The Iron-Responsive Fur/RyhB Regulatory Cascade Modulates the Shigella Outer Membrane Protease IcsP (original) (raw)
Abstract
Actin-based motility is central to the pathogenicity of the intracellular bacterial pathogen Shigella. Two Shigella outer membrane proteins, IcsA and IcsP, are required for efficient actin-based motility in the host cell cytoplasm, and the genes encoding both proteins are carried on the large virulence plasmid. IcsA triggers actin polymerization on the surface of the bacterium, leading to the formation of an actin tail that allows both intra- and intercellular spread. IcsP, an outer membrane protease, modulates the amount and distribution of the IcsA protein on the bacterial surface through proteolytic cleavage of IcsA. Transcription of icsP is increased in the presence of VirB, a DNA-binding protein that positively regulates many genes carried on the large virulence plasmid. In Shigella dysenteriae, the small regulatory RNA RyhB, which is a member of the iron-responsive Fur regulon, suppresses several virulence-associated phenotypes by downregulating levels of virB in response to iron limitation. Here we show that the Fur/RyhB regulatory pathway downregulates IcsP levels in response to low iron concentrations in Shigella flexneri and that this occurs at the level of transcription through the RyhB-dependent regulation of VirB. These observations demonstrate that in Shigella species the Fur/RyhB regulatory pathway provides a mechanism to finely tune the expression of icsP in response to the low concentrations of free iron predicted to be encountered within colonic epithelial cells.
INTRODUCTION
Shigella species are Gram-negative intracellular pathogens that cause severe and bloody diarrhea in humans and primates. It is estimated that the associated disease, shigellosis, is responsible for over 164 million infections each year, resulting in 1.1 million deaths worldwide (20). Transmission of shigellosis typically occurs via the fecal-oral route, although frequently intermediate steps may be involved, including person-to person contact, contaminated food and water, insect vectors, or human sexual practices (28).
Upon ingestion, shigellae transit through almost the entire alimentary canal before reaching the colonic epithelium, the site of infection. The passage of Shigella through the stomach and alimentary canal involves drastic pH swings, exposure to digestive enzymes, and a variety of other environmental stresses. Shigella species are adept at surviving these conditions as demonstrated by their incredibly low infectious dose, which has been reported to be as low as 10 to 100 bacteria in primate models of infection (8). A complete appreciation of how the diverse physical and chemical conditions encountered within the host lead to the coordinated expression of Shigella virulence genes has not yet been achieved.
Once Shigella reaches the large intestine, the bacteria invade cells of the colonic epithelium, escape the phagocytic vacuole, and spread from one cell to another by means of actin-based motility (reviewed in reference 38). A single bacterial protein, IcsA, is required for the actin-based motility of Shigella (15, 16). This surface-exposed outer membrane protein is encoded by a gene on the large Shigella virulence plasmid and triggers the formation of an actin tail on the surface of the bacterium, which propels the bacterium through the host cell cytoplasm and into adjacent cells (reviewed in reference 13).
IcsA is localized specifically to the old pole of Shigella (14). The unipolar distribution of IcsA is primarily achieved by cytoplasmic targeting of IcsA to the bacterial pole (6), yet once the IcsA polar cap has formed, it is maintained through the activity of the outer membrane protease IcsP (9, 40). In vitro, icsP mutants display aberrant localization of IcsA along the longitudinal axis of the bacterial cell (40), and Shigella strains that overexpress IcsP completely lack surface-associated IcsA and are unable to move using actin-based motility in tissue culture experiments (41, 45). The results of these experiments suggest that the icsP gene must be regulated in the host so that optimal amounts of this protease are present when Shigella enters the cytoplasm of colonic epithelial cells.
In contrast to icsA expression, which is primarily regulated by the transcriptional activator VirF (1, 21, 36), our previous studies have revealed that expression of icsP is regulated by VirB (4, 18, 46), a transcriptional activator whose expression is controlled directly by VirF. VirB, which directly controls the expression of many virulence-associated genes carried on the Shigella virulence plasmid (4, 21, 46), is maximally produced at 37°C, the temperature within the human host (30). Since all environments within the host are stably maintained at 37°C, we were interested in identifying additional site-specific environmental cues that influence the regulation of icsP expression that is required for Shigella to successfully establish and maintain an infection.
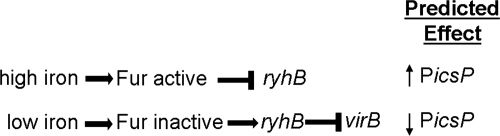
Recent work has demonstrated that virB mRNA levels in Shigella dysenteriae decrease when the organism is grown under iron-limiting conditions (27). This repression involves the iron-responsive small regulatory RNA RyhB, which is found in all four species of Shigella. The expression of ryhB is repressed by Fur, the global transcriptional regulator of iron homeostasis in enteric bacteria (23, 29). In the presence of iron, Fur complexes with iron, binds to its cognate DNA-binding sites, and represses transcription of many iron-responsive genes, including ryhB. Conversely, in the absence of iron, Fur-mediated repression is relieved, and RyhB is expressed, leading to a decrease in virB mRNA levels in the bacterial cell and ultimately a decrease in the transcription of at least some genes in the VirB regulon (27) (Fig. 1). On the basis of these observations, we designed experiments to test the hypothesis that the Fur/RyhB regulatory pathway finely tunes the regulation of icsP, a target of transcriptional regulation by VirB, and that the iron status of Shigella serves as an environmental cue that triggers precise control of IcsP production during the course of infection within the human host.
Fig. 1.
Regulatory pathway controlling the expression of virB in Shigella. In the presence of iron, the global iron-responsive transcriptional repressor Fur (3) binds to specific sequences within target bacterial promoters and represses the transcription of many genes, including ryhB (23, 29). We hypothesize that in the absence of RyhB production, virB expression proceeds uninhibited, and thus, transcription from the VirB-regulated icsP promoter is activated. Under conditions of iron limitation, Fur is inactive, and repression of ryhB expression is relieved. We hypothesize that under these conditions, increased production of RyhB decreases the expression of virB, and this downregulates expression from the icsP promoter.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Bacteria were grown routinely in Luria broth (LB) with aeration at 37°C. The following antibiotics were used at the indicated final concentrations: ampicillin, 100 μg ml−1; chloramphenicol, 25 μg ml−1; and gentamicin, 20 μg ml−1. Ethylenediamine-N,_N_′-bis(2-hydroxyphenylacetic acid) (EDDA) was deferrated as described previously (32) and added to the medium at a final concentration of 15 μg ml−1 to chelate iron. These concentrations of deferrated EDDA were found to slow the growth of Shigella cultures without inhibiting their growth completely, suggesting these cultures were experiencing iron starvation and mounting an iron starvation response.
Table 1.
Bacterial strains and plasmids used in this study
| Bacterial strain or plasmid | Description and/or relevant genotype or phenotypea | Reference or source |
|---|---|---|
| S. flexneri strains | ||
| 2457T | Wild type; serotype 2a | 12 |
| 2457T virB mutant (also called AWY3) | 2457T virB::Tn_5_; Knr | 46 |
| YSH6000 | Wild type; serotype 2a | 39 |
| M90T | Wild type; serotype 5a | 37 |
| SM100 | SA100 Strr | S. Selinger |
| SM100 Δ_fur_ mutant (also called SM1301) | SM100 Δ_fur_; Cmr | 29 |
| SM100 Δ_fur_ Δ_ryhB_ mutant (also called SM1302) | SM100 Δ_fur_ Δ_ryhB_; Cmr Knr | 29 |
| Plasmids | ||
| pryhB | ryhB in pQE2; Ampr | 27 |
| pQE2 | Qiagen cloning vector; Ampr | Qiagen |
| pHJW20 | icsP promoter region transcriptionally fused to lacZ; Cmr | 4 |
| pAFW01 | icsP promoter region transcriptionally fused to lacZ; Gmr; derived from pHJW20 | This study |
| pLIA05 | sitA promoter region transcriptionally fused to lacZ; Cmr | This study |
Construction of promoter fusion plasmids.
pLIA05 is derived from the P_icsP-lacZ_ reporter pHJW20 (4). The icsP promoter region was removed from pHJW20 using SalI and XbaI restriction enzymes. The sitA promoter region was amplified from Shigella flexneri strain 2457T using oligonucleotide primers 5′-CAAATTATCTAGAGCAGGTGAGGGCGAGGCA-3′ and 5′-GACTAGGTCGACTGTTGACGGCAGGAGCGAAC-3′, digested with SalI and XbaI restriction enzymes, and ligated with the pHJW20 SalI- and XbaI-restricted vector. The resulting plasmid, pLIA05, contains a 628-bp sitA promoter fragment comprised of 574 bp upstream and 36 bp downstream of the sitA coding region.
pAFW01 is derived from the P_icsP-lacZ_ reporter pHJW20 (4). An internal fragment of the chloramphenicol resistance gene was removed from pHJW20 using restriction enzyme AccIII and a gentamicin resistance cassette, restricted with XmaI, was subsequently inserted. The resulting plasmid confers gentamicin resistance.
Quantification of IcsP levels in Shigella.
Throughout these studies, IcsP production was measured between 5 and 7 h of growth because IcsP is maximally produced in wild-type cells grown under these conditions (H. J. Wing, unpublished work). The cells were routinely diluted 1:100 from an overnight culture and grown for 5 to 7 h in LB alone or LB supplemented with 200 μM isopropyl-β-d-thiogalactopyranoside (IPTG) or with the iron-specific chelator EDDA (15 μg ml−1). The cells were then harvested, and whole-cell protein extracts were prepared as described previously (41). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Equivalent amounts of protein were loaded by normalizing the harvest volume to cell density. Western blot analysis was performed with an affinity-purified IcsP rabbit antiserum (Pacific Immunology) and detected by chemiluminescence using a UVP BioSpectrum 410 imaging system. Integrated densitometry measurements were performed using the VisionWorksLS image acquisition and analysis software.
Quantification of promoter activity.
Promoter activity was determined by measuring β-galactosidase activity in strains carrying either pHJW20 (described in reference 4), its derivative pAFW01, or pLIA05 using the Miller protocol (25). To measure the effect of RyhB production on the activity of the icsP promoter, the cells were diluted 1:100 and grown for 7 h in either LB alone or LB supplemented with 200 μM IPTG prior to cell lysis. To measure the effect of iron availability on the activity of the sitA and icsP promoters, the cells were diluted 1:100 and grown for 7 h at 37°C in either LB alone or LB supplemented with 15 μg ml−1 EDDA to chelate iron prior to cell lysis. To measure icsP promoter activity in the different Shigella flexneri SM100 genetic backgrounds, the cells were diluted 1:100 and grown for 5 h at 37°C in LB prior to cell lysis. In all experiments, transcription was analyzed in three independent transformants, and assays were run in triplicate.
Quantification of mRNA levels by RT-PCR.
RNA was isolated from bacteria cultured for 5 to 7 h at 37°C in LB broth with the appropriate antibiotic and in the presence or absence of IPTG or EDDA. RNA was isolated from bacteria using an RNeasy Midi kit (Qiagen, Valencia, CA) per the product directions. Each RNA sample was then treated with 16 units of amplification-grade DNase I (Invitrogen, Carlsbad, CA), ethanol precipitated, and dried. The RNA pellet was resuspended in diethyl pyrocarbonate (DEPC)-treated water, and the nucleic acid concentration was measured using an ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). No more than 10 μg of total RNA was used to generate cDNA with the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA) per the product directions. Each cDNA sample was diluted 1:10 in water, and 5 μl was used as the template for each amplification reaction. iQ SYBR green supermix was used in all reaction mixtures with a final volume of 20 μl. All expression values were calculated using the ΔΔ_CT_ method, normalized to the level of rrsA measured in each sample and expressed relative to the value obtained from the indicated sample. All reaction mixtures were run in a Bio-Rad CFX96 real-time PCR (RT-PCR) system (Bio-Rad Laboratories, Hercules, CA) under reaction conditions optimized for each primer set. All primer sequences were designed using Beacon Designer 7.5 and are available upon request.
Statistical analyses.
Two-tailed, two-sample Student's t tests, assuming equal variance, were used throughout our studies to determine confidence levels.
RESULTS
RyhB regulates IcsP production in Shigella flexneri.
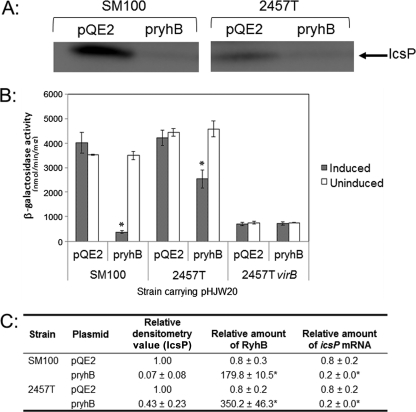
We first chose to examine the effect that expression of the small regulatory RNA RyhB has on IcsP protein levels in two Shigella flexneri strains. To do this, a plasmid, pryhB (27), carrying an inducible copy of the ryhB gene, or pQE2, the empty plasmid control, was introduced into wild-type S. flexneri strains SM100 and 2457T, and IcsP levels present in whole-cell extracts were measured by Western blot analysis following growth of each strain in the presence of inducer. Since RyhB negatively regulates virB expression (27) and VirB upregulates icsP expression (4, 46), we hypothesized that increased production of RyhB would lead to a decrease in the levels of IcsP. Although higher levels of IcsP were detected in the SM100 background than in the 2457T background, our data show that IcsP levels were dramatically decreased in both strains after induction of the small regulatory RNA RyhB (Fig. 2A and C). Similar observations were made with the S. flexneri serotype 2a strain, YSH6000, and the S. flexneri serotype 5 strain, M90T (see Fig. S1 in the supplemental material). These data indicate that increased production of RyhB decreases IcsP protein levels in all of the S. flexneri strains tested.
Fig. 2.
Production of RyhB decreases IcsP protein levels in two different Shigella flexneri strains (SM100 and 2457T). (A) Western blot analysis of total cellular protein harvested from wild-type Shigella strains carrying the vector control pQE2 or the inducible plasmid pryhB in the presence of inducer using an affinity-purified IcsP rabbit antiserum (densitometry values are shown in panel C). (B) β-Galactosidase expression from the P_icsP_-lacZ reporter pHJW20 measured in wild-type Shigella flexneri (2457T) and an isogenic strain lacking virB (AWY3) (46) after 7 h of growth (stationary phase). The cells were grown in the presence or absence of inducer. Values (bars) are the means ± standard deviations from the means (error bars) of three independent experiments. A statistical difference between the activity measured under induced and uninduced conditions (P value < 0.05) is indicated by an asterisk. (C) Densitometry of Western blots and relative amounts of RyhB and icsP mRNA under experimental conditions as judged by RT-PCR. Data are from three independent experiments. Values are expressed relative to the wild-type strain SM100 carrying pQE2 and grown in LB with inducer. Averages ± 1 standard deviation are shown. A statistically significant difference compared to the value obtained for each strain carrying the vector control pQE2 (P value of <0.05) is indicated by an asterisk.
RyhB regulation of the activity of the icsP promoter is mediated by VirB.
The activity of the icsP promoter is upregulated by the transcription factor VirB (46). Since RyhB decreases the levels of virB mRNA and VirB activity (27), we next reasoned that the activity of the icsP promoter would decrease when ryhB was expressed from an inducible plasmid in S. flexneri. To test this prediction, we made use of our low-copy P_icsP-lacZ_ reporter plasmid pHJW20 (4). Either the inducible ryhB expression plasmid pryhB or the control plasmid pQE2 was introduced into Shigella strains carrying pHJW20. Each strain was then grown to stationary phase with or without inducer, and β-galactosidase levels were measured.
Our data show that icsP promoter activity was significantly lower in both of the wild-type strains when ryhB expression was induced (Fig. 2B), although a more pronounced decrease was observed in S. flexneri SM100 than in S. flexneri 2457T under these experimental conditions (10-fold versus 2-fold, respectively), a difference that is likely due to strain variability. These data demonstrate that sequences within our P_icsP-lacZ_ reporter are sufficient to confer RyhB-dependent regulation onto the lacZ reporter gene. Furthermore, induction of ryhB had no effect on the activity of the icsP promoter in the virB mutant background. Taken together, these data are consistent with RyhB downregulating icsP promoter activity and strongly suggest that RyhB regulates IcsP levels through its regulation of VirB (27).
To further analyze our experimental system, we next chose to measure the relative abundance of RyhB and icsP mRNA in cells carrying either pryhB or pQE2 following growth under inducing conditions (conditions identical to those used for our Western blot analysis and our β-galactosidase assays; Fig. 2A and B). Our data show that RyhB levels were significantly increased in cells carrying pryhB and induced with IPTG (Fig. 2C). Under these conditions, regardless of the strain, a 5-fold decrease in icsP mRNA levels was observed (Fig. 2C). Together, these data demonstrate that both icsP promoter activity and icsP mRNA levels decrease significantly when the production of RyhB increases in S. flexneri.
Iron regulates the activity of the icsP promoter.
In all of our experiments described thus far, expression of ryhB had been from an inducible promoter. As a consequence, RyhB was likely to be present at superphysiological concentrations. To address this issue, we next chose to measure icsP promoter activity using growth conditions that trigger the expression of the native ryhB gene at physiologically relevant levels: icsP promoter activity was measured in the presence and absence of iron. Since expression of the Shigella ryhB gene is repressed by Fur when the regulator is complexed with iron (27, 29), growth of S. flexneri under iron-limiting conditions was expected to result in increased production of RyhB.
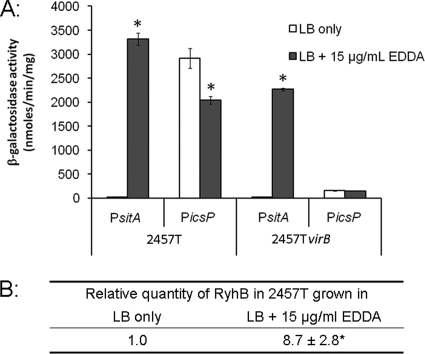
For these experiments, we chose to limit the available iron within the growth medium by the addition of the iron chelator EDDA (as described previously [29]). To ensure that appropriate levels of EDDA were being used in our assays, the construct pLIA05, carrying the S. flexneri sitA promoter fused to lacZ, was introduced into the wild-type S. flexneri strain 2457T. Although sitA promoter activity is known to increase under conditions of either iron or manganese limitation (33, 35), EDDA has approximately a 100-fold-greater affinity for Fe2+ (equilibrium constant, 6.45 × 109) than Mn2+ (5.13 × 107) (5), so the sitA promoter could be used to determine whether iron was limiting in our assays. Our data show that the sitA promoter is tightly repressed in cells grown in LB medium. In contrast, the activity of the sitA promoter significantly increases when cells are grown in LB containing 15 μg ml−1 of deferrated EDDA (Fig. 3A). These data indicate that the concentration of EDDA used in these experiments is sufficient to trigger an iron starvation response in Shigella.
Fig. 3.
The icsP promoter is downregulated in the absence of iron. (A) β-Galactosidase expression from pLIA05 (P_sitA-lacZ_ reporter; indicated as P_sitA_ in the figure) and pHJW20 (P_icsP-lacZ_ reporter; indicated as P_icsP_ in the figure) measured in wild-type Shigella flexneri (2457T) and an isogenic strain lacking virB (AWY3) (46) after 7 h of growth in either LB or LB supplemented with 15 μg ml−1 deferrated EDDA. Data represent the means of three independent experiments. Error bars represent standard deviations from the means. (B) Real-time PCR analysis of relative RyhB levels in S. flexneri wild-type strain 2457T grown for 7 h in LB alone or in LB supplemented with 15 μg/ml deferrated EDDA. All values were normalized to the level of rrsA mRNA in each sample, and the results are expressed relative to the value obtained for the wild-type strain grown in LB. The data are from four independent experiments. The average ± 1 standard deviation is shown. In both panels, a statistically significant difference compared to the value measured in LB alone (P value of <0.01) is indicated by an asterisk.
Having identified conditions that would induce an iron starvation response, we could next examine the effect of iron availability on the activity of the icsP promoter. Wild-type Shigella flexneri (2457T) and a virB mutant derivative, each carrying the P_icsP-lacZ_ reporter plasmid pHJW20, were grown in LB in the presence and absence of EDDA, and β-galactosidase levels were measured. Our data show that the activity of the icsP promoter decreased in the wild-type S. flexneri strain grown in LB containing EDDA (Fig. 3A), demonstrating that sequences within the P_icsP-lacZ_ reporter are sufficient to confer the EDDA-mediated regulation of icsP. Although the observed decrease in icsP promoter activity was small, the decrease was statistically significant (P value of <0.01) and is consistent with the fine tuning of gene expression that is classically imposed by small regulatory RNA molecules like RyhB. Furthermore, the activity of the icsP promoter in the virB mutant (AWY3) was unaffected by EDDA, suggesting that the regulation of icsP observed under these assay conditions is mediated by the regulation of VirB.
To further analyze this experimental system and to test the involvement of iron, we again chose to measure the relative abundance of the iron-responsive regulatory RNA RyhB (23, 27, 29) in S. flexneri 2457T under the conditions used in this assay. Our data show that RyhB increased by approximately 9-fold in the presence of EDDA, as judged by real-time PCR (RT-PCR) (Fig. 3B). Taken together, these data strongly suggest that physiological levels of RyhB produced by growth of the wild-type strain in iron-limiting conditions are sufficient to downregulate the icsP promoter in a VirB-dependent manner.
The Fur/RyhB regulatory pathway is the primary mediator of iron-dependent regulation of IcsP in Shigella.
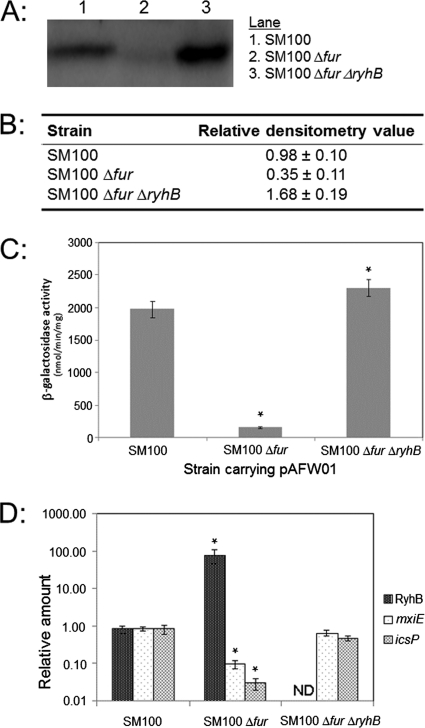
To further assess the involvement of iron in the regulation of icsP, we next chose to measure IcsP levels and icsP promoter activity in S. flexneri ryhB and fur mutants. To do this, three S. flexneri strains were used: SM100 (wild type), SM1301 (SM100 Δ_fur_), and a double mutant, SM1302 (SM100 Δ_fur_ Δ_ryhB_). IcsP levels present in whole-cell extracts were measured by Western blot analysis, and the activity of the icsP promoter was measured by using the P_icsP-lacZ_ fusion reporter plasmid pAFW01, which carries a gentamicin resistance cassette.
Our data show that IcsP protein levels were barely detected in the Shigella Δ_fur_ strain (3-fold lower than wild-type levels) and yet were approximately 5 times higher in the Δ_fur_ Δ_ryhB_ double mutant than in the Δ_fur_ mutant (1.7-fold higher than the level in wild-type cells; Fig. 4A and B). Similarly, the activity of the icsP promoter decreased to basal levels in the Shigella Δ_fur_ strain but was restored in the double mutant as judged by β-galactosidase assays (Fig. 4C). Consistent with the influence of Fur and RyhB on the activity of the icsP promoter, direct measurement of icsP mRNA levels by RT-PCR confirmed that icsP mRNA levels decrease in the Shigella Δ_fur_ strain and are restored in the double mutant (Fig. 4D). Additionally, an analysis of the relative abundance of RyhB in these backgrounds showed that RyhB levels are increased 100-fold in the absence of the fur gene (Fig. 4D), an increase similar to those detected when ryhB is induced from our inducible pryhB plasmid in the SM100 strain (Fig. 2C). Taken together, these data clearly show that both fur and ryhB are required for the precise iron-responsive regulation of the icsP promoter and that this regulation ultimately modulates IcsP levels in Shigella.
Fig. 4.
IcsP levels are significantly decreased in a Δ_fur_ mutant as compared to in wild-type Shigella and are restored in a Δ_fur_ Δ_ryhB_ double mutant. (A) Comparison of IcsP levels in wild-type Shigella (SM100) and isogenic mutant strains. IcsP was detected in total cellular protein by Western blot analysis using an affinity-purified IcsP rabbit antiserum. Experiments were repeated three times, and representative data are shown. (B) Densitometry of three independent Western blots similar to that shown in panel A. Values are expressed relative to S. flexneri SM100 grown in LB. Averages ± 1 standard deviation are shown. (C) β-Galactosidase expression from the P_icsP_-lacZ reporter pAFW01. Values (bars) are the means ± standard deviations from the means (error bars) of three independent experiments. (D) Real-time PCR analyses of RyhB levels in each strain background. All values were normalized to the level of rrsA mRNA in each sample, and the results are expressed relative to the value obtained for the wild-type strain grown in LB. The data represent four independent experiments. Averages ± 1 standard deviation are shown. In panels C and D, a statistically significant difference compared to the value measured in S. flexneri SM100 (P value of <0.05) is indicated by an asterisk. ND, nondetectable levels of target.
Since RyhB has been demonstrated to decrease the expression of other Shigella genes carried on the ipa-mxi-spa locus of the Shigella virulence plasmid (27), we next chose to measure the expression of mxiE under these experimental conditions, thereby allowing a comparison of the relative levels of RyhB-dependent regulation of mxiE and icsP and providing a positive control for the analysis performed using S. flexneri SM100 and its derivatives. Our data show that mxiE mRNA levels are decreased 8.5-fold in the fur mutant and are fully restored to wild-type levels in the fur ryhB double mutant (Fig. 4D). These data indicate that, under the conditions tested, icsP is more affected by the Fur/RyhB regulatory pathway than mxiE (27.6- versus 8.5-fold) and that expression of the well-characterized target mxiE is occurring as expected in each of our strains.
In summary, our data show that the Fur/RyhB regulatory pathway mediates the fine tuning of IcsP production in Shigella in response to iron availability. Under each condition where ryhB expression increases, namely, expression from an inducible plasmid, growth in iron-poor medium, or growth in the absence of Fur, icsP expression decreases. Furthermore, under each condition where ryhB expression decreases, namely, growth in iron-rich media or in the Δ_fur_ Δ_ryhB_ double mutant, icsP expression increases. These data suggest that there is an inverse relationship between ryhB expression and IcsP production and a direct relationship between iron availability and IcsP production in Shigella.
DISCUSSION
It is well documented that the icsP gene is regulated by VirB (21, 46), a transcription factor that is expressed upon a switch to 37°C (24, 42). In this work, we show that RyhB, an iron-responsive small regulatory RNA (23), downregulates IcsP production in four different strains and two different serotypes of S. flexneri (Fig. 2; see Fig. S1 in the supplemental material). Iron-dependent regulation of icsP expression is achieved through a multistep regulatory cascade involving Fur, RyhB, and VirB. While the degree of the effect varies from strain to strain (Fig. 2B), it is clear that the ultimate outcome of ryhB expression in S. flexneri strains is the same—the downregulation of IcsP production (Fig. 2A and Fig. S1). Furthermore, we demonstrate that icsP expression is downregulated primarily by the RyhB-dependent regulation of VirB, because promoter fusions to lacZ are also downregulated, and in the absence of VirB, expression of ryhB does not significantly affect icsP promoter activity. To our knowledge, this is the first evidence that icsP expression is linked to Fur, RyhB, and iron availability.
Although RyhB was originally implicated in the regulation of Shigella virulence genes in S. dysenteriae (27), icsP mRNA was not identified as having more than a 2-fold decrease during ryhB expression by microarray analysis under the conditions tested. Data collected recently in our laboratories however indicate that the icsP gene in S. dysenteriae is regulated by RyhB, leading to decreased icsP promoter activity and lower icsP mRNA levels as judged by RT -PCR (W. Broach, H. J. Wing, and E. R. Murphy, unpublished work). These data expand our findings and are consistent with the RyhB regulatory pathway modulating icsP expression in other Shigella species.
Within the human host, iron is tightly sequestered by high-affinity iron-binding proteins, such as transferrin or lactoferrin, the iron storage protein ferritin, or protoporphyrin rings, such as heme, as part of the innate immune defense system against bacterial infection (7, 31, 44). Iron sequestration results in concentrations of free iron estimated to be as low as 10−18 M in mammalian tissue (2), a concentration lower than that required for the survival of most bacterial pathogens.
For bacterial pathogens that are ingested with food and/or water, the transition to an iron-limited environment within the infected host is likely to be a relatively gradual one. Food substances typically contain freely available iron, which is readily solubilized in the stomach by the acidic conditions. In this form, iron can most readily be absorbed by cells of the duodenum and other regions of the small intestine (26), although the amount of iron absorbed is largely dependent upon the iron status of the mammal (11, 19). Based on these reports, as ingested iron is absorbed during transit through the gastrointestinal tract and is subsequently sequestered, less iron will be found in the large intestine than in the small intestine and less yet in the intracellular compartment of colonic epithelial cells (expanded upon in the next paragraph). It seems likely that bacteria, especially intracellular bacteria such as Shigella species, would detect this negative iron gradient and that this alteration in environmental iron concentration may influence the regulation of virulence gene expression.
Although little is known about the iron status within the cytoplasm of human cells, several lines of evidence suggest that iron limitation is encountered by Shigella in the intracellular compartment of epithelial cells. Specifically, multiple studies investigating the gene expression profile of Shigella growing within eukaryotic cells clearly demonstrate that the expression of several Fur-regulated genes increases within the intracellular environment (22, 34). Increased expression of Fur-regulated genes, such as sitA, sufA, and fhuA, indicates that the amount of free iron is insufficient to activate iron-dependent repression by Fur, thus supporting the conclusion that the intracellular environment is iron limited. Similarly, it has been documented that the expression of several genes within the VirB regulon is downregulated during the initial phase of intracellular growth (17) and during intracellular replication of Shigella (22). Given the findings that RyhB is expressed under iron-limited conditions (23) and represses the expression of virB (27), the observation that the expression of several genes within the VirB regulon is repressed during intracellular growth also supports the conclusion that the intracellular compartment of epithelial cells is an iron-poor environment. While these observations, which were made with cultured epithelial cells, certainly do not prove that the intracellular compartment of colonic epithelial cells within the human intestine is an iron-limiting environment for intracellular bacteria like Shigella, they are consistent with it being so.
Shigella species are not the only bacterial pathogens to downregulate virulence gene expression in response to iron limitation. In Salmonella enterica serovar Typhimurium, virulence genes carried on Salmonella pathogenicity island 1 (SPI-1), which carries genes classically involved in transversing the host cell epithelial layer, are regulated indirectly by Fur and downregulated under conditions of iron limitation (10, 43). To our knowledge, RyhB has not been implicated in this regulatory pathway; instead, Fur repression of the S. enterica hns gene lies at the center of this regulatory pathway (43). Regardless of the molecular pathway used to modulate gene expression in response to iron limitation, our study adds icsP to a growing list of bacterial virulence genes that are negatively regulated in response to iron limitation.
Since icsP is downregulated by the Fur/RyhB regulatory pathway in response to iron limitation, we propose the following model to describe how this regulation enhances the pathogenicity of Shigella in the human intestine. Within the lumen of the intestine, a relatively iron-rich environment compared to the intracellular compartment of colonic epithelial cells, Fur would be actively repressing ryhB, leading to the expression of virB and consequently icsP transcription. Production of IcsP in this environment may ensure that any IcsA produced within the lumen of the gut is actively cleared from the bacterial surface prior to invasion. Once invasion of the colonic epithelium occurs, downregulation of IcsP in response to iron limitation may ensure that IcsP is present at optimal levels so that appropriate levels of cell-associated IcsA are available for efficient actin-based motility. Future studies in our laboratory will address what effect iron limitation has on the regulation of icsA, the amount and distribution of cell-associated IcsA, and which, if any, iron-responsive pathways are involved.
In summary, our work shows that the Fur/RyhB regulatory pathway controls the expression of icsP, which encodes an outer membrane protease implicated in the modulation of IcsA and hence actin-based motility in Shigella. We propose that this regulatory pathway finely tunes the expression of icsP, allowing for optimal actin-based motility—a key process in Shigella pathogenesis.
Supplementary Material
Supplemental Material
ACKNOWLEDGMENTS
We thank E. Robleto and D. Basta for critical reading of the manuscript and W. Broach for sharing unpublished data on the effect of RyhB on icsP expression in S. dysenteriae.
This study was supported by NIH grant P20 RR-016464 from INBRE Program of the National Center for Research Resources by NIH grant R15 AI090573-01. L.A.A.A. was the recipient of an Undergraduate Research Opportunity Award from the NV INBRE program. E.R.M. was supported by the Ohio University Heritage College of Osteopathic Medicine and by an award from The Ohio University Research Committee.
Footnotes
▿
Published ahead of print on 22 August 2011.
REFERENCES
- 1.Adler B., et al. 1989. A dual transcriptional activation system for the 230 kb plasmid genes coding for virulence-associated antigens of Shigella flexneri. Mol. Microbiol. 3:627–635 [DOI] [PubMed] [Google Scholar]
- 2.Bullen J. J., Rogers H. J., Griffiths E. 1978. Role of iron in bacterial infection. Curr. Top. Microbiol. Immunol. 80:1–35 [DOI] [PubMed] [Google Scholar]
- 3.Carpenter B. M., Whitmire J. M., Merrell D. S. 2009. This is not your mother's repressor: the complex role of fur in pathogenesis. Infect. Immun. 77:2590–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castellanos M. I., et al. 2009. VirB alleviates H-NS repression of the icsP promoter in Shigella flexneri from sites over 1 kb upstream of the transcription start site. J. Bacteriol. 191:4047–4050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaberek S., Martell A. E. 1959. Organic sequestering agents. John Wiley & Sons, New York, NY [Google Scholar]
- 6.Charles M., Perez M., Kobil J. H., Goldberg M. B. 2001. Polar targeting of Shigella virulence factor IcsA in Enterobacteriacae and Vibrio. Proc. Natl. Acad. Sci. U. S. A. 98:9871–9876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chasteen N. D., Harrison P. M. 1999. Mineralization in ferritin: an efficient means of iron storage. J. Struct. Biol. 126:182–194 [DOI] [PubMed] [Google Scholar]
- 8.DuPont H. L., Levine M. M., Hornick R. B., Formal S. B. 1989. Inoculum size in shigellosis and implications for expected mode of transmission. J. Infect. Dis. 159:1126–1128 [DOI] [PubMed] [Google Scholar]
- 9.Egile C., d'Hauteville H., Parsot C., Sansonetti P. J. 1997. SopA, the outer membrane protease responsible for polar localization of IcsA in Shigella flexneri. Mol. Microbiol. 23:1063–1073 [DOI] [PubMed] [Google Scholar]
- 10.Ellermeier J. R., Slauch J. M. 2008. Fur regulates expression of the Salmonella pathogenicity island 1 type III secretion system through HilD. J. Bacteriol. 190:476–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finch C. 1994. Regulators of iron balance in humans. Blood 84:1697–1702 [PubMed] [Google Scholar]
- 12.Formal S. B., Dammin G. J., LaBrec E. H., Schneider H. 1958. Experimental Shigella infections: characteristics of a fatal infection produced in guinea pigs. J. Bacteriol. 75:604–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldberg M. B. 2001. Actin-based motility of intracellular microbial pathogens. Microbiol. Mol. Biol. Rev. 65:595–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg M. B., Barzu O., Parsot C., Sansonetti P. J. 1993. Unipolar localization and ATPase activity of IcsA, a Shigella flexneri protein involved in intracellular movement. Infect. Agents Dis. 2:210–211 [PubMed] [Google Scholar]
- 15.Goldberg M. B., Theriot J. A. 1995. Shigella flexneri surface protein IcsA is sufficient to direct actin-based motility. Proc. Natl. Acad. Sci. U. S. A. 92:6572–6576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldberg M. B., Theriot J. A., Sansonetti P. J. 1994. Regulation of surface presentation of IcsA, a Shigella protein essential to intracellular movement and spread, is growth phase dependent. Infect. Immun. 62:5664–5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Headley V. L., Payne S. M. 1990. Differential protein expression by Shigella flexneri in intracellular and extracellular environments. Proc. Natl. Acad. Sci. U. S. A. 87:4179–4183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hensley C. T., et al. 2011. Two promoters and two translation start sites control the expression of the Shigella flexneri outer membrane protease IcsP. Arch. Microbiol. 193:263–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan C. D., Kaplan J. 2009. Iron acquisition and transcriptional regulation. Chem. Rev. 109:4536–4552 [DOI] [PubMed] [Google Scholar]
- 20.Kotloff K. L., et al. 1999. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies, Bull. World Health Organ. 77:651–666 [PMC free article] [PubMed] [Google Scholar]
- 21.Le Gall T., et al. 2005. Analysis of virulence plasmid gene expression defines three classes of effectors in the type III secretion system of Shigella flexneri. Microbiology 151:951–962 [DOI] [PubMed] [Google Scholar]
- 22.Lucchini S., Liu H., Jin Q., Hinton J. C., Yu J. 2005. Transcriptional adaptation of Shigella flexneri during infection of macrophages and epithelial cells: insights into the strategies of a cytosolic bacterial pathogen. Infect. Immun. 73:88–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masse E., Gottesman S. 2002. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 99:4620–4625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maurelli A. T., Blackmon B., Curtiss III R. 1984. Temperature-dependent expression of virulence genes in Shigella species. Infect. Immun. 43:195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller J. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 26.Muir A., Hopfer U. 1985. Regional specificity of iron uptake by small intestinal brush-border membranes from normal and iron-deficient mice. Am. J. Physiol. 248:G376–G379 [DOI] [PubMed] [Google Scholar]
- 27.Murphy E. R., Payne S. M. 2007. RyhB, an iron-responsive small RNA molecule, regulates Shigella dysenteriae virulence. Infect. Immun. 75:3470–3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niyogi S. K. 2005. Shigellosis. J. Microbiol. 43:133–143 [PubMed] [Google Scholar]
- 29.Oglesby A. G., Murphy E. R., Iyer V. R., Payne S. M. 2005. Fur regulates acid resistance in Shigella flexneri via RyhB and ydeP. Mol. Microbiol. 58:1354–1367 [DOI] [PubMed] [Google Scholar]
- 30.Porter M. E. 1998. The regulation of virulence gene expression in Shigella flexneri. Ph.D. thesis. Trinity College, Dublin, Ireland [Google Scholar]
- 31.Ratledge C., Dover L. G. 2000. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 54:881–941 [DOI] [PubMed] [Google Scholar]
- 32.Rogers H. J. 1973. Iron-binding catechols and virulence in Escherichia coli. Infect. Immun. 7:445–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Runyen-Janecky L., Dazenski E., Hawkins S., Warner L. 2006. Role and regulation of the Shigella flexneri sit and MntH systems. Infect. Immun. 74:4666–4672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Runyen-Janecky L. J., Payne S. M. 2002. Identification of chromosomal Shigella flexneri genes induced by the eukaryotic intracellular environment. Infect. Immun. 70:4379–4388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Runyen-Janecky L. J., Reeves S. A., Gonzales E. G., Payne S. M. 2003. Contribution of the Shigella flexneri Sit, Iuc, and Feo iron acquisition systems to iron acquisition in vitro and in cultured cells. Infect. Immun. 71:1919–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakai T., Sasakawa C., Yoshikawa M. 1988. Expression of four virulence antigens of Shigella flexneri is positively regulated at the transcriptional level by the 30 kilodalton virF protein. Mol. Microbiol. 2:589–597 [DOI] [PubMed] [Google Scholar]
- 37.Sansonetti P. J., Kopecko D. J., Formal S. B. 1982. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect. Immun. 35:852–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sansonetti P. J., Tran Van Nhieu G., Egile C. 1999. Rupture of the intestinal epithelial barrier and mucosal invasion by Shigella flexneri. Clin. Infect. Dis. 28:466–475 [DOI] [PubMed] [Google Scholar]
- 39.Sasakawa C., et al. 1986. Molecular alteration of the 140-megadalton plasmid associated with loss of virulence and Congo red binding activity in Shigella flexneri. Infect. Immun. 51:470–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shere K. D., Sallustio S., Manessis A., D'Aversa T. G., Goldberg M. B. 1997. Disruption of IcsP, the major Shigella protease that cleaves IcsA, accelerates actin-based motility. Mol. Microbiol. 25:451–462 [DOI] [PubMed] [Google Scholar]
- 41.Steinhauer J., Agha R., Pham T., Varga A. W., Goldberg M. B. 1999. The unipolar Shigella surface protein IcsA is targeted directly to the bacterial old pole: IcsP cleavage of IcsA occurs over the entire bacterial surface. Mol. Microbiol. 32:367–377 [DOI] [PubMed] [Google Scholar]
- 42.Tobe T., Yoshikawa M., Mizuno T., Sasakawa C. 1993. Transcriptional control of the invasion regulatory gene virB of Shigella flexneri: activation by virF and repression by H-NS. J. Bacteriol. 175:6142–6149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Troxell B., et al. 2011. Fur negatively regulates hns and is required for the expression of HilA and virulence in Salmonella enterica serovar Typhimurium. J. Bacteriol. 193:497–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wandersman C., Delepelaire P. 2004. Bacterial iron sources: from siderophores to hemophores. Annu. Rev. Microbiol. 58:611–647 [DOI] [PubMed] [Google Scholar]
- 45.Wing H. J., Goldman S. R., Ally S., Goldberg M. B. 2005. Modulation of an outer membrane protease contributes to the virulence defect of Shigella flexneri strains carrying a mutation in the virK locus. Infect. Immun. 73:1217–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wing H. J., Yan A. W., Goldman S. R., Goldberg M. B. 2004. Regulation of IcsP, the outer membrane protease of the Shigella actin tail assembly protein IcsA, by virulence plasmid regulators VirF and VirB. J. Bacteriol. 186:699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material