Eukaryotic 5S rRNA biogenesis (original) (raw)
. Author manuscript; available in PMC: 2012 Feb 14.
Published in final edited form as: Wiley Interdiscip Rev RNA. 2011 Feb 25;2(4):523–533. doi: 10.1002/wrna.74
Abstract
The ribosome is a large complex containing both protein and RNA which must be assembled in a precise manner to allow proper functioning in the critical role of protein synthesis. 5S rRNA is the smallest of the RNA components of the ribosome, and although it has been studied for decades, we still do not have a clear understanding of its function within the complex ribosome machine. It is the only RNA species that binds ribosomal proteins prior to its assembly into the ribosome. Its transport into the nucleolus requires this interaction. Here we present an overview of some of the key findings concerning the structure and function of 5S rRNA and how its association with specific proteins impacts its localization and function.
INTRODUCTION
5S rRNA is a small RNA (120 nt) with a molecular mass of 40 kDa. The secondary and tertiary structures are generally conserved across phylogeny. The secondary structure is composed of five helices,1 four loops (two hairpin and two internal), and one hinge (Figure 1) which usually fold into a Y structure. 5S rRNA is unique among the rRNAs in that it forms a preribosomal particle with the ribosomal protein L5 in eukaryotes.2 Prior to joining the ribosome, 5S rRNA has a complicated biogenesis pathway that may involve several other proteins (Figure 2). Unlike the other eukaryotic rRNAs, it is not generally transcribed in the nucleolus. Therefore, its interaction with L5 in its function as a targeting protein may be required to bring it to the site of ribosomal assembly within the nucleolus (Figure 3). The presence of 5S rRNA is required for normal translation in most ribosomes although its presence in some mitochondrial ribosomes has not been demonstrated. 5S rRNA sits in the junction between the large ribosomal subunit (LSU or 60 S in eukaryotes) and small ribosomal subunit (SSU or 40 S in eukaryotes) and forms part of the central protuberance (CP).3 Although its exact function within the ribosome is unclear, it is thought to play a critical role in both protein–RNA and RNA–RNA interactions within the ribosome.
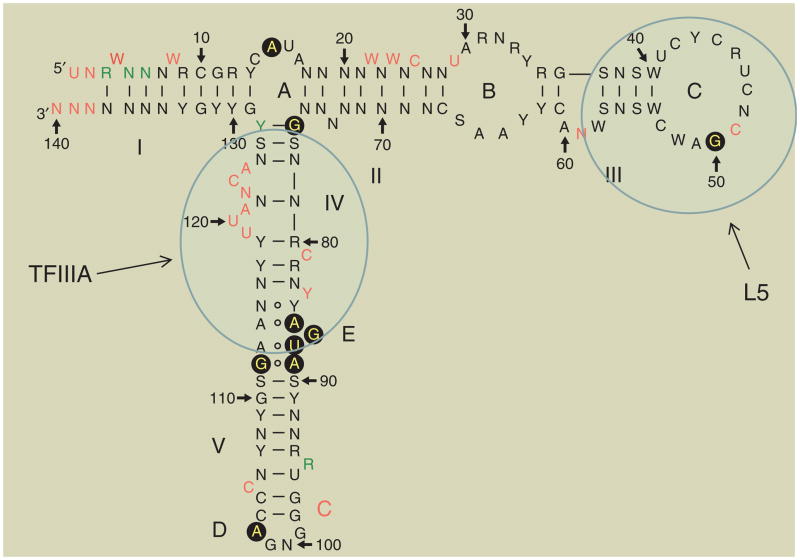
FIGURE 1.
Structure of 5S rRNA. Loops are labeled A–E and helices I–V. Translucent blue ovals represent contacts with TFIIIA and L5 (data used by permission of author).4
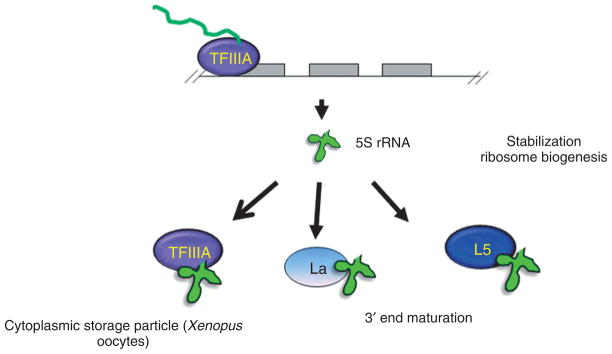
FIGURE 2.
Preribosomal interactions of 5S rRNA with its predominant binding partners. This figure indicates both the transient and more stable interaction of 5S rRNA (La, TFIIIA, and L5, respectively). The nuclear export of 5S rRNA can occur together with either TFIIIA or L5 but return to the nucleus occurs only in conjunction with L5.
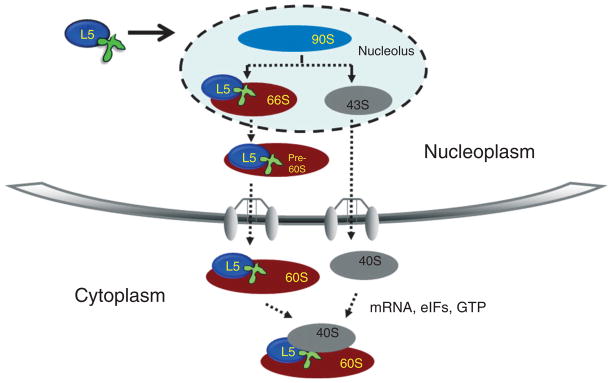
FIGURE 3.
Ribosomal biogenesis pathway. This diagram depicts the biogenesis of yeast 60S ribosomal subunits, including proteins that have known association at specific stages in this process. We have also included two of the known 60S subunit export factors: exportin 1 and Nmd3. Dashed arrows indicate known biogenesis and transport processes. (Reprinted with permission from Ref 5. Copyright 1999 American Society for Microbiology; and from Ref 6. Copyright 2009 American Society for Microbiology.)
5S rRNA Function
Early structural analysis of prokaryotic 5S rRNA led to speculation that a direct interaction of the–CGAAC–motif of 5S rRNA with the common arm of tRNA (GTΨCG) could lead to a transduction of rotational motion (coiling–uncoiling) in the 5S rRNA into linear motion (necessary for ‘walking’ along the mRNA).7 Prokaryotic 5S rRNA is indeed critical for tRNA binding to the aminoacyl–tRNA binding site of the ribosome.8 In Escherichia coli, it has been possible to reconstitute partially functional ribosomes without 5S rDNA, 5S rRNA, or 5S rRNA-binding proteins.9,10 However, cells with these defects exhibit growth defects and their ribosomal particles have limited ability to synthesize natural protein which is dramatically improved upon complementation with 5S rRNA.8 In Saccharomyces cerevisiae, mutant versions of 5S rRNA expressed in the absence of wild-type 5S rRNA led to inviable phenotypes.11 It has been proposed for yeast that, since 5S rRNA provides a physical connection between the different functional regions of the ribosome, it serves as a signal transducer to facilitate communication between these regions thus helping to direct their coordination of translation events.12 Furthermore, in E. coli the site of peptidyltransferase activity and the EF-G binding site may communicate through 5S rRNA by its interaction with LSU rRNA (varies in size from 23 to 28S depending on the organism).13 Recruitment of 5S rRNA is needed for the processing of the LSU rRNA and may assure that stoichiometric amounts of the other three rRNAs are present.14
5S rRNA Structure
5S rDNA genes are present in eukaryotic genomes as clusters of tandem repeats.15 The number of these genes is highly variable and their sequences have been used extensively as phylogenetic markers.16 The primary structure of the transcribed product, 5S rRNA, consists of approximately 120 nucleotides and has a molecular mass of 40 kDa. Several methodologies, including enzymatic and chemical probing,17,18 accessible base-specific chemical modifications,19 thermal melting analysis,18–21 and biochemical exchange of divergent or modified RNAs22 as well as theoretical base-pairing considerations18 have shed light on the secondary structure of 5S rRNA from various eukaryotic sources. It comprises five stems or helices (I–IV) and four loops (A–E; Figure 1). Two of these are hairpin loops (C and D), two are internal loops (B and E), and loop A acts as a hinge1 between three stems (I, II, and V). Helix III contains two conserved bulged adenosine residues.23,24 Helix II in Xenopus contains an extrahelical cytosine that points away from the duplex in two different configurations and which can establish major or minor groove contacts in base-triple formations, bridging RNA helices.25 Phylogenetic analysis of 5S rRNA has shown that helices I and III (Figure 1) are ancestral structural motifs.26
The secondary structure motifs fold into a characteristic tertiary structure. Loop A/B/C together with stems I/II/III form a ‘head’ or top domain, stems IV/V and loop E constitute a middle domain, and stems IV/loop D form a bottom or ‘toe’ domain.27 It has been hypothesized28 that helix V can also adopt a hairpin structure in the 5S rRNA gene, aiding in the formation of the transcription complex through interaction with TFIIIA. Structural elements have been described as more important than sequence elements in the binding to proteins (Figure 1).29
Assembly of 5S rRNA into the Ribosome
Whether 5S rRNA is immediately brought to the site of ribosomal assembly or arrives from a storage particle in the cytoplasm, it is brought to the nuclear pre-60S complex in a complex with the L5 ribosomal protein (Figures 2 and 3, discussed below). Reports differ on the stage at which 5S rRNA is integrated into the ribosome. Most recently, Woolford and colleagues30 have shown that they are associated in the very early 90S step in ribosomal assembly, although earlier work suggested that association occurred much later.31 In Trypanosoma brucei, it has been shown that 5S rRNA and L5 are both present in the 90S precursor particle.6 In S. cerevisiae, accessory factors Rpf2 and Rrs1 recruit 5S rRNA together with the ribosomal proteins L5 and L11 into the preribosomal 90S particle.30
5S rRNA Within the Ribosome
Several structural domains of 5S rRNA have been crystallized32 and X-ray scattering studies in solution have determined that free 5S rRNA is elongated in the absence of bound proteins but becomes more compact when it is in the ribosome33,34 (Figure 4). NMR studies performed on Xenopus laevis 5S rRNA have also shown that while loop E is fully folded in the absence of protein (approximating an A-formed helix), the junction of helix V and loop A is conformationally heterogenous in the free molecule.1 The major groove of the A-formed loop E is further kept open by an extrahelical guanosine that interacts with a reversed Hoogsteen A:U pair, presumably defining an interacting surface similar to the sarcin/ricin loop motif of 23S (LSU) rRNA.35–37 Most interactions with ribosomal proteins occur through the 2-amino group of a guanine in the minor groove. The major groove in A-form is more inaccessible and needs considerable distortion to provide intermolecular contacts.38
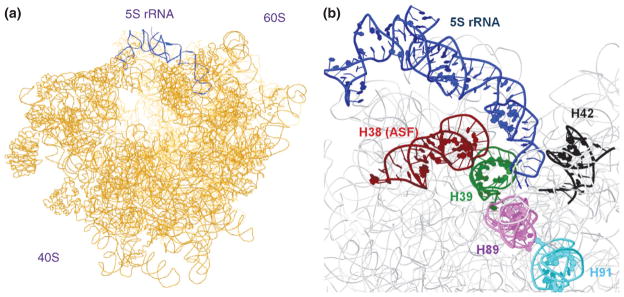
FIGURE 4.
Interactions of 5S rRNA within the ribosome. (a) Position of 5S rRNA (blue) in the CP of the ribosome. (b) Close-up view of the interactions between 5S rRNA (blue) and motifs in the LSU 26S rRNA; red: helix 38 (A-site finger), green: helix 39, black: helix 42, violet: helix 89, cyan: helix 91. PDB accession numbers: 3JYV, 3JYW, 3JYX.
Much of the structural information on 5S rRNA within the ribosome comes from bacteria, where 5S rRNA has been shown to form the CP of the 70S ribosomal subunit close to domains V and II of the 23S rRNA.39 Loop D contacts a cleft in the middle of helices 39 and 40 of 23S rRNA (domain II), whereas loop E and stem V contact helix 38 of 23S rRNA, the longest stem in the largest domain of this rRNA.38 However, most interactions between 5S rRNA and 23S rRNA occur indirectly, through ribosomal proteins. The bacterial 5S rRNA is linked to domains V of 23S rRNA through L5, L21e, and L10e and to domain II through L5, L21e, and L30.40
Through cryo-electron microscopy studies, it has observed that in S. cerevisiae 5S rRNA also sits in the CP of the 60S subunit and that it contacts helix 42 and helix 89 of the 28S rRNA (Figure 4), the former linking 5S rRNA to the GTPase associated center3,41 (involving contacts with protein factors and GTP hydrolysis stimulation). Helix 89 in turn contacts helix 91 which is linked to the elongation factor binding site through the sarcin/ricin loop of 28S rRNA. Therefore 5S rRNA, through direct or indirect contacts, reaches far into relevant functional sites in ribosomal subunit 60S (Figure 4).
5S rRNA and Ribosomal Biogenesis
Ribosomal biogenesis (Figure 3) is a process that requires the concerted processing and assembly of four ribosomal RNAs with over 80 ribosomal proteins.42,43 Recent large-scale proteomic approaches to the study of ribosome biogenesis have revealed an unexpectedly high number of enzymatic activities and _trans_-acting factors, including GTPases, ATPases, RNA exonucleases, RNA helicases, enzymes which modify RNA by methylation and pseudouridylation as well as many small RNAs that guide these processes.44,45 Biogenesis of both 60S and 40S subunits begins in the nucleolus46 with transcription of the 35S rRNA precursor47 by RNA polymerase I. In contrast, 5S rRNA is transcribed by RNA polymerase III from independent genes usually located in a different chromosomal locus. Yeast constitutes an exception, where 5S rRNA genes are interspersed between other nucleolar ribosomal genes, a situation that raises the question of whether and how their expression is affected by their positions within this locus. The individual subunit precursor particles, 66S (containing precursors to 25, 5.8, and 5S rRNAs) and 43S (containing precursors to 18S rRNA), are released from the 90S precursor upon cleavage of the 35S pre-rRNA.5,48 The two subunit precursors follow separate maturation pathways, although both are exported to the cytoplasm using the exportin 1 nuclear export pathway.49–51 The final steps of maturation occur in the cytoplasm, where joining of the 60S and 40S subunits occurs to form active 80S ribosomes.52,53
5S rRNA Processing
Processing of the 3′ ends of 5S rRNA involves participation of several yeast exonucleases (Rex1p, Rex2p, and Rex3p) with overlapping roles in the processing of other nuclear RNAs.54 Surveillance of readthrough transcripts and defective 5S rRNA species may occur by interaction with the Ro protein, a factor that binds a heterogeneous population of 69–112 nt RNA polymerase III transcripts.55 5S rRNA can be polyadenylated and subsequently degraded by the exosome.56,57 Nucleotides in eukaryotic 5S rRNA are rarely modified but there are reports of pseudouridinylation in some ascomycetes.58
PRERIBOSOMAL 5S rRNA BINDING PARTNERS
The La Protein
In eukaryotes, 5S rRNA metabolism begins with association of the primary transcript with the La protein (Figure 2).59–62 This association is transient, and only about 1–2% of free 5S rRNA containing particles contains the La protein.63,64 The La protein was first described in humans as an autoantigen but has since been found in all eukaryotes.62 La is an abundant protein of approximately 47 kDa that is primarily localized to the nucleus.65,66 It associates with a diverse group of RNAs which are transcribed by RNA pol III, including precursors to tRNAs and 5S rRNA. La interacts with these RNAs through recognition of their 3′ uridylates67 and functions as a chaperone in the maturation and stability of its RNA-binding partners62,68 and is thought to be involved in folding.
La is essential in flies and mammals but is not essential in yeast66 (except against a background of mutated Sm and Sm-like proteins involved in snRNP metabolism). It has been postulated that in S. cerevisiae, where La is missing, the cells are still viable because of the existence of unique alternative pathways.65 However, this is not simply a case of differences between unicellular eukaryotes and metazoans. RNA interference directed against the La homolog in T. brucei, an early branching unicellular eukaryote, causes a lethal phenotype in contrast to the results in yeast.69 It is unclear which of the many possible functions of La may be critical to the parasites’ survival but not required for the yeast cells to survive.
The La protein possesses both a La motif which is a winged-helix structure and an RRM1 which forms a typical RRM structure (Figure 5). Structural analysis of the La motif from human and trypanosomes indicates that it is conserved.70,71 Both the La and RRM motifs are required for La to bind UUUOH with high affinity,70,71 although La does not bind the substrate in the fashion typical for these motifs.72 La traffics to the nucleus via a nuclear localization signal (NLS) and also has a nuclear retention signal (NRE) as well as a nucleolar localization signal.
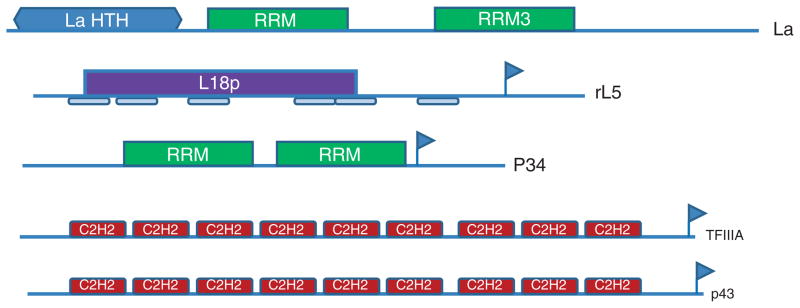
FIGURE 5.
Modular structure of eukaryotic characterized 5S rRNA binding proteins. Interpro signatures are as follows: L18p: IPR005484 Ribosomal_L18/L5; C2H2: IPR015880 Zn finger C2H2-like, LaHTH: IPR006630; RRM: IPR000504 (RNP1); RRM3: IPR014886; Vertical blue flags indicate nuclear export signals (NES) as defined by NetNES 1.1. The six rectangles beneath rL5 define the ribosomal L5 signature.
The Ribosomal L5 Protein
Following association with La and subsequent maturation, eukaryotic 5S rRNA associates with the ribosomal protein L5 (Figure 2). L5 functions to stabilize 5S rRNA73 and the levels of L5 also regulate the levels of 5S rRNA.74 5S rRNA is unique among the ribosomal RNAs because it can be found separately from the ribosome as part of a ribonucleoprotein particle (RNP). In bacteria there are three proteins found in this preribosomal complex: L5 (not the homolog of the eukaryotic L5), L18 (the eukaryotic L5 homolog), and, in some cases, L25. In contrast, in eukaryotes there is a single protein L5 (YL3 or Rpl5p in yeast, ortholog to bacterial L18). A complex of 5S rRNA and L5 can also be dissociated from the ribosome as was shown in early experiments.75 While other proteins can bind to 5S rRNA to translocate it to the cytoplasm or within the cytoplasm, only L5 can form a complex with 5S rRNA that can be transported to the nucleus for ribosomal assembly. Mutagenesis of the L5 protein or 5S rRNA can cause the RNP complex to be unstable and also render the assembled ribosomal subunits unstable64 supporting a critical role for the L5–5S rRNA complex. Phosphorylation of L5 by human CKII, a ubiquitous conserved Ser/Thr kinase, decreases binding to 5S rRNA76 and may play a regulatory function.
The 34 kDa L5 protein is localized to both the nucleus and the cytoplasm of eukaryotic cells and its dual localization plays an important part in the nucleocytoplasmic shuttling of L5–5S rRNA. Loss of L5 prevents the nuclear localization of 5S rRNA and affects subsequent ribosomal assembly. The NLS is complex, involving two distinct regions of the L5 sequence (aa 21–37 and aa 255–265), whereas the NES has been mapped to aa 101–11177
In S. cerevisiae L5, it has been postulated that a basic region toward the C-terminus is important in the binding to 5S rRNA2 and in effective ribosome biogenesis.78 These basic amino acids (R282, R285, and K289) lie on the same side of a putative _α_-helix. Single mutants of these residues interfere with 5S rRNA binding and double mutants are lethal. Eukaryotic 5S rRNA shows a high frequency of uncompensated G-U wobble pairs (pyrimidine at 5′ of G) that have been proposed to play a role in binding to L5, as well as to TFIIIA (see below).79
P34 and P37
In T. brucei, the causative agent of African trypanosomiasis, two novel, abundant- and closely related RNA-binding proteins, termed P34 and P37, have been identified.80,81 P34 and P37 (predicted 28.8 and 30.3 kDa, respectively), each contain three distinct domains as determined from their primary amino acid sequence (Figure 5). The N-terminal region consists of an alanine-, proline-, and lysine-rich domain which may serve a role in mediating protein–protein interactions. The internal regions of P34 and P37 contain two RBDs of the RRM family type, which consist of several basic amino acid residues. The last nine amino acids of the second RBD comprise a leucine-rich NES. The C-terminal portion is comprised of a lysine–lysine–aspartic acid-‘X’ motif, which includes both NLS and NES.
It has been shown that P34 and P37 are essential to the survival of both bloodstream forms (the stage present within the mammalian host) and procyclic forms (the replicative stage in the invertebrate) of T. brucei and the loss of these proteins leads to a 25-fold decrease in 5S rRNA.82 This change was specific to 5S rRNA and was not seen for other rRNAs. These results are similar to those observed in yeast cells where L5 plays a role in stabilizing 5S rRNA.73,74 Recent work has shown that P34 and P37 undergo nucleocytoplasmic shuttling6 as suggested by the presence of the NES and NLS (above). P34 and P37 have been shown to interact specifically with 5S rRNA in nuclear extracts6,83 and with 5S rRNA directly in in vitro filter binding assays (Ciganda and Williams, unpublished). Immunoprecipitation studies have shown that P34 and P37 also interact with T. brucei ribosomal protein L5 in nuclear extracts.6 These associations are indicative of potential role(s) of P34 and P37 in the stabilization and transport of 5S rRNA, and in the biogenesis of ribosomes, a process that remains poorly characterized. Moreover, in trypanosomes cells lacking P34 and P37 also show phenotypic alterations with respect to the formation of 80S ribosomes and overall cellular translation levels.84 Interestingly, these two 5S rRNA binding proteins are differentially expressed. P34 is dominantly expressed in the procyclic stage, while P37 is dominantly expressed in the bloodstream stage. It is not yet known if this reflects differences in function of these two proteins and their interaction with 5S rRNA between these stages. The discovery of additional, organism-specific 5S rRNA binding factors (see below, p43) expands our view of the interactions involved in 5S rRNA entry into the ribosomal biogenesis pathway originally described.
TFIIIA
TFIIIA has been carefully studied predominantly in its role as a transcription factor. It is a zinc finger protein (38.5 kDa) with nine C2H2 zinc finger repeats within the two thirds of the protein’s N-terminal (Figure 5). This region binds the internal control region of the 5S rDNA as well as mature 5S rRNA. Transcription of 5S rRNA requires TFIIIA, TFIIIB, and TFIIIC. TFIIIA has been identified in all eukaryotes to date, although it has not yet been identified in the genome of the kinetoplastids. In yeast, 5S rRNA transcription (but not stabilization) is the only essential function of TFIIIA. In the ascomycete Yarrowia lipolytica, TFIIIA-independent transcription of 5S rRNA can occur via dicistronic units of tRNA-5S rRNA.85 Xenopus TFIIIA interacts with approximately 50% of the 5S rRNA to form the 7S particle to function in transport of 5S rRNA from the nucleus to the cytoplasm for storage (see below). Fingers 4–7 bind RNA with high affinity (1 nM Kd) although the tertiary structure and some regions of secondary structure appear to be critical. Specific residues including a lysine in the _α_-helix of finger 4 and the threonine–tryptophan–threonine (TWT) in the _α_-helix of finger 6 are thought to be important to 5S rRNA binding through a central core comprised of loop A, helix V, region E, and helix IV, while finger 7 appears to bind helix II.86 This broad region of interaction suggests a role of TFIIIA in protecting 5S rRNA from turnover. Microinjection of mutant RNAs into Xenopus oocytes and localization studies have been used to correlate structural characteristics of 5S rRNA to domains necessary for nuclear import, present mainly in helix II, loop C, and loop E87 although no protein ligands have been identified for this specific role.
P43
Although TFIIIA in Xenopus oocytes binds to 50% of 5S rRNA as the 7S particle, a zinc finger protein p43 binds to the other 50% of the 5S rRNA in the form of a 42S RNP, together with a 48 kDa protein with EF-1_α_-like activity and tRNAs.88,89 TFIIIA and p43 do not share significant sequence homology, although p43, like TFIIIA, is a zinc finger protein (Figure 5) and both possess a TWT motif. Unlike TFIIIA, p43 does not bind directly to 5S rDNA.89 Zinc fingers 1–4 are essential for specific binding to 5S rRNA.90 Uncharacterized homologs of Xenopus P43 can be found in the genomes of Danio rerio (zebrafish) and Oryzias latipes (ricefish). Further studies on these factors are needed to clarify their roles and address the question of why these organisms need additional 5S rRNA binding proteins.
Mitochondrial 5S rRNA
Examination of mitochondrial ribosomes from many eukaryotes suggests that they lack 5S rRNA, although 5S rRNA has been reported in the mitochondria of a number of organisms (reviewed in Ref 91). No clear role for this mitochondrial 5S rRNA is yet defined. Although many mitochondrial genes including that for 5S rRNA have been transferred to the nucleus in the course of eukaryotic evolution, it is still present in the mitochondria of land plants and subsets of algae (reviewed in Ref 91). The existence of nuclearly encoded 5S rRNA in the mitochondria of eukaryotes has been a point of contention. Although some organisms have the mitochondrial gene for 5S rRNA and do not import nuclear 5S rRNA, mammalian mitochondria do import 5S rRNA.92,93 Helices I and IV (in particular G-U wobble pairs present in them) are necessary for high-efficiency import of human 5S rRNA into the mitochondrion.94
Cytoplasmic Storage of 5S rRNA
One of the most extensively characterized 5S rRNA pathways has been described for Xenopus oogenesis. Xenopus generates a huge excess of 5S rRNA in the early stages of oogenesis, the majority of which is found in the cytoplasm stored as a non-ribosomal RNP.95 Two types of 5S rRNA are present in these cells; a somatic type which is constitutively expressed and the oocyte specific type which is transcribed only in oogenesis.96 These two classes differ in sequence and appear to have differing affinity for TFIIIA and L5 with the oocyte specific type having a higher affinity for TFIIIA and the somatic having a higher affinity for L5. It is however the difference in abundance of the two proteins at different stages of oogenesis that has the greatest impact.
In the early stages, TFIIIA is present in excess of the 5S genes and is predominantly present in the cytoplasm as part of the 7S RNP. Later in oogenesis, TFIIIA levels drop and L5 levels rise in the middle stages of oogenesis concomitant with expression of other ribosomal components. 5S rRNA associates with L5 as part of the 5S RNP. Nuclear export of 5S rRNA appears to occur in association with either TFIIIA or L5 while the return to the nucleus in later stages of oogenesis requires association with L5 alone. This is because of the masking of the NLS of TFIIIA upon binding 5S rRNA.97–99
CONCLUSIONS
Although 5S rRNA has been studied for decades, both in its role in the ribosome and as a phylogenetic marker, we still have many unanswered questions concerning this small RNA. Even though the ribosome has garnered much attention since the awarding of the Nobel Prize in 2009, 5S rRNA remains the most elusive of all ribosomal RNAs regarding its precise function and how this correlates with structure. It is not immediately evident why 5S rRNA is necessary for eukaryotic translation, while its presence enhances but is not strictly required for prokaryotes. Whether or not it is present in eukaryotic mitochondria and what role it plays are still unresolved questions. We do not know the significance of the unusual chromosomal location of 5S rRNA genes in yeast and its implication for regulation. The lack of a requirement of the La protein in yeast raises the question of what alternative maturation pathways might be present in yeast but are not present in other unicellular eukaryotes requiring the La protein. We are also learning about previously unsuspected functions involving 5S rRNA, including its role in the p53 pathway. Finally, as the number of identified binding partners for this RNA increases (i.e., P43 and P34/P37), we begin to see that the 5S rRNA biogenesis pathway may not be as limited as once thought.
References
- 1.Lee BM, Xu J, Clarkson BK, Martinez-Yamout MA, Dyson HJ, Case DA, Gottesfeld JM, Wright PE. Induced fit and “lock and key” recognition of 5S RNA by zinc fingers of transcription factor IIIA. J Mol Biol. 2006;357:275–291. doi: 10.1016/j.jmb.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Deshmukh M, Stark J, Yeh LC, Lee JC, Woolford JLJ. Multiple regions of yeast ribosomal protein L1 are important for its interaction with 5S rRNA and assembly into ribosomes. J Biol Chem. 1995;270:30148–30156. doi: 10.1074/jbc.270.50.30148. [DOI] [PubMed] [Google Scholar]
- 3.Spahn CM, Beckmann R, Eswar N, Penczek PA, Sali A, Blobel G, Frank J. Structure of the 80S ribosome from Saccharomyces cerevisiae–tRNA-ribosome and subunit-subunit interactions. Cell. 2001;107:373–386. doi: 10.1016/s0092-8674(01)00539-6. [DOI] [PubMed] [Google Scholar]
- 4.Szymanski M, Barciszewska MZ, Erdmann VA, Barciszewski J. 5S Ribosomal RNA Database. Nucleic Acids Res. 2002;30:176–178. doi: 10.1093/nar/30.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kressler D, Linder P, De la Cruz J. Protein transacting factors involved in ribosome biogenesis in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:7897–7912. doi: 10.1128/mcb.19.12.7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prohaska K, Williams N. Assembly of the Trypanosoma brucei 60S ribosomal subunit nuclear export complex requires trypanosome-specific proteins P34 and P37. Eukaryot Cell. 2009;8:77–87. doi: 10.1128/EC.00234-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox GE, Woese CR. The architecture of 5S rRNA and its relation to function. J Mol Evol. 1975;6:61–76. doi: 10.1007/BF01732674. [DOI] [PubMed] [Google Scholar]
- 8.Dohme F, Nierhaus KH. Role of 5S RNA in assembly and function of the 50S subunit from Escherichia coli. Proc Natl Acad Sci USA. 1976;73:2221–2225. doi: 10.1073/pnas.73.7.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ammons D, Rampersad J, Fox GE. 5S rRNA gene deletions cause an unexpectedly high fitness loss in Escherichia coli. Nucleic Acids Res. 1999;27:637–642. doi: 10.1093/nar/27.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korepanov AP, Gongadze GM, Garber MB, Court DL, Bubunenko MG. Importance of the 5 S rRNA-binding ribosomal proteins for cell viability and translation in Escherichia coli. J Mol Biol. 2007;366:1199–1208. doi: 10.1016/j.jmb.2006.11.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiparisov S, Petrov A, Meskauskas A, Sergiev PV, Dontsova OA, Dinman JD. Structural and functional analysis of 5S rRNA in Saccharomyces cerevisiae. Mol Genet Genomics. 2005;274:235–247. doi: 10.1007/s00438-005-0020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith MW, Meskauskas A, Wang P, Sergiev PV, Dinman JD. Saturation mutagenesis of 5S rRNA in Saccharomyces cerevisiae. Mol Cell Biol. 2001;21:8264–8275. doi: 10.1128/MCB.21.24.8264-8275.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogdanov AA, Dontsova OA, Dokudovskaya SS, Lavrik IN. Structure and function of 5S rRNA in the ribosome. Biochem Cell Biol. 1995;73:869–876. doi: 10.1139/o95-094. [DOI] [PubMed] [Google Scholar]
- 14.Dechampesme AM, Koroleva O, Leger-Silvestre I, Gas N, Camier S. Assembly of 5S ribosomal RNA is required at a specific step of the pre-rRNA processing pathway. J Cell Biol. 1999;145:1369–1380. doi: 10.1083/jcb.145.7.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Douet J, Tourmente S. Transcription of the 5S rRNA heterochromatic genes is epigenetically controlled in Arabidopsis thaliana and Xenopus laevis. Heredity. 2007;99:5–13. doi: 10.1038/sj.hdy.6800964. [DOI] [PubMed] [Google Scholar]
- 16.Hunt LT, George DG, Yeh LS, Dayhoff MO. Evolution of prokaryote and eukaryote lines inferred from sequence evidence. Orig Life. 1984;14:657–664. doi: 10.1007/BF00933718. [DOI] [PubMed] [Google Scholar]
- 17.Chow CS, Hartmann KM, Rawlings SL, Huber PW, Barton JK. Delineation of structural domains in eukaryotic 5S rRNA with a rhodium probe. Biochem Int. 1992;31:3534–3542. doi: 10.1021/bi00128a030. [DOI] [PubMed] [Google Scholar]
- 18.Luehrsen KR, Fox GE. Secondary structure of eukaryotic cytoplasmic 5S ribosomal RNA. Proc Natl Acad Sci USA. 1981;78:2150–2154. doi: 10.1073/pnas.78.4.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pieler T, Digweed M, Bartsch M, Erdmann VA. Comparative structural analysis of cytoplasmic and chloroplastic 5S rRNA from spinach. Nucleic Acids Res. 1983;11:591–604. doi: 10.1093/nar/11.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pieler T, Schreiber A, Erdmann VA. Comparative structural analysis of eubacterial 5S rRNA by oxidation of adenines in the N-1 position. Nucleic Acids Res. 1984;12:3115–3126. doi: 10.1093/nar/12.7.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sneath B, Vary C, Pavlakis G, Vournakis J. Secondary structure of Tetrahymena thermophilia 5S ribosomal RNA as revealed by enzymatic digestion and micro-densitometric analysis. Nucleic Acids Res. 1986;14:1365–1378. doi: 10.1093/nar/14.3.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nazar R, Wildeman AG. Three helical domains form a protein binding site in the 5S RNA-protein complex from eukaryotic ribosomes. Nucleic Acids Res. 1983;11:3155–3168. doi: 10.1093/nar/11.10.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiNitto JP, Huber PW. A role for aromatic amino acids in the binding of Xenopus ribosomal protein L5 to 5S rRNA. Biochem. 2001;40:12645–12653. doi: 10.1021/bi011439m. [DOI] [PubMed] [Google Scholar]
- 24.Huber PW, Rife JP, Moore PB. The structure of helix III in Xenopus oocyte 5S rRNA: an RNA stem containing a two-nucleotide bulge. J Mol Biol. 2001;312:823–832. doi: 10.1006/jmbi.2001.4966. [DOI] [PubMed] [Google Scholar]
- 25.Xiong Y, Sundaralingam M. Two crystal forms of helix II of Xenopus laevis 5S rRNA with a cytosine bulge. RNA. 2000;6:1316–1324. doi: 10.1017/s135583820000090x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun FJ, Caetano-Anolles G. The evolutionary history of the structure of 5S ribosomal RNA. J Mol Evol. 2009;69:430–443. doi: 10.1007/s00239-009-9264-z. [DOI] [PubMed] [Google Scholar]
- 27.Dinman J. 5S rRNA: Structure and Function from Head to Toe. Int J Biomed Sci. 2005;1:2–7. [PMC free article] [PubMed] [Google Scholar]
- 28.Andersen J, Delihas N, Ikenaka K, Green PJ, Pines O, Ilercil O, Inouye M. The isolation and characterization of RNA coded by the micF gene in Escherichia coli. Nucleic Acids Res. 1987;15:2089–2101. doi: 10.1093/nar/15.5.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barciszewska MZ, Erdmann VA, Barciszewski J. Ribosomal 5S RNA: tertiary structure and interactions with proteins. Biol Rev Camb Philos Soc. 1996;71:1–25. doi: 10.1111/j.1469-185x.1996.tb00740.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Harnpicharnchai P, Jakovljevic J, Tang L, Guo Y, Oeffinger M, Rout M, Hiley S, Hughes T, Woolford J. Assembly factors Rpf2 and Rrs1 recruit 5S rRNA and ribosomal proteins rpL5 and rpL11 into nascent ribosomes. Genes Dev. 2007;21:2580–2592. doi: 10.1101/gad.1569307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warner JR, Soeiro R. Nascent ribosomes from HeLa cells. Proc Natl Acad Sci USA. 1967;58:1984–1990. doi: 10.1073/pnas.58.5.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nolte A, Klussman S, Lorenz S, Bald R, Betzel C, Dauter Z, Wilson K, Furste JP, Erdmann VA. Crystallization and preliminary diffraction studies of the structural domain E of Thermus flavus 5S rRNA. FEBS Lett. 1995;374:292–294. doi: 10.1016/0014-5793(95)01136-3. [DOI] [PubMed] [Google Scholar]
- 33.Funari SS, Rapp G, Perbandt M, Dierks K, Vallazza M, Betzel C, Erdmann VA, Svergun DI. Structure of free Thermus flavus 5S rRNA at 1. 3 nm resolution from synchrotron X-ray solution scattering. J Biol Chem. 2000;275:31283–31288. doi: 10.1074/jbc.M004974200. [DOI] [PubMed] [Google Scholar]
- 34.Skibinska L, Banachowicz E, Gapinski J, Patkowski A, Barciszewski J. Structural similarity of E. coli 5S rRNA in solution and within the ribosome. Biopolymers. 2004;73:316–325. doi: 10.1002/bip.10598. [DOI] [PubMed] [Google Scholar]
- 35.Scripture JB, Huber PW. Analysis of the binding of Xenopus ribosomal protein L5 to oocyte 5S rRNA. The major determinants of recognition are located in helix III-loop C. J Biol Chem. 1995;270:27358–27365. doi: 10.1074/jbc.270.45.27358. [DOI] [PubMed] [Google Scholar]
- 36.McBryant SJ, Veldhoen N, Gedulin B, Leresche A, Foster MP, Wright PE, Romaniuk PJ, Gottesfeld JM. Interaction of the RNA binding fingers of Xenopus transcription factor IIIA with specific regions of 5S ribosomal RNA. J Mol Biol. 1995;248:44–57. doi: 10.1006/jmbi.1995.0201. [DOI] [PubMed] [Google Scholar]
- 37.Correll CC, Freeborn B, Moore PB, Steitz TA. Metals, motifs, and recognition in the crystal structure of a 5S rRNA domain. Cell. 1997;91:705–712. doi: 10.1016/s0092-8674(00)80457-2. [DOI] [PubMed] [Google Scholar]
- 38.Klein DJ, Moore PB, Steitz TA. The roles of ribosomal proteins in the structure assembly, and evolution of the large ribosomal subunit. J Mol Biol. 2004;340:141–177. doi: 10.1016/j.jmb.2004.03.076. [DOI] [PubMed] [Google Scholar]
- 39.Harms J, Schluenzen F, Zarivach R, Bashan A, Gat S, Agmon I, Bartels H, Franceschi F, Yonath A. High resolution structure of the large ribosomal subunit from a mesophilic eubacterium. Cell. 2001;107:679–688. doi: 10.1016/s0092-8674(01)00546-3. [DOI] [PubMed] [Google Scholar]
- 40.Ban N, Nissen P, Hansen J, Moore P, Steitz T. The complete atomic structure of the large ribosomal subunit at 2. 4A resolution. Science. 2000;289:905–920. doi: 10.1126/science.289.5481.905. [DOI] [PubMed] [Google Scholar]
- 41.Spahn CM, Gomez-Lorenzo MG, Grassucci RA, Jorgensen R, Andersen GR, Beckmann R, Penczek PA, Ballesta JP, Frank J. Domain movements of elongation factor eEF2 and the eukaryotic 80S ribosome facilitate tRNA translocation. EMBO J. 2004;23:1008–1019. doi: 10.1038/sj.emboj.7600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henras AK, Soudet J, Gerus M, Lebaron S, Caizergues-Ferrer M, Mougin A, Henry Y. The post-transcriptional steps of eukaryotic ribosome biogenesis. Cell Mol Life Sci. 2008;65:2334–2359. doi: 10.1007/s00018-008-8027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fatica A, Tollervey D. Making ribosomes. Curr Opin Cell Biol. 2002;14:313–318. doi: 10.1016/s0955-0674(02)00336-8. [DOI] [PubMed] [Google Scholar]
- 44.Venema J, Tollervey D. Ribosome synthesis in Saccharomyces cerevisiae. Annu Rev Genet. 1999;33:261–311. doi: 10.1146/annurev.genet.33.1.261. [DOI] [PubMed] [Google Scholar]
- 45.Ahmad Y, Boisvert FM, Gregor P, Cobley A, Lamond AI. NOPdb: Nucleolar Proteome Database–2008 update. Nucleic Acids Res. 2009;37:D181–D184. doi: 10.1093/nar/gkn804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cmarko D, Smigova J, Minichova L, Popov A. Nucleolus: the ribosome factory. Histol Histopathol. 2008;23:1291–1298. doi: 10.14670/HH-23.1291. [DOI] [PubMed] [Google Scholar]
- 47.Grandi P, Rybin V, Bassler J, Petfalski E, Strauss D, Marzioch M, Schafer T, Kuster B, Tschochner H, Tollervey D, Gavin A, Hurt E. 90S preribosomes include the 35S pre-rRNA, the U3 snoRNP, and 40S subunit processing factors but predominantly lack 60S synthesis factors. Mol Cell. 2002;10:105–115. doi: 10.1016/s1097-2765(02)00579-8. [DOI] [PubMed] [Google Scholar]
- 48.Harnipicharnchai P, Jakovljevic J, Horsey E, Miles T, Roman J, Rout M, Meagher D, Imai B, Guo Y, Brame CJ, et al. Comparsion and functional characterization of yeast 66S ribosome assembly intermediates. Mol Cell. 2001;8:805–815. doi: 10.1016/s1097-2765(01)00344-6. [DOI] [PubMed] [Google Scholar]
- 49.Ho JH, Kallstron G, Johnson AW. Nascent 60S ribosomal subunits enter the free pool bound by Nmd3p. RNA. 2000;6:1625–1634. doi: 10.1017/s1355838200001291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gadal O, Strauss D, Kessl J, Trumpower B, Tollervey D, Hurt E. Nuclear export of 60s ribosomal subunits depends on Xpo1p and requires a nuclear export sequence-containing factor, Nmd3p, that associates with the large subunit protein Rpl10p. Mol Cell Biol. 2001;21:3405–3415. doi: 10.1128/MCB.21.10.3405-3415.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas F, Kutay U. Biogenesis and nuclear export of ribosomal subunits in higher eukaryotes depend on the CRM1 export pathway. J Cell Sci. 2003;116:2409–2419. doi: 10.1242/jcs.00464. [DOI] [PubMed] [Google Scholar]
- 52.Eisinger D, Dick F, Trumpower B. Qsr1p, a 60S ribosomal subunit protein, is required for joining of 40S and 60S subunits. Mol Cell Biol. 1997;17:5136–5145. doi: 10.1128/mcb.17.9.5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pestova T, Kolupaeva V, Lomakin B, Pilipenko E, Shatsky I, Agol V, Hellen C. Molecular mechanisms of translation initiation in eukaryotes. Proc Natl Acad Sci USA. 2001;98:7028–7036. doi: 10.1073/pnas.111145798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Hoof A, Lennertz P, Parker R. Three conserved members of the RNase D family have unique and overlapping functions in the processing of 5S, 5. 8S, U4, U5, RNase MRP and RNase P RNAs in yeast. EMBO J. 2000;19:1357–1365. doi: 10.1093/emboj/19.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Brien CA, Wolin SL. A possible role for the 60-kD Ro autoantigen in a discard pathway for defective 5S rRNA precursors. Genes Dev. 1994;8:2891–2903. doi: 10.1101/gad.8.23.2891. [DOI] [PubMed] [Google Scholar]
- 56.Kuai L, Fang F, Butler JS, Sherman F. Polyadenylation of rRNA in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2004;101:8581–8586. doi: 10.1073/pnas.0402888101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fulnecek J, Kovarik A. Low abundant spacer 5S rRNA transcripts are frequently polyadenylated in Nicotiana. Mol Genet Genomics. 2007;278:565–573. doi: 10.1007/s00438-007-0273-6. [DOI] [PubMed] [Google Scholar]
- 58.Miyazaki M. Studies on the nucleotide sequence of pseudouridine-containing 5S RNA from Saccharomyces cerevisiae. J Biochem. 1974;75:1407–1410. doi: 10.1093/oxfordjournals.jbchem.a130532. [DOI] [PubMed] [Google Scholar]
- 59.Guddat U, Bakken AH, Pieler T. Protein-mediated nuclear export of RNA: 5S rRNA containing small RNPs in Xenopus oocytes. Cell. 1990;60:619–628. doi: 10.1016/0092-8674(90)90665-2. [DOI] [PubMed] [Google Scholar]
- 60.Rinke J, Steitz JA. Precursor molecules of both human 5S ribosomal RNA and transfer RNAs are bound by a cellular protein reactive with anti-La lupus antibodies. Cell. 1982;29:149–159. doi: 10.1016/0092-8674(82)90099-x. [DOI] [PubMed] [Google Scholar]
- 61.Westermann S, Weber K. Cloning and recombinant expression of the La RNA-binding protein from Trypanosoma brucei. Biochim Biophys Acta. 2000;1492:483–487. doi: 10.1016/s0167-4781(00)00113-5. [DOI] [PubMed] [Google Scholar]
- 62.Wolin SL, Cedervall T. The La protein. Annu Rev Biochem. 2002;71:375–403. doi: 10.1146/annurev.biochem.71.090501.150003. [DOI] [PubMed] [Google Scholar]
- 63.Eichler DC, Craig N. Processing of eukaryotic ribosomal RNA. Prog Nucleic Acid Res Mol Biol. 1994;49:197–239. doi: 10.1016/s0079-6603(08)60051-3. [DOI] [PubMed] [Google Scholar]
- 64.Steitz JA, Berg C, Hendrick JP, La-Branche-Chabot H, Metspalu A, Rinke J, Yario T. A 5S rRNA/L5 complex is a precursor to ribosome assembly in mammalian cells. J Cell Biol. 1988;1988:545–556. doi: 10.1083/jcb.106.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoo CJ, Wolin SL. The yeast La protein is required for the 3′ endonucleolytic cleavage that matures tRNA precursors. Cell. 1997;89:393–402. doi: 10.1016/s0092-8674(00)80220-2. [DOI] [PubMed] [Google Scholar]
- 66.Yoo CJ, Wolin SL. La proteins from Drosophila melanogaster and Saccharomyces cerevisiae: a yeast homolog of the La autoantigen is dispensable for growth. Mol Cell Biol. 1994;14:5412–5424. doi: 10.1128/mcb.14.8.5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stefano JE. Purified lupus antigen La recognizes an oligouridylate stretch common to the 3′ termini of RNA polymerase III transcripts. Cell. 1984;36:145–154. doi: 10.1016/0092-8674(84)90083-7. [DOI] [PubMed] [Google Scholar]
- 68.Maraia RJ, Intine RV. Recognition of nascent RNA by the human La antigen: conserved and divergent features of structure and function. Mol Cell Biol. 2001;21:367–379. doi: 10.1128/MCB.21.2.367-379.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Foldynova-Trantirkova S, Paris Z, Sturm NR, Campbell DA, Lukes J. The Trypanosoma brucei La protein is a candidate poly(U) shield that impacts spliced leader RNA maturation and tRNA intron removal. Int J Parasitol. 2005;35:359–366. doi: 10.1016/j.ijpara.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 70.Alfano C, Sanfelice D, Babon J, Kelly G, Jacks A, Curry S, Conte MR. Structural analysis of cooperative RNA binding by the La motif and central RRM domain of human La protein. Nat Struct Mol Biol. 2004;11:323–329. doi: 10.1038/nsmb747. [DOI] [PubMed] [Google Scholar]
- 71.Dong G, Chakshusmathi G, Wolin SL, Reinisch KM. Structure of the La motif: a winged helix domain mediates RNA binding via a conserved aromatic patch. EMBO J. 2004;23:1000–1007. doi: 10.1038/sj.emboj.7600115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Teplova M, Yuan YR, Phan AT, Malinina L, Ilin S, Teplov A, Patel DJ. Structural basis for recognition and sequestration of UUU(OH) 3′ temini of nascent RNA polymerase III transcripts by La, a rheumatic disease autoantigen. Mol Cell. 2006;21:75–85. doi: 10.1016/j.molcel.2005.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee Y, Nazar RN. Ribosomal 5S rRNA maturation in Saccharomyces cerevisiae. J Biol Chem. 1997;272:15206–15212. doi: 10.1074/jbc.272.24.15206. [DOI] [PubMed] [Google Scholar]
- 74.Deshmukh M, Tsay YF, Paulovich AG, Woolford JL., Jr Yeast ribosomal protein L1 is required for the stability of newly synthesized 5S rRNA and the assembly of 60S ribosomal subunits. Mol Cell Biol. 1993;13:2835–2845. doi: 10.1128/mcb.13.5.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Isoda N, Tanaka T, Ishikawa K. Isolation of a 5S RNA-protein L5 complex from 60S subunits of rat liver ribosomes by cesium sulfate density-gradient equilibrium centrifugation. J Biochem. 1981;90:551–554. doi: 10.1093/oxfordjournals.jbchem.a133504. [DOI] [PubMed] [Google Scholar]
- 76.Park JW, Bae YS. Phosphorylation of ribosomal protein L5 by protein kinase CKII decreases its 5S rRNA binding activity. Biochem Biophys Res Commun. 1999;263:475–481. doi: 10.1006/bbrc.1999.1345. [DOI] [PubMed] [Google Scholar]
- 77.Rosorius O, Fries B, Stauber R, Hirschmann N, Bevec D, Hauber J. Human ribosomal protein L5 contains defined nuclear localization and export signals. J Biol Chem. 2000;275:12061–12068. doi: 10.1074/jbc.275.16.12061. [DOI] [PubMed] [Google Scholar]
- 78.Moradi H, Simoff I, Bartish G, Nygard O. Functional features of the C-terminal region of yeast ribosomal protein L5. Mol Gen Genomics. 2008;280:337–350. doi: 10.1007/s00438-008-0369-7. [DOI] [PubMed] [Google Scholar]
- 79.Szymanski M, Barciszewska MZ, Erdmann VA, Barciszewski J. An analysis of G-U base pair occurrence in eukaryotic 5S rRNAs. Mol Biol Evol. 2000;17:1194–1198. doi: 10.1093/oxfordjournals.molbev.a026402. [DOI] [PubMed] [Google Scholar]
- 80.Zhang J, Williams N. Purification, cloning and expression of two closely related Trypanosoma brucei nucleic acid binding proteins. Mol Biochem Parasitol. 1997;87:145–158. doi: 10.1016/s0166-6851(97)00060-1. [DOI] [PubMed] [Google Scholar]
- 81.Zhang J, Ruyechan W, Williams N. Developmental regulation of two nuclear RNA binding proteins, p34 and p37, from Trypanosoma brucei. Mol Biochem Parasitol. 1998;92:79–88. doi: 10.1016/s0166-6851(97)00228-4. [DOI] [PubMed] [Google Scholar]
- 82.Hellman K, Ciganda M, Brown S, Li J, Ruyechan W, Williams N. Two trypanosome-specific proteins are essential factors for 5S rRNA abundance and ribosomal assembly in Trypanosoma brucei. Eukaryot Cell. 2007;6:1766–1772. doi: 10.1128/EC.00119-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pitula J, Ruyechan WT, Williams N. Two novel RNA binding proteins from Trypanosoma brucei are associated with 5S rRNA. Biochem Biophys Res Commun. 2002;290:569–576. doi: 10.1006/bbrc.2001.6226. [DOI] [PubMed] [Google Scholar]
- 84.Hellman K, Prohaska K, Williams N. Trypanosoma brucei RNA binding proteins p34 and p37 mediate NOPP44/46 cellular localization via the exportin 1 pathway. Eukaryot Cell. 2007;6:2206–2213. doi: 10.1128/EC.00176-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Acker J, Ozanne C, Kachouri-Lafond R, Gaillardin C, Neuveglise C, Marck C. Dicistronic tRNA-5S rRNA genes in Yarrowia lipolytica: an alternative TFIIIA-independent way for expression of 5S rRNA genes. Nucleic Acids Res. 2008;36:5832–5844. doi: 10.1093/nar/gkn549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Neely LS, Lee BM, Xu J, Wright PE, Gottesfeld JM. Identification of a minimal domain of 5S ribosomal RNA sufficient for high affinity interactions with the RNA-specific zinc fingers of transcription factor IIIA. J Mol Biol. 1999;291:549–560. doi: 10.1006/jmbi.1999.2985. [DOI] [PubMed] [Google Scholar]
- 87.Allison LA, North MT, Murdoch KJ, Romaniuk PJ, Deschamps S, le Maire M. Structural requirements of 5S rRNA for nuclear transport, 7S ribonucleoprotein particle assembly, and 60S ribosomal subunit assembly in Xenopus oocytes. Mol Cell Biol. 1993;13:6819–6831. doi: 10.1128/mcb.13.11.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Picard B, le Maire M, Wegnez M, Denis H. Biochemical Research on oogenesis. Composition of the 42-S storage particles of Xenopus laevisoocytes. Eur J Biochem. 1980;109:359–368. doi: 10.1111/j.1432-1033.1980.tb04802.x. [DOI] [PubMed] [Google Scholar]
- 89.Zhang WQ, Romaniuk PJ. Characterization of the 5S RNA binding activity of Xenopus zinc finger protein p43. J Mol Biol. 1995;245:549–558. doi: 10.1006/jmbi.1994.0045. [DOI] [PubMed] [Google Scholar]
- 90.Bhatia SS, Weiss TC, Romaniuk PJ. Contribution of Individual Amino Acids to the 5S RNA Binding Activity of the Xenopus Zinc Finger Protein p43. Biochem. 2008;47:8398–8405. doi: 10.1021/bi800080c. [DOI] [PubMed] [Google Scholar]
- 91.Adams KL, Palmer JD. Evolution of mitochondrial gene content: gene loss and transfer to the nucleus. Mol Phylogenet Evol. 2003;29:380–395. doi: 10.1016/s1055-7903(03)00194-5. [DOI] [PubMed] [Google Scholar]
- 92.Yoshionari S, Koike T, Yokogawa T, Nishikawa K, Ueda T, Miura K, Watanabe K. Existence of nuclear-encoded 5S-rRNA in bovine mitochondria. FEBS Lett. 1994;338:137–142. doi: 10.1016/0014-5793(94)80351-x. [DOI] [PubMed] [Google Scholar]
- 93.Lang BF, Goff LJ, Gray MW. A 5S rRNA gene is present in the mitochondrial genome of the protist Reclinomonas americana but is absent from red algal mitochondrial DNA. J Mol Biol. 1996;261:407–413. doi: 10.1006/jmbi.1996.0486. [DOI] [PubMed] [Google Scholar]
- 94.Smirnov A, Tarassov I, Mager-Heckel AM, Letzelter M, Martin RP, Krasheninnikov IA, Entelis N. Two distinct structural elements of 5S rRNA are needed for its import into human mitochondria. RNA. 2008;14:749–759. doi: 10.1261/rna.952208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wormington WM, Brown DD. Onset of 5S RNA gene regulation during Xenopus embryogenesis. Dev Biol. 1983;99:248–257. doi: 10.1016/0012-1606(83)90273-7. [DOI] [PubMed] [Google Scholar]
- 96.Barciszewska MZ, Szymankski M, Erdmann VA, Barciszewski J. 5S Ribosomal RNA. Biomacromolecules. 2000:1. doi: 10.1021/bm000293o. [DOI] [PubMed] [Google Scholar]
- 97.Allison LA, North MT, Neville LA. Differential binding of oocyte-type and somatic-type 5S rRNA to TFIIIA and ribosomal protein L5 in Xenopus oocytes: specialization for storage versus mobilization. Dev Biol. 1995;168:284–295. doi: 10.1006/dbio.1995.1080. [DOI] [PubMed] [Google Scholar]
- 98.Allison LA, Romaniuk PJ, Bakken AH. RNA-protein interactions of stored 5S RNA with TFIIIA and ribosomal protein L5 during Xenopus oogenesis. Dev Biol. 1991;144:129–144. doi: 10.1016/0012-1606(91)90485-l. [DOI] [PubMed] [Google Scholar]
- 99.Claussen M, Rudt F, Pieler T. Functional modules in ribosomal protein L5 for ribonucleoprotein complex formation and nucleocytoplasmic transport. J Biol Chem. 1999;274:33951–33958. doi: 10.1074/jbc.274.48.33951. [DOI] [PubMed] [Google Scholar]