The essential Gcd10p–Gcd14p nuclear complex is required for 1-methyladenosine modification and maturation of initiator methionyl-tRNA (original) (raw)
Abstract
Gcd10p and Gcd14p are essential proteins required for the initiation of protein synthesis and translational repression of GCN4 mRNA. The phenotypes of gcd10 mutants were suppressed by high-copy-number IMT genes, encoding initiator methionyl tRNA (tRNAiMet), or LHP1, encoding the yeast homolog of the human La autoantigen. The gcd10-504 mutation led to a reduction in steady-state levels of mature tRNAiMet, attributable to increased turnover rather than decreased synthesis of pre-tRNAiMet. Remarkably, the lethality of a GCD10 deletion was suppressed by high-copy-number IMT4, indicating that its role in expression of mature tRNAiMet is the essential function of Gcd10p. A gcd14-2 mutant also showed reduced amounts of mature tRNAiMet, but in addition, displayed a defect in pre-tRNAiMet processing. Gcd10p and Gcd14p were found to be subunits of a protein complex with prominent nuclear localization, suggesting a direct role in tRNAiMet maturation. The chromatographic behavior of elongator and initiator tRNAMet on a RPC-5 column indicated that both species are altered structurally in gcd10Δ cells, and analysis of base modifications revealed that 1-methyladenosine (m1A) is undetectable in gcd10Δ tRNA. Interestingly, gcd10 and gcd14 mutations had no effect on processing or accumulation of elongator tRNAMet, which also contains m1A at position 58, suggesting a unique requirement for this base modification in initiator maturation.
Keywords: Gcd10p–Gcp14p nuclear complex, 1-methyladenosine, tRNA, initiator maturation
A key step in the initiation of protein synthesis in eukaryotic cells involves the binding of the methionyl initiator tRNAMet (Met–tRNAiMet) to the 40S ribosomal subunit to form a 43S preinitiation complex. Initiation factor 2 (eIF2) delivers Met–tRNAiMet to the ribosome in a ternary complex with GTP, and the eIF2 is released in an inactive binary complex with GDP. Exchange of the GDP bound to eIF2 for GTP is catalyzed by the guanine nucleotide exchange factor eIF2B. Phosphorylation of the α subunit of eIF2 (eIF2α) prevents the recycling of eIF2 by eIF2B, inhibiting the formation of eIF2 · GTP · Met–tRNAiMet ternary complexes (Hershey 1991; Hinnebusch 1996). In Saccharomyces cerevisiae, eIF2α is phosphorylated by the protein kinase Gcn2p when cells are deprived of an amino acid and this elicits increased translation of a specific mRNA encoding Gcn4p, a transcriptional activator of amino acid biosynthetic enzymes (Hinnebusch 1996).
Because translation of GCN4 is coupled inversely to the concentration of ternary complexes (Dever et al. 1995) mutations in subunits of eIF2 and eIF2B derepress GCN4 translation in cells lacking Gcn2p (for review, see Hinnebusch 1996). Mutations in GCD10 also derepress GCN4 translation in the absence of eIF2 phosphorylation by Gcn2p (Harashima and Hinnebusch 1986). GCD10 is essential and temperature-sensitive mutations in this gene inhibit general translation initiation at the restrictive temperature. It was found that Gcd10p copurified and coimmunoprecipitated with subunits of translation initiation factor eIF3 (Garcia-Barrio et al. 1995). Given their effect on GCN4 translation, it was proposed that gcd10 mutations reduce the ability of eIF3 to stimulate ternary complex binding to 40S ribosomes, mimicking the inhibition of ternary complex formation elicited by eIF2 phosphorylation. Recently, we purified a yeast eIF3 complex from a strain expressing a polyhistidine-tagged form of the _PRT1_-encoded subunit (Phan et al. 1998) and found that it contains all five S. cerevisiae proteins homologous to subunits of mammalian eIF3, but lacks Gcd10p. In addition, Gcd10p was not coimmunoprecipitated with an epitope-tagged version of the TIF34 subunit of yeast eIF3 (Asano et al. 1998; Phan et al. 1998). Together, these findings suggested that Gcd10p is not an integral subunit of yeast eIF3, and may have a distinct function involved in the formation of ternary complexes or their recruitment to the ribosome.
To identify the in vivo function of Gcd10p, we carried out a genetic analysis by isolating dosage suppressors of the temperature-sensitive phenotype of gcd10 mutations. Analysis of these suppressors revealed that maturation of initiator tRNAMet is defective in gcd10 mutants. Our biochemical results indicate that Gcd10p resides in a nuclear complex with Gcd14p, another factor involved in GCN4 translational control (Cuesta et al. 1998), and is required for 1-methyladenosine formation in yeast tRNA. Moreover, GCD10 was found to be nonessential in cells overexpressing initiator tRNAMet. It appears that the Gcd10p–Gcd14p complex is required specifically at the initiation step of translation because of a strong requirement for 1-methyladenosine at position 58 (m1A58) in the processing and accumulation of initiator tRNAMet.
Results
Genes encoding initiator tRNAMet or a La homolog are dosage suppressors of the growth defects of gcd10 mutants
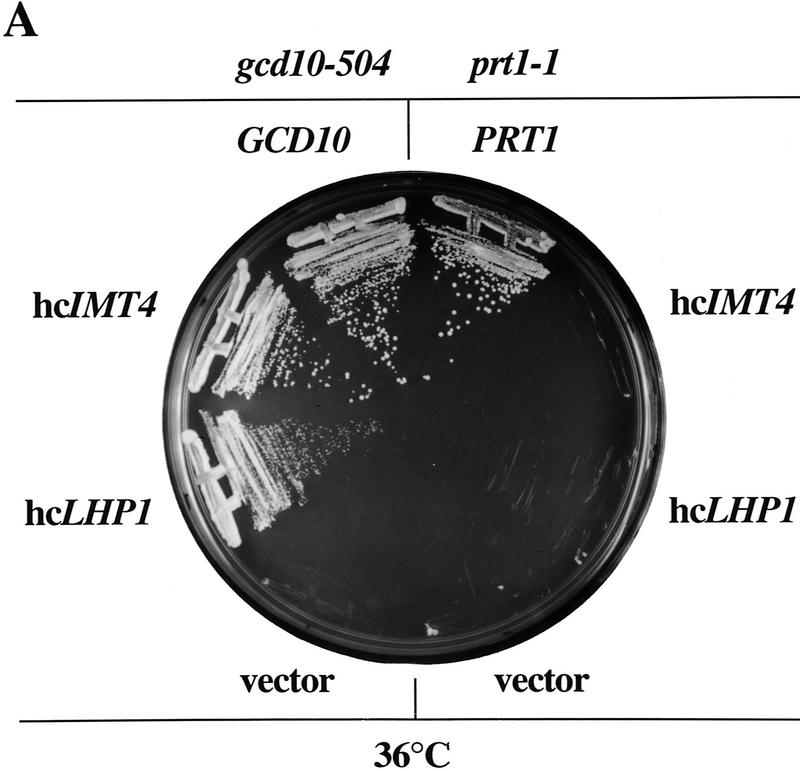
To identify functional interactions between Gcd10p and components of the translation initiation apparatus, we identified wild-type genes that in high-copy-number enable gcd10 mutants to grow at the nonpermissive temperature. Strains H2457(gcd10-504 gcn2-101) and Hm298 (gcd10-505 gcn2-101) were transformed with a high-copy yeast genomic library, and transformants were selected for growth at 36°C. Analysis of plasmids recovered from the ts+ transformants (described in Materials and Methods) led to the identification of five dosage suppressors, of which four are the genes encoding initiator tRNAMet, IMT1, IMT2, IMT3, and IMT4 (Cigan and Donahue 1986; Byström and Fink 1989). The remaining suppressor gene was identified as LHP1, encoding a homolog of the human La protein (Yoo and Wolin 1994) previously implicated in processing of tRNAs in yeast (Yoo and Wolin 1997) (Fig. 1A). All five genes were found to suppress the effects of gcd10 mutations on GCN4 translation in the following way. Because increased Gcn4p synthesis is required to derepress the histidine biosynthetic enzyme inhibited by 3-AT (His3p), gcn2 mutants are sensitive to 3-AT. gcd10 mutations lead to derepression of GCN4 translation in the absence of eIF2 phosphorylation by Gcn2p (Gcd− phenotype), conferring resistance to 3-AT (ATr) in a gcn2 strain background (Harashima and Hinnebusch 1986). All of the dosage suppressors overcame the 3-ATr phenotype of gcn2-101 gcd10-504 strain H2457 (data not shown), and thus appear to restore repression of GCN4 translation.
Figure 1.
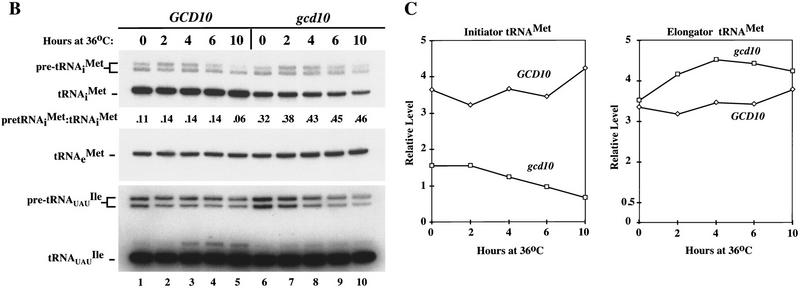
High-copy suppressors of gcd10-504 overcome a defect in accumulation of tRNAiMet (A) Transformants of strains H2457 (gcd10-504) and H1676 (prt1-1) containing low-copy plasmids bearing GCD10 (pMG107) (Garcia-Barrio et al. 1995) or PRT1 (p2625), respectively, or high-copy plasmids bearing IMT4 (pC44) (Cigan et al. 1988), LHP1 (p2626), or empty vector YEp24 were streaked for single colonies on minimally supplemented SD plates and incubated at 36°C for 2 days. (B) Northern blot analysis of total RNA (5 μg) isolated as described (Kohrer and Domdey 1991) from strain H2457 (gcd10-504) bearing GCD10 on low-copy plasmid pMG107 (GCD10 lanes) or empty vector YEp24 (gcd10 lanes) grown in supplemented SD at 26°C to mid-exponential phase (0 hr at 36°C) and shifted to 36°C for 2, 4, 6, or 10 hr. The RNAs were separated on an 8% polyacrylamide-_bis_-acrylamide (19:1), 8.3 m urea gel by electrophoresis and transferred to Hybond-N+ membranes (Amersham). The blot was probed using a radiolabeled oligonucleotide that hybridized specifically to tRNAiMet, stripped and reprobed with radiolabeled oligonucleotides specific for tRNAeMet or tRNAUAUIle. Direct quantitation of all hybridized probes was conducted by PhosphorImager analysis using a Storm 860 apparatus (Molecular Dynamics) and ImageQuant software. The positions of pre-tRNAiMet species, mature tRNAiMet, tRNAeMet, pre-tRNAUAUIle, and mature tRNAUAUIle are indicated at left. The relative intensities of the hybridization signals were quantified for mature tRNAiMet and pre-tRNAiMet , and the ratios of pre-tRNAiMet to mature tRNAiMet are listed under the appropriate lanes. The species that migrated just above mature tRNAUAUIle and accumulated at high temperature most likely represent spliced precursors still bearing the 3′ extension (O’Connor and Peebles 1991). (C) The relative intensities of the hybridization signals in B were quantified by PhosphorImager analysis of the autoradiograph and plotted against the time of incubation at 36°C.
The presence of IMT1, IMT2, and IMT4 in high copy suppressed the ts− phenotypes of the gcd10-504, gcd10-505, and gcd10-503 alleles as completely as did low-copy GCD10; however, high-copy-number IMT3 (hc_IMT3_) did not suppress fully the ts− phenotype of gcd10-505. High copy LHP1 only suppressed partially all three gcd10 alleles (data not shown). Only one additional copy of IMT4 or LHP1 on single-copy plasmids p2627 and 2628, respectively, did not suppress the phenotypes of gcd10-504 in H2457 (data not shown), suggesting that overexpression of tRNAiMet or Lhp1p is required for suppression.
We asked whether overexpression of tRNAiMet or Lhp1p would suppress general defects in translation initiation conferred by mutations in known initiation factors. PRT1 encodes the 90-kD subunit of eIF3 (Naranda et al. 1994) and prt1-1 mutants are impaired for translation initiation at the restrictive temperature both in vivo and in vitro (Feinberg et al. 1982). Unlike the gcd10 mutants, the ts− phenotype of a prt1-1 mutant was not suppressed by hc_IMT4_ or hc_LHP1_ (Fig. 1A). Furthermore, neither hc_IMT4_ nor hc_LHP1_ suppressed the growth defects of sui2-1 or gcd1-501 alleles, encoding defective subunits of eIF2α and eIF2Bγ, respectively (data not shown). These findings suggest that Gcd10p promotes translation initiation in a manner specifically involving initiator tRNAMet metabolism or function.
The abundance of mature initiator tRNAMet is reduced specifically in a gcd10-504 mutant
To examine whether initiator tRNAMet expression is reduced in gcd10 mutants, we used Northern blot analysis to measure the steady-state levels of tRNAiMet using sequences complementary to the tRNAiMet coding region as a probe. In addition to mature tRNAiMet, this probe hybridized to two larger species that appear to be precursors of tRNAiMet bearing different 5′ and 3′ terminal extensions encoded by the various IMT genes (see below). The amount of mature tRNAiMet in the gcd10 mutant was about twofold lower than in the wild type at 26°C, and only about one-sixth of the wild-type level after 10 hr at 36°C (Fig.1B,C; tRNAiMet). In contrast, levels of the putative tRNAiMet precursors were virtually indistinguishable between the mutant and wild-type strains (Fig. 1B; pre-tRNAiMet), leading to ratios of precursors to mature tRNAiMet three- to eightfold higher in the mutant over the course of the experiment (Fig. 1B). These results suggest that transcription of the IMT genes is not impaired in the gcd10 mutant but that a reduction in mature tRNAiMet abundance occurs at a post-transcriptional step.
Interestingly, the levels of mature tRNAHis and tRNACGASer were indistinguishable between the wild-type and gcd10 strains at the permissive and nonpermissive temperatures (data not shown), whereas we consistently observed a modest increase in mature elongator tRNAMet levels in the gcd10 mutant at 36°C (Fig. 1B,C; tRNAeMet). The level of pre-tRNAUAUIle decreased at 36°C slightly more in the gcd10 mutant than it did in the wild type (Fig. 1B; pre-tRNAUAUIle); however, the mature tRNAUAUIle levels were identical in the two strains at all temperatures. These findings suggest that the gcd10 mutation primarily reduces the accumulation of mature tRNAiMet.
To investigate whether the lowered expression of mature tRNAiMet in gcd10 cells could be a general response to reduced growth rates, we conducted Northern blot analysis on the temperature sensitive sui2-1 mutant, and compared the results to those shown in Figure 1B for the gcd10-504 strain (data not shown). The pre-tRNAiMet level was ∼34% lower in the sui2 mutant versus the SUI2 strain; however, the mature tRNAiMet level decreased by only 15% in the sui2 cells, leading to a small reduction in the precursor-to-mature ratio [0.08 (sui2) versus 0.16 (SUI2)]. Thus there was a much greater reduction in mature tRNAiMet levels in the gcd10 mutant (a factor of 6, Fig. 1B) versus the sui2 mutant (15%), although pre-tRNAiMet expression declined in the sui2 cells but not in the gcd10 cells. These data are consistent with the idea that gcd10 mutants are defective in maturation of pre-tRNAiMet at 36°C.
The dosage suppressors increase mature tRNAiMet levels in gcd10 mutants
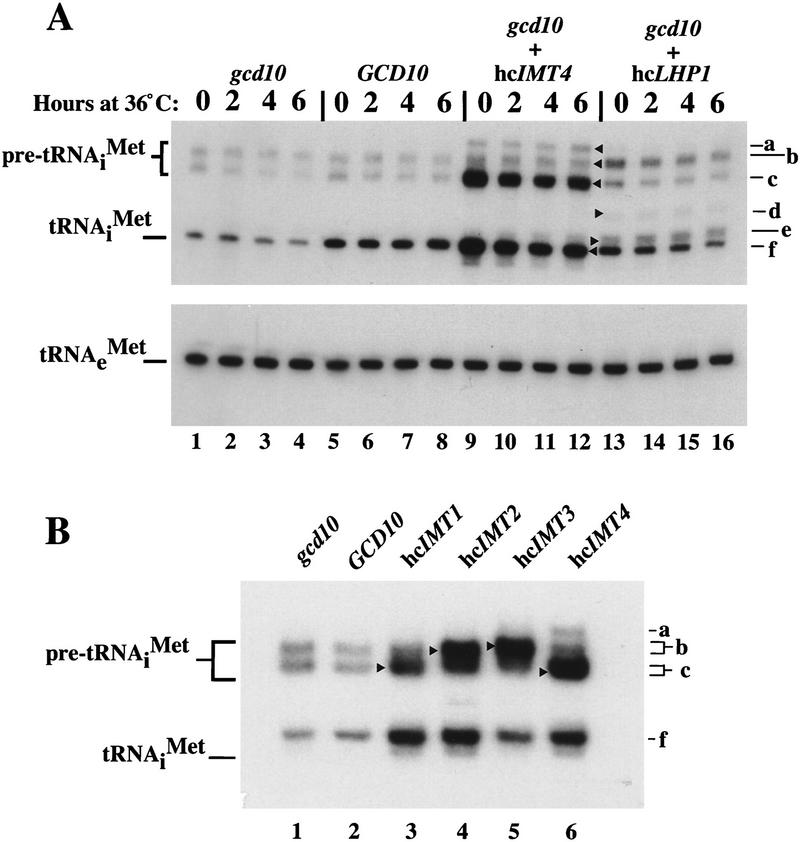
It seemed likely that suppression of gcd10 mutations by hc_IMT_ genes occurred by increasing the abundance of mature tRNAiMet. In accordance with this expectation, we found that hc_IMT4_ in the gcd10 mutant increased mature tRNAiMet to levels exceeding that observed in the isogenic gcd10 transformant containing a low-copy plasmid bearing GCD10 (Fig. 2A, cf. lanes 9–12 and lanes 5–8). The hc_IMT4_ transformant also showed increased amounts of the smaller of two putative tRNAiMet precursors detected in the wild-type strain plus an even slower migrating species not detected in wild type (Fig. 2A, cf. lanes 9–12 and lanes 5–8, species c and a, respectively).
Figure 2.
High-copy suppressors of gcd10 mutations lead to increased amounts of initiator tRNAMet and identification of pre-tRNAiMet species. (A) Northern blot analysis of total RNA (5 μg) isolated from transformants of strains H2457 (gcd10-504) carrying empty vector YEp24 (gcd10 lanes), low-copy plasmid pMG107 bearing GCD10 (GCD10 lanes), high-copy plasmid pC44 bearing IMT4 (gcd10 + hcIMT4 lanes), or high-copy plasmid p2626 bearing LHP1 (gcd10 + hc_LHP1_ lanes), grown at 26°C or 36°C for the indicated times, as described in Fig. 1. The blot was probed for tRNAiMet and stripped and reprobed for tRNAeMet as described in Fig. 1. Indicated with arrowheads inside the blot and labeled to the right are the presumed positions of pre-tRNAiMet containing 5′ and 3′ extensions encoded by IMT4 and terminating downstream of the usual termination site (a), pre-tRNAiMet containing 5′ and 3′ extensions encoded by IMT2 and IMT3 (b), pre-tRNAiMet containing 5′ and 3′ extensions encoded by IMT1 and IMT4 (c), processing intermediate of IMT3 containing the 3′ extension (d), processing intermediate containing partial 3′ extension (e), and mature tRNAiMet (f). (B) Northern blot analysis of total RNA (10 μg) isolated from H2457 (gcd10-504) transformants containing empty vector YEp24 (gcd10), low-copy plasmid pMG107 bearing GCD10, or a high-copy plasmid bearing one of the four IMT genes, as indicated above the blot, grown at 26°C as described in Fig. 1. Initiator tRNAMet was detected by hybridization as described above and in Fig. 1. Indicated with arrowheads inside the blot and labeled to the right are the positions of pre-tRNAiMet containing 5′ and 3′ extensions (a–c) and mature tRNAiMet (f), as in A.
Northern analysis of strains bearing hc_IMT1_, hc_IMT2_, hc_IMT3_, or hc_IMT4_ suggests that the larger putative precursors seen in wild-type strains derive primarily from IMT2 and IMT3, whereas the smaller species are produced from IMT1 and IMT4 (Fig. 2B, species b and c, respectively). In addition, we confirmed that the putative precursor overexpressed in the hc_IMT3_ transformant hybridized with probes containing only the 5′ and 3′ terminal extensions encoded at IMT3, and that the putative precursor c hybridized with sequences derived from upstream of the IMT4_-coding sequence (data not shown). The observed differences in pre-tRNAiMet sizes between the IMT genes can be explained by different locations of the transcription terminators either closer to (IMT1, IMT4) or farther away (IMT2, IMT3) from the 3′ end of the mature tRNA. The hc_IMT3 transformants contained relatively less mature tRNAiMet compared to the other hc_IMT_ transformants (Fig. 2B), which can explain why hc_IMT3_ suppressed the phenotypes of gcd10-505 less completely than did the other three hc_IMT_ genes (data not shown).
Hc_LHP1_ also increased the level of precursor and mature tRNAiMet in the gcd10 mutant (Fig. 2A, lanes 1–4 vs. lanes 13–16); however, mature tRNAiMet did not reach the wild-type level at 36°C (Fig. 2A, lane 16 vs. lane 8), explaining why hc_LHP1_ only suppressed partially the ts− phenotype of gcd10-504 (see Fig. 1A). Hc_LHP1_ increased differentially the two precursor species detectable in wild type (species b and c) and also led to the appearance of two novel species migrating slower than mature tRNAiMet (species d and e). The presence of species d and e suggests that Lhp1p overexpression interferes with exonucleolytic trimming of pre-tRNAiMet. There is evidence that Lhp1p blocks exonucleolytic trimming and promotes endonucleolytic cleavage of the 3′ trailer for certain other yeast tRNA families (Yoo and Wolin 1997). If this occurs for pre-tRNAiMet when Lhp1p is overexpressed, it could be responsible for the higher levels of full-length pre-tRNAiMet and mature tRNAiMet seen under these conditions, provided that endonucleolytic cleavage is more precise than exonucleolytic trimming in 3′ end maturation.
GCD10 is nonessential in yeast strains overexpressing initiator tRNAMet
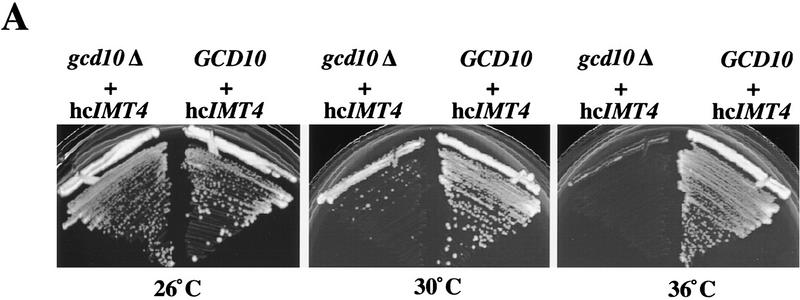
To test the possibility that overexpression of tRNAiMet would allow yeast cells to survive in the absence of Gcd10p, the gcd10Δ strain YJA143 containing wild-type GCD10 on a URA3 plasmid (p2702) was transformed with a high-copy LEU2 plasmid bearing IMT4 (p1775) (Dever et al. 1995). The URA3 GCD10 plasmid p2702 was readily eliminated from the resulting transformant by plasmid shuffling on medium containing 5-fluoro-orotic acid (5-FOA) (Boeke et al. 1987), whereas p2702 could not be lost from isogenic transformants containing an empty LEU2 vector. These results indicate that GCD10 is dispensable in cells overexpressing tRNAiMet. We verified by immunoblot analysis that one such strain (YJA146) containing hc_IMT4_ and lacking GCD10 had no detectable Gcd10p (data not shown).
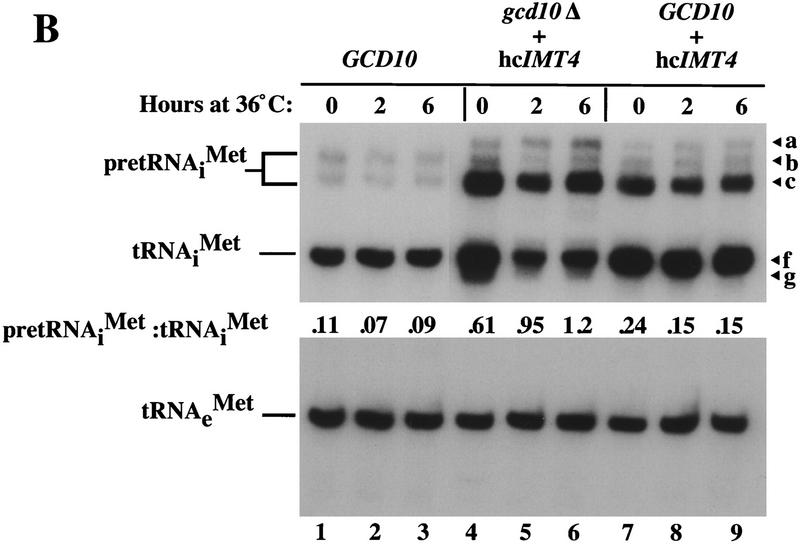
Although the gcd10Δ hc_IMT4_ strain is viable, it grows poorly at temperatures >26°C (Fig. 3A). We observed little difference in mature tRNAiMet levels between the isogenic gcd10Δ hc_IMT4_ and GCD10 hc_IMT4_ strains at 26°C (Fig. 3B, lanes 4,7). Thus, at low temperature, the requirement for GCD10 in mature tRNAiMet expression appears to be largely bypassed by overproduction of tRNAiMet. In contrast, mature tRNAiMet was reduced substantially after 2 or 6 hr at 36°C in the gcd10Δ strain (Fig. 3B, lanes 5,6 vs. 8,9), showing that GCD10 is required for accumulation of mature tRNAiMet at 36°C, even when tRNAiMet is being overexpressed. The gcd10Δ hc_IMT4_ transformant accumulated higher levels of pre-tRNAiMet than did the GCD10 hc_IMT4_ transformant (Fig. 3B, cf. lanes 4–6 and lanes 7–9), supporting the conclusion that Gcd10p is not required for efficient transcription of IMT4. In addition, the pre-tRNAiMet-to-mature tRNAiMet ratio was much greater in the gcd10Δ hc_IMT4_ transformant compared to the GCD10 hc_IMT4_ strain, particularly at 36°C (Fig. 3B). It is also noteworthy that the gcd10Δ hc_IMT4_ transformant accumulated species with mobilities greater than that of mature tRNAiMet, which were not observed in the GCD10 hc_IMT4_ transformant (Fig. 3B, cf. lanes 4–6 and lanes 7–9, species g). These observations suggest that Gcd10p is required for efficient processing of pre-tRNAiMet, particularly at elevated growth temperatures, and that in its absence, much of the unprocessed pre-tRNAiMet is degraded. The gcd10Δ hc_IMT4_ transformant showed no detectable reduction in the accumulation of other mature tRNAs when compared to the GCD10 + hc_IMT4_ transformant, including tRNAeMet (Fig. 3B; tRNAeMet), tRNAUAUIle, and tRNACGASer (data not shown).
Figure 3.
GCD10 is dispensable for cell viability in the presence of hc_IMT4_. (A) Growth of strain YJA146 (gcd10Δ + hc_IMT4_) and a transformant of its parental GCD10 strain BJ5464 bearing p1775 (GCD10 + hc_IMT4_) on YPD medium at 26°C for 3 days, and at 30°C or 36°C for 2 days. (B) Northern blot analysis of total RNA (7 μg) isolated from the same two strains described in A (gcd10Δ + hc_IMT4_ and GCD10 + hc_IMT4_ lanes) plus isogenic strain YJA143 containing the gcd10Δ chromosomal allele and single-copy GCD10 plasmid p2704 (GCD10). Strains were grown at 26°C or 36°C for 2 and 6 hr as described in Fig. 1. The membrane was probed for tRNAiMet and stripped and reprobed for tRNAeMet as described in Fig. 1. The different RNA species detected are indicated at left. The various forms of tRNAiMet species are labeled at right as in Fig. 2, with the addition of species g, which may be end-trimmed molecules lacking the CCA extension (see text). (C) Northern blot analysis of total RNA (5 μg) isolated from strain Hm296 (gcd14-2) containing wild-type GCD14 on single-copy plasmid pRC62 (GCD14 lanes) or empty vector YEp24 (gcd14 lanes), grown as described in Fig. 1. The membrane was probed for tRNAiMet and stripped and reprobed for tRNAeMet as described in Fig. 1. Indicated with arrowheads inside the blot and labeled to the right are the various tRNAiMet species described in Fig. 2 and above. The indicated ratios of pre-tRNAiMet to mature tRNAiMet were calculated from the relative intensities of hybridization signals quantitated by PhosphorImager analysis.
Despite the reduction in mature tRNAiMet expression at 36°C in the gcd10Δ hc_IMT4_ transformant, it still maintained levels comparable to that seen in isogenic wild-type cells grown under the same conditions (Fig. 3B, cf. lane 3 and lane 6, species f). The presence of species g in the gcd10Δ hc_IMT4_ transformant at 36°C suggests that mature tRNAiMet is either unstable, or is processed or modified incompletely, and may not be fully functional in translation. This could explain the inviability of this strain at high temperatures without the need to propose a second function for Gcd10p.
gcd14 mutants are defective for processing of initiator tRNAMet in vivo
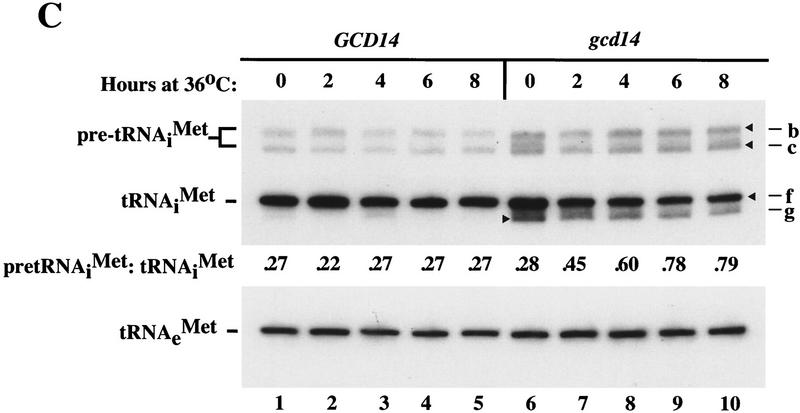
gcd14 mutants exhibit constitutively derepressed GCN4 translation (Gcd−) and are temperature sensitive for general translation, the same phenotypes described for gcd10 mutations (Cuesta et al. 1998). In addition, any of the four IMT genes or LHP1 on a high-copy plasmid suppressed the Gcd− and ts− phenotypes of gcd14 mutants (R. Cuesta, O. Calvo, J. Anderson, M. Garcia-Barrio, A. Hinnebusch, and M. Tamame, in prep.). In accordance with these findings, we found that mature tRNAiMet decreased by a factor of 1.7, whereas pre-tRNAiMet increased ∼1.5-fold in a gcd14-2 mutant after 8 hr at 36°C, increasing the ratio of precursor to mature tRNAiMet from 0.28 to 0.79 (Fig. 3C). These results suggest strongly that Gcd14p is required for processing of nascent tRNAiMet transcripts. Interestingly, a tRNAiMet species migrating faster than the wild-type mature form, exhibiting a similar mobility to the aberrant tRNAiMet seen in the gcd10Δ hc_IMT4_ transformants, is present in the gcd14-2 mutant under all conditions (Figs. 3B,C, species g). Thus, 5′- and 3′-trimmed tRNAiMet may be unstable, or incorrectly processed or modified, in gcd14-2 cells. As in the case of gcd10-504, the gcd14-2 mutation had no effect on the level or apparent length of elongator tRNAMet (Fig. 3C).
Gcd10p is required for the stability of total tRNAiMet in vivo
We used pulse–chase analysis to investigate whether the reduced amount of mature tRNAiMet in the gcd10-504 mutant results from rapid degradation of tRNAiMet transcripts. After incubating isogenic GCD10 and gcd10-504 strains for ∼2 hr at 36°C, cells were pulse-labeled with [3H]uracil for 1 hr, and chased for 5 hr with excess unlabeled uracil. Total RNA was isolated at different times and aliquots containing equivalent amounts of radioactivity were hybridized to immobilized oligonucleotides complementary to initiator tRNAMet or elongator tRNAMet. The proportion of 3H-labeled tRNAeMet in both mutant and wild-type cells, and of tRNAiMet in the wild-type strain, showed a small increase over the 5-hr chase period (Fig. 4A). Presumably, these increases occurred because the pool of uracil nucleotides chased slowly, allowing the proportion of label in stable RNA to increase relative to unstable mRNA. In contrast, the proportion of 3H-labeled tRNAiMet in the gcd10 mutant dropped by a factor of about two during the first hour of the chase and showed little additional change throughout the remaining 4-hr chase period (Fig. 4A). These results indicate that a large fraction of tRNAiMet transcripts made during the 1-hr pulse in the gcd10 mutant was very unstable, whereas the remainder was highly stable. As expected, the gcd10 mutation had no effect on the turnover of tRNAeMet.
Figure 4.
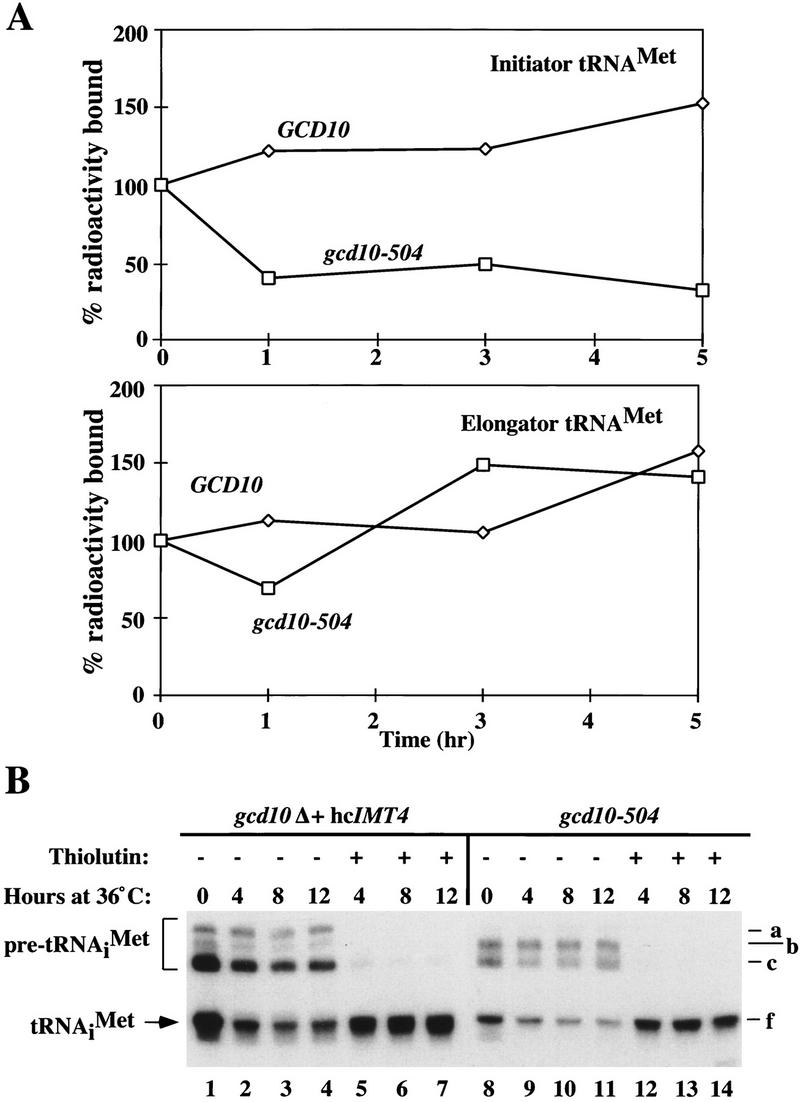
Evidence that newly synthesized initiator tRNAMet is unstable in gcd10 mutants. (A) Transformants of strain H2457 (gcd10-504) bearing the GCD10 plasmid pMG107 (GCD10) or vector YEp24 (gcd10-504) were grown in supplemented SD medium at 36°C for 2.25 hr before the addition of 5.0 mCi [5,6-3H]-uracil (37 Ci/mmole, 1 mCi/ml NEN). Cells were continuously labeled at 36°C for 60 min (pulse) after which 200-fold excess unlabeled uracil was added and incubation at 36°C was continued for 5 hr (chase). Total RNA was isolated from 2.0-ml aliquots at 0, 1, 3, and 5 hr after addition of unlabeled uracil and an amount of RNA representing equal cpms was hybridized to membrane-bound oligonucleotides complementary to full-length tRNAiMet (top) and tRNAeMet (bottom) in hybridization solution [500 mm NaCl, 24 mm NaH2PO4, 2.4 mm EDTA (pH 7.4), 30 % formamide, 5× Denhardt’s solution, 0.1% SDS] at 40°C for 2.5 days with constant mixing. After hybridization, filters were washed once in hybridization solution at 40°C for 30 min, once in 2× SSC, 0.1% SDS for 30 min at room temperature, once in 2× SSC for 30 min, and twice with 95% ethanol. Filters were dried and counted by liquid scintillation in Econo-fluor (Packard Chemical). The cpm bound to the membranes at each time point were corrected by subtracting the cpm bound to a third membrane containing a nonspecific oligonucleotide. The corrected counts per minute are expressed as the percentage of cpm bound to the membrane at the beginning of the chase (time = 0). (B) Strains YJA146 (gcd10Δ + hc_IMT4_) and a transformant of H2457 containing vector YEp24 (gcd10-504 + YEp24) were grown to mid-exponential phase at 26°C in SC or minimally supplemented SD medium and resuspended in the same medium prewarmed to 36°C containing 5 μg/ml thiolutin in DMSO or DMSO only. Northern blots of total RNA (10 μg) isolated from the strains at 26°C (0 hr at 36°C) or 36°C (4, 8, 12 hr at 36°C) with (+) or without (−) thiolutin treatment were probed with a labeled oligonucleotide that specifically hybridized to both pre-tRNAiMet and mature tRNAiMet, as described in Fig. 1. Labeled at right are various tRNAiMet species described in Figs. 2 and 3.
It seemed likely that the unstable pool of tRNAiMet transcripts detected by pulse–chase analysis in the gcd10-504 mutant represented nascent tRNAiMet molecules that were degraded rapidly in the nucleus. To eliminate the alternative possibility that mature tRNAiMet is unstable, we treated gcd10Δ and gcd10-504 cells with an inhibitor of Pol III transcription, thiolutin (Jimenez et al. 1973), coincident with the shift to 36°C to prevent cell division and new synthesis of pre-tRNAiMet transcripts at the nonpermissive temperature. As shown in Figure 4B, the untreated gcd10Δ and gcd10-504 cells showed the usual reduction in mature tRNAiMet after the temperature shift (lanes 1–4 and 8–11). In contrast, thiolutin treatment largely eliminated the reduction in mature tRNAiMet levels at 36°C (Fig. 4B, lanes 5–7 and 12–14). The disappearance of pre-tRNAiMet species after thiolutin treatment is the expected result of inhibiting IMT transcription without preventing processing (or degradation) of the preexisting pre-tRNAiMet transcripts. The fact that little or no change in mature tRNAiMet abundance occurred at 36°C in the presence of thiolutin suggests that the mature tRNAiMet present at the temperature shift is stable in gcd10-504 cells. The gcd10-504 mutant continues to grow at 36°C, albeit more slowly than the wild type, doubling in mass ∼2.5 times after the temperature shift. Thus, the reduction in mature tRNAiMet seen in the absence of thiolutin most likely occurs by dilution of stable preexisting mature tRNAiMet, coupled with a failure to produce new mature tRNAiMet during cell divisions at the nonpermissive temperature. We suggest that the unstable tRNAiMet molecules detected by pulse–chase analysis in the gcd10-504 mutant (Fig. 4A) are primarily nascent transcripts that are rapidly degraded in the nucleus.
Evidence that Gcd10p and Gcd14p are components of a heteromeric nuclear complex
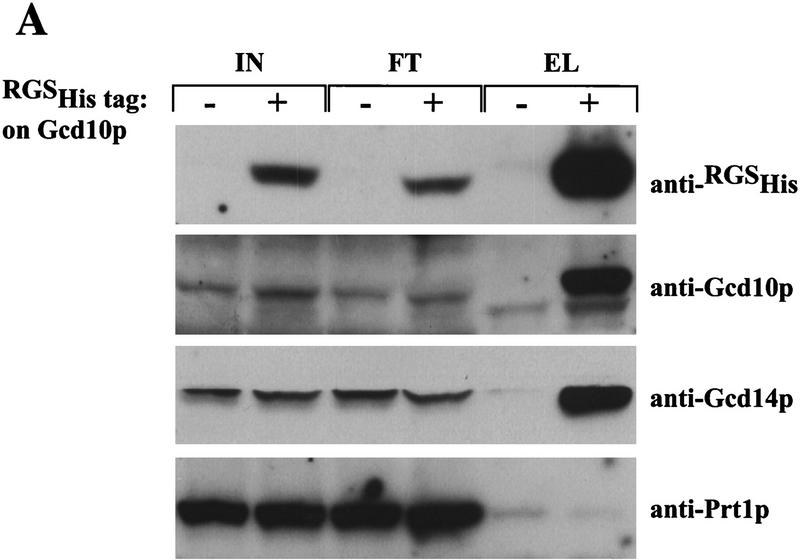
To investigate whether Gcd10p and Gcd14p are physically associated in vivo, we constructed a yeast strain expressing a polyhistidine-tagged form of Gcd10p (His–Gcd10p) to allow affinity purification of the protein. The GCD10–His allele encoding His–Gcd10p was indistinguishable from wild-type GCD10 in complementing gcd10 mutations in vivo (see Materials and Methods). As shown in Figure 5A, substantial fractions of both Gcd14p and Gcd10p in whole cell extracts were eluted from Ni+2–NTA agarose with the GCD10–His extract but not with the isogenic GCD10 extract. In contrast, the _PRT1_-encoded subunit of eIF3 (Naranda et al. 1994) in both extracts did not bind to the resin. These findings indicate that Gcd10p and Gcd14p are components of a heteromeric complex that is not stably associated with eIF3.
Figure 5.
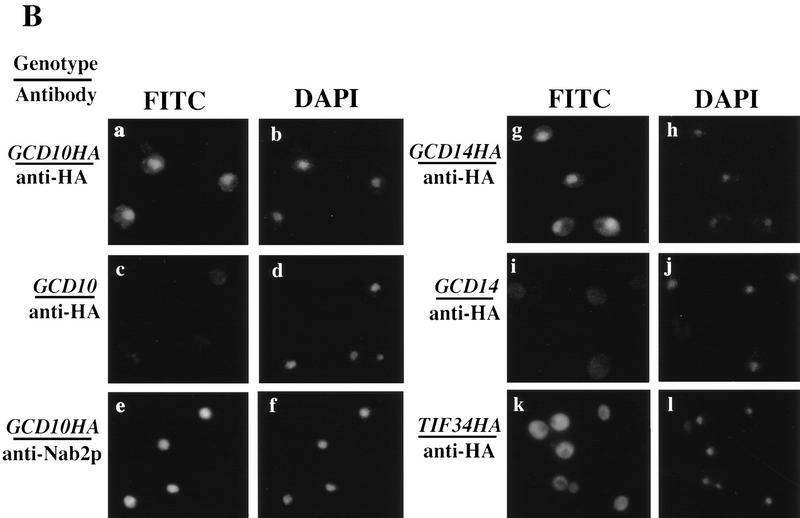
Gcd10p and Gcd14p form a stable nuclear complex in vivo. (A) Whole cell extracts were prepared from isogenic strains LPY251 (GCD10) and LPY252 (GCD10–His) containing wild-type and His-tagged Gcd10p, respectively, as described (Phan et al. 1998). Each clarified extract was batch-bound to 50 μl of Ni2+–agarose (Qiagen) in H2O (50% vol/vol) for 1 hr at 4°C. Proteins bound to Ni2+–agarose were collected by centrifugation at 3000 rpm for 2 min, washed four times with 300 μl of breaking buffer, and batch-eluted with 50 μl of breaking buffer containing 250 mm imidazole. Aliquots containing 10% of the input cell extracts (IN), 10% of the flowthrough wash (FT), and 100% of the eluate (EL) were resolved by SDS-PAGE and subjected to immunoblot analysis using monoclonal anti-RGSHis antibodies (1:500; Qiagen) directed against the tag on His–Gcd10p, and with polyclonal antibodies directed against Gcd10p (1:500), Gcd14p (1:500), or Prt1p(1:1000). (B) Indirect immunofluorescence was used to study the subcellular distribution of HA epitope-tagged forms of Gcd10p, Gcd14p, and Tif34p in strains YJA142 (GCD10–HA; a,b), YJA143 (GCD10; c,d), Hm296 bearing pRC64 (GCD14–HA; g,h), Hm296 bearing pRC62 (GCD14; i,j), and KAY8 (TIF34–HA; k,l), as described previously (Anderson et al. 1993). All antibodies were diluted in PBS, 5% non-fat dried milk. The affinity-purified 12CA5 monoclonal antibody against the HA epitope (at 20 μg/ml; Boehringer Mannheim) was used to probe strains expressing HA-tagged proteins and the isogenic control strains lacking tagged proteins (a,c,g,i,k). Monoclonal antibody 1E4 (at 1:750 dilution; Wilson et al. 1994) was used to detect Nab1p in strain YJA142 (e). Detection of the primary antibodies was accomplished using a fluorescein isothiocyanate (FITC)-conjugated secondary antibody (a,c,e,g,i,k) and the DNA distribution was visualized by DAPI (b,d,f,h,j,l).
As most steps in tRNA processing are believed to occur in the nucleus (Hopper and Martin 1992), we used indirect immunofluorescence to determine whether Gcd10p and Gcd14p are nuclear proteins. Toward this end, we constructed alleles of GCD10, GCD14, and TIF34 (encoding the 39-kD subunit of yeast eIF3; Naranda et al. 1997) tagged with the coding sequences for three tandem copies of the HA epitope. The tagged alleles were introduced on low-copy plasmids into yeast strains either lacking the cognate chromosomal allele, in the case of GCD10–HA (YJA142) and TIF34–HA (KAY8), or containing a temperature-sensitive allele in the case of GCD14–HA (see Materials and Methods). All three tagged alleles complemented the lethal effects of mutations in the corresponding genes as effectively as did the wild-type alleles (data not shown). In addition, the tagged proteins were expressed at levels similar to that of the wild-type proteins (data not shown). As expected for an integral subunit of eIF3, HA–Tif34p was found exclusively in the cytoplasm (Fig. 5B, k), whereas Nab1p showed diffuse nuclear staining characteristic of a nucleoplasmic protein (panel e) (Wilson et al. 1994). Both HA–Gcd10p and HA–Gcd14p showed prominent nuclear localization with staining indicative of nucleoplasmic factors (Fig. 5B, a,g). Because of the background staining with anti-HA antibodies of the control GCD10 and GCD14 strains (Fig. 5B, c,i), it was not possible to determine whether Gcd10p and Gcd14p are located in the cytoplasm in addition to the nucleus.
Gcd10p is required for the 1-methyladenosine modification of initiator tRNAMet at position 58
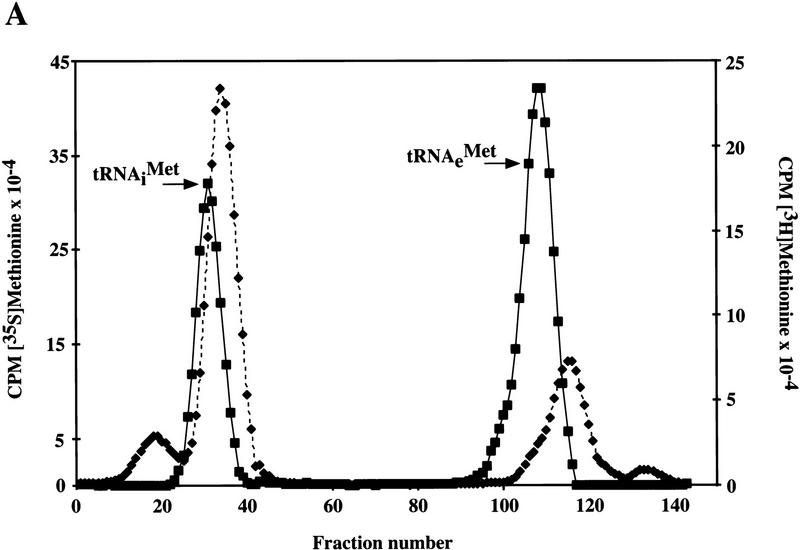
Gcd14p exhibits significant homology with _S_-adenosylmethionine-dependent methyltransferases (R. Cuesta, O. Calvo, J. Anderson, M. Garcia-Barrio, A. Hinnebusch, and M. Tamame, in prep.), suggesting the possibility that Gcd10p and Gcd14p are required for tRNAiMet methylation. To test this idea, total tRNA isolated from gcd10Δ and wild-type strains was aminoacylated with [35S]methionine or [3H]methionine, respectively, and the labeled tRNAs were chromatographed on a RPC-5 column (Kelmers and Heatherly 1971). It was shown previously that this technique can resolve tRNAs that differ by only a single methyl group (Diamond et al. 1993). As shown in Figure 6A, wild-type tRNA contains two peaks of methionine-accepting tRNAs corresponding to initiator (fractions 20–40) and elongator tRNAMet (fractions 95–120) (D. Hatfield, unpubl.). In the gcd10Δ tRNA, the majority of both initiator and elongator methionyltRNAMet were resolved into single peaks that eluted in later fractions than did the same isoacceptors from the wild type. The differences in methionyl-tRNAMet elution profiles might arise from differences in tRNA length, although this seems unlikely for the 3′ end because of the obligatory requirement of CCA termini for aminoacylation. In addition, we observed no differences in length of initiator or elongator tRNAMet between GCD10 and gcd10Δ tRNAs after separation on high resolution polyacrylamide gels and Northern blot analysis (data not shown). Together, these results provide strong evidence that Gcd10p and Gcd14p are required for modifying one or more bases in both forms of tRNAMet. Small amounts of the initiator and elongator methionyl-tRNAs from gcd10Δ cells eluted earlier (initiator) or later (elongator) than the major peaks (Fig. 6A). These minor peaks may represent methionine-accepting tRNAs lacking additional modifications, or exhibiting a collapsed tertiary structure, due to the absence of a Gcd10p-dependent modification.
Figure 6.
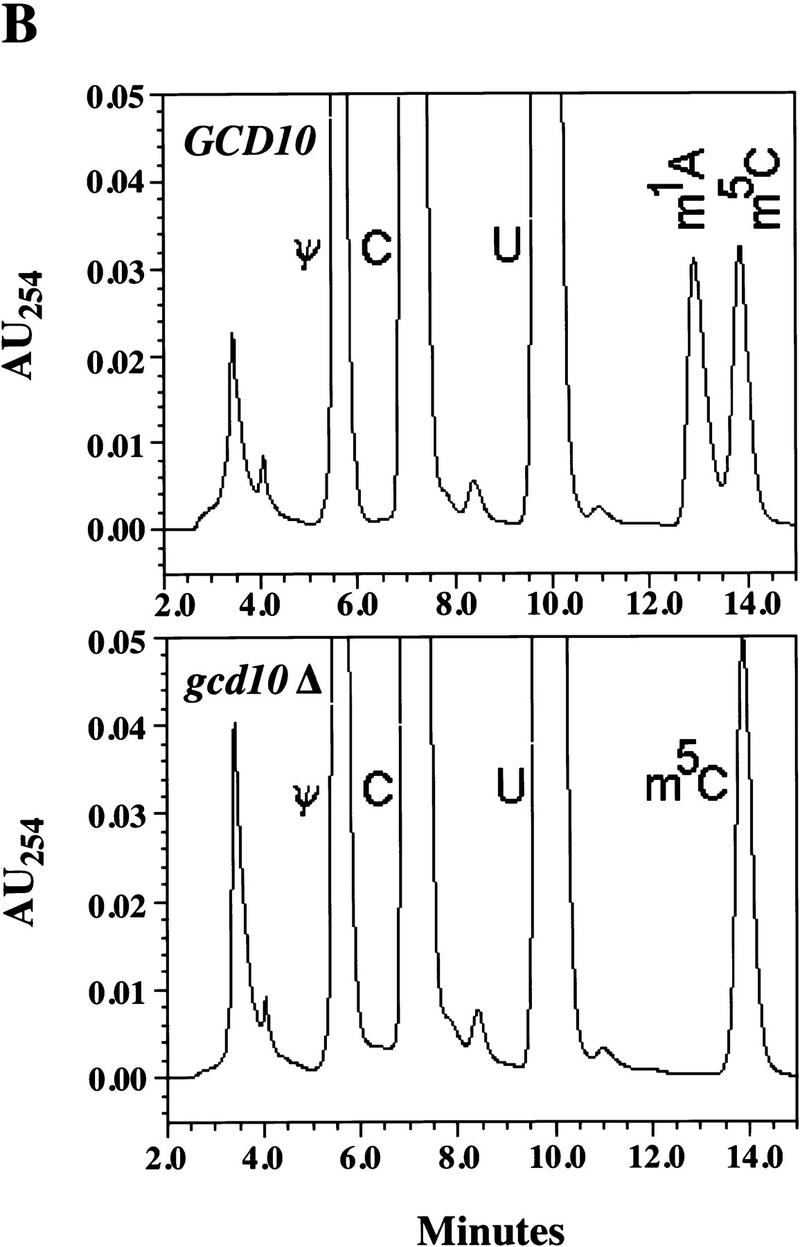
Evidence that methionine-accepting and other tRNAs from a gcd10Δ strain are hypomethylated. (A) Total tRNA isolated from YJA146 (gcd10Δ) and the p1775 transformant of Bj5464 (GCD10) was aminoacylated with [35S]methionine and [3H]methionine, respectively, and 500,000 cpm of each were chromatographed on a RPC-5 column. The radioactivity in each fraction (0.6 ml) was measured by liquid scintillation in 7 ml of Ecoscint A (National Diagnostics) and is plotted on different _y_-axes, as shown, against the fraction number. (█) Results obtained with wild-type tRNA (GCD10); (♦) results with gcd10Δ tRNA. The elution positions of the methionine-accepting tRNAs are indicated at the appropriate positions. (B) Transfer RNAs were digested to nucleosides and chromatographed by HPLC on a Supelcosil LC-18S column. Only the portion of the chromatogram (corresponding to retention times of 2–14.5 min) showing a difference between the GCD10 and gcd10Δ samples is shown here. The peak that is absent in the gcd10Δ sample was identified as m1A by several means (see text). The identities of other peaks in this portion of the chromatogram are indicated: (Θ) Pseudouridine; (C) cytidine; (U) uridine; (m1A) 1-methyladenosine; (m5C) 5-methylcytidine. (AU254) Absorbance units at 254 nm.
To examine directly whether the methionyl-tRNAs from gcd10Δ cells lack a modified base, total tRNA from the GCD10 and gcd10Δ strains was digested completely to nucleosides and separated by high-performance liquid chromatography (HPLC) (Gehrke and Kuo 1990). The resulting chromatograms were identical except for the absence of a single peak in the gcd10Δ sample with a retention time of 13.5 min, identified previously as 1-methyladenosine (Gehrke and Kuo 1990) (Fig. 6B). This assignment was confirmed in two ways. First, addition of synthetic m1A to wild-type or gcd10Δ tRNA hydrolyzates followed by HPLC analysis restored the missing peak to the gcd10Δ sample and led to a quantitative increase in the corresponding peak in the wild-type sample (data not shown). Second, the nucleoside present in the peak eluting at 13.5 min had the UV spectrum of m1A (Gehrke and Kuo 1990) and the same molecular mass as protonated m1A (282 Da) as determined by mass spectrometry using ionization electrospray (data not shown). These results strongly suggest that Gcd10p is required for the formation of 1-methyladenosine in tRNA. This base modification occurs at position 58 in initiator and elongator tRNAMet and in 16 other tRNAs, but is not found at any other positions in yeast tRNAs (Sprinzl et al. 1998).
Discussion
Previously, we identified Gcd10p genetically as a factor required for translational repression of GCN4 mRNA, indicating a role in the formation or utilization of ternary complexes. Using suppressor analysis, we have uncovered a function for Gcd10p in tRNAiMet maturation. After discovering that gcd10 mutations can be suppressed by extra copies of the IMT or LHP1 genes, we found that the level of mature tRNAiMet was reduced in a gcd10-504 mutant (Fig. 1). This was also true of a gcd14-2 mutant (Fig. 3C), which exhibits the same defect in GCN4 translation seen in gcd10 mutants (Cuesta et al. 1998) and can also be suppressed by hc_IMT_ or hc_LHP1_ (R. Cuesta, O. Calvo, J. Anderson, M. Garcia-Barrio, A. Hinnebusch, and M. Tamame, in prep.). The reductions in mature tRNAiMet levels in these mutants can be expected to diminish ternary complex formation, explaining their constitutive derepression of GCN4 translation. It was striking that overexpression of tRNAiMet suppressed the lethality of a gcd10Δ deletion (Fig. 3A), indicating that the essential function of GCD10 is to promote expression of mature tRNAiMet. In addition to the genetic links between Gcd10p and Gcd14p, both proteins show prominent nuclear localization (Fig. 5B) and are components of the same heteromeric protein complex (Fig. 5A; R. Cuesta, O. Calvo, J. Anderson, M. Garcia-Barrio, A. Hinnebusch, and M. Tamame, in prep.).
The gcd10-504 mutation did not lead to accumulation of pre-tRNAiMet or the appearance of novel tRNAiMet species, but only to reduced amounts of mature tRNAiMet (Fig. 1B). On the basis of the results of pulse–chase experiments indicating that a large fraction of newly synthesized tRNAiMet is unstable in gcd10-504 cells (Fig. 4), we concluded that the decrease in mature tRNAiMet arose from increased degradation rather than diminished synthesis of pre-tRNAiMet. This conclusion is consistent with our finding that in vitro transcription of IMT4 occurred at the same rates in extracts from gcd10Δ and GCD10 strains (J. Anderson and A.G. Hinnebusch, unpubl.). In the gcd14-2 mutant, accumulation of pre-tRNAiMet species containing both 5′ and 3′ extensions, plus an aberrant species shorter than mature tRNAiMet were observed in addition to a specific reduction in the amount of mature tRNAiMet (Fig. 3C). These findings strongly suggest that processing of pre-tRNAiMet is impaired by gcd14-2. Similar phenotypes were observed in the gcd10Δ hc_IMT4_ strain (Fig. 3B). Considering that Gcd10p and Gcd14p reside in the same complex, deletion of GCD10 may indirectly impair Gcd14p function. Because cleavage of the 5′ extension by RNase P generally precedes trimming of the 3′ extension (O’Connor and Peebles 1991), accumulation of pre-tRNAiMet in gcd14-2 and gcd10Δ hc_IMT4_ cells containing both 5′ and 3′ extensions (species b and c) suggests that trimming by RNase P occurs more slowly in these mutants. The predicted length of the novel tRNAiMet that migrates faster than mature tRNAiMet (species g) is consistent with tRNAs lacking the 3′ CCA. Thus, it is possible that CCA addition also occurs less efficiently in gcd14-2 and gcd10Δ hc_IMT4_ strains. We observed no significant differences in the rate of IMT3 transcription or the efficiency of pre-tRNAiMet processing between gcd14-2 and GCD14 extracts (J. Anderson and A.G. Hinnebusch, unpubl.), providing evidence that Gcd14p is not required for 5′- or 3′-end trimming of pre-tRNAiMet in vitro.
The fact that large fractions of Gcd10p and Gcd14p are found in the nucleus, where enzymes involved in modification (Rose et al. 1995; Simos et al. 1996) and processing (Clark and Abelson 1987) of tRNAs generally reside, suggests that they function directly in one or more aspects of tRNAiMet maturation. Gcd14p contains motifs (Kagan and Clarke 1994) common to _S_-adenosylmethionine-dependent methyltransferases (Cuesta et al. in prep.), raising the possibility that it methylates one of the bases in tRNAiMet. Experimental support for this hypothesis came from our finding that both initiator and elongator tRNAMet isolated from gcd10Δ cells eluted from a RPC-5 column in different positions than did the corresponding tRNAs from wild-type cells (Fig. 6A). As there are only four base methylations common to initiator and elongator methionine tRNAs, m2G10, m7G46, m5C48, and m1A58 (Sprinzl et al. 1998), these results led to the prediction that the Gcd10p–Gcd14p complex is required for one of these modifications. Analysis of all modified nucleosides in total tRNA (Fig. 6B) indicated that 1-methyladenosine, found at position 58 in 18 yeast tRNAs, was undetectable in gcd10Δ cells. Recently, we repeated the HPLC analysis of modified nucleosides in purified intitiator tRNAMet and again failed to detect m1A (J. Anderson, G.R. Björk, and A.G. Hinnebusch, unpubl.). Thus, we conclude that initiator tRNAMet produced in gcd10Δ cells lacks m1A58. Importantly, this residue plays a central role in determining a unique tertiary substructure not observed in elongator tRNAs, involving three residues unique to eukaryotic initiators, A60, A54, and A20 (Basavappa and Sigler 1991). Therefore, the absence of this modification might alter the tertiary structure of the initiator, and impair its processing and stability, without similarly affecting elongator tRNAMet and other tRNAs bearing m1A58. Several of the genes that function in modifying tRNAs have been cloned and none is essential for cell viability (Hopper and Martin 1992). If the Gcd10p–Gcd14p complex functions only in the production of m1A58, this would be the first instance of an essential tRNA modification in yeast.
In addition to showing a defect in processing, it appears that unprocessed pre-tRNAiMet molecules are degraded more rapidly in gcd10Δ mutants. These results suggest that Gcd10p is required to protect hypomethylated pre-tRNAiMet from degradation in addition to promoting the function of Gcd14p in m1A58 formation. The maturation of tRNAiMet occurs very inefficiently in gcd10Δ hc_IMT4_ cells grown at 36°C, such that mature tRNAiMet is not overproduced despite the increased copy number of IMT4 (Fig. 3B, cf. lanes 2 and 3 with lanes 5 and 6). This finding is consistent with the idea that Gcd10p stabilizes the conformation of hypomethylated pre-tRNAiMet, as the absence of Gcd10p would be expected to have more severe consequences at elevated temperatures where isomerization of pre-tRNAiMet to aberrant conformations should be favored. Furthermore, Gcd10p has strong RNA-binding activity (Garcia-Barrio et al. 1995), which might enable it to perform this proposed RNA chaperone function.
Recently, it was proposed that Lhp1p functions as an RNA chaperone in facilitating endonucleolytic trimming of the 3′ trailers of many yeast pre-tRNAs. Through binding to the poly(U) stretch at the 3′ end of pre-tRNA, Lhp1p would stabilize the conformation needed for endonucleolytic 3′-trimming and block access by 3′ → 5′ exonucleases (Yoo and Wolin 1997). Interestingly, a mutation in yeast tRNACGASer that renders its processing dependent on Lhp1p leads to degradation of processing intermediates when Lhp1p is depleted from cells (Yoo and Wolin 1997). This might resemble the situation in gcd10Δ mutants where the absence of m1A58, combined with loss of the putative Gcd10p chaperone function, would result in degradation of pre-tRNAiMet. Perhaps overexpression of Lhp1p in gcd10 mutants allows it to substitute partially for the chaperone function of Gcd10p, increasing the probability of accurate processing and protecting the hypomethylated pre-tRNAiMet from degradation.
It is conceivable that Gcd10p accompanies mature tRNAiMet from the nucleus to the cytoplasm, where it could promote formation of ternary complexes with eIF2 and GTP. This could explain why it copurified with eIF3 activity through several chromatographic separations (Garcia-Barrio et al. 1995), as stabilizing the ternary complex is one function ascribed to eIF3 (Peterson et al. 1979). Although we did not observe a stable interaction between Gcd10p and the PRT1 subunit of eIF3 under conditions where Gcd14p was tightly associated with Gcd10p (Fig. 5A), interaction between eIF3 and Gcd10p may be highly sensitive to differences in strain background, extract preparation, or purification scheme.
The gcd10-504 and gcd10Δ mutations had little or no effect on the levels of mature forms of several tRNAs, including elongator tRNAMet, tRNAHis, tRNAUAUIle, and tRNACGASer. Elongator tRNAMet contains m1A58, whereas tRNAHis does not, and the sequences of the other two tRNAs are unknown (Sprinzl et al. 1998). It will be interesting to determine whether expression of any other tRNAs containing m1A58 is impaired by gcd10 or gcd14 mutations. Given that the gcd10Δ mutant was rescued by overexpression of initiator tRNAMet, it is likely that Gcd10p and Gcd14p play an essential role in maturation and accumulation of only this tRNA. Accordingly, it is conceivable that these proteins provide a novel means of regulating translation initiation, whereby modulating the maturation and stability of pre-tRNAiMet in the nucleus would affect the formation of ternary complexes in the cytoplasm.
Materials and methods
Plasmid and yeast strain constructions
Table 1 contains the genotypes of all yeast strains used in this work. Details of plasmid constructions will be provided on request. Yeast strains YJA142, YJA143, and YJA146 were constructed by introducing plasmid p2705 (GCD10-HA) into strain BJ5464 (gift of E. Jones, Carnegie Mellon University, Pittsburgh, PA) and then deleting chromosomal GCD10 by transformation to Ura+ with a 6.6-kb _Xba_I–_Xho_I fragment containing the gcd10Δ::hisG::URA3::hisG allele (Alani et al. 1987) from pLPY1. A Ura− gcd10Δ::hisG derivative (YJA142) was selected by growth on SC medium containing 1 μg/ml 5-FOA (SC + FOA). YJA143 was constructed by replacing plasmid p2705 in YJA142 with plasmid p2704 by plasmid shuffling (Boeke et al. 1987). Strain YJA146 was constructed from YJA143 as described in the Results section. To construct strains LPY251 and LPY252, a diploid from a cross between CH1305 and K2348 (a gift from K. Nasmyth, Research Institute of Molecular Pathology, Vienna, Austria) was transformed with the 6.6-kb gcd10Δ::hisG::URA3::hisG fragment from pLPY1, and the gcd10Δ::hisG::URA3::hisG allele was converted to gcd10Δ::hisG as described above. One such Ura− strain, LPY25, was transformed to Ura+ with plasmid pLPY5, bearing GCD10 and URA3, sporulated and a Ura+ ascospore (LPY251B) was isolated by tetrad analysis. After introduction of the LEU2 plasmids pLPY2 or pLPY3 bearing GCD10 or GCD10–His, LPY251B was cured of plasmid pLPY5 on 5-FOA medium to produce LPY251 and LPY252.
Table 1.
Genotypes of yeast strains used in this study
| Strain | Genotype | Plasmid [name: relevant gene] | Source or reference |
|---|---|---|---|
| H2457 | MATα, gcd10-504, gcn2-101, his1-29, ura3-52, inol (HIS4–lacZ, ura3-52) | M. Garcia-Barrio (NIH) and M. Tamame | |
| H62 | MATα, gcd10-503, his1-29, gcn2-101, gcn3-101, ura3-52, (HIS4–lacZ, ura3-52) | Harashima and Hinnebusch (1986) | |
| Hm298 | MATa, gcd10-505, gcn2-101, his1-29, ura3-52, inol (HIS4–lacZ, ura3-52) | Garcia-Barrio et al. (1995) | |
| BJ5464 | MATα, ura3-52, trp1, leu2Δ1, his3Δ200, pep∷HIS4, prb1Δl‘1.6, can1 Gal+ | E. Jones | |
| YJA142 | MATα, gcd10Δ∷hisG, ura3-52, trp1, leu2Δ1, his3Δ200, pep∷HIS4, prb1Δl‘1.6, can1 Gal+ | [p2705:_GCD10HA_] | this work |
| YJA143 | MATα, gcd10Δ∷hisG, ura3-52, trp1, leu2Δ1, his3Δ200, pep∷HIS4, prb1Δl‘1.6, can1 Gal+ | [p2704:_GCD10_] | this work |
| YJA146 | MATα, gcd10Δ∷hisG, ura3-52, trp1, leu2Δ1, his3Δ200, pep∷HIS4, prb1Δl‘1.6, can1 Gal+ | [p1775:_IMT4_] | this work |
| Hm296 | MATα, gcd14-2, his1-29, gcn2-101, gcn3-101, ura3-52, inol (HIS4–lacZ, ura3-52) | Cuesta et al. (1998) | |
| H1676 | MATa, prt1-1, leu2-3; leu2-112, ura3-52 | M. Ramirez (NIH) and A.G. Hinnebusch | |
| TD-304-10 | MATa, leu2-3,-112, ura3-52, his4–303(ATT), sui2-1 | T. Donahue (Indiana University, Bloomington) | |
| H56 | MATα, gcd1-501, his 1-29, ura3-52, gcn2-101, gcn3-101, (HIS4–lacZ, ura3-52) | Harashima and Hinnebusch (1986) | |
| KAY8 | MATα, tif34Δ1, his1-29, gcn2-508, ura3-52, leu2-3,-112, (HIS4–lacZ, ura3-52) | [YCpL_TIF34HA_] | Asano et al. (1998) |
| CH1305 | MATa, ade3, leu2, ura3, lys2, can1, Gal+ | C. Holm (UCSD) | |
| K2348 | MATα, ade2-1, ade3, trp1-1, can1-100, leu2-3, his3-11,15, ura3, Gal+ psi+ | K. Nasmyth |
High copy suppressor analysis
Standard genetic techniques and culture media (SD, SC, and YPD) (Sherman et al. 1974) and the growth assays for sensitivity to 3-AT (Hinnebusch and Fink 1983) were described previously. Yeast strains H2457 and HM298 were transformed with a high-copy genomic library constructed in YEp24 (Carlson and Botstein 1982) and transformants were plated on minimally supplemented SD plates at 36°C. Plasmids were isolated from ts+ transformants as described previously (Hoffman and Winston 1987) and shown to confer a ts+ phenotype with reintroduction into H2457. The ends of the genomic inserts were sequenced using primers complementary to the sequences flanking the _Bam_HI site in YEp24, and compared to the complete S. cerevisiae sequence (http://genome-www.Stanford.edu/Saccharomyces/) to identify the end points of the genomic inserts. Tn10 insertion libraries were constructed for each plasmid as described previously (Huisman et al. 1987). A Tn10 insertion that destroyed the suppressor function of plasmid p2634 was found to interrupt the IMT3 gene. On the basis that the other three IMT genes were present on the genomic inserts in suppressor plasmids p2632 (IMT1), p2633 (IMT2), and p2635 (IMT4), the IMT coding regions in all four plasmids were disrupted by inserting a 604-bp _Bss_HII DNA fragment isolated from λ phage DNA into a unique Bss_HII site present in each gene, generating plasmids pJA104 (IMT2), pJA105 (IMT3), pJA106 (IMT4), and pJA107 (IMT1). None of the plasmids containing the disrupted IMT genes had suppressor activity in H2457, identifying the IMT genes as the dosage suppressors in plasmids p2623–p2625. A Tn_10 insertion that destroyed the suppressor function of p2636 interrupted the LHP1 open reading frame. Plasmid p2626 (LHP1) was created by inserting a 1.7-kb _Sal_I–_Pvu_II fragment containing LHP1 into YEp24 digested with _Sal_I and _Sma_I. p2626 showed suppressor activity similar to that of p2636, proving that LHP1 is the suppressor gene in p2636. The high-copy plasmids pC44 or p2626, bearing IMT4 or LHP1, respectively, were tested for the ability to suppress the ts− phenotypes of strains H1676 (prt1-1), H56 (gcd1-501), and TD304-10B (sui2-1) in comparison to the low-copy plasmids p2625, p256 (Hill and Struhl 1988), or p1234 (Vazquez de Aldana et al. 1993) bearing wild-type copies of PRT1, GCD1, or SUI2, respectively.
Analysis of tRNA modification
Total RNA was extracted from 300 grams (wet weight) of yeast cells grown in YPD medium (Rubin 1975) and tRNA was purified by DEAE–cellulose chromatography and deacylated (Hatfield et al. 1979), all as previously described. Transfer RNAs were aminoacylated with [3H]methionine (70 Ci/mmole, Amersham) or [35S]methionine (1000 Ci/mmole; Amersham) under conditions of limiting tRNA and the labeled tRNAs were fractionated by reversed-phase chromatography on a RPC-5 column (Kelmers and Heatherly 1971) essentially as described (Hatfield et al. 1979), except that elution of aminoacyl-tRNAs was carried out using a linear gradient of 0.45–0.65 m NaCl in the presence of 10 mm magnesium acetate. For HPLC analysis of base modifications, the tRNA was digested to nucleosides by nuclease P1 and alkaline phosphatase (Gehrke et al. 1982) and the hydrolyzate was analyzed by HPLC according to the method of Gehrke and Kuo (1990).
Acknowledgments
We thank Christopher Yoo and Sandra Wolin for instruction and advice on in vitro transcription of tRNA genes, Dolph Hatfield for his assistance and advice on the analysis of tRNA modification by RPC-5 chromatography, and Kerstin Jacobsson for HPLC analysis of modified nucleosides. We thank Bobbie Felix for help in preparation of the manuscript. G.R.B. was supported by Grants from the Swedish Cancer Society (project 680) and Swedish Natural Science Research Council (BU-2930).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked ‘advertisement’ in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL ahinnebusch@nih.gov; FAX (301) 496-6828.
References
- Alani E, Cao L, Kleckner N. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics. 1987;116:541–545. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JT, Paddy MR, Swanson MS. PUB1 is a major nuclear and cytoplasmic polyadenylated RNA-binding protein in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:6102–6113. doi: 10.1128/mcb.13.10.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano K, Phan L, Anderson J, Hinnebusch AG. Complex formation by all five homologues of mammalian translation initiation factor 3 subunits from yeast Saccharomyces cerevisiae. J Biol Chem. 1998;273:18573–18585. doi: 10.1074/jbc.273.29.18573. [DOI] [PubMed] [Google Scholar]
- Basavappa R, Sigler PB. The 3 Å crystal structure of yeast initiator tRNA: Functional implications in initiator/elongator discrimination. EMBO J. 1991;10:3105–3111. doi: 10.1002/j.1460-2075.1991.tb07864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke JD, Trueheart J, Natsoulis G, Fink GR. 5-Fluoroorotic acid as a selective agent in yeast molecular genes. Methods Enzymol. 1987;154:164–175. doi: 10.1016/0076-6879(87)54076-9. [DOI] [PubMed] [Google Scholar]
- Byström AS, Fink GR. A functional analysis of the repeated methionine initiator tRNA genes (IMT) in yeast. Mol Gen Genet. 1989;216:276–286. doi: 10.1007/BF00334366. [DOI] [PubMed] [Google Scholar]
- Carlson M, Botstein D. Two differentially regulated mRNAs with different 5′ ends encode secreted and intracellular forms of yeast invertase. Cell. 1982;28:145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- Cigan AM, Donahue TF. The methionine initiator tRNA genes of yeast. Gene. 1986;41:343–348. doi: 10.1016/0378-1119(86)90118-6. [DOI] [PubMed] [Google Scholar]
- Cigan AM, Feng L, Donahue TF. tRNAiMet functions in directing the scanning ribosome to the start site of translation. Science. 1988;242:93–97. doi: 10.1126/science.3051379. [DOI] [PubMed] [Google Scholar]
- Clark MW, Abelson J. The subnuclear localization of tRNA ligase in yeast. J Cell Biol. 1987;105:1515–1526. doi: 10.1083/jcb.105.4.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuesta R, Hinnebusch AG, Tamame M. Identification of GCD14 and GCD15, novel genes required for translational repression of GCN4 mRNA in Saccharomyces cerevisiae. Genetics. 1998;148:1007–1020. doi: 10.1093/genetics/148.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever TE, Yang W, Åström S, Byström AS, Hinnebusch AG. Modulation of tRNAiMet, eIF-2 and eIF-2B expression shows that GCN4 translation is inversely coupled to the level of eIF-2 · GTP · Met–tRNAiMet ternary complexes. Mol Cell Biol. 1995;15:6351–6363. doi: 10.1128/mcb.15.11.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond AM, Choi IS, Crain PF, Hashizume T, Pomerantz SC, Cruz R, Steer CJ, Hill KE, Burk RF, McCloskey JA, Hatfield DL. Dietary selenium affects methylation of the wobble nucleoside in the anticodon of selenocysteine tRNA[Ser]Sec*. J Biol Chem. 1993;268:14215–14223. [PubMed] [Google Scholar]
- Feinberg B, McLaughlin CS, Moldave K. Analysis of temperature-sensitive mutant ts187 of Saccharomyces cerevisiae altered in a component required for the initiation of protein synthesis. J Biol Chem. 1982;257:10846–10851. [PubMed] [Google Scholar]
- Garcia-Barrio MT, Naranda T, Cuesta R, Hinnebusch AG, Hershey JWB, Tamame M. GCD10, a translational repressor of GCN4, is the RNA-binding subunit of eukaryotic translation initiation factor-3. Genes & Dev. 1995;9:1781–1796. doi: 10.1101/gad.9.14.1781. [DOI] [PubMed] [Google Scholar]
- Gehrke CW, Kuo KC. Ribonucleoside analysis by reversed-phase high-performance liquid chromatography. In: Gehrke CW, Kuo KC, editors. Chromatography and modification of nucleosides. Amsterdam, The Netherlands: Elsevier; 1990. pp. A3–A71. [DOI] [PubMed] [Google Scholar]
- Gehrke CW, Kuo KC, McCune RA, Gerhardt KO, Agris PF. Quantitative enzymatic hydrolysis of tRNA: Reversed-phase high-performance liquid chromatography of tRNA nucleoside. J Chromatography. 1982;230:297–308. [PubMed] [Google Scholar]
- Harashima S, Hinnebusch AG. Multiple GCD genes required for repression of GCN4, a transcriptional activator of amino acid biosynthetic genes in Saccharomyces cerevisiae. Mol Cell Biol. 1986;6:3990–3998. doi: 10.1128/mcb.6.11.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield D, Matthews CR, Rice M. Aminoacyl-transfer RNA populations in mammalian cells chromatographic profiles and patterns of codon recognition. Biochim Biophys Acta. 1979;564:414–423. doi: 10.1016/0005-2787(79)90032-7. [DOI] [PubMed] [Google Scholar]
- Hershey JWB. Translational control in mammalian cells. Annu Rev Biochem. 1991;60:717–755. doi: 10.1146/annurev.bi.60.070191.003441. [DOI] [PubMed] [Google Scholar]
- Hill DE, Struhl K. Molecular characterization of GCD1, a yeast gene required for general control of amino acid biosynthesis and cell-cycle initiation. Nucleic Acids Res. 1988;16:9253–9265. doi: 10.1093/nar/16.19.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch AG. Translational control of GCN4: Gene-specific regulation by phosphorylation of eIF2. In: Hershey JWB, Mathews MB, Sonenberg N, editors. Translational control. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1996. pp. 199–244. [Google Scholar]
- Hinnebusch AG, Fink GR. Positive regulation in the general amino acid control of Saccharomyces cerevisiae. Proc Natl Acad Sci. 1983;80:5374–5378. doi: 10.1073/pnas.80.17.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman CS, Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Hopper AK, Martin NC. Processing of yeast cytoplasmic and mitochondrial precursor tRNAs. In: Jones EW, Pringle JR, Broach JR, editors. The molecular and cellular biology of the yeast Saccharomyces. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1992. pp. 99–141. [Google Scholar]
- Huisman O, Raymond W, Froehlich KU, Errada P, Kleckner N, Botstein D, Hoyt MA. A Tn10–lacZ–kanR–URA3 gene fusion transposon for insertion mutagenesis and fusion analysis of yeast and bacterial genes. Genetics. 1987;116:191–199. doi: 10.1093/genetics/116.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez A, Tipper DJ, Davies J. Mode of action of thiolutin, an inhibitor of macromolecular synthesis in Saccharomyces cerevisiae. Antimicrob Agents Chemother. 1973;3:729–738. doi: 10.1128/aac.3.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan RM, Clarke S. Widespread occurrence of three sequence motifs in diverse S-adenosylmethionine-dependent methyltransferases suggests a common structure for these enzymes. Arch Biochem Biophys. 1994;310:417–427. doi: 10.1006/abbi.1994.1187. [DOI] [PubMed] [Google Scholar]
- Kelmers AD, Heatherly DE. Columns for rapid chromatographic separation of small amounts of tracer-labeled transfer ribonucleic acids. Anal Biochem. 1971;44:486–495. doi: 10.1016/0003-2697(71)90236-3. [DOI] [PubMed] [Google Scholar]
- Kohrer K, Domdey H. Preparation of high molecular weight RNA. In: Guthrie C, Fink GR, editors. Methods in enzymology: Guide to yeast genetics and molecular biology. San Diego, CA: Academic Press; 1991. pp. 398–405. [DOI] [PubMed] [Google Scholar]
- Naranda T, Kainuma M, McMillan SE, Hershey JWB. The 39-kilodalton subunit of eukaryotic translation initiation factor 3 is essential for the complex’s integrity and for cell viability in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:145–153. doi: 10.1128/mcb.17.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranda T, MacMillan SE, Hershey JWB. Purified yeast translational initiation factor eIF-3 is an RNA-binding protein complex that contains the PRT1 protein. J Biol Chem. 1994;269:32286–32292. [PubMed] [Google Scholar]
- O’Connor JP, Peebles CL. In vivo pre-tRNA processing in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:425–439. doi: 10.1128/mcb.11.1.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DT, Merrick WC, Safer B. Binding and release of radiolabeled eukaroytic initiation factors 2 and 3 during 80 S initiation complex formation. J Biol Chem. 1979;254:2509–2519. [PubMed] [Google Scholar]
- Phan L, Zhang X, Asano K, Anderson J, Vornlocher HP, Greenberg JR, Qin J, Hinnebusch AG. Identification of a translation initiation factor 3 (eIF3) core complex, conserved in yeast and mammals, that interacts with eIF5. Mol Cell Biol. 1998;18:4935–4946. doi: 10.1128/mcb.18.8.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AM, Belford HG, Shen WC, Greer CL, Hopper AK, Martin NC. Location of N2,N2-dimethylguanosine-specific tRNA methyltransferase. Biochemie. 1995;77:45–53. doi: 10.1016/0300-9084(96)88103-x. [DOI] [PubMed] [Google Scholar]
- Rubin GM. Preparation of RNA and ribosomes from yeast. Methods Cell Biol. 1975;12:45–64. doi: 10.1016/s0091-679x(08)60951-6. [DOI] [PubMed] [Google Scholar]
- Sherman F, Fink GR, Lawrence CW. Methods of yeast genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1974. [Google Scholar]
- Simos G, Tekotte H, Grosjean H, Segref A, Sharma K, Tollervey D, Hurt EC. Nuclear pore proteins involved in the biogenesis of functional tRNA. EMBO J. 1996;15:2270–2284. [PMC free article] [PubMed] [Google Scholar]
- Sprinzl M, Horn C, Brown M, Ioudovitch A, Steinberg S. Compilation of tRNA sequences and sequenes of tRNA genes. Nucleic Acids Res. 1998;26:148–153. doi: 10.1093/nar/26.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez de Aldana CR, Dever TE, Hinnebusch AG. Mutations in the α subunit of eukaryotic translation initiation factor 2 (eIF-2α) that overcome the inhibitory effects of eIF-2α phosphorylation on translation initiation. Proc Natl Acad Sci. 1993;90:7215–7219. doi: 10.1073/pnas.90.15.7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Datar KV, Paddy MR, Swedlow JR, Swanson MS. Characterization of nuclear polyadenylated RNA-binding proteins in Saccharomyces cerevisiae. J Cell Biol. 1994;127:1173–1184. doi: 10.1083/jcb.127.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo CJ, Wolin SL. La proteins from Drosophila melanogaster and Saccharomyces cerevisiae: A yeast homolog of the La autoantigen is dispensable for growth. Mol Cell Biol. 1994;14:5412–5424. doi: 10.1128/mcb.14.8.5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— The yeast La protein is required for the 3′ endonucleolytic cleavage that matures tRNA precursors. Cell. 1997;89:393–402. doi: 10.1016/s0092-8674(00)80220-2. [DOI] [PubMed] [Google Scholar]