Tanslational Systems Biology: Introduction of an Engineering Approach to the Pathophysiology of the Burn Patient (original) (raw)
. Author manuscript; available in PMC: 2013 May 1.
Published in final edited form as: J Burn Care Res. 2008 Mar-Apr;29(2):277–285. doi: 10.1097/BCR.0b013e31816677c8
Abstract
The pathophysiology of the burn patient manifests the full spectrum of the complexity of the inflammatory response. In the acute phase, inflammation may have negative effects via capillary leak, the propagation of inhalation injury, and development of multiple organ failure. Attempts to mediate these processes remain a central subject of burn care research. Conversely, inflammation is a necessary prologue and component in the later stage processes of wound healing. Despite the volume of information concerning the cellular and molecular processes involved in inflammation, there exists a significant gap between the knowledge of mechanistic pathophysiology and the development of effective clinical therapeutic regimens. Translational systems biology (TSB) is the application of dynamic mathematical modeling and certain engineering principles to biological systems to integrate mechanism with phenomenon and, importantly, to revise clinical practice. This study will review the existing applications of TSB in the areas of inflammation and wound healing, relate them to specific areas of interest to the burn community, and present an integrated framework that links TSB with traditional burn research.
BURNS, BIOCOMPLEXITY, AND THE NEED FOR A TRANSLATIONAL METHODOLOGY
The burn community held its State of the Science meeting (Sponsored by the American Burn Association, the National Institutes of Health/National Institute of General Medical Sciences, the Shriners Hospitals for Children, the National Institute on Disability and Rehabilitation Research, the Department of Veterans Affairs and the U.S. Army Medical Materiel Command) from October 26–29, 2006, in order to determine the areas and foci of the community’s research emphasis for the decade to come. These areas were defined primarily by their clinical manifestations or situations, such as resuscitation issues, inhalation injury, multiple organ failure, and wound healing. However, discussions invariably included references to the investigation of means by which known or suspected cellular and molecular pathophysiologic mechanisms could be translated into effective clinical treatments. These discussions included both the process of discovery for new treatments and enhanced means of testing the efficacy of existing treatment strategies, particularly given the practical difficulties of executing definitive clinical trials. Both of these endeavors can be aided by the development of an “engineering approach” to the burn patient to help translate the knowledge of pathophysiologic mechanism to the level of clinical therapeutics. We will introduce an approach we have termed Translational Systems Biology (TSB) as a potential means to address the challenges of bio-complexity in the biomedical arena.
Biocomplexity describes the structure, organization and behavior of biological systems that exhibit the properties of complex systems. These properties include multiple interacting components, nonlinear dynamics due to multiple feedback loops, multiple levels of organization, robustness to perturbation and nonintuitive, paradoxical behavior.1–3 Other examples of complex systems include ecologies,4 social/political systems,5 economies,6 and cognition.7 The traditional paradigm of reductionist scientific analysis may not be sufficient for describing the behavior of complex biological systems.8–14 In the biomedical arena, this problem is manifest in the gap between the extent of mechanistic information regarding underlying cellular and molecular processes vs. the ability to translate that information to the level of system-wide behavior, particularly with respect to the development of effective therapeutics. The consequences of this discrepancy are most notable in diseases involving intrinsic systemic regulatory mechanisms, such as disorders of acute inflammation that include sepsis and burn injury. The Food and Drug Administration, in its “Critical Path” document15 and the National Institutes of Health, in its “Roadmap” statement16 have explicitly addressed this gap between mechanistic understanding at the basic science level and effective clinical regimens with respect to the future direction of biomedical research. The centrality and complexity of inflammation in both physiological and pathological stimuli from both without and within have been recently recognized in a new initiative of the NIH Roadmap (New Roadmap Emphasis Areas for 2008).17 These initiatives suggest that improved multidisciplinary approaches, with a focus on computational and mathematical simulation (in silico methods), are necessary to facilitate the translational aspect of biomedical research. In essence, both of these organizations are calling for the application of engineering methods of simulation and testing in the description of, and the development of manipulations for, complex biomedical processes. Lessons can be learned from the classical systems biology community,1 which has integrated computational, mathematical, and simulation technologies in a systems approach to cellular behavior. A similar approach needs to be applied to the translation from the molecule and cell to the organism and on to the clinical arena, and we suggest that TSB is an approach by which to meet this need. TSB involves using dynamic mathematical modeling based on mechanistic information generated in basic science research to simulate higher-level behaviors at the organ and organism level, thus creating a bridge between basic science, reductionist experimental data, and clinical phenomena. TSB aims to provide a mechanistic basis for pathophysiology based on integration of knowledge from the molecular and cellular level with the specific goal of facilitating clinical application. TSB is built on the premise that models be generated with the intent of rapid translational application in areas such as in silico clinical trials, patient diagnostics, and rational drug design and testing. The emphasis on reproducing clinically evident behavior represents a subtle but complimentary difference between TSB and the classic systems biology community. With its emphasis on concatenating the basic molecular mechanisms underlying gene regulation and control of cellular behavior, classical systems biology focuses on discovering “why” cells behave as they do.1,18–22 TSB, on the other hand, seeks to examine and explain “how” those mechanisms relate to behavior at the clinical level (Figure 1). It should be noted that these boundaries are generalizations, and that in practice there is crossover from both directions. However, it is important to emphasize that these two endeavors are complimentary in that classical systems biology often provides the underlying knowledge framework upon which the applied, or engineering, goal of TSB can be pursued. TSB groups draw on the organizational model of engineering teams by combining modeling expertise with domain expertise on both the underlying system mechanisms and the real-world applications, and by creating an iterative integrated environment for their interactions.23 In the interest of developing an engineering approach to the burn patient, we review TSB applications that have been directed at the acute inflammatory response (AIR), specifically related to sepsis, trauma, hemorrhagic shock, and wound healing.24–32 Many of the primary groups that have applied TSB to acute inflammation, shock, and critical care have coalesced into an organization named the Society of Complexity in Acute Illness (SCAI, Website at http://www.scai-med.org), which has provided a framework for the integration of TSB into the general research community.
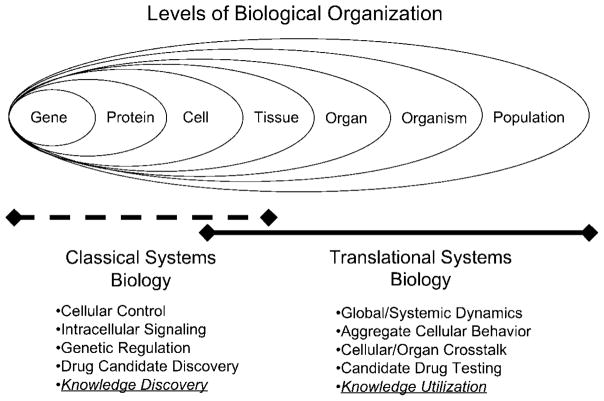
Figure 1.
Relationship between Translational Systems Biology and Classical Systems Biology: Biological Organization has a nested, hierarchical structure. Each hierarchy, however, is bounded to some epistemological degree due to the principles of biocomplexity. Classical systems biology generally focuses on the characterization and description of mechanisms of cellular control with an emphasis on genetic regulation and intracellular signaling. Translational systems biology attempts to use the knowledge generated at the subcellular level and extrapolates that knowledge to the type of systemic behavior seen in the clinical setting. These two approaches are complementary and generally intersect at the level of cellular and tissue behavior.
As we begin to describe this potential integration, we note that mathematical models have been used for over 20 years in the study of burns,33–45 and a large portion of these modeling studies might be termed directly translational.34,37,39,41–45 However, there is a paucity of mathematical modeling studies integrating the AIR and healing responses in burns.38,40,41,45 In addition, the methods and platforms used for modeling complex systems have evolved over that period of time. In the sections below, we will first describe some of the methods currently used in TSB. Next, we will present the application of TSB to sepsis as an example of how TSB can be implemented with respect to a particular problem. Finally, we will describe briefly how some of the research foci in the burn community may be addressed with TSB. We wish to emphasize that we are not proposing that the application of TSB in burn research will be a panacea. As with any emerging technology, a period of examination and assessment is required in order to determine how and where the new technology may be applied. This review is an initial step in that process.
DYNAMIC MATHEMATICAL MODELING: DEFINITION AND METHODS
The primary analytical/synthetic tool used in TSB is dynamic mathematical modeling. These models all involve a temporal component that allows them to evolve over time and simulate the behavior of a system based on mechanisms derived from empirical data from the basic science lab. Dynamic mathematical models can be distinguished from statistical mathematical models (such as regression fitting and cluster analysis) and network models, which are static representations of information and relationships. By incorporating both mechanism and behavior, dynamic mathematical models allow testing of manipulations and modulations of a system, a necessary component of the iterative process that underlies TSB.
The primary methods of dynamic mathematical modeling that have been thus far used in the TSB community are equation-based modeling (EBM)27–32,46–48 and agent-based modeling (ABM)24,25,49 The two forms of dynamic mathematical models have their relative strengths and weaknesses, but they are not mutually exclusive,50 and the utilization of both methods in the TSB community demonstrates a pragmatic, goal-directed approach that is not tied to a particular modeling platform.26 For a more detailed description of these methods see “Appendix.”
Regardless of their methodology, dynamic mathematical models fall into two general utilitarian categories: those intended for knowledge representation and augmenting understanding, and those intended to predict and pretest interventions. The ultimate goal is the latter situation, but in order for engineering-grade models/simulations to arise the earlier phase of development must be allowed to mature. It is important to emphasize that regardless the type of modeling used, such models are only as good as their constituent data and assumptions. Extreme care needs to be taken in the interpretation and evaluation of mathematical models,51 and it is particularly important to avoid petitio principii, the deductive logical fallacy of inferring a conclusion that has already been stated as an assumption, ie, “programming the proof.” There have been steps in developing guidelines toward an “evidence-based” method for their construction, emphasizing TSB as an iterative process, in which basic research informs the basis of models, which in turn provides questions and answers for additional bench research that is once again incorporated into the next generation of models.52 It bears noting, however, that the ability to represent, instantiate, and test existing hypotheses is extremely useful, and many initial applications in TSB focus on the ability to represent what is already known using dynamical simulations. With this concept in mind, the TSB community uses both ABM and EBM to study various aspects of the AIR, primarily thus far to demonstrate their capability as knowledge representation tools, but always with an eye towards the eventual goal of predictive, engineering-grade models. The range of TSB models related to inflammation spans from intracellular signaling to individual tissue/organ all the way to simulated clinical trials (specific examples and references below). This great diversity of computational simulations offers the promise to bridge the gap from bench to bedside, as we discuss in detail below.
APPLICATION OF TSB TO SEPSIS: A TEST CASE
TSB was, to a great degree, developed in response to the problem of sepsis, a prototypical “complex” disease state.8–14 Consequently, the bulk of existent TSB work has been focused on this subject. Below, we will track the course of development of TSB as applied to sepsis as an example of how these principles can be integrated into and augment existing trends in research.
There are three salient characteristics of the study of sepsis: 1) the distinctive clinical manifestations of the disease state, by which it was originally described; 2) the extent to which the intrinsic cellular and molecular processes are characterized and known to contribute to the disease state; and 3) the difficulty in linking 1 and 2 in terms of diagnosis, prognosis and therapy. While significant strides have been made in the systems-based profiling of patients with global inflammatory states (such as the work done within the context of the Inflammation and Host Response to Injury “Glue Grant”; www.gluegrant.org),53–60 there is still a need for a mechanistic means of translating basic science knowledge to global behavior. With this exigency in mind, the initial TSB models focused on conceptually characterizing the global dynamics of sepsis using models based on the “best” mechanistic knowledge available. The development proceeded in progressive fashion, initially focusing on the proinflammatory response to bacterial infection,46 then moving to the addition of anti-inflammatory mechanisms to account for the dynamic control needed to maintain homeostasis.47 Next, the phenomenon of endotoxin priming and tolerance was examined.48 In order to develop calibration and validation methodologies, a series of EBMs were matched to in vivo models of endotoxin administration in mice,29 where lethal doses of endotoxin were prospectively predicted using the model,28 and models of septic shock and adult respiratory distress syndrome in swine,61,62 where the model was used to predict circulating cytokine levels at various time points and severity of illness. Moreover, these models were coupled to novel data-fitting algorithms and circulating inflammation markers obtained from gene knockout mice in order to discern features underlying the altered inflammation in these animals.30 These models provided a “proof of concept” that the dynamics of sepsis could be captured mathematically and related to real-world situations, albeit in in vivo experimental preparations, but also recognizing that those in vivo models formed a vital core of existing research endeavors on sepsis.
The next step was the extension of these methods to humans, first in terms of characterization of clinically recognizable behavior,24,28,49 and then to retrospective simulations of clinical trials25,27 These two latter studies, despite being post hoc models of existing clinical trials, nevertheless provided useful insights that are consistent with the engineering paradigm of TSB. In the first case,25 the models were constructed with an emphasis on knowledge representation and instantiation. The fundamental assumptions of the model mirrored the state of the science at the time of the design of the initial anti-TNF and anti-IL-1 clinical trials; the computational model merely placed those assumptions transparently into a modeling framework that demonstrated the dynamic, systemic consequences of those assumptions. This type of model, then, can function as a tool in the early aspects of drug design, where the fundamental mechanistic basis of a proposed intervention can be tested in silico prior to further development. Conversely, the latter study27 provides a means by which the heterogeneity of host response and sepsis might be addressed in terms of evaluating the outcome of a clinical trial. The heterogeneity of septic patients is well recognized, and this computational model provides a means of providing finer-grained, mechanistic population subgroup analysis as an aid in selecting the types of patients to be included into a clinical trial. This work has been extended to identify and predict differential patient population responses based on geographical and demographic variables,62,63 and finally to predict prospectively the outcome of a clinical mediator-directed trial.64 This work was also applied to a purely predictive setting, for clinical trial simulation in the case of anthrax infection in the presence or absence of vaccination and antibiotics.65 It bears noting that these in silico trial models retain the same cellular and molecular mechanism-based framework as the earlier models of in vivo preparations. In fact, their improved predictive power arose from the addition of further basic mechanistic detail and more intensive calibration to clinically available mediator data.
The stepwise evolution of models, from conceptual hypothesis generation, to basic science/laboratory-based, to observational clinical, to prospective clinical prediction, spans the entire gamut of biomedical research. At each step there was close collaboration and communication between the modelers and the biomedical researchers, and the iterative fashion by which the TSB models were constructed and used never lost focus on the next necessary step in the research process. It is this progressive, directional iterative development that forms the hallmark of TSB.52
TARGETS FOR TSB IN THE BURN RESEARCH COMMUNITY
We will use some of the topic headings from the Basic Science sections from the State of the Science meeting and, using the TSB sepsis experience as a framework, propose potential applications of TSB to those particular areas. As such, the following section will take a necessarily speculative tone. Our intent is not to suggest that the application of TSB will provide an immediate solution for these challenges. Rather, our main goal is to make the burn community aware of possible resources and approaches that can potentially augment ongoing avenues of research. Furthermore, we suggest that the application of TSB can provide an integrated engineering framework consistent with the burn community’s tradition of a “research team approach” that has in the past already included mathematicians and modelers33–45 to address these areas of interest.
Resuscitation Issues
Despite the “formalization” of burn resuscitation formulas in the late 1960s, several issues persist. The acknowledgement of “fluid creep” in crystalloid resuscitation and its subsequent effect on the evolution of the burn wound and systemic response are significant areas of clinical interest.66 One potential conceptual focus could be on characterizing what differentiates burn injury from other proinflammatory stresses. Existing TSB global models of inflammation point to an effect of the degree of initial insult to the subsequent behavior of the system,24,28,31,48,49 but perhaps there are particular aspects of the mechanism of burn injury that affect the systemic inflammatory response. For instance, in no other type of inflammatory insult is the cellular mass of damaged tissue as great as in a burn. Does the degree and nature of the inflammatory response depend upon whether the precipitating factor is direct tissue damage, endothelial/neutrophil activation via ischemia/reperfusion (as seen in hemorrhagic shock), or macrophage response to endotoxin (as seen in gram negative sepsis)? TSB models exist for all three conditions,24,28,31 but there is currently no complete model of the presumed inflammatory pathophysiology of a burn patient. Early efforts at applying mathematical models to diverse aspects of the burn response33–45 could be coupled with the TSB models described above to define the mechanistic dynamics of burn injury and its response to resuscitation. Furthermore, the development of multiple TSB models of the acute response to burn injury and resuscitation might provide a means of rational, biologically mechanistic analysis of the genomic information generated by large-scale programs such as the Inflammation and Host Response to Injury “Glue Grant.”53–60 In this fashion TSB could provide a translational bridge between data on gene regulatory mechanisms and the clinical setting. TSB has already taken steps in this direction by linking hepatic transcriptome data to injury and hemorrhagic shock in a dynamic mathematical model 31.
Additionally, the diffuse capillary leak that is noted during the resuscitation phase has been the subject of several hypothesized interventions and changes in the classical crystalloid resuscitation. These strategies include the use of antioxidants such as Vitamin C,67 the use of hypertonic fluid,68,69 and the use and timing of colloid.70 Mechanistic evaluation of these modalities requires extensive understanding of the dynamics of cellular behavior, not only within the burn wound, but also at the end-organ level. Some of the presumed mechanisms by which some of these modalities are thought to have effect include endothelial activation and inflammatory cell trafficking, all phenomena for which TSB models exist.25,71 The extension of these TSB models to include the specific effects of Vitamin C and hypertonic saline, and integration of these mechanisms to calibrated global models, may provide a means to augment clinical trials that are currently constrained by limited patient availability and heterogeneity.
Inhalation Injury
Burn-related lung injury is one of the greatest causes of current mortality due to burn, and its pathophysiology with respect to the response of the airways to toxins, thermal injury, and burn-related inflammation are extremely well studied. However, the actual means of diagnosis of inhalation injury, as well as differentiation and characterization of the type of lung injury that will manifest, remains controversial. Therefore, research efforts focus on two main areas: 1) easy and early diagnosis of those patients who may have inhalation injury, and 2) therapy for inhalation injury. For the former, one goal would be to have a readily available means for molecular fingerprinting of the response of the lung to injury in a fashion that is predictive of disease course and outcome. As mentioned above, efforts of projects such as the Inflammation and Host Response to Injury “Glue Grant”53–60 provide invaluable information regarding the link between the genetic and genomic profile and the physiologic state of these types of patients, and are paving the road to being able to “personalize” medical therapy. However, the goal of “personalizing” diagnosis and care requires a substantial evolution in how these data are used. Current methods of evaluating genomic data to establish links between gene expression and clinical states are centered on statistical clustering and principal component analysis. These methods suggest correlative, rather than causal, hypotheses. The extrapolation of genomic/proteomic information beyond precollected data points requires some approximation of causality and mechanism by an analytic method. As mentioned above, TSB has already taken steps in this direction.31
In addition, existing TSB models that link systemic inflammation and lung injury/adult respiratory distress syndrome61,62,72 have the potential to be modified to include specific characteristics of inhalation injury, producing in silico testing platforms with which to examine the cellular and molecular dynamics of the evolution of inhalation injury. Furthermore, the TSB community has proposed modeling architectures intended to integrate multiple tissue/organ types and reproduce organ–organ cross talk,73,74 and these may prove to be fruitful platforms for examining the systemic effects of inhalation injury.
Wound Healing
The degree of actual tissue damage in burned patients, in addition to having the potential immediate effects noted above, also means that the processes of healing are at a level that would have significant global effects as compared with other types of systemic perturbation. Furthermore, the biology of wound healing is of significant interest in a much larger population of patients with smaller burns not subject to the tremendous systemic effects seen with large burns. The cellular and molecular effectors of wound healing are areas of great interest. Early simulation work on wound healing focused on epithelial proliferation and migration. Subsequently, the scope of these models was expanded to include the interactions between inflammation and healing.75–86 These types of computational models, if modified to reflect the characteristics of thermal/chemical/electrical injury, may provide insight into efforts to affect the acute biology of the burn wound, as well as project the subsequent consequences of those measures on subsequent healing and scarring. It is in this last area that TSB simulations might play a vital role. Since the “real-world” process takes such a long time, being able to test potential experimental interventions in silico, at a much faster rate, may rapidly accelerate the ability to study these interactions.
INTEGRATING TRANSLATIONAL SYSTEMS BIOLOGY INTO THE “BURN TEAM APPROACH”
What can TSB do for the burn research community? To illustrate an example, we can answer this question with another question: what is one of the greatest limitations to the translation of basic research to clinical burn therapy? One application of TSB to burn treatment lies in the fact that the number of severely burned patients in the developed world is declining. Consequently, it is difficult to design and carry out adequately powered, prospective, randomized clinical trials needed to “prove” clinical efficacy using current methods and trial structures. There are currently multiple clinical questions waiting for this test of “proof.” A short, noncomprehensive list would include: the use of colloids, hypertonic saline, and Vitamin C during resuscitation; metabolic manipulation with growth factors and specific nutrients; and manipulation of wound healing with various growth factors and skin substitutes. These are all pleuripotent and polymechanistic manipulations. Dynamic, mechanistic models such as those developed in TSB may be required to spearhead the development of effective therapies, with the ultimate goal of running in silico clinical trials as have already been done for sepsis60–62 and anthrax.63 However, TSB needs to further evolve as a burn research methodology if this goal is to be achieved.
Towards this end, we propose the integration of engineering principles into burn research at the outset of investigation and the discovery process, rather than at the point at which a particular discovery is ready for application. In doing so, we follow a traditional hallmark of the care of the burn patient: the concept of the “burn team” and the carryover of this paradigm into the area of burn research. TSB follows this “team concept” as it relies upon the integration of skills and expertise from different domains of science focused towards a single goal. The development of TSB as a field demonstrates the evolution towards a transdisciplinary, integrated research team and community.23
The method of concurrent, iterative simulation with more traditional research avenues will aid in addressing the volume and complexity of the data that are being generated and focus areas of investigation. We propose an investigatory structure that is oriented hierarchically along traditional research lines. Each category includes a traditional “wet lab” area of research, a focus group of simulation scientists and topics for TSB, and the eventual therapeutic output in terms of how this information may be used in the commercial/industrial setting. This structure is described in a consensus statement from the Society of Complexity in Acute Illness regarding the process of evidence-based dynamic mathematical modeling.52
This methodology, however, would benefit from a prototyping platform, and the burn research community represents a focused group that can implement this novel integrating framework of scientific knowledge and discovery. Given the cohesion and productivity at the recent State of the Science meeting with respect to identifying the overarching issues for the burn community in the forthcoming decade(s), there is an opportunity to augment the efficiency and productivity of the community’s research efforts by implementing this engineering framework in conjunction with the areas of focus identified at that conference. We hope that this review of the work in the TSB community will stimulate interactions that ultimately improve the care of the burn patient.
Acknowledgments
This work was supported in part by the National Institutes of Health grants R01-GM-67240-02, P50-GM-53789-08, R01-HL-76157-02, and R01-HL080926-01; National Institute on Disability and Rehabilitation Research grant H133E070024; as well as grants from the Commonwealth of Pennsylvania, the Pittsburgh Lifesciences Greenhouse, and the Pittsburgh Tissue Engineering Initiative.
The authors would like to acknowledge the contributions to this work of the following investigators, students, and postdoctoral fellows: Sven Zenker, Andres Torres, Patricio Polanco, Claudio Lagoa, Jose M. Prince, Ryan M. Levy, Judy Day, Angela Reynolds, Qi Mi, Nicole Li, Joshua Sullivan, Matthew Rosengart, Juan Carlos Puyana, Gary Nieman, David Carney, David Hackam, Jeffrey Upperman, Ruben Zamora, Katherine Verdolini, David L. Steed, John Bartels, Arie Baratt, Frederick D. Busche, Gregory Constantine, Ivan Yotov, David Swigon, Beatrice Riviere, Jonathan Rubin, Steve Chang, Mitchell P. Fink, Russell Delude, Edwin Dietch, Rena Feinman, Timothy R. Billiar, G. Bard Ermentrout, and Gilles Clermont. Additionally, several excellent technicians (Derek Barclay, David Gallo, and Binnie Betten) contributed to this work.
APPENDIX
The Primary Types of Dynamic Mathematical Modeling Used in Translational Systems Biology
Equation based modeling (EBM) has been the predominant modeling framework used in burn modeling studies.33–45 This methodology consists of assigning variables to various parameters within a system and writing differential equations that describe how those parameters change over time. These equations are linked to reproduce the dynamics of the system with respect to the selected parameters. Ordinary differential equations use time as the sole independent variable (assuming a well-mixed and homogeneous system), while partial differential equations incorporate spatial variables. Thus far, most EBMs used in the TSB community are ordinary differential equation-based.26,28,30–32,46–48
Though EBM is a more established modeling framework, agent-based modeling (ABM) has emerged as a major alternative for addressing features of complex biological systems.87 In ABMs the system is modeled as a set of components (agents), which can be grouped into populations (agent classes) based on similar intrinsic rules of behavior (agent-rules). While agents within a class will have the same rules for behavior, the behavior of the individual agents is varies because of differences in local conditions. The behavior of the simulation results from the aggregate interactions within the model.24,49,87 The analysis of ABM relies upon matching between the patterns of behavior between the simulation and the “real-world.”88
EBM has several advantages over ABM. Familiarity with EBM is more widespread and well established, and therefore expertise is more readily available within most mathematics departments. EBM facilitates formal mathematical analysis, and thus can provide insight into fundamental “laws” of behavior.89 EBM have the drawback that they can be daunting to non-mathematicians and are predicated on the assumption of a homogenously distributed system (for ordinary differential equations); thus, they may be less applicable in situations where spatial effects are important.26,89
There are several advantages of ABM. Such models map intuitively to biological phenomena, such as cells interacting within tissues and organs. Agent rule systems are often expressed as conditional statements (“if–then”), facilitating the translation from the results of basic science experiments into agent-rules. ABM has an intrinsically spatial component based on its reliance upon local interactions and environmental heterogeneity. The limitations of ABM are 1) they are computationally intensive; and 2) since there is often not a direct inferable relationship between the agent-rules and the system’s behavior, they can be difficult to quantitatively calibrate.26
References
- 1.Kitano H. Systems biology: a brief overview. Science. 2002;295:1662. doi: 10.1126/science.1069492. [DOI] [PubMed] [Google Scholar]
- 2.Csete ME, Doyle JC. Reverse engineering of biological complexity. Science. 2002;295:1664. doi: 10.1126/science.1069981. [DOI] [PubMed] [Google Scholar]
- 3.Doyle J, Csete M. Rules of engagement. Nature. 2007;446:860. doi: 10.1038/446860a. [DOI] [PubMed] [Google Scholar]
- 4.Kot M. Elements of mathematical ecology. Cambridge, UK: Cambridge University Press; 2001. [Google Scholar]
- 5.Anonymous. Models and methods in social network analysis (Structural analysis in the social sciences) Cambridge, UK: Cambridge University Press; 2005. [Google Scholar]
- 6.Anonymous. The economy as an evolving complex system, III: current perspectives and future directions (Santa Fe Institute Studies on the Sciences of Complexity) New York, NY: Oxford University Press; 2005. [Google Scholar]
- 7.Anonymous. The mind, the brain, and complex adaptive systems (Sante Fe Institute Studies on the Sciences of Complexity) Boston, MA: Addison Wesley Longman; 2006. [Google Scholar]
- 8.Buchman TG. Physiologic stability and physiologic state. J Trauma. 1996;41:599. doi: 10.1097/00005373-199610000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Seely AJ, Christou NV. Multiple organ dysfunction syndrome: exploring the paradigm of complex nonlinear systems [Review] Crit Care Med. 2000;28:2193. doi: 10.1097/00003246-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Marshall JC. Complexity, chaos, and incomprehensibility: parsing the biology of critical illness. Crit Care Med. 2000;28:2646. doi: 10.1097/00003246-200007000-00080. [DOI] [PubMed] [Google Scholar]
- 11.Neugebauer EA, Willy C, Sauerland S. Complexity and nonlinearity in shock research: reductionism or synthesis? Shock. 2001;16:252. doi: 10.1097/00024382-200116040-00003. [DOI] [PubMed] [Google Scholar]
- 12.Buchman TG, Cobb JP, Lapedes AS, Kepler TB. Complex systems analysis: a tool for shock research. Shock. 2001;16:248. doi: 10.1097/00024382-200116040-00002. [DOI] [PubMed] [Google Scholar]
- 13.Buchman TG. The community of the self. Nature. 2002;420:246. doi: 10.1038/nature01260. [DOI] [PubMed] [Google Scholar]
- 14.Buchman TG. Nonlinear dynamics, complex systems, and the pathobiology of critical illness. Curr Opin Crit Care. 2004;10:378. doi: 10.1097/01.ccx.0000139369.65817.b6. [DOI] [PubMed] [Google Scholar]
- 15.Food and Drug Administration. Innovation or Stagnation: Challenge and Opportunity on the Critical Path to New Medical Products. 2004. pp. 1–38. [Google Scholar]
- 16.NIH. [Accessed November 1, 2006];Roadmap for medical research: research teams. 2006 Available at: http://nihroadmap.nih.gov.
- 17.NIH. [Accessed January 30, 2008];New roadmap emphasis areas for 2008. Available at: http://nihroadmap.nih.gov/2008initiatives.asp.
- 18.Lengeler JW. Metabolic networks: a signal-oriented approach to cellular models. Biol Chem. 2000;381:911. doi: 10.1515/BC.2000.112. [DOI] [PubMed] [Google Scholar]
- 19.Smye SW, Clayton RH. Mathematical modelling for the new millennium: medicine by numbers. Med Eng Phys. 2002;24:565. doi: 10.1016/s1350-4533(02)00049-8. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein B, Faeder JR, Hlavacek WS. Mathematical and computational models of immune-receptor signalling. Nat Rev Immunol. 2004;4:445. doi: 10.1038/nri1374. [DOI] [PubMed] [Google Scholar]
- 21.Mogilner A, Wollman R, Civelekoglu-Scholey G, Scholey J. Modeling mitosis. Trends Cell Biol. 2006;16:88. doi: 10.1016/j.tcb.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Hlavacek WS, Faeder JR, Blinov ML, Posner RG, Hucka M, Fontana W. Rules for modeling signal-transduction systems. Sci STKE. 2006;2006(344):re6. doi: 10.1126/stke.3442006re6. [DOI] [PubMed] [Google Scholar]
- 23.An G, Hunt CA, Clermont G, Neugebauer E, Vodovotz Y. Challenges and rewards on the road to translational systems biology in acute illness: four case reports from interdisciplinary teams. J Crit Care. 2007;22:169–175. doi: 10.1016/j.jcrc.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.An G. Agent-based computer simulation and SIRS: building a bridge between basic science and clinical trials. Shock. 2001;16:266. doi: 10.1097/00024382-200116040-00006. [DOI] [PubMed] [Google Scholar]
- 25.An G. In-silico experiments of existing and hypothetical cytokine-directed clinical trials using agent based modeling. Crit Care Med. 2004;32:2050. doi: 10.1097/01.ccm.0000139707.13729.7d. [DOI] [PubMed] [Google Scholar]
- 26.Vodovotz Y, Clermont G, Chow C, An G. Mathematical models of the acute inflammatory response. Curr Opin Crit Care. 2004;10:383. doi: 10.1097/01.ccx.0000139360.30327.69. [DOI] [PubMed] [Google Scholar]
- 27.Clermont G, Bartels J, Kumar R, Constantine G, Vodovotz Y, Chow C. In silico design of clinical trials: a method coming of age. Crit Care Med. 2004;32:2061. doi: 10.1097/01.ccm.0000142394.28791.c3. [DOI] [PubMed] [Google Scholar]
- 28.Chow CC, Clermont G, Kumar R, et al. The acute inflammatory response in diverse shock states. Shock. 2005;24:74. doi: 10.1097/01.shk.0000168526.97716.f3. [DOI] [PubMed] [Google Scholar]
- 29.Vodovotz Y, Chow CC, Bartels J, et al. In silico models of acute inflammation in animals. Shock. 2006;26:235. doi: 10.1097/01.shk.0000225413.13866.fo. [DOI] [PubMed] [Google Scholar]
- 30.Prince JM, Levy RM, Bartels J, et al. In silico and in vivo approach to elucidate the inflammatory complexity of CD14-deficient mice. Mol Med. 2006;12:88. doi: 10.2119/2006-00012.Prince. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lagoa CE, Bartels J, Baratt A, et al. The role of initial trauma in the host’s response to injury and hemorrhage: insights from a comparison of mathematical simulations and hepatic transcriptomic analysis. Shock. 2006;26:592. doi: 10.1097/01.shk.0000232272.03602.0a. [DOI] [PubMed] [Google Scholar]
- 32.Ben David I, Price SE, Bortz DM, et al. Dynamics of intrapulmonary bacterial growth in a murine model of repeated microaspiration. Am J Respir Cell Mol Biol. 2005;33:476. doi: 10.1165/rcmb.2005-0053OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cantrell JH., Jr Ultrasonic determination of thermodynamic threshold parameters for irreversible cutaneous burns. J Acoust Soc Am. 1982;72:337. doi: 10.1121/1.388086. [DOI] [PubMed] [Google Scholar]
- 34.Roa LM, Gomez-Cia T, Cantero A. Analysis of burn injury by digital simulation. Burns Incl Therm Inj. 1988;14:201. doi: 10.1016/0305-4179(88)90039-3. [DOI] [PubMed] [Google Scholar]
- 35.Bert JL, Bowen BD, Gu X, Lund T, Reed RK. Microvascular exchange during burn injury, II. formulation and validation of a mathematical model. Circ Shock. 1989;28:199. [PubMed] [Google Scholar]
- 36.Bowen BD, Bert JL, Gu X, Lund T, Reed RK. Microvascular exchange during burn injury, III. implications of the model. Circ Shock. 1989;28:221. [PubMed] [Google Scholar]
- 37.Roa L, Gomez-Cia T, Cantero A. Pulmonary capillary dynamics and fluid distribution after burn and inhalation injury. Burns. 1990;16:25. doi: 10.1016/0305-4179(90)90202-8. [DOI] [PubMed] [Google Scholar]
- 38.Bondareva IB, Parfenov AS. A non-linear mathematical model for the in vivo evaluation of the RES phagocytic function. Medinfo. 1995;8(Pt 2):1091. [PubMed] [Google Scholar]
- 39.Bert J, Gyenge C, Bowen B, Reed R, Lund T. Fluid resuscitation following a burn injury: implications of a mathematical model of microvascular exchange. Burns. 1997;23:93. doi: 10.1016/s0305-4179(96)00115-5. [DOI] [PubMed] [Google Scholar]
- 40.Koerber AJ, King JR, Ward JP, Williams P, Croft JM, Sockett RE. A mathematical model of partial-thickness burn-wound infection by Pseudomonas aeruginosa: quorum sensing and the build-up to invasion. Bull Math Biol. 2002;64:239. doi: 10.1006/bulm.2001.0272. [DOI] [PubMed] [Google Scholar]
- 41.Rosinski M, Yarmush ML, Berthiaume F. Quantitative dynamics of in vivo bone marrow neutrophil production and egress in response to injury and infection. Ann Biomed Eng. 2004;32:1108. doi: 10.1114/b:abme.0000036647.81372.ce. [DOI] [PubMed] [Google Scholar]
- 42.Feng Q, Zhao-Yan H, Zheng-Kang Z, Li-Xing S. The establishment of the mathematical model of the 2nd degree burn injury of human tissues and its application. Conf Proc IEEE Eng Med Biol Soc. 2005;3:2918. doi: 10.1109/IEMBS.2005.1617085. [DOI] [PubMed] [Google Scholar]
- 43.Mercer GN, Sidhu HS. Modeling thermal burns due to air-bag deployment. Burns. 2005;31:977. doi: 10.1016/j.burns.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 44.Lv YG, Liu J, Zhang J. Theoretical evaluation of burns to the human respiratory tract due to inhalation of hot gas in the early stage of fires. Burns. 2006;32:436. doi: 10.1016/j.burns.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 45.Denman PK, McElwain DL, Harkin DG, Upton Z. Mathematical modelling of aerosolised skin grafts incorporating keratinocyte clonal subtypes. Bull Math Biol. 2007;69:157. doi: 10.1007/s11538-006-9082-z. [DOI] [PubMed] [Google Scholar]
- 46.Kumar R, Clermont G, Vodovotz Y, Chow CC. The dynamics of acute inflammation. J Theor Biol. 2004;230:145. doi: 10.1016/j.jtbi.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 47.Reynolds A, Rubin J, Clermont G, Day J, Vodovotz Y, Ermentrout GB. A reduced mathematical model of the acute inflammatory response: I. Derivation of model and analysis of anti-inflammation. J Theor Biol. 2006;242:220. doi: 10.1016/j.jtbi.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 48.Day J, Rubin J, Vodovotz Y, Chow CC, Reynolds A, Clermont G. A reduced mathematical model of the acute inflammatory response, II. capturing scenarios of repeated endotoxin administration. J Theor Biol. 2006;242:237. doi: 10.1016/j.jtbi.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 49.An G, Lee I. Complexity, emergence and pathophysiology: Using agent based computer simulation to characterize the non-adaptive inflammatory response (Manuscript # [344]) [Accessed January 31, 2008];Int J Complex Systems. 2000 Available at: http://www.interjournal.org.
- 50.Wakeland W, Macovsky L, An G. A hybrid simulation for studying acute inflammatory response. Proceedings of the 2007 Spring Simulation Multiconference/Agent-directed Simulation Symposium; Norfolk, VA. 2007. pp. 39–46. [Google Scholar]
- 51.Marshall JC. Through a glass darkly: the brave new world of in silico modeling. Crit Care Med. 2004;32:2157. doi: 10.1097/01.ccm.0000142935.34916.b5. [DOI] [PubMed] [Google Scholar]
- 52.Vodovotz Y, Clermont G, Hunt CA, et al. Evidence-based modeling of critical illness: an initial consensus from the Society for Complexity in Acute Illness. J Crit Care. 2007;22:77–84. doi: 10.1016/j.jcrc.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cobb JP, Laramie JM, Stormo GD, et al. Sepsis gene expression profiling: murine splenic compared with hepatic responses determined by using complementary DNA microarrays. Crit Care Med. 2002;30:2711. doi: 10.1097/00003246-200212000-00016. [DOI] [PubMed] [Google Scholar]
- 54.Chung TP, Laramie JM, Province M, Cobb JP. Functional genomics of critical illness and injury. Crit Care Med. 2002;30:S51. [PubMed] [Google Scholar]
- 55.Cobb JP, O’Keefe GE. Injury research in the genomic era. Lancet. 2004;363:2076. doi: 10.1016/S0140-6736(04)16460-X. [DOI] [PubMed] [Google Scholar]
- 56.Dasu MR, Cobb JP, Laramie JM, Chung TP, Spies M, Barrow RE. Gene expression profiles of livers from thermally injured rats. Gene. 2004;327:51. doi: 10.1016/j.gene.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 57.Calvano SE, Xiao W, Richards DR, et al. A network-based analysis of systemic inflammation in humans. Nature. 2005;438(7068):696. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- 58.Cobb JP, Mindrinos MN, Miller-Graziano C, et al. Application of genome-wide expression analysis to human health and disease. Proc Natl Acad Sci USA. 2005;102:4801. doi: 10.1073/pnas.0409768102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brownstein BH, Logvinenko T, Lederer JA, et al. Commonality and differences in leukocyte gene expression patterns among three models of inflammation and injury. Physiol Genomics. 2006;24:298. doi: 10.1152/physiolgenomics.00213.2005. [DOI] [PubMed] [Google Scholar]
- 60.Liu T, Qian WJ, Gritsenko MA, et al. High dynamic range characterization of the trauma patient plasma proteome. Mol Cell Proteomics. 2006;5:1899. doi: 10.1074/mcp.M600068-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nieman G, Bartels J, Wei J, et al. Mathematical simulation of inflammation in porcine septic shock and ARDS [abstract] Shock. 2005;23(Suppl 3):3. [Google Scholar]
- 62.Vodovotz Y. Deciphering the complexity of acute inflammation using mathematical models. Immunologic Res. 2006;36:237. doi: 10.1385/IR:36:1:237. [DOI] [PubMed] [Google Scholar]
- 63.Chang S, Busche F, Vodovotz Y, Clermont G, Fink M. Integrating environmental factors into a mathematical model to predict mortality of septic patients [abstract] Shock. 2005;23(Suppl 3):3. [Google Scholar]
- 64.Chang S, Baratt A, Clermont G, et al. Mathematical model predicting outcomes of sepsis patients treated with Xigris(R): ENHANCE trial. Shock. 2006;25:70. [Google Scholar]
- 65.Kumar R, Chow CC, Bartels J, Clermont G, Vodovotz Y. A mathematical simulation of the inflammatory response to anthrax infection. Shock. doi: 10.1097/SHK.0b013e318067da56. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Friedrich JB, Sullivan SR, Engrav LH, et al. Is supra-Baxter resuscitation in burn patients a new phenomenon? Burns. 2004;30:464–466. doi: 10.1016/j.burns.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 67.Tanaka H, Matsuda T, Miyagantani Y, Yukioka T, Matsuda H, Shimazaki S. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: a randomized, prospective study. Arch Surg. 2000;135:326. doi: 10.1001/archsurg.135.3.326. [DOI] [PubMed] [Google Scholar]
- 68.Oda J, Ueyama M, Yamashita K, et al. Hypertonic lactated saline resuscitation reduces the risk of abdominal compartment syndrome in severely burned patients. J Trauma. 2006;60:64. doi: 10.1097/01.ta.0000199431.66938.99. [DOI] [PubMed] [Google Scholar]
- 69.Horton JW, Maass DL, White DJ. Hypertonic saline dextran after burn injury decreases inflammatory cytokine responses to subsequent pneumonia-related sepsis. Am J Physiol Heart Circ Physiol. 2006;290:H1642. doi: 10.1152/ajpheart.00586.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hemington-Gorse SJ. Colloid or crystalloid for resuscitation of major burns. J Wound Care. 2005;14:256. doi: 10.12968/jowc.2005.14.6.26786. [DOI] [PubMed] [Google Scholar]
- 71.Bailey AM, Thorne BC, Peirce SM. Multi-cell agent-based simulation of the microvasculature to study the dynamics of circulating inflammatory cell trafficking. Ann Biomed Eng. doi: 10.1007/s10439-007-9266-1. In press. [DOI] [PubMed] [Google Scholar]
- 72.An G. Agent based models of pulmonary epithelial barrier function. Proc Am Thorac Soc. 2006;3:A309. [Google Scholar]
- 73.An G. Integrative modeling of inflammation and organ function using agent based modeling. Shock. 2006;26(Suppl 1):2. [Google Scholar]
- 74.Yan L, Hunt CA, Ropella GEP. In silico representation of the liver connecting function to anatomy, physiology and heterogeneous microenvironments. Proceedings of the 26th Annual International Conference of the IEEE EMBS; San Francisco, CA. 2004. pp. 853–856. [DOI] [PubMed] [Google Scholar]
- 75.Murray JD, Maini PK, Tranquillo R. Mechanochemical models for generating biological pattern and form in development. Physics Rep. 1988;171:59. [Google Scholar]
- 76.Murray JD. Mathematical biology. Heidelberg, Germany: Springer-Verlag; 1989. [Google Scholar]
- 77.Sherratt JA, Murray JD. Models of epidermal wound healing. Proc Biol Sci. 1990;241:29. doi: 10.1098/rspb.1990.0061. [DOI] [PubMed] [Google Scholar]
- 78.Tranquillo RT, Murray JD. Continuum model of fibroblast-driven wound contraction: inflammation-mediation. J Theor Biol. 1992;158:135. doi: 10.1016/s0022-5193(05)80715-5. [DOI] [PubMed] [Google Scholar]
- 79.Tranquillo RT, Murray JD. Mechanistic model of wound contraction. J Surg Res. 1993;55:233. doi: 10.1006/jsre.1993.1135. [DOI] [PubMed] [Google Scholar]
- 80.Cook J. A mathematical model for dermal wound healing: wound contraction and scar formation [dissertation] Seattle, Washington: University of Washington; 1995. [Google Scholar]
- 81.Olsen L, Sherratt JA, Maini PK. A mechanochemical model for adult dermal wound contraction and the permanence of the contracted tissue displacement profile. J Theor Biol. 1995;177:113. doi: 10.1006/jtbi.1995.0230. [DOI] [PubMed] [Google Scholar]
- 82.Dallon JC, Sherratt JA, Maini PK. Modeling the effects of transforming growth factor-beta on extracellular matrix alignment in dermal wound repair. Wound Repair Regen. 2001;9:278. doi: 10.1046/j.1524-475x.2001.00278.x. [DOI] [PubMed] [Google Scholar]
- 83.Sherratt JA, Dallon JC. Theoretical models of wound healing: past successes and future challenges. CR Biol. 2002;325:557. doi: 10.1016/s1631-0691(02)01464-6. [DOI] [PubMed] [Google Scholar]
- 84.Walker DC, Hill G, Wood SM, Smallwood RH, Southgate J. Agent-based computational modeling of epithelial cell monolayers: predicting the effect of exogenous calcium concentration on the rate of wound closure. IEEE Trans Nano-bioscience. 2004;3:153. doi: 10.1109/tnb.2004.833680. [DOI] [PubMed] [Google Scholar]
- 85.Walker DC, Southgate J, Hill G, et al. The epitheliome: agent-based modelling of the social behaviour of cells. Bio-systems. 2004;76:89. doi: 10.1016/j.biosystems.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 86.Mi Q, Rivière B, Clermont G, Steed DL, Vodovotz Y. Agent-based model of inflammation and wound healing: insights into diabetic foot ulcer pathology and the role of transforming growth factor-β1. Wound Rep Reg. 2007;15:671–682. doi: 10.1111/j.1524-475X.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 87.Ermentrout GB, Edelstein-Keshet L. Cellular automata approaches to biological modeling. J Theor Biol. 1993;160:97. doi: 10.1006/jtbi.1993.1007. [DOI] [PubMed] [Google Scholar]
- 88.Grimm V, Revilla E, Berger U, et al. Pattern-oriented modeling of agent-based complex systems: lessons from ecology. Science. 2005;310:987. doi: 10.1126/science.1116681. [DOI] [PubMed] [Google Scholar]
- 89.Seydel R. Practical bifurcation and stability analysis. New York: Springer; 1994. [Google Scholar]