Personalized Management of Hyperglycemia in Type 2 Diabetes: Reflections from a Diabetes Care Editors’ Expert Forum (original) (raw)
Abstract
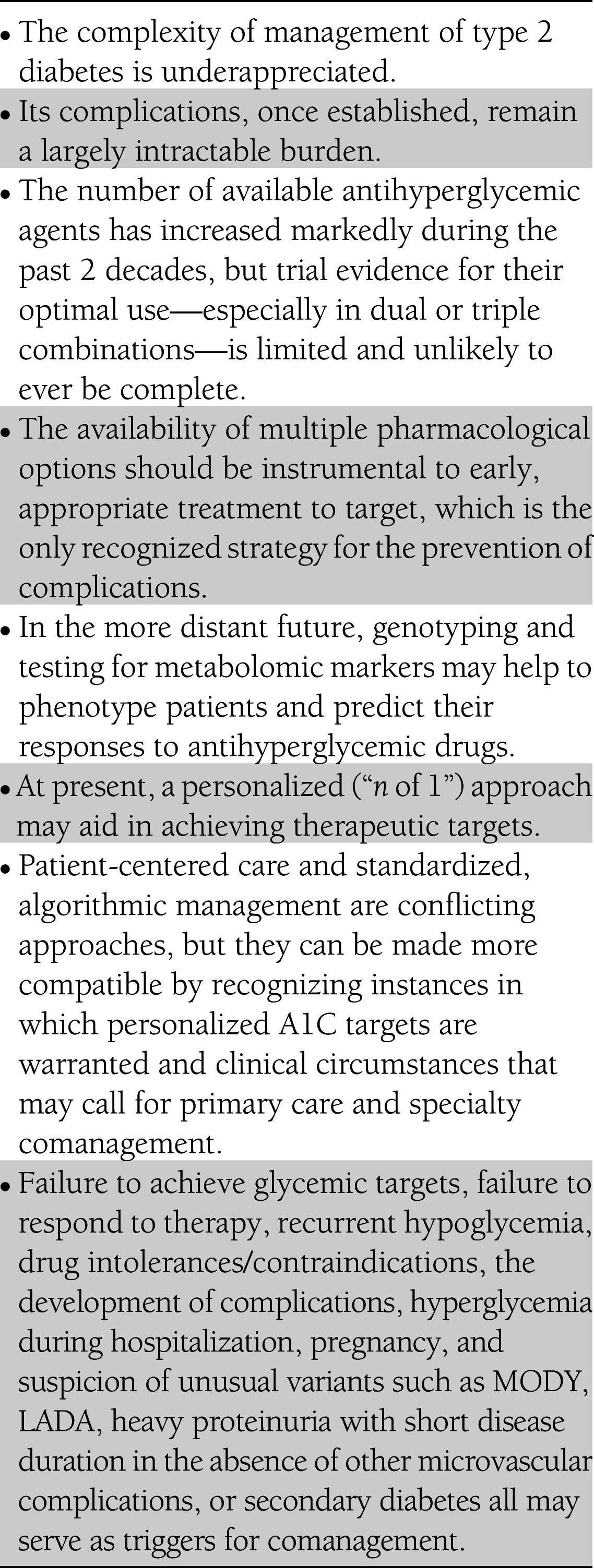
In June 2012, 13 thought leaders convened in a Diabetes Care Editors’ Expert Forum to discuss the concept of personalized medicine in the wake of a recently published American Diabetes Association/European Association for the Study of Diabetes position statement calling for a patient-centered approach to hyperglycemia management in type 2 diabetes. This article, an outgrowth of that forum, offers a clinical translation of the underlying issues that need to be considered for effectively personalizing diabetes care. The medical management of type 2 diabetes has become increasingly complex, and its complications remain a great burden to individual patients and the larger society. The burgeoning armamentarium of pharmacological agents for hyperglycemia management should aid clinicians in providing early treatment to delay or prevent these complications. However, trial evidence is limited for the optimal use of these agents, especially in dual or triple combinations. In the distant future, genotyping and testing for metabolomic markers may help us to better phenotype patients and predict their responses to antihyperglycemic drugs. For now, a personalized (“n of 1”) approach in which drugs are tested in a trial-and-error manner in each patient may be the most practical strategy for achieving therapeutic targets. Patient-centered care and standardized algorithmic management are conflicting approaches, but they can be made more compatible by recognizing instances in which personalized A1C targets are warranted and clinical circumstances that may call for comanagement by primary care and specialty clinicians.
In April 2012, the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) published a joint position statement titled “Management of Hyperglycemia in Type 2 Diabetes: A Patient-Centered Approach” (1). It was an important update to earlier guidelines (2–8), providing a thorough examination of the ever-more-complex therapeutic options for glycemic management, the benefits and risks of tight glycemic control, the efficacy and safety evidence for new drug classes, and the data supporting withdrawals of or restrictions on other agents. Furthermore, it placed great emphasis on patient-centered and personalized care.
These recommendations captured the attention of the Diabetes Care editorial team. On the one hand, the recommendations call for a more personalized approach, which, in theory, should be liberating for all health care providers (HCPs) involved in diabetes care. On the other hand, their “less prescriptive” nature has been viewed as providing insufficient guidance to some HCPs who may feel overwhelmed when trying to match the nuances of differences among the increasing number of antihyperglycemic medications to the nuances of each patient’s preferences and medical characteristics.
To explore these issues, we convened a Diabetes Care Editors’ Expert Forum in June 2012. Thirteen thought leaders from around the world convened and discussed approaches to personalized medicine, the rationale behind personalization in diabetes care, the tools necessary to implement such a strategy, and the current perceptions of personalized medicine. This narrative provides our view and clinical translation of the underlying issues that need to be considered for personalizing care and offers suggestions to stimulate future research in this area. Table 1 summarizes the main points discussed below.
Table 1.
Summary of the main points from the Diabetes Care Editors’ Expert Forum
PRACTICAL APPROACHES TO PERSONALIZED MEDICINE
From intervention trials to personalized targets
There can be little more than semantic differences among the terms “personalized medicine,” “patient-centered care,” and “clinical judgment.” Factors such as patients’ preferences, life expectancy, disease duration, comorbid conditions, socioeconomic status, and cognitive abilities have long played a role in the selection of optimal therapeutic options and, more recently, in the selection of therapeutic targets.
In 1998, the UK Prospective Diabetes Study (UKPDS) showed that treating patients with recently diagnosed type 2 diabetes reduced the risk of microvascular, but not macrovascular, complications (9). Of the three subsequent randomized controlled trials (RCTs) on glucose lowering and cardiovascular outcomes, two—ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation) and VADT (Veterans Affairs Diabetes Trial)—showed no statistically significant reduction in cardiovascular outcomes, while the glycemic intervention of the third—ACCORD (Action to Control Cardiovascular Risk in Diabetes)—was ended early because of increased mortality in participants randomized to intensive glycemic control (10–12). However, meta-analyses of the four intervention trials (UKPDS, ACCORD, ADVANCE, and VADT) have shown modest but statistically significant benefit of intensive glucose control on the risk for myocardial infarction, but not mortality (13).
Post hoc analyses seeking explanations for these results set the stage for today’s new emphasis on personalized care. Suggestions that adverse effects of individual therapeutic agents or severe hypoglycemia were directly implicated in causing cardiovascular events were not supported by these analyses but cannot be ruled out because efforts to capture hypoglycemic events were probably inadequate, especially in individuals with hypoglycemia unawareness (13). However, individuals assigned to intensive therapy who failed to improve control to A1C levels <7.0% (<53 mmol/mol) in ACCORD fared poorly and had more severe hypoglycemia, and severe hypoglycemia was noted to be a risk marker for a wide range of medical conditions in ADVANCE (14,15). It was also suggested that individuals with long-standing type 2 diabetes, existing cardiovascular disease (CVD), and other comorbidities were unable to achieve cardiovascular benefit from better glucose lowering within the timeframe of these studies (16).
Accordingly, these trials and their subsequent analyses raised important questions about rigid, algorithm-based, “glucocentric” approaches to therapy. One message, then, is that “one size does not fit all” for glucose targets, choice of therapy, or number of therapies used in combination. However, some questions pertinent to personalization remain unanswered. What were the characteristics of the small group of individuals in ACCORD who failed to respond to further glucose-lowering therapy but who contributed much of the excess case fatality (12)? Similarly, what can these studies teach us about patients who benefitted most from the interventions? Gaining insight into the pathophysiological, genetic, lifestyle, adherence, comorbidity, or other factors responsible for these disparate responses could improve our ability to effectively personalize therapy.
The 2012 ADA/EASD position statement still recommends an A1C goal of <7.0% (<53 mmol/mol) for most individuals with type 2 diabetes if it can be achieved safely in low-risk individuals with early diabetes or a relatively long life expectancy; it suggests an acceptance of higher A1C targets for individuals with a history of severe hypoglycemia, limited life expectancy, long-standing diabetes, or advanced micro- and macrovascular complications (1). Prior guidelines from multiple organizations (3–8) included recommendations about setting personalized glycemic targets based on phenotype and empirically matching “the right drugs to the right patients,” but without hard evidence to substantiate such an approach. Personalized treatment was articulated more vigorously in the new position statement (1).
The challenges of personalized care
Patient-centered personalized therapy, although appealing, may be difficult to implement without a good understanding of the ever-changing glucose-lowering armamentarium. β-Cell dysfunction is progressive in type 2 diabetes (9), and thus monotherapy, or even combinations of oral agents, is not likely to control hyperglycemia indefinitely (17), although the ORIGIN (Outcome Reduction With Initial Glargine Intervention) trial demonstrated sustained normoglycemia with basal insulin glargine plus metformin and near-normoglycemia even with standard therapy using metformin plus a sulfonylurea over a 6–7 year period in early type 2 diabetes (18). At this time, the processes of assessing β-cell function and providing reliable clinical decisions based on this factor are less than optimal. Furthermore, so-called evidence-based guidelines may be limited in their ability to be more prescriptive given the lack of clinical trial evidence from properly conducted long-term RCTs comparing the effects of various agents on clinically important outcomes. Clinical inertia is also a problem, and most clinicians do not alter their patients’ glucose-lowering regimens until A1C is significantly elevated (19). Developing and implementing personalized care plans may be especially daunting for those HCPs whose practice extends beyond diabetes alone and who must address these issues in the context of limited time and resources.
The need for translational tools
The task now at hand is clear: We should develop and make available tools that will enable effective translation of existing guidelines on targets and therapeutic options into practical clinical applications. It is one thing to assess the efficacy of an intervention within the context of a structured clinical trial setting, but entirely different to evaluate that intervention in ordinary clinical practices with resource variations, variable patient adherence, and sociodemographic and cultural differences. Thus, the translation of results from RCTs to real-world situations is not an exact science. Until more hard evidence becomes available, clinicians need well-structured and user-friendly evidence summaries that outline safe and effective processes for therapeutic intensification, while still allowing for the personalization of care.
Although such an undertaking is beyond the scope of this discussion, we are providing a starting point that may guide the development of such tools to aid HCPs in personalizing both targets and therapeutic regimens. For target-setting, suggestions have been made in the past (20,21). Another possible starting place might be the decision-making scale developed by Ismail-Beigi et al. (22) and adapted for inclusion in the ADA/EASD position statement (1). That scale includes seven parameters to consider when determining glycemic targets. Expanding it or providing some means of rating each parameter for individual patients could help clinicians to better weigh factors such as life expectancy, duration of diabetes, risk from hypoglycemia, comorbidities, and availability of support systems. Such a tool could assist clinicians in choosing targets and help to involve patients in the decision-making process in an easily understood manner.
Tools are also needed to help HCPs in selecting appropriate agents and intensifying therapy. The ADA/EASD position statement leaves treatment-goal decisions to clinicians and patients (1). However, some believe that because of the vast and expanding array of available drugs, there should be a systematic way to prioritize the selection of drugs in relation to their efficacy, safety, and cost. It is most important to emphasize that the percentage of patients who show sufficient clinical response to any of these drugs varies widely. Nonadherence to treatment regimens may be as high as 50% in patients with chronic diseases such as diabetes (23), often because of the patients’ lack of symptoms, negative emotions, and poor knowledge of their disease (24). Side effects are another cause of stopping or limiting treatment. Thus, patients must be adequately monitored, especially after changes to their treatment regimen, to evaluate whether they have reached targets and to ensure that there are no major side effects or adherence issues. This information is crucial to make informed decisions regarding whether to continue, change, or add to the therapy regimen.
STATE OF THE ART FOR PERSONALIZING MEDICINE
Personalized medicine can be defined in many ways. A shared decision-making approach that takes patient preferences and values into account in developing a management plan is widely endorsed. Another definition involves identifying a particular set of phenotypic and genotypic markers that would define ideal and nonideal therapies for individuals based, to whatever extent possible, on evidence rather than on clinical impressions. Perhaps the most relevant question is whether current science is at a stage where specific patient characteristics—genetic, pathophysiological, or phenotypic—might effectively guide us in more general diabetes practice.
Contributions from genetics: a distant hope
The field of genetics is not yet ready to contribute in these broader areas. Despite recent identification of monogenic forms of diabetes for which specific treatments seem to give benefit (25), for more typical type 2 diabetes, genetic information does not contribute greatly in guiding treatment choices. Recently, pharmacogenetic analysis has begun providing insights, finding possible links, for example, to poor responses to metformin (26,27) and glucagon-like peptide-1 (GLP-1) receptor agonists (28–30). Such research holds promise for eventually helping to identify individuals who are likely to be classified as “responders” or “nonresponders” to specific agents.
Human genome sequencing also offers some hope, but again, in the distant future (31). Because the development of diabetes, patients’ responses to available therapies, and the risks for complications are all multifactorial and probably involve numerous genes, the chances are small that specific mutations will turn out to be powerful markers of diabetes risk or of variable treatment responses. Even assuming a significant increase in pharmacogenetics research and decreases in the costs associated with genome sequencing, for the foreseeable future these efforts will not significantly improve our ability to predict, prevent, or diagnose diabetes or illuminate definitive pathways for selecting drug therapies for specific individuals.
What can we learn from pathophysiology?
Insulin resistance in the liver and muscle and islet β-cell failure represent the core pathophysiological defects in type 2 diabetes (32,33). Insulin resistance can often be demonstrated long before the onset of β-cell failure, but as long as the β-cells secrete sufficient amounts of insulin to offset the insulin resistance, glucose tolerance remains normal (32–36). With time, however, there is progressive β-cell failure, which leads to the development of impaired glucose tolerance and/or impaired fasting glucose and eventually type 2 diabetes (32–36). As the plasma insulin response declines, insulin resistance in the liver becomes manifest as an overproduction of glucose by the liver and the development of fasting hyperglycemia, while insulin resistance in muscle results in diminished glucose uptake and postprandial hyperglycemia (32,33).
Although the relative contributions of β-cell failure (possibly more severe in Asian populations) and insulin resistance (more severe in Westernized societies with a high prevalence of obesity) may vary among different ethnic groups (37), virtually all adults with type 2 diabetes have some combination of the two. Thus, antihyperglycemic agents that improve β-cell function and enhance hepatic and muscle insulin sensitivity may have a more durable effect in reducing A1C (38–45).
The importance of other pathophysiological disturbances in the development of type 2 diabetes is well recognized (32,33). These disturbances include
- Adipocyte insulin resistance, which leads to increased lipolysis, increased plasma free fatty acids, and eventual β-cell failure and muscle and hepatic insulin resistance (46)
- Excess glucagon secretion by α-cells and enhanced hepatic sensitivity to glucagon, leading to increased basal hepatic glucose production and impaired suppression of hepatic glucose production after meals (47,48)
- Dysfunction related to incretin hormones (GLP-1 and glucose-dependent insulinotropic peptide) (49), which are responsible for ∼50% of the insulin secreted in response to meals
- Possible renal adaptive mechanisms to hyperglycemia, which result in enhanced glucose reuptake leading to decreased urinary glucose clearance and the maintenance of established hyperglycemia (50)
- Central nervous system insensitivity to the anorectic effect of insulin and multiple neurotransmitter synaptic abnormalities resulting in excessive energy intake and obesity (33)
No single antihyperglycemic agent can correct all of these pathophysiological abnormalities. Thus, many patients may require multiple agents with different mechanisms of action to achieve their individualized A1C goal (33). Patients with type 2 diabetes who have a high initial A1C, in particular, may require two or more antihyperglycemic agents to achieve their A1C goal (1,4,7,8,33,51,52).
The precise choice of pharmacological agents to use remains a topic for debate, in part because of safety concerns involving several drug classes (53–55). But the basic point remains: To achieve durability of glycemic control, optimal regimens will likely need to address both insulin resistance and β-cell failure.
Does phenotype allow for personalized treatment?
The main characteristics that might influence approaches to treatment can be divided into two categories: patient features and disease features. Among the patient features are race/ethnicity, sex, age of onset or diagnosis, duration of diabetes, body weight, frailty/comorbidities, complications, propensity for side effects/drug tolerance, personality and aspirations, and psychosocial-economic context. Among the disease features are the balance between insulin deficiency and insulin insensitivity, fasting versus postprandial hyperglycemia, short versus long disease duration, and special circumstances such as maturity-onset diabetes of the young (MODY) or latent autoimmune diabetes in adulthood (LADA).
However, we are faced with a paucity of data on how patients with certain characteristics respond to specific therapies (56). We know that most glucose-lowering drugs for type 2 diabetes work in most patients. But we also know that there are nonresponders to any drug. Numerous post hoc studies have revealed some predictors of better responses, but the data are inconclusive (57–60). Furthermore, those response differences tend to be small, and the strongest predictor remains baseline A1C, with the patients with higher A1C levels responding with greater reductions although not necessarily attaining target levels (58,61).
Indeed, the most fruitful phenotypic considerations for personalizing care today may be patients’ propensity for side effects and tolerance of various medicines. There may be practical value to using a trial-and-error, or “n of 1,” approach (62) based on the anticipation of a drug’s efficacy (for example, “Pioglitazone will be highly effective in this very insulin-resistant patient”), a patient’s need for certain added benefits (“A GLP-1 receptor agonist will help control hyperglycemia and may encourage weight loss in this obese patient”), and concerns about adverse events (“I will not prescribe a sulfonylurea for this elderly patient who lives alone and had a severe hypoglycemic episode a few years ago”). This is becoming standard clinical procedure for diabetes, just as it is for hypertension and numerous other chronic diseases.
The challenge is how to proceed in more complex situations. How, for example, would one select an appropriate pharmacological regimen for a 68-year-old man with diabetes of 14 years’ duration who has coronary disease, obstructive sleep apnea, prostate cancer, and a history of possible pancreatitis; who is obese and has edema but no heart failure; who smokes and has a family history of bladder cancer; who has high fasting blood glucose and A1C levels; and who has some renal dysfunction and poorly controlled lipids? With so many competing comorbidities, what are this individual's targets and treatment options?
Ultimately, clinicians must develop highly personalized care regimens, and, in the absence of other conclusive evidence, “n of 1” trials may prove to be the best approach, providing strong evidence of therapy effectiveness and safety at the individual level and incorporating shared decision making with patients.
ARE ADEQUATE THERAPEUTIC TOOLS AVAILABLE NOW FOR PERSONALIZED DIABETES CARE?
Multiple glucose-lowering medication classes: freedom or confusion?
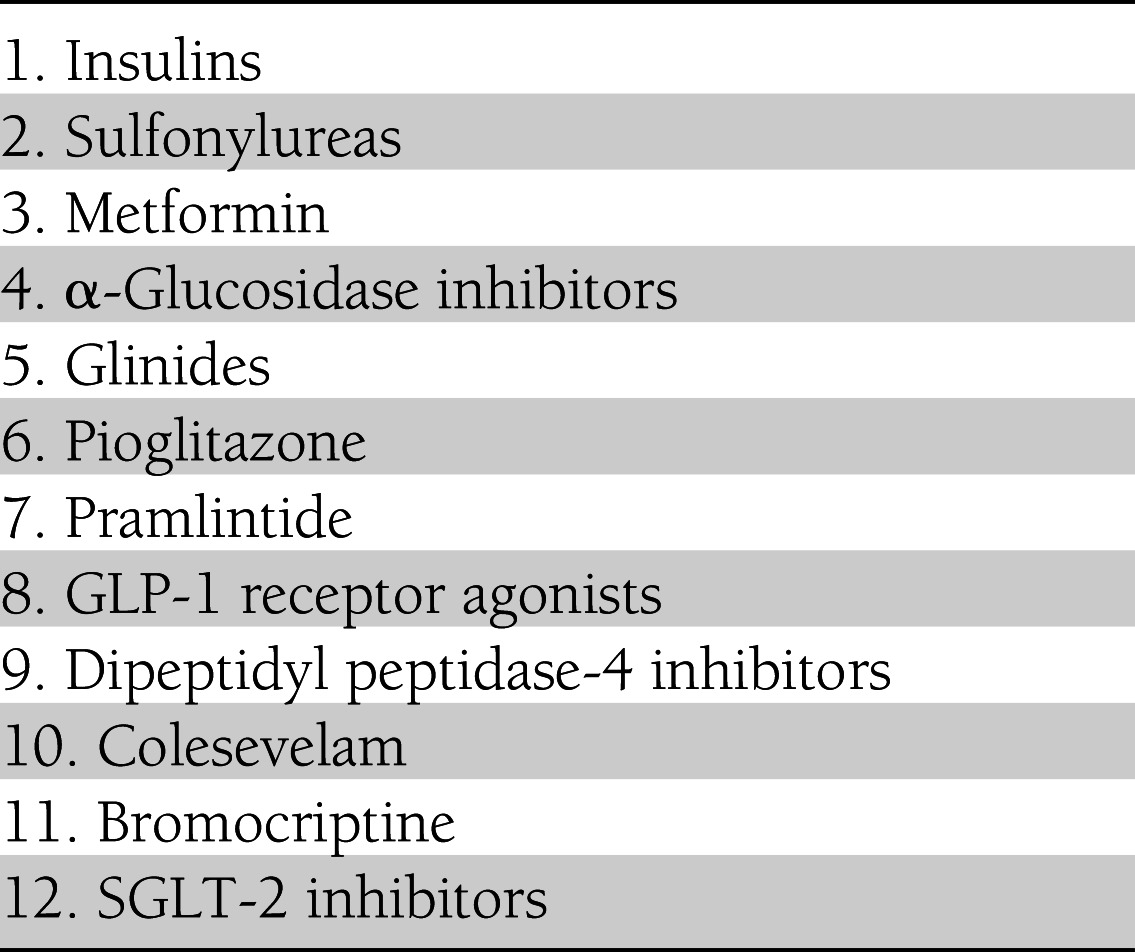
We now have numerous classes of antihyperglycemic therapies (Table 2) and more are expected to be licensed. Does this extensive arsenal provide us with more flexibility in designing personalized diabetes regimens, or does it make the task more difficult by multiplying the options? For specialists, the answer is no doubt the former. But for many primary care providers who must simultaneously stay abreast of developments in numerous fields of medicine, the expanding array of choices may, at times, seem intimidating.
Table 2.
Classes of antihyperglycemic agents
Recent meta-analyses have shown that there is not much difference among available therapies in glycemic control (e.g., A1C reduction and likelihood of achieving targets when adding an agent to metformin). However, when one considers other benefits, such as the risk of hypoglycemia and effects on body weight (63,64), there appears to be separation among the agents. In addition to these agents’ relative glycemic efficacy and effects on body weight and hypoglycemia, HCPs immersed in diabetes care must balance the potential benefits of each agent against concerns that have been raised regarding possible associations between various agents and the risk of developing other diseases (65–67).
Difficulties in making benefit-risk judgments are further amplified by the fact that marketing may seek to create demand for drugs that is out of proportion to their efficacy. In addition, there remains a general lack of adequate comparative and exploratory controlled trials between the medications available, not to mention a lack of research into phenotype- and pathophysiology-based regimens.
Developing a straightforward algorithm that narrows the field of viable options will clearly require more evidence than is currently available. Without such evidence, we can offer only opinion, albeit opinion based on an understanding of pathophysiology, epidemiology, pharmacodynamics, toxicology, and costs. Unfortunately, the studies needed to make evidence-based treatment decisions—those that involve comparisons among multiple agents and are adequately powered for important, long-term clinical outcomes—have, for the most part, not been performed.
The upcoming GRADE (Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study) trial will address some of these points (68). In addition, studies on how best to combine the various agents, as well as the optimal timing (early combination therapy vs. the traditional step-wise approach), are urgently needed.
Furthermore, even the most carefully considered set of guidelines is based on averages—average A1C-lowering effect, average efficacy, average risk of adverse effects—without adequate consideration of the confidence intervals around those averages. Averages fail to identify subpopulations that respond better and have better tolerance to specific agents, and without these data, evidence-based personalized advice cannot be provided. For now, all HCPs, whether in specialty or primary care settings, should test the efficacy and weigh the safety risks of any given drug in each patient, ideally trying options over a period of months to see how well they work at the individual level.
How will new and emerging therapies enhance our ability to personalize care?
To complicate future decision making, there are many new therapies in the research and development pipeline, including newer and longer-acting injectable incretin-based drugs, newer basal insulins, oral sodium-glucose cotransporter-2 (SGLT-2) inhibitors, agents targeting the various peroxisome proliferator–activated receptors, and free fatty acid receptor agonists.
It is hoped that pharmaceutical companies developing new glucose-lowering agents will focus on providing some added value beyond what is already available by addressing unmet clinical needs such as the effects leading to a reduction in CVD risk factors and meaningful cardiovascular and other outcomes. Arguably, we lack what we seek most in a diabetes treatment: definitive demonstration that an agent can safely lower A1C in a sustained and durable manner by definitively modifying disease progression, does so with minimal side effects (e.g., hypoglycemia), favorably improves CVD risk factors (e.g., weight, lipids, and blood pressure), and reduces cardiovascular and other morbidity and mortality.
As new drugs continue to be developed and submitted to regulatory agencies for approval, we should also consider the limitations of RCTs for informing a personalized approach to diabetes care (69,70). RCTs, at least as currently carried out, focus on selected populations and have restricted inclusion and exclusion criteria. They are generally of short duration, making it impossible to assess durability. They do not test individual responder rates and are not designed to identify responders who have a low safety risk. These trials are conducted in artificial environments, which pose problems for realistically measuring adherence. Finally, RCTs are not powered to assess subpopulations prospectively. Thus, efforts to personalize therapy are hindered by our reliance on trials that may be neither generalizable to the larger population nor individualized to specific patients.
Moving forward, there may be other informative data from these trials, not from the average results, but rather from outliers—the results from subjects who respond very well or not at all.
REGIONAL PERSPECTIVES ON PERSONALIZED MEDICINE
The questions, concerns, and practical considerations discussed here pose difficult challenges for diabetes HCPs throughout the world. Because diabetes is a burgeoning pandemic, it behooves us to understand the issues from an international perspective.
The viewpoint that personalized diabetes care may be too complex to be implemented in many care settings is common in Europe, as it is in the United States and elsewhere. In Italy, for example, the Renal Insufficiency And Cardiovascular Events (RIACE) multicenter study, which included 15,773 patients with type 2 diabetes attending hospital-based diabetes clinics, showed that 40% of patients were taking metformin, 15% were managed through diet only, 24% were on insulin, 18% were taking sulfonylureas, and 3% were taking thiazolidinediones (71). Strikingly, this pattern did not change with age or with renal function, duration of disease, or other stratifying criteria.
The story is much the same in other parts of the world, although patient characteristics differ. In China, key issues include rapid nutritional and lifestyle transitions, large patient populations, young age of onset, and heterogeneous phenotypes characterized by β-cell dysfunction, insulin resistance, and visceral obesity (72,73). High rates of kidney disease and diabetes-related cancer complicate diabetes care (72,74,75). All of these problems are compounded by a relative scarcity of research, low levels of awareness, an insufficient number of trained HCPs, and less-organized health care and financing systems.
Given the large population and finite resources, one may argue for using risk algorithms and biomarkers, including genetic variants, to identify high-risk subjects for early or intensified intervention, although the cost-effectiveness of such an approach will need to be formally tested. As elsewhere, patients with insulin-resistant features such as fatty liver, high triglycerides, and low HDL cholesterol may benefit from initial treatment with metformin, pioglitazone, and GLP-1 receptor agonists, whereas patients who are lean and face a long disease duration may benefit from dipeptidyl peptidase-4 (DPP-4) inhibitors or sulfonylureas with the early use of insulin. Other drugs such as α-glucosidase inhibitors and SGLT-2 inhibitors may help to lower A1C with a low risk of hypoglycemia and weight gain.
Although these phenotype-based therapies have a theoretical basis, clinical practice studies are needed to confirm their cost-effectiveness. There is also a need to empower medical and nonmedical personnel (diabetes educators) in clinics to collect patient data on demographics, risk factors, complications, social habits, emotional needs, self-care behaviors, compliance, expectations, and values to enable HCPs to personalize treatment goals, self-management strategies, and therapy regimens (76). These personnel should monitor patients’ adherence to treatment, as well as their achievement of treatment goals.
In the United States, attempts to implement a concept as expansive as personalized care quickly run up against two opposing traditions that permeate not only the field of medicine, but indeed the entire U.S. culture. The first, rooted in American industrialism, is standardization, exemplified by the processes of production line efficiency and continuous quality improvement. One recognizes this tradition in the vision of industrialist Henry J. Kaiser, who founded the prototype nonprofit health system Kaiser Permanente (77). The second tradition, embodied by the image of artist Norman Rockwell’s humble country doctor, is personalization. This is apparent in the teachings of Dr. Francis W. Peabody, whose seminal dissertation on patient care concluded, “The secret of care of the patient is caring for the patient,” (78) and in the work of Dr. Elliott P. Joslin, who wrote that “ . . . unless the physician takes care, he will fall into schematic ways and forget that it is the patient who comes for treatment and not the diabetes. Each is a case unto itself” (79).
Recent guidelines for diabetes care in the United States have fallen somewhere along a continuum between these traditions. The ADA Standards of Care (80) have sought to straddle the line, whereas the algorithm-based 2009 ADA/EASD consensus statement (2) leaned more toward standardization, and the 2012 ADA/EASD position statement (1) evolved more toward personalized care.
ENHANCING PERSONALIZED CARE THROUGH COMANAGEMENT
Research has yielded strong evidence in favor of fairly standardized treatment goals and an algorithmic initial therapy pathway involving lifestyle modification, metformin, and the eventual addition of other oral agents (sulfonylureas and basal insulin, in most cases). This approach allows many newly diagnosed patients to attain a reasonable blood glucose range and to maintain it for some period of time.
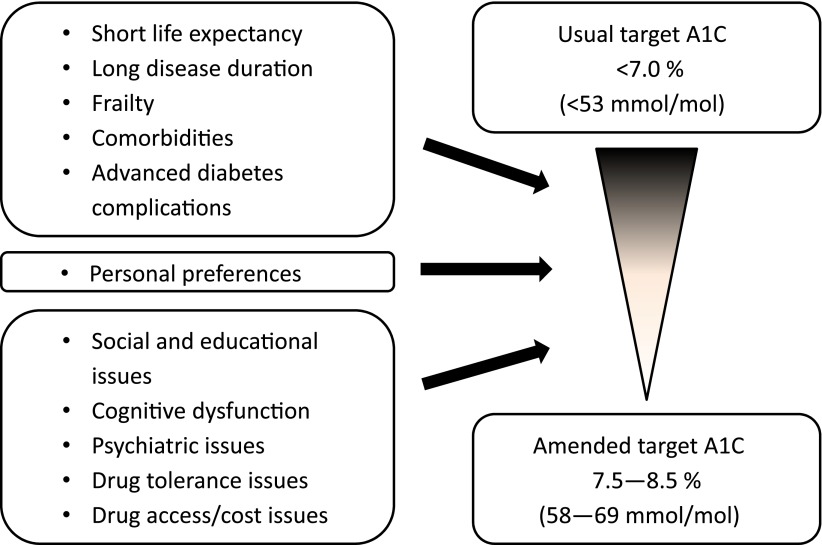
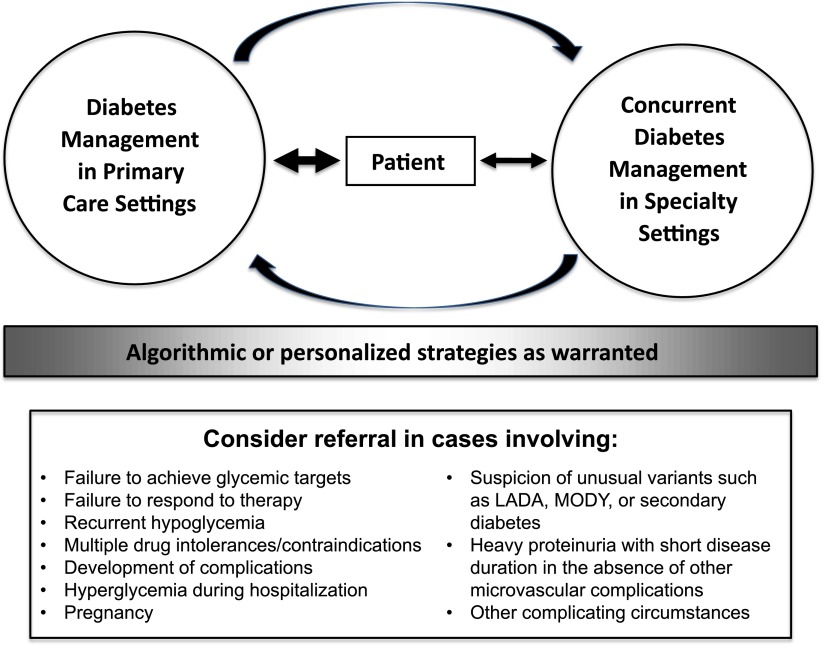
However, there will always be patients for whom the standard A1C target is not appropriate (Fig. 1). Likewise, patients’ clinical circumstances often become more complicated over time, at which point the core treatment algorithm must give way to a more personalized approach. In such situations, the ideal course of action would be a patient-centered comanagement approach involving primary and specialty care providers as well as diabetes educators, dietitians, psychologists, and other HCPs as warranted by individual patient needs. Figure 2 depicts such an approach, which could be invoked by specific triggers such as failure to respond to treatment (14,81), failure to attain A1C targets, drug intolerances or contraindications, severe hypoglycemia, hyperglycemia during hospitalization, pregnancy, suspicion of unusual variants such as LADA, MODY, or secondary diabetes, heavy proteinuria with short disease duration in the absence of other microvascular complications, or other complicating circumstances.
Figure 1.
Personalizing A1C targets for individuals with type 2 diabetes.
Figure 2.
A comanagement approach to personalized therapy for type 2 diabetes. The majority of patient care occurs in primary care settings with concurrent comanagement in specialty settings as warranted for individual patients. In such a model, the patient remains at the center of care, comanaging HCPs all provide algorithmic or personalized care as warranted, and communication occurs among all parties.
Regardless of the final form such a process takes, it seems clear that personalizing diabetes care will require improved cooperation and comanagement of patients among HCPs in various disciplines. In such a paradigm, algorithmic care would be both a useful starting place for most patients with type 2 diabetes and a framework on which to build more personalized therapy as needed.
CONCLUSIONS
Publication of the latest ADA/EASD position statement on type 2 diabetes management has generated strong interest in the concept of a personalized medical approach for individuals with diabetes (1). However, there are a multitude of pharmacological antihyperglycemic therapies now available, often with incomplete evidence concerning their long-term efficacy, effectiveness, tolerability, and safety. Accordingly, questions remain regarding the best ways to implement the recommendations of the position statement in the care of patients.
Emerging research in genetics, pathophysiology, metabolomics, and human behavior, as well as longer-term, randomized comparative trials could eventually yield new information to inform the personalization of care. In the meantime, we must develop tools to translate existing guidelines into practical clinical applications, and, more importantly, to develop processes that encourage the organized comanagement of patients by primary care providers, specialists, educators, dietitians, and other diabetes HCPs as patients’ unique needs and risks require. Another consideration is how well the tools we develop can be implemented around the globe given the differences in pathophysiology among ethnic groups, country-specific resources and medical care infrastructure, training level of providers, and knowledge of patients.
We hope these reflections have provided a broad overview of the evidence deficits and procedural challenges that will need to be overcome to ensure success in our efforts to implement effective, personalized therapy regimens for patients with type 2 diabetes.
Acknowledgments
I.R. has served on the advisory boards of AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Merck (MSD), and Novo Nordisk; as a consultant for Andromeda, AstraZeneca/BMS, Eli Lilly, HealOr, Insuline, Johnson & Johnson, Teva, and TransPharma; and as a member of the speaker’s bureau for AstraZeneca, Eli Lilly, Johnson & Johnson, Novo Nordisk, and Roche.
M.C.R. has received honoraria for consulting and/or research grant support through his institution from Amylin, Elcelyx, Eli Lilly, Sanofi, and Valeritas; these potential conflicts of interest have been reviewed and managed by Oregon Health and Science University.
J.R. has received grants or research support from Amylin, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, GlaxoSmithKline, Johnson & Johnson, Lexicon, MannKind, Merck, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi, and Takeda and has served on advisory boards for and received honoraria or consulting fees from Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, GlaxoSmithKline, Johnson & Johnson, Lexicon, MannKind, Novo Nordisk, Sanofi, and Takeda.
J.B.B. is an investigator and/or consultant without direct financial benefit under contracts between his employer and the following companies: Abbott, Amylin, Andromeda, AstraZeneca, Bayhill Therapeutics, BD Research Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Catabasis, Cebix, Diartis, Elcelyx, Eli Lilly, Exsulin, Genentech, GI Dynamics, GlaxoSmithKline, Halozyme, Hoffman-La Roche, Johnson & Johnson, LipoScience, Medtronic, Merck, Metabolic Solutions Development Company, Metabolon, Novan, Novella, Novartis, Novo Nordisk, Orexigen, Osiris, Pfizer, Rhythm, Sanofi, Spherix, Takeda, Tolerex, TransPharma, Veritas, and Verva.
S.E.I. has served as a consultant for Boehringer Ingelheim, Janssen, Merck, Novo Nordisk, and Takeda.
P.D.H. has received (or institutions with which he is associated have received) funding for his educational, advisory, and research activities from AstraZeneca/BMS Collaboration, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Janssen/Johnson & Johnson, Merck (MSD), Merck Serono, Novo Nordisk, Roche Diagnostics, Roche Pharmaceuticals, Sanofi, and Takeda.
S.D.P. has served as a consultant for AstraZeneca/BMS Collaboration, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Intarcia Therapeutics, Janssen/Johnson & Johnson, Merck (MSD), Merck Serono, Novartis, Novo Nordisk, Roche Pharmaceuticals, Sanofi, and Takeda and has received research support from Bristol-Myers Squibb, Merck (MSD), Novartis, Novo Nordisk, and Takeda.
E.F. has received honoraria for consulting and/or research grant support from AstraZeneca/BMS Collaboration, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, GlaxoSmithKline, Halozyme Therapeutics, Janssen/Johnson & Johnson, Merck (MSD), and Sanofi.
J.C.N.C. is a board member of the Asia Diabetes Foundation. She is a consultant for AstraZeneca, Bristol-Myers Squibb, Daiichi-Sankyo, GlaxoSmithKline, Merck (MSD), Pfizer, Qualigenics, and Sanofi. She has received honoraria, travel expenses, and/or payments for development of educational presentations from AstraZeneca, Bayer, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, GlaxoSmithKline, Merck Serono, Merck (MSD), Nestle Nutrition Institute, Novo Nordisk, Pfizer, Roche, Sanofi, and Takeda. Her institution, the Chinese University of Hong Kong, has received research grants from pharmaceutical companies for conducting clinical trials of drugs for individuals with diabetes and associated conditions.
L.A.L. has received research funding from, has provided continuing medical education on behalf of, and/or has acted as a consultant to AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Janssen, Merck, Novartis, Novo Nordisk, Roche, Sanofi, Servier, and Takeda.
D.L. is a consultant for AstraZeneca, Bristol-Myers Squibb, Janssen, Merck, and Sanofi.
R.D. serves on advisory boards or is a consultant for Amylin, Boehringer Ingelheim, Bristol-Myers Squibb, Lexicon, Novo Nordisk, and Takeda; receives grants from Amylin, Boehringer Ingelheim (pending), Bristol-Myers Squibb, and Takeda; and is a member of the speaker’s bureau for Novo Nordisk.
W.T.C. has served as a consultant for AstraZeneca, Bristol-Myers Squibb, Halozyme Therapeutics, Intarcia Therapeutics, Johnson & Johnson, Lexicon, and Sanofi and has served as a principal investigator on research studies awarded to his institution from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Johnson & Johnson, Lexicon, and MannKind.
Writing and editing support services for this article were provided by Debbie Kendall of Kendall Editorial in Richmond, Virginia.
This article contains no data or data analysis and therefore there is no guarantor of these. All authors contributed to the thinking behind and the writing of the manuscript.
Footnotes
A slide set summarizing this article is available online.
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35:1364–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association. European Association for the Study of Diabetes Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2009;52:17–30 [DOI] [PubMed] [Google Scholar]
- 3.Bergenstal RM, Bailey CJ, Kendall DM. Type 2 diabetes: assessing the relative risks and benefits of glucose-lowering medications. Am J Med 2010;123:374.e9–374.e18 [DOI] [PubMed]
- 4.International Diabetes Foundation Clinical Guidelines Task Force Global Guideline for Type 2 Diabetes. Brussels, International Diabetes Federation, 2012. Available from http://www.idf.org/global-guideline-type-2-diabetes-2012 Accessed 14 December 2012
- 5.Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 2009;15:540–559 [DOI] [PubMed] [Google Scholar]
- 6.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Canadian Journal of Diabetes 2008;32(Suppl. 1):S1–S201 [DOI] [PubMed] [Google Scholar]
- 7.National Institute for Health and Clinical Excellence Type 2 Diabetes: The Management of Type 2 Diabetes: NICE Clinical Guideline 87. London, National Institute for Health and Clinical Excellence, 2009 [Google Scholar]
- 8.Home P, Mant J, Diaz J, Turner C, Guideline Development Group Management of type 2 diabetes: summary of updated NICE guidance. BMJ 2008;336:1306–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 10.Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 11.Duckworth W, Abraira C, Moritz T, et al. VADT Investigators Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 12.Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Control Group. Turnbull FM, Abraira C, Anderson RJ, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009;52:2288–2298 [DOI] [PubMed] [Google Scholar]
- 14.Riddle MC, Ambrosius WT, Brillon DJ, et al. Action to Control Cardiovascular Risk in Diabetes Investigators Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care 2010;33:983–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zoungas S, Patel A, Chalmers J, et al. ADVANCE Collaborative Group Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010;363:1410–1418 [DOI] [PubMed] [Google Scholar]
- 16.Skyler JS, Bergenstal R, Bonow RO, et al. American Diabetes Association. American College of Cardiology Foundation. American Heart Association Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009;32:187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cook MN, Girman CJ, Stein PP, Alexander CM. Initial monotherapy with either metformin or sulphonylureas often fails to achieve or maintain current glycaemic goals in patients with type 2 diabetes in UK primary care. Diabet Med 2007;24:350–358 [DOI] [PubMed] [Google Scholar]
- 18.Gerstein HC, Bosch J, Dagenais GR, et al. ORIGIN Trial Investigators Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012;367:319–328 [DOI] [PubMed] [Google Scholar]
- 19.Karter AJ, Moffet HH, Liu J, et al. Glycemic response to newly initiated diabetes therapies. Am J Manag Care 2007;13:598–606 [PMC free article] [PubMed] [Google Scholar]
- 20.Pozzilli P, Leslie RD, Chan J, et al. The A1C and ABCD of glycaemia management in type 2 diabetes: a physician’s personalized approach. Diabetes Metab Res Rev 2010;26:239–244 [DOI] [PubMed] [Google Scholar]
- 21.Del Prato S, LaSalle J, Matthaei S, Bailey CJ, Global Partnership for Effective Diabetes Management Tailoring treatment to the individual in type 2 diabetes practical guidance from the Global Partnership for Effective Diabetes Management. Int J Clin Pract 2010;64:295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ismail-Beigi F, Moghissi E, Tiktin M, Hirsch IB, Inzucchi SE, Genuth S. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med 2011;154:554–559 [DOI] [PubMed] [Google Scholar]
- 23.Wu JY, Leung WY, Chang S, et al. Effectiveness of telephone counselling by a pharmacist in reducing mortality in patients receiving polypharmacy: randomised controlled trial. BMJ 2006;333:522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher EB, Chan JCN, Nan H, Sartorius N, Oldenburg B. Co-occurrence of diabetes and depression: conceptual considerations for an emerging global health challenge. J Affect Disord 2012;142(Suppl):.S56–S66 [DOI] [PubMed] [Google Scholar]
- 25.Greeley SA, John PM, Winn AN, et al. The cost-effectiveness of personalized genetic medicine: the case of genetic testing in neonatal diabetes. Diabetes Care 2011;34:622–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shu Y, Sheardown SA, Brown C, et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin Invest 2007;117:1422–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shu Y, Leabman MK, Feng B, et al. Pharmacogenetics Of Membrane Transporters Investigators Evolutionary conservation predicts function of variants of the human organic cation transporter, OCT1. Proc Natl Acad Sci USA 2003;100:5902–5907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schäfer SA, Müssig K, Staiger H, et al. A common genetic variant in WFS1 determines impaired glucagon-like peptide-1-induced insulin secretion. Diabetologia 2009;52:1075–1082 [DOI] [PubMed] [Google Scholar]
- 29.Müssig K, Staiger H, Machicao F, et al. Association of type 2 diabetes candidate polymorphisms in KCNQ1 with incretin and insulin secretion. Diabetes 2009;58:1715–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smushkin G, Sathananthan M, Sathananthan A, et al. Diabetes-associated common genetic variation and its association with GLP-1 concentrations and response to exogenous GLP-1. Diabetes 2012;61:1082–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen R, Mias GI, Li-Pook-Than J, et al. Personal omics profiling reveals dynamic molecular and medical phenotypes. Cell 2012;148:1293–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 1988;37:667–687 [DOI] [PubMed] [Google Scholar]
- 33.DeFronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 2005;90:493–500 [DOI] [PubMed] [Google Scholar]
- 35.Saad MF, Knowler WC, Pettitt DJ, Nelson RG, Mott DM, Bennett PH. The natural history of impaired glucose tolerance in the Pima Indians. N Engl J Med 1988;319:1500–1506 [DOI] [PubMed] [Google Scholar]
- 36.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 2006;55:1430–1435 [DOI] [PubMed] [Google Scholar]
- 37.Abdul-Ghani MA, Matsuda M, Sabbah M, et al. The relative contributions of insulin resistance and beta cell failure to the transition from normal to impaired glucose tolerance varies in different ethnic groups. Diabetes Metab Syndr 2007;1:105–112 [Google Scholar]
- 38.Bunck MC, Cornér A, Eliasson B, et al. Effects of exenatide on measures of β-cell function after 3 years in metformin-treated patients with type 2 diabetes. Diabetes Care 2011;34:2041–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Thiazolidinediones improve beta-cell function in type 2 diabetic patients. Am J Physiol Endocrinol Metab 2007;292:E871–E883 [DOI] [PubMed] [Google Scholar]
- 40.Cusi K, Consoli A, DeFronzo RA. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1996;81:4059–4067 [DOI] [PubMed] [Google Scholar]
- 41.Gastaldelli A, Miyazaki Y, Mahankali A, et al. The effect of pioglitazone on the liver: role of adiponectin. Diabetes Care 2006;29:2275–2281 [DOI] [PubMed] [Google Scholar]
- 42.Bajaj M, Baig R, Suraamornkul S, et al. Effects of pioglitazone on intramyocellular fat metabolism in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2010;95:1916–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008;24:275–286 [DOI] [PubMed] [Google Scholar]
- 44.DeFronzo RA, Tripathy D, Schwenke DC, et al. ACT NOW Study Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011;364:1104–1115 [DOI] [PubMed] [Google Scholar]
- 45.Kahn SE, Haffner SM, Heise MA, et al. ADOPT Study Group Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 46.Bays H, Mandarino L, DeFronzo RA. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J Clin Endocrinol Metab 2004;89:463–478 [DOI] [PubMed] [Google Scholar]
- 47.Unger RH, Aguilar-Parada E, Müller WA, Eisentraut AM. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest 1970;49:837–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsuda M, DeFronzo RA, Glass L, et al. Glucagon dose-response curve for hepatic glucose production and glucose disposal in type 2 diabetic patients and normal individuals. Metabolism 2002;51:1111–1119 [DOI] [PubMed] [Google Scholar]
- 49.Nauck MA, Vardarli I, Deacon CF, Holst JJ, Meier JJ. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: what is up, what is down? Diabetologia 2011;54:10–18 [DOI] [PubMed] [Google Scholar]
- 50.Abdul-Ghani MA, Norton L, DeFronzo RA. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev 2011;32:515–531 [DOI] [PubMed] [Google Scholar]
- 51.Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 2009;15:540–559 [DOI] [PubMed] [Google Scholar]
- 52.Harrison LB, Adams-Huet B, Raskin P, Lingvay I. β-Cell function preservation after 3.5 years of intensive diabetes therapy. Diabetes Care 2012;35:1406–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dormandy JA, Charbonnel B, Eckland DJ, et al. PROactive investigators Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005;366:1279–1289 [DOI] [PubMed] [Google Scholar]
- 54.Nissen SE, Wolski K. Rosiglitazone revisited: an updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch Intern Med 2010;170:1191–1201 [DOI] [PubMed] [Google Scholar]
- 55.Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care 2011;34:916–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith RJ, Nathan DM, Arslanian SA, Groop L, Rizza RA, Rotter JI. Individualizing therapies in type 2 diabetes mellitus based on patient characteristics: what we know and what we need to know. J Clin Endocrinol Metab 2010;95:1566–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Charpentier G, Vaur L, Halimi S, et al. DIAMETRE Predictors of response to glimepiride in patients with type 2 diabetes mellitus. Diabetes Metab 2001;27:563–571 [PubMed] [Google Scholar]
- 58.Bloomgarden ZT, Dodis R, Viscoli CM, Holmboe ES, Inzucchi SE. Lower baseline glycemia reduces apparent oral agent glucose-lowering efficacy: a meta-regression analysis. Diabetes Care 2006;29:2137–2139 [DOI] [PubMed] [Google Scholar]
- 59.Nichols GA, Alexander CM, Girman CJ, Kamal-Bahl SJ, Brown JB. Treatment escalation and rise in HbA1c following successful initial metformin therapy. Diabetes Care 2006;29:504–509 [DOI] [PubMed] [Google Scholar]
- 60.Tomioka S, Ogata H, Tamura Y, et al. Clinical characteristics influencing the effectiveness of metformin on Japanese type 2 diabetes receiving sulfonylureas. Endocr J 2007;54:247–253 [DOI] [PubMed] [Google Scholar]
- 61.Topp BG, Waters SB, Alexander CM. Differences in reported efficacy between oral anti-hyperglycemic agents largely reflect differences in baseline A1C. Poster presented at the 68th Scientific Sessions of the American Diabetes Association, 6–10 June 2008, at the Moscone Convention Center, San Francisco, California [Google Scholar]
- 62.Tsapas A, Matthews DR. N of 1 trials in diabetes: making individual therapeutic decisions. Diabetologia 2008;51:921–925 [DOI] [PubMed] [Google Scholar]
- 63.Phung OJ, Scholle JM, Talwar M, Coleman CI. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA 2010;303:1410–1418 [DOI] [PubMed] [Google Scholar]
- 64.Liu SC, Tu YK, Chien MN, Chien KL. Effect of antidiabetic agents added to metformin on glycaemic control, hypoglycaemia and weight change in patients with type 2 diabetes: a network meta-analysis. Diabetes Obes Metab 2012;14:810–820 [DOI] [PubMed] [Google Scholar]
- 65.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care 2010;33:1674–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Noel RA, Braun DK, Patterson RE, Bloomgren GL. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care 2009;32:834–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 2011;141:150–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.George Washington University Biostatistics Center The GRADE Study. Available from http://www2.bsc.gwu.edu/bsc/webpage.php?no=27 Accessed 14 December 2012
- 69.Woodcock J. The prospects for “personalized medicine” in drug development and drug therapy. Clin Pharmacol Ther 2007;81:164–169 [DOI] [PubMed] [Google Scholar]
- 70.Brown PM. Personalized medicine and comparative effectiveness research in an era of fixed budgets. EPMA J 2010;1:633–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Solini A, Penno G, Bonora E, et al. Renal Insufficiency And Cardiovascular Events (RIACE) Study Group Diverging association of reduced glomerular filtration rate and albuminuria with coronary and noncoronary events in patients with type 2 diabetes: the renal insufficiency and cardiovascular events (RIACE) Italian multicenter study. Diabetes Care 2012;35:143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 2009;301:2129–2140 [DOI] [PubMed] [Google Scholar]
- 73.Yoon KH, Lee JH, Kim JW, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet 2006;368:1681–1688 [DOI] [PubMed] [Google Scholar]
- 74.Yang XL, Ma RC, Chan JC. Meta-analysis of trial data may support a causal role of hyperglycaemia in cancer. Diabetologia 2011;54:709–710; author reply 711–712 [DOI] [PubMed] [Google Scholar]
- 75.Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese type II diabetic patients. Diabetologia 2002;45:85–96 [DOI] [PubMed] [Google Scholar]
- 76.So WY, Raboca J, Sobrepena L, et al. JADE Program Research Team Comprehensive risk assessments of diabetic patients from seven Asian countries: The Joint Asia Diabetes Evaluation (JADE) program. J Diabetes 2011;3:109–118 [DOI] [PubMed] [Google Scholar]
- 77.Kaiser Permanente Kaiser Permanente—more than 60 years of quality [article online]. Available from http://xnet.kp.org/newscenter/aboutkp/historyofkp.html Accessed 8 October 2012
- 78.Peabody FW. Landmark article March 19, 1927: The care of the patient. By Francis W. Peabody. JAMA 1984;252:813–818 [DOI] [PubMed] [Google Scholar]
- 79.Joslin EP. The Treatment of Diabetes Mellitus. 4th ed. Philadelphia, Lea & Febiger, 1928, p. 557 [Google Scholar]
- 80.American Diabetes Association Standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl. 1):S11–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Riddle MC, Karl DM. Individualizing targets and tactics for high-risk patients with type 2 diabetes: practical lessons from ACCORD and other cardiovascular trials. Diabetes Care 2012;35:2100–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]