In vitro anti-myeloma activity of the Aurora kinase inhibitor VE-465 (original) (raw)
. Author manuscript; available in PMC: 2013 Jun 4.
Abstract
This study characterized the preclinical anti-myeloma activity of VE465, a low molecular weight pan-Aurora kinase inhibitor. After 96-h drug exposure, several multiple myeloma (MM) cell lines were more sensitive to VE465 compared to non-malignant cells. The anti-MM activity of VE465 was maintained in the presence of interleukin-6 and, interestingly, enhanced by co-culture with stromal cells. However, primary MM cells were less responsive than cell lines. Combinations with dexamethasone (Dex), doxorubicin (Doxo), and bortezomib showed no antagonism. Our study highlights the potential role of the tumor micro-environment in modulating the activity of this drug class.
Introduction
The introduction of bortezomib (Richardson, et al 2005), thalidomide (Singhal, et al 1999) and lenalidomide (Richardson, et al 2006, Weber, et al 2007) into the therapeutic armamentarium for multiple myeloma (MM) has changed the management of this disease. Unfortunately, even patients who achieve prolonged remissions with these novel agents and their combinations eventually relapse, highlighting the importance of identifying additional therapeutic agents. The proliferation indices of MM cells tend to increase with disease progression. Therefore, Aurora kinase inhibitors, which target mechanisms regulating proliferation, may be active, especially in the setting of advanced disease.
Aurora kinases are a family of serine/threonine kinases, with an essential role in mitosis. Aurora kinase A (AURKA) is primarily involved in centrosome regulation and mitotic spindle formation, while Aurora kinase B (AURKB) acts to insure chromosome segregation and cytokinesis, and Aurora C plays a role similar to B, but is largely confined to mammalian testis (Carvajal, et al 2006). Aurora kinases have been implicated in a broad array of malignancies including colorectal, ovarian, and pancreatic cancers (Bischoff, et al 1998, Gritsko, et al 2003, Li, et al 2003). These functional studies, along with the documented pre-clinical activity of Aurora kinase inhibitors, such as Hesparadin, ZM447439, MK0457, and PHA-739358 (Carvajal, et al 2006), suggest that Aurora kinases represent legitimate targets.
Our previous studies have shown that histone deacetylase inhibition in MM suppresses Aurora kinase expression, suggesting that direct inhibition of Auroras with small molecule inhibitors may be beneficial for MM therapy (Mitsiades, et al 2004). We therefore characterized the anti-MM activity of VE465, a small molecule inhibitor of Aurora kinases A, B, & C.
Materials and Methods
Cell lines and Primary Samples
All human MM cell lines, primary MM patient cells, and HS-5 stroma were cultured as previously described (Mitsiades, et al 2001). Recombinant human Interleukin-6 (IL-6; R&D Systems; Minneapolis, MN) was added at 10ng/ml to cultures of MM patient cells. Primary MM cells from bone marrow (BM) aspirates of MM patients were acquired in accordance with an Institutional Review Board-approved protocol and processed as previously described (Mitsiades, et al 2001). VE465 was provided by Vertex Pharmaceuticals (Cambridge, MA) and Merck & Co (Boston, MA), dissolved in dimethyl sulfoxide (DMSO; Sigma, St. Louis, MO) and diluted in culture medium to concentrations stated in figures.
Cell Viability Assays
To determine activity of VE465 against MM cell lines, in the presence and absence of IL-6 (10 ng/mL), and in cell death commitment assays, cell viability was assessed by (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, as previously described (Mitsiades, et al 2001). For cell death commitment assays, MM.1S were treated with 0.5µM VE465 for 24–96 h, washed, and re-plated in drug-free media for an additional 72 h, and then viability was assessed by MTT assay. For primary MM cells, 10,000 cells per well were treated for 96 h and viability assessed by CellTiterGlo (Promega; Madison WI). For co-culture experiments, MM cells expressing a luciferase vector were cultured in the presence or absence of HS-5 cells in 96-well optical bottom tissue culture plates (Nunc, Rochester, NY). After 96 h of exposure to VE465, bioluminescence was measured using a Luminoskan Ascent Luminometer with Ascent Software (Labsystems, Finland), as previously described (McMillin, et al 2007). For cell cycle analysis, MM cells were cultured in the presence of VE465 or DMSO control for 8–96 h, washed with phosphate-buffered saline, fixed in 70% ethanol, and stained with solution of propidium iodide/RNase (Sigma) for 1 h. Cell cycle analysis was performed using an Epics flow cytometer (Beckmann-Coulter) and analyzed using FlowJo software (Treestar).
Statistics
The half maximal inhibitory concentration (IC50) values for VE465 treatments were calculated using online software (http://www.changbioscience.com/stat/ec50.html) and model a exp(−b x) + c.
Full information of materials and methods is available in the legends to Figures S1–S5.
Results & Discussion
In vitro activity of VE465 against MM cells and non-malignant cells
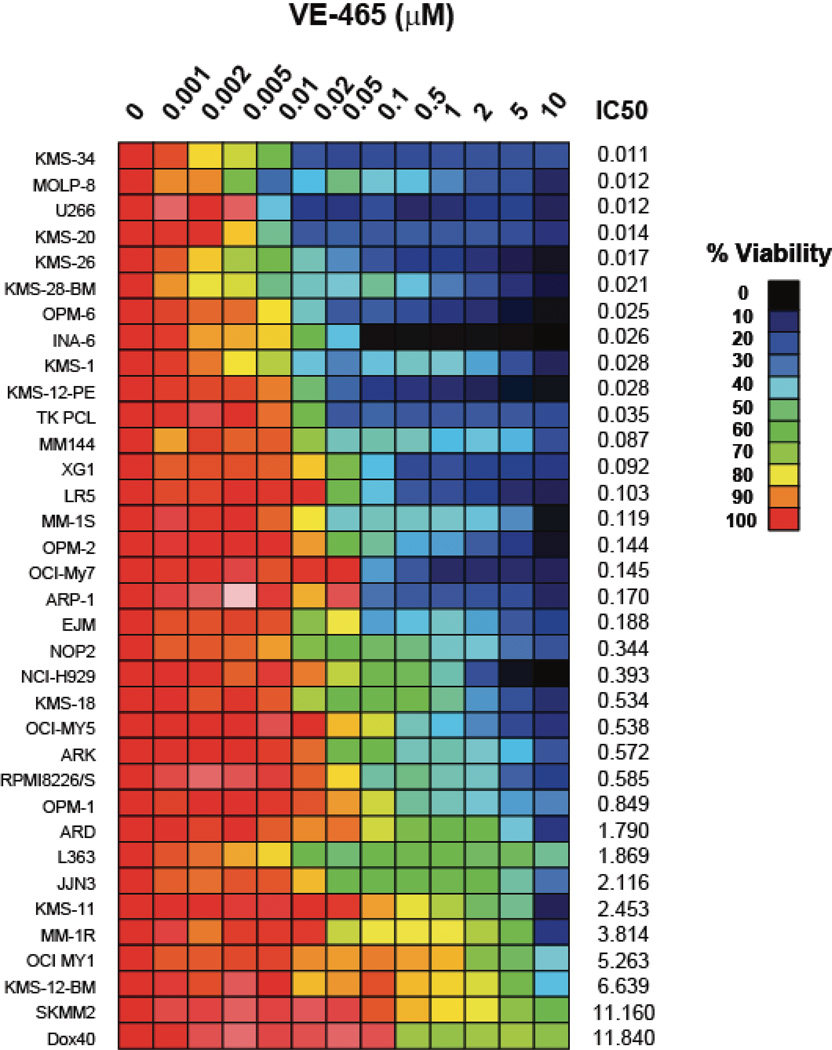
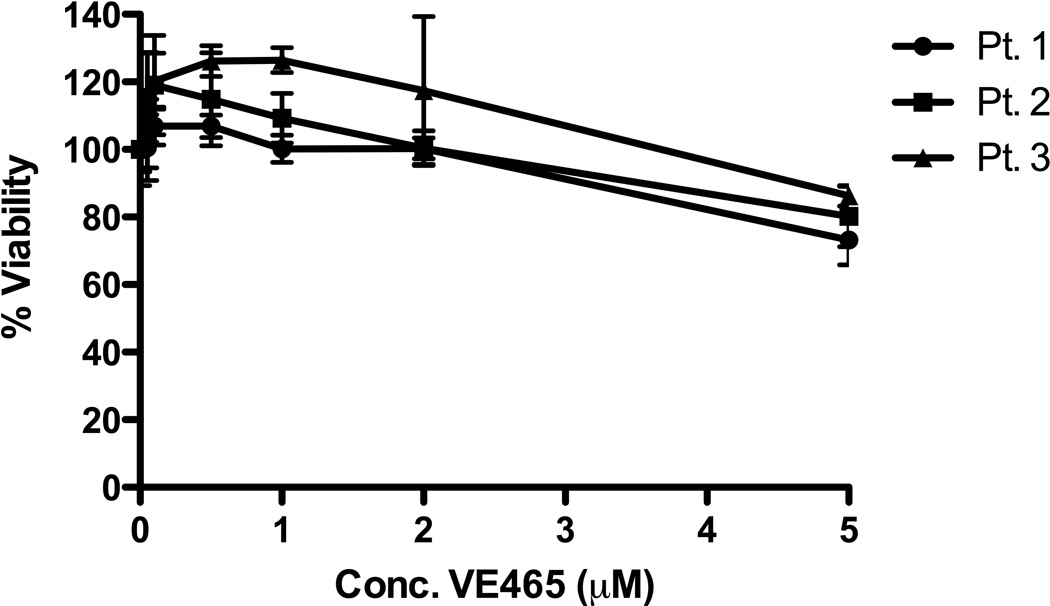
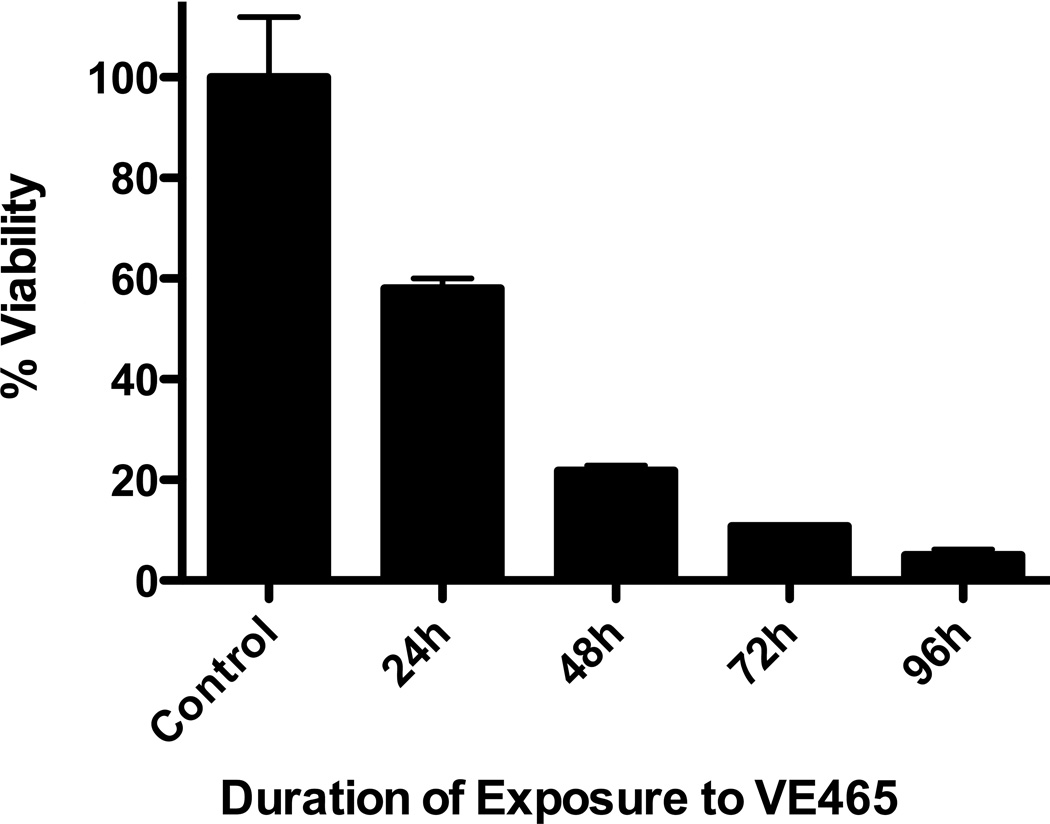
VE465 was active in vitro against a panel of human MM cell lines, including cell lines resistant to conventional and/or other novel anti-MM agents, such as Melphalan (LR5), Dex (U266), Doxo (Dox40), and lenalidomide (KMS11) (Fig 1A). A subset of cell lines had IC50 values at or below 400nM, a level that was achieved in patients enrolled in clinical trials of MK-047, a clinical analog of VE465 (Rubin, et al 2006) and were lower than IC50 values for non-malignant cells, such as phytohaemagglutinin-stimulated or unstimulated peripheral blood mononuclear cells (Fig S1A), HS-5 stromal cells, and THLE-3 hepatocytes (Fig S1B). In contrast, primary CD138+ plasma cells from MM patients, including those from patients with late stage disease had IC50 values >5µM (Figure 1B). The minimal exposure required to induce irreversible commitment of MM cells to death was assessed by cell death commitment assays, which revealed that 48-h exposure to 0.5µM VE465 was necessary to induce >50% killing in cell lines (Figure 1C). These data suggest that in order for the in vitro anti-MM activity of pan-Aurora inhibitors to translate to clinical efficacy, protracted exposure of tumours to drug would be necessary, especially considering the lower sensitivity of the patient samples tested thus far.
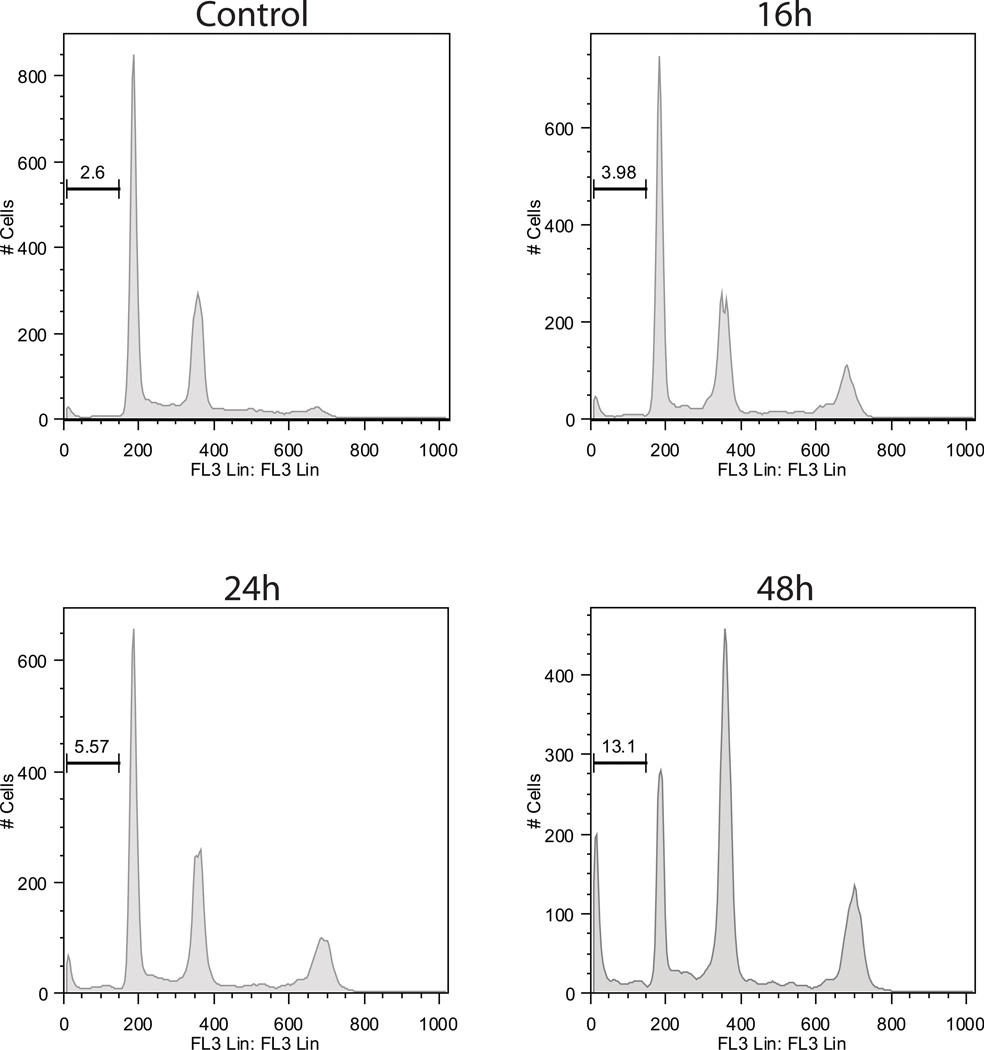
Figure 1. In vitro anti-myeloma activity of VE465.
Viability following treatment with VE465 on MM cell lines (N=3 replicates per condition) (A), and primary CD138+ plasma cells selected from bone marrow aspirates (N=6 replicates per condition) (B). Cell death commitment assay of MM.1S cells following treatment with VE465 (N=2 replicates per condition) (C). Cell Cycle Analysis of MM.1S cells treated with VE465 (single analysis per condition) (D). Data of panels A, B, and C, are presented as % viability compared to untreated controls.
Evaluation of cell cycle and apoptosis status in VE465–treated MM cells
VE465-treated MM cells exhibited a time-dependent increase in the G2/M peak and the sub-G1 population, as well as an increase in cells with 8N DNA content (Figure 1D & Fig S2). These findings are consistent with previously reported effects of pan-Aurora kinase inhibitors on cell cycle and cytokinesis (Chng, et al 2008, Evans, et al 2008, Shi, et al 2007), suggesting that treatment with VE465 initially prevents cell division leading to endo-duplication, followed eventually by DNA fragmentation. Annexin/propidium iodide staining (Fig S3), poly (ADP-ribose) polymerase and caspase-3, -8, -9 cleavage (Fig S4A) confirmed apoptotic cell death, which was accompanied with increase in Bax, decrease in Bcl-2, and no changes in Hsps-27, -70 and -90 (Fig S4B–D).
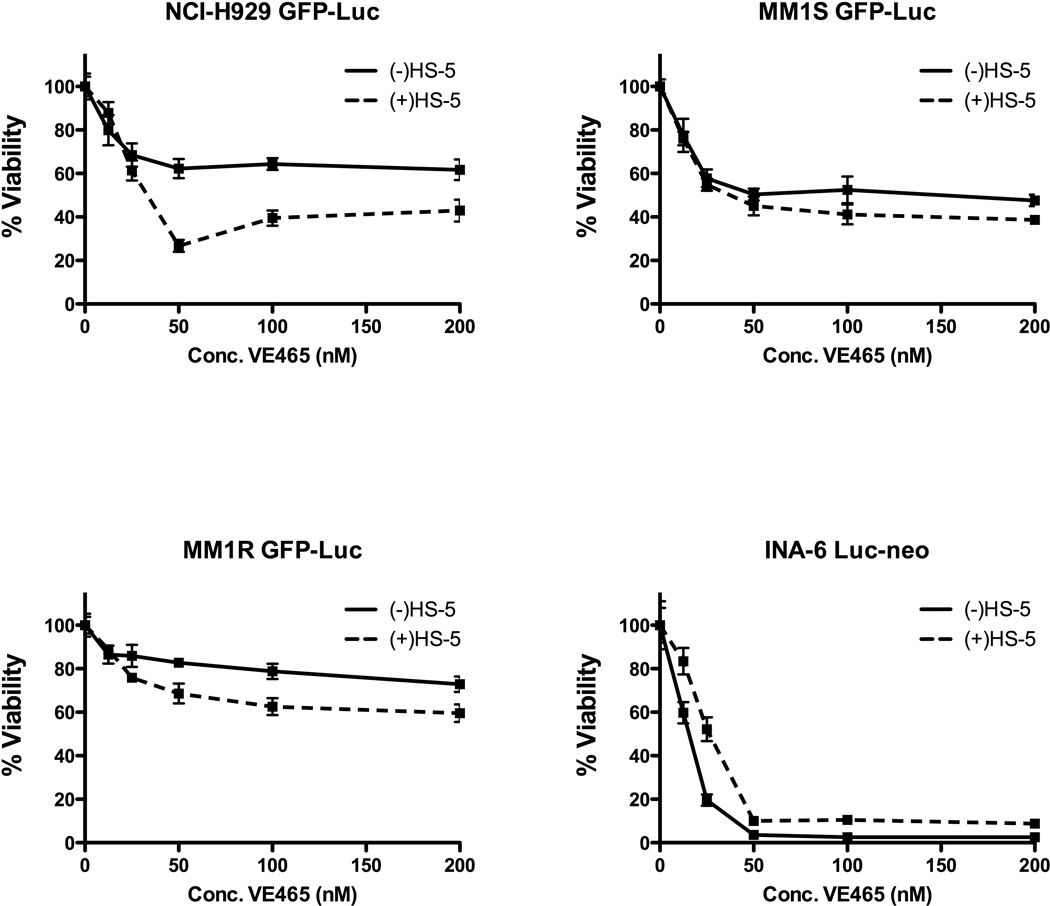
Activity of VE465 in the context of BM microenvironment
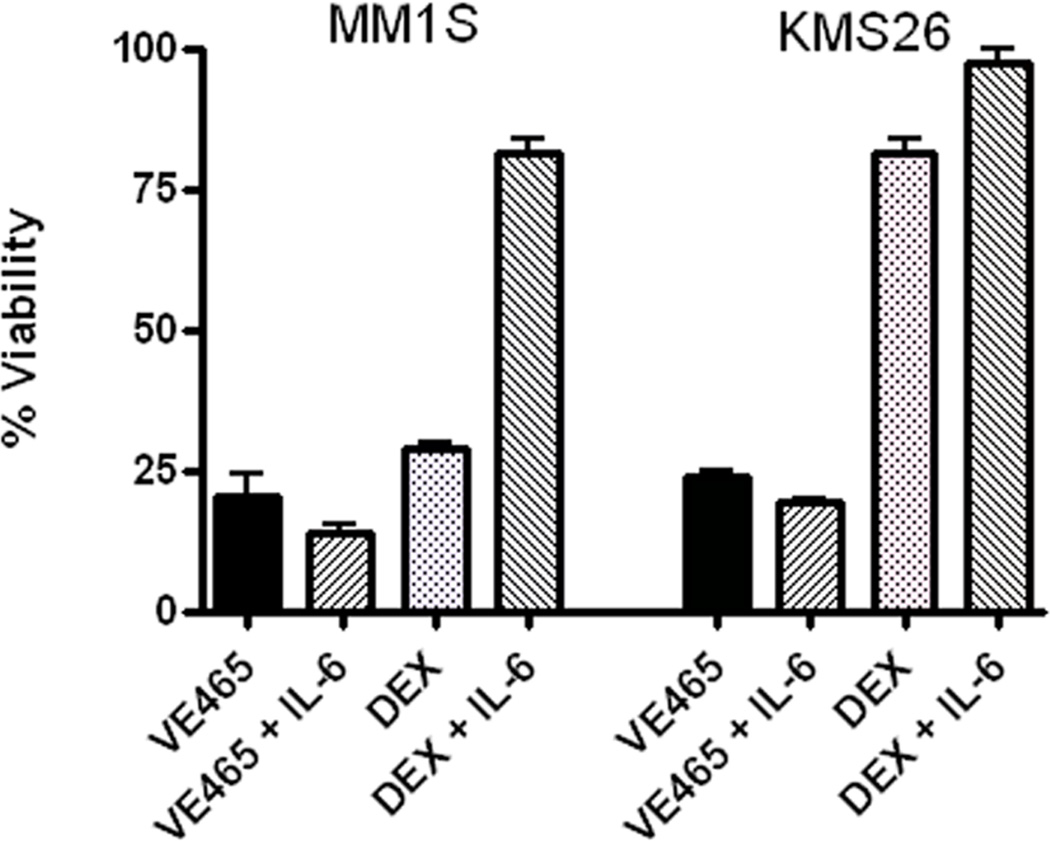
The present studies distinctly showed that co-culture with bone marrow stem cells (BMSCs) did not confer MM cell resistance against VE465. Interestingly, MM.1S, MM.1R and NCI-H929 cells exhibited a more potent response to low µM and/or sub-µM concentrations of VE645 when co-cultured with BMSCs (Figure 2A). Furthermore, the in vitro anti-MM activity of VE465 was not suppressed by IL-6, despite protecting the same MM cells from Dex (Figure 2B). How Aurora inhibition overcomes the protection of IL-6 is unclear at this time, but maybe due to off-target effects of VE465 on the IL-6R/STAT3 pathway, or simply the pro-apoptotic effects of Aurora inhibition.
Figure 2. VE465 overcomes protective effects of the bone marrow milieu.
Viability of MM cell lines treated with VE465 in the presence or absence of the bone-marrow stromal cell line HS-5 (N=5 replicates per condition) (A), or the presence or absence of exogenous IL-6 (10 ng/ml; N=3 replicates per condition) (B). Data are presented as % viability compared to untreated controls. DEX, dexamethasone
Combinations of VE465 with other anti-MM agents
VE465 combined with established anti-MM agents, including Dex, Doxo and bortezomib, achieved additive but not synergistic cytotoxicity on MM cells (Fig. S5). In addition, no antagonism was observed when VE465 treatment was in combination with vorinostat (SAHA; data not shown). Because VE465 induced cell cycle arrest with slow kinetics of action, synergy with cell-cycle dependent drugs may be difficult to achieve.
In summary, the broad panel of MM cell lines tested exhibited sensitivity to the Aurora kinase inhibitor VE465. However, the sensitivity of the MM cell lines was higher than that observed in primary MM cells from bone marrow aspirates. This kinase inhibitor was able to overcome the protective effects of the BM milieu on MM cells. Our study also highlighted other important considerations with regard to potential uses of Aurora kinase inhibition as a therapeutic strategy, including the duration of drug exposure required to observe the anti-MM activity of this drug class. Future studies should compare the anti-MM activity of isoform-selective vs. broad spectrum Aurora inhibitors..
Supplementary Material
Supp Fig s1-s5
Acknowledgments
Supported in part by the “Dunkin Donuts Rising Stars” Program at the Dana-Farber Cancer Institute (to C.S.M), the Chambers Medical Foundation (P.G.R and C.S.M.), the Richard J. Corman Fund (P.G.R and C.S.M.), the Cobb Family Fellowship (to D.W.M), and National Institutes of Health grants R01CA050947 (KCA and CSM) and PO-1-78378 (KCA).
REFERENCES
- Bischoff JR, Anderson L, Zhu Y, Mossie K, Ng L, Souza B, Schryver B, Flanagan P, Clairvoyant F, Ginther C, Chan CS, Novotny M, Slamon DJ, Plowman GD. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. Embo J. 1998;17:3052–3065. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal RD, Tse A, Schwartz GK. Aurora kinases: new targets for cancer therapy. Clin Cancer Res. 2006;12:6869–6875. doi: 10.1158/1078-0432.CCR-06-1405. [DOI] [PubMed] [Google Scholar]
- Chng WJ, Braggio E, Mulligan G, Bryant B, Remstein E, Valdez R, Dogan A, Fonseca R. The centrosome index is a powerful prognostic marker in myeloma and identifies a cohort of patients that might benefit from aurora kinase inhibition. Blood. 2008;111:1603–1609. doi: 10.1182/blood-2007-06-097774. [DOI] [PubMed] [Google Scholar]
- Evans R, Naber C, Steffler T, Checkland T, Keats J, Maxwell C, Perry T, Chau H, Belch A, Pilarski L, Reiman T. Aurora A kinase RNAi and small molecule inhibition of Aurora kinases with VE-465 induce apoptotic death in multiple myeloma cells. Leuk Lymphoma. 2008;49:559–569. doi: 10.1080/10428190701824544. [DOI] [PubMed] [Google Scholar]
- Gritsko TM, Coppola D, Paciga JE, Yang L, Sun M, Shelley SA, Fiorica JV, Nicosia SV, Cheng JQ. Activation and overexpression of centrosome kinase BTAK/Aurora-A in human ovarian cancer. Clin Cancer Res. 2003;9:1420–1426. [PubMed] [Google Scholar]
- Li D, Zhu J, Firozi PF, Abbruzzese JL, Evans DB, Cleary K, Friess H, Sen S. Overexpression of oncogenic STK15/BTAK/Aurora A kinase in human pancreatic cancer. Clin Cancer Res. 2003;9:991–997. [PubMed] [Google Scholar]
- McMillin D, Negri J, Hayden P, Weisberg E, Klippel S, Zurawska J, Mitsiades N, Anderson KC, Mitsiades CS. Compartment-specific bioluminescence imaging (CS-BLI): A high-throughput approach to identify novel anti-myeloma therapies that overcome the protection of stromal cells. Haematologica-the Hematology Journal. 2007;92:65–65. [Google Scholar]
- Mitsiades CS, Treon SP, Mitsiades N, Shima Y, Richardson P, Schlossman R, Hideshima T, Anderson KC. TRAIL/Apo2L ligand selectively induces apoptosis and overcomes drug resistance in multiple myeloma: therapeutic applications. Blood. 2001;98:795–804. doi: 10.1182/blood.v98.3.795. [DOI] [PubMed] [Google Scholar]
- Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Shringarpure R, Hideshima T, Akiyama M, Chauhan D, Munshi N, Gu X, Bailey C, Joseph M, Libermann TA, Richon VM, Marks PA, Anderson KC. Transcriptional signature of histone deacetylase inhibition in multiple myeloma: biological and clinical implications. Proc Natl Acad Sci U S A. 2004;101:540–545. doi: 10.1073/pnas.2536759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, San-Miguel JF, Blade J, Boccadoro M, Cavenagh J, Dalton WS, Boral AL, Esseltine DL, Porter JB, Schenkein D, Anderson KC. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Blood E, Mitsiades CS, Jagannath S, Zeldenrust SR, Alsina M, Schlossman RL, Rajkumar SV, Desikan KR, Hideshima T, Munshi NC, Kelly-Colson K, Doss D, McKenney ML, Gorelik S, Warren D, Freeman A, Rich R, Wu A, Olesnyckyj M, Wride K, Dalton WS, Zeldis J, Knight R, Weller E, Anderson KC. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458–3464. doi: 10.1182/blood-2006-04-015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin EH, Shapiro GI, Stein MN, Watson P, Bergstrom D, Xiao A, Clark JB, Freedman SJ, Eder JP. A phase I clinical and pharmacokinetic (PK) trial of the aurora kinase (AK) inhibitor MK-0457 in cancer patients. Journal of Clinical Oncology. 2006;24:123S–123S. [Google Scholar]
- Shi Y, Reiman T, Li W, Maxwell CA, Sen S, Pilarski L, Daniels TR, Penichet ML, Feldman R, Lichtenstein A. Targeting aurora kinases as therapy in multiple myeloma. Blood. 2007;109:3915–3921. doi: 10.1182/blood-2006-07-037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, Munshi N, Anaissie E, Wilson C, Dhodapkar M, Zeddis J, Barlogie B. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341:1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- Weber DM, Chen C, Niesvizky R, Wang M, Belch A, Stadtmauer EA, Siegel D, Borrello I, Rajkumar SV, Chanan-Khan AA, Lonial S, Yu Z, Patin J, Olesnyckyj M, Zeldis JB, Knight RD. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp Fig s1-s5