Hippocampal Focal Knockout of CBP Affects Specific Histone Modifications, Long-Term Potentiation, and Long-Term Memory (original) (raw)
Abstract
To identify the role of the histone acetyltransferase (HAT) CREB-binding protein (CBP) in neurons of the CA1 region of the hippocampus during memory formation, we examine the effects of a focal homozygous knockout of CBP on histone modifications, gene expression, synaptic plasticity, and long-term memory. We show that CBP is critical for the in vivo acetylation of lysines on histones H2B, H3, and H4. CBP's homolog p300 was unable to compensate for the loss of CBP. Neurons lacking CBP maintained phosphorylation of the transcription factor CREB, yet failed to activate CREB:CBP-mediated gene expression. Loss of CBP in dorsal CA1 of the hippocampus resulted in selective impairments to long-term potentiation and long-term memory for contextual fear and object recognition. Together, these results suggest a necessary role for specific chromatin modifications, selectively mediated by CBP in the consolidation of memories.
Keywords: CBP, histone acetylation, long-term memory, long-term potentiation
INTRODUCTION
Chromatin, the complex of DNA and associated proteins, is a physical barrier to transcription mechanisms. The manipulation of chromatin is critically involved in regulating gene expression for a number of neuronal processes, including the formation of long-term memory (Barrett and Wood, 2008). Chromatin-modifying complexes, which contain histone-modifying enzymes, regulate access to the underlying genomic DNA by relaxing chromatin structure and providing docking sites for additional regulatory factors (Kouzarides, 2007). The enzymes that regulate levels of histone acetylation are histone acetyltransferases (HATs) and histone deacetylases (HDACs). One such enzyme, the HAT CREB-binding protein (CBP) is the best studied of the three HATs implicated in the regulation of transcription during memory and synaptic plasticity (reviewed in Barrett and Wood, 2008).
To date, six different types of Cbp mutant mice have been generated to examine the role of CBP in memory (Oike et al, 1999; Bourtchouladze et al, 2003; Alarcon et al, 2004; Korzus et al, 2004; Wood et al, 2005, 2006; Chen et al, 2010). However, none of these Cbp genetically modified mice were designed to target a single brain region and only one generated a complete knockout of CBP, as a homozygous knockout of Cbp results in embryonic lethality (Tanaka et al, 1997). These mice, therefore, may have phenotypes reflecting residual CBP or compensatory mechanisms. To overcome current limitations, we have examined Cbp flox/flox mice, in which a focal deletion of Cbp can be generated by Cre recombinase introduced by adeno-associated virus (AAV-Cre). This approach allows us to generate a homozygous knockout of Cbp and limit the deletion to a specific brain region. Further, AAV-Cre can be introduced into adult mice, which limits potential developmental compensation, either pre- or postnatal, that may be observed if the deletion was induced earlier in life, such as in Cre-expressing mouse lines.
This approach allowed us to address key open questions regarding the role of CBP in histone acetylation and long-term memory. For example, in vitro and cell culture studies indicate that other HATs, such as p300, can acetylate many of the same residues as CBP. However, whether these HATs can compensate at specific residues in the brain for a lack of CBP is unknown. We also examined whether CREB phosphorylation can be uncoupled from CREB:CBP-mediated gene expression in vivo, as has been shown in cell culture (Chawla et al, 1998; Hardingham et al, 1999). Lastly, the effect of a homozygous deletion of Cbp on long-term potentiation (LTP) has never been examined. In this study, we addressed these questions and found that homozygous deletion of Cbp reveals a role for CBP in the regulation of a specific profile of histone modifications, hippocampal LTP, and hippocampus-dependent long-term memory. These findings provide new insight into CBP's in vivo role in the regulation of histone modification and gene expression required for long-term memory processes.
METHODS
Subjects and Surgical Procedures
CBP conditional knockout mice (Cbp flox/flox) were generated as described in detail in Kang-Decker et al (2004) and are maintained on a C57BL/6 background. Cre recombinase can be introduced to delete the sequence between the loxP sites, producing deletion of CBP (Kang-Decker et al, 2004; Kasper et al, 2006; Xu et al, 2006). At 2 weeks before the experiments, mice were anesthetized using isoflurane and infused with adeno-associated virus expressing Cre-recombinase (AAV-Cre), either serotype AAV2/2 (made in the lab of RWG), or AAV2/1 (purchased from Penn Vector Core; University of Pennsylvania; Kaspar et al, 2002). The infusion cannula was positioned over the hippocampus (AP −2.0 mm; ML ±1.5 mm from bregma) and lowered 0.2 mm/15 s to a depth of −1.5 mm (DV from bregma). It was allowed to sit for 2 min and then 1.0 μl of virus was injected at a rate of 6 μl/h and allowed to diffuse for 2 min. The cannula was removed 0.2 mm initially, followed by a 1 min pause, and then 0.1 mm/15 s. All deletions were confirmed by immunohistochemistry (as described below). Mice were 8–12 weeks old and had free access to food and water in their home cages. Lights were maintained on a 12 : 12 h light/dark cycle, with all behavioral testing performed during the light portion of the cycle. All experiments were conducted according to National Institutes of Health guidelines for animal care and use and were approved by the Institutional Animal Care and Use Committee of the University of California, Irvine.
Immunohistochemistry
Tissue preparation and immunohistochemical analysis was performed as described previously (Vecsey et al, 2007; Malvaez et al, 2010). Briefly, coronal sections of paraformaldehyde-fixed, cryoprotected brains were cut with a cryostat at a thickness of 20 μm at the level of the hippocampus and collected in PBS.
The following primary antibodies were used: anti-CBP (C-20; 1 : 1000), and anti-p300 (C-20; 1 : 1000) antibodies were purchased from Santa Cruz Biotechnology. Anti-H3K14ac (1 : 1000), anti-H4K12ac (1 : 500), and anti-phospho-CREB (1 : 1000) antibodies were purchased from Millipore. Anti-H4K8ac (1 : 1000) was purchased from Cell Signaling. Anti-H2BK12ac (1 : 500) was purchased from Abcam.
Floating sections were blocked for 1 h at room temperature in 8% normal goat serum (NGS, Jackson ImmunoResearch Laboratories) with 0.3% Triton X-100 in PBS and then incubated overnight at 4°C in 2% NGS, 0.3% Triton X-100 in PBS with primary antibody. The sections were then incubated for 2 h at room temperature with goat anti-rabbit IgG-FITC (1 : 1000, Chemicon International). All sections were washed three times for 5 min each in PBS before and after each incubation step and mounted on slides using ProLong Gold antifade reagent with DAPI (Invitrogen).
All images were acquired using an Olympus (BX51, Japan) microscope using a × 4 or × 20 objective, CCD camera (QImaging), QCapture Pro 6.0 software (QImaging), combined with ImageJ software (NIH).
Quantitative Real-Time RT-PCR
Quantitative real-time RT-PCR was performed to examine c-fos expression. Tissue was collected by taking 1 mm punches from dorsal hippocampal slices in the area of the focal deletion in Cbp flox/flox mice as confirmed by immunohistochemistry for CBP and equivalent regions in Cbp +/+ mice. RNA was isolated using RNeasy minikit (Qiagen, Carlsbad, CA). cDNA was made from 200 ng total RNA using the Transcriptor First Strand cDNA Synthesis kit (Roche Applied Sciences). Primers were derived from the Roche Universal ProbeLibrary: c-fos left primer 5′-ggggcaaagtagagcagcta-3′ c-fos right primer 5′-agctccctcctccgattc-3′ probe, 5′-atggctgc-3′ Cbp left primer 5′-caggcaggtgtttcacagg-3′ Cbp right primer 5′-gcatgttcagagggttaggg-3′ probe, 5′-cctggagc-3′. The c-fos and Cbp probes are conjugated to the dye FAM. GAPDH left primer 5′-atggtgaaggtcggtgtga-3′ right primer 5′-aatctccactttgccactgc-3′ probe 5′-tggcggtattgg-3′ GAPDH probe is conjugated to Lightcycler Yellow 555. The non-overlapping dyes and quencher on the reference gene allow for multiplexing in the Roche LightCycle 480 II machine (Roche Applied Sciences). All values were normalized to GAPDH expression levels. Analysis and statistics were performed using the Roche proprietary algorithms and REST 2009 software based on the Pfaffl method (Pfaffl, 2001, Pfaffl et al, 2002).
Contextual Fear Conditioning
Training and testing took place in four PhenoTyper conditioning chambers as previously described (Lattal et al, 2007). Mice were placed into the conditioning chamber and received a 2 s, 0.75 mA scrambled foot shock at 2.5 min after placement into the chamber. Mice were removed from the chamber after 3 min. During testing, mice received one 5 min exposure to the conditioned context in the absence of shock 1 h or 24 h after conditioning. Conditioning was assayed by measuring freezing behavior, the complete absence of movement (Fanselow, 1980). Freezing was scored during conditioning as well as testing.
Object Recognition
Training and testing for location-dependent object recognition (Roozendaal et al, 2010) and novel object recognition (Mumby et al, 2002, 2005; Winters et al, 2004; Balderas et al, 2008) was carried out as previously described (Roozendaal et al, 2010; Stefanko et al, 2009). Briefly, before training, mice were handled 1–2 min for 5 days and then habituated to the experimental apparatus (white rectangular open field, 30 × 23 × 21.5 cm) 5 min a day for 4 consecutive days in the absence of objects. During training, mice were placed into the experimental apparatus with two identical objects (A1 and A2; either 100 ml beakers, 2.5 cm diameter, 4 cm height; or large blue Lego blocks, 2.5 × 2.5 × 5 cm), and were allowed to explore for 10 min or 3 min (Stefanko et al, 2009). During the retention test, (90 min for short-term memory or 24 h for long-term memory), mice explored the experimental apparatus for 5 min. For the object recognition memory test (ORM), one familiar object (A3) and one novel object (B1) were placed in the same location as during training. For the object location memory test (OLM), one familiar object (A3) was placed in the middle of the box (a novel location) and another familiar object (A4) was placed in the same location as during training. All combinations and locations of objects were used in a balanced manner to reduce potential biases due to preference for particular locations or objects.
Slice Preparation and Recording
Hippocampal slices were prepared from Cbp flox/flox and Cbp _+/+_mice (approximately at 2 months of age). Following isoflurane anesthesia, mice were decapitated, and the brain was quickly removed and submerged in ice-cold, oxygenated dissection medium containing (mM): 124 NaCl, 3 KCl, 1.25 KH2PO4, 5 MgSO4, 0 CaCl2, 26 NaHCO3, and 10 glucose. Transverse hippocampal slices (300 μm) through the mid-third of the septotemporal axis of the hippocampus were prepared using a Leica vibrating tissue slicer (Model:VT1000S) before being transferred to an interface recording containing preheated artificial cerebrospinal fluid of the following composition (mM): 124 NaCl, 3 KCl, 1.25 KH2PO4, 1.5 MgSO4, 2.5 CaCl2, 26 NaHCO3, and 10 glucose and maintained at 31±1°C. Slices were continuously perfused with this solution at a rate of 1.75–2 ml/min, whereas the surface of the slices were exposed to warm, humidified 95% O2/5% CO2. Recordings began following at least 2 h of incubation.
Field excitatory postsynaptic potentials were recorded from CA1b stratum radiatum using a single glass pipette (2–3 MΩ). Bipolar stainless steel stimulation electrodes (25 μm diameter, FHC) were positioned at two sites (CA1a and CA1c) in the apical Schaffer collateral–commissural projections to provide activation of separate converging pathways of CA1b pyramidal cells. Pulses were administered in an alternating manner to the two electrodes at 0.05 Hz using a current that elicited a 50% maximal response. After establishing a 10–20 min stable baseline, LTP induced by delivering 5 or 10 ‘θ' bursts, with each burst consisting of four pulses at 100 Hz and the bursts themselves separated by 200 ms (ie, θ-burst stimulation or TBS). The stimulation intensity was not increased during TBS. Data were collected and digitized by NAC 2.0 Neurodata Acquisition System (Theta Burst Corporation, Irvine, CA) and stored on a disk.
RESULTS
Generation of Focal Homozygous Cbp Deletion Using Cbp flox/flox Mice and AAV-Cre
To generate a homozygous gene deletion of Cbp, we used mice carrying loxP sites flanking exon 9 of Cbp (Cbp flox/flox mice; Kang-Decker et al, 2004), in which Cre recombinase excises exon 9 of Cbp. Cre recombinase was delivered via adeno-associated virus (referred to as AAV-Cre). Cbp flox/flox mice have previously been shown to be indistinguishable from their wild-type littermates and to have a normal lifespan (Kang-Decker et al, 2004). We find no difference in CBP protein or mRNA in Cbp flox/flox mice not exposed to AAV-Cre (Supplementary Figure S1A, B). Cbp flox/flox and Cbp +/+ mice were administered intrahippocampal infusions of AAV-Cre (1 μl per side) serotype 2/2 (AAV2/2-Cre) or 2/1 (AAV2/1-Cre) to create a small (Figure 1a and b) and a large (Figure 1c and d) hippocampal CBP deletion, respectively. These serotypes, while having the same viral genome, have different coat proteins that allow them to transduce cells with different efficiencies (Burger et al, 2004) and therefore allow us to create deletions of CBP affecting more or less of the hippocampus. Both serotypes transduce neurons at a higher efficiency than other cell types (Burger et al, 2004). On the basis of the finding by Scammell et al (2003), showing that by 2 weeks post-AAV-Cre injection, a floxed gene can be deleted in the hippocampus, we chose to examine mice at 2 weeks post injection. We observed a complete deletion of CBP at every time point examined (at 2 weeks and at 1 month post-AAV-Cre infusion shown in Supplementary Figure S1C) and all behavior and immunohistochemistry was carried out between 2 and 6 weeks following AAV-Cre infusion. All deletions were verified with immunohistochemistry following behavior.
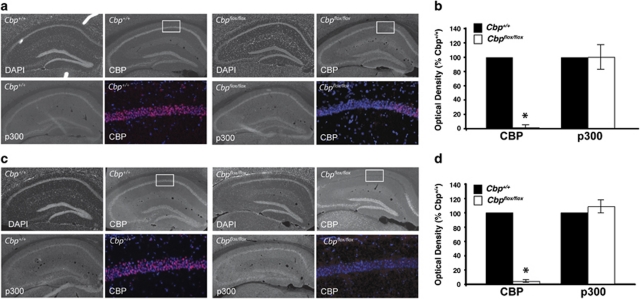
Figure 1.
AAV-Cre infusion in Cbp flox/flox mice induces a focal loss of CBP, but no change in expression of CBP homolog p300. Perfusion-fixed immunostained coronal brain slices were prepared from Cbp flox/flox and Cbp +/+ mice at 3–6 weeks following intrahippocampal infusions of AAV-Cre, either serotype 2/2 or 2/1 (AAV2/2-Cre or AAV2/1-Cre). Quantification of immunostaining is shown on right. (a) Representative images showing DAPI labeling and CBP and p300 immunoreactivity in hippocampi of AAV2/2-Cre infused Cbp +/+ and Cbp flox/flox mice. The color images are × 20 magnifications of the regions boxed in white, labeled with DAPI (blue), and CBP (pink). The Cbp +/+ mice displayed expression of CBP throughout CA1, CA3, and the dentate gyrus. However the Cbp flox/flox mice display a focal loss of CBP in area CA1 of the hippocampus, while exhibiting no difference in p300 expression in this region. (b) Quantification of CBP and p300 optical density. (c) Representative images showing DAPI labeling and CBP and p300 immunoreactivity in hippocampi of AAV2/1-Cre infused Cbp +/+ and Cbp flox/flox mice. The color images are × 20 magnifications of the regions boxed in white, labeled with DAPI (blue), and CBP (pink). The Cbp +/+ mice displayed expression of CBP throughout CA1, CA3, and the dentate gyrus. However the Cbp flox/flox mice display a loss of CBP, while exhibiting no difference in p300 expression in this region. (d) Quantification of CBP and p300 optical density. *p<0.05.
AAV2/2-Cre created a deletion limited to a portion of dorsal CA1 and did not affect the cortex. AAV2/1-Cre created a deletion that encompassed dorsal CA1, CA3, and the dentate gyrus, as well as more ventral CA1 and affected the region of the cortex closest to the deletion in the hippocampus (Supplementary Figure S1). This deletion does not induce cell death, indicated by the fact that nuclei are intact, as demonstrated by DAPI staining (Figure 1). Further, there is no indication of cell death by Fluoro-Jade B staining (Schmued and Hopkins, 2000a, 2000b; data not shown). AAV does not lead to an immune response or impair basal synaptic transmission (Scammell et al, 2003; see also Figure 4).
p300 is considered a functional homolog of CBP because of its high sequence similarity with CBP (Eckner et al, 1994) and overlapping interaction partners (Arany et al, 1995; Lundblad et al, 1995). p300 therefore may be expected to compensate for a lack of CBP. However, we observed no change in p300 in the region of CBP deletion (Figure 1) and therefore, similarly to findings in other CBP mutant mice (Kasper et al, 2006; Chen et al, 2010), it does not appear that p300 is being upregulated to compensate for a loss of CBP.
CBP is Involved in the Acetylation of Residues on Histones H2B, H3, and H4 in the Dorsal CA1
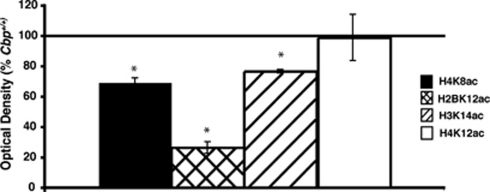
CBP has been shown in vitro to acetylate a number of histone residues involved in transcriptional regulation (Ogryzko et al, 1996; Kouzarides, 2007). We therefore examined the role of CBP in histone acetylation in vivo by assessing acetylation of specific histone residues in neurons lacking CBP. Acetylation of H3K14, H4K8, and H4K12 has been shown to correlate with learning (Levenson et al, 2004; Chwang et al, 2006, 2007; Peleg et al, 2010). Lysine 12 on H2B, lysine 14 on H3, lysine 8 on H4, known targets of CBP (Kouzarides, 2007), were all significantly reduced in the dorsal CA1 region of the hippocampus of Cbp flox/flox mice compared with Cbp +/+ mice (Figure 2). Lysine 12 on H4 is not known to be a target of CBP (Kouzarides, 2007) and was not different between Cbp flox/flox mice and Cbp +/+ mice (Figure 2). These results suggest that in vivo, in the hippocampus, CBP is critical for acetylation of specific histone residues, and that p300 is unable to fully compensate for the loss of CBP.
Figure 2.
Focal homozygous gene deletion of Cbp results in altered histone acetylation. Hippocampal sections from Cbp flox/flox and Cbp +/+ mice infused with AAV2/1-Cre adjacent to sections immunolabeled for CBP were immunolabeled for H4K8ac, H2BK12ac, H3K14ac, and H4K12ac. Optical density of × 20 magnifications of area CA1 of the hippocampus was quantified. *p<0.05.
CREB Phosphorylation is Uncoupled From CREB:CBP-Mediated Gene Expression In Vivo
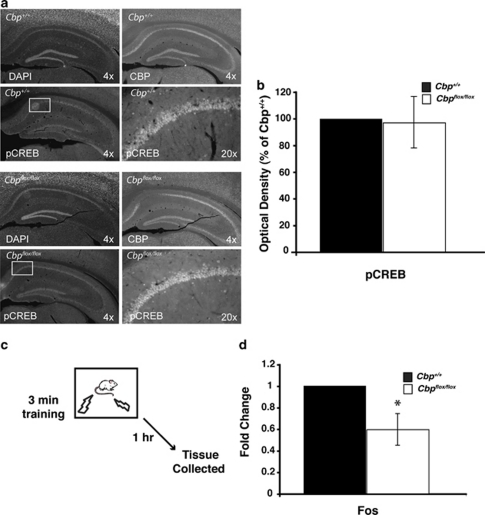
To determine whether signaling upstream of CBP and histone acetylation was affected by the deletion of CBP, we examined phosphorylation of CREB in Cbp flox/flox and Cbp +/+ mice 10 min following fear conditioning. This time point was chosen based on studies showing maximal phospho-CREB following fear conditioning in the hippocampus (Stanciu et al, 2001). Cbp flox/flox and Cbp +/+ mice received bilateral intrahippocampal AAV2/2-Cre infusions and 2 weeks later were trained in the contextual fear conditioning paradigm. At 10 min following training, mice were perfused. We observed no change in CREB phosphorylation (Figure 3a and b), indicating that signaling upstream of CBP is intact.
Figure 3.
Phosphorylation of CREB is unaltered while expression of c-fos is reduced following fear conditioning in the absence CBP. (a) Representative images showing DAPI labeling and pCREB immunoreactivity in hippocampi of Cbp +/+ and Cbp flox/flox mice. Mice were infused with AAV2/2-Cre and at 2 weeks later were fear conditioned. Perfusion-fixed immunostained coronal brain slices were prepared from mice killed at 10 min following fear conditioning. The Cbp +/+ mice displayed expression of CBP throughout CA1, CA3, and the dentate gyrus. However the Cbp flox/flox mice display a focal loss of CBP, but exhibit no difference in pCREB expression in this region (bottom right two panels). (b) Quantification of pCREB immunostaining indicates no difference in pCREB in Cbp +/+ and Cbp flox/flox mice in the region of Cbp deletion. (c) Mice were infused with AAV2/1-Cre and at 2 weeks later were fear conditioned. At 1 h following training, quantitative RT-PCR shows that c-fos expression is significantly decreased in the dorsal hippocampus of Cbp flox/flox mice compared with wild-type littermates. *p<0.05.
To determine whether CREB:CBP-mediated target gene expression is affected by the CBP deletion, we examined c-fos expression in Cbp flox/flox and Cbp +/+ mice at 1 h following contextual fear conditioning (Stanciu et al, 2001), as transcription of immediate early genes such as c-fos initiated by patterned synaptic activity is necessary for synaptic plasticity and long-term memory (reviewed in Alberini, 2009). Cbp flox/flox and Cbp +/+ mice received bilateral intrahippocampal AAV2/1-Cre infusions and at 2 weeks later were trained in the contextual fear conditioning paradigm. At 1 h following training (Figure 3c), tissue was collected by taking 1 mm punches from dorsal hippocampal slices in the area of the focal deletion in Cbp flox/flox mice (_n_=3), as confirmed by immunohistochemistry. Equivalent regions were analyzed from Cbp +/+ punches. In this experiment, AAV2/1 was used in order to generate larger deletion sizes, so that 1 mm punches could be taken from some slices and immunohistochemistry could be performed an adjacent slices to confirm the deletion. The expression of c-fos was significantly reduced after fear conditioning in Cbp flox/flox dorsal hippopcampus as compared with Cbp +/+ controls (Figure 3d). Together, these results suggest that although upstream signaling events appear normal, resulting in CREB phosphorylation, without CBP there is a failure to activate c-fos expression. This uncoupling between phospho-CREB and CREB:CBP-mediated gene expression is similar to previous observations showing this dissociation in cell culture (Chawla et al, 1998; Hardingham et al, 1999).
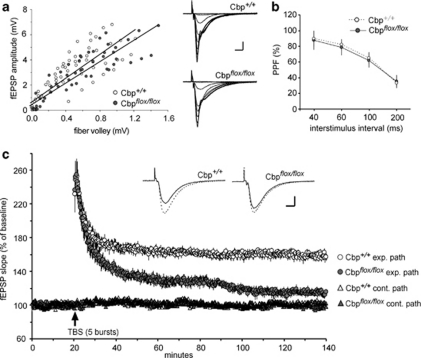
Focal Homozygous Cbp Deletion Using Cbp flox/flox Mice and AAV-Cre Impairs LTP in Hippocampus
Acute hippocampal slices were used to test for changes in synaptic physiology associated with focal deletion of Cbp using AAV2/1-Cre, as verified by immunohistochemistry for CBP following recording. Stimulation pulses were delivered to the Schaffer-commissural projections at 3 min and field EPSPs recorded from the proximal apical dendrites of field CA1b. Baseline synaptic transmission, as assayed by input/output curves (Figure 4a) and paired-pulse facilitation (Figure 4b), was not detectably different in the slices prepared from Cbp +/+ or Cbp flox/flox mice. However, LTP was clearly impaired in the latter group. A single train of five θ-bursts (TBS) produced a potentiation effect that decayed to levels well below that observed in Cbp +/+ slices (Figure 4c). The difference between the two groups in mean percent LTP at 2 h post TBS was highly significant (Cbp +/+: +58±11% relative to baseline; Cbp flox/flox: +14±10% _n_=7/group, p<0.001). Control (no TBS) inputs recorded on the same slices did not change from baseline over the 2-h test period in either group (Cbp _+/+_= −2±3% Cbp _flox/flox_=+3±6%), indicating that the deletion did not affect the synapse specificity of LTP or the stability of evoked EPSPs. Importantly, the pronounced potentiation recorded at 2 min post TBS was equivalent in the two groups (+144±47% Cbp +/+ and +144±50% relative to baseline from Cbp flox/flox). This strongly suggests that the complex signaling pathways underlying the induction and initial expression of LTP were left intact by CBP deletion. It follows from this that the essential problem produced by the deletion involves a failure of LTP to properly stabilize.
Figure 4.
Local deletion of CBP disrupts LTP in hippocampal slices. Field EPSPs evoked by stimulation of either of two electrodes placed in the Schaffer-commissural projections were recorded in CA1b stratum radiatum of slices prepared from Cbp +/+ and Cbp flox/flox mice infused with AAV2/1-Cre (_n_=7 slices/group). (a) Input/output curves compare amplitudes of the presynaptic fiber volley to thefield excitatory postsynaptic potentials (fEPSP) amplitude across a range of stimulation currents (10, 20, 30, 40, and 50 μA). Slopes for the linear regression lines were not detectably different between the two groups. Representative traces are shown at right (scale bar: 1 mV/5 ms). (b) Paired pulse facilitation of the initial slope of the synaptic response was comparable (40, 60, 100, and 200 ms inter-pulse intervals) in the two groups of slices. (c) A train of five θ-bursts delivered to one of the two stimulation electrodes (exp. path) produced stable potentiation in slices from Cbp+/+ mice (open circles) but not in Cbp flox/flox slices (dark circles) (p<0.001 at the end of the 2 h post-TBS recording session). Synaptic responses recorded in the pathway (cont. path) that did not receive TBS remained stable throughout the experiment for both groups (open triangle, Cbp++; dark triangle, Cpb floxflox). Insets show field excitatory postsynaptic potentials (fEPSP) traces collected during baseline testing (solid line) and at 2 h after TBS (dotted line). Scale: 1 mV/5 ms.
Past studies have overcome failures of LTP stabilization in learning impaired mouse models by using supra-threshold levels of θ-burst stimulation (Lauterborn et al, 2007). We tested for this effect in Cbp flox/flox mice using trains of 10 θ bursts to induce LTP. We found that the percent potentiation at 2 h post TBS (_n_=3; +18±5%) was about the same as that found at this time point in the 5 burst studies (_p_=0.56) but significantly reduced compared with LTP induced with 10 burst in slices from Cbp +/+ mice (_n_=5; +67±22% Cbp +/+, _p_=0.005). It is thus unlikely that the impairment involves a shift in the threshold for engaging the LTP consolidation machinery.
Long-Term Memory for Contextual Fear is Impaired in Mice With a Focal Hippocampal Deletion of CBP
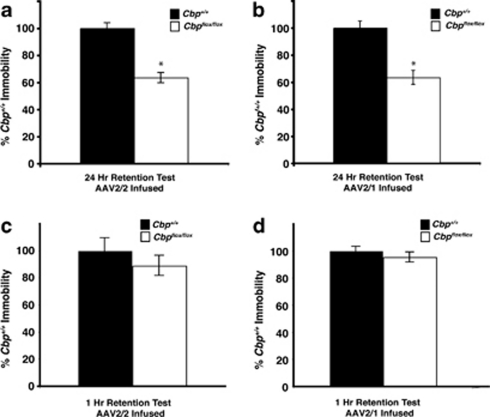
To examine the effect of a homozygous Cbp deletion in the dorsal hippocampus on memory formation, we measured the effect of small (using AAV2/2-Cre) and large (using AAV2/1-Cre) hippocampal CBP deletions on long-term memory for contextual fear conditioning. In contextual fear conditioning, mice learn to associate a foot shock with a context in a single training session (Maren and Quirk, 2004). Memory for this association is dependent on the hippocampus (Maren, 2001) and dorsal CA1 has been demonstrated to be involved in retrieval of contextual fear conditioning (Hunsaker and Kesner, 2008). Cbp flox/flox and Cbp +/+ mice were administered intrahippocampal infusions of AAV2/2-Cre or AAV2/1-Cre, fear conditioned at 2 weeks later, and tested either at 1 or 24 h following fear conditioning (different sets of mice used for 1 or 24 h time points). Deletions were verified with immunohistochemistry for CBP following behavior. Both Cbp flox/flox mice infused with AAV2/2 (Figure 5a) and those infused with AAV2/1 (Figure 5b) exhibited significantly reduced levels of freezing when re-exposed to the conditioned context during the 24 h test (p<0.05). There were, however, no differences between _Cbp_ _flox/flox_ mice and _Cbp_ _+/+_ controls not administered AAV-Cre when tested at 24 h after training (Supplementary Figure S1D). In a different set of mice, there were no differences during a 1 h test (_p_>0.05; Figure 5c and d). This experiment demonstrates that a focal CBP deletion in a subregion of CA1 is sufficient to impair long-term memory for contextual fear, but not short-term memory.
Figure 5.
CBP deletion in the hippocampus impairs long-term, but not short-term memory. Memory for contextual fear conditioning was measured as immobility and is displayed as percent of wild type. (a) Cbp +/+ and Cbp flox/flox mice were infused with AAV2/2-Cre and at 2 weeks later were trained and tested for contextual fear conditioning. Cbp flox/flox mice (_n_=9) exhibited a significant decrease in levels of freezing in a 24 h retention test compared with Cbp +/+ mice (_n_=8). *p<0.05. (b) Cbp flox/flox and Cbp +/+ mice were administered intrahippocampal infusions of AAV2/1-Cre. At 2 weeks later they were given contextual fear conditioning training. Cbp flox/flox mice (_n_=9) exhibited a significant decrease in levels of freezing in a 24 h retention test compared with Cbp +/+ mice (_n_=8). *p<0.05. (c) Cbp +/+ and Cbp flox/flox mice were infused with AAV2/2-Cre and at 2 weeks later were trained and tested at 1 h for contextual fear conditioning. Cbp flox/flox mice (_n_=9) and Cbp +/+ mice (_n_=9) were not significantly different. (d) Cbp +/+ and Cbp flox/flox mice were infused with AAV2/1-Cre and at 2 weeks later were trained and tested at 1 h for contextual fear conditioning. Cbp flox/flox mice (_n_=9) and Cbp +/+ mice (_n_=10) were not significantly different.
Long-Term Memory for Object Location, but not Memory for the Object Itself, is Impaired in Mice With a Focal Hippocampal Deletion of Cbp
To further understand the role of CBP in long-term memory formation, we examined the effect of a hippocampal homozygous Cbp deletion using two different object recognition tasks. As no differences were observed between Cbp flox/flox mice infused with AAV2/2-Cre and AAV2/1-Cre, Cbp flox/flox and Cbp +/+ mice were administered intrahippocampal infusions of AAV2/1-Cre. They were trained 2 weeks later, and tested at 24 h following training. During training, mice were placed in an arena with two identical objects for a 10-min training session, which we have previously shown to result in long-term memory (Stefanko et al, 2009; Roozendaal et al, 2010; Haettig et al, 2011), and then tested at 24 h later in the same arena with one familiar object moved to a novel location (OLM; Figure 6a). AAV2/1-Cre infused Cbp flox/flox mice exhibited no significant long-term memory for the object location as compared with Cbp +/+ mice (p<0.05; Figure 6b). However, _Cbp_ _flox/flox_ mice not infused with AAV-Cre exhibited long-term memory for the object location and were not significantly different than _Cbp_ _+/+_ controls (_p_>0.05; Supplementary Figure S1E).
Figure 6.
CBP deletion in the hippocampus leads to impairment in hippocampus-dependent location-dependent object recognition that is not rescued by HDAC inhibition. (a) Mice received 10 min training in an environment with two identical objects and received a retention test 24 h later for object location memory (OLM) in which one object is moved to a new location. (b) AAV2/1-Cre infused Cbp flox/flox (_n_=12) mice exhibit a significant 24 h long-term memory deficit (p<0.05) in a hippocampus-dependent object location recognition task as compared with Cbp +/+ mice (_n_=9). (c) Mice received 10 min training in an environment with two identical objects and received a 90 min OLM test, in which one object is moved to a new location. (d) AAV2/1-Cre infused Cbp flox/flox (_n_=7) mice exhibit normal 90 min short-term memory for a familiar location as compared with Cbp +/+ mice (_n_=8). (e) Mice received 10 min training in an environment with two identical objects and received a retention test at 24 h later for object recognition memory (ORM) in which one object is replaced with a novel one. (f) AAV2/1-Cre infused Cbp flox/flox mice (_n_=4) exhibit normal 24 h long-term memory for a familiar object as compared with Cbp +/+ mice (_n_=9) in a hippocampus-independent object recognition task. (g) Mice received subthreshold training (3 min) in an environment with two identical objects immediately followed by i.p. injection of sodium butyrate (NaBut) and received a retention test 24 h later in which one object is moved to a new location. (h) Cbp +/+ mice treated with NaBut (_n_=7) exhibited a significant preference for the novel object while Cbp +/+ mice treated with vehicle (_n_=11) and Cbp flox/flox mice treated with vehicle (_n_=9) or NaBut (_n_=9) did not show a significant preference. *p<0.05.
To examine whether short-term memory was affected by a hippocampal Cbp deletion, we trained a different group of AAV2/1-Cre infused Cbp flox/flox mice with two identical objects and tested at 90 min later for OLM (Figure 6c). AAV2/1-Cre infused Cbp flox/flox mice exhibited discrimination for the novel location that was not significantly different than that of Cbp +/+ mice (_p_>0.05; Figure 6d). This result indicates that Cbp flox/flox mice with a focal deletion of Cbp in the hippocampus have unimpaired short-term memory for object location.
We next examined whether the focal Cbp deletion affected long-term memory in a standard novel object recognition task (ORM; see Figure 6e). In this task, there is no change in context or object location, but one of the familiar objects is replaced with a novel object. The dorsal hippocampus has been shown to encode information regarding context and location (O'Keefe and Burgess, 1999; Fanselow, 2000; Maren and Holt, 2000; Smith and Mizumori, 2006), whereas other brain regions, such as insular cortex, have been shown to be critical for long-term memory for the object itself (Balderas et al, 2008; Roozendaal et al, 2010). This distinct neural circuitry for the ORM and OLM tasks can reveal the regional specificity of our Cbp deletions. In a recent study, we used these tasks to demonstrate that focal deletion of HDAC3 in the hippocampus of HDAC3flox/flox mice via AAV2/1-Cre affects long-term OLM, but not long-term memory for the object itself (ORM; McQuown et al, 2011).
Cbp flox/flox and Cbp +/+ mice were administered intrahippocampal infusions of AAV2/1-Cre, trained at 2 weeks later in ORM (see Figure 6e). Both Cbp flox/flox and Cbp +/+ mice showed a significant preference for the novel object and were not significantly different from each other (_p_>0.05; Figure 6f). These results demonstrate that focal deletions of CBP in the hippocampus impair long-term OLM, but not long-term memory for the object itself.
Long-Term Memory is not Rescued by Systemic Administration of an HDAC Inhibitor in Mice With a Focal Deletion of CBP
Previously our laboratory has shown that HDAC inhibition is able to enhance long-term memory and synaptic plasticity (Vecsey et al, 2007) and rescue long-term memory deficits in CBP mutant mice when the mice are tested on object recognition (Stefanko et al, 2009), but not when they are tested on object–location recognition (Haettig et al, 2011). We therefore examined the ability of the HDAC inhibitor sodium butyrate (NaBut) to ameliorate memory impairments in Cbp flox/flox mice. As before, Cbp flox/flox and Cbp +/+ mice were administered intrahippocampal infusions of AAV2/1-Cre, trained at 2 weeks later, and tested at 24 h following training. During training, mice were placed in an arena with two identical objects for a 3-min training session (Figure 6g). The 3-min training session was used because this subthreshold training does not lead to long-term memory formation in wild-type mice unless it is paired with HDAC inhibition (Stefanko et al, 2009; McQuown et al, 2011; Haettig et al, 2011). Immediately following training, mice were administered systemic injections of NaBut (1.2 g/kg). Mice were tested at 24 h later for object location recognition. NaBut administration led to expression of long-term memory in wild-type mice. However, there was no difference between vehicle and NaBut treated Cbp flox/flox mice (Figure 6h). These results further support the conclusion that CBP is critical for long-term memory formation in the hippocampus and even when histone deacetylation is inhibited, p300 cannot compensate for a loss of CBP.
DISCUSSION
Currently, there is little known about the actual histone modifications regulated by CBP in vivo, especially in the brain. Furthermore, no study to date has examined the effects of a complete focal deletion of CBP on modifications of specific histone residues in neurons and the subsequent impact on long-term memory. We demonstrate that a homozygous focal Cbp knockout in the dorsal hippocampus leads to impaired long-term memory but normal short-term memory and that systemic administration of an HDAC inhibitor does not rescue memory in these mice. In neurons in area CA1 of the hippocampus, this loss of CBP leads to a reduction in acetylation of several histone residues thought to be direct targets of CBP, which correlates with significantly reduced c-fos expression in the neurons of the focal deletion. These results indicate that CBP has unique functions in regulating histone modifications associated with long-term memory formation that cannot be compensated for by p300 in neurons of the hippocampus.
Although the HATs p300, PCAF, and CBP have all been implicated in the modification of histones necessary for the regulation of gene expression underlying memory formation, the specific role of each in this process is still unclear (Oliveira et al, 2006, 2007, 2011; Barrett and Wood, 2008; Maurice et al, 2008). The functions of p300 and CBP have been considered redundant until recently (Sterner and Berger, 2000) and although they have been previously shown to have non-overlapping functions (Kawasaki et al, 1998; Kung et al, 2000; Kasper et al, 2002; McManus and Hendzel, 2003), they are still often referred to as CBP/p300. Although both p300 and CBP have been shown to have similar targets in vitro (Ogryzko et al, 1996; Kouzarides, 2007), our data suggest p300 cannot fully compensate for CBP in vivo. p300 appears to be normally expressed in the hippocampus of Cbp flox/flox receiving AAV-Cre, similar to a previous study showing that Cbp flox/flox mice crossed with CaMKII_α_-Cre transgenic mice exhibit normal p300 expression in neurons lacking CBP (Kang-Decker et al, 2004; Chen et al, 2010). Supporting a previous behavioral study showing that CBP and p300 have different roles in motor learning, with CBP function being more critical for motor skill learning than p300 function (Oliveira et al, 2006), the findings reported here indicate that CBP and p300 may have unique functions in the brain, specifically in the hippocampus.
In the late 1990s, two studies demonstrated that the phosphorylation of CREB could be uncoupled from CREB-mediated gene expression (Chawla et al, 1998; Hardingham et al, 1999). Chawla et al (1998) proposed a model in which CREB:CBP-mediated gene expression requires two steps: The phosphorylation of CREB (resulting in the recruitment of CBP) and the phosphorylation of CBP for its activation. This model suggests how different signaling cascades may initiate different transcriptional pathways in spite of converging on a common transcription factor like CREB. Thus, even though upstream signaling may result in phosphorylation of CREB at serine 133 (see Figure 3a and b), such actions may be insufficient to activate CREB:CBP-mediated gene expression in the absence of CBP (Chawla et al, 1998; Hardingham et al, 1999; see Figure 3d). Loss of CBP resulted in a decrease in histone acetylation on multiple histones as well as decreased expression of c-fos, a well-known downstream target of CREB and CBP. These data agree with previous cell culture studies and also indicate that pCREB should not be used as an absolute marker of CREB-mediated gene expression.
Previously, we and others have found long-term memory impairments in CBP mutant mice (Oike et al, 1999; Bourtchouladze et al, 2003; Alarcon et al, 2004; Korzus et al, 2004; Wood et al, 2005, 2006; Chen et al, 2010). However, no study to date has examined a homozygous knockout of CBP in a site-specific and temporally defined manner. Our data demonstrate that a CBP focal deletion, whether large or small, leads to long-term, but not short-term, memory deficits. Mice with the large deletion exhibit impaired long-term memory for location-dependent object recognition, but normal long-term memory for object recognition in which the location was not changed. Further, systemic administration of the HDAC inhibitor NaBut was not able enhance memory in mice with a focal deletion of CBP, similar to findings previously published (Vecsey et al, 2007; Haettig et al, 2011). Together, these findings further support a role for CBP in hippocampus-dependent long-term memory formation.
Very recently, Chen et al (2010) found that CaMKII_α_-Cre driven deletion of Cbp in Cbp_flox/flox mice resulted in impairments in both short- and long-term memory. The impairments in short-term memory are particularly interesting as no other study examining genetically modified CBP mutant mice has found short-term memory impairments (Oike et al, 1999; Bourtchouladze et al, 2003; Alarcon et al, 2004; Korzus et al, 2004; Wood et al, 2005, 2006). Chen et al (2010) suggested this may be because a full CBP knockout had never been examined with regard to learning and memory before their study. However, it is possible that there are developmental confounds because of the use of CaMKII_α, which begins driving expression in all forebrain neurons after birth and reaches maximum expression over the next few weeks of life (Kojima et al, 1997). This could have consequences on the developing hippocampus considering a homozygous knockout of CBP is embryonic lethal (Tanaka et al, 1997). In this study, AAV-Cre was used to generate a complete CBP knockout in adult mice only in the dorsal hippocampus, thus reducing the possibility of confounding developmental or behavioral performance effects. Indeed, we observed no effect on basal synaptic transmission, upstream signaling events leading to phosphorylation of CREB, or short-term memory.
Our data demonstrate that a small focal CBP deletion in the dorsal CA1 area of the hippocampus is sufficient to disrupt long-term memory formation for contextual fear conditioning. Although it is difficult to directly compare the effects of the smaller and larger deletions, our data also suggest that a more extensive hippocampal CBP deletion does not create a larger disruption in memory. Compared with the smaller CBP deletions, larger CBP deletions described in this study not only affected more of area CA1, but CA3 and the dentate gyrus as well. Other studies have demonstrated different roles for different regions of the hippocampus in specific types of memory (Nakazawa et al, 2003; Lee and Kesner, 2004; Matus-Amat et al, 2004; Hunsaker and Kesner, 2008), and therefore it is interesting that our results indicate no difference between a deletion of CBP solely in area CA1 and one affecting multiple areas of the hippocampus. However, previous results indicate that while dorsal and ventral CA1 lesions as well as ventral CA3 lesions all impair memory for contextual fear conditioning, dorsal CA3 lesions do not (Hunsaker and Kesner, 2008). The larger deletions cover more of dorsal CA1 and extend into dorsal CA3 and while they also extend somewhat further ventrally, they are mainly restricted to the dorsal hippocampus. As such, our data fit well with lesion data, suggesting that in neurons that are critical for long-term memory, CBP is critical for the formation of this memory.
As predicted from the behavioral results, we found that CBP deletion results in a pronounced impairment to LTP in hippocampal field CA1b. The effect is surprisingly discrete: multiple measures of baseline transmission appeared normal, as did the induction and early expression of potentiation. The selective loss of LTP consolidation is fully consistent with our behavioral results indicating that the CBP:CREB system is not required for the initial phases of learning but instead has an essential role in transferring newly acquired information into long-term memory. The LTP findings also provide clues about the plasticity-related cellular mechanisms affected by the deletion. Past studies indicate that LTP consolidation involves rapid reorganization of the subsynaptic cytoskeleton (Fukazawa et al, 2003; Kramar et al, 2006) and a somewhat delayed protein synthesis event (Stanton and Sarvey, 1984; Frey and Morris, 1997; Nguyen and Kandel, 1997). The protracted time course over which potentiation decays in the Cbp knockouts is as expected for a defect in the synthesis stage of stabilization. Nguyen and Kandel (1997) showed that with a similar LTP induction, a transcription blocker resulted in impaired LTP, with a decay mirroring what we observe here. The LTP and basal synaptic transmission findings support our behavioral findings indicating that CBP is critical for long-term memory formation, but in adult neurons is not involved in basal synaptic function or short-term memory.
In summary, this study represents the first time a focal homozygous knockout of Cbp in a single region of the adult brain has been investigated, which is critically important, as it has revealed exciting functions of CBP. Although loss of CBP leaves upstream signaling events intact, indicated by unchanged phosphorylation of CREB, it disrupts downstream events such as the acetylation of histones, c-fos expression, and behavior. Together, these findings elucidate the mechanism by which CBP may be regulating coordinate gene regulation during memory formation.
Acknowledgments
This work was supported by the Whitehall Foundation and NIMH (R01MH081004; to MAW), the Department of Veterans Affairs and NIMH (R01MH080297; to RWG), and predoctoral NRSA fellowships (F31MH85494 to RMB; F31DA293682 to MM), the Office of Naval Research (MURI; to GL).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
Supplementary Figure S1
Supplementary Figure Legend
References
- Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, et al. Chromatin acetylation, memory, and LTP are impaired in CBP+/- mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. doi: 10.1016/j.neuron.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol Rev. 2009;89:121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany Z, Newsome D, Oldread E, Livingston DM, Eckner R. A family of transcriptional adaptor proteins targeted by the E1A oncoprotein. Nature. 1995;374:81–84. doi: 10.1038/374081a0. [DOI] [PubMed] [Google Scholar]
- Balderas I, Rodriguez-Ortiz CJ, Salgado-Tonda P, Chavez-Hurtado J, McGaugh JL, Bermudez-Rattoni F. The consolidation of object and context recognition memory involve different regions of the temporal lobe. Learn Mem. 2008;15:618–624. doi: 10.1101/lm.1028008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett RM, Wood MA. Beyond transcription factors: the role of chromatin modifying enzymes in regulating transcription required for memory. Learn Mem. 2008;15:460–467. doi: 10.1101/lm.917508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtchouladze R, Lidge R, Catapano R, Stanley J, Gossweiler S, Romashko D, et al. A mouse model of Rubinstein-Taybi syndrome: defective long-term memory is ameliorated by inhibitors of phosphodiesterase 4. Proc Natl Acad Sci USA. 2003;100:10518–10522. doi: 10.1073/pnas.1834280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, et al. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10:302–317. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Chawla S, Hardingham GE, Quinn DR, Bading H. CBP: a signal-regulated transcriptional coactivator controlled by nuclear calcium and CaM kinase IV. Science. 1998;281:1505–1509. doi: 10.1126/science.281.5382.1505. [DOI] [PubMed] [Google Scholar]
- Chen G, Zou X, Watanabe H, van Deursen JM, Shen J. CREB binding protein is required for both short-term and long-term memory formation. J Neurosci. 2010;30:13066–13077. doi: 10.1523/JNEUROSCI.2378-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chwang WB, O'Riordan KJ, Levenson JM, Sweatt JD. ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn Mem. 2006;13:322–328. doi: 10.1101/lm.152906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chwang WB, Arthur JS, Schumacher A, Sweatt JD. The nuclear kinase mitogen- and stress-activated protein kinase 1 regulates hippocampal chromatin remodeling in memory formation. J Neurosci. 2007;27:12732–12742. doi: 10.1523/JNEUROSCI.2522-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckner R, Arany Z, Ewen M, Sellers W, Livingston DM. The adenovirus E1A-associated 300-kD protein exhibits properties of a transcriptional coactivator and belongs to an evolutionarily conserved family. Cold Spring Harb Symp Quant Biol. 1994;59:85–95. doi: 10.1101/sqb.1994.059.01.012. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron. 2003;38:447–460. doi: 10.1016/s0896-6273(03)00206-x. [DOI] [PubMed] [Google Scholar]
- Haettig J, Stefanko DP, Multani ML, Figueroa DX, McQuown SC, Wood MA. HDAC inhibition modulates hippocampus-dependent long-term memory for object location in a CBP-dependent manner. Learn Mem. 2011;18:71–79. doi: 10.1101/lm.1986911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Chawla S, Cruzalegui FH, Bading H. Control of recruitment and transcription-activating function of CBP determines gene regulation by NMDA receptors and L-type calcium channels. Neuron. 1999;22:789–798. doi: 10.1016/s0896-6273(00)80737-0. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Kesner RP. Dissociations across the dorsal-ventral axis of CA3 and CA1 for encoding and retrieval of contextual and auditory-cued fear. Neurobiol Learn Mem. 2008;89:61–69. doi: 10.1016/j.nlm.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang-Decker N, Tong C, Boussouar F, Baker DJ, Xu W, Leontovich AA, et al. Loss of CBP causes T cell lymphomagenesis in synergy with p27Kip1 insufficiency. Cancer Cell. 2004;5:177–189. doi: 10.1016/s1535-6108(04)00022-4. [DOI] [PubMed] [Google Scholar]
- Kaspar BK, Vissel B, Bengoechea T, Crone S, Randolph-Moore L, Muller R, et al. Adeno-associated virus effectively mediates conditional gene modification in the brain. Proc Natl Acad Sci USA. 2002;99:2320–2325. doi: 10.1073/pnas.042678699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper LH, Boussouar F, Ney PA, Jackson CW, Rehg J, van Deursen JM, et al. A transcription-factor-binding surface of coactivator p300 is required for haematopoiesis. Nature. 2002;419:738–743. doi: 10.1038/nature01062. [DOI] [PubMed] [Google Scholar]
- Kasper LH, Fukuyama T, Biesen MA, Boussouar F, Tong C, de Pauw A, et al. Conditional knockout mice reveal distinct functions for the global transcriptional coactivators CBP and p300 in T-cell development. Mol Cell Biol. 2006;26:789–809. doi: 10.1128/MCB.26.3.789-809.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H, Eckner R, Yao TP, Taira K, Chiu R, Livingston DM, et al. Distinct roles of the co-activators p300 and CBP in retinoic-acid-induced F9-cell differentiation. Nature. 1998;393:284–289. doi: 10.1038/30538. [DOI] [PubMed] [Google Scholar]
- Kojima N, Wang J, Mansuy IM, Grant SG, Mayford M, Kandel ER. Rescuing impairment of long-term potentiation in fyn-deficient mice by introducing Fyn transgene. Proc Natl Acad Sci USA. 1997;94:4761–4765. doi: 10.1073/pnas.94.9.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42:961–972. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Kramar EA, Lin B, Rex CS, Gall CM, Lynch G. Integrin-driven actin polymerization consolidates long-term potentiation. Proc Natl Acad Sci USA. 2006;103:5579–5584. doi: 10.1073/pnas.0601354103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung AL, Rebel VI, Bronson RT, Ch'ng LE, Sieff CA, Livingston DM, et al. Gene dose-dependent control of hematopoiesis and hematologic tumor suppression by CBP. Genes Dev. 2000;14:272–277. [PMC free article] [PubMed] [Google Scholar]
- Lattal KM, Barrett RM, Wood MA. Systemic or intrahippocampal delivery of histone deacetylase inhibitors facilitates fear extinction. Behav Neurosci. 2007;121:1125–1131. doi: 10.1037/0735-7044.121.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterborn JC, Rex CS, Kramar E, Chen LY, Pandyarajan V, Lynch G, et al. Brain-derived neurotrophic factor rescues synaptic plasticity in a mouse model of fragile X syndrome. J Neurosci. 2007;27:10685–10694. doi: 10.1523/JNEUROSCI.2624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Differential contributions of dorsal hippocampal subregions to memory acquisition and retrieval in contextual fear-conditioning. Hippocampus. 2004;14:301–310. doi: 10.1002/hipo.10177. [DOI] [PubMed] [Google Scholar]
- Levenson JM, O'Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- Lundblad JR, Kwok RP, Laurance ME, Harter ML, Goodman RH. Adenoviral E1A-associated protein p300 as a functional homologue of the transcriptional co-activator CBP. Nature. 1995;374:85–88. doi: 10.1038/374085a0. [DOI] [PubMed] [Google Scholar]
- Malvaez M, Sanchis-Segura C, Vo D, Lattal KM, Wood MA. Modulation of chromatin modification facilitates extinction of cocaine-induced conditioned place preference. Biol Psychiatry. 2010;67:36–43. doi: 10.1016/j.biopsych.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S, Holt W. The hippocampus and contextual memory retrieval in Pavlovian conditioning. Behav Brain Res. 2000;110:97–108. doi: 10.1016/s0166-4328(99)00188-6. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J Neurosci. 2004;24:2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice T, Duclot F, Meunier J, Naert G, Givalois L, Meffre J, et al. Altered memory capacities and response to stress in p300/CBP-associated factor (PCAF) histone acetylase knockout mice. Neuropsychopharmacology. 2008;33:1584–1602. doi: 10.1038/sj.npp.1301551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus KJ, Hendzel MJ. Quantitative analysis of CBP- and P300-induced histone acetylations in vivo using native chromatin. Mol Cell Biol. 2003;23:7611–7627. doi: 10.1128/MCB.23.21.7611-7627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuown SC, Barrett RM, Matheos DP, Post R, Rogge GA, Alenghat T, et al. HDAC3 is a critical negative regulator of long-term memory formation. J Neurosci. 2011;31:764–774. doi: 10.1523/JNEUROSCI.5052-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG, Tremblay A, Lecluse V, Lehmann H. Hippocampal damage and anterograde object-recognition in rats after long retention intervals. Hippocampus. 2005;15:1050–1056. doi: 10.1002/hipo.20122. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Sun LD, Quirk MC, Rondi-Reig L, Wilson MA, Tonegawa S. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron. 2003;38:305–315. doi: 10.1016/s0896-6273(03)00165-x. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Kandel ER. Brief theta-burst stimulation induces a transcription-dependent late phase of LTP requiring cAMP in area CA1 of the mouse hippocampus. Learn Mem. 1997;4:230–243. doi: 10.1101/lm.4.2.230. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Burgess N. Theta activity, virtual navigation and the human hippocampus. Trends Cogn Sci. 1999;3:403–406. doi: 10.1016/s1364-6613(99)01396-0. [DOI] [PubMed] [Google Scholar]
- Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- Oike Y, Hata A, Mamiya T, Kaname T, Noda Y, Suzuki M, et al. Truncated CBP protein leads to classical Rubinstein-Taybi syndrome phenotypes in mice: implications for a dominant-negative mechanism. Hum Mol Genet. 1999;8:387–396. doi: 10.1093/hmg/8.3.387. [DOI] [PubMed] [Google Scholar]
- Oliveira AM, Abel T, Brindle PK, Wood MA. Differential role for CBP and p300 CREB-binding domain in motor skill learning. Behav Neurosci. 2006;120:724–729. doi: 10.1037/0735-7044.120.3.724. [DOI] [PubMed] [Google Scholar]
- Oliveira AM, Wood MA, McDonough CB, Abel T. Transgenic mice expressing an inhibitory truncated form of p300 exhibit long-term memory deficits. Learn Mem. 2007;14:564–572. doi: 10.1101/lm.656907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AM, Estevez MA, Hawk JD, Grimes S, Brindle PK, Abel T. Subregion-specific p300 conditional knock-out mice exhibit long-term memory impairments. Learn Mem. 2011;18:161–169. doi: 10.1101/lm.1939811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, et al. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;328:753–756. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29 doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30 doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Hernandez A, Cabrera SM, Hagewoud R, Malvaez M, Stefanko DP, et al. Membrane-associated glucocorticoid activity is necessary for modulation of long-term memory via chromatin modification. J Neurosci. 2010;30:5037–5046. doi: 10.1523/JNEUROSCI.5717-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell TE, Arrigoni E, Thompson MA, Ronan PJ, Saper CB, Greene RW. Focal deletion of the adenosine A1 receptor in adult mice using an adeno-associated viral vector. J Neurosci. 2003;23:5762–5770. doi: 10.1523/JNEUROSCI.23-13-05762.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000a;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade: novel fluorochromes for detecting toxicant-induced neuronal degeneration. Toxicol Pathol. 2000b;28:91–99. doi: 10.1177/019262330002800111. [DOI] [PubMed] [Google Scholar]
- Smith DM, Mizumori SJ. Learning-related development of context-specific neuronal responses to places and events: the hippocampal role in context processing. J Neurosci. 2006;26:3154–3163. doi: 10.1523/JNEUROSCI.3234-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanciu M, Radulovic J, Spiess J. Phosphorylated cAMP response element binding protein in the mouse brain after fear conditioning: relationship to Fos production. Brain Res Mol Brain Res. 2001;94:15–24. doi: 10.1016/s0169-328x(01)00174-7. [DOI] [PubMed] [Google Scholar]
- Stanton PK, Sarvey JM. Blockade of long-term potentiation in rat hippocampal CA1 region by inhibitors of protein synthesis. J Neurosci. 1984;4:3080–3088. doi: 10.1523/JNEUROSCI.04-12-03080.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA. Modulation of long-term memory for obejct recognition via HDAC inhibition. Proc Natl Acad Sci USA. 2009;106:9447–9452. doi: 10.1073/pnas.0903964106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner DE, Berger SL. Acetylation of histones and transcription-related factors. Microbiol Mol Biol Rev. 2000;64:435–459. doi: 10.1128/mmbr.64.2.435-459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Naruse I, Maekawa T, Masuya H, Shiroishi T, Ishii S. Abnormal skeletal patterning in embryos lacking a single Cbp allele: a partial similarity with Rubinstein-Taybi syndrome. Proc Natl Acad Sci USA. 1997;94:10215–10220. doi: 10.1073/pnas.94.19.10215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci. 2007;27:6128–6140. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J Neurosci. 2004;24:5901–5908. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MA, Attner MA, Oliveira AM, Brindle PK, Abel T. A transcription factor-binding domain of the coactivator CBP is essential for long-term memory and the expression of specific target genes. Learn Mem. 2006;13:609–617. doi: 10.1101/lm.213906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MA, Kaplan MP, Park A, Blanchard EJ, Oliveira AM, Lombardi TL, et al. Transgenic mice expressing a truncated form of CREB-binding protein (CBP) exhibit deficits in hippocampal synaptic plasticity and memory storage. Learn Mem. 2005;12:111–119. doi: 10.1101/lm.86605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Fukuyama T, Ney PA, Wang D, Rehg J, Boyd K, et al. Global transcriptional coactivators CREB-binding protein and p300 are highly essential collectively but not individually in peripheral B cells. Blood. 2006;107:4407–4416. doi: 10.1182/blood-2005-08-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure Legend