Identification of a protein altered in mutants resistant to microtubule inhibitors as a member of the major heat shock protein (hsp70) family (original) (raw)
Abstract
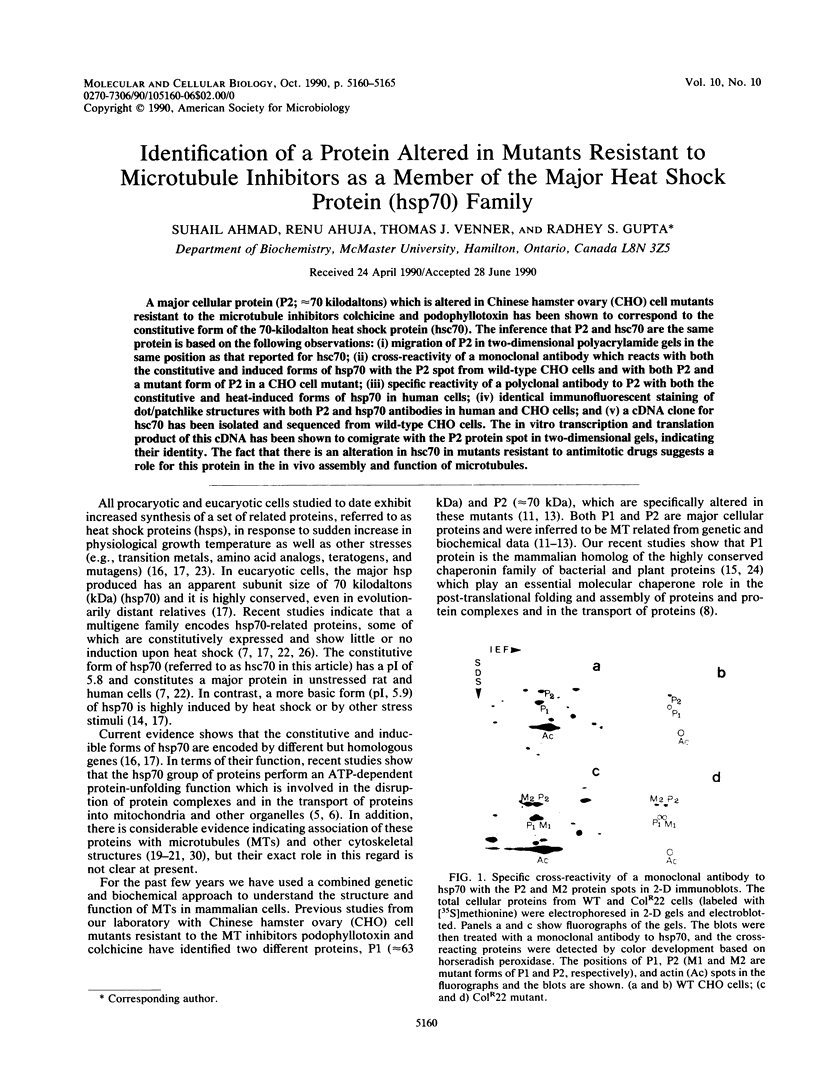
A major cellular protein (P2; approximately 70 kilodaltons) which is altered in Chinese hamster ovary (CHO) cell mutants resistant to the microtubule inhibitors colchicine and podophyllotoxin has been shown to correspond to the constitutive form of the 70-kilodalton heat shock protein (hsc70). The inference that P2 and hsc70 are the same protein is based on the following observations: (i) migration of P2 in two-dimensional polyacrylamide gels in the same position as that reported for hsc70; (ii) cross-reactivity of a monoclonal antibody which reacts with both the constitutive and induced forms of hsp70 with the P2 spot from wild-type CHO cells and with both P2 and a mutant form of P2 in a CHO cell mutant; (iii) specific reactivity of a polyclonal antibody to P2 with both the constitutive and heat-induced forms of hsp70 in human cells; (iv) identical immunofluorescent staining of dot/patchlike structures with both P2 and hsp70 antibodies in human and CHO cells; and (v) a cDNA clone for hsc70 has been isolated and sequenced from wild-type CHO cells. The in vitro transcription and translation product of this cDNA has been shown to comigrate with the P2 protein spot in two-dimensional gels, indicating their identity. The fact that there is an alteration in hsc70 in mutants resistant to antimitotic drugs suggests a role for this protein in the in vivo assembly and function of microtubules.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. L., Van Kersen I., Kraft P. E., Hahn G. M. Biochemical analysis of heat-resistant mouse tumor cell strains: a new member of the HSP70 family. Mol Cell Biol. 1989 Aug;9(8):3509–3516. doi: 10.1128/mcb.9.8.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aujame L. The major heat-shock protein hsp 68 is not induced by stress in mouse erythroleukemia cell lines. Biochem Cell Biol. 1988 Jul;66(7):691–701. doi: 10.1139/o88-079. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Lauridsen J. B., Basse B. Cell cycle-associated change in the expression of the proliferation-sensitive and heat-shock protein hs x 70 (IEF14): increased synthesis during mitosis. Exp Cell Res. 1988 Jul;177(1):176–185. doi: 10.1016/0014-4827(88)90035-3. [DOI] [PubMed] [Google Scholar]
- Chappell T. G., Welch W. J., Schlossman D. M., Palter K. B., Schlesinger M. J., Rothman J. E. Uncoating ATPase is a member of the 70 kilodalton family of stress proteins. Cell. 1986 Apr 11;45(1):3–13. doi: 10.1016/0092-8674(86)90532-5. [DOI] [PubMed] [Google Scholar]
- Chirico W. J., Waters M. G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 Apr 28;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Dworniczak B., Mirault M. E. Structure and expression of a human gene coding for a 71 kd heat shock 'cognate' protein. Nucleic Acids Res. 1987 Jul 10;15(13):5181–5197. doi: 10.1093/nar/15.13.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. J. The molecular chaperone concept. Semin Cell Biol. 1990 Feb;1(1):1–9. [PubMed] [Google Scholar]
- Fornace A. J., Jr, Alamo I., Jr, Hollander M. C., Lamoreaux E. Induction of heat shock protein transcripts and B2 transcripts by various stresses in Chinese hamster cells. Exp Cell Res. 1989 May;182(1):61–74. doi: 10.1016/0014-4827(89)90279-6. [DOI] [PubMed] [Google Scholar]
- Green L. A., Liem R. K. Beta-internexin is a microtubule-associated protein identical to the 70-kDa heat-shock cognate protein and the clathrin uncoating ATPase. J Biol Chem. 1989 Sep 15;264(26):15210–15215. [PubMed] [Google Scholar]
- Gupta R. S., Gupta R. Mutants of chinese hamster ovary cells affected in two different microtubule-associated proteins. Genetic and biochemical studies. J Biol Chem. 1984 Feb 10;259(3):1882–1890. [PubMed] [Google Scholar]
- Gupta R. S., Ho T. K., Moffat M. R., Gupta R. Podophyllotoxin-resistant mutants of Chinese hamster ovary cells. Alteration in a microtubule-associated protein. J Biol Chem. 1982 Jan 25;257(2):1071–1078. [PubMed] [Google Scholar]
- Gupta R. S., Venner T. J., Chopra A. Genetic and biochemical studies with mutants of mammalian cells affected in microtubule-related proteins other than tubulin: mitochondrial localization of a microtubule-related protein. Can J Biochem Cell Biol. 1985 Jun;63(6):489–502. doi: 10.1139/o85-068. [DOI] [PubMed] [Google Scholar]
- Hunt C., Morimoto R. I. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal S., Dudani A. K., Singh B., Harley C. B., Gupta R. S. Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol Cell Biol. 1989 May;9(5):2279–2283. doi: 10.1128/mcb.9.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Napolitano E. W., Pachter J. S., Chin S. S., Liem R. K. beta-Internexin, a ubiquitous intermediate filament-associated protein. J Cell Biol. 1985 Oct;101(4):1323–1331. doi: 10.1083/jcb.101.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano E. W., Pachter J. S., Liem R. K. Intracellular distribution of mammalian stress proteins. Effects of cytoskeletal-specific agents. J Biol Chem. 1987 Feb 5;262(4):1493–1504. [PubMed] [Google Scholar]
- O'Malley K., Mauron A., Barchas J. D., Kedes L. Constitutively expressed rat mRNA encoding a 70-kilodalton heat-shock-like protein. Mol Cell Biol. 1985 Dec;5(12):3476–3483. doi: 10.1128/mcb.5.12.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka K., Tanabe K., Nakamura H., Sato C. Possible cytoskeletal association of 69,000- and 68,000-dalton heat shock proteins and structural relations among heat shock proteins in murine mastocytoma cells. Radiat Res. 1986 Oct;108(1):34–42. [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Picketts D. J., Mayanil C. S., Gupta R. S. Molecular cloning of a Chinese hamster mitochondrial protein related to the "chaperonin" family of bacterial and plant proteins. J Biol Chem. 1989 Jul 15;264(20):12001–12008. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger P. K., Pelham H. R. Cloning and expression of a gene encoding hsc73, the major hsp70-like protein in unstressed rat cells. EMBO J. 1987 Apr;6(4):993–998. doi: 10.1002/j.1460-2075.1987.tb04850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Nuclear and nucleolar localization of the 72,000-dalton heat shock protein in heat-shocked mammalian cells. J Biol Chem. 1984 Apr 10;259(7):4501–4513. [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Purification of the major mammalian heat shock proteins. J Biol Chem. 1982 Dec 25;257(24):14949–14959. [PubMed] [Google Scholar]
- Welch W. J., Mizzen L. A. Characterization of the thermotolerant cell. II. Effects on the intracellular distribution of heat-shock protein 70, intermediate filaments, and small nuclear ribonucleoprotein complexes. J Cell Biol. 1988 Apr;106(4):1117–1130. doi: 10.1083/jcb.106.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatley S. A., Leung T., Hall C., Lim L. The brain 68-kilodalton microtubule-associated protein is a cognate form of the 70-kilodalton mammalian heat-shock protein and is present as a specific isoform in synaptosomal membranes. J Neurochem. 1986 Nov;47(5):1576–1583. doi: 10.1111/j.1471-4159.1986.tb00797.x. [DOI] [PubMed] [Google Scholar]