EUROGIN 2011 roadmap on prevention and treatment of HPV-related disease (original) (raw)
. Author manuscript; available in PMC: 2013 Nov 1.
Published in final edited form as: Int J Cancer. 2012 Jul 2;131(9):1969–1982. doi: 10.1002/ijc.27650
Abstract
The EUROGIN 2011 roadmap reviews the current burden of HPV (human papillomavirus)-related morbidity, as well as the evidence and potential practice recommendations regarding primary and secondary prevention and treatment of cancers and other disease associated with HPV infection.
HPV infection causes approximately 600,000 cases of cancer of the cervix, vulva, vagina, anus and oropharynx annually, as well as benign diseases such as genital warts and recurrent respiratory papillomatosis. Whereas the incidence of cervical cancer has been decreasing over recent decades, the incidence of anal and oropharyngeal carcinoma, for which there are no effective screening programs, has been rising over the last couple of decades.
Randomised trials have demonstrated improved efficacy of HPV-based compared to cytology-based cervical cancer screening. Defining the best algorithms to triage HPV-positive women, age ranges and screening intervals are priorities for pooled analyses and further research, whereas feasibility questions can be addressed through screening programmes.
HPV vaccination will reduce the burden of cervical precancer and probably also of invasive cervical and other HPV-related disease in women. Recent trials demonstrated that prophylactic vaccination also protects against anogenital HPV infection, ano-genital intraepithelial lesions and warts associated with vaccine types, in males; and anal HPV infection and anal intraepithelial neoplasia in MSM. HPV-related oropharyngeal cancer could be treated less aggressively because of better survival compared to cancers of the oropharynx unrelated to HPV.
Key findings in the field of cervical cancer prevention should now be translated in cost-effective strategies, following an organised approach integrating primary and secondary prevention, according to scientific evidence but adapted to the local situation with particular attention to regions with the highest burden of disease.
Keywords: cervical cancer, vulvar cancer, anal cancer, penile cancer, head & neck cancer, genital warts, incidence, mortality, human papillomavirus, HPV, screening, vaccination
INTRODUCTION
A multidisciplinary group of experts from five continents have summarised the highlights of the last EUROGIN conference entitled “HPV Associated Diseases and Cancer: From Reality Now to the Future” (Lisbon, Portugal; 8-11 May, 2011). As in previous three EUROGIN reports, the fourth EUROGIN Roadmap updates knowledge on the current burden and recent trends of cervical cancer and discusses the development of new policies incorporating HPV-based cervical cancer screening in developed and developing countries. In addition, this fourth Eurogin Roadmap describes recent experiences and early effects of HPV vaccine introduction and addresses also the primary prevention of precursors of vulvar, anal and penile cancer, experimental treatment of vulvar intraepithelial neoplasia, potential screening for anal cancer in high-risk groups and the prevention of anogenital disease through male circumcision. Finally, particular attention is focused on the increased incidence of HPV-related oropharyngeal cancer and new prognostic insights which encourage treatment modifications in HPV-positive patients with oropharyngeal squamous cell carcinoma (OSCC).
DISEASES RELATED TO HPV INFECTION
hrHPV infection is causally related to cancer of the cervix, vagina, vulva, anal canal, penis and oropharynx1.
Cervical cancer
HPV is detectable in virtually 100% of cervical cancer cases2, although individual studies may show lower estimates which are generally explained by technical issues. HPV16 is the most common type and combined with HPV18 account for over 70% of all cases of cervical cancer3,4.
Other ano-genital (pre-)cancers
HPV may cause over 70% of all cancers of vagina and anus, whereas HPV attribution for penile and vulva cancers is lower ranging from 40% to 47% (Table 1). Most vulvar cancers (92%) are squamous cell carcinomas5. HPV prevalence is high in vulvar intraepithelial neoplasia (VIN) (>80%) and in invasive vulvar cancers of the basaloid/warty type (86%) but only 6% in keratinizing squamous vulvar carcinoma6,7,8. HPV16 accounts for 85% of HPV-positive vulvar cancers.
Table 1.
Cancers associated with high-risk HPV infection and with HPV16 and 18 infection.
| Number of cancers | |||||
|---|---|---|---|---|---|
| Site | Attributableto hrHPV | Of whichHPV16/18 | Total | Attributableto hrHPV | Attributableto HPV16/18 |
| Cervix | 100% 2 | 71% 4 | 529,500 19 | 529,500 | 375,945 |
| Penis | 47% 9 | 74% 9 | 26,300 23 | 12,361 | 9,098 |
| Vulva | 40% 95 | 93% 95 | 30,000 23 | 12,000 | 11,100 |
| Vagina | 70% 95 | 93% 95 | 15,000 23 | 10,500 | 9,750 |
| Anus (female) | 84% 95 | 94% 95 | 15,900 23 | 13,356 | 12,561 |
| Anus (male) | 84% 95 | 94% 95 | 14,5900 23 | 12,180 | 11,455 |
| Oro-pharynx (female) | 19% 96† | 89% 13 | 12,900 97 | 2,394 | 2,138 |
| Oro-pharynx (male) | 19% 96† | 89% 13 | 48,900 97 | 9,291 | 8,299 |
| All sites (females) | 9.4% | 6.8% | 6,044,710 | 567,750 | 411,494 |
| All sites (males) | 0.5% | 0.4% | 6,617,844 | 33,832 | 28,852 |
| All sites (both sexes) | 4.8% | 3.5% | 12,662,554 | 601,582 | 440,346 |
Approximately 95% of invasive penile cancers are squamous cell carcinomas (SCC)9,10. HPV is commonly detected in basaloid and warty tumours, but is less common in keratinizing and verrucous tumours. Approximately 60-100% of penile intraepithelial neoplasia (PIN) lesions are HPV DNA positive. In invasive penile tumours, HPV16 was the most common type detected (40%), followed by HPV6 (22%), HPV52 (15%), and HPV11 (4%)11.
In a recent study, HPV DNA was found in 97% of 366 anal cancers. HPV 16 was the most prevalent genotype (75%). HPV16 or18 were found in 78% of all cases12.
Oropharyngeal cancer
HPV attribution for oropharynx cancers varies between studies and anatomical sub-sites (5-70%)13. A recent meta-analysis showed that HPV prevalence in head-and-neck tumours increased significantly from 41% prior to 2000 to 72% after 2004 and that HPV16 accounted for 96% of HPV-positive OSCC14. Further, HPV prevalence was higher among OSCC in North-America (60%) versus Europe (40%) and all other regions (33%). Interestingly, regional differences were significant only prior to 2000. Trends were independent of methods used for HPV detection. It appears that within two decades, HPV has replaced tobacco and alcohol as the major cause of OSCC in North-America and Western-Europe14.
Cancer of the oral cavity
The role for HPV in the pathogenesis of oral cavity carcinomas remains controversial. A meta-analysis of the association between oral HPV infection and oral cavity SCC and potentially malignant disorders was performed15. It was estimated that any oral HPV or HPV16 infection confers a four-fold increase in the odds of developing oral cavity cancer (OR=3.98, 95%CI:2.62-6.02 and OR=3.86, 95%CI:2.16-6.87, respectively). A similar four-fold increase in the odds of potentially malignant oral lesions was also observed. The causal relation between oral cancer or precancerous conditions cannot be established with certainty since misclassification of OSCC as oral cavity cancers and alternative explanations cannot be excluded. Moreover, other recent large case-control studies reported no association between HPV and oral cavity carcinoma16. Further research is needed to clarify the etiological role of HPV in oral cancers.
Lesions associated with low-risk HPV
Genital warts are largely attributable to HPV types 6 and 11 although co-infections with hr-HPV are also frequently detected17. These two HPV types also cause the majority of RRP18.
BURDEN OF HPV-RELATED DISEASE
Cervical cancer
Approximately 530,000 new cases of cervical cancer were estimated for 200818. This number could increase to ~665,000 by 2020, if current trends and demographic effects are taken into account. Cervical cancer is the third most common cancer in women worldwide and the second most common in developing regions (www.who.int/hpvcentre).18,19
Approximately 47% of new annual cervical cancer cases are diagnosed in women aged <50 years, whereas this proportion is only 26% for all cancers. Eighty-six percent of the global burden occurs in less developed regions, where it accounts for 13% of all cancers in women19. Cervical cancer is the most common cancer in women in Sub-Saharan Africa, South-Central Asia and Melanesia. Incidence rates are low (world age-standardised incidence rate [ASIR] <6 per 100,000) in Western-Asia, North-America and Australia/New-Zealand19.
Worldwide, the ratio of mortality to incidence is 52%. An estimated 275,000 women died from cervical cancer in 2008, about 88% of which occurred in less developed regions19. Overall, 0.9% of women die from the disease before the age of 75 years.
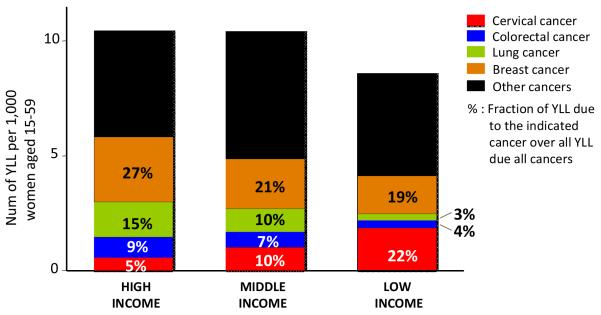
Cervical cancer contributed 3.4 million years of life lost (YLL) worldwide in 2004, and was the greatest single cause of YLL from cancer in women from low-income countries accounting for 20% of premature cancer deaths (22% in women aged 15-59 years) (see Figure 1)16. Cervical cancer is a paradigm of global health disparity; it takes a toll on young women from the poorest countries and the most disadvantaged populations.
Figure 1.
Years of life lost (YLL) lost to cancer in women aged 15-59 y by income of the country.
Cancer of the vulva and the vagina
An estimated 30,000 and 15,000 new cases of cancer of the vulva and the vagina, respectively, occur annually (ASIR=0.2-1.6/100,000 and 0.3-0.5/100,000, worldwide)20. Vulvar cancer accounts for approximately 4% of gynaecological malignancies21. The incidence of vulvar cancer and VIN has been reported to increase in recent years, particularly among younger women22.
Anal cancer
Globally, there are about 30,400 new cases every year23. Since the 1970s, the incidence of anal cancer has been increasing in developed countries by about 2% per year in the general population24. The median age of diagnosis of anal cancer is 57 years among men and 68 years among women. Anal cancer is more common in certain high-risk groups; these include: MSM (men having sex with men) 25, anyone with a history of anal warts or high-grade CIN/VIN/cervical or vulvovaginal cancer; immunosuppressed populations, including those with human immunodeficiency virus (HIV) infection and organ graft recipients)26.
In the general population, anal cancer affects more women than men23. Between 1998 and 2003, in the United States, the average annual incidence of anal cancer was 1.0/100,000 among men and 1.5/100,00027 among women. Between 2003 and 2007, the incidence of anal cancer had risen to 1.4/100,000 among men and 1.8/100,000 among women. The incidence of anal cancer among MSM was estimated to be as high as 37/100,000 prior to the onset of the HIV epidemic28, and is even higher among HIV-seropositive MSM29. The advent of antiretroviral therapy has not led to a reduction in the incidence of anal cancer30. The incidence may continue to increase as this population lives longer with HIV disease.
Penile cancer
Globally, the annual burden for penile cancer has been estimated to be 26,300 cases23 with incidence rates strongly correlating with those of cervical cancer31. Invasive penile cancer is rare and most commonly affects men aged 50-70 years. Incidence of penile cancer in the US is highest among Hispanics and men who live in the Southern US or areas with high levels of poverty32. Incidence is also higher in less developed countries, where penile cancer accounts for up to 10% of male cancers in some parts of Africa, South America and Asia10. PIN lesions are rare.
Oropharyngeal cancer
About 137,000 new cases of cancer of the pharynx (excluding nasopharynx) and 96,000 associated deaths occurred worldwide in 200823. The majority of head and neck cancers are associated with high tobacco and alcohol consumption. HPV has been mainly associated with the oropharynx (e.g. tonsil and tongue base)33. In these locations, HPV detection ranges from 5-64%, making overall HPV burden difficult to estimate16,34. High and increasing prevalence rates have been reported recently in the US, Canada, the Netherlands, Finland, Sweden, United Kingdom and Australia. Increased practice of oral sex has been postulated as an explanation in these societies where smoking, a major risk factor, is decreasing although the natural history is still unclear.
Incidence rates for OSCC and tonsillar cancer, in particular, have significantly increased over the last three decades in several countries. Through direct analyses of tumours, HPV is considered as the underlying cause of this increase in the US35, Sweden36 and Australia14. In the US, incidence rates for HPV-positive OSCC increased by 225% from 1988 to 2004, whereas rates for HPV-negative cancer declined by 50%35. Similar trends were observed in Sweden, where the proportion of HPV-positive OSCC increased from ~23 to 93% from 1970 to 200736. In all countries, rates increased more sharply in younger birth cohorts, consistent with the hypothesis that sexual behavioural changes have led to increased HPV exposure while, concomitantly, tobacco exposure has declined.
Genital warts
Two to eleven of sexually active men and women in the general population of the US or European countries report ever being diagnosed with genital warts37-39. Incidence rates vary from 1 to 2 per 1000 person-years with highest rates in 16-24 year-old females (up to 1% episodes per annum) and slightly lower rates in 25-29 year old males40-42.
PREVENTION OF CERVICAL CANCER
Screening in high resource settings
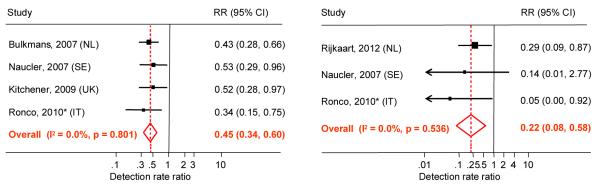
Recently, randomised controlled trials (RCTs) have provided evidence that HPV-based screening is more effective than cytology-based cervical screening43. In Europe, four randomised trials consistently showed, in the second screening round, a significant reduction in the incidence of CIN3+ (average relative risk [RR] of 0.45; 95%CI 0.34-0.60)44, and even of even of invasive cancer (average RR=0.22; 95%CI 0.08-0.58 [3 trials]) by screening with a validated HPV assay compared with cytology (Figure 2)45-49. The specificity of HPV-based screening is lower than screening with cytology, but this loss of specificity could be minimised by avoiding HPV screening in young women, using more specific HPV tests, and by appropriate triage algorithms. Most currently available evidence from RCTs indicates that reflex cytology could be recommended for triage of HPV-positive women. Other candidate markers for triage, which could be considered, but for which evidence is today still insufficient, are: restricted HPV genotyping (types 16 and 18), p16 immunocytochemistry or p16Ki67 double staining. Also HPV screening using a more specific test such as the APTIMA RNA assay50 or Hybrid Capture-2 at a higher viral load cut-off51 increases specificity and PPV with no or a small loss in cross-sectional sensitivity51. The results from the RCTs suggest that HPV screening in women older than 30-35 years, followed by cytology triage of HPV-positive women does not cause substantial increases in diagnostic work-up and over-treatment. This knowledge can now be transferred into pilot implementation in organised and quality-controlled programmes to demonstrate feasibility. Further research is needed to optimise the screening protocols with HPV, such as age to start and screening intervals. The planned pooled analysis of individual data of the RCTs will be crucial for these points. The Netherlands is the first country with an official recommendation to introduce HPV-based primary screening.
Figure 2.
Meta-analysis of the main outcomes from randomised trials comparing HPV- and cytology-based cervical cancer screening. Relative detection rate of CIN3+ (left panel) and cervical cancer (right panel), observed in the second screening round among women who were HPV-negative versus cytology-negative at enrolment. * restricted to women 35 years or older.
Management of screen-positive women
Management of HPV-positive women requires further research. Recent interesting results from the combined use of genotyping and cytology are available52. However, comparison with other possible markers, such as p16 and mRNA, both in terms of cross-sectional and longitudinal accuracy, is needed to find optimal strategies for diagnostic work-up53.
Testing for hr-HPV DNA has been shown to be an efficient triage tool for ASC-US cytology in the framework of cytology-based screening54 and has been widely implemented in clinical practice. However, the high prevalence of hr-HPV DNA among women with LSIL results limits the utility of hr-HPV testing for this cytology category54. Among women with ASC-US, those positive for HPV16 or HPV18 have the highest risk of high grade CIN compared to those positive for other hr-types55, potentially warranting different management strategies. Several biomarkers, including hr-HPV RNA and cellular proliferation markers have been evaluated for cytology triage. In triage of ASC-US, p16INK4a and the APTIMA-mRNA assay showed higher specificity and similar sensitivity compared to HC2. In LSIL triage, both tests showed increased specificity but, sensitivity for cervical precancer was lower for p16INK4a but similar for APTIMA 56,57. Correct ascertainment of high grade CIN in women referred for abnormal screening test results can be compromised at the level of colposcopy and at the level of cervical histology. Increasing the number of biopsies during colposcopic evaluation improves the detection of CIN358,59. There is an ongoing debate as to whether taking multiple random, or multiple directed biopsies, is the more efficient approach. The incremental benefit of taking multiple directed biopsies is currently being evaluated in the NCI-led Biopsy Study. Structured colposcopy teaching has been also suggested to improve colposcopic accuracy. Recently, it was demonstrated that evaluation of cervical histology in conjunction with p16 staining improves reproducibility and can achieve similar accuracy as expert pathologist adjudication of conventional histology slides60,61.
Screening in low resource settings
Cervical cancer prevention efforts in the past 15 years have focussed on alternative technologies to cytology screening and approaches allowing management of screen-positive women at the same time as the screening visit (“screen and treat”).
An RCT, conducted in South-Africa, used HPV testing with HC2 and VIA testing in un-screened women aged 35-65 years62. In Arms 1 and 2, all HPV- and VIA-positive women, respectively, were treated with cryotherapy without colposcopy/histology confirmation. In Arm 3 (control), management was delayed. After a follow-up of 36 months, there was a sustained significant decrease in the detection of CIN2+ lesions in arm 1 (1.5%) and arm 2 (3.8%), compared to the control arm (5.6%), corresponding with a risk ratio of 0.27 (95%CI:0.17-0.43) and 0.68 (95%CI:0.50:0.92), respectively.
Another landmark RCT enrolled 131,746 Indian women aged 30-59 years who were assigned to screening with 1) HPV testing with HC2, 2) cytological testing, 3) VIA or 4) routine care without screening as the control group63. Women who had positive tests underwent colposcopy with directed biopsies and those with cervical cancer precursors were treated. The 8-year cumulative incidence of cervical cancer stage-2 or higher and death rates from cervical cancer were significantly reduced in women screened with HC2 (hazard ratios of 0.47, 95%CI:0.32-0.69 and 0.52, 95%CI;0.33-0.83, respectively), whereas no significant reductions were observed in the VIA or cytology arms. Further, the age-standardised incidence rate of invasive cancer among women who had negative test results with cytological or VIA testing was more than four times greater the rate among HPV-negative women.
These data provide evidence for the superior performance of HPV DNA testing as a primary screening compared to VIA and cytology and demonstrated feasibility and effectiveness of screen and treatment approaches.
Recently, a large population-based screening program was set up in China, and currently covers 10 million women aged 35-59 years who are offered screening with cytology or VIA64. The low-cost careHPV assay, which can be easily used in field conditions, was shown to have a sensitivity and specificity for detection of CIN2+ (90 and 84%, respectively) comparable to HC2 which requires laboratory infrastructure65. These results are encouraging and may enable the use of HPV testing in developing countries at an affordable cost.
HPV vaccination
Vaccination coverage
According to the WHO (2010), 33 countries are using the HPV vaccine as part of their national immunization programme, mainly in developed countries. Coverage rates come from a variety of sources and will be standardised through the WHO. They are highest in countries with organised programmes, usually though school-based delivery (see Table 2).
Table 2.
HPV vaccination policies and coverage (for the third dose) of prophylactic HPV vaccination in a selection of developed countries (web table)
| Country | Region | Organisation | System | Targetgroup | Period | Vaccine | Definitioncoverage | Coverage(3rddose) | Reportdate | Source |
|---|---|---|---|---|---|---|---|---|---|---|
| Australia | Wholecountry | Organised, school-based | Routine | 12-13 y | Since2009 | 4-valent | 12-13 y*14-15 y | 73%72% | Mar/11 | http://www.health.gov.au/internet/immunise/publishing.nsf/Content/immunise-hpv |
| Organised, school-based +GPs+community providers | Catch-up | 12-26 y | 2007-2009 | 4-valent | 16-17 y18-19 y20-26 y | 66%38%30% | Mar/11 | |||
| Belgium | Wholecountry | Opportunistic (partiallyreimbursed) | On pre-scriptionbyphysician | 12-18 y | Since Nov2007: 12-15 y;since Dec2008: 12-18 y | 2 & 4-valent | Dec, 2009C1991C1992C1993C1994C1995 | 10%69%64%51%37% | Oct/11 | WIV/IMA 2011; Arbyn,Gynecol Obstet Invest2010; Simoens, Fabri etal, Eurosurveillance2009Lefevere, Vaccine 2012 |
| FlemishCommunity | Organised | Routine | 1st yrsecondaryschool; (GPs,paediatricians) | Since Sep2010 | 4-valent | C1998(school yr2010-11)* | 83% | Oct/11 | www.zorg-en-gezondheid.be/HPV/ | |
| FrenchCommunity | Organised | Routine | 2nd yrsecondaryschool | Planned tostart inSep 2012 | 2-valent | - | - | - | www.sante.cfwb.be | |
| Canada | BritishColumbia | Organised | Routine | Grade 6 and9 | SinceSeptember2008 | 4-valent | Grade 6(2008)Grade 9(2008) | 62%62% | - | |
| Quebec | Organised | Routine | Grade 4 and9. Doses atmonths 0 and2 and year 5 | SinceSeptember2008 | 4-valent | Grade 4,1st 2doses(2008) | 80% | - | - | |
| Grade 9,3rd dose(2008) | 81% | |||||||||
| Ontario | Organised | Routine | Grade 8 | Since Sept2007 | 4-valent | - | - | - | ||
| Denmark | Wholecountry | Organised | Routine,via GPs | 12 y | Since Jan2009 | 4-valent | C1993C1994C1995 | 77%82%83% | May/11 | www.ssi.dk.EPI-NEWS,National Surveillance ofCommunicable Diseases,Statens Serum Institut,Dept. of Epidemiology,Copenhagen, No. 18,2011 |
| Catch-up,via GPs | Cohorts1993-95 Oct08- Dec 10(13-16 y) | Since Jan2009 | 4-valent | C1996C1997 | 79%70% | May/11 | ||||
| France | Wholecountry | Opportunistic (partiallyreimbursed) | On pre-scriptionbyphysician | Priority: 14 yAdolescents15-23 y ifnot or <1 yafter startsexualactivity | Since Jul2007 | 4-valent* | C1991*C1992C1993C1994 | 25%28%24%15% | Aug/11 | http://www.invs.sante.fr/publications/2010/ |
| the Nether-lands | Wholecountry | Organised: masscampaigns by GGDs* | Routine | 12 y | Since2010 | 2-valent | C1997 | 52% | Feb/11 | www.rivm.nl |
| Catch-up | Cohorts1993-96 (age13-17) | In 2010only | 2-valent | C1993-96 | 52% | Feb/11 | ||||
| New Zealand | Wholecountry | Organised | Routine | 13 y | Schoolyr2009-10:Schooly2010-11 | 4-valent | C1997C1998 | 49%34% | Oct/11 | |
| NewZealand | Wholecountry | Organised by GPs | Catch-up | Girls 14-20 y | 2008-11 | 4-valent | C1990-91C1992-96 | 41%49% | Oct/11 | |
| Spain | Wholecountry | Organised (schoolbased or via GPs) | Routine | 1-year cohortin the 12-14(last yrprimaryschool) | Since2009 | 2-valent& 4-valent | Schoolyear2009-10,12-14 y | :64% | Sep/2011 | www.msc.es/profesionales/saludPublica/prevPromocion/vacunaciones/coberturas.htm |
| UK | Scotland | Organised, school-based | Routine | 2nd yrsecondaryschool (aged~12-13 y) | Since Sep2008 | 2-valent | School yr2009-10 | 87% | Aug/11 | www.isdscotland.org/ |
| Organised, school-based | Catch-up | 4th & 5th yrsecondaryschool (aged~14-16y) | Sep 2008-Sep 2011 | 2-valent | School yr2009-10 | 80% | Aug/11 | |||
| Routine& catch-upcombined | All targetsgroup above* | Since Sep2008 | 2-valent | C1990C1991C1992C1993C1994C1995C1996 | 32%51%69%68%80%89%86% | Feb/11 | ||||
| UK | England | Organised, school-based | Routine | 12-13 y | Sinceschoolyear2008/09 | 2-valent | ~C1996~C1997 | 84%76% | Dec/10 | http://www.dh.gov.uk/health/category/publications/ |
| Organised, school-based+GPs+communitycentres | Catch-up | 13-18 y | Schoolyears2009/10&2010/11 | 2-valent | ~C1991~C1992~C1993~C1994~C1995 | 47%39%42%69%69% | Dec/10 | |||
| USA | Wholecountry | Opportunistic throughproviders offices(partially reimbursed) | Routine | Priority:11-12 y | Since Jan2007 | 4-valent | (2010; age atinterview)13-17 y13 y14 y15 y16 y17 y | 32%23%31%32%37%38% | Aug/11 | National ImmunizationSurvey (chart-verifiedsurvey) |
| Opportunisticthrough providers offices(partially reimbursed) | Catch-up | 13-26 y | Sep 2008-Sep 2011 | 4-valent | (2009)19-26 y | 17% | Aug/11 | National Health InterviewSurvey,http://www.cdc.gov/vaccines/stats-surv/nhis/2009-nhis.htm#04 |
Pilot introduction in developing countries has proven successful through donor programs. For example, in April 2011, Rwanda started nationwide HPV, school-based vaccination (6th grade of primary level) and in out-of-school girls aged 12 years through health centres, reaching virtually complete coverage for the first dose. In the Americas, Panama, and Mexico have included HPV vaccination in their immunisation programmes:and Argentina, Guyana, Peru, and Suriname have been planning to implement national programs in 2011 66.
Impact of vaccination
With high HPV vaccination coverage for 12-17-year-olds, Australia has observed early effects. In sentinel sexually transmitted disease clinics, a 77% reduction in genital warts was observed amongst vaccine age eligible females as well as a 44% decrease among unvaccinated but age-matched heterosexual males between 2007 and 201067. A significant reduction in genital warts of 25% amongst older (non vaccine eligible) heterosexual men is also becoming apparent, suggesting increasing herd immunity68. Trend analysis of data from the Victorian Cervical Cytology Registry has indicated a decline in the incidence of high-grade CIN2+ in women under the age of 18 years between 2007 and 2009, but no similar declines in low-grade CIN or in older women69. Whilst linkage the individual level is required to confirm that this ecological correlation is due to vaccination, the early observed decline is promising and in agreement with pre-vaccination predictions70.
When vaccinated cohorts will reach the target age currently defined for screening, screening policies may require adaptation with less frequent screening and more specific HPV-based screening methods71.
Evidence-based guidelines for cervical cancer prevention
Systematic reviews on new screening and vaccination strategies are often conducted simultaneously in several countries and institutions. This results in multiplication of resources, dilution of competencies, and sometimes yields contradictory findings, generating confusion among stakeholders, health professionals and the general public. International coordination is needed involving specialists skilled in health-technology assessment, HPV epidemiology and clinical experts, allowing for balanced interests72.
PRIMARY PREVENTION AND TREATMENT OF VULVAR PRECANCEROUS LESIONS
In 2004, the International Society for the Study of Vulvar Disease (ISSVD) revised vulvar precancer terminology according to the recognition of two forms of vulvar squamous cell cancer, one related to HPV, termed VIN usual type, as it is the most frequent form of VIN, and one not related to HPV, termed differentiated VIN 73. HPV related precancer lesions were thus collated into a single category, which includes what was previously categorised as VIN2 or VIN3, and VIN1 was excluded because it represents HPV infection and the term lacks reproducibility. Therefore trials including only VIN2/3 patients will be termed simply as “VIN”.
High protection against HPV16/18-related VIN or worse disease has been shown in a pooled analysis of randomised prophylactic vaccination trials with quadrivalent HPV vaccine (100% in baseline HPV16/18-negative women, and 62% in women including those who were HPV16/18 positive at baseline)74.
Currently, no evidence is available supporting screening for VIN or vulvar cancer. In addition, after surgical treatment of VIN, poorer quality of life and sexual function75 and recurrence are frequently reported 76. Randomised trials have demonstrated that topical treatment of VIN with imiquimod reduces lesion size77,78, however side effects were common.
Favourable results have been reported from randomised trials evaluating the therapeutic effect of vaccination of HPV16-positive VIN patients, using E6 and E7 peptides or fusion HPV16 E6E7L2 protein primed by topical imiquimod treatment79,80.
PRIMARY AND SECONDARY PREVENTION OF ANAL CANCER
Prevention efforts fall into two categories: screening for and treatment of high-grade anal intraepithelial neoplasia (HGAIN**,** AIN grade 2 or 3), the anal cancer precursor, and prevention of anal HPV infection through HPV vaccination. Screening for anal cancer and HGAIN is proposed for high-risk groups but not for the general population. The main argument in favour of screening is the analogy with, and success of screening and treatment for CIN to prevent cervical cancer. The primary argument against anal screening is the absence of studies showing that HGAIN treatment reduces the incidence of anal cancer. It is critical to set up such trials as well as studies on biomarkers to predict progression from HGAIN to cancer 81.
Currently, the primary screening tool for anal HPV-associated diseases is anal cytology, with referral of screen-positive individuals for high resolution anoscopy and anal biopsy, with treatment decisions based on the grade of AIN. HGAIN can be treated using a variety of approaches depending on size and location. Some clinicians screen high-risk patients with standard anoscopy82.
HPV vaccination holds promise for the reduction of the incidence of anal cancer in the long term. A recent RCT in HIV-negative MSM has shown that the quadrivalent vaccine has 74.9% efficacy against HGAIN (95%CI:8.8-95.4) in the per-protocol population and 54.2% (95%CI:18.0-75.3) in the intention-to-treat population83. Prevention of AIN and anal cancer was approved by the U.S. Food and Drug Administration (FDA) as an indication for the quadrivalent HPV vaccine in men and women aged 9-26 years84. The bivalent vaccine was recently shown to reduce the risk of acquiring anal HPV infection in women85, but has not yet been studied for efficacy against AIN. It will likely be several decades before a reduction in anal cancer is detected among the vaccinated population.
PREVENTION AND TREATMENT OF HPV-RELATED MALE GENITAL LESIONS
Anogenital warts are the most common clinical manifestation of HPV infection86. Though they are benign and not associated with mortality, they are a source of psychosocial distress and can cause physical discomfort including pain, bleeding and itching. Genital warts are highly infectious; approximately 65% of people whose sexual partner has genital warts will develop warts themselves. Warts appear between 3 weeks and 8 months after an HPV infection87,88. Although perhaps 20-30% of genital warts spontaneously regress, recurrence of warts is common, resulting in high medical costs for treatments. A high lifetime number of female sexual partners significantly increase the risk of genital warts, while frequent condom use was protective in some, but not all studies.
Prevention of genital HPV infection and genital warts through vaccination
In a phase III trial in men aged 16-26 years, the efficacy of the quadrivalent vaccine against HPV-6/11/16/18 related external genital lesions (EGLs) in the intent-to-treat population was high (65.5%, 95%CI:45.8-78.6), as was efficacy against development of EGL regardless of HPV type (60.2%, 95%CI:40.8-73.8)89. In the per protocol population, vaccination reduced the incidence of HPV-6/11/16/18-related EGLs by 90.4% (95%CI:69.2-98.1). Efficacy against genital warts in this population was 89.4% (95%CI:65.5-97.9). In addition, the vaccine protected against HPV-6/11/16/18-related persistent infection.
Prevention of genital HPV infection and disease through circumcision
Circumcision at young age has long been known to be associated with a decreased risk of penile cancer. Recent RCTs showed that adult male circumcision resulted in ~50% decreased incidence of HIV infection, as well as a significant lower incidence of. penile hr-HPV infection in both HIV-negative and -positive men, and in female partners of HIV-negative men but not in the female partners of HIV-positive men90. Therefore circumcision of neonatal boys and adult males contributes directly to HPV control, as well as to the control of other sexually transmitted diseases acting as co-factors for HPV transmission.
PRIMARY PREVENTION, DIAGNOSIS AND TREATMENT OF HPV-RELATED OROPHARYNGEAL CANCER
HPV and prognosis of oropharyngeal cancer
Tumour HPV status is now established as a significant predictor of survival for patients with loco-regionally advanced OSCC91 corresponding with a 60% lower risk of death, equivalent to a 30% difference in absolute five-year survival34. The survival difference is attributable to multiple factors: younger age, higher performance status, less co-morbidities among HPV-positive patients, increased response rates to both cisplatin-based chemotherapy and radiotherapy and lower risk of second primary tumours34. Importantly, a history of ≥10 pack-years of cigarette smoking reduces survival for HPV-positive patients. Treatment strategies for the low-risk group (HPV-positive/<10 pack-years) are now investigating whether treatment intensity and thus long-term morbidity can be reduced without compromising survival. By contrast, strategies to improve survival for the other risk-groups include addition of molecularly targeted agents to the platform of concurrent cisplatin-based chemoradiotherapy. Clinical trials are now stratified by tumour HPV status. Furthermore, routine testing of OSCC tumour HPV status is now recommended in US guidelines.
Diagnostic challenges in the diagnosis of OSCC
Introduction of HPV testing in the clinic has been hindered by the absence of validated assays. HPV in situ hybridization (ISH) or a surrogate of HPV E7 oncoprotein function, p16 immunohistochemistry (IHC), were most frequently used in trials that established HPV as a prognostic factor. Available algorithms in the literature with sensitivity and specificity for HPV16 E6/7 oncogene expression (the gold standard) approaching 100% have combined p16 IHC with PCR detection of HPV DNA in fresh frozen tumour and are therefore unlikely to be feasible in a routine pathology laboratory92. p16 IHC has shown high sensitivity (≥90%) and moderate-to-high (>80%) specificity for HPV16 E6 mRNA expression as well as high inter-reader agreement82,93. Commercially available ISH assays show variable sensitivity and specificity estimates94,93. In the future, the decreased prevalence of HPV16/18-related precancer resulting from prophylactic vaccination will warrant more specific and less frequent screening.
Future directions
Areas for future research include: (1) the role of HPV in non-oropharyngeal cancers of the head and neck; (2) the molecular underpinnings for the improved response rates to chemotherapy and radiotherapy for HPV-positive patients; (3) the prevalence and distribution of oral HPV infection in the population; (4) the natural history of oral HPV infection; (5) the efficacy of HPV vaccines in preventing oral HPV16 infections; (6) the potential utility of oral HPV testing for screening; (7) the precise characterisation of HPV-positive premalignant lesions, and (8) identification of novel surrogate markers of HPV infections and/or HPV-induced (pre-)malignant lesions.
CONCLUSIONS
The EUROGIN roadmaps represent a continuing effort to update and interpret information on primary and secondary prevention of cervical cancer. This year the roadmap widened its focus and also addressed the burden and prevention, diagnosis and treatment of other HPV-related disease.
HPV infection causes approximately 600,000 cases of cancer of the cervix, vulva, vagina, penis, anus and oropharynx annually, as well as benign diseases such as genital warts and RRP. Whereas the incidence of cervical cancer has been decreasing over recent decades, the incidence of other HPV-related cancer for which there are no effective screening programs has been rising over the last decades.
Cervical cancer screening effectiveness may be improved by replacing frequent cytology with HPV screening of women aged 30-35 years or older every 5 to 8 years, using validated assays. Defining the best triage algorithms, age ranges and screening intervals are priorities for research. The specificity of HPV-based screening could be improved by using more specific tests or by applying more specific triage strategies (for instance higher viral load cutoffs, mRNA testing, genotyping, p16 and other biomarkers).
HPV vaccination will reduce the burden of cervical precancer and probably also of invasive cervical and other HPV-related disease in women. In the future, the decreased prevalence of HPV16/18-related precancer resulting from prophylactic vaccination will warrant less frequent and more specific screening.
These promising findings should now be translated in cost-effective strategies, by preference following an organised approach integrating primary and secondary prevention, according to scientific evidence and adapted to the local situation with particular attention for regions with the highest burden of disease.
Acknowledgements
MA received financial support from: (1) Directorate of SANCO of the European Commission, Luxembourg, Grand-Duchy of Luxembourg), through the ECCG project (European Cooperation on development and implementation of Cancer screening and prevention Guidelines, the IARC, Lyon, France and through the EUROCHIP-3 Network (Istituto Nazionale dei Tumori, Milan, Italy); (2) the 7th Framework Programme of DG Research of the European Commission through the PREHDICT project (grant No. 242061, coordinated by the Vrije Universiteit Amsterdam, the Netherlands) and the HPV-AHEAD project (FP7-HEALTH-2011-282562,coordinated by IARC); (3) the Belgian Foundation Against Cancer (Brussels, Belgium).
NW was supported by the Intramural Research Program of the National Cancer Institute (Bethesda, USA).
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflicts of interest
MA: see acknowledgements. Participation at Eurogin conference (Lisbon 2011) funded by organisers of the conference
SdS has an unrestricted grant from Merck and has had occasional grants for assistance to scientific meetings from GSK, MSD and Qiagen.
MSa: no conflict of interest.
MSi: consultancy with salary to IEO with the following companies: Qiagen, GSK, Sanofi Pasteur, MTM labs, Roche Diagnostics, Innogenetics.
JP: no conflict of interest declared.
CL has acted as a consultant for SPMSD, and received travel grants from SPMSD & GSK.
MG: Merck (funding and consulting), GSK (consulting).
LB: no conflict of interest declared.
GR: no conflict of interest declared.
NW: no conflict of interest declared.
JBr: is an investigator on an Australian Research Council Linkage Grant, for which CSL Biotherapies is a partner organisation and was a chief investigator on a study of HPV prevalence in Australian women, which was funded by a grant from the Cooperative Research Centre for Aboriginal Health, as well as education grants in aid from GlaxoSmithKline and CSL Limited.
YLQ: no conflict of interest declared.
LD has received honoraria from Merck and Glaxosmithkline for appearing on various speaker fora. And has conducted research funded by both organisations.
JBe: no conflict of interest declared.
LA: no conflict of interest declared.
AG: Merck (Speaker Bureau, consult, grant funding), GSK (grant funding).
MT: no conflict of interest declared.
JM has participated to Steering Committees at Merck, and to the Advisory Board of Sanofi Pasteur MSD, Gen-Probe, Qiagen, and Roche Diagnostics.
References
- 1.IARC working group on the evaluation of carcinogenic risks to humans. Human Papillomaviruses. IARC Monogr Eval Carcinog Risks Hum. 2007;90:1–689. [PMC free article] [PubMed] [Google Scholar]
- 2.Walboomers JM, Jacobs MV, Manos M, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJLM, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 3.Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer. 2011;128:927–35. doi: 10.1002/ijc.25396. [DOI] [PubMed] [Google Scholar]
- 4.de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR, Vallejos CS, de Ruiz PA, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–56. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 5.Madeleine MM, Daling JR. Cancers of the vulva and vagina. In: Schottenfeld D, Fraumeni JF Jr., editors. Cancer epidemiology and prevention. Oxford University Press; New York: 2006. pp. 1068–1074. [Google Scholar]
- 6.Hording U, Daugaard S, Junge J, Lundvall F. Human papillomaviruses and multifocal genital neoplasia. Int J Gynecol Pathol. 1996;15:230–4. doi: 10.1097/00004347-199607000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Kim YT, Thomas NF, Kessis TD, Wilkinson EJ, Hedrick L, Cho KR. p53 mutations and clonality in vulvar carcinomas and squamous hyperplasias: evidence suggesting that squamous hyperplasias do not serve as direct precursors of human papillomavirus-negative vulvar carcinomas. Hum Pathol. 1996;27:389–95. doi: 10.1016/s0046-8177(96)90113-6. [DOI] [PubMed] [Google Scholar]
- 8.Toki T, Kurman RJ, Park JS, Kessis T, Daniel RW, Shah KV. Probable nonpapillomavirus etiology of squamous cell carcinoma of the vulva in older women: a clinicopathologic study using in situ hybridization and polymerase chain reaction. Int J Gynecol Pathol. 1991;10:107–25. doi: 10.1097/00004347-199104000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Miralles-Guri C, Bruni L, Cubilla AL, Castellsague X, Bosch FX, de SS. Human papillomavirus prevalence and type distribution in penile carcinoma. J Clin Pathol. 2009;62:870–8. doi: 10.1136/jcp.2008.063149. [DOI] [PubMed] [Google Scholar]
- 10.Bleeker MC, Heideman DA, Snijders PJ, Horenblas S, Dillner J, Meijer CJ. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol. 2009;27:141–50. doi: 10.1007/s00345-008-0302-z. [DOI] [PubMed] [Google Scholar]
- 11.Rubin MA, Kleter B, Zhou M, Ayala G, Cubilla AL, Quint WG, Pirog EC. Detection and typing of human papillomavirus DNA in penile carcinoma: evidence for multiple independent pathways of penile carcinogenesis. Am J Pathol. 2001;159:1211–8. doi: 10.1016/S0002-9440(10)62506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abramowitz L, Jacquard AC, Jaroud F, Haesebaert J, Siproudhis L, Pradat P, Aynaud O, Leocmach Y, Soubeyrand B, Dachez R, Riethmuller D, Mougin C, et al. Human papillomavirus genotype distribution in anal cancer in France: the EDiTH V study. Int J Cancer. 2011;129:433–9. doi: 10.1002/ijc.25671. [DOI] [PubMed] [Google Scholar]
- 13.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467–75. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 14.Mehanna H, Beech T, Nicholson T. The prevalence of human papillomavirus in oropharyngeal and non-oropharyngeal head and neck cancer - a systematic review and meta-analysis of trends by time and region. Head Neck. 2011 doi: 10.1002/hed.22015. * [DOI] [PubMed] [Google Scholar]
- 15.Syrjanen S, Lodi G, von B I, Aliko A, Arduino P, Campisi G, Challacombe S, Ficarra G, Flaitz C, Zhou HM, Maeda H, Miller C, et al. Human papillomaviruses in oral carcinoma and oral potentially malignant disorders: a systematic review. Oral Dis. 2011;17(Suppl 1):58–72. doi: 10.1111/j.1601-0825.2011.01792.x. [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro KB, Levi JE, Pawlita M, Koifman S, Matos E, Eluf-Neto J, Wunsch-Filho V, Curado MP, Shangina O, Zaridze D, Szeszenia-Dabrowska N, Lissowska J, et al. Low human papillomavirus prevalence in head and neck cancer: results from two large case-control studies in high-incidence regions. Int J Epidemiol. 2011;40:489–502. doi: 10.1093/ije/dyq249. [DOI] [PubMed] [Google Scholar]
- 17.Brown DR, Schroeder JM, Bryan JT, Stoler MH, Fife KH. Detection of multiple human papillomavirus types in Condylomata acuminata lesions from otherwise healthy and immunosuppressed patients. J Clin Microbiol. 1999;37:3316–22. doi: 10.1128/jcm.37.10.3316-3322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giuliano AR, Tortolero-Luna G, Ferrer E, Burchell AN, de Sanjose S, Kruger-Kjaer S, Munoz N, Schiffman M, Bosch FX. Epidemiology of Human Papillomavirus infection in Men, Cancers other than Cervical and Benign Conditions. Vaccine. 2008;26S:K17–K28. doi: 10.1016/j.vaccine.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arbyn M, Castellsagué X, de Sanjosé S, Bruni L, Saraiya M, Bray F, Ferlay J. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011;22:2675–86. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 20.Bruni L, Diaz M. Burden of disease: recent estimates and limitations; 27th International Papillomavirus Conference and Clinical Workshop; Berlin. 2011. [Google Scholar]
- 21.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 22.Jones RW, Baranyai J, Stables S. Trends in squamous cell carcinoma of the vulva: the influence of vulvar intraepithelial neoplasia. Obstet Gynecol. 1997;90:448–52. doi: 10.1016/s0029-7844(97)00298-6. [DOI] [PubMed] [Google Scholar]
- 23.Ferlay J, Parkin DM, Curado MP. Volumes I to IX. IARC; Lyon, France: 2010. Cancer incidence in five continents. * * [Google Scholar]
- 24.Johnson LG, Madeleine MM, Newcomer LM, Schwartz SM, Daling JR. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973-2000. Cancer. 2004;101:281–8. doi: 10.1002/cncr.20364. [DOI] [PubMed] [Google Scholar]
- 25.Machalek DA, Poynten M, Jin F, Fairley CK, Farnsworth A, Garland SM, Hillman RJ, Petoumenos K, Roberts J, Tabrizi SN, Templeton DJ, Grulich AE. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol. 2012 doi: 10.1016/S1470-2045(12)70080-3. [DOI] [PubMed] [Google Scholar]
- 26.Penn I. Cancer in the immunosuppressed organ recipient. Transplant Proc. 1991;23:1771–2. [PubMed] [Google Scholar]
- 27.Watson M, Saraiya M, Ahmed F, Cardinez CJ, Reichman ME, Weir HK, Richards TB. Using population-based cancer registry data to assess the burden of human papillomavirus-associated cancers in the United States: Overview of methods. Cancer. 2008;113:2841–54. doi: 10.1002/cncr.23758. [DOI] [PubMed] [Google Scholar]
- 28.Daling JR, Weiss NS, Klopfenstein LL, Cochran LE, Chow WH, Daifuku R. Correlates of homosexual behavior and the incidence of anal cancer. JAMA. 1982;247:1988–90. [PubMed] [Google Scholar]
- 29.Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst. 2000;92:1500–10. doi: 10.1093/jnci/92.18.1500. [DOI] [PubMed] [Google Scholar]
- 30.Crum-Cianflone NF, Hullsiek KH, Marconi VC, Ganesan A, Weintrob A, Barthel RV, Agan BK. Anal cancers among HIV-infected persons: HAART is not slowing rising incidence. AIDS. 2010;24:535–43. doi: 10.1097/QAD.0b013e328331f6e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24(Suppl 3):S11–S25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- 32.Hernandez BY, Barnholtz-Sloan J, German RR, Giuliano A, Goodman MT, King JB, Negoita S, Villalon-Gomez JM. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer. 2008;113:2883–91. doi: 10.1002/cncr.23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.IARC Monograph Working Group, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; Vol 90: Human Papillomaviruses; Lyon: IARCPress. 2007.p. 670. [Google Scholar]
- 34.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug-Saber M, Cozen W, Liu L, Lynch CF, et al. Human Papillomavirus and Rising Oropharyngeal Cancer Incidence in the United States. J Clin Oncol. 2011 doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nasman A, Attner P, Hammarstedt L, Du J, Eriksson M, Giraud G, Ahrlund-Richter S, Marklund L, Romanitan M, Lindquist D, Ramqvist T, Lindholm J, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer. 2009;125:362–6. doi: 10.1002/ijc.24339. [DOI] [PubMed] [Google Scholar]
- 37.Dinh TH, Sternberg M, Dunne EF, Markowitz LE. Genital warts among 18- to 59-year-olds in the United States, national health and nutrition examination survey, 1999--2004. Sex Transm Dis. 2008;35:357–60. doi: 10.1097/OLQ.0b013e3181632d61. [DOI] [PubMed] [Google Scholar]
- 38.Hartwig S, Syrjanen S, Dominiak-Felden G, Brotons M, Castellsague X. Estimation of the epidemiological burden of human papillomavirus-related cancers and non-malignant diseases in men in Europe: a review. BMC Cancer. 2012;12:30. doi: 10.1186/1471-2407-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kjaer SK, Tran TN, Sparen P, Tryggvadottir L, Munk C, Dasbach E, Liaw KL, Nygard J, Nygard M. The burden of genital warts: a study of nearly 70,000 women from the general female population in the 4 Nordic countries. J Infect Dis. 2007;196:1447–54. doi: 10.1086/522863. [DOI] [PubMed] [Google Scholar]
- 40.Kraut AA, Schink T, Schulze-Rath R, Mikolajczyk RT, Garbe E. Incidence of anogenital warts in Germany: a population-based cohort study. BMC Infect Dis. 2010;10:360. doi: 10.1186/1471-2334-10-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoy T, Singhal PK, Willey VJ, Insinga RP. Assessing incidence and economic burden of genital warts with data from a US commercially insured population. Curr Med Res Opin. 2009;25:2343–51. doi: 10.1185/03007990903136378. [DOI] [PubMed] [Google Scholar]
- 42.Desai S, Wetten S, Woodhall SC, Peters L, Hughes G, Soldan K. Genital warts and cost of care in England. Sex Transm Infect. 2011;87:464–8. doi: 10.1136/sti.2010.048421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franceschi S, Denny L, Irwin KL, Jeronimo J, Lopalco PL, Monsonego J, Peto J, Ronco G, Sasieni P, Wheeler CM. Eurogin 2010 roadmap on cervical cancer prevention. Int J Cancer. 2011;128:2765–4. doi: 10.1002/ijc.25915. [DOI] [PubMed] [Google Scholar]
- 44.Arbyn M, Ronco G, Meijer CJLM, Naucler P. Trials comparing cytology with human papillomavirus screening. Lancet Oncol. 2009;10:935–6. doi: 10.1016/S1470-2045(09)70296-7. [DOI] [PubMed] [Google Scholar]
- 45.Bulkmans N, Berkhof J, Rozendaal L, van Kemenade F, Boeke A, Bulk S, Voorhorst F, Verheijen R, van Groningen K, Boon M, Ruitinga W, van Ballegooijen M, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet. 2007;370:796–802. doi: 10.1016/S0140-6736(07)61450-0. [DOI] [PubMed] [Google Scholar]
- 46.Rijkaart DC, Berkhof J, Rozendaal L, van Kemenade FJ, Bulkmans NW, Heideman DA, Kenter GG, Cuzick J, Snijders PJ, Meijer CJ. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2011 doi: 10.1016/S1470-2045(11)70296-0. [DOI] [PubMed] [Google Scholar]
- 47.Naucler P, Ryd W, Tornberg S, Strand A, Wadell G, Elfgren K, Radberg T, Strander B, Forslund O, Hansson BG, Rylander E, Dillner J. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357:1589–97. doi: 10.1056/NEJMoa073204. [DOI] [PubMed] [Google Scholar]
- 48.Kitchener HC, Almonte M, Thomson C, Wheeler P, Sargent A, Stoykova B, Gilham C, Baysson H, Roberts C, Dowie R, Desai M, Mather J, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 2009;10:672–82. doi: 10.1016/S1470-2045(09)70156-1. [DOI] [PubMed] [Google Scholar]
- 49.Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Dalla PP, Del Mistro A, Ghiringhello B, Girlando S, Gillio-Tos A, De Marco L, Naldoni C, Pierotti P, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249–57. doi: 10.1016/S1470-2045(09)70360-2. [DOI] [PubMed] [Google Scholar]
- 50.Monsonego J, Hudgens MG, Zerat L, Zerat JC, Syrjanen K, Halfon P, Ruiz F, Smith JS. Evaluation of oncogenic human papillomavirus RNA and DNA tests with liquid based cytology in primary cervical cancer screening (The FASE study) Int J Cancer. 2011;129:691–701. doi: 10.1002/ijc.25726. [DOI] [PubMed] [Google Scholar]
- 51.Rebolj M, Bonde J, Njor SH, Lynge E. Human papillomavirus testing in primary cervical screening and the cut-off level for hybrid capture 2 tests: systematic review. BMJ. 2011;342:d2757. doi: 10.1136/bmj.d2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Castle PE, Stoler MH, Wright TC, Jr., Sharma A, Wright TL, Behrens CM. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol. 2011;12:880–90. doi: 10.1016/S1470-2045(11)70188-7. [DOI] [PubMed] [Google Scholar]
- 53.Ronco G, Franceschi S, Segnan N. HPV16 and HPV18 genotyping in cervical cancer screening. Lancet Oncol. 2011;12:831–2. doi: 10.1016/S1470-2045(11)70195-4. [DOI] [PubMed] [Google Scholar]
- 54.Arbyn M, Martin-Hirsch P, Buntinx F, Van Ranst M, Paraskevaidis E, Dillner J. Triage of women with equivocal or low-grade cervical cytology results. A meta-analysis of the HPV test positivity rate. J Cell Mol Med. 2009;13:648–59. doi: 10.1111/j.1582-4934.2008.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stoler MH, Wright TC, Jr., Sharma A, Apple R, Gutekunst K, Wright TL. High-Risk Human Papillomavirus Testing in Women With ASC-US Cytology: Results From the ATHENA HPV Study. Am J Clin Pathol. 2011;135:468–75. doi: 10.1309/AJCPZ5JY6FCVNMOT. [DOI] [PubMed] [Google Scholar]
- 56.Roelens J, Reuschenbach M, von Knebel-Doeberitz M, Wentzensen N, Bergeron C, Arbyn M. p16INK4a immunocytochemistry versus HPV testing for triage of women with minor cytological abnormalities: A systematic review and meta-analysis. Cancer. 2012 doi: 10.1002/cncy.21205. accepted for-publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arbyn M, Schneider A, Gissmann L, Kaufmann AM. Highlights of the 27th International Papillomavirus Conference and Clinical Workshop (Berlin, 17-22 September 2011) - Part 2: Applied Clinical Science. Future Virol. 2012;7:19–24. [Google Scholar]
- 58.Pretorius RG, Belinson JL, Burchette RJ, Hu S, Zhang X, Qiao YL. Regardless of Skill, Performing More Biopsies Increases the Sensitivity of Colposcopy. J Low Genit Tract Dis. 2011 doi: 10.1097/LGT.0b013e3181fb4547. * [DOI] [PubMed] [Google Scholar]
- 59.Stoler MH, Vichnin MD, Ferenczy A, Ferris DG, Perez G, Paavonen J, Joura EA, Djursing H, Sigurdsson K, Jefferson L, Alvarez F, Sings HL, et al. The accuracy of colposcopic biopsy: analyses from the placebo arm of the Gardasil clinical trials. Int J Cancer. 2011;128:1354–62. doi: 10.1002/ijc.25470. [DOI] [PubMed] [Google Scholar]
- 60.Bergeron C, Ordi J, Schmidt D, Trunk MJ, Keller T, Ridder R. Conjunctive p16INK4a testing significantly increases accuracy in diagnosing high-grade cervical intraepithelial neoplasia. Am J Clin Pathol. 2010;133:395–406. doi: 10.1309/AJCPXSVCDZ3D5MZM. [DOI] [PubMed] [Google Scholar]
- 61.Dijkstra MG, Heideman DA, de Roy SC, Rozendaal L, Berkhof J, van KK, van GK, Snijders PJ, Meijer CJ, van Kemenade FJ. p16(INK4a) immunostaining as an alternative to histology review for reliable grading of cervical intraepithelial lesions. J Clin Pathol. 2010;63:972–7. doi: 10.1136/jcp.2010.078634. [DOI] [PubMed] [Google Scholar]
- 62.Denny L, Kuhn L, Hu CC, Tsai WY, Wright TC., Jr. Human papillomavirus-based cervical cancer prevention: long-term results of a randomized screening trial. J Natl Cancer Inst. 2010;102:1557–67. doi: 10.1093/jnci/djq342. [DOI] [PubMed] [Google Scholar]
- 63.Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM, Hingmire S, Malvi SG, Thorat R, Kothari A, Chinoy R, Kelkar R, et al. HPV screening for cervical cancer in Rural India. N Engl J Med. 2009;360:1385–94. doi: 10.1056/NEJMoa0808516. [DOI] [PubMed] [Google Scholar]
- 64.The Lancet. Women’s health in rural China. Lancet. 2009;374:358. doi: 10.1016/S0140-6736(09)61394-5. [DOI] [PubMed] [Google Scholar]
- 65.Qiao YL, Sellors JW, Eder PS, Bao YP, Lim JM, Zhao FH, Weigl B, Zhang WH, Peck RB, Li L, Cheng F, Pan QJ, et al. A new HPV-DNA test for cervical-cancer screening in developing regions: a cross-sectional study of clinical accuracy in rural China. Lancet Oncol. 2008;9:929–36. doi: 10.1016/S1470-2045(08)70210-9. [DOI] [PubMed] [Google Scholar]
- 66.Meites E, Vicari AS, Dilsa LG, Deeks S, Mireles SPC, San Roman MLL, Romero RE, Mulato MD, Richardson López-Collada V, de Moltó Y, de Hewitt IS, Saraiya M, et al. Progress toward implementation of human papillomavirus vaccination--the Americas, 2006-2010. MMWR Morb Mortal Wkly Rep. 2011;60:1382–4. [PubMed] [Google Scholar]
- 67.Donovan B, Franklin N, Guy R, Grulich AE, Regan DG, Ali H, Wand H, Fairley CK. Quadrivalent human papillomavirus vaccination and trends in genital warts in Australia: analysis of national sentinel surveillance data. Lancet Infect Dis. 2011;11:39–44. doi: 10.1016/S1473-3099(10)70225-5. [DOI] [PubMed] [Google Scholar]
- 68.Arbyn M, Schneider A, Kaufmann AM, Gissmann L. Highlights of the 27th International Papillomavirus Conference and Clinical Workshop (Berlin, 17-22 September 2011) - Part 3: Epidemiology and Public Health. Future Virol. 2012;7:127–33. [Google Scholar]
- 69.Brotherton JM, Fridman M, May CL, Chappell G, Saville AM, Gertig DM. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet. 2011;377:2085–92. doi: 10.1016/S0140-6736(11)60551-5. [DOI] [PubMed] [Google Scholar]
- 70.Winer RL, Kiviat NB, Hughes JP, Adam DE, Lee SK, Kuypers JM, Koutsky LA. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005;191:731–8. doi: 10.1086/427557. [DOI] [PubMed] [Google Scholar]
- 71.Franco EL, Cuzick J. Cervical cancer screening following prophylactic human papillomavirus vaccination. Vaccine. 2008;26(Suppl 1):A16–A23. doi: 10.1016/j.vaccine.2007.11.069. [DOI] [PubMed] [Google Scholar]
- 72.Arbyn M, Cuzick J. International agreement to join forces in synthesizing evidence on new methods for cervical cancer prevention. Cancer Lett. 2009;278:1–2. doi: 10.1016/j.canlet.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 73.Sideri M, Jones RW, Wilkinson EJ, Preti M, Heller DS, Scurry J, Haefner H, Neill S. Squamous vulvar intraepithelial neoplasia: 2004 modified terminology, ISSVD Vulvar Oncology Subcommittee. J Reprod Med. 2005;50:807–10. [PubMed] [Google Scholar]
- 74.Joura EA, Leodolter S, Hernandez-Avila M, Wheeler CM, Perez G, Koutsky LA, Garland SM, Harper DM, Tang GW, Ferris DG, Steben M, Jones RW, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369:1693–702. doi: 10.1016/S0140-6736(07)60777-6. [DOI] [PubMed] [Google Scholar]
- 75.Likes WM, Stegbauer C, Tillmanns T, Pruett J. Pilot study of sexual function and quality of life after excision for vulvar intraepithelial neoplasia. J Reprod Med. 2007;52:23–7. [PubMed] [Google Scholar]
- 76.Todd RW, Luesley DM. Medical management of vulvar intraepithelial neoplasia. J Low Genit Tract Dis. 2005;9:206–12. doi: 10.1097/01.lgt.0000179858.21833.0d. [DOI] [PubMed] [Google Scholar]
- 77.Mathiesen O, Buus SK, Cramers M. Topical imiquimod can reverse vulvar intraepithelial neoplasia: a randomised, double-blinded study. Gynecol Oncol. 2007;107:219–22. doi: 10.1016/j.ygyno.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 78.van Seters M, Van Beurden M, ten Kate FJ, Beckmann I, Ewing PC, Eijkemans MJ, Kagie MJ, Meijer CJ, Aaronson NK, Kleinjan A, Heijmans-Antonissen C, Zijlstra FJ, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod. N Engl J Med. 2008;358:1465–73. doi: 10.1056/NEJMoa072685. [DOI] [PubMed] [Google Scholar]
- 79.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Essahsah F, Fathers LM, Offringa R, Drijfhout JW, Wafelman AR, Oostendorp J, et al. Vaccination against HPV-16 Oncoproteins for Vulvar Intraepithelial Neoplasia. N Engl J Med. 2009;361:1838–47. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 80.Daayana S, Elkord E, Winters U, Pawlita M, Roden R, Stern PL, Kitchener HC. Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. Br J Cancer. 2010;102:1129–36. doi: 10.1038/sj.bjc.6605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wentzensen N. Screening for anal cancer: endpoints needed. Lancet Oncol. 2012 doi: 10.1016/S1470-2045(12)70101-8. [DOI] [PubMed] [Google Scholar]
- 82.Abramowitz L, Benabderrahmane D, Baron G, Walker F, Yeni P, Duval X. Systematic evaluation and description of anal pathology in HIV-infected patients during the HAART era. Dis Colon Rectum. 2009;52:1130–6. doi: 10.1007/DCR.0b013e3181a65f5f. [DOI] [PubMed] [Google Scholar]
- 83.Palefsky JM, Giuliano AR, Goldstone SE, Moreira ED, Jr., Aranda C, Jessen H, Hillman R, Ferris D, Coutlee F, Stoler M, Marshall JB, Radley D, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576–85. doi: 10.1056/NEJMoa1010971. [DOI] [PubMed] [Google Scholar]
- 84.FDA . FDA approves HPV vaccine to prevent anal cancer. 2012. [Google Scholar]
- 85.Kreimer AR, Gonzalez P, Katki HA, Porras C, Schiffman M, Rodriguez AC, Solomon D, Jimenez S, Schiller JT, Lowy DR, van Doorn LJ, Struijk L, et al. Efficacy of a bivalent HPV 16/18 vaccine against anal HPV 16/18 infection among young women: a nested analysis within the Costa Rica Vaccine Trial. Lancet Oncol. 2011;12:862–70. doi: 10.1016/S1470-2045(11)70213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Anic GM, Giuliano AR. Genital HPV infection and related lesions in men. Prev Med. 2011;53(Suppl 1):S36–S41. doi: 10.1016/j.ypmed.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lacey CJ, Lowndes CM, Shah KV. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine. 2006;24:35–41. doi: 10.1016/j.vaccine.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 88.Garland SM, Steben M, Sings HL, James M, Lu S, Railkar R, Barr E, Haupt RM, Joura EA. Natural History of Genital Warts: Analysis of the Placebo Arm of 2 Randomized Phase III Trials of a Quadrivalent Human Papillomavirus (Types 6, 11, 16, and 18) Vaccine. J Infect Dis. 2009 doi: 10.1086/597071. [DOI] [PubMed] [Google Scholar]
- 89.Giuliano AR, Palefsky JM, Goldstone S, Moreira ED, Penny ME, Aranda C, Vardas E, Moi H, Jessen H, Hillman R, Chang YH, Ferris D, et al. Efficacy of Quadrivalent HPV Vaccine against HPV Infection and Disease in Males. N Engl J Med. 2011;364:401–11. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tobian AA, Gray RH. Male foreskin and oncogenic human papillomavirus infection in men and their female partners. Future Microbiol. 2011;6:739–45. doi: 10.2217/fmb.11.59. [DOI] [PubMed] [Google Scholar]
- 91.Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27:1992–8. doi: 10.1200/JCO.2008.20.2853. [DOI] [PubMed] [Google Scholar]
- 92.Thavaraj S, Stokes A, Guerra E, Bible J, Halligan E, Long A, Okpokam A, Sloan P, Odell E, Robinson M. Evaluation of human papillomavirus testing for squamous cell carcinoma of the tonsil in clinical practice. J Clin Pathol. 2011;64:308–12. doi: 10.1136/jcp.2010.088450. [DOI] [PubMed] [Google Scholar]
- 93.Schlecht NF, Brandwein-Gensler M, Nuovo GJ, Li M, Dunne A, Kawachi N, Smith RV, Burk RD, Prystowsky MB. A comparison of clinically utilized human papillomavirus detection methods in head and neck cancer. Mod Pathol. 2011;24:1295–305. doi: 10.1038/modpathol.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schache AG, Liloglou T, Risk JM, Filia A, Jones TM, Sheard J, Woolgar JA, Helliwell TR, Triantafyllou A, Robinson M, Sloan P, Harvey-Woodworth C, et al. Evaluation of human papilloma virus diagnostic testing in oropharyngeal squamous cell carcinoma: sensitivity, specificity, and prognostic discrimination. Clin Cancer Res. 2011;17:6262–71. doi: 10.1158/1078-0432.CCR-11-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer. 2009;124:1626–36. doi: 10.1002/ijc.24116. [DOI] [PubMed] [Google Scholar]
- 96.De Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Plummer M. The global burden of cancers attributable to infections in the year 2008: a review and synthetic analysis. Lancet Oncol. 2012;13 doi: 10.1016/S1470-2045(12)70137-7. in-press. [DOI] [PubMed] [Google Scholar]
- 97.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]