Targeted gene disruption in somatic zebrafish cells using engineered TALENs (original) (raw)
. Author manuscript; available in PMC: 2012 Feb 5.
Published in final edited form as: Nat Biotechnol. 2011 Aug 5;29(8):697–698. doi: 10.1038/nbt.1934
To the Editor:
Miller et al. recently described a TALE nuclease architecture for performing efficient genome editing1. The authors demonstrated that TALE nucleases, composed of an engineered array of TALE repeats fused to the non-specific _Fok_I cleavage domain, could be used to introduce targeted double-stranded breaks (DSBs) in human cells with high efficiency. Repair of these DSBs by normal DNA repair mechanisms such as non-homologous end-joining (NHEJ) or homologous recombination (HR) enables introduction of alterations at or near the site of the break. A single 34 amino acid TALE repeat binds to one bp of DNA and repeats that bind each of the four DNA bases have been described2, 3. These modules can be assembled into arrays capable of binding extended DNA sequences. TALE nucleases may have advantages over engineered zinc finger nucleases (ZFNs) due to the relative ease with which they can be designed and their potential ability to be targeted to a wide range of sequences (with target sites reported to be as frequent as 1 in 35 bps of random DNA sequence4).
We sought to determine whether the TALE nuclease framework described by Miller et al. could also be used to efficiently modify endogenous genes in zebrafish. Previous studies have shown that error-prone repair of ZFN-induced DSBs by NHEJ can result in the efficient introduction of small insertions or deletions (indels) at cleavage sites in endogenous zebrafish genes5–7. These indels frequently result in frameshift knockout mutations that can be passed through the germline to create mutant fish5–9. ZFN technology has enabled reverse genetics studies to be performed in zebrafish, a capability that did not previously exist. However, engineering ZFNs can be challenging due to the need to account for context-dependent effects among individual fingers in an array. In addition, although many zebrafish genes can be targeted with ZFNs made by publicly available methods that account for context-dependence7, 10, it can in some instances be difficult to target within some genes in zebrafish due to the currently limited targeting range of publicly available ZFN engineering platforms. Thus, if TALE nucleases could be used to introduce targeted mutations in zebrafish, this platform would provide an important additional capability for this model organism.
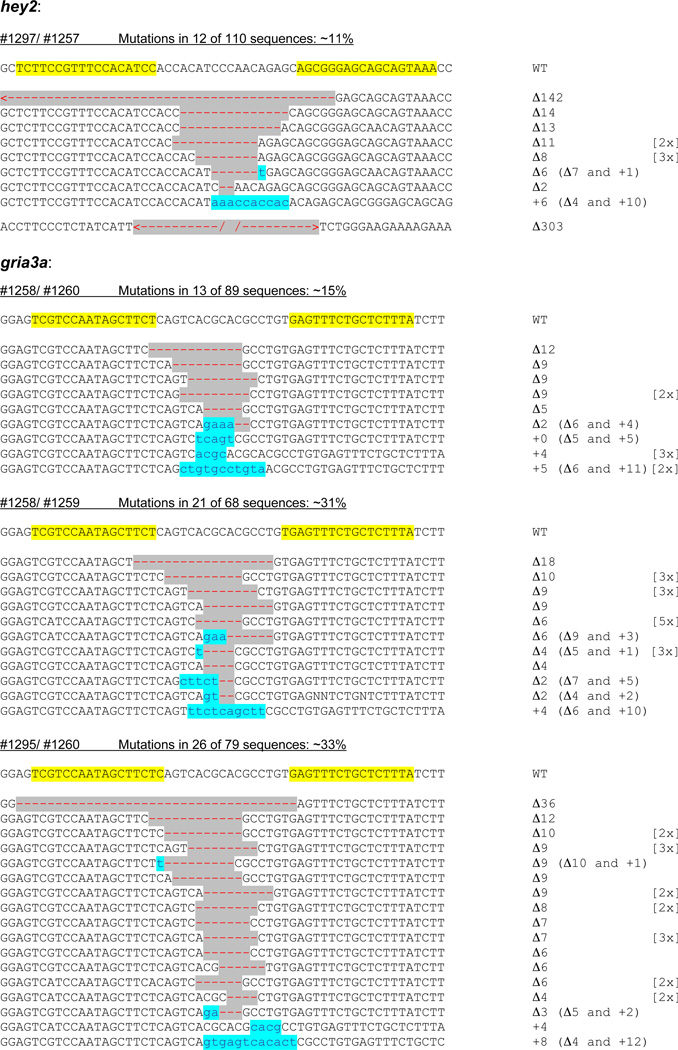
To test the ability of TALE nucleases to function in zebrafish, we targeted DNA sequences in two endogenous zebrafish genes gria3a and hey2 (Figure 1). To avoid confounding effects that might affect binding and cleavage of DNA sites by TALE nucleases (e.g.--chromatin structure or DNA methylation), we chose to target sequences that we had efficiently altered previously in zebrafish using engineered ZFNs (Supplementary Figures 1 and 2). Using an iterative assembly approach (Supplementary Methods), we constructed four TALE nuclease monomers to partially overlapping sites in the gria3a gene and two TALE nuclease monomers to a site in the hey2 gene (Figure 1 and Supplementary Figure 3). These six TALE nuclease monomers all harbor the wild-type _Fok_I cleavage domain (Supplementary Figures 4 and 5) and can be paired in combinations to make three TALE nuclease dimers to the gria3a gene and one TALE nuclease dimer to the hey2 gene (Figure 1). We injected RNAs encoding the various TALE nuclease pairs into one-cell stage zebrafish embryos and determined the frequency of NHEJ-mediated mutagenesis at the target site by sequence analysis of alleles from pooled injected embryos (Supplementary Methods, Supplementary Figs. 6–10 and Supplementary Table 1). As shown in Figure 1, we found that all four pairs of TALE nucleases induced targeted indels with high mutation frequencies ranging from 11 to 33%. These frequencies are comparable to what we obtained with ZFNs targeted to DNA sequences in the same vicinity of the gene (Supplementary Figure 1); however, we note that TALE nucleases harbor wild-type _Fok_I domains whereas the ZFNs harbor obligate heterodimeric _Fok_I domains11. Although small indels were typically observed with the TALE nucleases, some large deletions (up to 303 basepairs) were also found (Figure 1).
Figure 1. Target sequences, frequencies of mutations, and sequences of mutations induced by TALE nucleases in embryonic zebrafish cells.
For each pair of TALE nucleases, the wild-type (WT) target sequence is shown at the top with the intended target sites of the TALE nucleases marked in yellow. Deletions are indicated by gray highlighted red dashes and insertions by blue highlighted lower case blue letters. The sizes of the insertions (+) or deletions (Δ) are indicated to the right of each mutant allele. The number of times that each mutant allele was isolated is shown in brackets. Mutation frequencies are calculated as the number of mutant alleles isolated/the total number of alleles analyzed. For the hey2 gene, we also identified two larger deletions 142 and 303 bps in length that extend substantially beyond the intended target sites of the TALE nucleases.
To assess the toxicity of our engineered TALE nucleases, we scored the percentages of dead and deformed embryos that resulted from mRNA microinjections (Supplementary Figure 11). Although we cannot directly compare these results with the microinjections of ZFNs due to the differences between the _Fok_I endonuclease domains used (EL/KK heterodimeric _Fok_I11 for ZFNs versus wild-type _Fok_I for the TALE nucleases) and the specific sequences targeted, the toxicity we observed with injection of 600 pg of TALE nuclease mRNAs (ranging between 40–80%) appears similar to that observed with 400–500 pg of mRNAs encoding ZFNs targeted to sequences in the same vicinity and to other genes (Supplementary Figure 12 and Reference 7).
An important future experiment will be to demonstrate germline transmission of TALE nuclease-induced mutations. Given that the frequencies of mutation and the extent of toxicities we observe are similar to what we have seen with ZFNs, we expect that TALE nuclease-induced mutations should be efficiently passed through the germline to progeny and we are currently conducting experiments to test this prediction. Successful germline transmission of these mutations will be critical for using TALE nucleases to perform reverse genetics in zebrafish. Progeny fish bearing TALE nuclease-induced mutations, unlike founder F0 fish, will not be mosaic (i.e.--these fish will have uniform mutation of all cells in the organism); such mutant fish will enable determination of whether both mono-allelic and bi-allelic alterations of a gene are possible and will provide a more straightforward background on which to perform analysis of off-target effects.
In summary, we show that the TALE nuclease framework described by Miller et al. can be used to efficiently introduce targeted indel mutations in endogenous zebrafish genes at the somatic cell level. Although in this study we chose two genomic loci that have been successfully targeted with ZFNs before, all six TALE nuclease monomers we constructed showed high mutagenesis activities when tested in various pairwise combinations, suggesting that the TALE nuclease framework is also highly robust and effective in zebrafish. As is the case with ZFNs, the complete genome-wide spectrum of off-target mutations introduced by TALE nucleases remains unknown. However, expression of the TALE nucleases we made in zebrafish does not show toxicity substantially different from that observed with expression of ZFNs, suggesting that the magnitude of off-target effects may be comparable with the two types of nucleases. In principle, off-target mutations made by TALE nucleases can be removed by out-crossing the founder assuming that they are not tightly linked to the intended mutation. In addition, mutant phenotypes could also be confirmed by generation of a second mutant allele using nucleases targeted to a different site. For mutagenesis of genes in zebrafish (and other model organisms such as C. elegans12), TALE nucleases may offer potential advantages over ZFNs because they can be easily and quickly assembled in a modular fashion and they can potentially target a greater range of DNA sequences. Thus, we expect that the ability to utilize both ZFNs and TALE nucleases should enable any researcher to rapidly and easily create targeted mutations in their endogenous zebrafish gene of interest.
Supplementary Material
1
Acknowledgments
We thank Daniel Voytas and Adam Bogdanove for helpful discussions in the early stages of this project and Jonathan Foley, Morgan Maeder, Stacey Thibodeau-Beganny, Feng Zhang, Michelle Christian, and Daniel Voytas for help with characterizing the _hey2_- and _gria3a_-targeted ZFN pairs. This work was supported by NIH R01 GM088040 (J.K.J. & R.T.P.), NIH Director’s Pioneer Award DP1 OD006862 (J.K.J.), NIH T32 CA009216 (J.D.S.), NIH K01 AG031300 (J.-R.J.Y.), the Jim and Ann Orr MGH Research Scholar award (J.K.J.), and the Charles and Ann Sanders MGH Research Scholar award (R.T.P.).
Footnotes
Author Contributions
J.D.S., R.T.P., J.K.J. and J.-R.J.Y. conceived the study. J.D.S., C.K., D.R. and J.K.J. designed and constructed the TALE nucleases. J.D.S., L.C., C.K. and D.R. performed the experiments. L.C. and J.-R.J.Y. analyzed the mutation results. J.D.S., L.C., R.T.P., J.K.J. and J.-R.J.Y wrote the paper.
Competing Financial Interests
The authors declare no competing financial interests.
References
- 1.Miller JC, et al. A TALE nuclease architecture for efficient genome editing. Nat Biotechnol. 2011;29:143–148. doi: 10.1038/nbt.1755. [DOI] [PubMed] [Google Scholar]
- 2.Moscou MJ, Bogdanove AJ. A simple cipher governs DNA recognition by TAL effectors. Science. 2009;326:1501. doi: 10.1126/science.1178817. [DOI] [PubMed] [Google Scholar]
- 3.Boch J, et al. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326:1509–1512. doi: 10.1126/science.1178811. [DOI] [PubMed] [Google Scholar]
- 4.Cermak T, et al. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 2011 Apr 14; doi: 10.1093/nar/gkr218. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meng X, Noyes MB, Zhu LJ, Lawson ND, Wolfe SA. Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nat Biotechnol. 2008;26:695–701. doi: 10.1038/nbt1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doyon Y, et al. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat Biotechnol. 2008;26:702–708. doi: 10.1038/nbt1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foley JE, et al. Rapid mutation of endogenous zebrafish genes using zinc finger nucleases made by Oligomerized Pool ENgineering (OPEN) PLoS One. 2009;4:e4348. doi: 10.1371/journal.pone.0004348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cifuentes D, et al. A novel miRNA processing pathway independent of Dicer requires Argonaute2 catalytic activity. Science. 328:1694–1698. doi: 10.1126/science.1190809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siekmann AF, Standley C, Fogarty KE, Wolfe SA, Lawson ND. Chemokine signaling guides regional patterning of the first embryonic artery. Genes Dev. 2009;23:2272–2277. doi: 10.1101/gad.1813509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sander JD, et al. Selection-free zinc-finger-nuclease engineering by context-dependent assembly (CoDA) Nat Methods. 2011;8:67–69. doi: 10.1038/nmeth.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller JC, et al. An improved zinc-finger nuclease architecture for highly specific genome editing. Nat Biotechnol. 2007;25:778–785. doi: 10.1038/nbt1319. [DOI] [PubMed] [Google Scholar]
- 12.Wood AJ, et al. Targeted Genome Editing Across Species Using ZFNs and TALENs. Science. 2011 Jun 23; doi: 10.1126/science.1207773. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1