(n-3) Fatty Acids and Cardiovascular Health: Are Effects of EPA and DHA Shared or Complementary? (original) (raw)
Abstract
Considerable research supports cardiovascular benefits of consuming omega-3 PUFA, also known as (n-3) PUFA, from fish or fish oil. Whether individual long-chain (n-3) PUFA have shared or complementary effects is not well established. We reviewed evidence for dietary and endogenous sources and cardiovascular effects on biologic pathways, physiologic risk factors, and clinical endpoints of EPA [20:5(n-3)], docosapentaenoic acid [DPA, 22:5(n-3)], and DHA [22:6(n-3)]. DHA requires direct dietary consumption, with little synthesis from or retroconversion to DPA or EPA. Whereas EPA is also largely derived from direct consumption, EPA can also be synthesized in small amounts from plant (n-3) precursors, especially stearidonic acid. In contrast, DPA appears principally derived from endogenous elongation from EPA, and DPA can also undergo retroconversion back to EPA. In experimental and animal models, both EPA and DHA modulate several relevant biologic pathways, with evidence for some differential benefits. In humans, both fatty acids lower TG levels and, based on more limited studies, favorably affect cardiac diastolic filling, arterial compliance, and some metrics of inflammation and oxidative stress. All three (n-3) PUFA reduce ex vivo platelet aggregation and DHA also modestly increases LDL and HDL particle size; the clinical relevance of such findings is uncertain. Combined EPA+DHA or DPA+DHA levels are associated with lower risk of fatal cardiac events and DHA with lower risk of atrial fibrillation, suggesting direct or indirect benefits of DHA for cardiac arrhythmias (although not excluding similar benefits of EPA or DPA). Conversely, EPA and DPA, but not DHA, are associated with lower risk of nonfatal cardiovascular endpoints in some studies, and purified EPA reduced risk of nonfatal coronary syndromes in one large clinical trial. Overall, for many cardiovascular pathways and outcomes, identified studies of individual (n-3) PUFA were relatively limited, especially for DPA. Nonetheless, the present evidence suggests that EPA and DHA have both shared and complementary benefits. Based on current evidence, increasing consumption of either would be advantageous compared to little or no consumption. Focusing on their combined consumption remains most prudent given the potential for complementary effects and the existing more robust literature on cardiovascular benefits of their combined consumption as fish or fish oil for cardiovascular benefits.
Introduction
Evidence from multiple research paradigms, including in vitro studies, animal experiments, observational studies, and clinical trials, supports the cardiovascular benefits of long-chain (n-3) PUFA, especially for fatal CHD9 events (1). The major long-chain (n-3) PUFA are EPA [20:5(n-3)] and DHA [22:6(n-3)], principally derived from seafood consumption (Fig. 1). Circulating levels of DPA [22:5(n-3)], another long-chain (n-3) PUFA, are unrelated to dietary consumption but correlate with circulating EPA levels (2, 3), suggesting endogenous synthesis from EPA. Whereas consumption of EPA and DHA from fish or fish oil has cardiovascular benefits in observational studies and experimental trials, these two fatty acids are nearly always consumed, and their health effects investigated, as a combination or sum. Thus, relatively little is known about the potentially distinct health effects of EPA compared to DHA and even less is known about DPA.
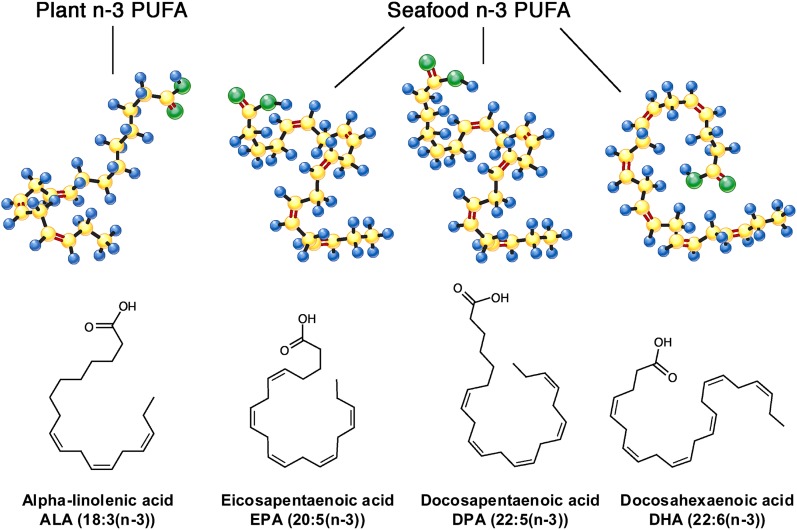
FIGURE 1.
Major (n-3) PUFA. Major (n-3) PUFA include ALA, EPA, DPA, and docosahexaenoic acid (DHA). ALA is the plant-derived (n-3) PUFA, found in certain seeds, nuts, and their oils. Evidence for its independent cardiovascular effects is still relatively limited. EPA and DHA are the major long-chain (n-3) PUFA derived from seafood consumption. DPA is another long-chain (n-3) PUFA that is contained in smaller amounts in seafood and also synthesized endogenously from EPA. The long hydrocarbon backbones, multiple double bonds, and location of the first double bond in the (n-3) position result in complex and unique 3-dimensional configurations that contribute to the singular biologic properties of these fatty acids. Figure reproduced with permission from (1). ALA, α-linolenic acid; DPA, docosapentaenoic acid.
In addition to scientific interest, whether or not these individual long-chain (n-3) PUFA have shared or complementary cardiovascular effects is of public health relevance. For example, after infancy, DHA cannot be synthesized from EPA or DPA to any appreciable extent (4–6). Thus, if DHA has particular or unique cardiovascular benefits, then its direct consumption would be essential. On the other hand, EPA can be synthesized in small amounts from the plant-derived (n-3) PUFA, ALA [18:3(n-3)] (4, 5). Whereas only a few types of seeds, nuts, and their oils contain appreciable amounts of ALA, e.g., flaxseed, canola, walnuts, butternuts, soybeans, and mustard, these plant sources are still more plentiful on a global scale than is seafood. Consequently, even the limited conversion of ALA to EPA could be quite important in certain populations, such as those with low seafood consumption, if EPA (and/or its metabolite DPA) possessed specific cardiovascular benefits independently of DHA. Scientists are also developing purified (e.g., algal-derived) preparations of EPA and DHA as well as modified plant seeds enriched with stearidonic acid [18:4(n-3)], a plant-derived (n-3) PUFA that is much more readily converted to EPA after consumption. Given the lower cost, greater availability, and potentially lower environmental impact of many plant seeds and oils compared to seafood, such sources of long-chain (n-3) PUFA could become quite important. Understanding whether cardiovascular effects of different long-chain (n-3) PUFA are shared or complementary is therefore of importance to inform guidance for both their individual and combined consumption.
We reviewed the evidence for shared or distinct cardiovascular effects of individual long-chain (n-3) PUFA, including EPA, its metabolite DPA, and DHA, including their dietary and endogenous sources and their effects on biologic pathways, physiologic risk factors, and clinical endpoints. We focused on evidence from controlled trials in humans wherever possible. We did not review in detail the cardiovascular effects of combined EPA+DHA consumption or the potential cardiovascular effects of ALA consumption, which were previously summarized (1, 7, 8).
Dietary and Endogenous Sources
The major dietary sources of EPA, DPA, and DHA are summarized in Table 1 (1, 9). Enzyme pathways also exist in humans to endogenously synthesize each of these fatty acids from their (n-3) PUFA precursors, i.e., from ALA to stearidonic acid to EPA to DPA to DHA.
TABLE 1.
Major dietary sources of long-chain (n-3) PUFA1
| EPA | DPA2 | DHA | Combined EPA+DHA | |
|---|---|---|---|---|
| mg/100 g | ||||
| Anchovy | 763 | 41 | 1292 | 2055 |
| Herring, Atlantic | 909 | 71 | 1105 | 2014 |
| Salmon, farmed | 862 | 393 | 1104 | 1966 |
| Salmon, wild | 411 | 368 | 1429 | 1840 |
| Mackerel, Atlantic | 504 | 106 | 699 | 1203 |
| Bluefish | 323 | 79 | 665 | 988 |
| Sardines, Atlantic | 473 | 0 | 509 | 982 |
| Trout | 259 | 235 | 677 | 936 |
| Golden bass (tilefish) | 172 | 143 | 733 | 905 |
| Swordfish | 127 | 168 | 772 | 899 |
| Tuna, white (albacore) | 233 | 18 | 629 | 862 |
| Mussels | 276 | 44 | 506 | 782 |
| Striped bass | 169 | 0 | 585 | 754 |
| Shark | 258 | 89 | 431 | 689 |
| Pollock, Atlantic | 91 | 28 | 451 | 542 |
| Oysters, wild | 274 | 16 | 210 | 484 |
| King mackerel | 174 | 22 | 227 | 401 |
| Tuna, light (skipjack) | 91 | 17 | 237 | 328 |
| Snapper | 48 | 22 | 273 | 321 |
| Flounder and sole | 168 | 34 | 132 | 300 |
| Clams | 138 | 104 | 146 | 284 |
| Grouper | 35 | 17 | 213 | 248 |
| Halibut | 80 | 20 | 155 | 235 |
| Lobster | 117 | 6 | 78 | 195 |
| Scallops | 72 | 5 | 104 | 176 |
| Blue crab | 101 | 9 | 67 | 168 |
| Cod, Pacific | 42 | 5 | 118 | 160 |
| Shrimp | 50 | 5 | 52 | 102 |
| Catfish, farmed | 20 | 18 | 69 | 89 |
| Eggs | 0 | 7 | 58 | 58 |
| Chicken breast | 10 | 10 | 20 | 30 |
| Beef | 2 | 4 | 1 | 3 |
| Pork | 0 | 10 | 2 | 2 |
In considering dietary compared to endogenous sources, several points are relevant. First, the endogenous synthesis of DHA from its (n-3) precursors is extremely low (4, 5), so its levels appear largely determined by direct dietary consumption. In contrast, circulating DPA levels do not correlate with fish consumption but do correlate with EPA levels (2, 3), implying that DPA levels in humans are principally derived from endogenous elongation from EPA. Consistent with this, in 3 randomized controlled trials, supplementation with 4 g/d of purified EPA, but not DHA, significantly raised (more than doubled) serum phospholipid DPA levels (10–12). DPA can also be retroconverted to EPA; in contrast, retroconversion of DHA to DPA appears quite limited (6). Interestingly, DHA supplementation modestly lowers DPA and correspondingly raises EPA levels, suggesting possible inhibition by DHA of the conversion of EPA to DPA (or stimulation of the retroconversion of DPA to EPA) (10–12).
Similar to DHA, levels of EPA are primarily determined by dietary sources. However, some limited synthesis of EPA from ALA also does occur in adults (4, 5). The rate-limiting step appears to be the conversion of ALA to stearidonic acid, because direct stearidonic acid consumption results in much more substantial conversion to EPA (13, 14). Thus, in addition to direct dietary consumption, EPA (and consequently DPA) can also be gained in small amounts from relatively high consumption of certain ALA-containing plant seeds and oils, or in more substantial amounts from consumption of modified seeds or seed oils enriched with the (n-3) PUFA stearidonic acid.
Many research studies of fish or fish oil consumption have been performed. The majority evaluated combinations of EPA and DHA and will not be reviewed herein, because these studies do not allow inference about potentially different health effects of each individual (n-3) PUFA. It should be emphasized that fish and other seafood also contain specific amino acids, vitamin D, selenium, and other minerals and elements (15–17) that could contribute to cardiovascular benefits. Nevertheless, a multitude of experimental and clinical studies with fish oil alone, or even with purified EPA or DHA, demonstrate the clear bioactivity and potency of these fatty acids (1), and thus it is appropriate to conclude that a substantial part of the cardiovascular benefits of fish consumption relate to the (n-3) PUFA content. The evidence for potential cardiovascular effects of each individual long-chain (n-3) PUFA is reviewed below.
Biologic Pathways
(n-3) PUFA play diverse roles in membrane structure and function, tissue metabolism, and genetic regulation. Experimentally demonstrated effects include modulation of cell and organelle membrane structure and function; modulation of ion channels and cellular electrophysiology; regulation of nuclear receptors and transcription factors; modulation of arachidonic acid-derived eicosanoids; and production of recently identified (n-3) PUFA-derived metabolites (1). Many such studies have evaluated EPA and DHA separately and some also evaluated DPA; the relevance of such cell culture and animal experimental effects to clinical effects of these fatty acids remains unclear. We review illustrative examples of such studies.
Membrane physiochemistry
With long hydrocarbon chains and multiple double bonds, both EPA and DHA alter lipid membrane properties. Due to its longer hydrocarbon chain length and higher degree of unsaturation, DHA may be especially effective in altering lipid membrane structure and function (18). For example, in rat aortic endothelial cells, DHA incorporation increased membrane fluidity more so than EPA (19). In cultured mouse lymphocytes, DHA but not EPA modified the distribution and size of lipid rafts, specialized lipid domains in cellular membranes with major regulatory roles on protein function and signaling events (20). In contrast, in cultured human T-cells, EPA also affected lipid raft composition and function; these latter studies did not evaluate possible differences in activity between EPA and DHA (21, 22).
We did not identify studies investigating the effects of DPA on membrane properties. Some studies have evaluated (n-6) DPA, a similar fatty acid but with the first double bond in the (n-6) position, and found it to have smaller effects on membrane fluidity and protein function compared with DHA (23, 24). Such findings, however, may have little relevance to the effects of (n-3) DPA.
Ion channel effects
In both cell culture and animal experiments, (n-3) PUFA alter the function of membrane ion channels, including Na+, L-type Ca2+, and Na+-Ca2+ exchangers (25–32). In human myocardial membranes, DHA is present at 5- to 10-fold higher concentrations than EPA and 2- to 3-fold higher concentrations than DPA (33, 34). This has led researchers to focus on DHA’s effects for protection against cardiac arrhythmias. On the other hand, relative concentrations of myocardial EPA are most responsive to dietary changes; in a human feeding study, combined consumption of 0.6 g/d EPA and 0.4 g/d DHA for 6 mo produced a >300% increase in myocardial EPA but only a 50% increase in myocardial DHA, whereas myocardial DPA levels were unaffected (33). Similar findings were seen using higher dietary doses (34). Animal experiments of dietary (n-3) PUFA and ion channel activities have not separately evaluated effects of EPA, DPA, or DHA (35–39).
In various cellular models, both EPA and DHA in their nonesterified (free) form alter membrane ion channel function (28, 32, 40, 41). We did not identify any ion channel studies evaluating effects of DPA. In these experiments, EPA and DHA possessed different potency of effects depending upon the target ion channel. For example, in isolated human atrial myocytes, DHA more strongly inhibited the ultra-rapid, delayed-rectifier K+ current (IC50 = 17.5 _μ_mol/L for EPA; 4.3 _μ_mol/L for DHA), but EPA more strongly inhibited the voltage-gated Na+ current than did DHA (IC50 = 10.8 _μ_mol/L for EPA; 41.2 _μ_mol/L for DHA) (28). Such effects might be most relevant to circulating levels of nonesterified (n-3) PUFA in humans, for which limited information exists. In one study among healthy individuals, plasma nonesterified EPA, DPA, and DHA levels were 0.4, 0.8, and 1.5 _μ_mol/L, respectively (42, 43). Following 6 wk of supplementation with 20 g/d seal oil (containing 1.3 g EPA, 0.8 g DPA, and 1.7 g DHA), these levels increased to 1.7, 1.4, and 4.7 _μ_mol/L, respectively (43).
As described above, both EPA and DHA alter membrane and lipid raft composition and function. Many ion channels localize in lipid rafts, and physical properties of membranes and lipid rafts modulate ion channel activities (44). Molecular interactions between (n-3) PUFA and ion channels may be delicate. In human cardiac cells, a single amino acid point mutation in the Na+ channel α-subunit significantly reduced the inhibitory effect of both EPA and DHA, although similar findings for oleic and stearic acids raise concerns for specificity of this experimental system (29). Amino acid substitutions in subunits of the kainate receptor, a neuronal ion channel, induced susceptibility to inhibition by DHA (45, 46); these studies did not investigate EPA.
DHA may also alter membrane protein function via close-range interactions (47, 48), potentially related to the property of DHA to efficiently pack adjacent to membrane proteins (49). In molecular simulation studies, DHA formed tight associations with rhodopsin (a prototypical G-protein-coupled membrane receptor, the primary visual light receptor) in a limited number of specific locations, which may facilitate the transition of the protein into its active form (48). Potential close-range interaction effects of EPA or DPA were not reported in these studies.
Nuclear receptors and transcription factors
Long-chain (n-3) PUFA are natural ligands of several nuclear receptors that regulate gene expression, including PPAR, hepatic nuclear factor, liver X receptors, and retinoid X receptors, and also alter expression of transcription factors such as sterol regulatory element binding-protein and carbohydrate response element binding-protein (50). Resulting effects on gene expression contribute to the physiologic effects of (n-3) PUFA, e.g., on lipid metabolism and inflammation.
Experimental evidence suggests both some shared and other differential effects of individual (n-3) PUFA on these pathways. For example, both EPA and DHA induced PPARα activity in rat primary hepatocytes but with stronger effects of EPA; DPA was largely ineffective (51). Based on conformational analyses, EPA also appeared to be a stronger activator than DHA of PPARδ (52). In contrast, acyl-CoA forms of EPA and DHA had similar binding affinity for hepatic nuclear factor-4α (53). Similarly, both EPA and DHA inhibited sterol regulatory element binding-protein 1-c gene expression (54). However, only DHA, not EPA, also reduced nuclear abundance of the protein product by increasing its targeting for degradation via a 26S proteasome-dependent pathway (54). Although the relative effect of DPA was not evaluated in most of these studies, a limited number of animal models demonstrate that, similar to EPA and DHA, DPA reduces the expression of lipogenic genes (6).
Arachidonic acid metabolites
The (n-6) PUFA arachidonic acid gives rise to several classes of eicosanoids, including 2-series PG, thromboxanes, 4-series leukotrienes, epoxyeicosatrienoic acids, and lipoxins, that possess a diverse array of both proinflammatory and cardioprotective functions (55, 56). In human studies, (n-3) PUFA consumption decreased ex vivo leukocyte synthesis of several of these eicosanoids (e.g., PG-E2, leukotriene-B4, thromboxane-B2) (57) as well as circulating concentrations of epoxyeicosatrienoic acids (58). Such effects potentially arise from long-chain (n-3) PUFA, replacing membrane arachidonic acid, modulating genes responsible for eicosanoid synthesis, and competing with arachidonic acid as substrates for metabolic enzymes (59–61).
The various (n-3) PUFA appear to share at least some of these effects but with evidence for differential specificity depending on the particular enzymatic pathway. For instance, both EPA and DHA are metabolized by cytochrome P450 2J2, the major enzyme in the heart for generation of epoxyeicosatrienoic acids from arachidonic acid, to analogous (n-3) fatty acid metabolites with conversion rates that are 9-fold and 2-fold higher, respectively, than for arachidonic acid (62). EPA, but not DHA, can also act as a substrate for cyclooxygenase-1 and 5-lipoxygenase to generate 3-series PG and 5-series leukotrienes that in some models have attenuated proinflammatory properties compared to their arachidonate-derived counterparts (55).
DPA is also metabolized by lipoxygenases (63, 64), and, in human platelets, DPA reduced the synthesis of thromboxane-B2 from arachidonic acid (64). In animal and experimental models, DPA inhibited ex vivo collagen-stimulated platelet aggregation, thromboxane production, and cyclooxygenase-1 activity, with more potent dose-response effects than either EPA or DHA (6).
Evidence of effects of individual (n-3) PUFA on production of potentially cardioprotective arachidonate-derived eicosanoids, such as epoxyeicosatrienoic acids and lipoxins, is at a very early stage. One recent study in ovariectomized rats reported that after receiving EPA or DHA ethyl ester supplementation for 4 mo, EPA completely blocked, whereas DHA only reduced, bone marrow levels of lipoxins (65).
Newly identified (n-3) PUFA metabolites
In addition to PG and leukotrienes, (n-3) PUFA are precursors to SPM, a recently identified class of lipid molecules that are not only antiinflammatory but also promote active resolution of inflammatory events (66). Both EPA and DHA give rise to resolvins, one class of SPM that protects against prolonged inflammation and tissue injury in several animal models, including peritonitis and ischemia-reperfusion (66). However, only DHA is metabolized to other classes of SPM, such as neuroprotectin-D1 and maresins, that could confer unique inflammation-resolving properties of DHA (67, 68). In animal models, neuroprotectin-D1 promotes neuronal cell survival and may contribute to protective effects of DHA against ischemic stroke (67, 69). Effects of neuroprotectin-D1 and maresins in cardiac models of injury have not yet been reported. Novel cyclooxygenase-2 and dehydrogenase-synthesized electrophilic oxo-derivatives of DHA and DPA were also recently identified that exhibited genetic modulatory and antiinflammatory effects in cultured murine macrophages (70).
EPA and DHA are also metabolized by cytochrome-P450 enzymes to generate MEFA, which have potent beneficial properties in experimental and animal studies (71). For instance, in rat ventricular myocytes, MEFA derived from either EPA or DHA possessed nearly 1000-fold greater potency than the parent (n-3) PUFA for reducing the spontaneous beating rate induced by Ca2+ overload (71). In human bronchi, an EPA-derived MEFA displayed potent antiinflammatory activity, an effect related to PPARγ activation (72). MEFA derived from EPA and DHA also robustly activated calcium-activated K+ channels, mediating porcine coronary and mesenteric microvessel dilation (73, 74). MEFA derived from either EPA or DHA were similarly potent, with <10 pmol/L concentration achieving 50% dilation of the microvessels (73, 74).
Physiologic Risk Factors
The combined consumption of EPA+DHA is known to favorably affect a wide range of risk factors, including lowering TG levels, improving vascular and cardiac hemodynamics and function, and possibly improving endothelial function (1). Long-chain (n-3) PUFA also have antiinflammatory and antithrombotic effects, although it remains unclear whether such effects are clinically meaningful at usual dietary or low-dose supplement doses (e.g., <2 g/d) (1).
The individual effects of EPA and DHA on cardiovascular risk factors were previously reviewed by Mori and Woodman (75), largely based on research published before 2000. We took advantage of the studies in that report as a starting point and reviewed that evidence together with additional research published since 2000 for individual effects of EPA and DHA (and DPA, when possible) on several key physiologic risk factors.
Lipids
Five randomized controlled trials published before 2003 (75) and several more recent trials (76–81) evaluated the effects of EPA and/or DHA alone on blood lipids. Doses ranged from ~1.5 to 5 g/d (typically 3–4 g/d) and durations of treatment from 4 to 20 wk (typically 6–8 wk). These trials were generally small; the largest two comprised 234 and 93 subjects and all others were fewer than 75 subjects each. Both EPA and DHA reduced TG levels in these trials, with larger effects of DHA. For example, in three trials among 224 healthy men, 74 healthy men and women, and 56 overweight hyperlipidemic men, DHA reduced TG levels by 18, 31, and 33%, respectively, and EPA reduced TG levels by 12, 16, and 21%, respectively, compared with baseline (P < 0.05 each) (10, 11, 79). Effects on other lipid fractions and apolipoproteins in these trials were less consistent and generally small. For instance, in two trials, DHA slightly raised HDL-cholesterol and/or apoA-1 levels compared with EPA; neither (n-3) PUFA significantly affected LDL-cholesterol and/or ApoB (10, 79). Conversely, in others trials, DHA raised LDL-cholesterol concentrations, primarily driven by an increase in particle size rather than number (11, 77, 78, 81). Additionally, although total HDL-cholesterol concentrations were not appreciably altered in most trials, subfractions were affected when evaluated in some trials, with DHA raising HDL2-cholesterol and DHA and/or EPA lowering HDL3-cholesterol (11, 78, 81). Interestingly, in multivariable-adjusted cross-sectional analyses evaluating habitual dietary consumption among nearly 3000 older adults, only plasma phospholipid EPA levels were positively associated with HDL-cholesterol; DPA levels were inversely associated and DHA levels were unassociated (2).
Only one large, long-term trial of an individual (n-3) PUFA has been reported. In this trial, 16,397 Japanese patients with hypercholesterolemia were randomized to receive statin therapy alone vs. statin therapy plus EPA 1.8 g/d (open-label; i.e., no EPA placebo), for an average of 4.6 y (82). TG levels decreased by 9% in the statin+EPA group compared with 4% in the statin-only group (between-group P < 0.0001). Changes in total cholesterol, LDL-cholesterol, and HDL-cholesterol were similar between the two groups.
Overall, the evidence demonstrate that both EPA and DHA lower TG, with larger effects seen with DHA. Based on a limited number of trials, DHA also increases LDL particle size and concentrations of large HDL2-cholesterol. The clinical relevance of such modest and not always consistent differential effects of EPA compared to DHA on LDL and/or HDL is unclear. At the least, such differences indicate at least partly differing mechanistic pathways of these fatty acids on lipid metabolism.
Vascular and cardiac hemodynamics
The combined consumption of EPA+DHA is known to lower resting heart rate and BP (1). In multivariable-adjusted cross-sectional analyses among nearly 3000 older adults, plasma phospholipid EPA was not significantly associated with resting heart rate or BP, DPA was associated with a nonsignificant trend toward lower resting heart rate, and DHA was inversely associated with resting heart rate and a trend toward lower BP (2). Controlled trials are consistent with these observational findings of habitual dietary intake. In several trials, DHA lowered resting heart rate and/or BP (78, 83–86). In contrast, in five short-term controlled trials, EPA did not significantly alter resting BP levels (75) nor, in one trial, 24-h ambulatory BP or heart rate (83). In contrast, DHA reduced 24-h ambulatory BP and heart rate (83). In another small trial (n = 59), mean 24-h ambulatory heart rate was not significantly affected by either EPA or DHA (4 g/d each), but statistical power may have been limited (87). In a larger trial among 224 healthy men, supplementation with either DHA or EPA (4 g/d each) produced similar reductions (~2 bpm) in resting heart rate (85). However, when achieved circulating (n-3) PUFA levels were assessed in this trial, only changes in plasma phospholipid DHA, but not EPA, were inversely associated with changes in heart rate (85). In a meta-analysis regression model based on 31 controlled trials, both DHA and EPA were estimated to reduce BP (e.g., per 1 g/d, 1.5- and 0.9-mm Hg reductions in systolic BP, respectively) (88). However, all these trials used combined EPA and DHA, so the regression model would be unlikely to adequately separate their individual effects. Overall, it is clear that DHA lowers both BP and heart rate. EPA appears unlikely to have significant BP-lowering effects, and evidence for effects of EPA on heart rate are equivocal and based on few trials.
Combined EPA and DHA consumption improves cardiac diastolic filling (1). In a 7-wk controlled trial, supplementation with either DHA or EPA (4 g/d each) led to similar improvements in metrics of early left ventricular filling (P = 0.06 and 0.08) (85). In a trial of 38 older overweight adults, both EPA and DHA (3 g/d each for 7 wk) similarly improved systemic arterial compliance, as assessed by aortic flow and peripheral pressures (89).
Endothelial function
In a recent systematic review, 33 trials were identified that tested the effects of (n-3) PUFA on fasting or postprandial endothelial function (90). In some trials, fish or fish oil consumption improved various functional measures of endothelial function or NO-related metrics (e.g., endothelial nitric oxide synthase gene expression) and in some trials, especially those among older individuals, circulating biomarkers of endothelial dysfunction were also reduced. However, the overall evidence for effects of (n-3) PUFA on endothelial function was inconsistent, with evidence limited by mixed doses, durations, populations treated, and outcomes evaluated. Only 4 of these studies (80, 84, 91, 92) as well as two additional relevant trials we identified (93, 94) separately tested the effects of EPA and/or DHA.
Four of these studies evaluated circulating biomarkers of endothelial dysfunction; none showed effects of either EPA or DHA alone. Among 93 generally healthy men, different doses of EPA (1.35, 2.7, and 4.05 g/d for 12 wk each) had no effects on soluble E-selectin, VCAM-1, or ICAM-1; in subgroup analyses, the highest EPA dose actually increased soluble E-selectin in younger men (80). Among 34 patients with hyperlipidemia, DHA (3 g/d for 13 wk) did not affect levels of soluble E-Selectin, VCAM-1, or ICAM-1 (93). Similarly, neither EPA nor DHA (3 g/d for 3 wk each) altered concentrations of endothelin-1 or soluble E-selectin, VCAM-1, or ICAM-1 among 48 generally healthy young adults (91). In a trial among 46 healthy older participants, DHA (0.7 g/d for 12 wk) did not affect levels of soluble E-selectin, VCAM-1, or ICAM-1; interestingly, fish oil (combined 0.7 g/d EPA + 0.3 g/d DHA) did reduce both soluble E-selectin and VCAM-1 (92). In a cross-sectional observational analysis among ~280 women, total plasma EPA, DPA, and DHA were each associated with lower levels of soluble E-selectin, but not ICAM-1 or VCAM-1 (3).
Two trials evaluated functional metrics of endothelial function. Among patients with variant angina, EPA 1.8 g/d for 4 mo reduced acetylcholine-stimulated coronary vasospasm in normal but not spastic coronary segments; DHA was not evaluated (94). Several limitations make it difficult to interpret these findings, including the open-label design (no blinding or placebo), small size (n = 22), and observed benefits only in certain subtypes of arteries. Among 40 men with hyperlipidemia, DHA but not EPA (~4 g/d for 6 wk each) improved acetylcholine-stimulated forearm blood flow, enhanced arterial vasodilatory responses to sodium nitroprusside, and attenuated norepinephrine-stimulated vasoconstriction (84).
Overall, the limited and mixed evidence precludes strong conclusions about the effects of EPA or DHA on endothelial function. Fish or fish oil consumption may have some benefits, but further investigation is needed to confirm such effects.
Inflammation and oxidative stress
Biologic effects of (n-3) PUFA could alter several inflammatory and oxidative stress pathways (see above). In multivariable-adjusted cross-sectional analyses among nearly 3000 older adults, plasma phospholipid EPA and DPA, but not DHA, were each inversely associated with C-reactive protein and fibrinogen levels (2). In multivariable-adjusted cross-sectional analyses among ~280 women, total plasma DPA, but not EPA or DHA, was robustly associated with lower levels of IL-6; neither EPA, DPA, nor DHA were associated with C-reactive protein levels (3).
Among 34 patients with hyperlipidemia, DHA (3 g/d for 13 wk) reduced levels of IL-6 (−23%) and C-reactive protein (−15%) and increased matrix metalloproteinase-2 levels (+7%) (93). No effects were seen on plasma levels of other cytokines, including IL-1b, IL-2, IL-8, IL-10, or TNFα (93). Among 51 patients with type 2 diabetes, EPA and DHA (4 g/d for 6 wk each) each produced no significant changes in IL-6 or C-reactive protein, nonsignificant trends toward reduction of TNFα, and significant 20% reductions in urinary F2-isoprostanes, a biomarker of oxidative stress (12). Similarly, in another trial performed by these researchers among 56 participants with hyperlipidemia, EPA and DHA (4 g/d for 6 wk each) each produced ~27% reductions in urinary F2-isoprostanes (95). In contrast, an uncontrolled intervention among 12 healthy adults demonstrated increased urinary F2-isoprostanes with DHA supplementation (1.6 g/d) (96).
In sum, both EPA and DHA appear to have at least some beneficial effects on inflammation and oxidative stress, but few well-designed studies in humans have assessed their potentially separate effects.
Thrombosis and coagulation
In a prior review, several uncontrolled studies were identified in which EPA supplementation reduced ex-vivo platelet aggregation in response to collagen (75). Similar effects were seen with DHA in an uncontrolled intervention among 12 healthy adults, with dose-dependent effects up to 1.6 g/d (96). In another uncontrolled study using blood drawn from healthy adults, EPA, DPA, and DHA (1.0 mmol/L each) were each equally effective in reducing in vitro platelet aggregation in women, but DPA and DHA were less effective in men (97).
Few controlled trials have been performed. One trial (n = 30) found that a single consumed dose of either EPA or DHA influenced ex vivo platelet aggregation over 24 h but with potential interaction by both time and gender (98). In contrast, among 51 diabetic participants, DHA, but not EPA (4 g/d for 6 wk each), reduced ex vivo collagen-stimulated platelet aggregation and, consistent with this, platelet-derived thromboxane-B2 levels (99). Neither EPA nor DHA produced significant changes in platelet aggregation in response to platelet activating factor (which is only partly thromboxane dependent) nor changes in several fibrinolytic markers (e.g., plasminogen activator inhibitor-1 antigen, tissue plasminogen activator antigen, etc.) (99). Several other controlled trials demonstrated a similar lack of effects of either EPA or DHA on various markers of fibrinolytic function (75).
Clinical Endpoints
In observational studies and randomized trials of clinical endpoints, benefits of fish or fish oil consumption are most consistent and robust for fatal CHD events (1), suggesting pathways of benefits related to risk of fatal cardiac arrhythmias. Nearly all of these studies have evaluated the combination of EPA and DHA (plus, implicitly, the small amounts of DPA found in fish or fish oil or synthesized from EPA). Several observational biomarker studies have shown combined EPA+DHA or DPA+DHA levels to be inversely associated with risk of sudden death or fatal CHD (100–103). Although these studies did not separately evaluate individual (n-3) PUFA, the concentrations of circulating DHA are typically several fold higher than EPA or DPA, suggesting that such summed associations were driven by DHA levels. Given that DHA is also present in much higher concentrations in myocardial membranes compared with EPA or DPA (33, 34), such findings could represent a specific cardiac benefit of DHA for cardiac arrhythmias. Consistent with this, in a prospective evaluation of atrial arrhythmias that evaluated individual (n-3) PUFA, plasma DHA, but not EPA or DPA, levels were significantly associated with lower risk of incident atrial fibrillation (104). Together, these findings suggest that DHA may be more closely linked to lower risk of cardiac arrhythmias than EPA or DPA. On the other hand, both dietary sources and circulating and tissue levels of EPA and DHA are positively correlated, so apparent specificity for DHA could simply be an artifact of its higher biomarker levels, and similar effects of EPA or DPA on clinical arrhythmias cannot be excluded.
A limited number of biomarker studies have evaluated how individual (n-3) PUFA levels relate to nonfatal clinical endpoints. In small, retrospective, case-control studies of nonfatal MI, biomarker levels of EPA, DPA, and DHA were each lower in cases than in controls (105, 106). In another small, retrospective, case-control study, only higher serum EPA and DPA, but not DHA, were associated with lower risk of nonfatal MI (107). In a large retrospective case-control study, adipose DHA was not associated with nonfatal MI; EPA was not evaluated (108). These retrospective studies were often limited by only modest control for potential confounding covariates and, more importantly, by possible control selection bias given their large retrospective designs.
In a small prospective study of incident CHD, both serum DPA and DHA were associated with lower risk; serum EPA was associated with a nonsignificant trend toward lower risk (109). However, there were few total events (n = 94 cases), fatal compared to nonfatal events were not separately evaluated, and minimal adjustments were made for potential confounding factors. In two larger prospective observational studies, higher circulating concentrations of both EPA and DPA were associated with lower multivariable-adjusted risk of nonfatal cardiovascular events, including MI (3) and incident congestive heart failure (2), after adjustment for other risk factors. Circulating DHA levels were not significantly associated with these nonfatal outcomes in these large prospective studies (2, 3).
In a large, open-label, randomized controlled trial among Japanese patients with elevated cholesterol levels, purified EPA at a dose of 1.8 g/d for ~5 y reduced the risk of nonfatal coronary syndromes [RR = 0.81 (95% CI = 0.68–0.96)], including similar lower risk or trends toward lower risk of unstable angina, nonfatal MI, and coronary revascularization (82). Effects were not seen on fatal CHD, but background rates of CHD mortality were extremely low in this trial, likely resulting from high population consumption in Japan of dietary (n-3) PUFA from seafood (110). The open-label design (neither patients nor their doctors were blinded) could have biased the results, leading to overestimation of benefits for nonfatal coronary events. Nonetheless, the findings from this large, long-term trial support the results of observational studies evaluating biomarker EPA levels and nonfatal coronary events.
In sum, these findings suggest that EPA (and its metabolite DPA) reduce the risk of nonfatal cardiovascular outcomes. In biomarker studies, DHA levels appear less strongly related to such nonfatal endpoints. Randomized controlled trials of purified DPA or DHA and clinical cardiovascular events have not been reported.
Dietary Guidelines
A variety of governmental, scientific, and international organizations have established target or minimum consumption levels of fish and (n-3) PUFA (Table 2) (111–125). For long-chain (n-3) PUFA, most guidelines are for combined EPA+DHA and are based on primary prevention of cardiovascular disease, in particular CHD mortality, in the general population. Generally, these guidelines converge on a recommended minimum consumption of 250–500 mg/d of combined EPA+DHA. Average intakes in most countries, including in the US (126), are much lower. Most guidelines do not make separate specific recommendations about EPA compared to DHA consumption in the general population, and none make recommendations about DPA consumption.
TABLE 2.
National and international guidelines for consumption of (n-3) PUFA in the general population1
| Recommendations | ||||
|---|---|---|---|---|
| Source | Year | EPA+DHA | ALA | Total (n-3) PUFA |
| International Society for the Study of Fatty Acids and Lipids Workshop (111) | 1999 | Target: 650 mg/d Adequate: ≥220 mg/d EPA Adequate: ≥220 mg/d DHA | Target: 2.2 g/d | Target: 1.3% energy |
| European Commission Eurodiet Core Report (112) | 2000 | Target: 200 mg/d | Target: 2 g/d | |
| Health Council of Netherlands (113) | 2001 | Adequate: 200 mg/d | Adequate: 1% energy | |
| US National Academy of Sciences (114) | 2002 | AMDR: 0.06–0.12% energy | AMDR2: 0.6–1.2% energy | |
| French Agency for Food Environmental and Occupational Health Safety Omega-3 Report (115) | 2003 | Target: 400–500 mg/d, including AA; 100–120 mg/d DHA | Target: 1.6–2 g/d | Target: 1% energy |
| European Society of Cardiology (116) | 2003 | Recommendation2: ~1 g/d | ||
| Joint United Nations FAO/WHO Expert Consultation. Diet, Nutrition and the Prevention of Chronic Diseases (117) | 2003 | Target: 400–1000 mg/d (1–2 fish servings/wk) | Target: 1–2% energy | |
| International Society for the Study of Fatty Acids and Lipids Policy Statement 3 (118) | 2004 | Minimum: 500 mg/d | Target: 0.7% energy | |
| United Kingdom Scientific Advisory Committee on Nutrition (119) | 2004 | Minimum: 450 mg/d (≥2 fish servings/wk) | ||
| AHA (120–122) | 2002, 2006, 2010 | Minimum: Two 100-g fish servings/wk, especially oily fish ~1 g/d2 | ||
| National Health and Medical Research Council (Australia and New Zealand) (123) | 2006 | Adequate: 90–160 mg/d Target: 430–610 mg/d | Adequate: 0.8–1.3 g/d Target: 2.7 g/d | |
| United Nations FAO Report on Fats and Fatty Acids in Human Nutrition (124) | 2008 | AMDR3: 250–2000 mg/d | Minimum: 0.5% energy | AMDR: 0.5–2% energy |
| USDA, 2010 Dietary Guidelines for Americans (125) | 2010 | Minimum: Two 113-g fish servings/wk, providing an average of ≥250 mg/d | 0.6–1.2% energy |
Conclusion
In conclusion, whereas the types and numbers of studies for many outcomes are limited, the current evidence indicates that EPA and DHA have some collective but other potentially complementary cardiovascular benefits (Table 3). In experimental studies and animal models, both fatty acids modulate a variety of relevant biologic pathways, with several lines of evidence suggesting at least some differential benefits. In human studies, both fatty acids lower TG levels and collagen-stimulated platelet aggregation and, based on limited numbers of studies, favorably affect cardiac diastolic filling, arterial compliance, and at least some metrics of inflammation and oxidative stress. DHA also modestly increases the proportions of larger LDL and HDL cholesterol particles, although clinical relevance of these findings is unclear. Less is known about the effects of DPA on biologic pathways or physiologic risk factors; a few observational studies suggest potential benefits for inflammation.
TABLE 3.
Evidence for cardiovascular effects of individual long-chain (n-3) PUFA in human studies1
| EPA | DHA | DPA | |
|---|---|---|---|
| Physiologic risk factors | |||
| Lipids | ↓ TG levels | ↓ TG levels | — |
| ↓ HDL3 cholesterol2 | ↑ LDL particle size | ||
| ↑ HDL2 cholesterol | |||
| Vascular and cardiac hemodynamics | Minimal BP effects | ↓ BP | — |
| ? Heart rate effects | ↓ Heart rate | ||
| ↑ Cardiac diastolic filling2 | ↑ Cardiac diastolic filling2 | ||
| ↑ Arterial compliance2 | ↑ Arterial compliance2 | ||
| Endothelial function | No clear effects2 | No clear effects2 | — |
| Inflammation and oxidative stress | ↓ Inflammation, mixed results2 | ↓ Inflammation, mixed results2 | ↓ Inflammation, mixed results23 |
| ↓ Oxidative stress, mixed results2 | ↓ Oxidative stress, mixed results2 | ||
| Thrombosis and coagulation | ↓ Collagen-stimulated platelet aggregation2 | ↓ Collagen-stimulated platelet aggregation2 | ↓ Collagen-stimulated platelet aggregation2 |
| Otherwise minimal effects on thrombosis or coagulation | Otherwise minimal effects on thrombosis or coagulation | ||
| Clinical endpoints | |||
| Fatal CHD and sudden death | — | ↓ Risk4 | —4 |
| Atrial fibrillation | No clear effects23 | ↓ Risk23 | — |
| Nonfatal coronary syndromes | ↓ Risk | No clear effects23 | ↓ Risk23 |
| Congestive heart failure | ↓ Risk23 | No clear effects23 | ↓ Risk23 |
Combined EPA+DHA or DPA+DHA biomarker levels are consistently associated with lower risk of fatal cardiac events, and DHA biomarker levels with lower risk of atrial fibrillation in one report, suggesting direct or indirect benefits of DHA for cardiac arrhythmias (while not excluding similar benefits of EPA or DPA). In contrast, EPA and DPA but not DHA are linked to lower risk of nonfatal cardiovascular endpoints in limited numbers of observational studies, and purified EPA lowered risk of nonfatal coronary syndromes in one large clinical trial.
Clearly, additional experimental, observational, and clinical studies are needed to elucidate the shared and complementary effects of these individual long-chain (n-3) PUFA. Available data are insufficient to make specific quantitative recommendations about their individual intakes or any ratio of their intakes for preventing cardiovascular disease. Nonetheless, the evidence suggests that EPA and DHA each independently provide at least some cardiovascular benefits. DPA may also have some positive effects, but its circulating levels appear to depend on endogenous conversion from EPA, and effects of direct DPA consumption are unknown. Based on these findings, increasing the consumption of either EPA or DHA would be advantageous compared to little or no consumption of either. Focusing on their combined consumption would be most prudent, given the evidence for at least some complementary rather than shared effects and, more importantly, given the much larger body of literature demonstrating experimental, physiologic, and clinical benefits of their combined consumption as fish or fish oil.
Acknowledgments
Dr. Wu thanks the National Heart Foundation of Australia for postdoctoral fellowship support. D.M. and J.H.Y.W. designed and conducted the research, interpreted data, and wrote the manuscript. Both authors have read and approved the final manuscript.
Footnotes
2
Supported by the National Heart, Lung, and Blood Institute, NIH (RC2-HL-101816). Dr. Wu was supported by the National Heart Foundation of Australia.
3
Author disclosures: D. Mozaffarian reports receiving research grants from GlaxoSmithKline, Sigma Tau, Pronova, and the NIH for an investigator-initiated, not-for-profit clinical trial of fish oil; travel reimbursement, honoraria, or consulting fees from Aramark, Unilever, SPRIM, Nutrition Impact, Bunge, and FoodMinds for topics related to diet and cardiovascular health; and royalties from UpToDate for an online chapter on fish oil. D. Mozaffarian received travel funds and an honorarium from the American Society for Nutrition for giving a presentation and writing a paper for this symposium. J. H. Y. Wu, no conflicts of interest.
9
Abbreviations used: ALA, α-linolenic acid; BP, blood pressure; CHD, coronary heart disease; DPA, docosapentaenoic acid; ICAM, inter-cellular adhesion molecule; MEFA, mono-epoxides derived from (n-3) fatty acids; MI, myocardial infarction; SPM, specialized pro-resolving mediator; VCAM, vascular cell adhesion molecule.
Literature Cited
- 1.Mozaffarian D, Wu JHY. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;57: 1697–9 [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Lemaitre RN, King IB, Song X, Spiegelman D, Sacks FM, Rimm EB, Siscovick DS. Circulating long-chain omega-3 fatty acids and incidence of congestive heart failure in older adults: the cardiovascular health study: a cohort study. Ann Intern Med. 2011;155:160–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun Q, Ma J, Campos H, Rexrode KM, Albert CM, Mozaffarian D, Hu FB. Blood concentrations of individual long-chain n-3 fatty acids and risk of nonfatal myocardial infarction. Am J Clin Nutr. 2008;88:216–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burdge G. Alpha-linolenic acid metabolism in men and women: nutritional and biological implications. Curr Opin Clin Nutr Metab Care. 2004;7:137–44 [DOI] [PubMed] [Google Scholar]
- 5.Pawlosky RJ, Hibbeln JR, Novotny JA, Salem N., Jr Physiological compartmental analysis of alpha-linolenic acid metabolism in adult humans. J Lipid Res. 2001;42:1257–65 [PubMed] [Google Scholar]
- 6.Kaur G, Cameron-Smith D, Garg M, Sinclair AJ. Docosapentaenoic acid (22:5n-3): a review of its biological effects. Prog Lipid Res. 2011;50:28–34 [DOI] [PubMed] [Google Scholar]
- 7.Mozaffarian D. Does alpha-linolenic acid intake reduce the risk of coronary heart disease? A review of the evidence. Altern Ther Health Med. 2005;11:24–30; quiz 31, 79. [PubMed] [Google Scholar]
- 8.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–99 [DOI] [PubMed] [Google Scholar]
- 9.Artinian NT, Fletcher GF, Mozaffarian D, Kris-Etherton P, Van Horn L, Lichtenstein AH, Kumanyika S, Kraus WE, Fleg JL, Redeker NS, et al. Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:406–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimsgaard S, Bonaa KH, Hansen JB, Nordoy A. Highly purified eicosapentaenoic acid and docosahexaenoic acid in humans have similar triacylglycerol-lowering effects but divergent effects on serum fatty acids. Am J Clin Nutr. 1997;66:649–59 [DOI] [PubMed] [Google Scholar]
- 11.Mori TA, Burke V, Puddey IB, Watts GF, O'Neal DN, Best JD, Beilin LJ. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am J Clin Nutr. 2000;71:1085–94 [DOI] [PubMed] [Google Scholar]
- 12.Mori TA, Woodman RJ, Burke V, Puddey IB, Croft KD, Beilin LJ. Effect of eicosapentaenoic acid and docosahexaenoic acid on oxidative stress and inflammatory markers in treated-hypertensive type 2 diabetic subjects. Free Radic Biol Med. 2003;35:772–81 [DOI] [PubMed] [Google Scholar]
- 13.James MJ, Ursin VM, Cleland LG. Metabolism of stearidonic acid in human subjects: comparison with the metabolism of other n-3 fatty acids. Am J Clin Nutr. 2003;77:1140–5 [DOI] [PubMed] [Google Scholar]
- 14.Lemke SL, Vicini JL, Su H, Goldstein DA, Nemeth MA, Krul ES, Harris WS. Dietary intake of stearidonic acid-enriched soybean oil increases the omega-3 index: randomized, double-blind clinical study of efficacy and safety. Am J Clin Nutr. 2010;92:766–75 [DOI] [PubMed] [Google Scholar]
- 15.Roos N, Wahab MA, Chamnan C, Thilsted SH. The role of fish in food-based strategies to combat vitamin A and mineral deficiencies in developing countries. J Nutr. 2007;137:1106–9 [DOI] [PubMed] [Google Scholar]
- 16.Fox TE, Van den Heuvel EG, Atherton CA, Dainty JR, Lewis DJ, Langford NJ, Crews HM, Luten JB, Lorentzen M, Sieling FW, et al. Bioavailability of selenium from fish, yeast and selenate: a comparative study in humans using stable isotopes. Eur J Clin Nutr. 2004;58:343–49 [DOI] [PubMed] [Google Scholar]
- 17.Holden JM, Lemar LE, Exler J. Vitamin D in foods: development of the US Department of Agriculture database. Am J Clin Nutr. 2008;87:1092S–6S [DOI] [PubMed] [Google Scholar]
- 18.Wassall SR, Stillwell W. Docosahexaenoic acid domains: the ultimate non-raft membrane domain. Chem Phys Lipids. 2008;153:57–63 [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto M, Hossain S, Yamasaki H, Yazawa K, Masumura S. Effects of eicosapentaenoic acid and docosahexaenoic acid on plasma membrane fluidity of aortic endothelial cells. Lipids. 1999;34:1297–304 [DOI] [PubMed] [Google Scholar]
- 20.Shaikh SR, Rockett BD, Salameh M, Carraway K. Docosahexaenoic acid modifies the clustering and size of lipid rafts and the lateral organization and surface expression of MHC class I of EL4 cells. J Nutr. 2009;139:1632–9 [DOI] [PubMed] [Google Scholar]
- 21.Geyeregger R, Zeyda M, Zlabinger GJ, Waldhausl W, Stulnig TM. Polyunsaturated fatty acids interfere with formation of the immunological synapse. J Leukoc Biol. 2005;77:680–8 [DOI] [PubMed] [Google Scholar]
- 22.Stulnig TM, Huber J, Leitinger N, Imre EM, Angelisova P, Nowotny P, Waldhausl W. Polyunsaturated eicosapentaenoic acid displaces proteins from membrane rafts by altering raft lipid composition. J Biol Chem. 2001;276:37335–40 [DOI] [PubMed] [Google Scholar]
- 23.Eldho NV, Feller SE, Tristram-Nagle S, Polozov IV, Gawrisch K. Polyunsaturated docosahexaenoic vs docosapentaenoic acid-differences in lipid matrix properties from the loss of one double bond. J Am Chem Soc. 2003;125:6409–21 [DOI] [PubMed] [Google Scholar]
- 24.Niu SL, Mitchell DC, Lim SY, Wen ZM, Kim HY, Salem N, Jr, Litman BJ. Reduced G protein-coupled signaling efficiency in retinal rod outer segments in response to n-3 fatty acid deficiency. J Biol Chem. 2004;279:31098–104 [DOI] [PubMed] [Google Scholar]
- 25.Xiao YF, Ma L, Wang SY, Josephson ME, Wang GK, Morgan JP, Leaf A. Potent block of inactivation-deficient Na+ channels by n-3 polyunsaturated fatty acids. Am J Physiol Cell Physiol. 2006;290:C362–70 [DOI] [PubMed] [Google Scholar]
- 26.Ander BP, Hurtado C, Raposo CS, Maddaford TG, Deniset JF, Hryshko LV, Pierce GN, Lukas A. Differential sensitivities of the NCX1.1 and NCX1.3 isoforms of the Na+-Ca2+ exchanger to alpha-linolenic acid. Cardiovasc Res. 2007;73:395–403 [DOI] [PubMed] [Google Scholar]
- 27.Ferrier GR, Redondo I, Zhu J, Murphy MG. Differential effects of docosahexaenoic acid on contractions and L-type Ca2+ current in adult cardiac myocytes. Cardiovasc Res. 2002;54:601–10 [DOI] [PubMed] [Google Scholar]
- 28.Li GR, Sun HY, Zhang XH, Cheng LC, Chiu SW, Tse HF, Lau CP. Omega-3 polyunsaturated fatty acids inhibit transient outward and ultra-rapid delayed rectifier K+currents and Na+current in human atrial myocytes. Cardiovasc Res. 2009;81:286–93 [DOI] [PubMed] [Google Scholar]
- 29.Xiao YF, Ke Q, Wang SY, Auktor K, Yang Y, Wang GK, Morgan JP, Leaf A. Single point mutations affect fatty acid block of human myocardial sodium channel alpha subunit Na+ channels. Proc Natl Acad Sci USA. 2001;98:3606–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao YF, Ke Q, Chen Y, Morgan JP, Leaf A. Inhibitory effect of n-3 fish oil fatty acids on cardiac Na+/Ca2+ exchange currents in HEK293t cells. Biochem Biophys Res Commun. 2004;321:116–23 [DOI] [PubMed] [Google Scholar]
- 31.Dujardin KS, Dumotier B, David M, Guizy M, Valenzuela C, Hondeghem LM. Ultrafast sodium channel block by dietary fish oil prevents dofetilide-induced ventricular arrhythmias in rabbit hearts. Am J Physiol Heart Circ Physiol. 2008;295:H1414–21 [DOI] [PubMed] [Google Scholar]
- 32.Leifert WR, McMurchie EJ, Saint DA. Inhibition of cardiac sodium currents in adult rat myocytes by n-3 polyunsaturated fatty acids. J Physiol. 1999;520:671–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris WS, Sands SA, Windsor SL, Ali HA, Stevens TL, Magalski A, Porter CB, Borkon AM. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients: correlation with erythrocytes and response to supplementation. Circulation. 2004;110:1645–9 [DOI] [PubMed] [Google Scholar]
- 34.Metcalf RG, James MJ, Gibson RA, Edwards JR, Stubberfield J, Stuklis R, Roberts-Thomson K, Young GD, Cleland LG. Effects of fish-oil supplementation on myocardial fatty acids in humans. Am J Clin Nutr. 2007;85:1222–8 [DOI] [PubMed] [Google Scholar]
- 35.Coronel R, Wilms-Schopman FJ, Den Ruijter HM, Belterman CN, Schumacher CA, Opthof T, Hovenier R, Lemmens AG, Terpstra AH, Katan MB, et al. Dietary n-3 fatty acids promote arrhythmias during acute regional myocardial ischemia in isolated pig hearts. Cardiovasc Res. 2007;73:386–94 [DOI] [PubMed] [Google Scholar]
- 36.Verkerk AO, van Ginneken AC, Berecki G, den Ruijter HM, Schumacher CA, Veldkamp MW, Baartscheer A, Casini S, Opthof T, Hovenier R, et al. Incorporated sarcolemmal fish oil fatty acids shorten pig ventricular action potentials. Cardiovasc Res. 2006;70:509–20 [DOI] [PubMed] [Google Scholar]
- 37.Leifert WR, Jahangiri A, Saint DA, McMurchie EJ. Effects of dietary n-3 fatty acids on contractility, Na+ and K+ currents in a rat cardiomyocyte model of arrhythmia. J Nutr Biochem. 2000;11:382–92 [DOI] [PubMed] [Google Scholar]
- 38.Berecki G, Den Ruijter HM, Verkerk AO, Schumacher CA, Baartscheer A, Bakker D, Boukens BJ, van Ginneken AC, Fiolet JW, Opthof T, et al. Dietary fish oil reduces the incidence of triggered arrhythmias in pig ventricular myocytes. Heart Rhythm. 2007;4:1452–60 [DOI] [PubMed] [Google Scholar]
- 39.Billman GE, Nishijima Y, Belevych AE, Terentyev D, Xu Y, Haizlip KM, Monasky MM, Hiranandani N, Harris WS, Gyorke S, et al. Effects of dietary omega-3 fatty acids on ventricular function in dogs with healed myocardial infarctions: in vivo and in vitro studies. Am J Physiol Heart Circ Physiol. 2010;298:H1219–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Honen BN, Saint DA, Laver DR. Suppression of calcium sparks in rat ventricular myocytes and direct inhibition of sheep cardiac RyR channels by EPA, DHA and oleic acid. J Membr Biol. 2003;196:95–103 [DOI] [PubMed] [Google Scholar]
- 41.Den Ruijter HM, Berecki G, Verkerk AO, Bakker D, Baartscheer A, Schumacher CA, Belterman CN, de Jonge N, Fiolet JW, Brouwer IA, et al. Acute administration of fish oil inhibits triggered activity in isolated myocytes from rabbits and patients with heart failure. Circulation. 2008;117:536–44 [DOI] [PubMed] [Google Scholar]
- 42.Conquer JA, Holub BJ. Effect of supplementation with different doses of DHA on the levels of circulating DHA as non-esterified fatty acid in subjects of Asian Indian background. J Lipid Res. 1998;39:286–92 [PubMed] [Google Scholar]
- 43.Conquer JA, Cheryk LA, Chan E, Gentry PA, Holub BJ. Effect of supplementation with dietary seal oil on selected cardiovascular risk factors and hemostatic variables in healthy male subjects. Thromb Res. 1999;96:239–50 [DOI] [PubMed] [Google Scholar]
- 44.Dart C. Lipid microdomains and the regulation of ion channel function. J Physiol. 2010;588:3169–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilding TJ, Chen K, Huettner JE. Fatty acid modulation and polyamine block of GluK2 kainate receptors analyzed by scanning mutagenesis. J Gen Physiol. 2010;136:339–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilding TJ, Zhou Y, Huettner JE. Q/R site editing controls kainate receptor inhibition by membrane fatty acids. J Neurosci. 2005;25:9470–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soubias O, Teague WE, Gawrisch K. Evidence for specificity in lipid-rhodopsin interactions. J Biol Chem. 2006;281:33233–41 [DOI] [PubMed] [Google Scholar]
- 48.Grossfield A, Feller SE, Pitman MC. A role for direct interactions in the modulation of rhodopsin by omega-3 polyunsaturated lipids. Proc Natl Acad Sci USA. 2006;103:4888–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bruno MJ, Koeppe RE II, Andersen OS. Docosahexaenoic acid alters bilayer elastic properties. Proc Natl Acad Sci USA. 2007;104:9638–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jump DB. N-3 polyunsaturated fatty acid regulation of hepatic gene transcription. Curr Opin Lipidol. 2008;19:242–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pawar A, Jump DB. Unsaturated fatty acid regulation of peroxisome proliferator-activated receptor alpha activity in rat primary hepatocytes. J Biol Chem. 2003;278:35931–9 [DOI] [PubMed] [Google Scholar]
- 52.Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci USA. 1997;94:4312–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hertz R, Magenheim J, Berman I, Bar-Tana J. Fatty acyl-CoA thioesters are ligands of hepatic nuclear factor-4alpha. Nature. 1998;392:512–6 [DOI] [PubMed] [Google Scholar]
- 54.Botolin D, Wang Y, Christian B, Jump DB. Docosahexaneoic acid (22:6,n-3) regulates rat hepatocyte SREBP-1 nuclear abundance by Erk- and 26S proteasome-dependent pathways. J Lipid Res. 2006;47:181–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calder PC. Polyunsaturated fatty acids and inflammation. Biochem Soc Trans. 2005;33:423–7 [DOI] [PubMed] [Google Scholar]
- 56.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8:794–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Roos B, Mavrommatis Y, Brouwer IA. Long-chain n-3 polyunsaturated fatty acids: new insights into mechanisms relating to inflammation and coronary heart disease. Br J Pharmacol. 2009;158:413–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shearer GC, Harris WS, Pedersen TL, Newman JW. Detection of omega-3 oxylipins in human plasma and response to treatment with omega-3 acid ethyl esters. J Lipid Res. 2010;51:2074–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wada M, DeLong CJ, Hong YH, Rieke CJ, Song I, Sidhu RS, Yuan C, Warnock M, Schmaier AH, Yokoyama C, et al. Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived versus eicosapentaenoic acid-derived substrates and products. J Biol Chem. 2007;282:22254–66 [DOI] [PubMed] [Google Scholar]
- 60.Lands WE. Biosynthesis of prostaglandins. Annu Rev Nutr. 1991;11:41–60 [DOI] [PubMed] [Google Scholar]
- 61.Massaro M, Habib A, Lubrano L, Del Turco S, Lazzerini G, Bourcier T, Weksler BB, De Caterina R. The omega-3 fatty acid docosahexaenoate attenuates endothelial cyclooxygenase-2 induction through both NADP(H) oxidase and PKC epsilon inhibition. Proc Natl Acad Sci USA. 2006;103:15184–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arnold C, Konkel A, Fischer R, Schunck WH. Cytochrome P450-dependent metabolism of omega-6 and omega-3 long-chain polyunsaturated fatty acids. Pharmacol Rep. 2010;62:536–47 [DOI] [PubMed] [Google Scholar]
- 63.Dangi B, Obeng M, Nauroth JM, Teymourlouei M, Needham M, Raman K, Arterburn LM. Biogenic synthesis, purification, and chemical characterization of anti-inflammatory resolvins derived from docosapentaenoic acid (DPAn-6). J Biol Chem. 2009;284:14744–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Careaga MM, Sprecher H. Synthesis of two hydroxy fatty acids from 7,10,13,16,19-docosapentaenoic acid by human platelets. J Biol Chem. 1984;259:14413–7 [PubMed] [Google Scholar]
- 65.Poulsen RC, Gotlinger KH, Serhan CN, Kruger MC. Identification of inflammatory and proresolving lipid mediators in bone marrow and their lipidomic profiles with ovariectomy and omega-3 intake. Am J Hematol. 2008;83:437–45 [DOI] [PubMed] [Google Scholar]
- 66.Spite M, Serhan CN. Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circ Res. 2010;107:1170–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang C, Bazan NG. Lipid-mediated cell signaling protects against injury and neurodegeneration. J Nutr. 2010;140:858–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. 2009;206:15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–17 [DOI] [PubMed] [Google Scholar]
- 70.Groeger AL, Cipollina C, Cole MP, Woodcock SR, Bonacci G, Rudolph TK, Rudolph V, Freeman BA, Schopfer FJ. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat Chem Biol. 2010;6:433–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arnold C, Markovic M, Blossey K, Wallukat G, Fischer R, Dechend R, Konkel A, von Schacky C, Luft FC, Muller DN, et al. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of {omega}-3 fatty acids. J Biol Chem. 2010;285:32720–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morin C, Sirois M, Echave V, Albadine R, Rousseau E. 17,18-epoxyeicosatetraenoic acid targets PPARgamma and p38 mitogen-activated protein kinase to mediate its anti-inflammatory effects in the lung: role of soluble epoxide hydrolase. Am J Respir Cell Mol Biol. 2010;43:564–75 [DOI] [PubMed] [Google Scholar]
- 73.Ye D, Zhang D, Oltman C, Dellsperger K, Lee HC, VanRollins M. Cytochrome p-450 epoxygenase metabolites of docosahexaenoate potently dilate coronary arterioles by activating large-conductance calcium-activated potassium channels. J Pharmacol Exp Ther. 2002;303:768–76 [DOI] [PubMed] [Google Scholar]
- 74.Zhang Y, Oltman CL, Lu T, Lee HC, Dellsperger KC, VanRollins M. EET homologs potently dilate coronary microvessels and activate BK(Ca) channels. Am J Physiol Heart Circ Physiol. 2001;280:H2430–40 [DOI] [PubMed] [Google Scholar]
- 75.Mori TA, Woodman RJ. The independent effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular risk factors in humans. Curr Opin Clin Nutr Metab Care. 2006;9:95–104 [DOI] [PubMed] [Google Scholar]
- 76.Buckley R, Shewring B, Turner R, Yaqoob P, Minihane AM. Circulating triacylglycerol and apoE levels in response to EPA and docosahexaenoic acid supplementation in adult human subjects. Br J Nutr. 2004;92:477–83 [DOI] [PubMed] [Google Scholar]
- 77.Maki KC, Van Elswyk ME, McCarthy D, Hess SP, Veith PE, Bell M, Subbaiah P, Davidson MH. Lipid responses to a dietary docosahexaenoic acid supplement in men and women with below average levels of high density lipoprotein cholesterol. J Am Coll Nutr. 2005;24:189–99 [DOI] [PubMed] [Google Scholar]
- 78.Kelley DS, Siegel D, Vemuri M, Mackey BE. Docosahexaenoic acid supplementation improves fasting and postprandial lipid profiles in hypertriglyceridemic men. Am J Clin Nutr. 2007;86:324–33 [DOI] [PubMed] [Google Scholar]
- 79.Egert S, Kannenberg F, Somoza V, Erbersdobler HF, Wahrburg U. Dietary alpha-linolenic acid, EPA, and DHA have differential effects on LDL fatty acid composition but similar effects on serum lipid profiles in normolipidemic humans. J Nutr. 2009;139:861–8 [DOI] [PubMed] [Google Scholar]
- 80.Cazzola R, Russo-Volpe S, Miles EA, Rees D, Banerjee T, Roynette CE, Wells SJ, Goua M, Wahle KW, Calder PC, et al. Age- and dose-dependent effects of an eicosapentaenoic acid-rich oil on cardiovascular risk factors in healthy male subjects. Atherosclerosis. 2007;193:159–67 [DOI] [PubMed] [Google Scholar]
- 81.Neff LM, Culiner J, Cunningham-Rundles S, Seidman C, Meehan D, Maturi J, Wittkowski KM, Levine B, Breslow JL. Algal docosahexaenoic acid affects plasma lipoprotein particle size distribution in overweight and obese adults. J Nutr. 2011;141:207–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–8 [DOI] [PubMed] [Google Scholar]
- 83.Mori TA, Bao DQ, Burke V, Puddey IB, Beilin LJ. Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans. Hypertension. 1999;34:253–60 [DOI] [PubMed] [Google Scholar]
- 84.Mori TA, Watts GF, Burke V, Hilme E, Puddey IB, Beilin LJ. Differential effects of eicosapentaenoic acid and docosahexaenoic acid on vascular reactivity of the forearm microcirculation in hyperlipidemic, overweight men. Circulation. 2000;102:1264–9 [DOI] [PubMed] [Google Scholar]
- 85.Grimsgaard S, Bonaa KH, Hansen JB, Myhre ES. Effects of highly purified eicosapentaenoic acid and docosahexaenoic acid on hemodynamics in humans. Am J Clin Nutr. 1998;68:52–9 [DOI] [PubMed] [Google Scholar]
- 86.Theobald HE, Goodall AH, Sattar N, Talbot DC, Chowienczyk PJ, Sanders TA. Low-dose docosahexaenoic acid lowers diastolic blood pressure in middle-aged men and women. J Nutr. 2007;137:973–8 [DOI] [PubMed] [Google Scholar]
- 87.Woodman RJ, Mori TA, Burke V, Puddey IB, Watts GF, Beilin LJ. Effects of purified eicosapentaenoic and docosahexaenoic acids on glycemic control, blood pressure, and serum lipids in type 2 diabetic patients with treated hypertension. Am J Clin Nutr. 2002;76:1007–15 [DOI] [PubMed] [Google Scholar]
- 88.Morris MC, Sacks F, Rosner B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation. 1993;88:523–33 [DOI] [PubMed] [Google Scholar]
- 89.Nestel P, Shige H, Pomeroy S, Cehun M, Abbey M, Raederstorff D. The n-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid increase systemic arterial compliance in humans. Am J Clin Nutr. 2002;76:326–30 [DOI] [PubMed] [Google Scholar]
- 90.Egert S, Stehle P. Impact of n-3 fatty acids on endothelial function: results from human interventions studies. Curr Opin Clin Nutr Metab Care. 2011;14:121–31 [DOI] [PubMed] [Google Scholar]
- 91.Egert S, Rassoul F, Boesch-Saadatmandi C, Richter V, Rimbach G, Erbersdobler HF. Effects of controlled diets enriched with alpha-linolenic acid, eicosapentaenoic acid or docosahexaenoic acid on soluble adhesion molecules and endothelin-1 concentrations in healthy volunteers. CTNR. 2007;5:189–195. [Google Scholar]
- 92.Thies F, Miles EA, Nebe-von-Caron G, Powell JR, Hurst TL, Newsholme EA, Calder PC. Influence of dietary supplementation with long-chain n-3 or n-6 polyunsaturated fatty acids on blood inflammatory cell populations and functions and on plasma soluble adhesion molecules in healthy adults. Lipids. 2001;36:1183–93 [DOI] [PubMed] [Google Scholar]
- 93.Kelley DS, Siegel D, Fedor DM, Adkins Y, Mackey BE. DHA supplementation decreases serum C-reactive protein and other markers of inflammation in hypertriglyceridemic men. J Nutr. 2009;139:495–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yamamoto H, Yoshimura H, Noma M, Suzuki S, Kai H, Tajimi T, Sugihara M, Kikuchi Y. Improvement of coronary vasomotion with eicosapentaenoic acid does not inhibit acetylcholine-induced coronary vasospasm in patients with variant angina. Jpn Circ J. 1995;59:608–16 [DOI] [PubMed] [Google Scholar]
- 95.Mori TA, Puddey IB, Burke V, Croft KD, Dunstan DW, Rivera JH, Beilin LJ. doi: 10.1179/rer.2000.5.1.45. Effect of omega 3 fatty acids on oxidative stress in humans: GC-MS measurement of urinary F2-isoprostane excretion. Redox Rep. 2000;5:45–6. [DOI] [PubMed] [Google Scholar]
- 96.Guillot N, Caillet E, Laville M, Calzada C, Lagarde M, Vericel E. Increasing intakes of the long-chain omega-3 docosahexaenoic acid: effects on platelet functions and redox status in healthy men. FASEB J. 2009;23:2909–16 [DOI] [PubMed] [Google Scholar]
- 97.Phang M, Garg ML, Sinclair AJ. Inhibition of platelet aggregation by omega-3 polyunsaturated fatty acids is gender specific-redefining platelet response to fish oils. Prostaglandins Leukot Essent Fatty Acids. 2009;81:35–40 [DOI] [PubMed] [Google Scholar]
- 98.Phang M, Sinclair AJ, Lincz LF, Garg ML. doi: 10.1016/j.numecd.2010.04.012. Gender-specific inhibition of platelet aggregation following omega-3 fatty acid supplementation. Nutr Metab Cardiovasc Dis. Epub 2010 Aug 11. [DOI] [PubMed] [Google Scholar]
- 99.Woodman RJ, Mori TA, Burke V, Puddey IB, Barden A, Watts GF, Beilin LJ. Effects of purified eicosapentaenoic acid and docosahexaenoic acid on platelet, fibrinolytic and vascular function in hypertensive type 2 diabetic patients. Atherosclerosis. 2003;166:85–93 [DOI] [PubMed] [Google Scholar]
- 100.Siscovick DS, Raghunathan TE, King I, Weinmann S, Wicklund KG, Albright J, Bovbjerg V, Arbogast P, Smith H, Kushi LH, et al. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA. 1995;274:1363–7 [DOI] [PubMed] [Google Scholar]
- 101.Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, Ma J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–8 [DOI] [PubMed] [Google Scholar]
- 102.Lemaitre RN, King IB, Mozaffarian D, Kuller LH, Tracy RP, Siscovick DS. n-3 Polyunsaturated fatty acids, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: the Cardiovascular Health Study. Am J Clin Nutr. 2003;77:319–25 [DOI] [PubMed] [Google Scholar]
- 103.Virtanen JK, Voutilainen S, Rissanen TH, Mursu J, Tuomainen TP, Korhonen MJ, Valkonen VP, Seppanen K, Laukkanen JA, Salonen JT. Mercury, fish oils, and risk of acute coronary events and cardiovascular disease, coronary heart disease, and all-cause mortality in men in eastern Finland. Arterioscler Thromb Vasc Biol. 2005;25:228–33 [DOI] [PubMed] [Google Scholar]
- 104.Virtanen JK, Mursu J, Voutilainen S, Tuomainen TP. Serum long-chain n-3 polyunsaturated fatty acids and risk of hospital diagnosis of atrial fibrillation in men. Circulation. 2009;120:2315–21 [DOI] [PubMed] [Google Scholar]
- 105.Pedersen JI, Ringstad J, Almendingen K, Haugen TS, Stensvold I, Thelle DS. Adipose tissue fatty acids and risk of myocardial infarction: a case-control study. Eur J Clin Nutr. 2000;54:618–25 [DOI] [PubMed] [Google Scholar]
- 106.Yli-Jama P, Meyer HE, Ringstad J, Pedersen JI. Serum free fatty acid pattern and risk of myocardial infarction: a case-control study. J Intern Med. 2002;251:19–28 [DOI] [PubMed] [Google Scholar]
- 107.Oda E, Hatada K, Katoh K, Kodama M, Nakamura Y, Aizawa Y. A case-control pilot study on n-3 polyunsaturated fatty acid as a negative risk factor for myocardial infarction. Int Heart J. 2005;46:583–91 [DOI] [PubMed] [Google Scholar]
- 108.Guallar E, Aro A, Jimenez FJ, Martin-Moreno JM, Salminen I, van't Veer P, Kardinaal AF, Gomez-Aracena J, Martin BC, Kohlmeier L, et al. Omega-3 fatty acids in adipose tissue and risk of myocardial infarction: the EURAMIC study. Arterioscler Thromb Vasc Biol. 1999;19:1111–8 [DOI] [PubMed] [Google Scholar]
- 109.Simon JA, Hodgkins ML, Browner WS, Neuhaus JM, Bernert JT, Jr, Hulley SB. Serum fatty acids and the risk of coronary heart disease. Am J Epidemiol. 1995;142:469–76 [DOI] [PubMed] [Google Scholar]
- 110.Mozaffarian D. JELIS, fish oil, and cardiac events. Lancet. 2007;369:1062–3 [DOI] [PubMed] [Google Scholar]
- 111.Simopoulos AP, Leaf A, Salem N., Jr Essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. Ann Nutr Metab. 1999;43:127–30 [DOI] [PubMed] [Google Scholar]
- 112.European Commission. Eurodiet Core Report; 2000 [cited 2010 Nov 17]. Available from: http://ec.europa.eu/health/archive/ph_determinants/life_style/nutrition/report01_en.pdf.
- 113.Health Council of the Netherlands. Dietary reference intakes: energy, proteins, fats, and digestible carbohydrates. Publication no. 2001/19. The Hague: Health Council of the Netherlands; 2001. [Google Scholar]
- 114.United States National Academy of Sciences Food and Nutrition Board. The dietary reference intakes for energy, carbohydrates, fiber, fat, protein and amino acids (macronutrients). Washington: National Academy Press; 2002. p. 45–6. [Google Scholar]
- French Agency for Food Environmental and Occupational Health Safety. Omega 3 Report; 2003 [cited 2010 Nov 17]. Available from: http://www.afssa.fr/Documents/NUT-Ra-omega3EN.pdf.
- 116.Van de Werf F, Ardissino D, Betriu A, Cokkinos DV, Falk E, Fox KA, Julian D, Lengyel M, Neumann FJ, Ruzyllo W, et al. Management of acute myocardial infarction in patients presenting with ST-segment elevation. The Task Force on the Management of Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2003;24:28–66 [DOI] [PubMed] [Google Scholar]
- Joint WHO/FAO Expert Consultation. Diet, nutrition and the prevention of chronic diseases. WHO Technical Report Series no. 916. Geneva: WHO; 2003. p. 89#x201390. [PubMed]
- 118.International Society for the Study of Fatty Acids and Lipids. ISSFAL Policy Statement 3. Recommendations for intake of polyunsaturated fatty acids in healthy adults; June, 2004 [cited 2010 Nov 17]. Available from: http://www.issfal.org/images/stories/pdfs/PUFAIntakeReccomdFinalReport.pdf.
- 119.Scientific Advisory Committee on Nutrition. Advice on fish consumption: benefits and risks; 2004 [cited 2010 Nov 17]. Available from: http://www.food.gov.uk/news/newsarchive/2004/jun/fishreport2004.
- 120.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–57 [DOI] [PubMed] [Google Scholar]
- 121.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96 [DOI] [PubMed] [Google Scholar]
- 122.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaseli GF, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613 [DOI] [PubMed] [Google Scholar]
- 123.National Health and Medical Research Council. Nutrient reference values for Australia and New Zealand including recommended dietary intakes; 2006 [cited 2010 Nov 17]. Available from: http://www.nhmrc.gov.au/publications/synopses/n35syn.htm.
- Joint FAO/WHO Expert Consultation on fats and fatty acids in human nutrition; 2008 [cited 2010 Nov 17]. Available from: http://www.who.int/nutrition/topics/FFA_summary_rec_conclusion.pdf.
- USDA and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th ed. Washington, DC: U.S. Government Printing Office; 2010. [DOI] [PMC free article] [PubMed]
- 126.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, et al. Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–209 [DOI] [PMC free article] [PubMed] [Google Scholar]