Secular Changes in U.S. Prediabetes Prevalence Defined by Hemoglobin A1c and Fasting Plasma Glucose: National Health and Nutrition Examination Surveys, 1999–2010 (original) (raw)
Abstract
OBJECTIVE
Using a nationally representative sample of the civilian noninstitutionalized U.S. population, we estimated prediabetes prevalence and its changes during 1999–2010.
RESEARCH DESIGN AND METHODS
Data were from 19,182 nonpregnant individuals aged ≥12 years who participated in the 1999–2010 National Health and Nutrition Examination Surveys. We defined prediabetes as hemoglobin A1c (A1C) 5.7 to <6.5% (39 to <48 mmol/mol, A1C5.7) or fasting plasma glucose (FPG) 100 to <126 mg/dL (impaired fasting glucose [IFG]). We estimated the prevalence of prediabetes, A1C5.7, and IFG for 1999–2002, 2003–2006, and 2007–2010. We calculated estimates age-standardized to the 2000 U.S. census population and used logistic regression to compute estimates adjusted for age, sex, race/ethnicity, poverty-to-income ratio, and BMI. Participants with self-reported diabetes, A1C ≥6.5% (≥48 mmol/mol), or FPG ≥126 mg/dL were included.
RESULTS
Among those aged ≥12 years, age-adjusted prediabetes prevalence increased from 27.4% (95% CI 25.1–29.7) in 1999–2002 to 34.1% (32.5–35.8) in 2007–2010. Among adults aged ≥18 years, the prevalence increased from 29.2% (26.8–31.8) to 36.2% (34.5–38.0). As single measures among individuals aged ≥12 years, A1C5.7 prevalence increased from 9.5% (8.4–10.8) to 17.8% (16.6–19.0), a relative increase of 87%, whereas IFG remained stable. These prevalence changes were similar among the total population, across subgroups, and after controlling for covariates.
CONCLUSIONS
During 1999–2010, U.S. prediabetes prevalence increased because of increases in A1C5.7. Continuous monitoring of prediabetes is needed to identify, quantify, and characterize the population of high-risk individuals targeted for ongoing diabetes primary prevention efforts.
Diabetes is a major public health problem in the U.S., affecting 25.8 million people in 2011 (1). In addition, a condition known as prediabetes is associated with an increased risk of developing diabetes. An expert committee convened by the America Diabetes Association defined prediabetes as a fasting plasma glucose (FPG) level of 100 to 125 mg/dL (impaired fasting glucose [IFG]), a 2-h plasma glucose level after a 75-g oral glucose tolerance test of 140 to 199 mg/dL (impaired glucose tolerance [IGT]), or hemoglobin A1c (A1C) 5.7 to <6.5% (39 to <48 mmol/mol) (2). In 2010, 79 million U.S. adults had prediabetes based on IFG or elevated A1C criteria (1). Fortunately, the Diabetes Prevention Program of the National Institutes of Health and other studies have shown that among adults with elevated glucose levels, type 2 diabetes can be delayed or prevented (3–6). Further, recent studies have shown that effective lifestyle-based interventions can be successfully implemented in community-based settings (7–9), potentially reaching disparate populations (10–13). As the public health community seeks to address the urgent problem of diabetes prevention with broad implementation of proven measures, there is a strong need for reliable surveillance data to identify, measure, and characterize populations who could benefit from such interventions and help assess the effectiveness of prevention efforts.
Prediabetes prevalence estimates vary according to the type and combination of glycemic tests in use, the demographic characteristics of the population being measured, and other factors (14). Although increases in diagnosed and total diabetes in the U.S. are well documented, no increases in the prevalence of prediabetes have been detected (15). However, there have been several changes in the measurement of prediabetes since 2003, including changes in FPG criteria from 110 to 100 mg/dL and the introduction of A1C prediabetes cut points (16,17). Changes have also occurred in the demographic distribution of the U.S. population (18). To examine whether these changes may have been accompanied by corresponding changes in the prevalence of prediabetes, we estimated the prevalence of prediabetes in the U.S. for three time periods: 1999–2002, 2003–2006, and 2007–2010. We compared prevalence estimates obtained using two different measures of glycemic status—alone and combined—and obtained estimates for a range of subpopulations defined by sociodemographic and obesity status.
RESEARCH DESIGN AND METHODS
Data source and population
We used data from the 1999–2010 National Health and Nutrition Examination Surveys (NHANES), a repeated cross-sectional survey representative of the civilian, noninstitutionalized U.S. population, conducted by the National Center for Health Statistics (NCHS) (19–21) of the Centers for Disease Control and Prevention (CDC). NHANES is conducted in independent, 2-year cycles. To produce reliable estimates, NCHS recommends that data from two or more cycles be combined for analysis (21). Response rates were similar across the six cycles conducted from 1999 through 2010, ranging from 75 to 80% (19). The survey protocol was approved by the NCHS Institutional Review Board. Written informed consent was obtained from all participants aged ≥18 years; written parental consent was obtained for those aged <18 years.
From 1999 through 2006, NHANES used a stratified, multistage design with oversampling of adolescents, older adults, African Americans, Mexican Americans, low-income non-Hispanic whites, and pregnant women (20). Beginning in 2007, the sampling methodology was modified to provide estimates for specific age ranges, oversample Hispanics, and discontinue the oversampling of adolescents and pregnant women (21). NHANES participants completed a household interview, followed by a physical examination and interviews at a mobile examination center (MEC). Participants were randomly assigned to a morning or afternoon/evening session for their MEC appointment. Participants assigned to the morning sessions were instructed to fast for 9 h. Those who were unable to fast (i.e., those taking insulin or oral medications for diabetes, with hemophilia, with chemotherapy safety exclusions, etc.) were excluded from the morning MEC session.
During 1999–2010, 44,535 individuals aged ≥12 years were interviewed in NHANES. For our analysis, we included morning MEC session participants who had fasted 8 to <24 h and had complete data for A1C and FPG, as well as all individuals with self-reported diagnosed diabetes from any MEC session (n = 19,778). After excluding 596 pregnant women, a total of 19,182 participants were included in the analytic sample.
Measures
The A1C assays used high-performance liquid chromatography (HPLC) methods performed on instruments certified by the National Glycohemoglobin Standardization Program (19). Final reportable A1C results were standardized to the reference method used for the Diabetes Control and Complications Trial (22). During 1999–2010, three types of changes occurred in the A1C measurement methods used in NHANES: 1) two instrument changes (Primus I, Model CLC330 [1999–2004] to Tosoh A1C 2.2 Plus [2005–2006] to Tosoh A1C G7 [2007–2010], Primus Corporation, Kansas City, MO, and Tosoh Bioscience, Inc., South San Francisco, CA), 2) a laboratory site change (Missouri [1999–2004] to Minnesota [2005–2010]), and 3) an HPLC method change (boronate-affinity [1999–2004] to nonporous ion-exchange [2005–2010]). However, per NCHS recommendation, we analyzed the A1C data without any correction for these laboratory changes (23).
FPG measurements were performed using a hexokinase enzymatic method (19). During 2005–2010, there were two changes in instruments used to measure glucose levels (Roche Cobas Mira [1999–2004] to Roche/Hitachi 911 [2005–2006] to Roche/Hitachi Modular P [2007–2010]; Roche Diagnostics, Inc., Indianapolis, IN). We applied Deming regression equations, as recommended by NCHS, to data from 2005–2006 (FPG × 0.9835) and 2007–2010 (0.9835 × [FPG − 1.139]) to ensure comparability to earlier years of NHANES glucose data (24,25).
Prediabetes and diabetes.
We defined prediabetes as having A1C5.7 to <6.5% (39 to <48 mmol/mol, A1C5.7) or FPG 100 to <126 mg/dL (IFG). Individuals with A1C ≥6.5% (≥48 mmol/mol), FPG ≥126 mg/dL, or those with self-reported diagnosed diabetes were classified as having diabetes (n = 3,939).
Other measures.
Survey staff conducted standardized measurements of weight and height, from which BMI was calculated as weight (kg)/height (m)2. Using CDC’s 2000 BMI-for-age growth charts, we defined youth aged <20 years as being overweight from the sex-specific 85th percentile up to the 95th percentile and as being obese at the sex-specific 95th percentile or greater (26). Adults aged ≥20 years with BMIs of <25.0, 25.0–29.9, and ≥30 kg/m2 were defined as underweight/normal, overweight, and obese, respectively. Other variables included in this study were age (years), sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other race/ethnicity), and poverty-to-income ratio (PIR; ratio of family income to federal poverty thresholds, specific to family size, year, and state).
Statistical analysis
We imputed missing data for PIR (n = 1,544) and BMI (n = 376) using the PROC MI procedure in SAS 9.2 software (SAS Institute, Inc., Cary, NC). All dependent, independent, and design variables were included in the imputation model. We reported combined estimates using five imputed datasets. To examine how characteristics of the U.S. population differed during the study period 1999–2010, we reported selected descriptive statistics for consecutive 2-year survey periods. After excluding participants with diagnosed or undiagnosed diabetes, we examined the distributions of A1C and FPG values during 12 years using empirically estimated cumulative distribution functions for each 2-year period. Including participants with diagnosed or undiagnosed diabetes in the denominator, we estimated the prevalence of prediabetes, A1C5.7, and IFG for three periods: 1999–2002, 2003–2006, and 2007–2010.
For each measure, we compared age-adjusted prevalences for the three periods using an overall F test for equal proportions and t tests for the following contrasts: 1999–2002 versus 2003–2006, 2003–2006 versus 2007–2010, and 1999–2002 versus 2007–2010. Age-adjusted estimates were computed using the direct method, standardized to the 2000 U.S. census population with four age groups: 12–17, 18–44, 45–64, and ≥65 years. To examine changes independent of major risk factors for prediabetes, we also used multivariable logistic regression to estimate adjusted prevalences (predictive margins) for the total U.S. population aged ≥12 years and for selected subgroups, controlling for age, sex, race/ethnicity, PIR, BMI, and survey period. First-order interactions of survey period with each sociodemographic variable and BMI were tested with Satterthwaite-adjusted F statistics (27). Appropriate sampling weights were used to ensure that the sum of the sample weights was equivalent to the total U.S. population (15) and to account for the NHANES complex sampling design and nonresponse. We did not account for multiple comparisons due to the observational nature of the study (28). P values of < 0.05 were considered statistically significant. All analyses were performed using SAS-callable SUDAAN 10.0.1 software (RTI International, Research Triangle Park, NC).
RESULTS
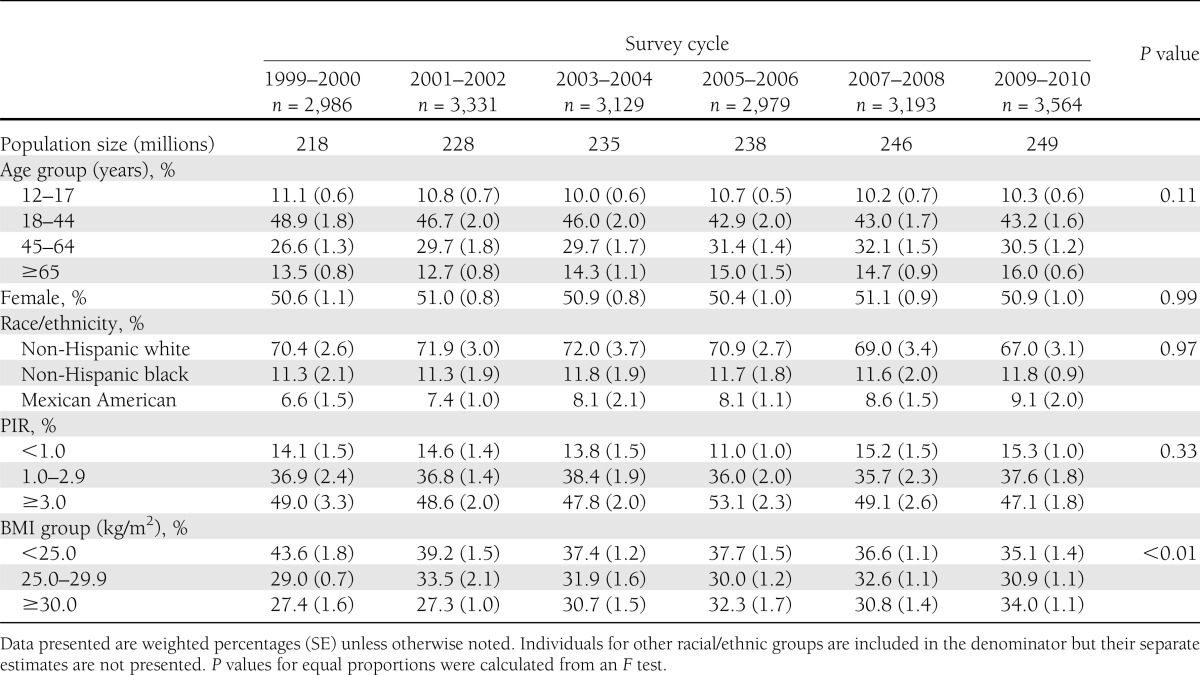
For the U.S. population aged ≥12 years, the distributions of age, sex, racial/ethnic, and income groups changed little, whereas the proportion of individuals classified as normal weight declined by 8.5 percentage points (ppts) and as obese increased by 6.6 ppts (Table 1).
Table 1.
Characteristics of U.S. population by survey cycle, NHANES 1999–2010
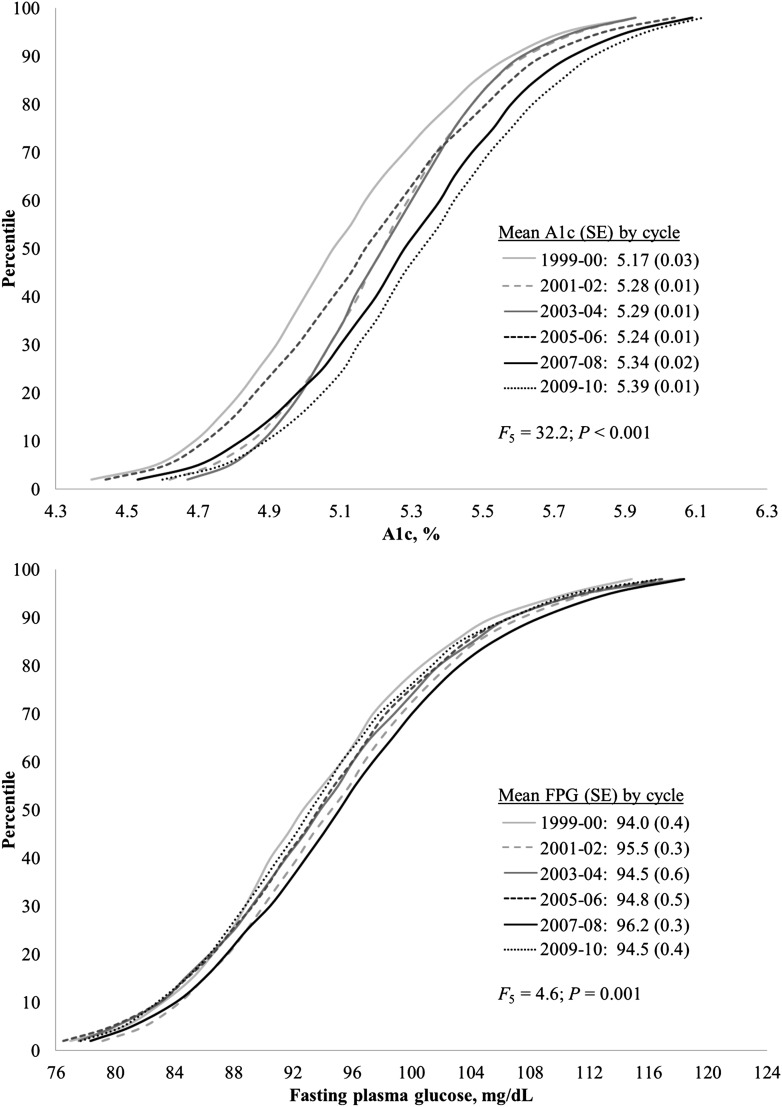
As shown in Fig. 1, among the U.S. population aged ≥12 years without diabetes, A1C distributions shifted slightly higher over the six survey cycles, with mean A1C levels increasing from ∼5.2% to 5.4% (33 to 36 mmol/mol). Throughout the 12-year period, distributional shifts for FPG were of a lesser relative magnitude than those of A1C, as mean FPG values increased from 94.0 mg/dL in 1999–2002 to 96.2 mg/dL in 2007–2008, but then declined to 94.5 mg/dL in 2009–2010. Cumulative distributions for A1C and FPG values stratified by age, sex, and BMI showed similar patterns as the overall distributions (data not shown).
Figure 1.
Cumulative distributions of A1C and fasting plasma glucose values for the U.S. population aged ≥12 years without diabetes for each survey cycle: 1999–2000, 2001–2002, 2003–2004, 2005–2006, 2007–2008, and 2009–2010. Estimates were weighted to the U.S. population. P value for equality of means was calculated from F test with five degrees of freedom (_F_5). A1C units can be converted to mmol/mol using the equation: (10.93 × A1C) – 23.50.
Table 2 reports the age-adjusted prevalence of prediabetes, A1C5.7, and IFG across the three 4-year survey periods. During 1999–2010, the proportion of individuals with neither diabetes nor prediabetes declined by ∼8 ppts (P < 0.001). The prevalence of prediabetes increased significantly from 27.4% (crude: 27.5% [95% CI, 25.1–30.0]) in 1999–2002 to 34.1% (35.1 [33.3–36.9]) in 2007–2010. A1C5.7 increased from 9.5% (9.6 [8.4–11.0]) to 17.8% (18.6 [17.2–20.0]), but IFG was relatively stable (23.9 [21.6–26.3] to 26.6 [24.7–28.6]). Among adults aged ≥18 years, age-adjusted prevalences in 2007–2010 were 36.2% (37.3 [35.4–39.2]) for prediabetes, 19.3% (20.2 [18.7–21.8]) for A1C5.7, and 27.5% (28.2 [26.2–30.3]) for IFG. In all, no significant prevalence changes occurred between the first two time periods; whereas changes in prediabetes and A1C5.7 between the second two time periods accounted for the significant net changes across all three survey periods.
Table 2.
Age-adjusted prevalence of prediabetes, A1C5.7, and IFG for the U.S. population, NHANES survey period, 1999–2010
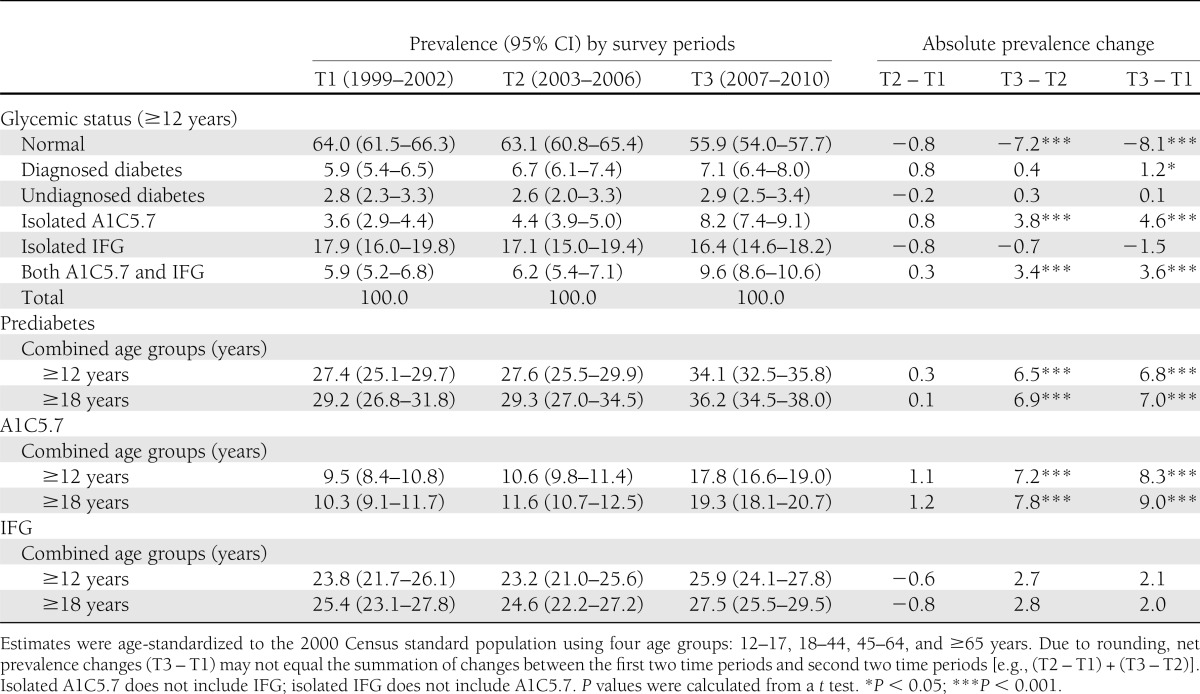
Table 3 reports the multivariate-adjusted prevalence of prediabetes by sociodemographic and BMI subgroups. For individuals aged ≥12 years, the overall prediabetes prevalence increased from 28.3 to 34.3% (P < 0.001). Among adults aged ≥18 years, the prevalence increased from 30.2 to 36.5% (P < 0.001). The proportion of the U.S. population with prediabetes was highest in 2007–2010 for all subgroups compared with previous time periods. Prediabetes was more prevalent among older adults, males, those with income below the federal poverty level, and obese individuals than among other groups.
Table 3.
Multivariate-adjusted prevalence†of prediabetes and prevalence change for the U.S. population aged 12 years and older by sociodemographic characteristics and BMI, NHANES, 1999–2010
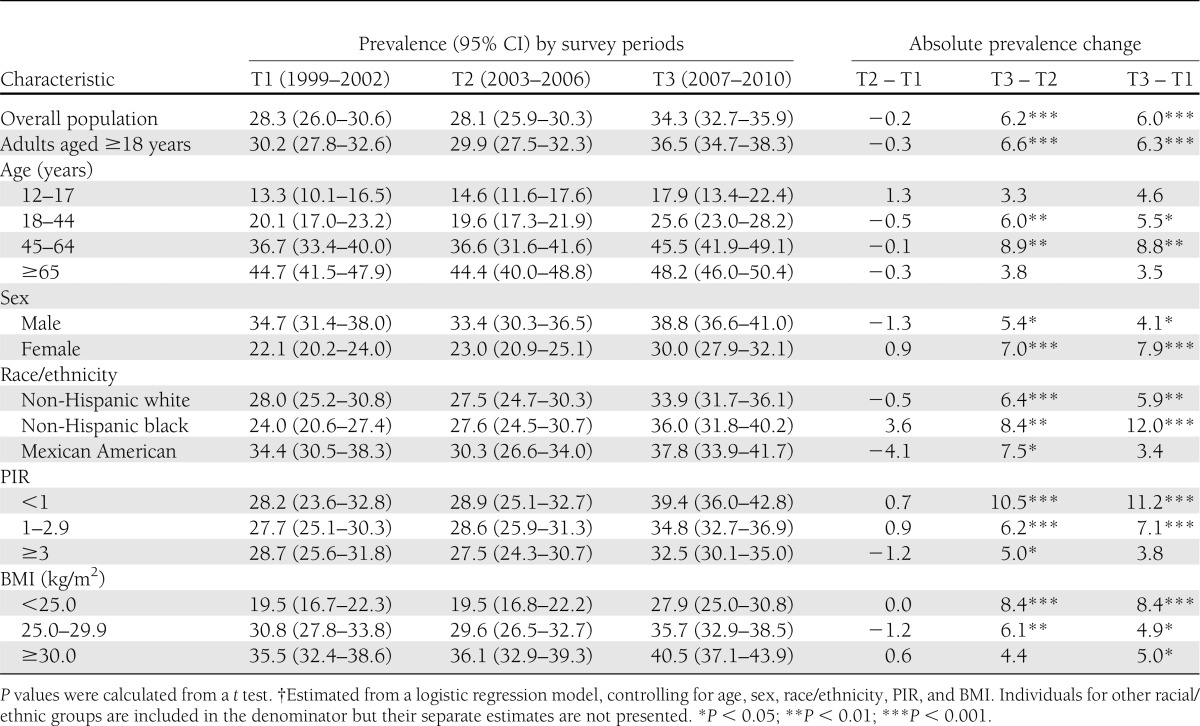
Prediabetes prevalence was significantly higher among young- and middle-aged adults during 2007–2010 compared with 2003–2006 or 1999–2002. No observed prevalence changes were observed in prediabetes for the youngest and oldest age groups. The percentage of the population with prediabetes increased significantly for both sexes, with greater increases from the earliest time period to the last for females (7.9 ppts) than males (4.1 ppts). These prevalence changes by sex account for the reduction in the gender disparity over the study period when prediabetes was 1.6, 1.5, and 1.3 times higher for males than for females in 1999–2002, 2003–2006, and 2007–2010, respectively. For racial/ethnic subgroups, increases in prediabetes prevalence from 1999–2002 to 2007–2010 were observed only for non-Hispanic whites (5.9 ppts) and non-Hispanic blacks (12.0 ppts). However, although Mexican Americans had higher prediabetes prevalence in 1999–2002 than did non-Hispanic whites and non-Hispanic blacks, in 2007–2010 there were no statistical differences in prediabetes prevalence by race/ethnicity. For those in the low- and middle-PIR groups, the percentage of individuals with prediabetes increased by 11.2 and 7.1 ppts, respectively. In addition, significant prevalence changes of 8.4, 4.9, and 5.0 ppts were noted for those in the normal weight, overweight, and obese groups.
Supplementary Table 1 reports multivariate-adjusted prevalences of A1C5.7 and IFG by sociodemographic and BMI subgroups. During the 12-year period, the magnitude of absolute prevalence changes across subgroups was greater for A1C5.7 alone (range 2.1 to 12 ppts) than IFG alone (−4.2 to 5.2 ppts). However, the proportion of individuals with IFG was consistently higher than the proportion with A1C5.7. Increases in A1C5.7 prevalence were significant for all subgroups. A significant interaction was also observed between survey period and BMI group (P = 0.03), with a relatively muted magnitude of prevalence change among individuals in the obese category compared with the normal and overweight categories. Changes in IFG prevalence were significant only for Mexican Americans (−7.4 ppts during 1999–2006; 6.0 ppts during 2003–2010), those below the federal poverty level (6.3 ppts during 2003–2010), and those in the normal BMI range (3.8 ppts during 2003–2010).
CONCLUSIONS
In the U.S., A1C and FPG are both used to estimate prevalence and monitor trends in prediabetes (1,14,15), and each is commonly used in clinical diagnosis (29,30). Using a sample representing the U.S. civilian, noninstitutionalized population, we found a 21% increase in prediabetes prevalence from 1999 through 2010. To our knowledge, this is the first study using a nationally representative sample to examine contiguous changes in prediabetes prevalence during the first decade of the 21st century. In addition to revealing an increase in prediabetes prevalence, our results identified diverse demographic subgroups that might benefit from targeted diabetes primary prevention programs that promote a healthy lifestyle, including weight loss and maintenance, physical activity, and healthy diet.
Few nationally representative studies of the U.S. population have compared prediabetes prevalence estimates defined by A1C and FPG (14,15), and NHANES serves as the national data source for the studies that are available. Only one other study estimated national trends in prediabetes prevalence. Cowie et al. (15) reported estimates of prediabetes prevalence as measured by IFG or IGT for adults aged ≥20 years across population subgroups from NHANES 1988–1994 through 2005–2006. They found no substantial changes in prediabetes prevalence except for a significant decline among Mexican Americans (15). Besides sociodemographic and compositional changes in the U.S. population, differences in estimates between our study and the studies of Cowie et al. (15) and James et al. (14) may reflect having additional years of data as well as varying laboratory methodology and sampling frames within the NHANES surveys during the monitoring periods.
Although it is important to recognize the overall changes in prediabetes prevalence when A1C and FPG criteria are combined, it is equally important to understand trends for A1C5.7 and IFG as single measures, which may follow clinical and economic preferences and, in some cases, may vary by population subgroups. Changes between 2003–2006 and 2007–2010 explained most of the shift in prediabetes prevalence over the entire time period, which was primarily driven by the dramatic increase in the prevalence of A1C5.7 in all subgroups between these periods. Combined with stable, albeit higher, IFG prevalences over time, these A1C5.7 prevalence changes yielded a net change of 6 ppts in overall prediabetes prevalence. These trends in prediabetes, A1C5.7, and IFG persisted despite adjustment for BMI and sociodemographic factors known to be associated with prediabetes.
Certain population subgroups have a greater burden of prediabetes, which has implications for developing and delivering interventions. We observed increases in prediabetes prevalence for most subgroups. However, prevalence changes were most worrisome for non-Hispanic black individuals and those below the federal poverty level; both groups had increases of more than 11 ppts. Interestingly, prediabetes prevalence for the older adult population appeared stable over time; however, by 2007–2010, ∼48% of the population aged ≥65 years had prediabetes. A recent review describes problems to be encountered with this rapidly expanding age group, including the finding that almost 8 in 10 older adults in the U.S. have some form of dysglycemia (31).
Accounting for age, race/ethnicity, and other characteristics, the proportion of U.S. adolescent girls and women with prediabetes is growing at a rate nearly twice that of males, accounting for the reduction in the gender disparity over the last 12 years. This finding is of concern, because offspring of these women may have an increased risk of diabetes after being exposed to hyperglycemia in utero (32). Aggregation of the two glycemic measures appears to have “washed out” ethnic differences in A1C5.7 and IFG, so that by 2007–2010, the prevalence of prediabetes was similar for the three ethnic groups in our study. Future work will monitor prediabetes as an aggregate condition and continue to examine the disproportionate burden of A1C5.7 and IFG among some ethnic groups in the U.S.
For each BMI group, there was a prevalence change of at least 4.9 ppts from 1999 through 2010 for prediabetes, yet the subpopulation with a normal BMI experienced the greatest relative increase in prevalence, which apparently arose from a more than doubling of A1C5.7. We are not aware of any reports using data other than NHANES of increasing prediabetes trends for those in the normal-weight category. Future research should attempt to replicate this finding and explore possible explanations for this worrisome trend among those with a normal BMI.
A major strength of our study is that it used nationally representative samples of the U.S. noninstitutionalized population with the potential to explore many diverse subgroups. Standardized protocols allowed for comparability across survey periods, and we used consistent prediabetes criteria despite definitional changes for prediabetes since 2003 (16,17).
However, there were also a number of limitations. First, this study used cross-sectional data with single measurements of A1C and FPG, which may have resulted in misclassification of prediabetes because of potential problems with intraindividual variability. Second, nonrandom error due to known sampling changes during the 2003–2010 NHANES monitoring period may have spuriously affected A1C or FPG values and, consequently, yielded inconsistent patterns of prediabetes prevalence. Third, methodological changes in A1C and FPG measurement occurred after the 2003–2004 NHANES cycle, including a new laboratory site, assay method enhancements, and instrument upgrades (23–25). In aggregate, these changes may have contributed to distributional shifts in A1C and FPG; however, the effect of survey design changes on A1C was ruled out by NCHS (23). Further calibration panels, fully representative crossover studies, and other standardized quality-control procedures for both measures may be warranted. To reduce the effect of these limitations, we used multiple years of data and combinations of measures to help stabilize our prediabetes estimates.
In summary, although it may be difficult to disentangle all possible explanations for the increase in prediabetes prevalence, this study highlights the importance of consistent population surveillance to monitor trends in diabetes risk. The recent emphasis on defining and deploying multiple laboratory measures for prediabetes and diabetes testing underscores the need for good data on the distribution of these risk factors in the population. Surveillance data are essential as well for targeting, implementing, and evaluating large-scale programs for diabetes primary prevention, such as the National Diabetes Prevention Program led by the CDC (33).
Differences in traditional factors, such as aging of the population and slight increases in BMI over time (31,34), might explain prevalence trends, so we controlled for them in the analysis. There may be other factors—real, but unidentified—that could explain the increase in prediabetes prevalence. For example, one might consider changes in physical activity to help explain the increase in overall prediabetes prevalence, but this cannot be explored because NHANES instituted a new physical activity questionnaire in 2007–2008 (35). As such, we need more data to substantiate the patterns in prediabetes prevalence that we are observing, especially among those of normal body weight and in high-risk, vulnerable populations requiring special targeted interventions (10–13). Our research and surveillance efforts depend on the availability of consistently measured outcomes. Future changes in survey methodology and data collection may affect our interpretation of estimates, disparities, and trends in unforeseen ways. However, the matching of surveillance data with the development and delivery of targeted interventions can potentiate broad prevention efforts that reach appropriate populations, as well as monitor progress to ensure accountability in an era of limited resources.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
K.M.B. developed the study concept and design, conducted statistical analyses, and wrote the manuscript. S.H.S. and C.J.C. guided statistical analyses and participated in revision of the manuscript. G.I., C.C.C., E.W.G., L.S.G., and D.B.R. guided the analyses, contributed to the discussion, and edited the manuscript for important intellectual content. Y.J.C. and D.E.W. guided the analyses and reviewed and edited the manuscript. K.M.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
See accompanying commentary, p. 2139.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1.Centers for Disease Control and Prevention National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011 [Google Scholar]
- 2.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2012;35(Suppl. 1):S64–S71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 5.Tuomilehto J, Lindström J, Eriksson JG, et al. Finnish Diabetes Prevention Study Group Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 6.Vermunt PW, Milder IE, Wielaard F, de Vries JH, van Oers HA, Westert GP. Lifestyle counseling for type 2 diabetes risk reduction in Dutch primary care: results of the APHRODITE study after 0.5 and 1.5 years. Diabetes Care 2011;34:1919–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Program into the community. The DEPLOY Pilot Study. Am J Prev Med 2008;35:357–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amundson HA, Butcher MK, Gohdes D, et al. Montana Cardiovascular Disease and Diabetes Prevention Program Workgroup Translating the diabetes prevention program into practice in the general community: findings from the Montana Cardiovascular Disease and Diabetes Prevention Program. Diabetes Educ 2009;35:209–210, 213–214, 216–220 passim [DOI] [PubMed] [Google Scholar]
- 9.Kramer MK, Kriska AM, Venditti EM, et al. Translating the Diabetes Prevention Program: a comprehensive model for prevention training and program delivery. Am J Prev Med 2009;37:505–511 [DOI] [PubMed] [Google Scholar]
- 10.Seidel MC, Powell RO, Zgibor JC, Siminerio LM, Piatt GA. Translating the Diabetes Prevention Program into an urban medically underserved community: a nonrandomized prospective intervention study. Diabetes Care 2008;31:684–689 [DOI] [PubMed] [Google Scholar]
- 11.Faridi Z, Shuval K, Njike VY, et al. PREDICT Project Working Group Partners reducing effects of diabetes (PREDICT): a diabetes prevention physical activity and dietary intervention through African-American churches. Health Educ Res 2010;25:306–315 [DOI] [PubMed] [Google Scholar]
- 12.Vadheim LM, Brewer KA, Kassner DR, et al. Effectiveness of a lifestyle intervention program among persons at high risk for cardiovascular disease and diabetes in a rural community. J Rural Health 2010;26:266–272 [DOI] [PubMed] [Google Scholar]
- 13.Jaber LA, Pinelli NR, Brown MB, et al. Feasibility of group lifestyle intervention for diabetes prevention in Arab Americans. Diabetes Res Clin Pract 2011;91:307–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James C, Bullard KM, Rolka DB, et al. Implications of alternative definitions of prediabetes for prevalence in U.S. adults. Diabetes Care 2011;34:387–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988-1994 and 2005-2006. Diabetes Care 2009;32:287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genuth S, Alberti KG, Bennett P, et al. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160–3167 [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association Standards of medical care in diabetes—2010. Diabetes Care 2010;33(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shrestha LB, Heisler EJ. The changing demographic profile of the United States. [Internet] Washington, DC, Congressional Research Service. Available from http://www.fas.org/sgp/crs/misc/RL32701.pdf Accessed 29 October 2012
- 19.Centers for Disease Control and Prevention. National Center for Health Statistics. National Health and Nutrition Examination Survey [Internet]. Questionnaires, datasets and related documentation. Available from http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm Accessed 29 June 2012
- 20.Centers for Disease Control and Prevention. National Center for Health Statistics. National Health and Nutrition Examination Survey [Internet]. The National Health and Nutrition Examination Survey: Sample Design, 1999–2006. Hyattsville, MD, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2012. Available from http://www.cdc.gov/nchs/data/series/sr_02/sr02_155.pdf Accessed 29 October 2012
- 21.Centers for Disease Control and Prevention. National Center for Health Statistics. National Health and Nutrition Examination Survey [Internet]. Analytic note regarding 2007–2010 survey design changes and combining data across other survey cycles. Hyattsville, MD, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011. Available from http://www.cdc.gov/nchs/data/nhanes/analyticnote_2007-2010.pdf Accessed 30 July 2012
- 22.Steffes M, Cleary P, Goldstein D, et al. Hemoglobin A1c measurements over nearly two decades: sustaining comparable values throughout the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications Study. Clin Chem 2005;51:753–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. National Center for Health Statistics. National Health and Nutrition Examination Survey [Internet]. Updated advisory for NHANES Hemoglobin A1c (glycohemoglobin) data. Hyattsville, MD, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2012. Available from http://www.cdc.gov/nchs/data/nhanes/A1c_webnotice.pdf Accessed 30 July 2012
- 24.Centers for Disease Control and Prevention. National Center for Health Statistics. National Health and Nutrition Examination Survey [Internet]. 2005–2006 Lab methods. Available from http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/lab_methods_05_06.htm Accessed 29 June 2012
- 25.Centers for Disease Control and Prevention. National Center for Health Statistics. National Health and Nutrition Examination Survey [Internet]. 2007–2008 Lab methods. Available from http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/lab_methods_07_08.htm Accessed 29 June 2012
- 26.Ogden CL, Flegal KM. Changes in terminology for childhood overweight and obesity. Natl Health Stat Rep; no 25. Hyattsville, MD, National Center for Health Statistics, 2010 [PubMed] [Google Scholar]
- 27.Skinner CJ, Holt D, Smith TMF. Analysis of Complex Surveys. Chichester, England, John Wiley & Sons, 1989 [Google Scholar]
- 28.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43–46 [PubMed] [Google Scholar]
- 29.Herman WH, Cohen RM. Racial and ethnic differences in the relationship between HbA1c and blood glucose: implications for the diagnosis of diabetes. J Clin Endocrinol Metab 2012;97:1067–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inzucchi SE. Clinical practice. Diagnosis of diabetes. N Engl J Med 2012;367:542–550 [DOI] [PubMed] [Google Scholar]
- 31.Caspersen CJ, Thomas GD, Boseman LA, Beckles GL, Albright AL. Aging, diabetes, and the public health system in the United States. Am J Public Health 2012;102:1482–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clausen TD, Mathiesen ER, Hansen T, et al. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care 2008;31:340–346 [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. Division of Diabetes Translation, National Center for Chronic Disease Prevention and Health Promotion [Internet]. National Diabetes Prevention Program Registry of Recognized Programs. Available from http://www.cdc.gov/diabetes/prevention/recognition/registry.htm Accessed 1 August 2012
- 34.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 2012;307:491–497 [DOI] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. National Center for Health Statistics. National Health and Nutrition Examination Survey [Internet]. 2007–2008 physical activity. Data documentation, codebook, and frequencies. Available from http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/PAQ_E.htm Accessed 3 February 2013