Serotonergic regulation of neuronal excitability in the prefrontal cortex (original) (raw)
. Author manuscript; available in PMC: 2012 Sep 1.
Abstract
The cerebral cortex receives a dense serotonergic innervation originating predominantly from the dorsal raphe nucleus. This innervation regulates cortical functioning by activating multiple serotonin receptors that are differentially expressed by pyramidal cells and interneurons. Electrophysiological studies in the prefrontal cortex indicate that receptors of the 5-HT1A and 5-HT2A subtypes are the main serotonin receptors regulating membrane excitability in pyramidal cells. Most pyramidal cells in layer V coexpress 5-HT1A and 5-HT2A receptors that together regulate how these neurons encode excitatory input into neuronal firing. In contrast, a subset of large pyramidal cells of deep layer V appears to express exclusively 5-HT2A receptors that depolarize and excite these cells. Serotonin also depolarizes and excites at least two classes of GABAergic interneurons by acting on 5-HT3 and 5-HT2A receptors. The differential expression of serotonin receptors in different pyramidal cells and interneurons is consistent with a growing appreciation of the anatomical, molecular and functional heterogeneity of pyramidal cells and interneurons of the cerebral cortex. These find ings begin to lay the ground for a cellular-level understanding of the serotonergic regulation of the prefrontal cortex.
Keywords: Serotonin, 5-HT1A receptor, 5-HT2A receptor, pyramidal cell, GABArgic interneuron, prefrontal cortex
1. Introduction
The cerebral cortex, including the prefrontal cortex, receives a robust serotonergic innervation that is thought to contribute to the subcortical regulation of cortical function (Fuxe, 1965; Lidov et al., 1980; Jacobs and Azmitia, 1992). Furthermore, numerous clinical and preclinical studies point to a role for this innervation in neuropsychiatric disorders and/ or their pharmacotherapeutic treatment (Henninger, 2000). However, exactly how serotonin regulates cortical function is only partly understood. This stems from the complexity of the problem, which needs to be tackled at multiple levels, and from technical limitations that have slowed advances in this field. In this review we focus on recent advances in our understanding of the electrophysiological actions of serotonin in the rodent cerebral cortex, with a special focus on cellular electrophysiology of serotonin in the prefrontal cortex, which is the focus of the author’s work.
2. A brief primer on the cerebral cortex
Neurons of the cerebral cortex can be divided into two main types: glutamate-secreting “excitatory” cells, mostly but not exclusively of the pyramidal cell type, and “inhibitory” GABA-secreting interneurons. Pyramidal cells are the most abundant cell type in the cortex and are organized in band s of varying cell density which define the cortical cell layers. The neocortex has been divided traditionally into six layers with layer I being the most superficial and layer VI the deepest. However many regions of what we recognize as cerebral cortex contains fewer layers. Most notably for the purposes of this review, much of the anterior cortex including the prefrontal cortex, is agranular, that is to say it lacks a defined layer IV.
Cell fating studies have now convincingly demonstrated that the laminar cytoarchitecture of the cerebral cortex and its functional organization are grounded in the development of the cerebral cortex. Pyramidal cells are born in the ventricular surface and migrate radially through the developing cortical plate towards the brain surface to produce the cortical layers. The migration pattern of the pyramidal cells follows an “inside-out” progression such that the earliest born cells are located in the deepest layers of cortex while later born cells are found at progressively more superficial levels (reviewed by Rakic, 2006). Recent studies indicate that as the progenitor cells divide, to give rise to pyramidal cells, they execute a rolling genetic program that generates a series of pyramidal neuron lineages that segregate radially giving rise to the cortical lamination (Molyneaux et al., 2007; Leone et al., 2008). GABAergic interneurons, in contrast, are born predominantly in the ganglionic eminence, at least in rodents, and migrate into cortex following a more complex program that makes their final laminar position less dependent on birthdate than for pyramidal cells (Butt et al., 2005; Miyoshi and Fishell, 2010).
In addition to the cellular and laminar heterogeneity outlined above, the cerebral cortex also exhibits considerable regional heterogeneity. This is widely recognized in our habitual parcelation of cortex into functional domains such as visual, primary motor, somatosensory or prefrontal cortices. These different regions were originally defined based upon functional studies and subsequently refined based upon thalamic connectivity. More recent studies however have focused on the molecular determinants underlying areal specialization and have begun to identify the signaling clues that help define different areas of cortex (O’Leary and Sahara, 2008; Rakic et al., 2009). This areal and laminar specialization results in considerable heterogeneity in the properties of pyramidal cells and interneurons and their connectivity and regulation by subcortical afferents. Of course this diversity needs to be considered when trying to understand the effects of serotonin in this region.
3. The serotonergic innervation of the cerebral cortex
The cerebral cortex receives a substantial serotonergic innervation originating predominantly from the dorsal and, to a lesser extent, the median raphe nuclei (O’Hearn and Molliver, 1984; Wilson and Molliver, 1991a; Wilson and Molliver, 1991b; Hoover and Vertes, 2007). This innervation is surprisingly widespread and, at least in rodents, exhibits only moderate variations in terms of areal and laminar density (Lidov et al., 1980). Receptor autoradiographic and/ or in situ hybridization studies have also documented the presence of most serotonin receptor subtypes and their mRNAs in the cerebral cortex (Mengod et al., 2006). However the distribution of these receptors exhibits substantial areal, laminar and cellular heterogeneity. For example, among 5-HT1 receptors, 5-HT1A and 5-HT1B receptor mRNAs are widely distributed in cortex while the expression of 5-HT1D receptor mRNA appears is more restricted and is confined predominantly to the entorhinal cortex (Pompeiano et al., 1992; Bruinvels et al., 1994; Bonaventure et al., 1998). Similarly, among 5-HT2 receptors, 5-HT2A receptor mRNA and 5-HT2A receptors are strongly expressed in cortex along a steep anteroposterior gradient (Mengod et al., 1990)(Figure 1) while 5-HT2C receptors exhibit a much more restricted distribution. Similarly, although both 5-HT1A and 5-HT2A receptors are coexpressed in the medial prefrontal cortex, their laminar distribution is only partly congruent. This heterogeneity is consistent with the cellular heterogeneity within the cerebral cortex discussed above, and emphasizes the need to focus on understanding the effects of serotonin at the level of defined cell types in well defined anatomical regions.
Figure 1. 5-HT2A receptors expression in the cerebral cortex.
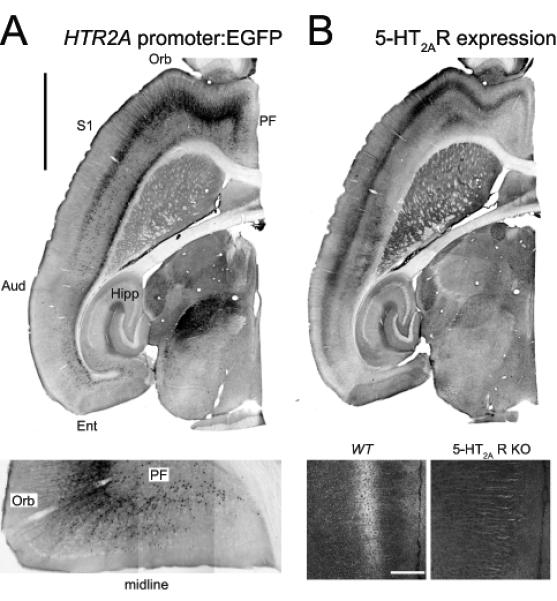
A. Expression of the 5-HT2A receptor gene (HTR2A) in the mouse forebrain as revealed in a BAC transgenic mouse expressing EGFP under the control of the 5-HT2A receptor promoter. Calibration bar: 2 mm. Aud: auditory cortex, Ent: entorhinal cortex, Orb: orbital cortex, PF: prefrontal cortex, S1: primary somatosensory cortex. Lower panel. Close up image depicting EGFP expression in an horizontal section through the prefrontal and orbital cortices. B. 5-HT2A receptor expression as determined using an affinity purified anti-5-HT2A receptor antibody. Notice the strong correspondence in EGFP in panel A and 5-HT2A receptor expression in Panel B. Lower panel: 5-HT2A receptor staining in the prefrontal cortex of a wild type (wt) mouse and a 5-HT2A receptor knockout (5-HT2AR KO) mouse. Calibration bar 200 μm. (Modified from Weber and Andrade, 2010).
4. The electrophysiology of serotonin in the rat prefrontal cortex
While the effects of serotonin on the physiology of the cerebral cortex are likely mediated at multiple levels, one important component is how serotonin regulates the electrical excitability of different cortical neurons and how this results in the regulation of neuronal networks. This is obviously a complex question that is made more difficult by our limited understanding of the cellular heterogeneity and synaptic architecture of cortex. This limitation notwithstanding, during the last decade considerable progress has been made in our understanding of the effects of serotonin in various cortical regions. Here we focus on the effects of serotonin in the rodent prefrontal cortex, which is probably where the effects of serotonin have been most thoroughly studied.
4.1. Serotonin regulation of pyramidal cell excitability
Administration of serotonin to cortical brain slices has been shown to elicit hyperpolarizing/ inhibitory and depolarizing/ excitatory effects on pyramidal and non-pyramidal cells as well as to modulate active conductances. It is now widely recognized that this diversity reflects the ability of serotonin to activate multiple serotonin receptors which are differentially expressed in different types of pyramidal and non-pyramidal cells in the cerebral cortex. In addition, serotonin has been show n to increase spontaneous excitatory and inhibitory synaptic transmission suggesting activation of intracortical circuits.
The effects of serotonin in prefrontal cortex have been studied most extensively in pyramidal cells of layer V. The most obvious direct effect of serotonin on these cells, in juvenile and adult rodent brain slices, is a hyperpolarization that is mediated by the activation of receptors of the 5-HT1A subtype (Araneda and Andrade, 1991; Beique et al., 2004)(Figure 2). A 5-HT1A receptor-mediated inhibition is also seen in vivo after stimulation of the dorsal raphe nucleus suggesting that these receptors can be activated by the release of endogenous serotonin (Margos-Bosch et al., 2004). It is now widely appreciated that 5-HT1A receptor induced hyperpolarizations are mediated by the activation of Kir3.x channels secondary to the liberation of Gβγ from heterotrimeric G proteins of the Gαi/ o subfamily (Andrade et al., 1986; Hibino et al., 2010). This is likely to also be the case in the prefrontal cortex. The observation of 5-HT1A receptor-mediated hyperpolarizations in layer V is consistent with the robust exp ression of 5-HT1A receptors in layers V and VI of the prefrontal cortex (Pompeiano et al., 1992)
Figure 2. 5-HT1A and 5-HT2 receptor-mediated responses in different neuronal subtypes in layer V of the prefrontal cortex.
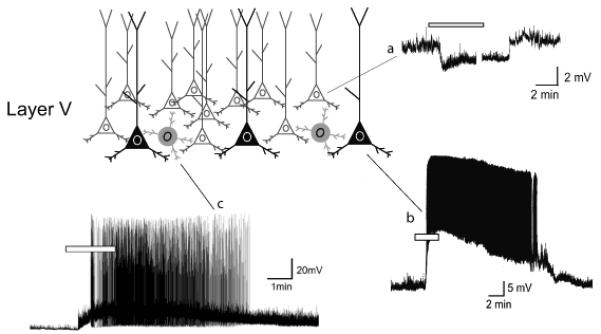
Activation of 5-HT1A receptors elicits a membrane hyperpolarization in many if not most pyramidal cells of layer V of the rodent prefrontal cortex (a). In contrast, activation of 5-HT2A receptors depolarizes a subset of pyramidal cells in deep layer V (b) and also a subset of GABAergic interneurons (c).
Pyramidal cells of layer V also express 5-HT2A receptors that, when activated by serotonin, induce a slow membrane depolarization that is accompanied by an inhibition of the slow calcium-activated afterhyperpolarization expressed in many cells of the layer V (Araneda and Andrade, 1991; Villalobos et al., 2005). Both of these effects increase membrane excitability and account for the ability of serotonin to excite at least a subset of layer V pyramidal neurons (Beique et al., 2007) (Figure 2). Unfortunately, at the present time little is known about the mechanism underlying the 5-HT2A receptor- induced depolarization or inhibition of the slow after hyperpolarization. Based upon previous work on other G_α_q-11-coupled receptors in these cells we can speculate that the 5-HT2A receptor depolarization may reflect the activation of nonselective cation currents (Araneda and Andrade, 1991; Haj-Dahmane and Andrade, 1996). Similarly, while the slow after hyperpolarization is known to reflect the activation of potassium currents downstream from calcium influx, little is know n currently about the identity of the channels carrying this current or the molecular signaling cascade used by 5-HT2A receptors to inhibit it.
The presence of robust 5-HT2A receptor-mediated electrophysiological effects in layer V is consistent with results obtained using in situ hybridization and receptor autoradiographic methods showing expression of 5-HT2A receptor mRNA and 5-HT2A binding sites in layer V of the prefrontal cortex (Mengod et al., 1990; Wright et al., 1995). It is also consistent with more recent results obtained using genetic approaches to map the cellular expression of the Htr2A gene, which codes the 5-HT2A receptor, and immunohistochemical approaches to map 5-HT2A receptor distribution in cortex (Weber and Andrade, 2010)(Figure 1).
Interestingly, 5-HT1A and 5-HT2A receptors appear to be coexpressed in a large fraction of pyramidal cells (Araneda and And rade, 1991; Beique et al., 2004; Margos-Bosch et al., 2004). In these neurons administration of serotonin results in a membrane hyperpolarization or outward current that is often followed by a slow membrane depolarization or inward current. Concurrent with these effects, 5-HT2A receptor also signals the inhibition of the slow after hyperpolarization (Villalobos et al., 2005). On the surface the coexpression of “inhibitory” 5-HT1A and “excitatory” 5-HT2A receptors would appear to make little functional sense. However we have argued that the “inhibitory” and “excitatory” categories are not suitable to describe the modulatory effects signaled by these G protein-coupled receptors. A more likely possibility is that 5-HT1A and 5-HT2A receptors regulate in a cooperative manner how pyramidal neurons encode excitatory inputs into action potential firing. Specifically, we have proposed that 5-HT2A receptors modulate neuronal gain by controlling the slow after hyperpolarization while 5-HT1A receptors control the input intensity range over which the cell will encode excitatory inputs into firing activity by regulating the cell’s membrane potential, (Araneda and Andrade, 1991; Zhang and Arsenault, 2005; Higgs et al., 2006).
4.2. Serotonin regulation of glutamate release
In concert with these direct effects of serotonin on pyramidal cell excitability, early studies in the prefrontal cortex identified a very strong 5-HT2A receptor-induced increase in spontaneous glutamate-mediated excitatory synaptic activity (Aghajanian and Marek, 1997; Marek and Aghajanian, 1998; Lambe et al., 2000). This effect was initially thought to reflect retrograde signaling by 5-HT2A receptors leading to the excitation of thalamocortical afferents (Lambe and Aghajanian, 2001). However recent work taking advantage of newly developed pharmacological and molecular interventions have failed to support this possibility (Beique et al., 2007). Instead, these studies suggest that, while a large fraction of pyramidal cells of layer V express both 5-HT1A and 5-HT2A receptors, a subpopulation of pyramid al cells located in d eep layer V expresses solely 5-HT2A receptors. These cells are strongly depolarized and excited by serotonin (Figure 2) and, since cortical pyramidal cells are extensively interconected, excitation of this second population of pyramidal cells in layer V probably accounts, at least in part, for the 5-HT2A receptor-mediated increase in glutamate release (Beique et al., 2007). Unfortunately the identity of this second population of pyramidal cells is currently unknown. Cell fating studies have previously shown in other regions of cortex that expression of the transcription factor Fezf2 helps define two populations of pyramidal cells in layer V, one giving rise to corticocortical projections and the other to corticofugal projections (Molyneaux et al., 2007; Leone et al., 2008). It is tempting to speculate that these two cell types may correspond to the two cell types defined by their serotonin responsiveness.
Much less is known about the effects of serotonin on pyramidal cells outside of layer V. Pyramidal cells of layer II and layer III give rise to ipsilateral and contralateral corticocortical projections and recent work suggest that these cells express predominantly 5-HT1A receptors signaling a membrane hyperpolarization/ outward current (Goodfellow et al., 2009). Little is currently known about the effects of serotonin in layer VI.
4.3. Serotonin regulation of GABAergic interneuron excitability
GABAergic interneurons, as a class, represents approximately a fourth of a ll neurons in the cerebral cortex. These cells form a diverse group that has been subdivided into more than a dozen subtypes based upon electrophysiological properties, anatomical features and molecular markers (Klausberger and Somogyi, 2008). Early studies showed that ad ministration of serotonin to slices of the cerebral cortex, including the prefrontal regions, results in large enhancements in ongoing spontaneous GABAergic synaptic transmission (Zhou and Hablitz, 1999). This suggested that serotonin excites at least a subpopulation of GABAergic interneurons (Figure 2). Subsequent studies have shown that this increase in synaptic spontaneous synaptic activity is mediated by the activation of 5-HT2A and 5-HT3 receptors, which are expressed predominantly on fast spiking parvalbumin expressing (Weber and Andrade, 2010) and vasoactive intestinal peptide/ cholecystokinin expressing interneurons (Ferezou et al., 2002) respectively. Direct recording from these two cell types have confirmed that serotonin indeed depolarizes and excites at least subsets of these two interneuron subtypes (Ferezou et al., 2002; Weber and Andrade, 2010). Serotonin can also inhibit fast spiking interneurons by activating 5-HT1A receptors (Puig et al., 2010). However, at the present time, it is not known if 5-HT1A receptors are coexpressed with 5-HT2A receptors in a subgroup of fast spiking interneurons or whether these receptors are expressed in different subgroups of fast spiking interneurons.
5. Effect of serotonin during cortical development
Cortical neurons and cortical networks undergo extensive changes during postnatal development. The early postnatal period is characterized by extensive synaptogenesis followed by synaptic refinement and pyramidal cells and interneurons undergo extensive morphological and electrophysiological maturation during this developmental epoch. Not surprisingly, this cortical maturation is accompanied by changes in the electrophysiological effects signaled by serotonin.
In the case of the prefrontal cortex, administration of serotonin to pyramidal cells of layer V of the prefrontal cortex during the first two postnatal weeks results in a consistent membrane depolarization or inward current that is capable of inducing robust neuronal excitations (Zhang, 2003; Beique et al., 2004). This depolarization is primarily mediated by serotonin receptors of the 5-HT7 subtype, with only a secondary contribution of 5-HT2A receptors. Thus the effects of serotonin on layer V of the prefrontal cortex during the immediate postnatal period differ markedly from those seen in juvenile and adult animals. Remarkably, this robust serotonin-induced d epolarization wanes during the third postnatal week as pyramidal cells of layer V cease to express 5-HT7 receptors (Beique et al., 2004). At the same time, pyramidal cells of layer V begin to express 5-HT1A receptors, which leads to the appearance of the serotonin-induced hyperpolarization that characterizes the adult response (Beique et al., 2004). This developmental progression, however, is not seen in all cells of prefrontal cortex. In particular, pyramidal cells of layer II-III have been reported to be consistently inhibited by serotonin acting via 5-HT1A receptors throughout postnatal development (Goodfellow et al., 2009).
These coordinated changes in serotonin receptor expression during development suggest a potentially important roles for serotonin in the maturation of cortical circuits. Consistent with this idea, behavioral or genetic manipulations leading to changes in serotonin receptor expression during development have been shown to have profound consequences on adult behavior (Gross et al., 2002; Goodfellow et al., 2009). Unfortunately at the present time little is known about the precise mechanisms by which changes in receptor expression regulates the maturation of cortical circuits. The postnatal period is characterized by extensive synaptogenesis followed by synaptic refinement and pruning (O’Leary and Koester, 1993) and previous studies have shown that neuronal and synaptic activity play a key role in the specification (and strength) of synaptic connections during this period (Maffei and Turrigiano, 2008). Therefore it is possible that serotonin may regulate the maturation of cortical circuits through its ability to modulate neuronal excitability. In addition, metabotropic serotonin receptors can also control a multitude of intracellular processes including cytoskeletal dynamics and gene expression, processes that are thought to play important roles controlling synapse formation and elimination. Sorting between the relative contributions of these different factors will be an important task for future studies.
6. Unresolved issues
The studies outlined above provide the beginnings of a mechanistic description of the effects of serotonin in the prefrontal cortex. However they suffer from a number of shortcomings that need to be addressed before we can truly understand how serotonin regulates the functioning of this region. First, as outlined above, studies to date have only scratched the surface in terms of incorporating our growing awareness of pyramidal and interneu ron cell heterogeneity into efforts to understand what serotonin does in cortex. Second , it is also now abundantly clear that to fully understand the effects of serotonin in cortex we will need to understand not only how serotonin regulates specific cell types but also how serotonin regulates the function of the cortical networks. This task will require not only the elucidation of the effects of serotonin on membrane excitability in different cells but also on synaptic transmission, synaptic connectivity and beyond. Finally, and perhaps most importantly, it will be essential to understand how synaptically released serotonin regulates the neurons and circuits of the cerebral cortex. Fortunately recent technical and conceptual advances have begun to lay the ground for addressing these issues. This gives hope that we will see at least partial answers to these questions in a not too distant future.
Acknowledgement
Work in the author’s laboratory is supported by NIH grant MH43987.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aghajanian GK, Marek GJ. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997;36:589–599. doi: 10.1016/s0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Andrade R, Malenka RC, Nicoll RA. A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science. 1986;234:1261–1265. doi: 10.1126/science.2430334. [DOI] [PubMed] [Google Scholar]
- Araneda R, Andrade R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine 1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- Beique JC, Campbell B, Perring P, Hamblin MW, Walker P, Mladenovic L, Andrade R. Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors. J. Neurosci. 2004;24:4807–4817. doi: 10.1523/JNEUROSCI.5113-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beique JC, Imad M, Mladenovic L, Gingrich JA, Andrade R. Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2007;104:9870–9875. doi: 10.1073/pnas.0700436104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventure P, Voorn P, Luyten WH, Leysen JE. 5HT1B and 5HT1D receptor mRNA differential co-localization with peptide mRNA in the guinea pig trigeminal ganglion. Neuroreport. 1998;9:641–645. doi: 10.1097/00001756-199803090-00015. [DOI] [PubMed] [Google Scholar]
- Bruinvels AT, Landwehrmeyer B, Gustafson EL, Durkin MM, Mengod G, Branchek TA, Hoyer D, Palacios JM. Localization of 5-HT1B, 5-HT1D alpha, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology. 1994;33:367–386. doi: 10.1016/0028-3908(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Ferezou I, Cauli B, Hill EL, Rossier J, Hamel E, Lambolez B. 5-HT3 receptors mediate serotonergic fast synaptic excitation of neocortical vasoactive intestinal peptide/cholecystokinin interneurons. J. Neurosci. 2002;22:7389–7397. doi: 10.1523/JNEUROSCI.22-17-07389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K. Evidence for the existence of monoamine neurons in the central nervous system. IV. Distribution of monoamine nerve terminals in the central nervous system. Acta Physiol Scand. Suppl. 1965;64:39–85. [PubMed] [Google Scholar]
- Goodfellow NM, Benekareddy M, Vaidya VA, Lambe EK. Layer II/III of the prefrontal cortex: Inhibition by the serotonin 5-HT1A receptor in development and stress. J. Neurosci. 2009;29:10094–10103. doi: 10.1523/JNEUROSCI.1960-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, Beck S, Hen R. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- Haj-Dahmane S, Andrade R. Muscarinic activation of a voltage-dependent cation nonselective current in rat association cortex. J. Neurosci. 1996;16:3848–3861. doi: 10.1523/JNEUROSCI.16-12-03848.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henninger GR. Indoleamines: The role of serotonin in clinical disorders. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology - The fourth generation of progress. Lippincott Williams and Wilkins; Philadelphia: 2000. [Google Scholar]
- Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- Higgs MH, Slee SJ, Spain WJ. Diversity of gain modulation by noise in neocortical neurons: regulation by the slow afterhyperpolarization conductance. J. Neurosci. 2006;26:8787–8799. doi: 10.1523/JNEUROSCI.1792-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct. Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Aghajanian GK. The role of Kv1.2-containing potassium channels in serotonin-induced glutamate release from thalamocortical terminals in rat frontal cortex. J. Neurosci. 2001;21:9955–9963. doi: 10.1523/JNEUROSCI.21-24-09955.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Goldman-Rakic PS, Aghajanian GK. Serotonin induces EPSCs preferentially in layer V pyramidal neurons of the frontal cortex in the rat. Cereb. Cortex. 2000;10:974–980. doi: 10.1093/cercor/10.10.974. [DOI] [PubMed] [Google Scholar]
- Leone DP, Srinivasan K, Chen B, Alcamo E, McConnell SK. The determination of projection neuron identity in the developing cerebral cortex. Curr. Opin. Neurobiol. 2008;18:28–35. doi: 10.1016/j.conb.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidov HG, Grzanna R, Molliver ME. The serotonin innervation of the cerebral cortex in the rat--an immunohistochemical analysis. Neuroscience. 1980;5:207–227. doi: 10.1016/0306-4522(80)90099-8. [DOI] [PubMed] [Google Scholar]
- Maffei A, Turrigiano G. The age of plasticity: developmental regulation of synaptic plasticity in neocortical microcircuits. Prog. Brain Res. 2008;169:211–223. doi: 10.1016/S0079-6123(07)00012-X. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Aghajanian GK. 5-Hydroxytryptamine-induced excitatory postsynaptic currents in neocortical layer V pyramidal cells: suppression by muopiate receptor activation. Neuroscience. 1998;86:485–497. doi: 10.1016/s0306-4522(98)00043-8. [DOI] [PubMed] [Google Scholar]
- margos-Bosch M, Bortolozzi A, Puig MV, Serrats J, Adell A, Celada P, Toth M, Mengod G, Artigas F. Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cereb. Cortex. 2004;14:281–299. doi: 10.1093/cercor/bhg128. [DOI] [PubMed] [Google Scholar]
- Mengod G, Pompeiano M, Martinez-Mir MI, Palacios JM. Localization of the mRNA for the 5-HT2 receptor by in situ hybridization histochemistry. Correlation with the distribution of receptor sites. Brain Res. 1990;524:139–143. doi: 10.1016/0006-8993(90)90502-3. [DOI] [PubMed] [Google Scholar]
- Mengod G, Vilaro MT, Cortes R, Lopez-Jimenez JF, Raurich A, Palacios JM. Chemical neuroanatomy of 5-HT receptors subtypes in the mammalian brain. In: Roth BL, editor. The serotonin Receptors. Humana Press; Totowa, New Jersey: 2006. pp. 319–364. [Google Scholar]
- Miyoshi G, Fishell G. GABAergic Interneuron Lineages Selectively Sort into Specific Cortical Layers during Early Postnatal Development. Cereb. Cortex. 2010 doi: 10.1093/cercor/bhq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat. Rev. Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- O’Hearn E, Molliver ME. Organization of raphe-cortical projections in rat: a quantitative retrograde study. Brain Res. Bull. 1984;13:709–726. doi: 10.1016/0361-9230(84)90232-6. [DOI] [PubMed] [Google Scholar]
- O’Leary DD, Koester SE. Development of projection neuron types, axon pathways, and patterned connections of the mammalian cortex. Neuron. 1993;10:991–1006. doi: 10.1016/0896-6273(93)90049-w. [DOI] [PubMed] [Google Scholar]
- O’Leary DD, Sahara S. Genetic regulation of arealization of the neocortex. Curr. Opin. Neurobiol. 2008;18:90–100. doi: 10.1016/j.conb.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J. Neurosci. 1992;12:440–453. doi: 10.1523/JNEUROSCI.12-02-00440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig MV, Watakabe A, Ushimaru M, Yamamori T, Kawaguchi Y. Serotonin modulates fast-spiking interneuron and synchronous activity in the rat prefrontal cortex through 5-HT1A and 5-HT2A receptors. J. Neurosci. 2010;30:2211–2222. doi: 10.1523/JNEUROSCI.3335-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. A century of progress in corticoneurogenesis: from silver impregnation to genetic engineering. Cereb. Cortex. 2006;16(Suppl 1):i3–17. doi: 10.1093/cercor/bhk036. [DOI] [PubMed] [Google Scholar]
- Rakic P, Ayoub AE, Breunig JJ, Dominguez MH. Decision by division: making cortical maps. Trends Neurosci. 2009;32:291–301. doi: 10.1016/j.tins.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos C, Beique JC, Gingrich JA, Andrade R. Serotonergic regulation of calcium-activated potassium currents in rodent prefrontal cortex. Eur. J. Neurosci. 2005;22:1120–1126. doi: 10.1111/j.1460-9568.2005.04307.x. [DOI] [PubMed] [Google Scholar]
- Weber ET, Andrade R. Htr2a Gene and 5-HT(2A) Receptor Expression in the Cerebral Cortex Studied Using Genetically Modified Mice. Front Neurosci. 2010:4. doi: 10.3389/fnins.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, Molliver ME. The organization of serotonergic projections to cerebral cortex in primates: regional distribution of axon terminals. Neuroscience. 1991a;44:537–553. doi: 10.1016/0306-4522(91)90076-z. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Molliver ME. The organization of serotonergic projections to cerebral cortex in primates: retrograde transport studies. Neuroscience. 1991b;44:555–570. doi: 10.1016/0306-4522(91)90077-2. [DOI] [PubMed] [Google Scholar]
- Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J. Comp Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- Zhang ZW. Serotonin induces tonic firing in layer V pyramidal neurons of rat prefrontal cortex during postnatal development. J. Neurosci. 2003;23:3373–3384. doi: 10.1523/JNEUROSCI.23-08-03373.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZW, Arsenault D. Gain modulation by serotonin in pyramidal neurones of the rat prefrontal cortex. J. Physiol. 2005;566:379–394. doi: 10.1113/jphysiol.2005.086066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Hablitz JJ. Activation of serotonin receptors modulates synaptic transmission in rat cerebral cortex. J. Neurophysiol. 1999;82:2989–2999. doi: 10.1152/jn.1999.82.6.2989. [DOI] [PubMed] [Google Scholar]