Requirement for Atr in phosphorylation of Chk1 and cell cycle regulation in response to DNA replication blocks and UV-damaged DNA in Xenopus egg extracts (original) (raw)
Abstract
The checkpoint kinase Xchk1 becomes phosphorylated in Xenopus egg extracts in response to DNA replication blocks or UV-damaged DNA. Xchk1 is also required for the cell cycle delay that is induced by unreplicated or UV-damaged DNA. In this report, we have removed the Xenopus homolog of ATR (Xatr) from egg extracts by immunodepletion. In Xatr-depleted extracts, the checkpoint-associated phosphorylation of Xchk1 is abolished, and the cell cycle delay induced by replication blocks is strongly compromised. Xatr from egg extracts phosphorylated recombinant Xchk1 in vitro, but not a mutant form of Xchk1 (Xchk1-4AQ) containing nonphosphorylatable residues in its four conserved SQ/TQ motifs. Recombinant human ATR, but not a kinase-inactive mutant, phosphorylated the same sites in Xchk1. Furthermore, the Xchk1-4AQ mutant was found to be defective in mediating a checkpoint response in egg extracts. These findings suggest that Xchk1 is a functionally important target of Xatr during a checkpoint response to unreplicated or UV-damaged DNA.
Keywords: Atr, Chk1, phosphorylation, replication checkpoint, mitosis
During the cell cycle, the fidelity of DNA synthesis and the occurrence of DNA damage are monitored by checkpoint pathways, which ensure that chromosomal DNA is accurately replicated and transmitted in an undamaged form to daughter cells (Elledge 1996; O'Connell et al. 2000). Many components of such checkpoint pathways have been identified from yeast to vertebrates. In the fission yeast Schizosaccharomyces pombe, a group of eight proteins (Rad1, Rad3, Rad9, Rad17, Rad26, Hus1, Cut5, and Crb2) is thought to participate in detecting structures characteristic of DNA damage and/or incomplete DNA replication. Homologous components exist in the budding yeast Saccharomyces cerevisiae and higher eukaryotes, including Xenopus and humans (Elledge 1996). Among these sensor proteins, Rad3 encodes a protein kinase that plays a pivotal role in checkpoint signaling (Bentley et al. 1996; O'Connell et al. 2000). Its budding yeast and human homologs are Mec1 and ATR, respectively (Cimprich et al. 1996; Keegan et al. 1996). Rad3, Mec1, and ATR belong to a larger subfamily of protein kinases with a phosphoinositide kinase (PIK)-related domain at the C terminus. A member of this family, ATM, is mutated in the disease ataxia telangiectasia (AT; Lavin and Shiloh 1997). Cells derived from AT patients exhibit defective checkpoint responses to DNA damage induced by ionizing radiation (IR). The budding yeast homolog of ATM, Tel1, is also involved in damage responses (Sanchez et al. 1996). Another member, DNA-PKcs, is well characterized as a protein kinase that binds to DNA and is activated by double-stranded DNA ends (Smith and Jackson 1999).
After detection of damaged DNA or specific DNA-replication structures, a signal is transduced to effector molecules, including the protein kinases Chk1 and Cds1 (Elledge 1996). Yeast Rad3 or Mec1 are absolutely required for signaling through the effector kinases Chk1 and Cds1/Rad53, which are phosphorylated because of the presence of DNA damage or replication blocks. In human cells, ATM controls the phosphorylation of the Cds1 homolog Chk2 after exposure to ionizing radiation but not ultraviolet (UV) light or DNA replication blocks (Matsuoka et al. 1998; Blasina et al. 1999a; Brown et al. 1999; Chaturvedi et al. 1999; Tominaga et al. 1999). In contrast to ATM, ATR is essential for early embryonic development of mice (Brown and Baltimore 2000; de Klein et al. 2000). Overexpression of a kinase-inactive ATR in human fibroblasts causes increased sensitivity to IR, UV, and hydroxyurea (HU) and abrogates the cell cycle arrest after DNA damage (Cliby et al. 1998; Wright et al. 1998). Recently, evidence has been presented that ATR regulates the phosphorylation of human Chk1 in response to DNA damage (Liu et al. 2000). Both ATM and ATR phosphorylate p53, and ATM phosphorylates Nbs1 and Brca1 (Banin et al. 1998; Canman et al. 1998; Cortez et al. 1999; Tibbetts et al. 1999; Gatei et al. 2000; Lim et al. 2000). Nonetheless, the functional effects of these phosphorylations have yet to be elucidated. Chk1 and Chk2/Cds1 phosphorylate Cdc25C at a 14–3–3 binding site, leading to its cytoplasmic sequestration through the binding of 14–3–3 proteins (Kumagai and Dunphy 1999; Lopez-Girona et al. 1999; Yang et al. 1999; Zeng and Piwnica-Worms 1999). Chk1 and Chk2/Cds1 also phosphorylate p53 on Ser 20, resulting in stabilization of p53 (Chehab et al. 2000; Hirao et al. 2000; Shieh et al. 2000). The p53 target gene product 14–3–3ς, induced by DNA damage through the function of p53, interacts with Cdc2-cyclin B1 in the cytoplasm (Chan et al. 1999). The cytoplasmic sequestration of Cdc25C and Cdc2-cyclin B1 appears to contribute, at least in part, to DNA checkpoint-induced cell cycle arrest mechanisms.
Previously, by using Xenopus egg extracts, we have demonstrated that the effector kinases Cds1 and Chk1 respond to quite different signals from the genome. Xenopus Cds1 (Xcds1) becomes phosphorylated and activated in response to DNA molecules with double-stranded ends (Guo and Dunphy 2000), whereas Xenopus Chk1 (Xchk1) responds to DNA replication blocks and UV damage (Kumagai et al. 1998). Similar, but less restricted, responses of Cds1 and Chk1 to DNA signals have been observed in other vertebrates, including mice and humans (Sanchez et al. 1997; Matsuoka et al. 1998; Blasina et al. 1999a; Brown et al. 1999; Tominaga et al. 1999; Hirao et al. 2000; Takai et al. 2000; Liu et al. 2000). To explore the role of Atr in these pathways, we have cloned and characterized a Xenopus homolog of ATR (Xatr). Xatr binds to DNA molecules in egg extracts, and this form of Xatr displays greatly increased kinase activity. Immunodepletion of Xatr from egg extracts abolishes the response of Xchk1 to incompletely replicated and UV-damaged DNA, whereas Xcds1 still responds to double-stranded DNA ends in Xatr-depleted extracts. We present evidence that Xchk1 is a direct and functionally important target of Xatr in the unreplicated/UV-damaged DNA checkpoint(s).
Results
Isolation of Xenopus Atr
To investigate the function of ATR in cell cycle regulation, we have isolated a cDNA encoding the Xenopus homolog of ATR (Xatr) by using a degenerate polymerase chain reaction (PCR) approach. Through multiple rounds of library screening, three overlapping Xenopus oocyte cDNAs were isolated that contain ∼90% of the COOH-terminal coding region and the 3′ untranslated region. The NH2-terminal 10% of the coding sequence and the 5′ untranslated region (∼1.2 kb) were obtained by reverse transcription–coupled PCR using Xenopus oocyte total RNA as template. The four cDNA sequences were ligated to generate a product that encodes a 301-kD protein containing 2654 amino acids. As the 5′ untranslated region of this cDNA contains three in-frame translation termination codons, its open-reading frame most likely represents the full-length coding region. Xatr is most related to human ATR (70% identical and 80% similar) and is 29%, 28%, and 23% identical to Drosophila melanogaster Mei-41, S. pombe Rad3, and S. cerevisiae Mec1, respectively. The COOH-terminal kinase domain, shown in Figure 1A, is the most conserved region among the ATR homologs.
Figure 1.
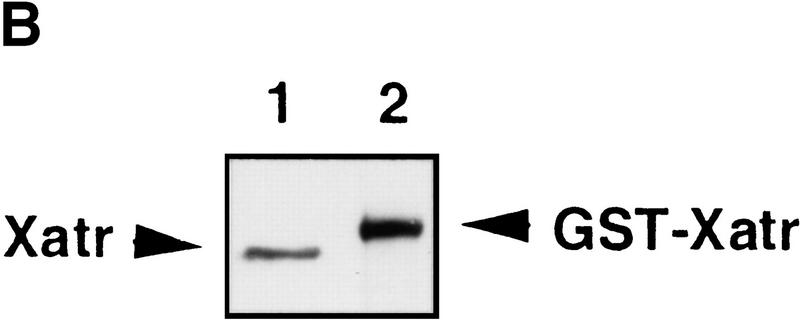
Sequence of Xatr. (A) The COOH-terminal sequence. ATR homologs were aligned by using the PrettyPlot function of the GCG program. Identical residues are boxed. Sequences that were used to design the degenerate PCR primers are underlined. GenBank accession number for Xatr is AF223644. (B) Immunoblot analysis of Xatr and GST–Xatr. Endogenous Xatr in egg extracts (lane 1) and purified GST–Xatr (lane 2) were detected by immunoblotting with anti-Xatr antibodies against His6-Xatr(2351–2654).
Xatr binds to DNA in egg extracts
To functionally characterize Xatr, polyclonal antibodies were generated by using either the truncated fusion protein His6-Xatr (2351–2654) or a 14 amino acid peptide (EKTNPKPGTRGEPK, residues 1617–1630) as the antigen. Affinity-purified antibodies recognized a ∼300-kD protein in Xenopus egg extracts (Fig. 1B, lane 1). The antibodies also recognized a recombinant GST (glutathione _S_-transferase)-Xatr fusion protein expressed in budding yeast (Fig. 1B, lane 2). GST–Xatr migrated about 30 kD larger than endogenous Xatr on SDS-PAGE gels, which corroborates the fact that the Xatr cDNA is full length.
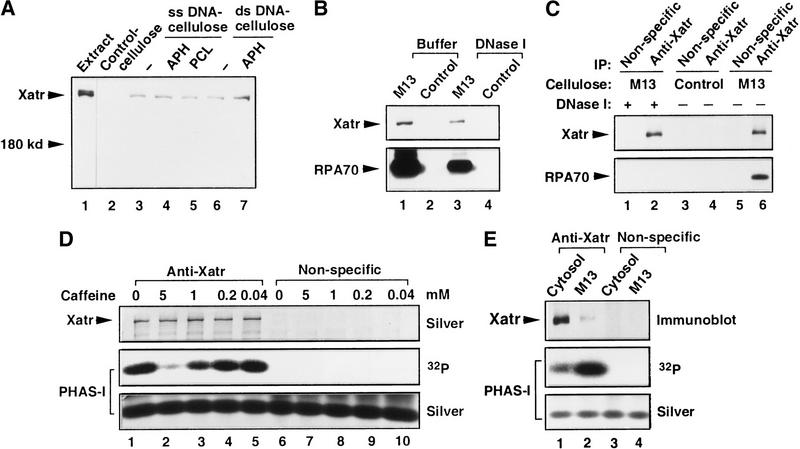
A noteworthy characteristic of the PIK-related protein kinases ATM, ATR, and DNA-PKcs is that they bind to DNA and/or associate with chromosomes, although it remains to be determined whether this association represents direct contact. DNA-PKcs binds to DNA through two accessory proteins Ku70 and Ku80, and its kinase activity is greatly increased by double-stranded DNA ends (Smith and Jackson 1999). It has been reported that both ATR and ATM are associated with meiotic chromosomes (Keegan et al. 1996). Evidence has also been presented that ATM binds to double-stranded DNA and that this interaction is enhanced by treatment of the DNA with ionizing radiation or restriction enzymes (Smith et al. 1999; Suzuki et al. 1999). To test whether Xatr is bound to DNA molecules in Xenopus egg extracts, we incubated DNA cellulose (M13 single-stranded DNA or pBluescript plasmid DNA) with interphase egg cytosol. After extensive washing, the Xatr protein was found to associate with both types of DNA cellulose but not with control cellulose (Fig. 2A). Addition of the DNA polymerase inhibitor aphidicolin or protease inhibitors to cytosol did not significantly enhance the binding. The association of Xatr with DNA cellulose was reduced if the resin was treated with DNaseI after incubation with cytosol (Fig. 2B), indicating that DNA digestion had partially released Xatr. As a control, RPA70, the largest subunit of the RPA complex, which binds tightly to single- and double-stranded DNA in egg extracts (Adachi and Laemmli 1992), also specifically bound to DNA cellulose, and its binding was reduced by DNaseI treatment (Fig. 2B).
Figure 2.
Characterization of Xatr. (A) Xatr binds to DNA cellulose. Control cellulose (lane 2), single-stranded DNA cellulose (lanes 3–5), or double-stranded DNA cellulose (lanes 6,7) were incubated with 50 μL of cytosol in the absence or presence of aphidicolin (APH; 100 μg/mL; lanes 4,7) or protease inhibitors (PCL; 100 μg/mL each of pepstatin, chymostatin, and leupeptin; lane 5). Washed cellulose beads were boiled in 80 μL of gel loading buffer, half of which was subjected to immunoblot analysis for Xatr protein. Lane 1 depicts Xatr in the egg extract. (B) DNaseI digestion partially released Xatr and RPA70 from DNA cellulose. Single-stranded DNA cellulose (lanes 1,3) and control cellulose (lanes 2,4) that had been incubated with cytosol and washed were incubated with buffer alone (lanes 1,2) or DNaseI (lanes 3,4). The proteins that still bound to cellulose were separated by SDS-PAGE and transferred to a PVDF membrane. The Xatr and RPA70 proteins were detected by immunoblot. (C) DNA mediated the coimmunoprecipitation of Xatr and RPA70. Proteins associated with single-stranded DNA cellulose (lanes 1,2,5,6) or control cellulose (lanes 3,4) were released with either DNaseI treatment (lanes 1,2) or 0.5% NP-40 (lanes 3–6). The released proteins were incubated with either anti-Xatr antibodies or nonspecific IgG. The immunoprecipitates were subjected to immunoblot analysis with anti-Xatr (upper panel) and anti-RPA70 antibodies (lower panel). (D) Sensitivity of Xatr-associated kinase activity to caffeine. Kinase assays were performed by incubating the anti-Xatr immunoprecipitates (lanes 1–5) or control immunoprecipitates (lanes 6–10) with PHAS-I (1 μg) and 0–5 mM caffeine, as indicated. Proteins were separated by SDS-PAGE and visualized by silver staining. Phosphorylation of PHAS-I was detected by autoradiography. (E) Xatr released from DNA cellulose displays increased kinase activity. Xatr was immunoprecipitated from cytosol (lane 1) or from DNA cellulose-associated proteins that were released by DNaseI treatment (lane 2). Xatr-associated kinase activity was measured by using PHAS-I as the substrate. As a control, nonspecific IgG did not immunoprecipitate any kinase activity toward PHAS-I from either cytosol or DNA cellulose eluates (lanes 3,4). Xatr immunoprecipitated from both M13 DNA cellulose and pBluescript DNA cellulose eluates displayed similarly elevated kinase activity. Shown here is the result obtained with M13 DNA cellulose.
Because DNA interacts with cellulose through physical adsorption, it can be released by a variety of conditions, under which tightly bound proteins may still associate with DNA and might be coimmunoprecipitated because of bridging DNA. Indeed, RPA70 was found to coimmunoprecipitate with Xatr in 0.5% NP-40 eluates (Fig. 2C). In contrast, RPA70 could not be coimmunoprecipitated with Xatr after elution from DNA cellulose by DNaseI treatment (Fig. 2C).
Enhanced kinase activity of Xatr isolated with DNA cellulose
Next, we characterized the kinase activity of Xatr and its potential regulation by DNA. By using affinity purified antibodies, we found that anti-Xatr immunoprecipitates from Xenopus egg extracts contained a kinase activity that phosphorylated the model substrate protein PHAS-I in vitro (Fig. 2D, lane 1). Control immunoprecipitates with nonspecific IgG did not contain this activity (Fig. 2D, lane 6). Furthermore, the Xatr-associated kinase activity was efficiently inhibited by caffeine (half maximal inhibition at about 0.9 mM caffeine; Fig. 2D), which is consistent with the reports that the PIK-related kinases ATR, ATM, and TOR are sensitive to caffeine, though to different extents (Blasina et al. 1999b; Hall-Jackson et al. 1999; Sarkaria et al. 1999). Significantly, caffeine also inhibits the phosphorylation of Xchk1 in response to unreplicated DNA in aphidicolin-treated Xenopus egg extracts at essentially the same half-maximally effective dose (data not shown), indicating that Xatr and the kinase activity that phosphorylates Xchk1 in these extracts display similar sensitivities to caffeine. To investigate the role of binding to DNA on Xatr-associated kinase activity, we incubated egg cytosol with DNA cellulose to allow the binding of Xatr, digested the DNA with DNaseI, and then immunoprecipitated the released Xatr with anti-Xatr antibodies. Anti-Xatr immunoprecipitates prepared in this manner displayed approximately 10- to 20-fold higher kinase activity toward PHAS-I than Xatr that was immunoprecipitated directly from egg cytosol (Fig. 2E). Addition of DNA templates (e.g., M13 and pBluescript) directly to anti-Xatr immunoprecipitates from egg cytosol did not result in elevation of Xatr-associated kinase activity (data not shown). Therefore, the DNA-cellulose procedure either results in activation of Xatr or is useful for the isolation of a population of Xatr that is already activated in the extracts.
Response of Xchk1 to DNA replication blocks or UV-damaged DNA is dependent on Xatr
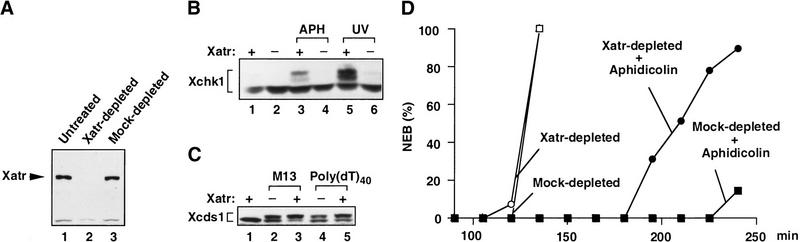
To study the signaling pathways between Xatr and potential downstream effectors, we immunodepleted Xatr from Xenopus egg extracts (Fig. 3). As shown in Figure 3A, Xatr could be completely removed from these extracts with polyclonal anti-Xatr antibodies. Normally, the Xchk1 protein in the nuclear fraction of egg extracts undergoes extensive phosphorylation in response to the DNA polymerase inhibitor aphidicolin or to the presence of UV-damaged DNA (Kumagai et al. 1998; Fig. 3B). However, in Xatr-depleted extracts, the phosphorylation of Xchk1 in the presence of aphidicolin or UV-damaged DNA was completely abolished in comparison with mock-depleted extracts (Fig. 3B). In parallel, we assessed the role of Xatr in the phosphorylation of the Xenopus Cds1 homolog Xcds1. As described recently, Xcds1 responds to DNA templates distinct from those that elicit phosphorylation of Xchk1 (Guo and Dunphy 2000). Xcds1 is not affected by unreplicated or UV-damaged DNA. Instead, Xcds1, but not Xchk1, becomes highly phosphorylated in response to linearized plasmids, double-stranded oligonucleotides, M13 DNA, and poly(dT)40. As M13 DNA, which is partially nicked, and poly(dT)40 are efficiently replicated to a double-stranded form in egg extracts, it appears that only DNA molecules with double-stranded ends can bring about the phosphorylation of Xcds1 (Guo and Dunphy 2000). As shown in Figure 3C, the phosphorylation of Xcds1 in response to M13 DNA or poly(dT)40 was not abolished by removal of Xatr from egg extracts. Therefore, immunodepletion of Xatr causes a selective defect in the phosphorylation of Xchk1.
Figure 3.
The effects of Xatr immunodepletion. (A) Immunodepletion of Xatr. Egg extracts depleted with affinity-purified anti-Xatr antibodies (made against His6-Xatr(2351–2654); lane 2) or nonspecific rabbit IgG (lane 3), along with untreated extract (lane 1), were analyzed for Xatr protein by immunoblotting. (B) Effects of Xatr depletion on the phosphorylation of Xchk1. Extracts depleted of Xatr (lanes 2,4,6) or mock-depleted extracts (lanes 1,3,5) containing sperm nuclei (2000 nuclei/μL; lanes 1,2), sperm nuclei (2000 nuclei/μL) and aphidicolin (100 μg/mL; lanes 3,4), or UV-damaged nuclei (2000 nuclei/μL; lanes 5,6) were incubated at 23°C for 100 min in the presence of 100 μg/mL of cycloheximide. Xchk1 present in the nuclear fraction was detected by immunoblot analysis. (C) Effects of Xatr depletion on the phosphorylation of Xcds1. Extracts depleted of Xatr (lanes 2,4) or mock-depleted extracts (lanes 1,3,5) containing no DNA (lane 1), M13 DNA (10 ng/μL; lanes 2,3), or poly(dT)40 (50 ng/μL; lanes 4,5) were incubated at 23°C for 100 min in the presence of 100 μg/mL of cycloheximide before analysis for Xcds1 protein by immunoblotting. (D) Immunodepletion of Xatr compromised the cell cycle delay induced by DNA replication blocks. Xatr-depleted egg extracts (open circles, filled circles) or mock-depleted extracts (open squares, filled squares) were activated with CaCl2 before the addition of sperm nuclei (500 nuclei/μL; open circles, open squares) or both sperm nuclei (1000 nuclei/μL) and aphidicolin (100 μg/mL) (filled circles, filled squares). The timing of nuclear envelope breakdown (NEB) was monitored by microscopy.
To pursue these observations further, we examined whether immunodepletion of Xatr would affect cell cycle progression in egg extracts undergoing a checkpoint delay. For this purpose, we incubated Xatr-depleted extracts or mock-depleted extracts in the absence or presence of aphidicolin (Fig. 3D). We observed that the Xatr-depleted extracts treated with aphidicolin entered mitosis much sooner than mock-depleted extracts containing this replication inhibitor, indicating that removal of Xatr had significantly compromised the checkpoint response to unreplicated DNA. Interestingly, however, aphidicolin still induced a partial delay in Xatr-depleted extracts in comparison with extracts lacking aphidicolin. As described previously, this characteristic is shared with aphidicolin-treated extracts from which Xchk1 has been immunodepleted, as the replication checkpoint in this system involves both Xchk1-dependent and Xchk1-independent pathways (Kumagai et al. 1998). Thus, the response of Xatr-depleted extracts to DNA replication blocks is strongly compromised but not completely abolished, as is also the case for Xchk1-depleted extracts.
Xatr and human ATR phosphorylate Xchk1 in vitro
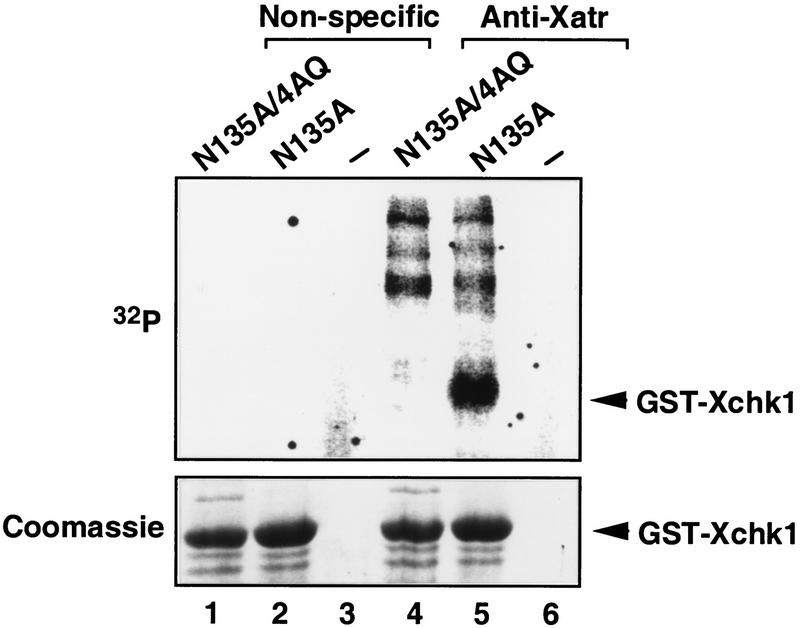
As the checkpoint-associated phosphorylation of Xchk1 did not occur in Xatr-depleted extracts, we asked whether Xchk1 could be a substrate of Xatr. To investigate this possibility, we incubated a kinase-inactive GST-Xchk1-N135A protein with Xatr that had been immunoprecipitated from DNA cellulose eluates. As shown in Fig. 4, the GST-Xchk1-N135A protein was strongly phosphorylated by anti-Xatr immunoprecipitates, but not by control immunoprecipitates. It is known that DNA-PKcs, ATM, and ATR display a preference to phosphorylate SQ or TQ motifs in their substrates (Kim et al. 1999). Xchk1 contains one TQ (Thr 314) and three SQ (Ser 344, Ser 356, Ser 365) sequences in a 52–amino acid region. To assess whether Xatr could phosphorylate these sites, we prepared a modified version of GST-Xchk1-N135A in which Thr 314, Ser 344, Ser 356, and Ser 365 were all mutated to alanine. The resulting GST-Xchk1-N135A-4AQ protein could not serve as a substrate for Xatr-associated kinase activity (Fig. 4, lane 4).
Figure 4.
Phosphorylation of bacterially expressed, full-length GST-Xchk1 by Xatr in vitro. Xatr immunoprecipitated from eluates of DNA (pBluescript) cellulose was incubated with GST-Xchk1-N135A (lane 5), GST-Xchk1-N135A-4AQ (lane 4), or no substrate (lane 6) in the presence of 32P-ATP. The corresponding immunoprecipitates with nonspecific IgG were assayed in lanes 1–3. The proteins were separated by SDS-PAGE and visualized by Coomassie blue staining (bottom panel). Phosphorylated proteins were detected by autoradiography (top panel).
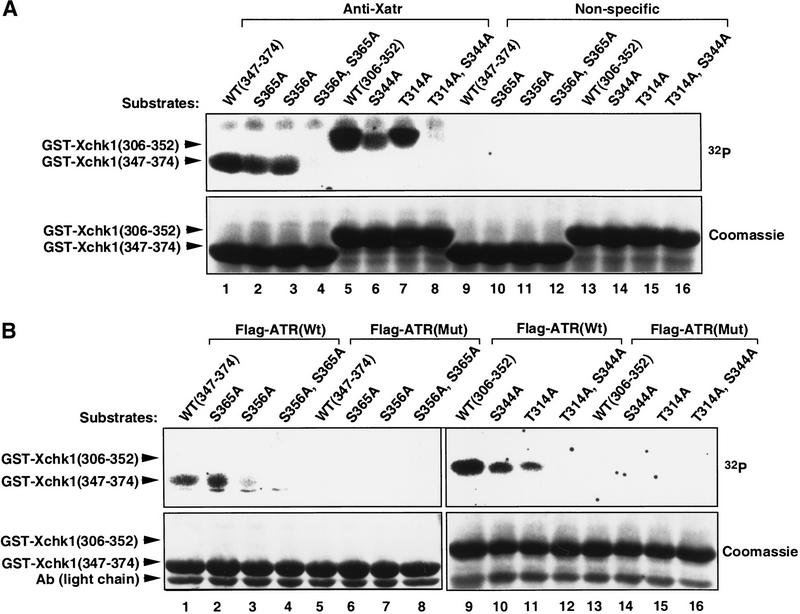
To define more precisely which residues of Xchk1 were required for phosphorylation, we prepared a series of GST-Xchk1 fusion peptides. Initially, two peptides, Xchk1(306–352) and Xchk1(347–374), which contain Thr 314 and Ser 344 and Ser 356 and Ser 365, respectively, were fused to GST and expressed in Escherichia coli. Both GST-Xchk1(306–352) and GST-Xchk1(347–374) were extensively phosphorylated by the anti-Xatr immunoprecipitates, though GST-Xchk1(306–352) seemed to be a better substrate (Fig. 5A, lanes 1,5). Next, we mutated one or both of the S/T residues in the SQ/TQ motifs of each peptide to alanine. Alteration of one such sequence to AQ significantly reduced the phosphorylation, whereas mutagenesis of both sequences to AQ completely abolished phosphorylation of each peptide (Fig. 5A). Thus, it appears that Thr 314, Ser 344, Ser 356, and Ser 365 can all undergo phosphorylation in this in vitro kinase assay. Both GST-Xchk1(306–352) and GST-Xchk1(347–374) were also phosphorylated by a recombinant human Flag-tagged ATR (Fig. 5B, lanes 1,9; Canman et al. 1998) but not a kinase-inactive mutant ATR (Fig. 5B, lanes 5,13), both of which were isolated from human 293T cell extracts. Similar to Xatr, human ATR showed a higher activity toward GST-Xchk1(306–352) than GST-Xchk1(347–374). Likewise, the phosphorylation was reduced or abolished by mutating one or both SQ/TQ motifs in each peptide to AQ (Fig. 5B). Since recombinant human ATR and immunoprecipitated Xatr but not kinase-inactive human ATR phosphorylate Xchk1 at the same sites, this function appears to represent a well-conserved, intrinsic activity of the ATR family.
Figure 5.
Xatr and human ATR phosphorylate Xchk1 at SQ/TQ sites. (A) The fusion proteins GST-Xchk1(347–374), GST-Xchk1(306–352), and the indicated SQ/TQ→AQ mutants of these proteins were tested as substrates in kinase assays with anti-Xatr and control immunoprecipitates that were prepared exactly as described in Figure 4. (B) Phosphorylation of Xchk1 by recombinant human ATR. Wild-type (Wt) or kinase-inactive (Mut) Flag-tagged ATR was isolated from 293T cells as described in Materials and Methods. Each ATR preparation (Wt or Mut) was aliquoted into nine samples, eight of which were used in kinase assays with GST-Xchk1(306–352), GST-Xchk1(347–374), and the indicated SQ/TQ mutant fusion proteins as substrates. Immunoblotting with anti-Flag antibodies indicated that the amount of Flag-tagged ATR protein (Wt or Mut) in each preparation was comparable.
Phosphorylation of Xchk1 in egg extracts is required for the DNA replication checkpoint
To evaluate the contribution of the SQ/TQ domain to the function of Xchk1, we carried out the following experiments. First, we examined the phosphorylation of these motifs in Xenopus egg extracts undergoing a checkpoint delay. As one approach, 35S-labeled wild-type Xchk1 and various SQ/TQ mutant Xchk1 proteins were incubated in egg extracts that either contained or lacked aphidicolin. Subsequently, the radiolabeled Xchk1 proteins were isolated from the nuclear fraction of the extracts and analyzed for phosphorylation-dependent shifts in mobility during gel electrophoresis. As shown in Figure 6A, in the case of the S344A and S356A mutants, the aphidicolin-induced phosphorylation of Xchk1 was strongly reduced. The T314A and S365A mutants still underwent substantial phosphorylation under these conditions. Finally, the checkpoint-dependent upshift in electrophoretic mobility was abolished in the Xchk1-4AQ mutant in which Thr 314, Ser 344, Ser 356, and Ser 365 were all changed to alanine.
Figure 6.
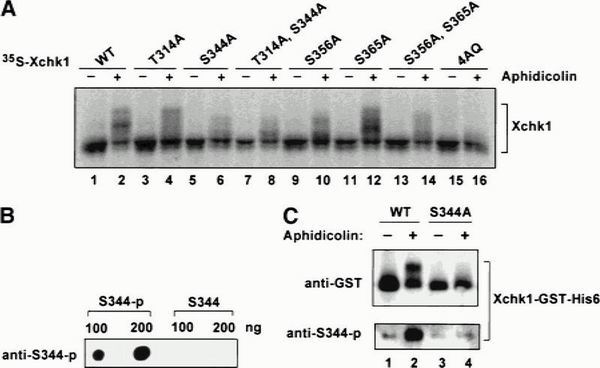
Phosphorylation of the SQ/TQ domain of Xchk1 in Xenopus egg extracts. (A) Modification of wild-type (WT) and various mutant 35S-labeled Xchk1 proteins in egg extracts. The indicated 35S-labeled Xchk1 proteins (WT, T314A, S344A, T314A/S344A, S356A, S365A, S356A/S365A, and 4AQ) were prepared in reticulocyte lysates as described (Kumagai et al. 1998) and incubated in interphase extracts containing sperm nuclei (1000/μL) in the absence (−) or presence (+) of 100 μg/mL aphidicolin. (B) Characterization of anti-S344-p antibodies. Antibodies that recognize phosphorylated Ser 344 were produced as described in Materials and Methods. The antibodies were tested by immunoblotting a peptide containing phosphorylated Ser 344 (S344-p) and the corresponding nonphosphorylated version of the same peptide (S344) that were spotted onto nitrocellulose (100 and 200 ng of each peptide were used). (C) Detection of phosphorylated Xchk1 with anti-S344-p antibodies. Xchk1-WT-GST-His6 (lanes 1,2) and Xchk1-S344A-GST-His6 (lanes 3,4) were incubated in egg extracts in the absence (lanes 1,3) or presence of aphidicolin (lanes 2,4). After 90 min, the recombinant Xchk1 proteins were reisolated with glutathione agarose and immunoblotted with either anti-GST antibodies (top panel) or anti-S344-p antibodies (bottom panel).
In another method, we used antiphosphopeptide antibodies to monitor the phosphorylation of Ser 344 in the presence and absence of aphidicolin (Fig. 6B,C). The Ser 344 site was chosen because mutagenesis of this residue caused a severe reduction in the aphidicolin-induced mobility shift of Xchk1. Also, Ser 344 resides in the most highly conserved of the SQ/TQ motifs among the Xenopus, human, and Drosophila Chk1 proteins. For this experiment, we used recombinant, baculovirus-expressed Xchk1-WT-GST-His6 and Xchk1-S344A-GST-His6 proteins that were double-tagged at the C-terminal end. Xchk1-WT-GST-His6 and Xchk1-S344A-GST-His6 were incubated in egg extracts in the absence or presence of aphidicolin for 90 min, reisolated with glutathione agarose, and then subjected to immunoblotting with either anti-GST antibodies to detect recombinant Xchk1 (Fig. 6C, top panel) or anti-S344-p antibodies to detect phosphorylation of Ser 344 (Fig. 6C, bottom panel). The results of this analysis indicated that wild-type Xchk1 is phosphorylated on Ser 344 in the presence of but not in the absence of aphidicolin (Fig. 6C, bottom, lanes 1,2). In contrast, the anti-S344-p antibodies did not react with the S344A mutant of Xchk1 in either condition (Fig. 6C, bottom, lanes 3,4).
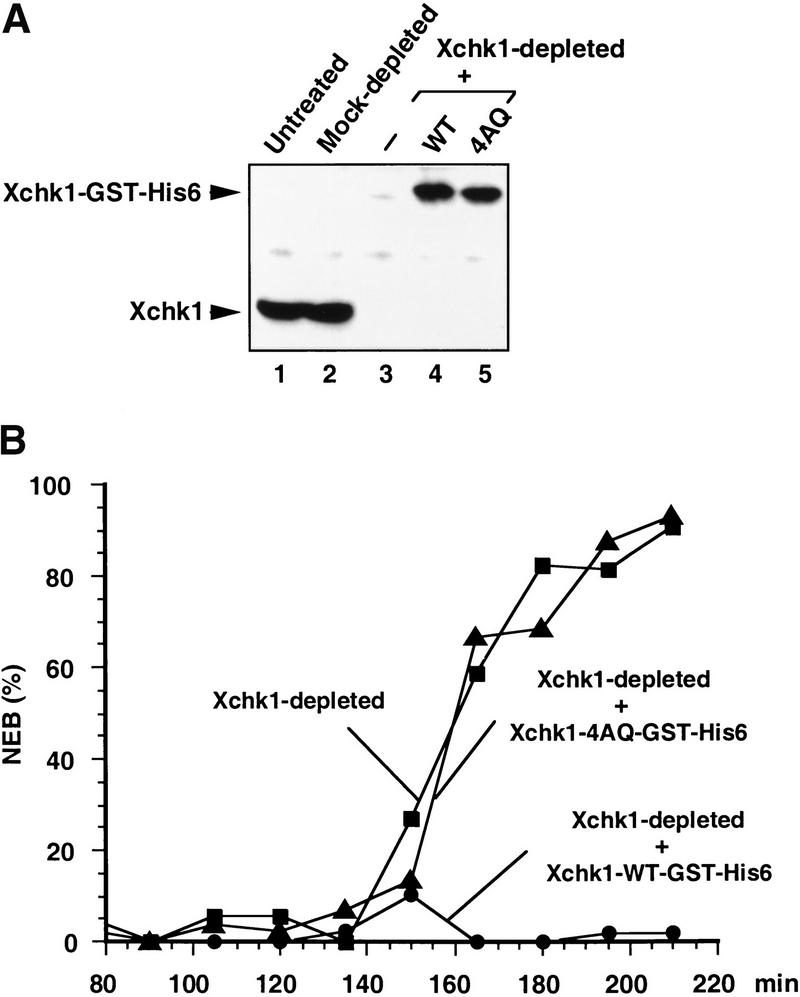
To assess the physiological significance of phosphorylation at the SQ/TQ motifs, we examined whether the 4AQ mutant could function in the DNA replication checkpoint in egg extracts. For this purpose, we removed endogenous Xchk1 completely from egg extracts by immunodepletion with anti-Xchk1 antibodies (Fig. 7A, lane 3). Next, we divided the Xchk1-depleted extract into three aliquots and added baculovirus-expressed wild-type Xchk1, the Xchk1-4AQ mutant, or no additional protein (Fig. 7A, lanes 3–5). Finally, we treated the extracts with aphidicolin and monitored the timing of mitosis (Fig. 7B). Consistent with previous results, the extract containing no Xchk1 entered mitosis inappropriately (Kumagai et al. 1998). As expected, the depleted extract that was restored with recombinant wild-type Xchk1 remained in interphase for at least 3.5 h (Kumagai et al. 1998). By contrast, the extract containing the recombinant Xchk1-4AQ mutant underwent mitosis at the same time as the extract lacking Xchk1, indicating that this mutant of Xchk1 cannot respond to the presence of incompletely replicated DNA.
Figure 7.
The Xchk1-4AQ mutant is defective in mediating the DNA replication checkpoint. (A) Xenopus egg extracts (lane 1) were immunodepleted with control antibodies (lane 2) or anti-Xchk1 antibodies (lanes 3–5). Next, no recombinant protein (lane 3), Xchk1-WT-GST-His6 (lane 4), or Xchk1-4AQ-GST-His6 (lane 5) was added back to the Xchk1-depleted extracts. The extracts were analyzed by immunoblotting with anti-Xchk1 antibodies. (B) Egg extracts lacking endogenous Xchk1 but containing Xchk1-WT-GST-His6 (filled circles), Xchk1–4AQ-GST-His6 (filled triangles), or no additional recombinant protein (filled squares) were activated with CaCl2 before the addition of sperm nuclei (1000 nuclei/μL) and aphidicolin (100 μg/mL). The timing of nuclear envelope breakdown (NEB) was monitored by microscopy. Half-maximal NEB occurred at 90 min in control extracts lacking aphidicolin.
Discussion
In this report, we have provided evidence that Xchk1 is a direct target of Xatr during a checkpoint response to unreplicated or UV-damaged DNA. Immunodepletion of Xatr from Xenopus egg extracts abolishes the phosphorylation of Xchk1 that occurs when chromosomal DNA replication is blocked by treatment with aphidicolin or when the DNA is damaged by exposure to UV radiation. Furthermore, removal of Xatr from egg extracts greatly compromises the cell cycle delay that is induced by DNA replication blocks. Both Xatr and human ATR phosphorylate Xchk1 in vitro at a number of SQ/TQ sites. Moreover, a mutant of Xchk1 that lacks these sites, and thus can not serve as substrate for Xatr, is unable to act as an effector of the DNA replication checkpoint in Xenopus egg extracts. Taken together, these findings indicate that Xchk1 is a functionally critical substrate of Xatr.
The cellular response to damaged or incompletely replicated DNA includes a group of proteins that are highly conserved from yeast to humans. The functions of these proteins have been analyzed by treating cells with DNA damaging agents, such as methylmethane sulfonate (MMS, which creates single- and double-stranded DNA breaks), IR (which induces double-stranded breaks effectively), and UV light (which leads to the formation of pyrimidine dimers; Lindahl and Wood 1999). Agents such as HU and aphidicolin elicit DNA replication blocks initially and may subsequently induce DNA damage. The respective roles of the effector kinases Chk1 and Cds1 in response to the DNA signals generated by such agents or treatments display differences between lower eukaryotes and metazoans. In fission yeast, for example, Chk1 normally responds to DNA damage (induced by MMS, IR, and UV), but not to inhibition of replication with HU, except in cells lacking Cds1 (Walworth and Bernards 1996; Brondello et al. 1999). Conversely, fission yeast Cds1 is activated by blockage of DNA replication (Murakami and Okayama 1995; Lindsay et al. 1998; Brondello et al. 1999) but is also regulated by DNA damage that is inflicted during S-phase.
The situation appears to be somewhat different in metazoans. For instance, Xchk1 in Xenopus egg extracts responds to aphidicolin and UV-treated DNA, which is strongly impaired for replication (Kumagai et al. 1998), but not double-stranded DNA ends (Guo and Dunphy 2000). Likewise, the Drosophila Chk1 homolog Grapes is required for the replication checkpoint in early embryos (Fogarty et al. 1997; Sibon et al. 1997). In embryonic mouse cells lacking Chk1, checkpoint responses to IR, UV, and aphidicolin are compromised (Liu et al. 2000; Takai et al. 2000).
A variety of observations have indicated that vertebrate Cds1 homologs operate in pathways that are involved in sensing double-stranded DNA breaks. In the human system, Chk2/Cds1 is activated on exposure of cells to IR, UV light, and HU. However, the IR-induced activation of Chk2/Cds1 is the strongest, and this response is abolished in cells deficient for ATM, which is clearly involved in a double-stranded DNA break pathway (Matsuoka et al. 1998; Brown et al. 1999). Significantly, the weaker phosphorylation of Chk2/Cds1 in response to HU and UV light is independent of ATM. Optimal phosphorylation of other ATM substrates such as p53 and Brca1 also appears to require double-stranded breaks (Banin et al. 1998; Canman et al. 1998; Cortez et al. 1999). Taken together, these investigations indicate that ATM controls the activation of Chk2/Cds1 in response to double-stranded DNA breaks.
In Xenopus egg extracts, the Cds1 homolog Xcds1 undergoes activation in response to DNA templates, including relatively simple oligonucleotides, that contain double-stranded ends (Guo and Dunphy 2000). Xcds1 does not respond to the presence of aphidicolin (DNA replication blocks) or UV-treated DNA, which does not undergo replication (Kumagai et al. 1998). Because Xatr functions upstream of Xchk1, which does not respond to double-stranded DNA ends, the signal that the Xatr-dependent pathway recognizes is probably distinct from double-stranded breaks. Xatr binds well to either single-stranded or double-stranded DNA cellulose in Xenopus egg extracts. M13 phage DNA and the plasmid pBluescript were used as the single- and double-stranded DNA templates, respectively. Double-stranded DNA ends do not appear to be important for the binding of Xatr to DNA because digestion of the double-stranded plasmid with restriction enzymes did not promote the association of Xatr with the cellulose resin (data not shown). In contrast, double-stranded breaks enhance the interaction of ATM with DNA (Smith et al. 1999; Suzuki et al. 1999), providing another line of evidence that ATM recognizes this structure.
In Xenopus egg extracts, single-stranded DNA is a very good template for replication (Mechali and Harland 1982). However, double-stranded DNA can undergo strand separation in these extracts (Gaudette and Benbow 1986). Thus, it remains to be determined what structure Xatr actually recognizes, directly or indirectly, and how this interaction leads to the regulation of Xatr. This structure might resemble a signal that accumulates in aphidicolin-treated nuclei in Xenopus egg extracts. Exposure to aphidicolin does not block the firing of replication origins in Xenopus sperm chromatin but, rather, arrests DNA synthesis at some point subsequent to the priming stage (Mahbubani et al. 1997). Thus, some aspects of stalled DNA replication forks may lead to the Xatr-dependent phosphorylation of Xchk1.
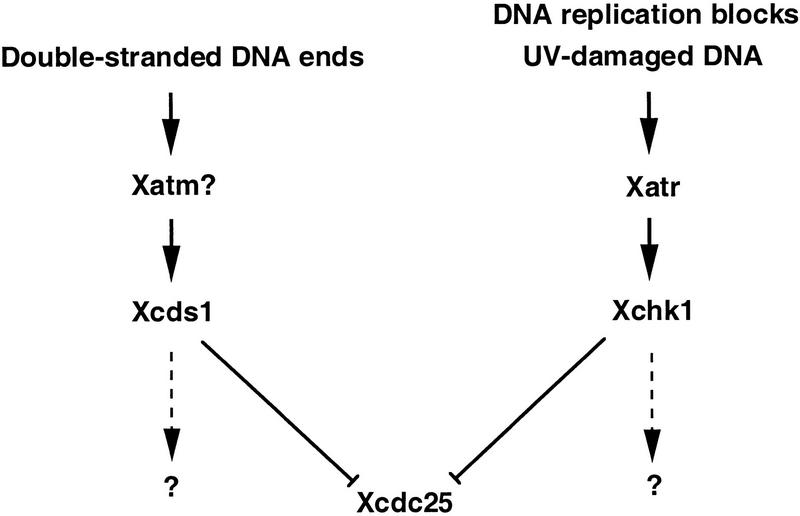
There appears to be significant insulation between the Chk1 and Cds1 pathways in this vertebrate system, although it is possible that there is some cross-talk that is beyond our detection. As shown here, Xatr is an upstream regulator of Xchk1 (Fig. 8). Xatr appears not to be a major regulator of Xcds1. In the human system, ATM controls Chk2/Cds1 and the response to double-stranded DNA breaks (Matsuoka et al. 1998; Brown et al. 1999; Tominaga et al. 1999; Chehab et al. 2000; Hirao et al. 2000; Shieh et al. 2000). It is not known whether Xcds1 is a target of Xenopus Atm (Robertson et al. 1999), but this seems plausible. Both the DNA replication blocks → Atr → Chk1 and double-stranded DNA breaks → Atm → Cds1 pathways appear to possess Cdc25 as one ultimate regulatory target. However, some insulation might be required if these pathways had other separate targets that must respond to certain DNA structures but need to ignore others.
Figure 8.
Model for checkpoint signaling pathways in the Xenopus egg extract system.
In conclusion, we have provided evidence that Xatr regulates Xchk1 during operation of the checkpoint that detects incompletely replicated or UV-damaged DNA. Xatr phosphorylates Xchk1 at a number of conserved SQ/TQ motifs, especially the Ser 344 site. Immunodepletion of either Xatr or Xchk1 from egg extracts abrogates the DNA replication checkpoint to a similar extent. Similarly, egg extracts containing a mutant of Xchk1 that can not be phosphorylated by Xatr are indistinguishable from Xchk1-depleted extracts in their responsiveness to unreplicated DNA. These observations provide a strong argument that Xatr and Xchk1 are functionally linked in a common regulatory pathway. The involvement of Xatr and Xchk1 in a vital function such as monitoring the success of DNA replication would be consistent with the fact that both ATR and Chk1 are essential for early embryonic viability in the mouse (Brown and Baltimore 2000; de Klein et al. 2000; Liu et al. 2000; Takai et al. 2000).
Materials and methods
Cloning of a cDNA encoding Xatr
An internal fragment (140 bp) of a cDNA encoding Xatr was obtained by PCR using the degenerate oligonucleotides CCG GAATTGA(T/C)GCI(A/C)GI(C/T)TIATGG and CGCGGATC CICC(A/G)CA(C/T)TCITC(A/G)TT, which were designed according to conserved areas in ATR homologs (Fig. 1). The PCR reactions contained Xenopus oocyte cDNAs as template, 50 pmole of the degenerate oligonucleotides, 200 μM of dNTPs, and 0.5 units of Taq polymerase in the buffer supplied by the manufacturer (GIBCO BRL). PCR reactions were heated at 94°C for 2 min, followed by 30 cycles of amplification. Each cycle consisted of segments of 94°C for 1 min, 45°C for 1 min, and 72°C for 1 min. An extra 10 min were added to the 72°C extension step for the last cycle. The 140-bp probe was used to screen a Xenopus oocyte cDNA library (Mueller et al. 1995). A 3-kb clone was isolated that includes the COOH-terminal sequence of Xatr and 3′ untranslated region. By using the 5′ end sequence (150 bp) of the 3-kb clone as the probe, a 1.1-kb overlapping clone was isolated from the same library. The 5′ end sequence (150 bp) of the 1.1-kb fragment was labeled with 32P and used to screen another Xenopus oocyte cDNA library (Kinoshita et al. 1995). A 4-kb clone was obtained that overlaps with the 1.1-kb fragment but not with the 3-kb clone. The NH2-terminal sequence of Xatr and 5′ untranslated region were subsequently cloned by using the 5′ RACE system (GIBCO BRL).
Antibody production
An _Nde_I-_Eco_RI fragment encoding amino acids 2351–2654 of Xatr was produced by PCR and cloned into pET3 (Novagen). The His6-Xatr(2351–2654) protein encoded by this plasmid was expressed in E. coli, isolated with nickel agarose, purified further by SDS-PAGE, and used for production of antibodies at a commercial facility (Covance Research Products). Antibodies against an internal peptide of Xatr (EKTNPKPGTRGEPK, residues 1617–1630) were generated at another commercial facility (Zymed Laboratories).
Preparation of DNA cellulose and egg extracts
M13 single-stranded DNA was prepared according to a protocol from the mutagenesis kit from Amersham (oligonucleotide-directed in vitro mutagenesis system version 2). The pBS plasmids were prepared by an alkaline lysis protocol (Sambrook et al. 1989). To prepare DNA cellulose, 1 mg of M13 single-stranded DNA or pBluescript plasmid DNA dissolved in 1 mL of TE buffer (10 mM Tris-HCl, 1 mM EDTA at pH 8.0) was incubated with 0.3 g of dry, clean cellulose for 5 min at 23°C before lyophilization for 18 h. For naked control cellulose, 1 mL of TE buffer containing no DNA was incubated with cellulose under the same conditions. The thoroughly dried powder was resuspended in 20 volumes of TE buffer and incubated at 4°C for 24 h. DNA cellulose was washed twice with 10 volumes of TE and frozen at −70°C.
Xenopus cytostatic factor (CSF)-arrested egg extracts were prepared from unactivated eggs in M-phase as described (Murray 1991). CaCl2 (0.4 mM) was added to drive these extracts into interphase. In some cases, extracts were arrested in interphase with cycloheximide (100 μg/mL). Interphase cytosol was prepared by centrifugation at 260,000_g_ for 1.5 h at 4°C.
Binding of Xatr to DNA cellulose
To allow the binding of Xatr to DNA cellulose, 25–100 μL of DNA cellulose was incubated with 50–500 μL of interphase egg cytosol for 40 min at 23°C with constant rocking. After centrifugation, the cytosol supernatant was removed and the cellulose beads were washed for four times with 1 mL of wash buffer (10 mM HEPES at pH 7.5, 150 mM NaCl, 0.05% NP-40, 30 mM β-glycerolphosphate, 0.1 mM Na3VO4, 0.1 mM phenylmethylsulfonyl fluoride, and 10 μg/mL each of pepstatin, chymostatin, and leupeptin). Washed cellulose beads were digested with DNaseI (4 U/mL) at 23°C for 10 min. After centrifugation, the supernatant was saved for immunoprecipitation of Xatr.
Immunodepletion of Xatr and Xchk1 from egg extracts
M-phase extracts (100 μL) were incubated with 20 μg of affinity-purified anti-Xatr antibodies or 10 μg of anti-Xchk1 antibodies bound to 10 μL of Affiprep protein A beads (Bio-Rad Laboratories) at 4°C for 50 min. The same amount of control rabbit IgG (Zymed Laboratories) was used for mock depletion. After the incubation, the beads were removed by centrifugation. The supernatants were treated again under the same conditions to ensure that Xatr or Xchk1 was quantitatively removed from the extracts.
Immunoprecipitation and kinase assays
Polyclonal antibodies against the peptide EKTNPKPGTRGEPK were used for immunoprecipitating Xatr from either egg cytosol or DNA cellulose eluates. Immunoprecipitations and kinase assays were performed as described (Guo and Dunphy 2000).
Preparation of various recombinant Xchk1 proteins
Individual and combination mutants in which Thr 314, Ser 344, Ser 356, and Ser 365 were changed to alanine were prepared by one or more rounds of mutagenesis of the pBS-Xchk1 plasmid (Kumagai et al. 1998) using the QuikChange kit (Stratagene) and the appropriate oligonucleotides. 35S-labeled versions of wild-type and mutant Xchk1 proteins were prepared using the TNT in vitro transcription/translation system (Promega). DNA fragments encoding Xchk1-N135A, Xchk1-N135A-4AQ, and various wild-type and mutated peptides encompassing the SQ/TQ domain of Xchk1 were subcloned into the _Bam_HI and _Eco_RI sites of pGEX-2T (Amersham Pharmacia Biotech). GST-Xchk1-N135A, GST-Xchk1-N135A-4AQ, and the various GST fusion proteins containing fragments of Xchk1 were isolated from E. coli according to a published protocol (Frangioni and Neel 1993). The production of Xchk1-WT-GST-His6, Xchk1-S344A-GST-His6, and Xchk1-4AQ-GST-His6, which are double tagged at the C-terminal end, in baculovirus-infected Sf9 cells will be described elsewhere.
Detection of Xchk1 with anti-phosphopeptide antibodies
Interphase extracts (200 μL) containing 100 μg/mL cycloheximide and 3 μM tautomycin were incubated with 2 μg of Xchk1-WT-GST-His6 or Xchk1-S344A-GST-His6 in the presence or absence of 100 μg/mL aphidicolin and 3000 sperm nuclei per μL of extract, as indicated. After an incubation of 90 min, 400 μL of dilution buffer (10 mM Hepes at pH 7.5, 150 mM NaCl, 20 mM β-glycerolphosphate, 2.5 mM EGTA, and 0.1% CHAPS) was added. The recombinant Xchk1 proteins were isolated with glutathione agarose, washed four times with dilution buffer, eluted by boiling for 5 min with SDS gel sample buffer, subjected to SDS-PAGE, and immunoblotted either with anti-GST antibodies (Santa Cruz Biotechnology) to detect recombinant Xchk1 or with anti-S344-p antibodies to detect phosphorylation of Ser 344. Anti-S344-p antibodies were produced against a peptide [CGKGISFS(p)QPAAPDNM], which is phosphorylated on Ser 344, at a commercial facility (Covance Research Products). The antibodies were affinity purified as described (Lim et al. 2000). A nonphosphorylated version of the same peptide was used to remove antibodies that do not require phosphorylation of Ser 344 for binding.
Miscellaneous
To block chromosomal DNA replication, aphidicolin (dissolved in DMSO at 10 mg/mL) was added to egg extracts to a final concentration of 100 μg/mL. To produce the GST–Xatr fusion protein, a _Bam_HI-_Sal_I fragment containing the full-length Xatr coding sequence was subcloned into the yeast expression vector pEG(KT) (Mitchell et al. 1993). The resulting plasmid pEG(KT)-Xatr was transformed into the host strain JD51 (_MATa/MAT_α ura3_–_52/ura3_–_52 leu2_–_3,112/leu2_–_3,112 his3_–Δ_200/his3_–Δ_200 trp1_Δ_63/trp1_Δ_63 lys2_–_801/lys2_–_801; Dohmen et al. 1995). Expression and isolation of the GST–Xatr protein were performed according to established procedures (Mitchell et al. 1993). The recombinant GST–Xatr protein is not catalytically active. Transfection of 293T cells with constructs encoding wild-type or kinase-inactive Flag-tagged ATR, immunoprecipitation of Flag-tagged ATR proteins, and kinase assays were performed as described (Canman et al. 1998).
Acknowledgments
We thank members of the Dunphy lab for suggestions throughout this work; K.A. Cimprich for human ATR constructs; A. Murray and J. Minshull for the Xenopus cDNA library; B. W. Wu for helpful advice on 293T cell transfection; and I. Barta, A. Varshavsky, and F. Sherman for yeast strains and expression vectors. This work was supported in part by a grant from the National Institutes of Health. Z.G. is an associate and W.G.D. an investigator in the Howard Hughes Medical Institute, respectively.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL dunphy@cco.caltech.edu; FAX (626) 795-7563.
References
- Adachi Y, Laemmli UK. Identification of nuclear pre-replication centers poised for DNA synthesis in Xenopus egg extracts: Immunolocalization study of replication protein A. J Cell Biol. 1992;119:1–15. doi: 10.1083/jcb.119.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banin S, Moyal L, Shieh S, Taya Y, Anderson CW, Chessa L, Smorodinsky NI, Prives C, Reiss Y, Shiloh Y, et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- Bentley NJ, Holtzman DA, Flaggs G, Keegan KS, DeMaggio A, Ford JC, Hoekstra M, Carr AM. The Schizosaccharomyces pombe rad3 checkpoint gene. EMBO J. 1996;15:6641–6651. [PMC free article] [PubMed] [Google Scholar]
- Blasina A, de Weyer IV, Laus MC, Luyten WH, Parker AE, McGowan CH. A human homolog of the checkpoint kinase Cds1 directly inhibits Cdc25 phosphatase. Curr Biol. 1999a;9:1–10. doi: 10.1016/s0960-9822(99)80041-4. [DOI] [PubMed] [Google Scholar]
- Blasina A, Price BD, Turenne GA, McGowan CH. Caffeine inhibits the checkpoint kinase ATM. Curr Biol. 1999b;9:1135–1138. doi: 10.1016/s0960-9822(99)80486-2. [DOI] [PubMed] [Google Scholar]
- Brondello JM, Boddy MN, Furnari B, Russell P. Basis for the checkpoint signal specificity that regulates Chk1 and Cds1 protein kinases. Mol Cell Biol. 1999;19:4262–4269. doi: 10.1128/mcb.19.6.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AL, Lee CH, Schwarz JK, Mitiku N, Piwnica-Worms H, Chung JH. A human Cds1-related kinase that functions downstream of ATM protein in the cellular response to DNA damage. Proc Natl Acad Sci. 1999;96:3745–3750. doi: 10.1073/pnas.96.7.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EJ, Baltimore D. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes & Dev. 2000;14:397–402. [PMC free article] [PubMed] [Google Scholar]
- Canman CE, Lim DS, Cimprich KA, Taya Y, Tamai K, Sakaguchi K, Appella E, Kastan MB, Siliciano JD. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- Chan TA, Hermeking H, Lengauer C, Kinzler KW, Vogelstein B. 14–3–3ς is required to prevent mitotic catastrophe after DNA damage. Nature. 1999;401:616–620. doi: 10.1038/44188. [DOI] [PubMed] [Google Scholar]
- Chaturvedi P, Eng WK, Zhu Y, Mattern MR, Mishra R, Hurle MR, Zhang X, Annan RS, Lu Q, Faucette LF, et al. Mammalian Chk2 is a downstream effector of the ATM-dependent DNA damage checkpoint pathway. Oncogene. 1999;18:4047–4054. doi: 10.1038/sj.onc.1202925. [DOI] [PubMed] [Google Scholar]
- Chehab NH, Malikzay A, Appel M, Halazonetis TD. Chk2/hCds1 functions as a DNA damage checkpoint in G(1) by stabilizing p53. Genes & Dev. 2000;14:278–288. [PMC free article] [PubMed] [Google Scholar]
- Cimprich KA, Shin TB, Keith CT, Schreiber SL. cDNA cloning and gene mapping of a candidate human cell cycle checkpoint protein. Proc Natl Acad Sci. 1996;93:2850–2855. doi: 10.1073/pnas.93.7.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliby WA, Roberts CJ, Cimprich KA, Stringer CM, Lamb JR, Schreiber SL, Friend SH. Overexpression of a kinase-inactive ATR protein causes sensitivity to DNA-damaging agents and defects in cell cycle checkpoints. EMBO J. 1998;17:159–169. doi: 10.1093/emboj/17.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez D, Wang Y, Qin J, Elledge SJ. Requirement of ATM-dependent phosphorylation of Brca1 in the DNA damage response to double-strand breaks. Science. 1999;286:1162–1166. doi: 10.1126/science.286.5442.1162. [DOI] [PubMed] [Google Scholar]
- de Klein A, Muijtjens M, van Os R, Verhoeven Y, Smit B, Carr AM, Lehmann AR, Hoeijmakers JH. Targeted disruption of the cell-cycle checkpoint gene ATR leads to early embryonic lethality in mice. Curr Biol. 2000;10:479–482. doi: 10.1016/s0960-9822(00)00447-4. [DOI] [PubMed] [Google Scholar]
- Dohmen RJ, Stappen R, McGrath JP, Forrova H, Kolarov J, Goffeau A, Varshavsky A. An essential yeast gene encoding a homolog of ubiquitin-activating enzyme. J Biol Chem. 1995;270:18099–18109. doi: 10.1074/jbc.270.30.18099. [DOI] [PubMed] [Google Scholar]
- Elledge SJ. Cell cycle checkpoints: Preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- Fogarty P, Campbell SD, Abu-Shumays R, Phalle BS, Yu KR, Uy GL, Goldberg ML, Sullivan W. The Drosophila grapes gene is related to checkpoint gene Chk1/Rad27 and is required for late syncytial division fidelity. Curr Biol. 1997;7:418–426. doi: 10.1016/s0960-9822(06)00189-8. [DOI] [PubMed] [Google Scholar]
- Frangioni JV, Neel BG. Solubilization and purification of enzymatically active glutathione S-transferase (pGEX) fusion proteins. Anal Biochem. 1993;210:179–187. doi: 10.1006/abio.1993.1170. [DOI] [PubMed] [Google Scholar]
- Gatei M, Young D, Cerosaletti KM, Desai-Mehta A, Spring K, Kozlov S, Lavin M F, Gatti R A, Concannon P, Khanna K. ATM-dependent phosphorylation of nibrin in response to radiation exposure. Nat Genet. 2000;25:115–119. doi: 10.1038/75508. [DOI] [PubMed] [Google Scholar]
- Gaudette MF, Benbow RM. Replication forks are underrepresented in chromosomal DNA of Xenopus laevis embryos. Proc Natl Acad Sci. 1986;83:5953–5957. doi: 10.1073/pnas.83.16.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Dunphy WG. Response of Xenopus Cds1 in cell-free extracts to DNA templates with double-stranded ends. Mol Biol Cell. 2000;11:1535–1546. doi: 10.1091/mbc.11.5.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Jackson CA, Cross DA, Morrice N, Smythe C. ATR is a caffeine-sensitive, DNA-activated protein kinase with a substrate specificity distinct from DNA-PK. Oncogene. 1999;18:6707–6713. doi: 10.1038/sj.onc.1203077. [DOI] [PubMed] [Google Scholar]
- Hirao A, Kong YY, Matsuoka S, Wakeham A, Ruland J, Yoshida H, Liu D, Elledge SJ, Mak TW. DNA damage–induced activation of p53 by the checkpoint kinase chk2. Science. 2000;287:1824–1827. doi: 10.1126/science.287.5459.1824. [DOI] [PubMed] [Google Scholar]
- Keegan KS, Holtzman DA, Plug AW, Christenson ER, Brainerd EE, Flaggs G, Bentley NJ, Taylor EM, Meyn MS, Moss SB, et al. The Atr and Atm protein kinases associate with different sites along meiotically pairing chromosomes. Genes & Dev. 1996;10:2423–2437. doi: 10.1101/gad.10.19.2423. [DOI] [PubMed] [Google Scholar]
- Kim ST, Lim DS, Canman CE, Kastan MB. Substrate specificities and identification of putative substrates of ATM kinase family members. J Biol Chem. 1999;274:37538–37543. doi: 10.1074/jbc.274.53.37538. [DOI] [PubMed] [Google Scholar]
- Kinoshita N, Minshull J, Kirschner MW. The identification of two novel ligands of the FGF receptor by a yeast screening method and their activity in Xenopus development. Cell. 1995;83:621–630. doi: 10.1016/0092-8674(95)90102-7. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Binding of 14–3–3 proteins and nuclear export control the intracellular localization of the mitotic inducer Cdc25. Genes & Dev. 1999;13:1067–1072. doi: 10.1101/gad.13.9.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, Guo Z, Emami KH, Wang SX, Dunphy WG. The Xenopus Chk1 protein kinase mediates a caffeine-sensitive pathway of checkpoint control in cell-free extracts. J Cell Biol. 1998;142:1559–1569. doi: 10.1083/jcb.142.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin MF, Shiloh Y. The genetic defect in ataxia-telangiectasia. Annu Rev Immunol. 1997;15:177–202. doi: 10.1146/annurev.immunol.15.1.177. [DOI] [PubMed] [Google Scholar]
- Lim DS, Kim ST, Xu B, Maser RS, Lin J, Petrini JH, Kastan MB. ATM phosphorylates p95/Nbs1 in an S-phase checkpoint pathway. Nature. 2000;404:613–617. doi: 10.1038/35007091. [DOI] [PubMed] [Google Scholar]
- Lindahl T, Wood RD. Quality control by DNA repair. Science. 1999;286:1897–1905. doi: 10.1126/science.286.5446.1897. [DOI] [PubMed] [Google Scholar]
- Lindsay HD, Griffiths DJ, Edwards RJ, Christensen PU, Murray JM, Osman F, Walworth N, Carr AM. S-phase-specific activation of Cds1 kinase defines a subpathway of the checkpoint response in Schizosaccharomyces pombe. Genes & Dev. 1998;12:382–395. doi: 10.1101/gad.12.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Guntuku S, Cui X-S, Matsuoka S, Cortez D, Tamai K, Luo G, Carattini-Rivera S, DeMayo F, Bradley A, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G2/M DNA damage checkpoint. Genes & Dev. 2000;14:1448–1459. [PMC free article] [PubMed] [Google Scholar]
- Lopez-Girona A, Furnari B, Mondesert O, Russell P. Nuclear localization of Cdc25 is regulated by DNA damage and a 14–3–3 protein. Nature. 1999;397:172–175. doi: 10.1038/16488. [DOI] [PubMed] [Google Scholar]
- Mahbubani HM, Chong JP, Chevalier S, Thommes P, Blow JJ. Cell cycle regulation of the replication licensing system: Involvement of a Cdk-dependent inhibitor. J Cell Biol. 1997;136:125–135. doi: 10.1083/jcb.136.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- Mechali M, Harland RM. DNA synthesis in a cell-free system from Xenopus eggs: Priming and elongation on single-stranded DNA in vitro. Cell. 1982;30:93–101. doi: 10.1016/0092-8674(82)90015-0. [DOI] [PubMed] [Google Scholar]
- Mitchell DA, Marshall TK, Deschenes RJ. Vectors for the inducible overexpression of glutathione S-transferase fusion proteins in yeast. Yeast. 1993;9:715–722. doi: 10.1002/yea.320090705. [DOI] [PubMed] [Google Scholar]
- Mueller PR, Coleman TR, Dunphy WG. Cell cycle regulation of a Xenopus Wee1-like kinase. Mol Biol Cell. 1995;6:119–134. doi: 10.1091/mbc.6.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami H, Okayama H. A kinase from fission yeast responsible for blocking mitosis in S phase. Nature. 1995;374:817–819. doi: 10.1038/374817a0. [DOI] [PubMed] [Google Scholar]
- Murray AW. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- O'Connell MJ, Walworth NC, Carr AM. The G2-phase DNA-damage checkpoint. Trends Cell Biol. 2000;10:296–303. doi: 10.1016/s0962-8924(00)01773-6. [DOI] [PubMed] [Google Scholar]
- Robertson K, Hensey C, Gautier J. Isolation and characterization of Xenopus ATM (X-ATM): Expression, localization, and complex formation during oogenesis and early development. Oncogene. 1999;18:7070–7079. doi: 10.1038/sj.onc.1203194. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Sanchez Y, Desany BA, Jones WJ, Liu Q, Wang B, Elledge SJ. Regulation of RAD53 by the ATM-like kinases MEC1 and TEL1 in yeast cell cycle checkpoint pathways. Science. 1996;271:357–360. doi: 10.1126/science.271.5247.357. [DOI] [PubMed] [Google Scholar]
- Sanchez Y, Wong C, Thoma RS, Richman R, Wu Z, Piwnica-Worms H, Elledge SJ. Conservation of the Chk1 checkpoint pathway in mammals: Linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- Sarkaria JN, Busby EC, Tibbetts RS, Roos P, Taya Y, Karnitz LM, Abraham RT. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 1999;59:4375–4382. [PubMed] [Google Scholar]
- Shieh SY, Ahn J, Tamai K, Taya Y, Prives C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes & Dev. 2000;14:289–300. [PMC free article] [PubMed] [Google Scholar]
- Sibon OC, Stevenson VA, Theurkauf WE. DNA-replication checkpoint control at the Drosophila midblastula transition. Nature. 1997;388:93–97. doi: 10.1038/40439. [DOI] [PubMed] [Google Scholar]
- Smith GC, Jackson SP. The DNA-dependent protein kinase. Genes & Dev. 1999;13:916–934. doi: 10.1101/gad.13.8.916. [DOI] [PubMed] [Google Scholar]
- Smith GC, Cary RB, Lakin ND, Hann BC, Teo SH, Chen DJ, Jackson SP. Purification and DNA binding properties of the ataxia-telangiectasia gene product ATM. Proc Natl Acad Sci. 1999;96:11134–11139. doi: 10.1073/pnas.96.20.11134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Kodama S, Watanabe M. Recruitment of ATM protein to double strand DNA irradiated with ionizing radiation. J Biol Chem. 1999;274:25571–25575. doi: 10.1074/jbc.274.36.25571. [DOI] [PubMed] [Google Scholar]
- Takai H, Tominaga K, Motoyama N, Minamishima YA, Nagahama H, Tsukiyama T, Ikeda K, Nakayama K, Nakanishi M, Nakayama K. Aberrant cell cycle checkpoint function and early embryonic death in Chk1−/− mice. Genes & Dev. 2000;14:1439–1447. [PMC free article] [PubMed] [Google Scholar]
- Tibbetts RS, Brumbaugh KM, Williams JM, Sarkaria JN, Cliby WA, Shieh SY, Taya Y, Prives C, Abraham RT. A role for ATR in the DNA damage-induced phosphorylation of p53. Genes & Dev. 1999;13:152–157. doi: 10.1101/gad.13.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga K, Morisaki H, Kaneko Y, Fujimoto A, Tanaka T, Ohtsubo M, Hirai M, Okayama H, Ikeda K, Nakanishi M. Role of human Cds1 (Chk2) kinase in DNA damage checkpoint and its regulation by p53. J Biol Chem. 1999;274:31463–31467. doi: 10.1074/jbc.274.44.31463. [DOI] [PubMed] [Google Scholar]
- Walworth NC, Bernards R. Rad-dependent response of the Chk1-encoded protein kinase at the DNA damage checkpoint. Science. 1996;271:353–356. doi: 10.1126/science.271.5247.353. [DOI] [PubMed] [Google Scholar]
- Wright JA, Keegan KS, Herendeen DR, Bentley NJ, Carr AM, Hoekstra MF, Concannon P. Protein kinase mutants of human ATR increase sensitivity to UV and ionizing radiation and abrogate cell cycle checkpoint control. Proc Natl Acad Sci. 1998;95:7445–7450. doi: 10.1073/pnas.95.13.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Winkler K, Yoshida M, Kornbluth S. Maintenance of G2 arrest in the Xenopus oocyte: A role for 14–3–3-mediated inhibition of Cdc25 nuclear import. EMBO J. 1999;18:2174–2183. doi: 10.1093/emboj/18.8.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Piwnica-Worms H. DNA damage and replication checkpoints in fission yeast require nuclear exclusion of the Cdc25 phosphatase via 14–3–3 binding. Mol Cell Biol. 1999;19:7410–7419. doi: 10.1128/mcb.19.11.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]