Selection of self-reactive T cells in the thymus (original) (raw)
. Author manuscript; available in PMC: 2012 Dec 10.
Abstract
On the whole, the healthy adaptive immune system is responsive to foreign antigens, and tolerant to self. However many individual lymphocytes have, and even require, substantial self-reactivity for their particular functions in immunity. In this review, we discuss several populations of lymphocytes that are thought to experience agonist stimulation through the TCR during selection: nTreg cells, iNKT cells, nIELs, and nTh17s. We discuss the nature of this self-reactivity, how it compares with conventional T cells, and why it is important for overall immune health. We also outline molecular pathways unique to each lineage, and consider possible commonalities to their development and survival.
Keywords: Tolerance, Thymic selection, invariant natural killer T cell, intraepithelial lymphocytes, regulatory T cell, natural Th17
The central paradigm of low self-reactivity shaping and maintaining the naïve CD4 and CD8 T cell repertoire
The development of a functional repertoire of T cells requires TCR specificity based selection events that initiate in the thymus. The most abundant products of this process are conventional naïve CD4 and CD8 αβ T cells, produced at a rate of approximately 1–2x106/day in a young mouse. The combined actions of positive (1) and negative (2) selection produce a T cell repertoire that is both MHC restricted and non-self reactive. Selection initiates at the double positive (DP) stage in the specialized microenvironment of the thymic cortex. DP thymocytes are acutely sensitive to TCR stimulation (3, 4), and the bulk of evidence suggests that this sensitivity facilitates the perception of low affinity self-peptide/MHC ligands presented by cortical epithelial cells. Although the precise affinity range (5), abundance (1), and uniqueness of the low affinity positive selection ligands (6) continues to be debated, most agree that perception of high affinity ligands at this stage will trigger clonal deletion by direct induction of apoptosis. Failure to receive the appropriate TCR signal required for positive selection results in a default “death by neglect” program (see Figure 1).
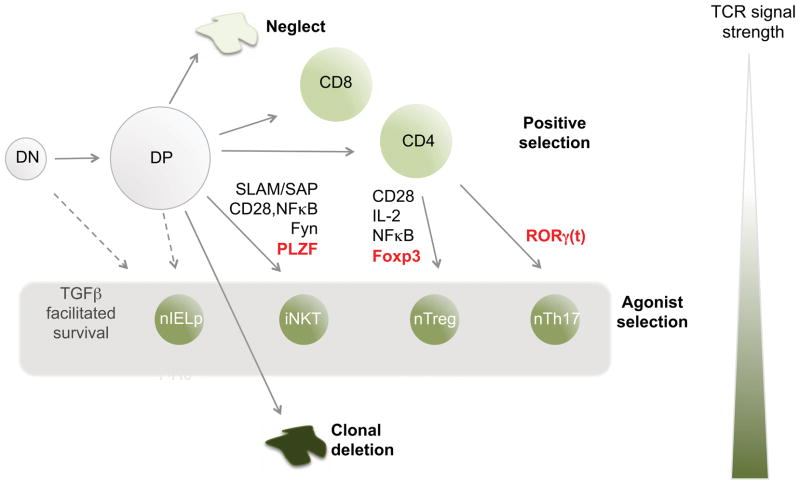
Figure 1. Proposed agonist selection of unique subsets of TCRαβ + T cells in the thymus.
The thymus generates a large pool of immature thymocyte progenitors that express clonally distinct αβ TCRs (DP). The reactivity of these TCRs for self-MHC ligands (shown by color scale, darker green being more reactive) plays a defining role in fate. Cells that do not express a TCR, or express a TCR with no ability to react with thymic ligands will die by neglect. Those with low affinity undergo positive selection to become CD4+ helper or CD8+ killer cells and the major cellular products of the thymus. Those with high affinity undergo clonal deletion to preserve self-tolerance. However, several smaller sub-populations of lymphocytes also develop in the thymus: the precursors to CD8αα+ intraepithelial lymphocytes (nIELp), invariant NK T cells (iNKT), natural FoxP3+ regulatory T cells (nTreg) and natural T helper cells that can produce IL-17 (nTh17). Evidence summarized in this review suggests that these four subsets experience stronger interactions with self-ligands during development (agonist selection). Each of these subsets requires distinct molecular factors (some of which are listed). They arise at distinct stages; can be Class I, Class II, or CD1d restricted; and can express either or no co-receptor. However, their commonalities include: 1) an activated/memory phenotype, 2) evidence for stimulation by high affinity (agonist) self-ligands during development, 3) a requirement for TGFβ, and 4) regulatory function in immunity.
The most widely used models for studying selection have employed TCR transgenic mice that also express the corresponding cognate antigen (7). These models clearly show the deletion of DP thymocytes following interactions with high affinity self-antigens. Although these models have suggested that clonal deletion is massively efficient (8), approaches that measure the polyclonal repertoire specific to a given self-antigen suggest more modest reductions (9, 10). Indeed no one has accurately estimated the fraction of the positively selected repertoire that is clonally deleted. Although estimates of 50–65% have been made, they are limited by their underlying assumptions, e.g. that only bone marrow derived cells mediate clonal deletion (11) or that the repertoire selected on a single pMHC is representative of the whole repertoire (12, 13).
T cells that have undergone both positive and negative selection then leave the thymus and enter the periphery where they persist by receiving homeostatic signals. A diverse naïve T cell repertoire is maintained by interactions with low affinity self-peptide-MHC molecules and limited IL-7 in the periphery (14, 15). When animals are lymphopenic, these same interactions facilitate cellular proliferation—so called lymphopenia-induced proliferation or LIP — likely due to an increase in the amount of available IL-7. It is thought that the TCR affinity of the naïve T cell for self-peptide-MHC molecules dictates the rate of LIP, with the most highly self-reactive T cells undergoing the greatest expansion. However, the role of TCR affinity for self in LIP mainly derives from the study of TCR transgenics, where the “affinity for self” is inferred based on a combination of the rate of proliferation, selection efficiency in the thymus, and the level of CD5 expression (16, 17). As apparent from the somewhat circular logic applied here, the field greatly needs tools to more directly gauge the TCR affinity for self, particularly amongst polyclonal populations.
Evidence for high TCR self-reactivity in certain T cell populations: agonist selection
T cell precursors that interact with high affinity for self-peptides are generally deleted. However, not only do some cells seem to escape this fate, their specific function in the immune system depends on these so-called “agonist” interactions. These include natural T regulatory (nTreg) cells, invariant natural killer T (iNKT) cells, natural CD8αα+ intraepithelial T cells that reside in the gut (nIEL), and natural T helper 17 (nTh17) cells. What is the evidence that these subsets have higher self-reactivity?
nTreg cell self reactivity
nTreg cells play an important role in suppressing autoreactivity and are hypothesized to have self-reactive TCRs although to date, no one has identified a specific self-peptide ligand(s) of a naturally occurring nTreg cell (18, 19). Nonetheless, the higher self-reactivity of nTreg cells has been inferred from a number of different lines of evidence. First, nTreg cells in the thymus have an activated phenotype highlighted by elevated expression of the activation marker CD25, the α chain of the IL-2 receptor. They also have intermediate levels of CD44, a surface protein associated with antigenic experience in C57BL/6 mice, and express GITR. This activated phenotype indirectly suggests that nTreg cells have encountered their cognate antigen during development (Table I). Further evidence supporting agonist selection came from experiments using TCR transgenic models. In a landmark study, Jordan and colleagues showed that nTreg cell development was enhanced in TS1 TCR transgenic mice (specific for influenza hemagglutinin (HA)) when they were crossed to HA transgenic mice (20). Furthermore, when the TS1 TCR was altered to have low affinity for HA, thymocytes no longer developed into nTreg cells. However, HA specific T cells (TS1 TCR) were deleted when they were crossed to mice that expressed HA encoded by a different transgene, suggesting that where and when the self-peptide is expressed impacts the fate of the T cell (21). This general observation was subsequently made in several TCR transgenic strain combinations (reviewed in (22)), but not all. Additionally, TCR transgenic mice with TCRs specific for a foreign antigen crossed to a RAG deficient background generally lack nTreg cells unless they are crossed to mice that express the cognate antigen.
Table I.
Evidence for high self-reactivity in T lymphocyte subpopulations
| Subset | Phenotype | Specificity | Repertoire | TCR transgenic systems | Other | GFP level Nur77GFP mice |
|---|---|---|---|---|---|---|
| nTreg | CD44hiCD25+GITR+ | MHCII/self-peptides (generally rare?, tissue-specific?) | Diverse | MCHII restricted TCR Tgs with antigen increase Treg cells | Rapid expansion in lymphopenic recipients | High in progenitorsHigh in mature cells |
| iNKT | CD44hiCD69+ | CD1d-self lipids (iGb3 and others?) | Oligoclonal | Vα14-Jα18 TCR transgenics have reduced thymic cellularity | Peripheral cells can produce cytokines in response to self lipid/CD1d | High in progenitorsLow in mature cells |
| CD8αα + nIEL | CD44intCD69+ | Unknown | Oligoclonal | Increased in MHCI TCR Tgs with antigen | Gut iEL exhibit high basal calcium flux | Low in mature cells |
| nTh17 | CD44hiα4β1+CCR6+ | MHCII/self peptides uncharacterized | ? | Increased in MHCII TCR Tgs with antigen | Not determined |
The idea that nTreg TCRs have higher reactivity for self would imply that the nTreg TCR repertoire would be distinct from conventional T cells. Indeed, several studies showed that the TCR repertoire of nTreg cells was diverse and predominantly distinct from that of non-nTreg cells (23–25). However, there is some overlap, from which it has been argued that self-reactivity can play only a limited role in nTreg cell development (26), and generally highlights the difficulty of making precise inferences about development based solely on repertoire studies.
It has been difficult to directly demonstrate that the T cell receptors expressed by nTreg cells are self-reactive. nTreg cells do not proliferate in a standard mixed lymphocyte reaction as they are inherently non-proliferative, and overt self-reactivity was not detected in T cell hybridomas expressing nTreg TCR (26). However, T cells transduced with nTreg TCR were shown to undergo enhanced homeostatic expansion in normal or lymphopenic recipients (23, 27). These observations suggest that nTreg cell self-reactivity is either limited to rare tissue specific antigens or is of an intermediate, weaker level, compared to TCR reactivity to foreign antigens. As might be expected, the creation of nTreg TCR transgenic mice provided interesting insight into nTreg cell development. Two groups independently created transgenic mice with TCR genes cloned from naturally occurring nTreg cells (28, 29). Their analysis showed that precursor frequency dramatically affected the likelihood that a given progenitor would develop into a Foxp3+ nTreg cell. Efficient nTreg cell development only occurred at very low precursor frequencies, suggesting that nTreg cells compete for crucial developmental factor(s). The limiting factor could be a rare high affinity self-antigen, or it could be a TCR extrinsic factor (such as IL-2 or co-stimulation, discussed below).
Recently, a novel fluorescent reporter mouse was created where GFP was inserted into the start site of a TCR immediate early gene (Nr4a1 or Nur77) (30). Analysis of this mouse suggested that GFP levels specifically reflect TCR signal strength in T cells. In such Nur77GFP mice, polyclonal nTreg cells showed a higher level of GFP than non-nTreg cells, consistent with an overall higher self-reactivity of the nTreg cell repertoire. Interestingly, when crossed to nTreg TCR transgenic mice, GFP levels were only higher when the precursor frequency was very low, suggesting that nTreg clones compete for rare self-ligands during development.
Overall, the picture emerging is that at the population level, nTreg cells do have an overall enhanced self-reactivity compared to conventional T cells. However, each TCR does not have a digital propensity to become a nTreg or conventional T cell. Rather, their development is probabilistic, with the likelihood that a given TCR will become a nTreg cell being dependent on cellular competition and possibly other environmental factors (discussed below) (31). This contrasts with clonal deletion, which is thought to have a sharp and well-defined affinity threshold (5), and is thought not to be greatly influenced by competition or environmental factors. However, it should be noted that much of the work on clonal deletion thresholds has been done with Class I restricted CD8 T cell progenitors, whereas nTreg cell development is a Class II restricted event. Indeed, a critical unanswered issue is whether nTreg cell development and clonal deletion occur at similar or distinct thresholds. Was a nTreg cell progenitor “rescued” from clonal deletion? Or is the self-reactivity of nTreg cells, while high compared to non-nTreg cells, still beneath the threshold for clonal elimination? Further work examining clonal deletion and nTreg cell selection in the same experimental systems will be needed in the future to address this question.
iNKT cell self-reactivity
iNKT cells are a minor lineage of T cells that express a semi-invariant TCR and recognize lipid antigens in the context of the MHC class I –like molecule, CD1d (32). They are important regulators of immunity, but unlike nTreg cells, they can either suppress or enhance immune responses, depending on what cytokines they produce, and where (33). iNKT cells develop from DP progenitors and are thought to undergo positive and negative selection similar to conventional T cell progenitors, except in response to self-lipid antigens, and not self-peptide antigens (34). Like nTreg cells, iNKT cells are thought to experience agonist selection in the thymus. They have an antigen-experienced phenotype, being CD44 positive, but are not CD25 positive. They express intermediate levels of the activation marker CD69, and diverse expression of NK cell markers, including NK1.1, DX5, and NKG2D (Table I) (35). iNKT TCR transgenic mice have a markedly smaller thymus in the presence of CD1d than in the absence (36) suggesting that some clonal deletion occurs in addition to iNKT cell maturation during development, consistent with the notion of agonist selection. Nonetheless, iNKT cells can be further deleted by exposure to high affinity exogenous lipids like αGalCer or DC overexpressing CD1d (37). Thus it is possible that iNKT cell selection is similar to that proposed for nTreg cell selection, where agonist lipids select iNKT cells but very strong agonists can lead to deletion.
Like nTreg cells, the precise self-ligands involved in iNKT cell selection are not completely defined, although there is more knowledge about this for iNKT cells than for nTreg cells. The expression of CD1d specifically on DP thymocytes is required for iNKT cell selection and development (38). Bendelac and colleagues identified one potential iNKT cell selecting ligand expressed in the thymus as isoglobotrihexosylceramide (iGb3) (39). Mice deficient in β-hexosaminidase B, which is important for the production of iGb3 in lysosomes, showed a major defect in iNKT cell development and thus argued the importance of iGb3 in iNKT cell development. However, other work suggested that iGb3 is not the only glycosphingolipid deficient in mice lacking β-hexosaminidase B, which have a broad defect in glycosphingolipid processing (40). Mice deficient in iGb3 synthase, which is crucial for iGb3 biosynthesis, have normal iNKT cell development (41). Yet iGb3 shapes the repertoire in a manner similar to that which occurs naturally (42). Thus iGb3 may not be the sole selecting ligand, but is likely to be a relevant self-ligand in vivo. iGb3 pulsed DCs can stimulate CD69 expression and proliferation in Vα14 Tg mice (42), IL-2 production in iNKT hybridomas (or human iNKT cells) (39, 43), and activation of iNKT cells in vivo (44), thus it may be considered an agonist ligand, yet iGb3/CD1d tetramers do not stain iNKT cells (39). Furthermore, data suggest that APCs do not consistently display the same set of lipid ligands, and that activation associated changes in cellular lipid metabolism can alter the display (44). Further work is needed to understand the nature and timing of self-lipid recognition in iNKT cell selection and homeostasis.
In support of an agonist selection mechanism, iNKT cells developing in the thymus expressed a high level of GFP in Nur77GFP mice. Interestingly, the level of GFP in peripheral iNKT cells was very low (30). It is possible that iNKT cells see an agonist self-ligand displayed by DP in the thymus, but that same ligand may not be constitutively displayed in the periphery. Alternatively, iNKT cells in the periphery may have become desensitized to their selecting ligand(s). Interestingly, while the development of iNKT cells requires CD1d, their survival and function does not (45).
CD8αα+ T cell self-reactivity
Intraepithelial lymphocytes (IELs) of the gut are enriched in T cells that express CD8αα homodimers (CD8αα+ T cells), recently referred to as natural IEL or nIEL (46). They are thought to play an important role in immunity and tolerance at the mucosal surfaces of the body. CD8αα+ T cells can express either γδ or αβ TCRs. We will focus here on TCRαβ + CD8αα+ nIEL cells, since their specificity and selection has been more widely studied.
The site(s) of nIEL T cell development is still a matter of debate (47–49). Several studies suggested that the development of nIEL can occur through an extrathymic pathway (50–52). However, compelling evidence suggests that nIEL can and typically do arise from thymic precursors (53–56). It should also be noted that nIEL have remarkable homeostatic proliferation capacity, a feature that can complicate interpretation of various studies (57). Overall, it seems likely that multiple pathways exist for nIEL development, which are more or less prominent under certain conditions or at different periods in development.
nIEL reside between the epithelial cells, most prominently in the small intestine. Like nTreg and iNKT cells, nIEL have an activated phenotype (58) (Table I). Studies using TCR transgenic models suggested that the selection of TCRαβ + nIEL is dependent on agonist interactions in the thymus (54, 59–63). Furthermore, DP thymocytes from TCR transgenic mice that are exposed to high doses of their cognate antigen can differentiate into cells that appear similar to nIEL in thymic organ cultures (64–66, 67, Hogquist, 1998 #176). However, it is unclear if the normal thymic precursors for polyclonal nIEL encounter agonist ligands in the thymus. This is difficult to study because there are no definitive means to identify nIEL precursors in the thymus. One study showed that TL tetramers identified a subset of DP thymocytes that was enriched in progenitors (54). However, other studies contest the notion that nIEL develop via a thymic DP progenitor, instead arguing that CD44+ TCR- DN cells give rise to nIEL (68, 69). Furthermore, if the precursors to nIEL do encounter agonist ligands in the thymus, do they continue this recognition in the tissue? nIEL were recently shown to display high basal calcium levels and be refractory to TCR-dependent calcium-flux induction. Blocking the TCR on γδ+ nIEL in vivo led to a decrease of basal calcium suggesting that γδ+ nIEL, at least, are constantly being triggered through the TCR (70).
nTh17 T cell self-reactivity
Mature naïve CD4 T cells can differentiate into several different subsets depending on the microenvironment during stimulation. In the presence of TGF-β +IL-6 they differentiate into Th17 cells (71). Th17 cells secrete a variety of cytokines including IL-17A/F and express the transcription factors RORγt and RORα. Th17 cells are important in protection against extracellular bacteria however aberrant responses to self-antigen can lead to a variety of autoimmune diseases (72). Recently Craft and colleagues have shown evidence of IL-17 producing cells that arise developmentally, in the thymus. These naturally occurring Th17 cells (nTh17) are enriched in the presence of high affinity ligands, illustrated by the use of double transgenic mice (Table I) (73). Similar cells in the thymus and periphery of normal unimmunized mice express an activated phenotype, suggesting that nTh17 cells are selected by agonist peptides.
Finally, it is worth noting that the γδ T cell repertoire is also thought to embody substantial self-reactivity (74). Indeed some γδ T cell subpopulations bear remarkable similarity to αβ T cell subsets, e.g. γδ NKT cells and αβ NKT cells (75). However, less is understood about the specificity of γδ T cells in general, and we refer the reader to other sources for further information (76).
Measuring self-reactivity
Accurately measuring the self-reactivity of polyclonal cell populations has presented a major challenge to understanding T cell homeostasis and regulation. Cell surface markers of T cell activation have been used in this regard. The transmembrane C-type lectin CD69 is arguably the most widely accepted marker of TCR activation, as expression is low in naïve T cells and rapidly induced in an ERK dependent fashion after TCR activation. Consistent with its potential utility as a marker of self-reactivity, some agonist selected T cell populations do constitutively express CD69 (see Table I). However, CD69 up-regulation is not specific for TCR signaling. Inflammatory stimuli, like those that induce type I interferon and toll-like receptor signaling, result in widespread up-regulation of CD69 on lymphocytes (88). Thus it is unclear if the expression of CD69 on iNKT cells and IELs reflects their constitutive perception of self-stimuli, or of other micro environmental stimuli, e.g. through NK-receptors, TLR or other pattern recognition receptors. For example, CpG induced CD69, but not GFP expression, in Nur77GFP mice (30), and other evidence suggested that antigen specific nIEL express CD69 even in the absence of antigen (89). Thus CD69 has utility as a marker of acute TCR activation in some contexts, but is of limited utility for analysis of polyclonal populations, particularly during infection or in specific tissue environments.
The cell surface glycoprotein CD5 is expressed on thymocytes and mature T cells. Its expression is low on DN thymocytes whereas DP and single positive (SP) thymocytes have intermediate to high levels of CD5 (90). High CD5 expression requires peptide-MHC contact and it has been suggested that the expression of CD5 correlates with the avidity of TCR-MHC-ligand interactions based on the observation that CD5 expression parallels TCR signal strength in thymocytes (90) and was reduced on CD4 T cells when they were deprived of Class II (91) or CD8 T cells deprived of Class I (92). Interestingly, a recent study showed that naïve T cells expressing the highest level of CD5 (and presumably the most self-reactive) were hypersensitive to cytokines (93). Thus CD5 may prove to be a valid experimental means to distinguish self-reactivity in the polyclonal repertoire. Consistent with this, CD5 levels tend to correlate with GFP levels in naïve T cells of Nur77GFP mice (GLS and KAH unpublished data), and nTreg cells express a higher level of CD5 than conventional T cells (94). However, it is not yet clear if CD5 is regulated exclusively by the TCR, or in all T cell subsets, such as iNKT cells (95) or nIELs (96). Furthermore, there is evidence that CD5 plays a role in negatively regulating TCR signaling (97, 98), thus CD5 levels presumably both reflect and alter TCR self-reactivity.
The recent development of a Nur77GFP mouse strain suggests its potential to be a useful tool in studying self-reactivity. GFP expression in the Nur77GFP mice specifically reflected the signal strength of TCR-MHC interactions without being influenced by inflammatory stimuli (30). As discussed above, both nTreg and iNKT cells showed increased GFP levels during selection in the thymus (30). Thus further investigation of other agonist selected cell types including CD8αα+ nIEL and nTh17 cells, and of autoreactive and anergic T cells in various models, using the Nur77GFP mice may be interesting in the future. In summary, there is likely no single perfect means to measure self-reactivity in lymphocyte populations, although combinations of the markers discussed above may be useful.
Molecular mechanisms of agonist selection
Although nTreg cells, iNKT cells, and CD8αα nIELs are selected by agonist interactions, each cell type requires unique cellular and molecular factors to develop. Thus it is not merely the TCR interaction that specifies fate, but the context it is encountered in. Key cytokines and co-stimulatory factors are provided in distinct thymic microenvironments, both to favor survival in the face of strong TCR stimuli, and to specify a functionally unique differentiation program. These unique combinations lead to the expression of distinct transcription factors, which play a dominant role in programming each T cell lineage as outlined in the following sections.
Unique factors in nTreg cell development
One key requirement for nTreg cell development is the interaction of CD28 with its B7 family member ligands (Figure 3). Mice deficient in the co-stimulatory molecule CD28 or CD28 ligands B7-1/B7-2 have decreased thymic nTreg cell numbers and percentages (99–101). The role that co-stimulation plays in nTreg cell development is not completely clear. Some reports suggest that co-stimulation provides a quantitative signal (along with TCR stimulation) that drives a T cell to develop into a nTreg cell. Other models suggest that co-stimulation prevents negative selection and supports nTreg cell development (reviewed in (22)). Regardless, it is clear that CD28/B7 interactions are required for nTreg cell development. B7.1 and B7.2 are primarily expressed on thymic APC, including DC and epithelial cells, and it is interesting to consider the possibility that different thymic APC are involved in selecting nTreg cells versus clonal deletion. A variety of experimental data exist on this topic, also reviewed recently in (22). To date, the collective data do not support a simple model whereby one APC is specialized for nTreg cell induction and another for deletion. However future experiments testing this in more physiologic contexts may change the picture.
Figure 3. Unique aspects of nTreg cell development in the thymus.
A) A double positive (DP) progenitor is positively selected upon interacting with peptides presented by cortical epithelial cells. In this model, we propose that nTreg and conventional T cells are selected in a similar manner in the cortex, i.e. via low affinity interactions with abundant self-peptides. A given clone might recognize multiple distinct self-peptides in this affinity range. B) After positive selection, progenitors migrate from the cortex to medulla and differentiate to the semi-mature (HSAhi, CD62Llo) stage. If a progenitor encounters self-peptides displayed by B7.1/2+ medullary APC (mTEC or DC) with high affinity, this triggers the upregulation of CD25. C) A CD25+ Foxp3− nTreg cell precursors requires IL-2 for survival and differentiation to the mature Foxp3+ nTreg cell stage. D) Mature, self-reactive nTreg cells emigrate from the thymus to populate the peripheral lymphoid organs. E) In the periphery, nTreg cells continue to perceive higher affinity self-ligands (presumably same or similar to peptide B) and regulate self-tolerance in the steady state.
NFκB is a transcription factor downstream of several pathways, but most notably in the case of nTreg cells, the CD28/B7 pathway. Therefore, it was not surprising that the development of nTreg cells also required NFκB activation. Specifically the NFκB family member c-Rel, but not NFκB1, is critical for nTreg cell development in the thymus (reviewed in (102)).
Another requirement for nTreg cell development is cytokine signaling, most prominently IL-2. Mice deficient in IL-2Rβ have a significant decrease in nTreg cells (103). The phenotype of mice deficient in IL-2 is less dramatic however, with only a 50 percent decrease of nTreg cells in the thymus (104, 105), but this is due to redundancy with other common γ chain cytokines like IL-15 (106). Thus, IL-2 plays a critical role in nTreg cell development and function (107). A two-step model has been proposed where TCR engagement leads to the expression of the high affinity IL-2 receptor, which ultimately leads to IL-2 induced Foxp3 expression and nTreg cell commitment (Figure 3, steps B & C) (108). Mice that express constitutively active STAT5, a transcription factor downstream of IL-2 signaling, further support the importance of IL-2 in nTreg cell development (109). These mice have increased nTreg cell numbers and a more diverse nTreg cell TCR pool that confirms that IL-2 acts primarily on pre-instructed nTreg precursors.
The master transcription factor for nTreg cells is forkhead box P3 (Foxp3). Foxp3 is not only required for their suppressive capabilities, but also for their development. Mice deficient in Foxp3 or scurfy mice, which have mutated Foxp3 gene, developed lethal multi-organ inflammation. The development of multi-organ inflammation was attributed to a defect in thymic nTreg cell development, demonstrated by a specific deletion of Foxp3 in T cells. Furthermore, adoptive transfer of Foxp3+ T cells into neonates protected Foxp3 deficient mice from their autoimmune pathology (110, 111). Altogether, it is clear that nTreg cells require Foxp3 for development and it is thought to seal their functional fate.
Unique factors in iNKT cell development
The development of conventional T cells requires antigen presentation by thymic epithelial cells, whereas iNKT cells require lipid presentation by CD1d positive cortical thymocytes (32). Cortical thymocytes not only present lipids to iNKT cells, but also provide many co-stimulatory signals necessary for commitment and development.
The SLAM signaling pathway plays a major role in iNKT cell development (Figure 4). The SLAM family surface receptors SLAM1 and SLAM6 are expressed on cortical thymocytes and mediate iNKT cell positive selection. Therefore, mice deficient in both SLAM1 and SLAM6 have a defect in iNKT cells (112). Furthermore, mice that lack the SLAM adaptor protein (SAP), which is required for SLAM signaling, are deficient in iNKT cells (113–115). The importance of the SLAM/SAP pathway was also highlighted in a study where MHC class II expression was restricted to cortical thymocytes (116). Conventional MHC class II restricted T cells positively selected by the thymocytes had a phenotype very similar to iNKT cells, and was SAP dependent (117) further suggesting that the SLAM/SAP signaling provided by the cortical thymocytes drives development of this unique lineage (Figure 4).
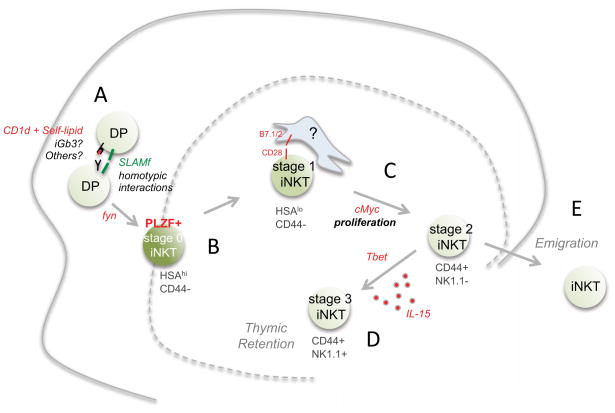
Figure 4. Unique aspects of iNKT cell development in the thymus.
A) iNKT cells arise from rare DP progenitors that express a Vα14-Jα18 TCR. Interaction with hematopoetic APC (DP cells) that express CD1d molecules and stimulatory self-lipids, facilitated by SLAM family member interactions, initiate positive selection through the src family kinase fyn. B) The earliest post-selection iNKT cell is an HSAhi “stage 0” cell, which already express the iNKT lineage transcription factor PLZF. As the progenitor develops, it downregulates HSA (stage 1), and upregulates CD44 (stage 2). C) During this time it undergoes cellular proliferation requiring cMyc. The cellular interactions that iNKT cells make in the medulla are not well characterized, but CD28 interaction with B7.1/2+ APC is required for iNKT cell expansion. D) Stage 2 iNKT cells upregulate Tbet and respond to IL-15. The most mature (stage 3) iNKT cell expresses NK1.1 and is retained in the thymus for long periods. E) Alternatively, stage 2 iNKT cells can emigrate from the thymus and populate the spleen and liver. Some evidence suggests that peripheral iNKT cells do not continuously perceive stimulatory self-lipid. Rather, APC can be activated during infection to display stimulatory self-lipids and thus activate iNKT cells.
Downstream of TCR/SLAM interactions are several signaling molecules including the Src tyrosine kinase Fyn. Fyn is required for iNKT cell development, therefore mice deficient in Fyn lack iNKT cells (118, 119). In addition to the Fyn signaling pathway NFκB is critical for iNKT cell development. NFκB is required for downstream signaling of the TCR as well as inhibiting apoptosis. Inhibition of several different NFκB family members (by conditional knockout mice or inhibitors) has significant influence on iNKT cell development (120–123). Early growth response 2 (Egr2) is a transcription factor downstream of the calcineurin-NFAT pathway that is activated upon TCR stimulation. It is required for efficient positive selection, maturation and survival of iNKT cells (124).
Finally, the transcription factor PLZF is expressed in iNKT cells directly after positive selection. It is now clear that PLZF is a master transcription factor for iNKT cells and is required for both their maturation and function (125, 126). Furthermore, iNKT cells from mice deficient in PLZF lose their activated phenotype, have a defect in trafficking, and have impaired effector functions (127). More studies will be required to establish precisely how PLZF is induced in iNKT cells and what are its direct gene targets.
Unique factors in nTh17 cell and CD8αα T cell development
The unique factors required for the development and selection of nTh17 cells and CD8αα+ T cells have yet to be uncovered. It would be tempting to hypothesize that the critical factor required for nTh17 cell development/selection is RORγt, the master regulator of differentiated Th17 cells (128). It has been published that nTh17 cells express RORγt; however, the requirement for RORγt in nTh17 cell development has yet to be established (73). It will be important to dissect RORγt targets in both Th17 cells and nTh17 cells to determine if they are similar or distinct. The unique factors involved in CD8αα+ T cell development are even less clear. Additional studies will need to be done in order to understand the signaling and interactions involved for development and maturation of these cells.
TGF-β common survival/development mechanism in thymus?
The development of nTreg cells, iNKT cells, nTh17 cells, and nIEL occurs in the thymus under conditions that normally drive clonal deletion. It is possible that factors specific for the thymic environment might promote an increased survival threshold in these cell types. One potential common mechanism for survival in the thymus is the cytokine TGF-β (Figure 1). TGF-β is a cytokine that has pleiotropic regulatory effects on many different cell types. Ouyang et al. showed that blocking TGF-β signaling in the thymus led to increased deletion of nTreg cells (129). This sensitivity to apoptosis was also observed when thymocytes from TGF-β RII –deficient mice were stimulated in vitro with anti-CD3. These results as well as the significant reduction of developing nTreg cells in the TGF-β RII-deficient mice suggest that TGF-β plays an important role in nTreg cell development and survival in the thymus (129).
iNKT cell development also requires TGF-β signaling in the thymus. iNKT cells from the thymus express increased levels of both TGF-βRI and TGF-βRII. Furthermore, it was shown, using mice deficient in TGF-β signaling components, that TGF-β is required for protection against apoptosis, lineage expansion, and maturation of iNKT cells (130).
Recent work by Chen and colleagues provided evidence that the TGF-β plays an important role in nIEL development (131). Mice deficient in TGF-β, TGF-β RI or the downstream factor Smad3 have significantly reduced numbers of CD8αα+ nIEL. Furthermore, mice deficient in TGF-β have reduced frequency of a putative nIEL precursor in the thymus, showing that TGF-β signaling plays a critical role in nIEL development.
The recently characterization of nTh17 cells also highlighted the importance of TGF-β in development (73). Using TGF-β R DN mice, Marks et al. showed the development of thymic Th17 cells requires TGF-β signaling.
The requirement for TGF-β in developing nTreg cells, iNKT cells, nTh17 cells, and nIEL provides a potential common mechanism of survival of agonist-selected cells (Figure 1). It is not clear if TGF-β plays a role in differentiation or of cells selected by agonist ligands, or merely a survival role. Further studies will be needed to address this and to elucidate the targets of TGF-β signaling. It is possible that common factors other than TGF-β, not yet elucidated, are also important in survival of cells following agonist interactions.
Functional significance of self-reactivity in peripheral T cell homeostasis and immunity
The usefulness of lymphocytes with reactivity to self-antigens is two-fold: they can both promote immunological tolerance and enhance immunity to foreign antigens. nTreg cells provide a classic example of the ability of self-reactive cells to promote immunological tolerance. Ablation of nTreg cells results in massive lympho- and myelo-proliferation. Mature nTreg cells require stimulation via the TCR to exert their suppressive effects in the periphery, although once activated, can exhibit non-specific suppression (132). Indeed, the high GFP levels in peripheral nTreg cells from Nur77GFP mice suggested that nTreg cells continually encounter stimulatory self-peptides (30)(Figure 3). That certain nTreg cell TCR specificities are more common in one anatomic site than another is consistent with continued TCR ligand recognition driving tissue localization (27). Several effector mechanisms have been elaborated for nTreg cells, including production of suppressive cytokines, reducing stimulatory properties of DC, and conversion of inflammatory ATP to adenosine. It is thought that because of their activated phenotype, nTreg cells are more responsive to self-antigens than naïve T cells and could compete for interactions with APC. Interestingly, there is evidence that nTreg cells integrate environmental cues with self-reactivity to tailor the regulatory response to the type of tissue or to the type of inflammatory response going on (133).
iNKT cells can alter immune responses in multiple ways depending on the type of cytokine response they produce (33), and are often considered “innate immune” cells, as their rapid production of cytokines early during infection can alter immunity. iNKT cells offer protection from certain infections (134), but also may contribute to autoimmune disease (135) and allergic hypersensitivity (136). Some species of pathogens produce lipids that can directly activate iNKT cells through the antigen receptor, including Sphingomonas and Borrelia burgdorferi. However, iNKT cells become activated during many other bacterial, viral, and fungal infections that are not thought to involve a foreign lipid that stimulates through the iNKT T cell receptor (134). Data are emerging to support the idea that during infection, APC display stimulatory self-lipids to activate iNKT cells, particularly in combination with inflammatory cytokines (137–140). For example, iNKT cell activation during Salmonella infection could be blocked by antibodies to CD1d, despite the fact that the bacteria themselves do not produce stimulatory lipids (137). Recent evidence suggests that this may be accomplished through TLR induced alterations in endogenous lipid catabolism (44). Thus, iNKT cells may represent a scenario where strong TCR stimulation is involved in selection in the thymus, but it does not continue in the periphery (Figure 4). During infection or inflammation, APC can be triggered to display the same or similar stimulatory lipids and thereby activate iNKT cells.
The specific functions of CD8αα+ nIEL are rather poorly defined to date. It is presumed that they survey their environment for danger signals and respond via the production of inflammatory cytokines, cytolysis, or tissue repair. Mice deficient in TCRγδ cells are more susceptible to colitis suggesting that TCRγδ nIEL have an important role in the homeostasis of the epithelium (141, 142). Also, TCRαβ nIEL were capable of preventing development of inflammatory bowel disease (IBD) (143). Is TCR recognition important for this role? nIEL in the intestine clearly exist in a partially activated state (144, 145). They express CD69, various cytolytic molecules, and rapidly produce IFNγ (146). However, despite their partially activated state, they proliferate poorly in response to mitogenic signals. Furthermore, it is not known if the activated state is a consequence of antigen receptor stimulation or other environmental stimuli. Further work including the development of IEL TCR transgenic models may be required to fully understand the role of TCR specificity in IEL function.
Since they were only described recently, little is known about the function of nTh17 cells. However, they were shown to be able to mediate host protection in the liver following toxin-induced hepatitis (73). Protection was mediated through secretion of the cytokine IL-22, previously shown to be protective in the liver (147). Future experiments will be also be needed to understand if TCR recognition of self-peptides is involved in nTh17 function.
In summary, the thymus produces several minor T cell lineages that nonetheless play crucial roles in immunity. The requirement for perception of strong TCR signals during development in the thymus unifies these lineages. The cytokine TGFβ may play a key role in the ability of progenitors to survive such strong signals. However, beyond that, there are very few commonalities in the factors involved in differentiation. Each lineage expresses distinct key transcription factors, which dictate unique tissue localization, effector functions, and roles in immunity.
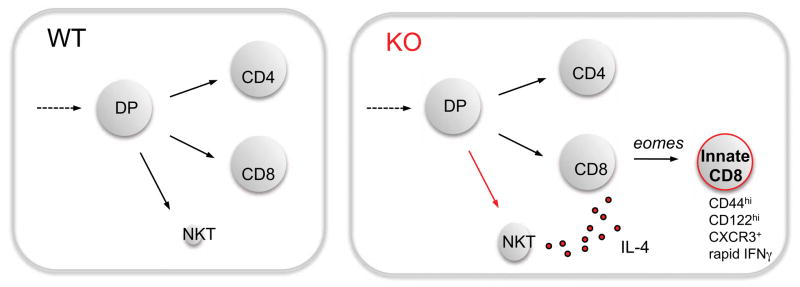
Figure 2. Cross talk between developing iNKT and CD8SP progenitors in the thymus generates innate CD8 T cells.
The iNKT, CD4 helper and CD8 killer lineages normally develop largely independently (WT, left panel). However, in some gene deficient or mutant mice (ITK, KLF2, CDP, Id3, and SLP76Y145F) (KO, right panel) and in some inbred strains (like BALB/c), iNKT cells that constitutively produce IL-4 are expanded. IL-4 acts on developing CD8 lineage cells to upregulate the transcription factor eomes and a number of downstream target genes. This leads to the generation of CD8 T cells with a memory phenotype and capacity to rapidly produce cytokines.
Side bar. The special case of “innate CD8” T cells.
Another unusual population of T cells has sometimes been considered under the umbrella of agonist selection are innate CD8 T cells (77, 78). These cells were first studied in ITK deficient mice, where CD8 SP thymocytes accumulate with a memory phenotype and ability to rapidly produce cytokines (79, 80). Like iNKT cells, the development of this population required selection on hematopoietic cells (80), CD28, and SAP (81). One model was that ITK deficiency increased TCR signaling thresholds such that only those cells with agonist interactions with self would survive (82). Interestingly, subsequent studies identified an expanded population of innate CD8 T cells in multiple other gene deficient or mutant mice, including KLF2, CBP, Id3, and SLP76Y145F (83), several of which would not be predicted to alter TCR signaling thresholds. Key insight came from analysis of innate CD8 T cells in KLF2 deficient mice, where wild-type cells in a KLF2 deficient environment also acquired an innate CD8 T cell phenotype, suggesting an indirect or bystander effect of soluble factors, and not a cell-intrinsic effect (84). Further studies confirmed a similar indirect mechanism operates in ITK, CBP (85), Id3 (86), and SLP76Y145F mice (87). Interestingly, the innate CD8 phenotype results from IL-4 over-produced by αβ or γδ iNKT cells, which expand in all of these strains of mice (83) (Figure 2). In fact, the requirement for iNKT cell produced IL-4 explains why the effect is dependent on hematopoietic cell selection, CD28, and SAP, and not because CD8 T cells themselves have an altered recognition of MHC. In fact, the level of Nur77GFP in CD8 T cells is not increased in KLF2 KO chimeras, where they acquire the innate CD8 phenotype (Y.J. Lee and K.A. Hogquist, unpublished data). Altogether these data show that innate CD8 T cells, although they bear phenotypic and functional resemblance to iNKT cells, and to CD8α+ T cells (64), are not a product of agonist selection in the thymus.
Acknowledgments
The authors would like to thank Keli Holzapfel for critical review of the manuscript. We apologize to the many colleagues whose work is relevant to this topic but could not be cited because of space limitations. This work was supported by NIH RO1AI39560 and XXX.
Glossary
Agonist selection
positive selection of T cells by agonist ligand, in which cells with overtly self-reactive TCRs are directed into a mature T cell lineage
Nur77 (Nr4a1)
immediate early gene up-regulated by TCR stimulation
LIP
lymphopenia-induced proliferation
Clonal deletion
process where self-reactive specific T and B cells are eliminated from the repertoire
iGb3
isoglobotrihexosylceramide
nTh17 cell
natural IL-17 producing CD4 T helper cell that arises in the thymus via agonist selection
nIEL
natural intraepithelial lymphocytes that expresses CD8αα homodimers and resides in mucosal surfaces of the body
iNKT
invariant natural killer T cell that expresses semi-invariant TCRs and recognizes lipid antigens in the context of CD1d
Footnotes
Disclosure Statement
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
References
- 1.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–76. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 2.Palmer E. Negative selection--clearing out the bad apples from the T-cell repertoire. Nat Rev Immunol. 2003;3:383–91. doi: 10.1038/nri1085. [DOI] [PubMed] [Google Scholar]
- 3.Davey GM, Schober SL, Endrizzi BT, Dutcher AK, Jameson SC, Hogquist KA. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. J Exp Med. 1998;188:1867–74. doi: 10.1084/jem.188.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucas B, Stefanova I, Yasutomo K, Dautigny N, Germain RN. Divergent changes in the sensitivity of maturing T cells to structurally related ligands underlies formation of a useful T cell repertoire. Immunity. 1999;10:367–76. doi: 10.1016/s1074-7613(00)80036-9. [DOI] [PubMed] [Google Scholar]
- 5.Palmer E, Naeher D. Affinity threshold for thymic selection through a T-cell receptor-co-receptor zipper. Nat Rev Immunol. 2009;9:207–13. doi: 10.1038/nri2469. [DOI] [PubMed] [Google Scholar]
- 6.Takahama Y, Nitta T, Mat Ripen A, Nitta S, Murata S, Tanaka K. Role of thymic cortex-specific self-peptides in positive selection of T cells. Semin Immunol. 2010;22:287–93. doi: 10.1016/j.smim.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 7.McCaughtry TM, Hogquist KA. Central tolerance: what have we learned from mice? Semin Immunopathol. 2008;30:399–409. doi: 10.1007/s00281-008-0137-0. [DOI] [PubMed] [Google Scholar]
- 8.Peterson DA, DiPaolo RJ, Kanagawa O, Unanue ER. Cutting edge: negative selection of immature thymocytes by a few peptide-MHC complexes: differential sensitivity of immature and mature T cells. J Immunol. 1999;162:3117–20. [PubMed] [Google Scholar]
- 9.Zehn D, Bevan MJ. T cells with low avidity for a tissue-restricted antigen routinely evade central and peripheral tolerance and cause autoimmunity. Immunity. 2006;25:261–70. doi: 10.1016/j.immuni.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouneaud C, Kourilsky P, Bousso P. Impact of negative selection on the T cell repertoire reactive to a self-peptide: a large fraction of T cell clones escapes clonal deletion. Immunity. 2000;13:829–40. doi: 10.1016/s1074-7613(00)00080-7. [DOI] [PubMed] [Google Scholar]
- 11.van Meerwijk JP, Marguerat S, Lees RK, Germain RN, Fowlkes BJ, MacDonald HR. Quantitative impact of thymic clonal deletion on the T cell repertoire. J Exp Med. 1997;185:377–83. doi: 10.1084/jem.185.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ignatowicz L, Kappler J, Marrack P. The repertoire of T cells shaped by a single MHC/peptide ligand. Cell. 1996;84:521–9. doi: 10.1016/s0092-8674(00)81028-4. [DOI] [PubMed] [Google Scholar]
- 13.Fukui Y, Ishimoto T, Utsuyama M, Gyotoku T, Koga T, Nakao K, Hirokawa K, Katsuki M, Sasazuki T. Positive and negative CD4+ thymocyte selection by a single MHC class II/peptide ligand affected by its expression level in the thymus. Immunity. 1997;6:401–10. doi: 10.1016/s1074-7613(00)80283-6. [DOI] [PubMed] [Google Scholar]
- 14.Boyman O, Purton JF, Surh CD, Sprent J. Cytokines and T-cell homeostasis. Curr Opin Immunol. 2007;19:320–6. doi: 10.1016/j.coi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Takada K, Jameson SC. Naive T cell homeostasis: from awareness of space to a sense of place. Nat Rev Immunol. 2009;9:823–32. doi: 10.1038/nri2657. [DOI] [PubMed] [Google Scholar]
- 16.Kassiotis G, Zamoyska R, Stockinger B. Involvement of avidity for major histocompatibility complex in homeostasis of naive and memory T cells. J Exp Med. 2003;197:1007–16. doi: 10.1084/jem.20021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–62. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–26. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 19.Shevach EM. Regulatory T cells in autoimmmunity*. Annu Rev Immunol. 2000;18:423–49. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 20.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–6. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 21.Caton AJ, Cozzo C, Larkin J, 3rd, Lerman MA, Boesteanu A, Jordan MS. CD4(+) CD25(+) regulatory T cell selection. Ann N Y Acad Sci. 2004;1029:101–14. doi: 10.1196/annals.1309.028. [DOI] [PubMed] [Google Scholar]
- 22.Wirnsberger G, Hinterberger M, Klein L. Regulatory T-cell differentiation versus clonal deletion of autoreactive thymocytes. Immunol Cell Biol. 2011;89:45–53. doi: 10.1038/icb.2010.123. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–77. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Wong J, Obst R, Correia-Neves M, Losyev G, Mathis D, Benoist C. Adaptation of TCR repertoires to self-peptides in regulatory and nonregulatory CD4+ T cells. J Immunol. 2007;178:7032–41. doi: 10.4049/jimmunol.178.11.7032. [DOI] [PubMed] [Google Scholar]
- 25.Pacholczyk R, Ignatowicz H, Kraj P, Ignatowicz L. Origin and T cell receptor diversity of Foxp3+CD4+CD25+ T cells. Immunity. 2006;25:249–59. doi: 10.1016/j.immuni.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 26.Pacholczyk R, Kern J, Singh N, Iwashima M, Kraj P, Ignatowicz L. Nonself-antigens are the cognate specificities of Foxp3+ regulatory T cells. Immunity. 2007;27:493–504. doi: 10.1016/j.immuni.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7:401–10. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 28.Bautista JL, Lio CW, Lathrop SK, Forbush K, Liang Y, Luo J, Rudensky AY, Hsieh CS. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat Immunol. 2009;10:610–7. doi: 10.1038/ni.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leung MW, Shen S, Lafaille JJ. TCR-dependent differentiation of thymic Foxp3+ cells is limited to small clonal sizes. J Exp Med. 2009;206:2121–30. doi: 10.1084/jem.20091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–89. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lio CW, Hsieh CS. Becoming self-aware: the thymic education of regulatory T cells. Curr Opin Immunol. 2011;23:213–9. doi: 10.1016/j.coi.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 33.Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of the immune system. Curr Opin Immunol. 2008;20:358–68. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 35.Godfrey DI, Berzins SP. Control points in NKT-cell development. Nat Rev Immunol. 2007;7:505–18. doi: 10.1038/nri2116. [DOI] [PubMed] [Google Scholar]
- 36.Bendelac A, Hunziker RD, Lantz O. Increased interleukin 4 and immunoglobulin E production in transgenic mice overexpressing NK1 T cells. J Exp Med. 1996;184:1285–93. doi: 10.1084/jem.184.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chun T, Page MJ, Gapin L, Matsuda JL, Xu H, Nguyen H, Kang HS, Stanic AK, Joyce S, Koltun WA, Chorney MJ, Kronenberg M, Wang CR. CD1d-expressing dendritic cells but not thymic epithelial cells can mediate negative selection of NKT cells. J Exp Med. 2003;197:907–18. doi: 10.1084/jem.20021366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J Exp Med. 1995;182:2091–6. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou D, Mattner J, Cantu C, 3rd, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, Teneberg S, Wang D, Proia RL, Levery SB, Savage PB, Teyton L, Bendelac A. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–9. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 40.Gadola SD, Koch M, Marles-Wright J, Lissin NM, Shepherd D, Matulis G, Harlos K, Villiger PM, Stuart DI, Jakobsen BK, Cerundolo V, Jones EY. Structure and binding kinetics of three different human CD1d-alpha-galactosylceramide-specific T cell receptors. J Exp Med. 2006;203:699–710. doi: 10.1084/jem.20052369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Porubsky S, Speak AO, Luckow B, Cerundolo V, Platt FM, Grone HJ. Normal development and function of invariant natural killer T cells in mice with isoglobotrihexosylceramide (iGb3) deficiency. Proc Natl Acad Sci U S A. 2007;104:5977–82. doi: 10.1073/pnas.0611139104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei DG, Curran SA, Savage PB, Teyton L, Bendelac A. Mechanisms imposing the Vbeta bias of Valpha14 natural killer T cells and consequences for microbial glycolipid recognition. J Exp Med. 2006;203:1197–207. doi: 10.1084/jem.20060418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xia C, Yao Q, Schumann J, Rossy E, Chen W, Zhu L, Zhang W, De Libero G, Wang PG. Synthesis and biological evaluation of alpha-galactosylceramide (KRN7000) and isoglobotrihexosylceramide (iGb3) Bioorg Med Chem Lett. 2006;16:2195–9. doi: 10.1016/j.bmcl.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 44.Darmoise A, Teneberg S, Bouzonville L, Brady RO, Beck M, Kaufmann SH, Winau F. Lysosomal alpha-galactosidase controls the generation of self lipid antigens for natural killer T cells. Immunity. 2010;33:216–28. doi: 10.1016/j.immuni.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuda JL, Gapin L, Sidobre S, Kieper WC, Tan JT, Ceredig R, Surh CD, Kronenberg M. Homeostasis of V alpha 14i NKT cells. Nat Immunol. 2002;3:966–74. doi: 10.1038/ni837. [DOI] [PubMed] [Google Scholar]
- 46.Cheroutre H, Lambolez F, Mucida D. The light and dark sides ofintestinal intraepithelial lymphocytes. Nat Rev Immunol. 2011;11:445–56. doi: 10.1038/nri3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayday A, Gibbons D. Brokering the peace: the origin of intestinal T cells. Mucosal Immunol. 2008;1:172–4. doi: 10.1038/mi.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peaudecerf L, Rocha B. Role of the gut as a primary lymphoid organ. Immunol Lett. 2011 doi: 10.1016/j.imlet.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 49.Cheroutre H, Lambolez F. The thymus chapter in the life of gut-specific intra epithelial lymphocytes. Curr Opin Immunol. 2008;20:185–91. doi: 10.1016/j.coi.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanamori Y, Ishimaru K, Nanno M, Maki K, Ikuta K, Nariuchi H, Ishikawa H. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+ IL-7R+ Thy1+ lympho-hemopoietic progenitors develop. J Exp Med. 1996;184:1449–59. doi: 10.1084/jem.184.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saito H, Kanamori Y, Takemori T, Nariuchi H, Kubota E, Takahashi-Iwanaga H, Iwanaga T, Ishikawa H. Generation of intestinal T cells from progenitors residing in gut cryptopatches. Science. 1998;280:275–8. doi: 10.1126/science.280.5361.275. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki K, Oida T, Hamada H, Hitotsumatsu O, Watanabe M, Hibi T, Yamamoto H, Kubota E, Kaminogawa S, Ishikawa H. Gut cryptopatches: direct evidence of extrathymic anatomical sites for intestinal T lymphopoiesis. Immunity. 2000;13:691–702. doi: 10.1016/s1074-7613(00)00068-6. [DOI] [PubMed] [Google Scholar]
- 53.Lefrancois L, Olson S. A novel pathway of thymus-directed T lymphocyte maturation. J Immunol. 1994;153:987–95. [PubMed] [Google Scholar]
- 54.Leishman AJ, Gapin L, Capone M, Palmer E, MacDonald HR, Kronenberg M, Cheroutre H. Precursors of functional MHC class I-or class II-restricted CD8alphaalpha(+) T cells are positively selected in the thymus by agonist self-peptides. Immunity. 2002;16:355–64. doi: 10.1016/s1074-7613(02)00284-4. [DOI] [PubMed] [Google Scholar]
- 55.Guy-Grand D, Azogui O, Celli S, Darche S, Nussenzweig MC, Kourilsky P, Vassalli P. Extrathymic T cell lymphopoiesis: ontogeny and contribution to gut intraepithelial lymphocytes in athymic and euthymic mice. J Exp Med. 2003;197:333–41. doi: 10.1084/jem.20021639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science. 2004;305:248–51. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- 57.Hendricks DW, Fink PJ. Uneven colonization of the lymphoid periphery by T cells that undergo early TCR{alpha} rearrangements. J Immunol. 2009;182:4267–74. doi: 10.4049/jimmunol.0804180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Camerini V, Panwala C, Kronenberg M. Regional specialization of the mucosal immune system. Intraepithelial lymphocytes of the large intestine have a different phenotype and function than those of the small intestine. J Immunol. 1993;151:1765–76. [PubMed] [Google Scholar]
- 59.Cruz D, Sydora BC, Hetzel K, Yakoub G, Kronenberg M, Cheroutre H. An opposite pattern of selection of a single T cell antigen receptor in the thymus and among intraepithelial lymphocytes. J Exp Med. 1998;188:255–65. doi: 10.1084/jem.188.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guehler SR, Bluestone JA, Barrett TA. Immune deviation of 2C transgenic intraepithelial lymphocytes in antigen-bearing hosts. J Exp Med. 1996;184:493–503. doi: 10.1084/jem.184.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levelt CN, de Jong YP, Mizoguchi E, O’Farrelly C, Bhan AK, Tonegawa S, Terhorst C, Simpson SJ. High-and low-affinity single-peptide/MHC ligands have distinct effects on the development of mucosal CD8alphaalpha and CD8alphabeta T lymphocytes. Proc Natl Acad Sci U S A. 1999;96:5628–33. doi: 10.1073/pnas.96.10.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guy-Grand D, Pardigon N, Darche S, Lantz O, Kourilsky P, Vassalli P. Contribution of double-negative thymic precursors to CD8alpha alpha (+) intraepithelial lymphocytes of the gut in mice bearing TCR transgenes. Eur J Immunol. 2001;31:2593–602. doi: 10.1002/1521-4141(200109)31:9<2593::aid-immu2593>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 63.Rocha B, von Boehmer H, Guy-Grand D. Selection of intraepithelial lymphocytes with CD8 alpha/alpha co-receptors by self-antigen in the murine gut. Proc Natl Acad Sci U S A. 1992;89:5336–40. doi: 10.1073/pnas.89.12.5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamagata T, Mathis D, Benoist C. Self-reactivity in thymic double-positive cells commits cells to a CD8 alpha alpha lineage with characteristics of innate immune cells. Nat Immunol. 2004;5:597–605. doi: 10.1038/ni1070. [DOI] [PubMed] [Google Scholar]
- 65.Barnden MJ, Heath WR, Carbone FR. Down-modulation of CD8 beta-chain in response to an altered peptide ligand enables developing thymocytes to escape negative selection. Cell Immunol. 1997;175:111–9. doi: 10.1006/cimm.1996.1054. [DOI] [PubMed] [Google Scholar]
- 66.Chidgey A, Boyd R. Agonist peptide modulates T cell selection thresholds through qualitative and quantitative shifts in CD8 co-receptor expression. Int Immunol. 1997;9:1527–36. doi: 10.1093/intimm/9.10.1527. [DOI] [PubMed] [Google Scholar]
- 67.Mintern JD, Maurice MM, Ploegh HL, Schott E. Thymic selection and peripheral activation of CD8 T cells by the same class I MHC/peptide complex. J Immunol. 2004;172:699–708. doi: 10.4049/jimmunol.172.1.699. [DOI] [PubMed] [Google Scholar]
- 68.Lambolez F, Arcangeli ML, Joret AM, Pasqualetto V, Cordier C, Di Santo JP, Rocha B, Ezine S. The thymus exports long-lived fully committed T cell precursors that can colonize primary lymphoid organs. Nat Immunol. 2006;7:76–82. doi: 10.1038/ni1293. [DOI] [PubMed] [Google Scholar]
- 69.Peaudecerf L, dos Santos PR, Boudil A, Ezine S, Pardigon N, Rocha B. The role of the gut as a primary lymphoid organ: CD8alphaalpha intraepithelial T lymphocytes in euthymic mice derive from very immature CD44+ thymocyte precursors. Mucosal Immunol. 2011;4:93–101. doi: 10.1038/mi.2010.47. [DOI] [PubMed] [Google Scholar]
- 70.Malinarich FH, Grabski E, Worbs T, Chennupati V, Haas JD, Schmitz S, Candia E, Quera R, Malissen B, Forster R, Hermoso M, Prinz I. Constant TCR triggering suggests that the TCR expressed on intestinal intraepithelial gammadelta T cells is functional in vivo. Eur J Immunol. 2010;40:3378–88. doi: 10.1002/eji.201040727. [DOI] [PubMed] [Google Scholar]
- 71.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–89. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 72.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 73.Marks BR, Nowyhed HN, Choi JY, Poholek AC, Odegard JM, Flavell RA, Craft J. Thymic self-reactivity selects natural interleukin 17-producing T cells that can regulate peripheral inflammation. Nat Immunol. 2009;10:1125–32. doi: 10.1038/ni.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hayes SM, Laird RM, Love PE. Beyond alphabeta/gammadelta lineage commitment: TCR signal strength regulates gammadelta T cell maturation and effector fate. Semin Immunol. 2010;22:247–51. doi: 10.1016/j.smim.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qi Q, Kannan AK, August A. Tec family kinases: Itk signaling and the development of NKT alphabeta and gammadelta T cells. FEBS J. 2011 doi: 10.1111/j.1742-4658.2011.08074.x. [DOI] [PubMed] [Google Scholar]
- 76.Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184–96. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 77.Prince AL, Yin CC, Enos ME, Felices M, Berg LJ. The Tec kinases Itk and Rlk regulate conventional versus innate T-cell development. Immunol Rev. 2009;228:115–31. doi: 10.1111/j.1600-065X.2008.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baldwin TA, Hogquist KA, Jameson SC. The fourth way? Harnessing aggressive tendencies in the thymus. J Immunol. 2004;173:6515–20. doi: 10.4049/jimmunol.173.11.6515. [DOI] [PubMed] [Google Scholar]
- 79.Atherly LO, Lucas JA, Felices M, Yin CC, Reiner SL, Berg LJ. The Tec family tyrosine kinases Itk and Rlk regulate the development of conventional CD8+ T cells. Immunity. 2006;25:79–91. doi: 10.1016/j.immuni.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 80.Broussard C, Fleischacker C, Horai R, Chetana M, Venegas AM, Sharp LL, Hedrick SM, Fowlkes BJ, Schwartzberg PL. Altered development of CD8+ T cell lineages in mice deficient for the Tec kinases Itk and Rlk. Immunity. 2006;25:93–104. doi: 10.1016/j.immuni.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 81.Horai R, Mueller KL, Handon RA, Cannons JL, Anderson SM, Kirby MR, Schwartzberg PL. Requirements for selection of conventional and innate T lymphocyte lineages. Immunity. 2007;27:775–85. doi: 10.1016/j.immuni.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schaeffer EM, Broussard C, Debnath J, Anderson S, McVicar DW, Schwartzberg PL. Tec family kinases modulate thresholds for thymocyte development and selection. J Exp Med. 2000;192:987–1000. doi: 10.1084/jem.192.7.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee YJ, Jameson SC, Hogquist KA. Alternative memory in the CD8 T cell lineage. Trends Immunol. 2011;32:50–6. doi: 10.1016/j.it.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weinreich MA, Takada K, Skon C, Reiner SL, Jameson SC, Hogquist KA. KLF2 transcription-factor deficiency in T cells results in unrestrained cytokine production and upregulation of bystander chemokine receptors. Immunity. 2009;31:122–30. doi: 10.1016/j.immuni.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weinreich MA, Odumade OA, Jameson SC, Hogquist KA. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat Immunol. 2010;11:709–16. doi: 10.1038/ni.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Verykokakis M, Boos MD, Bendelac A, Kee BL. SAP protein-dependent natural killer T-like cells regulate the development of CD8(+) T cells with innate lymphocyte characteristics. Immunity. 2010;33:203–15. doi: 10.1016/j.immuni.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gordon SM, Carty SA, Kim JS, Zou T, Smith-Garvin J, Alonzo ES, Haimm E, Sant’Angelo DB, Koretzky GA, Reiner SL, Jordan MS. Requirements for eomesodermin and promyelocytic leukemia zinc finger in the development of innate-like CD8+ T cells. J Immunol. 2011;186:4573–8. doi: 10.4049/jimmunol.1100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–4. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 89.Goodman T, Lefrancois L. Intraepithelial lymphocytes. Anatomical site, not T cell receptor form, dictates phenotype and function. J Exp Med. 1989;170:1569–81. doi: 10.1084/jem.170.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med. 1998;188:2301–11. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smith K, Seddon B, Purbhoo MA, Zamoyska R, Fisher AG, Merkenschlager M. Sensory adaptation in naive peripheral CD4 T cells. J Exp Med. 2001;194:1253–61. doi: 10.1084/jem.194.9.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Takada K, Jameson SC. Self-class I MHC molecules support survival of naive CD8 T cells, but depress their functional sensitivity through regulation of CD8 expression levels. J Exp Med. 2009;206:2253–69. doi: 10.1084/jem.20082553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cho JH, Kim HO, Surh CD, Sprent J. T cell receptor-dependent regulation of lipid rafts controls naive CD8+ T cell homeostasis. Immunity. 2010;32:214–26. doi: 10.1016/j.immuni.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ordonez-Rueda D, Lozano F, Sarukhan A, Raman C, Garcia-Zepeda EA, Soldevila G. Increased numbers of thymic and peripheral CD4+ CD25+Foxp3+ cells in the absence of CD5 signaling. Eur J Immunol. 2009;39:2233–47. doi: 10.1002/eji.200839053. [DOI] [PubMed] [Google Scholar]
- 95.Masuda K, Makino Y, Cui J, Ito T, Tokuhisa T, Takahama Y, Koseki H, Tsuchida K, Koike T, Moriya H, Amano M, Taniguchi M. Phenotypes and invariant alpha beta TCR expression of peripheral V alpha 14+ NK T cells. J Immunol. 1997;158:2076–82. [PubMed] [Google Scholar]
- 96.Muller S, Jungo M, Aichele P, Mueller C. CD5-CD8alpha beta intestinal intraepithelial lymphocytes (IEL) are induced to express CD5 upon antigen-specific activation: CD5-and CD5+ CD8 alpha beta IEL do not represent separate T cell lineages. Eur J Immunol. 1997;27:1756–61. doi: 10.1002/eji.1830270724. [DOI] [PubMed] [Google Scholar]
- 97.Tarakhovsky A, Kanner SB, Hombach J, Ledbetter JA, Muller W, Killeen N, Rajewsky K. A role for CD5 in TCR-mediated signal transduction and thymocyte selection. Science. 1995;269:535–7. doi: 10.1126/science.7542801. [DOI] [PubMed] [Google Scholar]
- 98.Azzam HS, DeJarnette JB, Huang K, Emmons R, Park CS, Sommers CL, El-Khoury D, Shores EW, Love PE. Fine tuning of TCR signaling by CD5. J Immunol. 2001;166:5464–72. doi: 10.4049/jimmunol.166.9.5464. [DOI] [PubMed] [Google Scholar]
- 99.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–40. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 100.Tang Q, Henriksen KJ, Boden EK, Tooley AJ, Ye J, Subudhi SK, Zheng XX, Strom TB, Bluestone JA. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:3348–52. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 101.Lohr J, Knoechel B, Kahn EC, Abbas AK. Role of B7 in T cell tolerance. J Immunol. 2004;173:5028–35. doi: 10.4049/jimmunol.173.8.5028. [DOI] [PubMed] [Google Scholar]
- 102.Hori S. c-Rel: a pioneer in directing regulatory T-cell lineage commitment? Eur J Immunol. 2010;40:664–7. doi: 10.1002/eji.201040372. [DOI] [PubMed] [Google Scholar]
- 103.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–78. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 104.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–51. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 105.D’Cruz LM, Klein L. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat Immunol. 2005;6:1152–9. doi: 10.1038/ni1264. [DOI] [PubMed] [Google Scholar]
- 106.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–90. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 107.Burchill MA, Yang J, Vang KB, Farrar MA. Interleukin-2 receptor signaling in regulatory T cell development and homeostasis. Immunol Lett. 2007;114:1–8. doi: 10.1016/j.imlet.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–11. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Burchill MA, Yang J, Vang KB, Moon JJ, Chu HH, Lio CW, Vegoe AL, Hsieh CS, Jenkins MK, Farrar MA. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 2008;28:112–21. doi: 10.1016/j.immuni.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–41. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 111.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 112.Griewank K, Borowski C, Rietdijk S, Wang N, Julien A, Wei DG, Mamchak AA, Terhorst C, Bendelac A. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007;27:751–62. doi: 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pasquier B, Yin L, Fondaneche MC, Relouzat F, Bloch-Queyrat C, Lambert N, Fischer A, de Saint-Basile G, Latour S. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J Exp Med. 2005;201:695–701. doi: 10.1084/jem.20042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nichols KE, Hom J, Gong SY, Ganguly A, Ma CS, Cannons JL, Tangye SG, Schwartzberg PL, Koretzky GA, Stein PL. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat Med. 2005;11:340–5. doi: 10.1038/nm1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chung B, Aoukaty A, Dutz J, Terhorst C, Tan R. Signaling lymphocytic activation molecule-associated protein controls NKT cell functions. J Immunol. 2005;174:3153–7. doi: 10.4049/jimmunol.174.6.3153. [DOI] [PubMed] [Google Scholar]
- 116.Li W, Kim MG, Gourley TS, McCarthy BP, Sant’Angelo DB, Chang CH. An alternate pathway for CD4 Tcell development: thymocyte-expressed MHC class II selects a distinct T cell population. Immunity. 2005;23:375–86. doi: 10.1016/j.immuni.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 117.Li W, Sofi MH, Rietdijk S, Wang N, Terhorst C, Chang CH. The SLAM-associated protein signaling pathway is required for development of CD4+ T cells selected by homotypic thymocyte interaction. Immunity. 2007;27:763–74. doi: 10.1016/j.immuni.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eberl G, Lowin-Kropf B, MacDonald HR. Cutting edge: NKT cell development is selectively impaired in Fyn-deficient mice. J Immunol. 1999;163:4091–4. [PubMed] [Google Scholar]
- 119.Gadue P, Morton N, Stein PL. The Src family tyrosine kinase Fyn regulates natural killer T cell development. J Exp Med. 1999;190:1189–96. doi: 10.1084/jem.190.8.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stanic AK, Bezbradica JS, Park JJ, Van Kaer L, Boothby MR, Joyce S. Cutting edge: the ontogeny and functionof Va14Ja18 natural T lymphocytes require signal processing by protein kinase C theta and NF-kappa B. J Immunol. 2004;172:4667–71. doi: 10.4049/jimmunol.172.8.4667. [DOI] [PubMed] [Google Scholar]
- 121.Elewaut D, Shaikh RB, Hammond KJ, De Winter H, Leishman AJ, Sidobre S, Turovskaya O, Prigozy TI, Ma L, Banks TA, Lo D, Ware CF, Cheroutre H, Kronenberg M. NIK-dependent RelB activation defines a unique signaling pathway for the development of V alpha 14i NKT cells. J Exp Med. 2003;197:1623–33. doi: 10.1084/jem.20030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schmidt-Supprian M, Tian J, Grant EP, Pasparakis M, Maehr R, Ovaa H, Ploegh HL, Coyle AJ, Rajewsky K. Differential dependence of CD4+CD25+ regulatory and natural killer-like T cells on signals leading to NF-kappaB activation. Proc Natl Acad Sci U S A. 2004;101:4566–71. doi: 10.1073/pnas.0400885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sivakumar V, Hammond KJ, Howells N, Pfeffer K, Weih F. Differential requirement for Rel/nuclear factor kappa B family members in natural killer T cell development. J Exp Med. 2003;197:1613–21. doi: 10.1084/jem.20022234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lazarevic V, Zullo AJ, Schweitzer MN, Staton TL, Gallo EM, Crabtree GR, Glimcher LH. The gene encoding early growth response 2, a target of the transcription factor NFAT, is required for the development and maturation of natural killer T cells. Nat Immunol. 2009;10:306–13. doi: 10.1038/ni.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, Lantz O, Bendelac A. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, Chua K, Eidson M, Kim HJ, Im JS, Pandolfi PP, Sant’Angelo DB. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol. 2008;9:1055–64. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kovalovsky D, Alonzo ES, Uche OU, Eidson M, Nichols KE, Sant’Angelo DB. PLZF induces the spontaneous acquisition of memory/effector functions in T cells independently of NKT cell-related signals. J Immunol. 2010;184:6746–55. doi: 10.4049/jimmunol.1000776. [DOI] [PubMed] [Google Scholar]
- 128.Ivanov, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 129.Ouyang W, Beckett O, Ma Q, Li MO. Transforming growth factor-beta signaling curbs thymic negative selection promoting regulatory T cell development. Immunity. 2010;32:642–53. doi: 10.1016/j.immuni.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Doisne JM, Bartholin L, Yan KP, Garcia CN, Duarte N, Le Luduec JB, Vincent D, Cyprian F, Horvat B, Martel S, Rimokh R, Losson R, Benlagha K, Marie JC. iNKT cell development is orchestrated by different branches of TGF-beta signaling. J Exp Med. 2009;206:1365–78. doi: 10.1084/jem.20090127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Konkel JE, Maruyama T, Carpenter AC, Xiong Y, Zamarron BF, Hall BE, Kulkarni AB, Zhang P, Bosselut R, Chen W. Control of the development of CD8alphaalpha+ intestinal intraepithelial lymphocytes by TGF-beta. Nat Immunol. 2011;12:312–9. doi: 10.1038/ni.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ohkura N, Sakaguchi S. Regulatory T cells: roles of T cell receptor for their development and function. Semin Immunopathol. 2010;32:95–106. doi: 10.1007/s00281-010-0200-5. [DOI] [PubMed] [Google Scholar]
- 133.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–58. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 134.Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol. 2007;5:405–17. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- 135.Jahng AW, Maricic I, Pedersen B, Burdin N, Naidenko O, Kronenberg M, Koezuka Y, Kumar V. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1789–99. doi: 10.1084/jem.194.12.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Umetsu DT, Dekruyff RH. Natural killer T cells are important in the pathogenesis of asthma: the many pathways to asthma. J Allergy Clin Immunol. 2010;125:975–9. doi: 10.1016/j.jaci.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–7. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 138.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, 3rd, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewind O, Walker D, Beutler B, Teyton L, Savage PB, Bendelac A. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–9. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 139.Duthie MS, Kahn M, White M, Kapur RP, Kahn SJ. Both CD1d antigen presentation and interleukin-12 are required to activate natural killer T cells during Trypanosoma cruzi infection. Infect Immun. 2005;73:1890–4. doi: 10.1128/IAI.73.3.1890-1894.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Brigl M, Tatituri RV, Watts GF, Bhowruth V, Leadbetter EA, Barton N, Cohen NR, Hsu FF, Besra GS, Brenner MB. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J Exp Med. 2011;208:1163–77. doi: 10.1084/jem.20102555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc Natl Acad Sci U S A. 2002;99:14338–43. doi: 10.1073/pnas.212290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Inagaki-Ohara K, Chinen T, Matsuzaki G, Sasaki A, Sakamoto Y, Hiromatsu K, Nakamura-Uchiyama F, Nawa Y, Yoshimura A. Mucosal T cells bearing TCRgammadelta play a protective role in intestinal inflammation. J Immunol. 2004;173:1390–8. doi: 10.4049/jimmunol.173.2.1390. [DOI] [PubMed] [Google Scholar]
- 143.Poussier P, Ning T, Banerjee D, Julius M. A unique subset of self-specific intraintestinal T cells maintains gut integrity. J Exp Med. 2002;195:1491–7. doi: 10.1084/jem.20011793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Denning TL, Granger SW, Mucida D, Graddy R, Leclercq G, Zhang W, Honey K, Rasmussen JP, Cheroutre H, Rudensky AY, Kronenberg M. Mouse TCRalphabeta+CD8alphaalpha intraepithelial lymphocytes express genes that down-regulate their antigen reactivity and suppress immune responses. J Immunol. 2007;178:4230–9. doi: 10.4049/jimmunol.178.7.4230. [DOI] [PubMed] [Google Scholar]
- 145.Shires J, Theodoridis E, Hayday AC. Biological insights into TCRgammadelta+ and TCRalphabeta+ intraepithelial lymphocytes provided by serial analysis of gene expression (SAGE) Immunity. 2001;15:419–34. doi: 10.1016/s1074-7613(01)00192-3. [DOI] [PubMed] [Google Scholar]
- 146.Montufar-Solis D, Garza T, Klein JR. T-cell activation in the intestinal mucosa. Immunol Rev. 2007;215:189–201. doi: 10.1111/j.1600-065X.2006.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647–59. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]