Regulation of cell–cell adhesion by the cadherin–catenin complex (original) (raw)
. Author manuscript; available in PMC: 2012 Jun 6.
Published in final edited form as: Biochem Soc Trans. 2008 Apr;36(Pt 2):149–155. doi: 10.1042/BST0360149
Abstract
Ca2+-dependent cell–cell adhesion is regulated by the cadherin family of cell adhesion proteins. Cadherins form _trans-_interactions on opposing cell surfaces which result in weak cell–cell adhesion. Stronger cell–cell adhesion occurs by clustering of cadherins and through changes in the organization of the actin cytoskeleton. Although cadherins were thought to bind directly to the actin cytoskeleton through cytoplasmic proteins, termed _α_- and _β_-catenin, recent studies with purified proteins indicate that the interaction is not direct, and instead an allosteric switch in _α_-catenin may mediate actin cytoskeleton reorganization. Organization and function of the cadherin–catenin complex are additionally regulated by phosphorylation and endocytosis. Direct studies of cell–cell adhesion has revealed that the cadherin–catenin complex and the underlying actin cytoskeleton undergo a series of reorganizations that are controlled by the Rho GTPases, Rac1 and RhoA, that result in the expansion and completion of cell–cell adhesion. In the present article, in vitro protein assembly studies and live-cell studies of de novo cell–cell adhesion are discussed in the context of how the cadherin–catenin complex and the actin cytoskeleton regulate cell-cell adhesion.
Keywords: actin, cadherin, catenin, cell–cell adhesion, cytoskeleton, Rho GTPase
Overview
Cell–cell adhesion is involved in all aspects of tissue morphogenesis in multicellular organisms, including regulating cell shape, movement and sorting into complex organizations in tissues and organs [1,2]. In addition to dynamic changes in cell–cell contacts, tissue morphogenesis requires remodelling of the actin cytoskeleton to effect changes in cell shape and dynamics. Thus insight into mechanisms that regulate cellular dynamics during tissue morphogenesis requires not only an understanding of cell–cell adhesion, but also an understanding of how the actin cytoskeleton is remodelled.
Epithelial cell–cell adhesion is mediated by a variety of membrane proteins, including classical cadherins, claudins/ occludin, nectin and desmosomal cadherins [1-4]. Classical cadherins are required to initiate cell–cell contacts, and other adhesion protein complexes subsequently assemble, including the tight junction, which controls paracellular diffusion [5], and desmosomes, which maintain the structural continuum of the epithelium [6].
Cadherins are single-membrane-spanning proteins with a divergent extracellular domain of five repeats and a conserved cytoplasmic domain [7]. Binding between extracellular domains, which requires Ca2+ for protein conformation [8], is thought to involve multiple _cis_-dimers of cadherin [9] that form _trans_-oligomers between cadherins on opposing cell surfaces [10]. Binding between cadherin extracellular domains is weak [10], but strong cell–cell adhesion develops during lateral clustering of cadherins (see below). Clustering of cadherins was thought to depend on linkage through cytoplasmic catenins to the actin cytoskeleton since _β_-catenin binds directly to both the cadherin cytoplasmic domain [11] and to the actin-binding protein _α_-catenin [12], and it was assumed, but had not been shown directly, that _α_-catenin simply linked the E-cadherin–_β_-catenin complex to the actin cytoskeleton [13,14]. However, recent studies have shown that interactions between the cadherin–catenin complex and the actin cytoskeleton are more complex and dynamic [15] (Figure 1).
Figure 1. Protein-protein interactions between cadherins, catenins and the actin cytoskeleton.
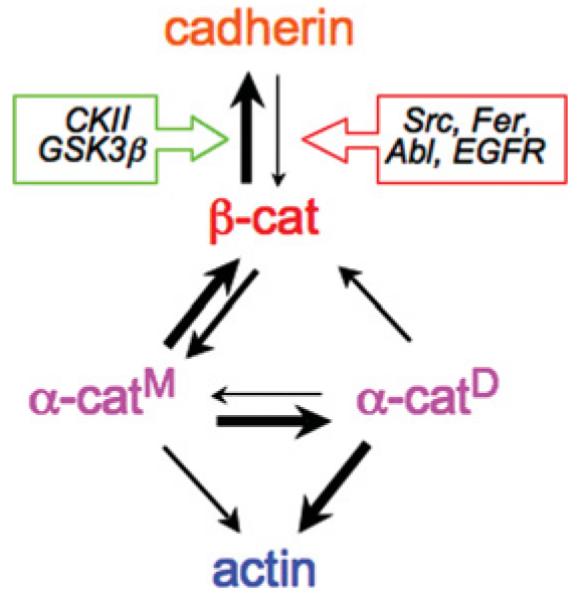
Protein interactions formed between cadherin, _β_-catenin (_β_-cat), _α_-catenin monomers (_α_-catM), _α_-catenin dimers (_α_-catD) and actin. Differences in the thickness of the arrows represent strengths of protein-protein interactions (i.e. increased thickness shows increased binding). The interaction between cadherin and β_-catenin is regulated by kinases that increase (green box: CK2 and GSK3_β) or decrease (red box: Src, Fer, Abl and EGFR) the binding affinity.
In addition to roles in cell–cell adhesion, the actin cytoskeleton plays important roles in regulating plasma membrane dynamics, cell migration and cell shape through the local activation of nucleators of actin polymerization such as the Arp2/3 (actin-related protein 2/3) complex [16]. The actin cytoskeleton has different organizations during cell migration (branched actin arrays [16]) and at cell–cell contacts (parallel actin bundles [17]), but it is unclear how actin polymerization and organization are regulated when migratory cells form cadherin-mediated cell–cell adhesion and become quiescent. A reversal of these phenotypes also occurs during development and wound healing, and in diseases such as cancer when dynamic cell movement is (re-)initiated as stationary contacting cells are induced to become migratory. Several mechanisms may be involved in regulating cell–cell adhesion, including changes in strengths of interactions within the cadherin–catenin complex through phosphorylation, endocytosis of cadherins and by local regulation of the actin cytoskeleton by _α_-catenin. These regulatory mechanisms are summarized and placed in the context of recent studies of de novo adhesion between pairs of epithelial cells.
Regulation of the cadherin–catenin complex by phosphorylation and endocytosis
The structural integrity of the cadherin–catenin complex is positively and negatively regulated by kinases that are often up-regulated during dynamic cell movements in development and in cancer. Three serine residues in the cadherin cytoplasmic domain (Ser684, Ser686 and Ser692) are phosphorylated by the protein kinases CK2 and GSK3_β_ (glycogen synthase kinase 3_β_), which creates additional interactions with _β_-catenin resulting in a large increase in the affinity of the interaction (picomolar affinity [11]). In contrast, tyrosine phosphorylation of _β_-catenin at Tyr489 or Tyr654 disrupts binding to cadherin, and at Tyr142 disrupts binding to _α_-catenin [18]. Src phosphorylates _β_-catenin at Tyr654 [19]. Other tyrosine kinases phosphorylate _β_-catenin at Tyr489 (Abl [20]), Tyr654 {EGFR (epidermal growth factor receptor) [21]} and Tyr142 (Fer [22]) (Figure 1).
Tyrosine kinase phosphorylation of _β_-catenin is balanced by protein tyrosine phosphatases that bind _β_-catenin and cadherin. The non-receptor protein tyrosine phosphatase PTP1B (protein tyrosine phosphatase 1B) regulates cadherin-based adhesion by binding directly to the cadherin cytoplasmic domain and dephosphorylating _β_-catenin at Tyr654 [23]. Phosphorylation of PTP1B at Tyr152 is required for the interaction with cadherin [20]. The tyrosine kinase Fer appears to be responsible for phosphorylating PTP1B. Fer binds to the cadherin-binding protein p120, which would promote binding of PTP1B to cadherin and dephosphorylation of _β_-catenin at Tyr654. Inhibiting the p120–Fer interaction prevents dephosphorylation of _β_-catenin at Tyr654 and disrupts the cadherin–catenin complex [24].
Although a considerable amount is known about sites of phosphorylation in the cadherin–catenin complex and about activated kinases such as Src perturbing cell–cell adhesion (for example, see [25]), little is known about how specific kinases are activated or target the complex during cell–cell adhesion. Recent studies of the inactivation of N-cadherin following binding of the secreted axon guidance cue Slit to its receptor, Robo, have shown a regulatory role of _β_-catenin phosphorylation on cell–cell adhesion [26]. In this case, a complex of Slit-bound Robo, Abl tyrosine kinase and N-cadherin-associated _β_-catenin is formed; Abl-mediated phosphorylation of _β_-catenin at Tyr489 results in uncoupling of the _β_-catenin-N-cadherin complex. The result of _β_-catenin phosphorylation is the loss of N-cadherin function, and targeting of phospho-Tyr489-_β_-catenin to the nucleus where it activates gene expression [26]. Further studies, however, are needed to examine changes in the phosphorylation status of the cadherin–catenin complex during initiation and loss of cell–cell adhesion in other cell types, particularly epithelial cells.
In addition to _β_-catenin, another related protein termed p120-catenin binds to the juxtamembrane region of the cytoplasmic domain of classical cadherins [27]. Like _β_-catenin, p120-catenin binding to cadherin is regulated by phosphorylation. Phosphorylation of p120-catenin increases binding affinity to E-cadherin [22]. Association of p120-catenin with E-cadherin has been proposed to stabilize E-cadherin at the plasma membrane during the formation of cell–cell contacts [28]. siRNA (small interfering RNA)-mediated knockdown of p120-catenin [29] and competitive expression of other cadherins [30] suggest that p120-catenin increases the retention of the cadherin complex at the plasma membrane and prevents cadherin internalization and degradation.
One mechanism of targeting cadherin for degradation involves Hakai, an E3-ubiquitin ligase, which binds E-cadherin in a Src phosphorylation-dependent manner [31]. Expression of Hakai increased both the ubiquitination and rate of E-cadherin endocytosis [31], but it is not known whether p120-catenin binding is involved in this degradation pathway. It is important to note, however, that loss of p120-catenin in an E-cadherin-null background has also been shown to increase cell–cell adhesion, raising the possibility that p120-catenin plays additional roles in modulating cell–cell adhesion [32].
In vitro analysis of cadherin, catenin and actin interactions
Although the cadherin–catenin complex includes the actin-binding protein _α_-catenin [13], it had not been shown whether the complex bound directly to the actin cytoskeleton, as had been generally assumed [14]. This model has now been tested directly with purified proteins [33,34]. The results of this analysis showed that the association of the cadherin–catenin complex with the actin cytoskeleton is more complex than previously thought (reviewed in [15]). Although a 1:1:1 stoichiometric complex of cadherin (cytoplasmic domain), _β_-catenin and _α_-catenin could be reconstituted in vitro, as reported previously [12], an interaction of the complex with actin filaments could not be demonstrated. Furthermore, direct comparison of the dynamics of the cadherin–catenin complex with those of actin in whole epithelial cells using FRAP (fluorescence recovery after photobleaching) showed that, although E-cadherin, _β_-catenin and _α_-catenin had similar recovery profiles and mobile fractions, actin immediately adjacent to cell–cell contacts was much more dynamic [34]. These results appeared to be in conflict with a previous study showing that _α_-catenin is just an actin-binding and -bundling protein bound to cadhenin [13]. However, a resolution of these apparently disparate results was the finding that _α_-catenin exists as either a monomer or a homodimer, and that the homodimer has a higher affinity for actin filaments than the monomer; conversely, the _α_-catenin monomer has a higher affinity for _β_-cadherin bound to cadherin than the homodimer [33] (Figure 1). Two key experiments underscored these conclusions: (i) a chimaeric monomeric protein comprising the _α_-catenin-binding domain of _β_-catenin fused to _α_-catenin, which folds to form an intermolecular complex similar to that of the _β_-catenin-_α_-catenin heterodimer, did not bind actin filaments; and (ii) when a preassembled purified ternary complex of cadherin (cytoplasmic domain), _β_-catenin and _α_-catenin was incubated with actin filaments, only _α_-catenin, but not the remaining cadherin–catenin complex, pelleted with actin filaments; under these experimental conditions, the source of the actin-binding fraction of _α_-catenin must have been the pre-assembled cadherin–catenin complex, indicating that _α_-catenin can dissociate from the complex, presumably homodimerize and bind actin filaments [33].
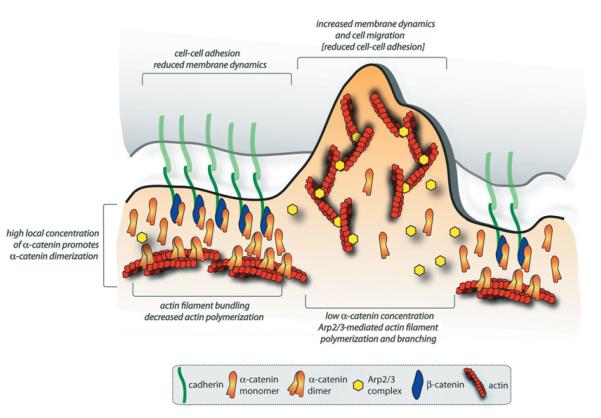
In addition to the fact that _α_-catenin homodimers bind (and bundle) actin filaments, additional studies revealed that _α_-catenin homodimers suppress Arp2/3-mediated actin polymerization by competing directly with the Arp2/3 complex for binding to actin filaments [33]; recall that actin bundles are associated with strong cell–cell contacts [17], whereas Arp2/3-mediated actin polymerization is required for dynamic membrane organization (lamellipodia) and cell migration [16]. These results suggest new roles for _α_-catenin in local regulation of actin assembly and organization at sites of cell–cell adhesion (see below and Figure 2).
Figure 2. A model for regulation of cytoskeleton and membrane dynamics by the cadherin–catenin complex.
See the text for details.
Dynamics of the cadherin–catenin complex and actin cytoskeleton during de novo cell–cell adhesion
High-resolution live-cell imaging of normal MDCK (Madin–Darby canine kidney) epithelial cells showed that functional E-cadherin–GFP (green fluorescent protein) and associated catenins are immediately recruited to initial cell–cell contacts where they become progressively immobilized into puncta, more of which are added as the contact expands laterally [35]. In a model in which actin filaments are associated directly with the cadherin–catenin complex, it would be expected that actin filaments would become concentrated and reorganized around cadherin–catenin puncta as the cell–cell contact expanded. Indeed, actin cables have been observed to impinge on cadherin puncta during formation of cell–cell adhesion by keratinocytes [36], a stratified epithelium, although these actin cables were present during cell migration before cell–cell adhesion and appeared to coalesce and sharpen further upon retraction of lamellae along the cell–cell contact [37]. Analysis of sites of actin filament assembly indicated that new assembly was occurring at the tips of filaments that appeared to be closely associated with cadherin puncta [36].
On the other hand, studies of simple epithelia, such as MDCK cells, revealed that the cortical bundle of actin associated with the periphery of migrating cells initially frayed and then dissociated beneath sites of cell–cell contact [38,39]. This resulted in relatively few actin filaments associated directly with sites of cell–cell adhesion; those that remained appeared to be mostly associated with dynamic lamellipodia [38,39] that were generally localized to the periphery of the expanding cell–cell contact ([38], see also [40]) and contained Arp3–GFP [39]. The ends of the cortical actin bundle localized to the edges of the expanding MDCK cell–cell contact and eventually relocated to the periphery of the adhering cells. Analysis of sites of actin filament assembly in MDCK cell–cell contacts revealed little if any new assembly within the established cell–cell contact that contained the cadherin–catenin complex, but new assembly occurred at the periphery of the contact where the ends of the cortical actin bundle are located and integrin-based cell adhesion complexes are also concentrated [39]; it would be interesting to determine whether the sites of actin assembly at cadherin puncta in adhering keratinocytes (see above) were also associated with integrin-based adhesions. It remains unclear whether differences in the location of actin filaments and sites of actin assembly between stratified epithelial keratinocytes and simple epithelial MDCK cells are due to cell-type difference, perhaps associated with different functions of the cells; clearly, further work is needed to identify where actin assembly occurs and how actin assembly is regulated locally and excluded from other sites.
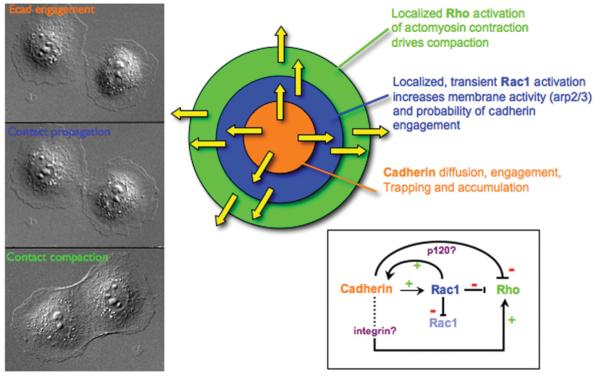
Analysis of the expansion of cell–cell contacts in MDCK cells indicated diverse roles for the actin cytoskeleton in cell–cell adhesion, including localized lamellipodia activity involved in initiating contact between adjacent cells, and actomyosin contraction in later stages of cell–cell adhesion (compaction) [39]. Lamellipodia activity is mediated by Rac1 control of actin dynamics by locally regulating the activity of actin nucleators, such as the Arp2/3 complex [16]. Rac1 is activated upon E-cadherin adhesion [41,42] and Rac1 protein co-localizes with E-cadherin during initial cell–cell adhesion [38,42]. Direct analysis of subcellular sites of Rac1 activity, using FRET (fluorescence resonance energy transfer), revealed that Rac1 activity is restricted to regions of newly forming cell–cell contacts, but was suppressed in regions of established cell–cell contacts, resulting in a zone of Rac1 activity at the edges of the expanding cell–cell contact [39]. Diminished Rac1 activity, and hence membrane dynamics, in the newly formed cell–cell contact might allow weak _trans_-interactions between E-cadherin on opposing membranes to be maintained. At present, it is not known how Rac1 is localized to, and selectively activated at, new cell–cell adhesions. Rac1 activation may be mediated directly by local activation of PI3K (phosphoinositide 3-kinase) [43] and accumulation of phosphoinositides that recruit guanine-exchange factors [43,44]; indeed, Rac1 distribution at the edge of expanding MDCK cell–cell contacts is similar to that of PH (pleckstrin homology)-Akt-GFP, a readout for phosphoinositides [38] (Figure 3). However, PI3K activity is not required for either Rac1 or E-cadherin accumulation at cell–cell contacts [38,45] suggesting that Rac1 recruitment and activation might also involve interactions with protein complexes associated with cell–cell adhesion [46].
Figure 3. A model for propagation of cell–cell adhesion in which two zones of Rho family GTPase activity are restricted to the edges of the cell–cell contact as it expands laterally.
Left: cell–cell adhesion between pairs of MDCK cells involves initial E-cadherin engagement, followed by propagation and finally compaction of the contact. Upper-right: the initial zone comprises a zone of cadherin engagement (orange); a zone of active Rac1 and its downstream effectors, the Arp2/3 complex and lamellipodia, localized to de novo contacts between cells at the edges of the zone of cadherin engagement (blue); and the zone of RhoA and its downstream effector actomyosin contractility, restricted to the edges of the contact and is required to drive expansion and completion of cell–cell adhesion (green). The activity zone of Rac1 is transient and rapidly diminishes as cadherin accumulates, but a new round of activation occurs at the periphery of the contacting membranes that would push the membranes together to initiate new E-cadherin adhesion. These sequential signalling zones comprising E-cadherin accumulation, Rac1-induced lamellipodia and RhoA-induced actomyosin contraction co-ordinate the induction, propagation and expansion of the cell–cell contact. Lower-right: a model representing regulatory interactions between cadherin, p120-catenin, Rho GTPases and integrins (-, negative regulation; +, positive regulation) (see the text for details).
If the cadherin–catenin complex is not bound directly to the actin cytoskeleton as indicated by in vitro studies with purified proteins (see above), how might the actin-binding/bundling protein _α_-catenin regulate these local changes in actin organization during initial cell–cell adhesion? It has been suggested that changes in actin organization are due to the formation and local concentration of _α_-catenin dimers [15,33] (Figure 2). Clustering of the cadherin–catenin complex upon cell–cell adhesion, perhaps by diffusion-mediated trapping [47], would result in an increase in the local concentration of _α_-catenin sufficient for dimerization of _α_-catenin, from monomer dissociating from the cadherin–catenin complex [33] and in the cytoplasm. _α_-Catenin homodimers could then locally inhibit Arp2/3-mediated actin polymerization [33], which would result in local changes in the branched organization of the actin cytoskeleton, and decreases in actin polymerization [39] and membrane (lamellipodia) dynamics [38] (Figure 2). Although analyses of MDCK cell–cell adhesion are consistent with these ideas of _α_-catenin functions, further studies are needed to test whether _α_-catenin dimers indeed form at cell–cell contacts, and whether _α_-catenin dimers are involved directly in the decrease in actin polymerization and membrane activity observed in live cells.
Observations of simple epithelial MDCK cell–cell adhesion showed that the latter stages of contact formation appeared to require an active process involving actomyosin contraction. Previous studies have reported that activated (phospho-) myosin II localized to cell–cell contacts and that disruption of regulatory pathways controlling myosin II activation affected the maintenance and reformation of disrupted cell–cell contacts in confluent cell monolayers [48-50]. However, more recent studies analysed the spatiotemporal regulation of RhoA activity using FRET and actomyosin contractility during de novo cell–cell adhesion between pairs of cells [39]. These studies revealed that active RhoA and phosphomyosin II were excluded from the centre of the contact and restricted to the cortical actin bundle in a zone at the outside edges of cell–cell contacts, where G-actin was also incorporated (see above and Figure 3). To define the directionality of actomyosin contractility, low concentrations of latrunculin D were used to cap the barbed ends of actin filaments and then the distribution of actin filaments was observed; these studies revealed that actomyosin contractile forces were directed outwards and backwards from the cell–cell contact [39]. This direction of actomyosin contraction would have the effect of pulling the edges of the contacting membranes outwards to fully expand the contact to the width of the cells; in the opposite direction to that assumed to occur during resealing of cell–cell contacts in cell monolayers [48,49,51], indicating that different contractile mechanisms are involved during formation of de novo cell–cell adhesion and reformation of cell–cell contacts between cells in established monolayers. At present, it is not clear how the barbed ends of the cortical actin bundle are anchored at the edges of the contact during de novo cell–cell adhesion, although high-resolution TIRF (total internal reflection fluorescence) microscopy indicates that they are closely localized with cadherin–catenin complexes and integrin-based focal adhesions [39]. Since the cadherin–catenin complex does not bind actin directly [33,34] it is possible that, at these sites, actin may be anchored by integrin-based focal adhesions [52], but further studies on actin linkages to integrins and cross-talk with the cadherin–catenin complex are required to resolve the mechanism(s) involved.
At present it is unclear how RhoA activity is localized to the outer edges of the expanding cell–cell contact. RhoA activation and actomyosin contraction could be induced by local clustering of integrin-mediated adhesions at the edge of the cell–cell contact [52]. Alternatively, or in combination, RhoA activity could be suppressed in the centre of the expanding contact by p120-catenin localized with cadherin along the contact [53]; however, further studies will be needed to test these possibilities directly.
Future perspectives
Previous studies of the structural and functional organization of the cadherin–catenin complex have revealed that the cadherin–catenin complex is not simply linked directly to the actin cytoskeleton, but may, through newly uncovered roles for _α_-catenin, locally regulate actin cytoskeleton organization and polymerization by the Arp2/3 complex [33,34]. Live-cell analysis of MDCK cell–cell adhesion has begun to test these new properties of the cadherin–catenin complex. These studies indicate that the actin cytoskeleton does not directly associate with, or polymerize around the cadherin–catenin complex, but instead undergoes dramatic reorganization as the contact expands [34,54]. In MDCK cells, the reorganization of the actin cytoskeleton coincides with decreases in actin polymerization and membrane dynamics at stabilized cell–cell contacts, which are consistent with decreased Arp2/3 complex activity in regions of high concentrations of the cadherin–catenin complex [39]; further studies are required, however, to test whether _α_-catenin dimers are involved directly in regulating Arp2/3 and membrane activity. Localized and transient up-regulation of membrane dynamics by Rac1, and actomyosin contraction by RhoA, appears to initialize further cell–cell contacts and maximally expand the contact respectively. Despite these new insights, much remains unknown, including: definitive evidence of _α_-catenin dimerization from clustered cadherin–catenin complexes; roles for _α_-catenin homodimers in locally regulating actin and membrane dynamics; and mechanisms locally up-regulating and then suppressing Rho family GTPases. In addition, it is unclear how changes in cadherin–catenin phosphorylation or endocytosis are regulated under normal conditions of cell–cell adhesion, or how these processes might affect protein dynamics and functions of the cadherin–catenin complex. Further studies of protein dynamics in live cells, and manipulation of different pools of _α_-catenin may provide further molecular insights into functions of the cadherin–catenin complex in regulating cell–cell adhesion.
Acknowledgments
Work from the Nelson laboratory is supported by the NIH (National Institutes of Health) (grant GM35527).
Abbreviations used
Arp2/3
actin-related protein 2/3
EGFR
epidermal growth factor receptor
FRET
fluorescence resonance energy transfer
GFP
green fluorescent protein
GSK3_β_
glycogen synthase kinase 3_β_
MDCK
Madin-Darby canine kidney
PI3K
phosphoinositide 3-kinase
PTP1B
protein tyrosine phosphatase 1B.
References
- 1.Takeichi M. Morphogenetic roles of classic cadherins. Curr. Opin. Cell Biol. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- 2.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 3.Takai Y, Nakanishi H. Nectin and afadin: novel organizers of intercellular junctions. J. Cell Sci. 2003;116:17–27. doi: 10.1242/jcs.00167. [DOI] [PubMed] [Google Scholar]
- 4.Yamada S, Nelson WJ. Synapses: sites of cell recognition, adhesion and functional specification. Annu. Rev. Biochem. 2007;76:267–294. doi: 10.1146/annurev.biochem.75.103004.142811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu. Rev. Physiol. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- 6.Getsios S, Huen AC, Green KJ. Working out the strength and flexibility of desmosomes. Nat. Rev. Mol. Cell Biol. 2004;5:271–281. doi: 10.1038/nrm1356. [DOI] [PubMed] [Google Scholar]
- 7.Gumbiner BM. Regulation of cadherin adhesive activity. J. Cell Biol. 2000;148:399–404. doi: 10.1083/jcb.148.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pertz O, Bozic D, Koch AW, Fauser C, Brancaccio A, Engel J. A new crystal structure, Ca2+ dependence and mutational analysis reveal molecular details of E-cadherin homoassociation. EMBO J. 1999;18:1738–1747. doi: 10.1093/emboj/18.7.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brieher WM, Yap AS, Gumbiner BM. Lateral dimerization is required for the homophilic binding activity of C-cadherin. J. Cell Biol. 1996;135:487–496. doi: 10.1083/jcb.135.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen CP, Posy S, Ben-Shaul A, Shapiro L, Honig BH. Specificity of cell-cell adhesion by classical cadherins: critical role for low-affinity dimerization through β-strand swapping. Proc. Natl. Acad. Sci. U.S.A. 2005;102:8531–8536. doi: 10.1073/pnas.0503319102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huber AH, Weis WI. The structure of the β-catenin/ E-cadherin complex and the molecular basis of diverse ligand recognition by β-catenin. Cell. 2001;105:391–402. doi: 10.1016/s0092-8674(01)00330-0. [DOI] [PubMed] [Google Scholar]
- 12.Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J. Cell Sci. 1994;107:3655–3663. doi: 10.1242/jcs.107.12.3655. [DOI] [PubMed] [Google Scholar]
- 13.Rimmimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. α1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc. Natl. Acad. Sci. U.S.A. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gates J, Peifer M. Can 1000 reviews be wrong? Actin, α-catenin, and adherens junctions. Cell. 2005;123:769–772. doi: 10.1016/j.cell.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Weis WI, Nelson WJ. Re-solving the cadherin-catenin-actin conundrum. J. Biol. Chem. 2006;281:35593–35599. doi: 10.1074/jbc.R600027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 17.Hirokawa N, Keller TC, 3rd, Chasan R, Mooseker MS. Mechanism of brush border contractility studied by the quick-freeze, deep-etch method. J. Cell Biol. 1983;96:1325–1336. doi: 10.1083/jcb.96.5.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lilien J, Balsamo J, Arregui C, Xu G. Turn-off, drop-out: functional state switching of cadherins. Dev. Dyn. 2002;224:18–29. doi: 10.1002/dvdy.10087. [DOI] [PubMed] [Google Scholar]
- 19.Piedra J, Martinez D, Castano J, Miravet S, Dunach M, de Herreros AG. Regulation of β-catenin structure and activity by tyrosine phosphorylation. J. Biol. Chem. 2001;276:20436–20443. doi: 10.1074/jbc.M100194200. [DOI] [PubMed] [Google Scholar]
- 20.Rhee J, Lilien J, Balsamo J. Essential tyrosine residues for interaction of the non-receptor protein-tyrosine phosphatase PTP1B with N-cadherin. J. Biol. Chem. 2001;276:6640–6644. doi: 10.1074/jbc.M007656200. [DOI] [PubMed] [Google Scholar]
- 21.Hoschuetzky H, Aberle H, Kemler R. β-Catenin mediates the interaction of the cadherin-catenin complex with epidermal growth factor receptor. J. Cell Biol. 1994;127:1375–1380. doi: 10.1083/jcb.127.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piedra J, Miravet S, Castano J, Palmer HG, Heisterkamp N, Garcia de Herreros A, Dunach M. p120 catenin-associated Fer and Fyn tyrosine kinases regulate β-catenin Tyr-142 phosphorylation and β-catenin-α-catenin interaction. Mol. Cell. Biol. 2003;23:2287–2297. doi: 10.1128/MCB.23.7.2287-2297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balsamo J, Leung T, Ernst H, Zanin MK, Hoffman S, Lilien J. Regulated binding of PTP1B-like phosphatase to N-cadherin: control of cadherin-mediated adhesion by dephosphorylation of β-catenin. J. Cell Biol. 1996;134:801–813. doi: 10.1083/jcb.134.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu G, Craig AW, Greer P, Miller M, Anastasiadis PZ, Lilien J, Balsamo J. Continuous association of cadherin with β-catenin requires the non-receptor tyrosine-kinase Fer. J. Cell Sci. 2004;117:3207–3219. doi: 10.1242/jcs.01174. [DOI] [PubMed] [Google Scholar]
- 25.Matsuyoshi N, Hamaguchi M, Taniguchi S, Nagafuchi A, Tsukita S, Takeichi M. Cadherin-mediated cell-cell adhesion is perturbed by v-src tyrosine phosphorylation in metastatic fibroblasts. J. Cell Biol. 1992;118:703–714. doi: 10.1083/jcb.118.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhee J, Buchan T, Zukerberg L, Lilien J, Balsamo J. Cables links Robo-bound Abl kinase to N-cadherin-bound β-catenin to mediate Slit-induced modulation of adhesion and transcription. Nat. Cell Biol. 2007;9:883–892. doi: 10.1038/ncb1614. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds AB, Roczniak-Ferguson A. Emerging roles of p120-catenin in cell adhesion and cancer. Oncogene. 2004;23:7947–7956. doi: 10.1038/sj.onc.1208161. [DOI] [PubMed] [Google Scholar]
- 28.Thoreson MA, Anastasiadis PZ, Daniel JM, Ireton RC, Wheelock MJ, Johnson KR, Hummingbird DK, Reynolds AB. Selective uncoupling of p120ctn from E-cadherin disrupts strong adhesion. J. Cell Biol. 2000;148:189–202. doi: 10.1083/jcb.148.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J. Cell Biol. 2003;163:525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maeda Y, Johnson E, Mandal SH, Lawson KR, Keim SA, Svoboda RA, Caplan S, Wahl JK, 3rd, Wheelock MJ, Johnson KR. Expression of inappropriate cadherins by epithelial tumor cells promotes endocytosis and degradation of E-cadherin via competition for p120ctn. Oncogene. 2006;25:4595–4604. doi: 10.1038/sj.onc.1209396. [DOI] [PubMed] [Google Scholar]
- 31.Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrerns J, Sommer T, Birchmeier W. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat. Cell Biol. 2002;4:222–231. doi: 10.1038/ncb758. [DOI] [PubMed] [Google Scholar]
- 32.Yanagisawa M, Anastasiadis P. p120 catenin is essential for mesenchymal cadherin-mediated regulation of cell motility and invasivenes. J. Cell Biol. 2006;174:1087–1096. doi: 10.1083/jcb.200605022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. α-Catenin is a molecular switch that binds E-cadherin-β-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–90135. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adams CL, Chen YT, Smith SJ, Nelson WJ. Mechanisms of epithelial cell-cell adhesion and cell compaction revealed by high-resolution tracking of E-cadherin-green fluorescent protein. J. Cell Biol. 1998;142:1105–1119. doi: 10.1083/jcb.142.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- 37.Vaezi A, Bauer C, Vasioukhin V, Fuchs E. Actin cable dynamics and Rho/Rock orchestrate a polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium. Dev. Cell. 2002;3:367–381. doi: 10.1016/s1534-5807(02)00259-9. [DOI] [PubMed] [Google Scholar]
- 38.Ehrlich JS, Hansen MD, Nelson WJ. Spatio-temporal regulation of Rac1 localization and lamellipodia dynamics during epithelial cell-cell adhesion. Dev. Cell. 2002;3:259–270. doi: 10.1016/s1534-5807(02)00216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J. Cell Biol. 2007;178:517–527. doi: 10.1083/jcb.200701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krendel MF, Bonder EM. Analysis of actin filament bundle dynamics during contact formation in live epithelial cells. Cell Motil. Cytoskeleton. 1999;43:296–309. doi: 10.1002/(SICI)1097-0169(1999)43:4<296::AID-CM3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 41.Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J. Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakagawa M, Fukata M, Yamaga M, Itoh N, Kaibuchi K. Recruitment and activation of Rac1 by the formation of E-cadherin-mediated cell-cell adhesion sites. J. Cell Sci. 2001;114:1829–1838. doi: 10.1242/jcs.114.10.1829. [DOI] [PubMed] [Google Scholar]
- 43.Kovacs EM, Ali RG, McCormack AJ, Yap AS. E-cadherin homophilic ligation directly signals through Rac and phosphatidylinositol 3-kinase to regulate adhesive contacts. J. Biol. Chem. 2002;277:6708–6718. doi: 10.1074/jbc.M109640200. [DOI] [PubMed] [Google Scholar]
- 44.Malliri A, van Es S, Huveneers S, Collard JG. The Rac exchange factor Tiam1 is required for the establishment and maintenance of cadherin-based adhesions. J. Biol. Chem. 2004;279:30092–30098. doi: 10.1074/jbc.M401192200. [DOI] [PubMed] [Google Scholar]
- 45.Nakajima Y, Okamoto M, Nishimura H, Obata K, Kitano H, Sugita M, Matsuyama T. Neuronal expression of mint1 and mint2, novel multimodular proteins, in adult murine brain. Brain Res. 2001;92:27–42. doi: 10.1016/s0169-328x(01)00126-7. [DOI] [PubMed] [Google Scholar]
- 46.Hansen MD, Ehrlich JS, Nelson WJ. Molecular mechanism for orienting membrane and actin dynamics to nascent cell-cell contacts in epithelial cells. J. Biol. Chem. 2002;277:45371–45376. doi: 10.1074/jbc.M207747200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singer SJ. In: In Surface Membrane Receptors: Interface Between Cells and Their Environment. Bradshaw RA, Frazier WA, Merrell RC, Gottlieb DI, Hogue-Angeletti RA, editors. Plenum Press; New York: 1976. pp. 1–24. [Google Scholar]
- 48.Ivanov AI, Hunt D, Utech M, Nusrat A, Parkos CA. Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol. Biol. Cell. 2005;16:2636–2650. doi: 10.1091/mbc.E05-01-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krendel M, Gloushankova NA, Bonder EM, Feder HH, Vasiliev JM, Gelfand IM. Myosin-dependent contractile activity of the actin cytoskeleton modulates the spatial organization of cell-cell contacts in cultured epitheliocytes. Proc. Natl. Acad. Sci. U.S.A. 1999;96:9666–9670. doi: 10.1073/pnas.96.17.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shewan AM, Maddugoda M, Kraemer A, Stehbens SJ, Verma S, Kovacs EM, Yap AS. Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell-cell contacts. Mol. Biol. Cell. 2005;16:4531–4542. doi: 10.1091/mbc.E05-04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J, Betson M, Erasmus J, Zeikos K, Bailly M, Cramer LP, Braga VM. Actin at cell-cell junctions is composed of two dynamic and functional populations. J. Cell Sci. 2005;118:5549–5562. doi: 10.1242/jcs.02639. [DOI] [PubMed] [Google Scholar]
- 52.DeMali KA, Wennerberg K, Burridge K. Integrin signaling to the actin cytoskeleton. Curr. Opin. Cell Biol. 2003;15:572–582. doi: 10.1016/s0955-0674(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 53.Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, Reynolds AB. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell. 2006;127:1027–1039. doi: 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 54.Perez TD, Tamada M, Sheetz MP, Nelson WJ. Immediate-early signaling induced by E-cadherin engagement and adhesion. J. Biol. Chem. 2007;283:5014–5022. doi: 10.1074/jbc.M705209200. [DOI] [PMC free article] [PubMed] [Google Scholar]