneath D, Nagai R, Wilson S, Ingram AJ: Low-intensity warfarin is ineffective for the prevention of PTFE graft failure in patients on hemodialysis: A randomized controlled trial. J Am Soc Nephrol 13: 2331–2337, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Price PA, Faus SA, Williamson MK: Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arterioscler Thromb Vasc Biol 18: 1400–1407, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Koos R, Mahnken AH, Muhlenbruch G, Brandenburg V, Pflueger B, Wildberger JE, Kuhl HP: Relation of oral anticoagulation to cardiac valvular and coronary calcium assessed by multislice spiral computed tomography. Am J Cardiol 96: 747–749, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Shea MK, O'Donnell CJ, Hoffmann U, Dallal GE, Dawson-Hughes B, Ordovas JM, Price PA, Williamson MK, Booth SL: Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am J Clin Nutr 89: 1799–1807, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geleijnse JM, Vermeer C, Grobbee DE, Schurgers LJ, Knapen MH, van dM I, Hofman A, Witteman JC: Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: The Rotterdam Study. J Nutr 134: 3100–3105, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Gast GC, de Roos NM, Sluijs I, Bots ML, Beulens JW, Geleijnse JM, Witteman JC, Grobbee DE, Peeters PH, van der Schouw YT: A high menaquinone intake reduces the incidence of coronary heart disease. Nutr Metab Cardiovasc Dis 19: 504–510, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Schlieper G, Kruger T, Djuric Z, Damjanovic T, Markovic N, Schurgers LJ, Brandenburg VM, Westenfeld R, Dimkovic S, Ketteler M, Grootendorst DC, Dekker FW, Floege J, Dimkovic N: Vascular access calcification predicts mortality in hemodialysis patients. Kidney Int 74: 1582–1587, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Wang AY, Wang M, Woo J, Lam CW, Li PK, Lui SF, Sanderson JE: Cardiac valve calcification as an important predictor for all-cause mortality and cardiovascular mortality in long-term peritoneal dialysis patients: A prospective study. J Am Soc Nephrol 14: 159–168, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Bellasi A, Ferramosca E, Muntner P, Ratti C, Wildman RP, Block GA, Raggi P: Correlation of simple imaging tests and coronary artery calcium measured by computed tomography in hemodialysis patients. Kidney Int 70: 1623–1628, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Grosley BM, Hirschauer C, Chambrette B, Bezeaud A, Amiral J: Specific measurement of hypocarboxylated prothrombin in plasma or serum and application to the diagnosis of hepatocellular carcinoma. J Lab Clin Med 127: 553–564, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Cranenburg EC, Vermeer C, Koos R, Boumans ML, Hackeng TM, Bouwman FG, Kwaijtaal M, Brandenburg VM, Ketteler M, Schurgers LJ: The circulating inactive form of matrix Gla protein (ucMGP) as a biomarker for cardiovascular calcification. J Vasc Res 45: 427–436, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Cranenburg EC, Brandenburg VM, Vermeer C, Stenger M, Muhlenbruch G, Mahnken AH, Gladziwa U, Ketteler M, Schurgers LJ: Uncarboxylated matrix Gla protein (ucMGP) is associated with coronary artery calcification in haemodialysis patients. Thromb Haemost 101: 359–366, 2009 [PubMed] [Google Scholar]