SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors (original) (raw)
. Author manuscript; available in PMC: 2014 Jan 3.
Published in final edited form as: Cell Stem Cell. 2013 Jul 3;13(1):102–116. doi: 10.1016/j.stem.2013.05.014
SUMMARY
Hematopoietic stem cells (HSCs) and multipotent hematopoietic progenitors (MPPs) are routinely isolated using various markers but remain heterogeneous. Here we show that four SLAM family markers, CD150, CD48, CD229, and CD244, can distinguish HSCs and MPPs from restricted progenitors and subdivide them into a hierarchy of functionally distinct subpopulations with stepwise changes in cell-cycle status, self-renewal, and reconstituting potential. CD229 expression largely distinguished lymphoid-biased HSCs from rarely-dividing myeloid-biased HSCs, enabling prospective enrichment of these HSC subsets. Differences in CD229 and CD244 expression resolved CD150−CD48−/lowLineage−/lowSca-1+c-Kit+ cells into a hierarchy of highly-purified MPPs that retained erythroid and platelet potential but exhibited progressive changes in mitotic activity and reconstituting potential. Use of these markers, and reconstitution assays, showed that conditional deletion of Scf from endothelial cells and perivascular stromal cells eliminated the vast majority of bone marrow HSCs, including nearly all CD229−/low HSCs, demonstrating that quiescent HSCs are maintained by a perivascular niche.
INTRODUCTION
A number of markers permit the prospective identification and isolation of hematopoietic stem cells (HSCs) and multipotent progenitors (MPPs) (Bryder et al., 2006). In the adult mouse, all multipotent cells are contained in the Lineage−/lowSca-1+c-Kit+ (LSK) fraction of bone marrow cells, though this population is very heterogeneous (Spangrude et al., 1988; Ikuta and Weissman, 1992; Uchida et al., 1994). Higher levels of HSC purity can be achieved by selecting the Thy-1low subset of Lineage−/lowSca-1+ cells (Spangrude et al., 1988) and by distinguishing cells with different levels of Mac-1 and CD4 expression: HSCs are Thy1lowSca-1+Lineage−Mac-1−CD4−c-Kit+ while MPPs are Thy1loSca-1+Lineage−Mac-1lowCD4low (Morrison and Weissman, 1994; Morrison et al., 1997). Higher levels of HSC purity are also achieved by selecting the subset of LSK cells that is CD34 negative/low (Osawa et al., 1996), Flt3 negative (Christensen and Weissman, 2001; Adolfsson et al., 2005; Yang et al., 2005), or that effluxes Hoechst 33342 (Goodell et al., 1996).
HSCs can also be isolated using the SLAM family markers CD150 (also known as Slamf1) and CD48 (Slamf2) as CD150+CD48− cells (Forsberg et al., 2005; Kiel et al., 2005; Kim et al., 2006; Yilmaz et al., 2006; Kiel et al., 2008). Addition of LSK markers to these SLAM family markers only modestly increases HSC purity but is useful to confirm the purity of the cells during sorting (Kiel et al., 2005; Kim et al., 2006; Yilmaz et al., 2006; Kiel et al., 2008). MPPs can be isolated by selecting C150−CD48−LSK cells (Kiel et al., 2008). Consistent with this, the self-renewal and reconstituting potentials of HSCs decline as CD150 expression levels decline (Papathanasiou et al., 2009; Beerman et al., 2010; Morita et al., 2010).
Despite markers that can give high levels of HSC and MPP purity, HSC and MPP populations remain functionally heterogeneous. Most C150+CD48−LSK HSCs are in G0 and only 3% are in S/G2/M phase of the cell cycle, indicating that this population contains very few cycling cells (Kiel et al., 2007). Nonetheless, the quiescent cells in this population are heterogeneous with respect to the rate at which they enter cycle over time, with about 80% going into cycle every 12 days and about 20% of the cells entering cycle every 100 days (Wilson et al., 2008; Foudi et al., 2009). HSCs are also heterogeneous with respect to the ratio of myeloid/lymphoid cells they generate upon transplantation into irradiated mice (Muller-Sieburg et al., 2002; Dykstra et al., 2007; Kent et al., 2009; Beerman et al., 2010; Challen et al., 2010; Morita et al., 2010) and with respect to their self-renewal potential upon transplantation (Ema et al., 2005; Benveniste et al., 2010; Morita et al., 2010). However, limitations in the ability to purify each subset of HSCs has meant that some subsets of HSCs have often been characterized based on retrospective analyses of reconstitution patterns in irradiated mice (Copley et al., 2012).
MPPs are heterogeneous with respect to reconstitution kinetics in irradiated mice and the types of blood cells they produce (Morrison and Weissman, 1994; Morrison et al., 1997; Adolfsson et al., 2005; Yang et al., 2005; Forsberg et al., 2006). However, many of the MPP populations that have been studied are relatively impure: many cells must be transplanted to detectably reconstitute irradiated mice. The dependence on such heterogeneous populations has confounded the ability to characterize these cells - new markers are required.
The inability to resolve distinct subpopulations of HSCs has impeded our ability to characterize their niche. HSCs reside in a perivascular niche in which endothelial cells and perivascular stromal cells promote HSC maintenance by producing SCF and Cxcl12 (Sugiyama et al., 2006; Mendez-Ferrer et al., 2010; Ding et al., 2012; Greenbaum et al., 2012; Ding and Morrison, 2013). Conditional deletion of Scf or Cxcl12 from osteoblasts did not affect HSC frequency or function (Ding et al., 2012; Greenbaum et al., 2012; Ding and Morrison, 2013). However, conditional deletion of Scf or Cxcl12 from endothelial cells or from Leptin Receptor-expressing perivascular stromal cells did deplete bone marrow HSCs. Conditional deletion of Scf from both endothelial cells and perivascular stromal cells in Tie2-Cre; Lepr-Cre; Scffl/− mice eliminated most HSCs based on surface marker phenotype (Ding et al., 2012). However, others have speculated that only “activated” HSCs reside in a perivascular niche. Without markers to distinguish “activated” HSCs, this idea has been impossible to directly test.
In this study we extended our analysis of the SLAM family and found that four SLAM family markers, CD150 (Slamf1), CD48 (Slamf2), CD229 (Slamf3), and CD244 (Slamf4), subdivided adult mouse bone marrow LSK cells into seven functionally-distinct fractions of cells: two that contained HSCs, three that contained MPPs, and two that contained restricted hematopoietic progenitors (HPCs). These fractions formed a hierarchy that exhibited progressive changes in cell cycle kinetics, self-renewal capacity, and reconstituting potential. These markers thus allowed us to distinguish different subsets of HSCs and MPPs while prospectively isolating each population to a high degree of purity. Use of these markers, along with reconstitution assays, showed that the vast majority of phenotypically and functionally identifiable HSCs were lost when Scf was conditionally deleted from endothelial cells and perivascular stromal cells in the bone marrow of adult Tie2-Cre; Lepr-Cre; Scffl/− mice. Quiescent HSCs therefore depend upon a perivascular niche for their maintenance.
RESULTS
CD150, CD48, CD229 and CD244 subdivide LSK cells into distinct fractions
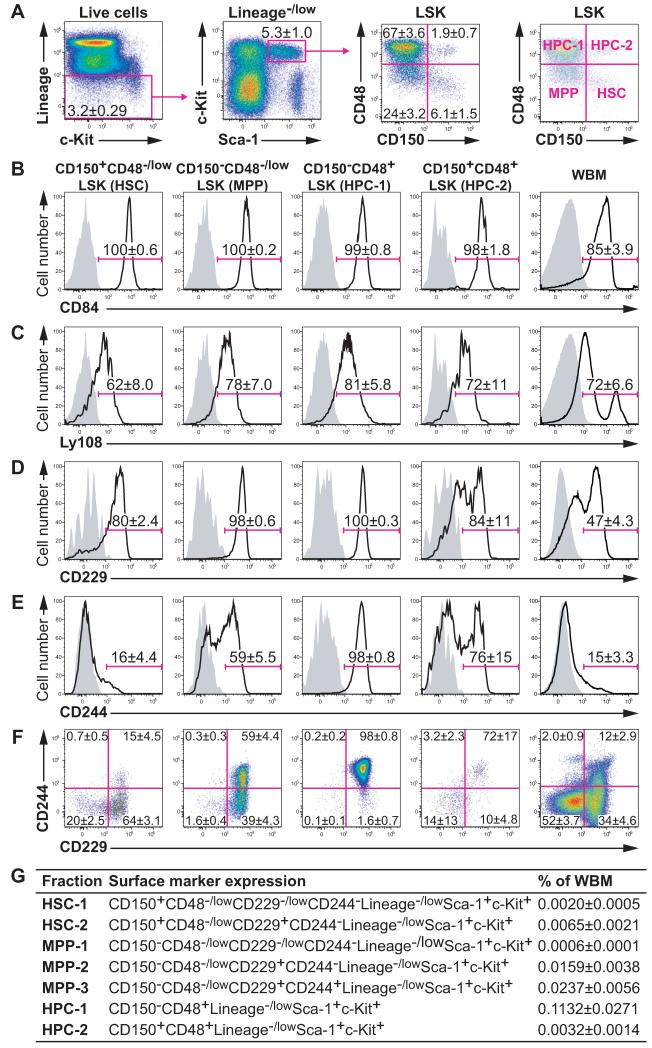
Lineage−/lowSca-1+c-kit+ (LSK) cells can be subdivided into four fractions based on the expression of CD150 and CD48 (Figure 1A): HSCs are CD150+CD48−LSK, MPPs are CD150−CD48−LSK, and CD150−CD48+LSK (HPC-1) and CD150+CD48+LSK (HPC-2) cells contain heterogeneous restricted progenitors (Kiel et al., 2005; Kiel et al., 2008). In the present study, we investigated the expression of CD229 (Slamf3), CD244 (Slamf4), CD84 (Slamf5), and Ly108 (Slamf6). CD84 and Ly108 were similarly expressed by almost all LSK cells (Figures 1B and 1C). In contrast, CD229 was heterogeneously expressed by HSCs and HPC-2 cells (Figure 1D) and CD244 was heterogeneously expressed by HSCs, MPPs, and HPC-2 cells (Figure 1E). Figure S1 shows the expression of CD229, CD244, CD84, and Ly108 on other subpopulations of hematopoietic progenitors.
Figure 1. Expression of the SLAM family markers CD84, Ly108, CD229, and CD244 by LSK hematopoietic stem and progenitor cells.
(A) Gating strategy for LSK cells (left and middle left panels) and expression of CD150 and CD48 by LSK cells (middle right panel). The CD150+CD48−/lowLSK, CD150−CD48−/lowLSK, CD150−CD48+LSK, and CD150+CD48+LSK fractions were labeled as HSC, MPP, HPC-1, and HPC-2, respectively (right panel). Doublets, red blood cells, and dead cells were excluded prior to analysis.
(B-E) Cell surface staining for CD84/Slamf5 (B), Ly108/Slamf6 (C), CD229/Slamf3 (D), and CD244/Slamf4 (E). Solid lines indicate staining with antibodies against the indicated antigens and shaded areas indicate background fluorescence in controls stained with all antibodies except for the indicated antigen.
(F) Flow cytometry plots showing combined CD229 and CD244 staining in each cell fraction.
(G) Summary of LSK stem and progenitor cell fractions subdivided according to CD229 and CD244 staining. Data represent mean ± S.D. from 3 (B and C) or 11 independent experiments (A, D-G). See also Figures S1 and S2.
Differences in CD229 and CD244 expression subdivided CD150+CD48−/lowLSK HSCs into two fractions and CD150−CD48−/lowLSK MPPs into three fractions. CD150−CD48+LSK HPC-1 cells were almost entirely CD229+CD244+ (Figure 1F). CD150+CD48+LSK HPC-2 cells were heterogeneous for CD229 and CD244 but we did not separately analyze the two fractions because they were too rare (Figure 1F). We also did not characterize CD229+CD244+ HSCs because they nearly disappeared when we used stringent HSC gates (data not shown). To assess whether SLAM family markers distinguished functionally different subpopulations of HSCs and MPPs we focused on CD150+CD48−/lowCD229−/low CD244−LSK HSC-1 cells, CD150+CD48−/lowCD229+CD244−LSK HSC-2 cells, CD150−CD48−/lowCD229−/lowCD244−LSK MPP-1 cells, CD150−CD48−/lowCD229+CD244−LSK MPP-2 cells, CD150−CD48−/lowCD229+CD244+LSK MPP-3 cells, CD150−CD48+LSK HPC-1 cells, and CD150+CD48+LSK HPC-2 cells (Figure 1G).
We first analyzed the expression of markers used previously to distinguish HSCs and MPPs including CD34, Flt3 (also known as Flk2), Thy1, EPCR, and ESAM (Spangrude et al., 1988; Osawa et al., 1996; Christensen and Weissman, 2001; Adolfsson et al., 2005; Yang et al., 2005; Balazs et al., 2006; Ooi et al., 2009; Yokota et al., 2009). The HSC-1 population contained more CD34−/lowFlt3− cells than HSC-2 cells, suggesting that HSC-1 cells were more primitive (Figure S2A). The MPP-1, MPP-2, and MPP-3 fractions contained primarily CD34−/lowFlt3− cells, CD34+Flt3−/+ cells, and CD34+Flt3+ cells, respectively, suggesting that MPP-1 cells were most primitive and MPP-3 cells were least primitive (Figure S2A). Almost all cells in the HSC-1, HSC-2, MPP-1, MPP-2, and MPP-3 fractions expressed Thy1, EPCR, and ESAM, though the expression levels were higher in HSCs than in MPPs (Figures S2B-S2D).
Different cell-cycle distributions among SLAM-defined HSC and MPP populations
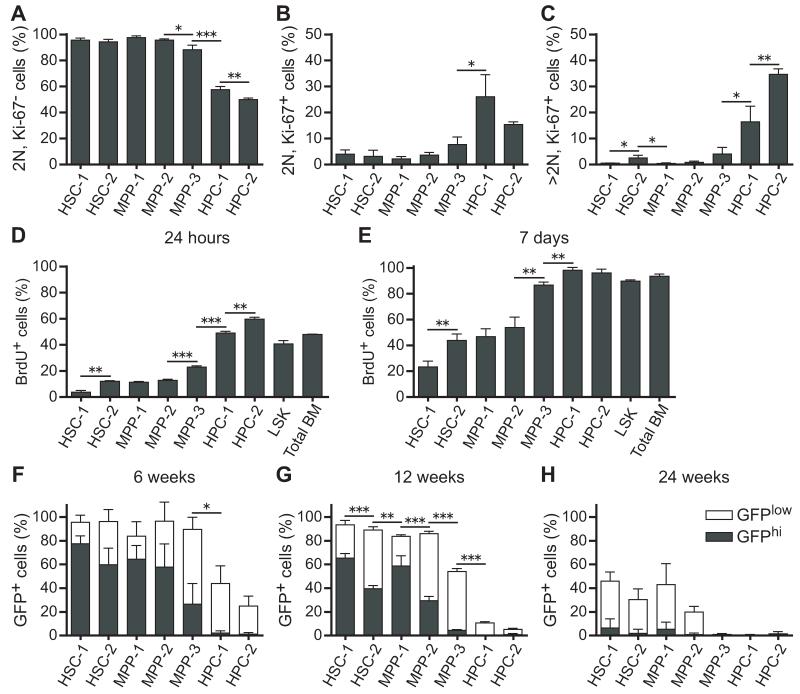
We used Ki-67 and propidium iodide (PI) staining to estimate the frequencies of G0 (Ki-67−; 2N DNA content), G1(Ki-67+; 2N DNA content), and S/G2/M (Ki-67+; >2N DNA content) cells in each population (Figure S3A). According to these markers, more than 90% of HSC-1, HSC-2, MPP-1, and MPP-2 cells were in G0 at any one time, but significantly fewer MPP-3 cells were in G0 (88±3%), and the HPC-1 and HPC-2 fractions were actively cycling (Figures 2A-2C). The frequencies of cells in G1 and S/G2/M generally increased as cells transitioned through the HSC-2, MPP-1, MPP-2, MPP-3, HPC-1, and HPC-2 fractions (Figures 2A-2C). The only exception was that HSC-2 cells included a slightly higher frequency of cells in S/G2/M (2.5±1.1%) as compared to MPP-1 and MPP-2 cells (both <1%) (Figures 2A-2C).
Figure 2. Cell cycle status of each hematopoietic stem and progenitor cell fraction.
(A-C) The frequency of cells in G0 (A; Ki-67−, 2N DNA content), G1 (B; Ki-67+, 2N DNA content), and S/G2/M (C; Ki-67+, >2N DNA content) phases of the cell cycle.
(D and E) The frequency of BrdU+ cells in each fraction after 24 hours (D) or 7 days (E) of BrdU administration.
(F-H) Expression of histone H2B-GFP was induced in hematopoietic cells by doxycycline administration for 6 weeks then chased for 6 weeks (F), 12 weeks (G), or 24 weeks (H) without doxycycline to monitor the rate of H2B-GFP dilution. Filled and open bars represent the proportion of GFPhi and GFPlo cells, respectively, in each population. The gating scheme used to identify GFPhi and GFPlo cells is shown in Figure S2. Data represent mean ± S.D. from 2 independent experiments with a total of 4 mice (F and G) or 3 independent experiments with a total of 7 mice (A-E, H). *p<0.05; **p<0.01; ***p<0.001 by Student’s _t_-test. Statistical analyses in F-H compared only the frequency of GFPhi cells among cell populations. See also Figure S3.
We next administered 5-bromo-deoxyuridine (BrdU) for 24 hours or for seven days to mice then analyzed each subpopulation of HSCs/MPPs. The rate of BrdU incorporation increased progressively from the HSC-1 fraction through the HPC-2 population (Figures 2D and 2E). HSC-1 cells were most highly quiescent, with only 3.7±1.5% (mean±SD) and 23±5% BrdU+ cells after 24 hours and 7 days, respectively (Figures 2D and 2E). The rates of BrdU incorporation into HSC-2, MPP-1, and MPP-2 cells was significantly higher. Almost all MPP-3, HPC-1, and HPC-2 cells incorporated BrdU over a 7 day period (Figures 2D and 2E).
We also used histone H2B-green fluorescent protein (H2B-GFP) label retention to monitor the division history of HSC/MPP subpopulations (Wilson et al., 2008; Foudi et al., 2009). The Col1A1-H2B-GFP; Rosa26-M2-rtTA double transgenic mice (Foudi et al., 2009) were administered doxycycline or 6 weeks to label all hematopoietic cells with H2B-GFP then chased for 6, 12, or 24 weeks without doxycycline to monitor the rate at which H2B-GFP was lost over time. Figures S3B-S3D show the gates used to distinguish GFPhigh from GFPlow cells. After 6 weeks of chase, most HSC-1, HSC-2, MPP-1, and MPP-2 cells retained high levels of GFP but the frequency of GFPhigh cells decreased progressively among these populations (Figure 2F). In contrast, few MPP-3 cells retained high levels of GFP and the HPC-1 and HPC-2 populations lost almost all GFPhigh cells (Figure 2F). After 12 weeks of chase, HSC-1 cells included significantly more GFPhigh cells than HSC-2 cells, and MPP-1 cells included significantly more GFPhigh cells than MPP-2 cells (Figures 2G). Our data are thus consistent with previously published results (Morrison and Weissman, 1994; Foudi et al., 2009) in indicating that many MPPs divide at similar rates as HSCs. Similar trends were observed after 24 weeks of chase, though by this time few cells in any population retained high levels of GFP (Figure 2H).
HSC-1 and HSC-2 contain a hierarchy of long-term multilineage reconstituting cells
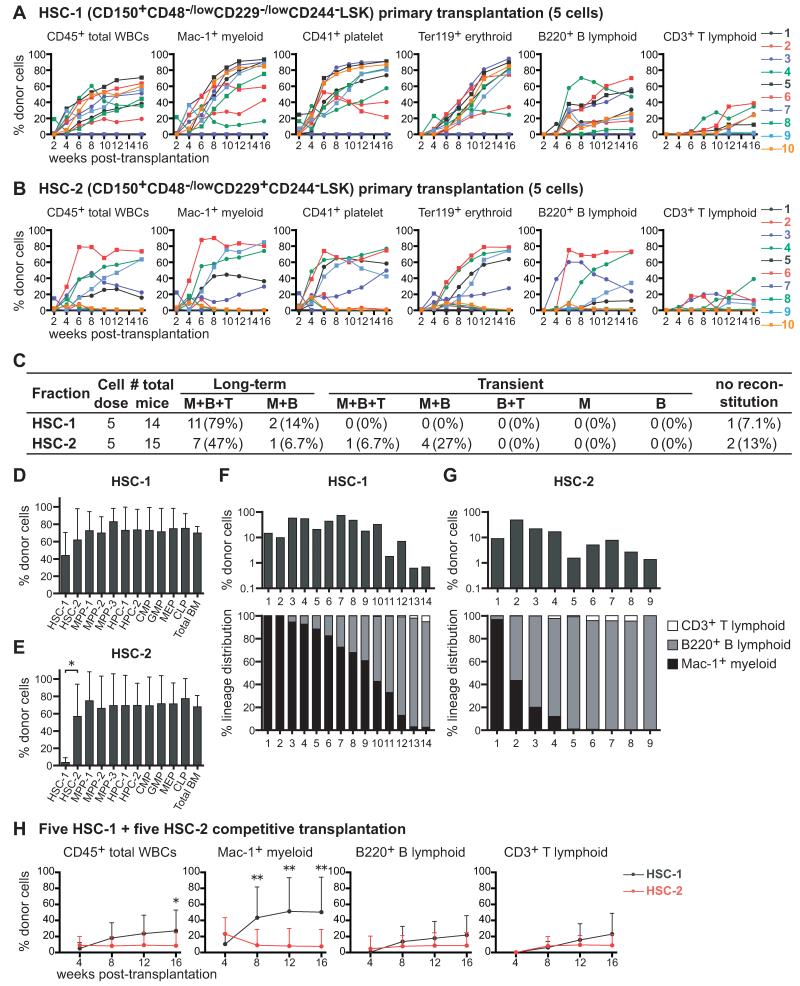
Five HSC-1 or HSC-2 cells isolated from UBC-GFP mice were transplanted into irradiated recipient mice along with 200,000 recipient bone marrow cells. UBC-GFP donors were used to assess donor contributions to erythrocyte and platelet reconstitution, in addition to myeloid, B, and T cells (Figures S4A and S4B). Both fractions gave long-term multilineage reconstitution in most recipients (Figures 3A and 3B). Ninety-three percent of recipients that received HSC-1 cells had long-term myeloid and lymphoid reconstitution by donor cells (Figure 3C). Fifty-four percent of recipients that received HSC-2 cells had long-term myeloid and lymphoid reconstitution (Figure 3C). Another 34% of recipients of HSC-2 cells exhibited transient reconstitution by myeloid and lymphoid cells, suggesting that HSC-2 cells represent a transitional population between long-term self-renewing HSCs and transiently self-renewing MPPs.
Figure 3. The HSC-1 and HSC-2 populations form a hierarchy of long-term multilineage reconstituting cells.
(A and B) Long-term competitive reconstitution assay in which 5 GFP+ donor cells from the HSC-1 (A) or HSC-2 (B) population were transplanted into irradiated recipient mice along with 200,000 recipient bone marrow cells. Each line represents the frequency of donor-derived cells in the blood of a single recipient.
(C) Summary of the results from the primary transplants in panels A and B. Recipients were considered long-term reconstituted if donor myeloid cells were more than 1% of blood cells for at least 16 weeks after transplantation. M, Mac-1+ myeloid cells; B, B220+ B cells; T, CD3+ T cells.
(D and E) The frequencies of donor-derived hematopoietic stem/progenitor cells in the bone marrow of mice that were long-term reconstituted by 5 HSC-1 (D) or 5 HSC-2 (E) cells at 17 to 25 weeks after primary transplantation.
(F and G) Clonal analysis of HSC-1 and HSC-2 cells. Single GFP+ HSC-1 (F) or HSC-2 (G) cells were transplanted into 30 irradiated recipient mice per population along with 200,000 recipient bone marrow cells. Some of these mice were long-term multilineage reconstituted and some were transiently multilineage reconstituted.
(H) Long-term competitive reconstitution assay in which 5 CD45.1+ HSC-1 cells and 5 CD45.2+ HSC-2 cells were transplanted into irradiated CD45.1+CD45.2+ recipient mice along with 200,000 CD45.1+CD45.2+ bone marrow cells. Data represent mean ± S.D. from one experiment with a total of 13 recipient mice per treatment. Data in other panels are from 2 (A, B, D, E) or 3 (C, G) independent experiments. *p<0.05; **p<0.01 by student _t_-test. See also Figure S4.
All recipients of 5 HSC-1 cells had donor-derived HSC-1, HSC-2, MPP-1, MPP-2, MPP-3, HPC-1, HPC-2, CMP, GMP, MEP, and CLP cells in their bone marrow (Figure 3D; see Figure S4C for individual mice). Consistent with our cell cycle analysis (Figure 2), this suggests that HSC-1 includes the most primitive HSCs. Recipients of 5 HSC-2 cells had donor-derived HSC-2 cells in their bone marrow in addition to MPP-1, MPP-2, MPP-3, HPC-1, HPC-2, CMP, GMP, MEP, and CLP cells (5 of 5 mice tested; Figures 3E and S4D); however, recipients of HSC-2 cells usually did not have donor-derived HSC-1 cells (Figure S4D). This suggests that HSC-1 cells give rise to HSC-2 cells but that HSC-2 cells have little capacity to form HSC-1 cells.
We also transplanted single HSC-1 or HSC-2 cells along with 200,000 bone marrow cells into irradiated mice. Fourteen of 30 mice that received one HSC-1 cell were reconstituted by donor cells 16 weeks after transplantation, and twelve of these mice exhibited long-term multilineage reconstitution, suggesting that at least 40% of HSC-1 cells are HSCs. The reconstituted recipients of HSC-1 cells exhibited high levels of donor cells at 16 weeks after transplantation (27±24% of nucleated blood cells, mean±SD), usually myeloid-biased reconstitution (Figure 3F). Nine of 30 mice that received one HSC-2 cell were reconstituted by donor cells 16 weeks after transplantation, usually lymphoid-biased reconstitution (Figure 3G). Only four of these recipients were long-term multilineage reconstituted, suggesting that at least 13% of HSC-2 cells were HSCs. The reconstituted recipients of HSC-2 cells had lower levels of donor cells (13±15% of blood cells, mean±SD) than recipients of HSC-1 cells (Figure 3G).
Based on the criteria used to distinguish myeloid-biased, balanced, and lymphoid balanced clones (Muller-Sieburg et al., 2002), 79% of HSC-1 cells were myeloid-biased, 7% were balanced, and 14% were lymphoid-biased (Figure S4E). Among HSC-2 cells, 22% were myeloid-biased, 22% were balanced, and 56% were lymphoid-biased (Figure S4E). Based on the criteria used by Dykstra et al. (Dykstra et al., 2007), the HSC-1 population contained 57% α cells, 21% β cells, 7% γ cells, and 14% δ cells (Figure S4F). The HSC-2 population contained 11% α cells, 11% β cells, 22% γ cells, and 56% δ cells (Figure S4F). SLAM family markers thus make it possible to prospectively enrich subpopulations of HSCs that were retrospectively analyzed in prior studies based on reconstitution patterns in irradiated mice.
We also transplanted 5 CD45.1+ HSC-1 cells and 5 CD45.2+ HSC-2 cells into irradiated CD45.1+CD45.2+ (heterozygous) recipient mice along with 200,000 CD45.1+CD45.2+ recipient whole bone marrow cells. As expected, HSC-1 cells gave significantly higher levels of overall reconstitution, particularly in the myeloid lineage (Figure 3H).
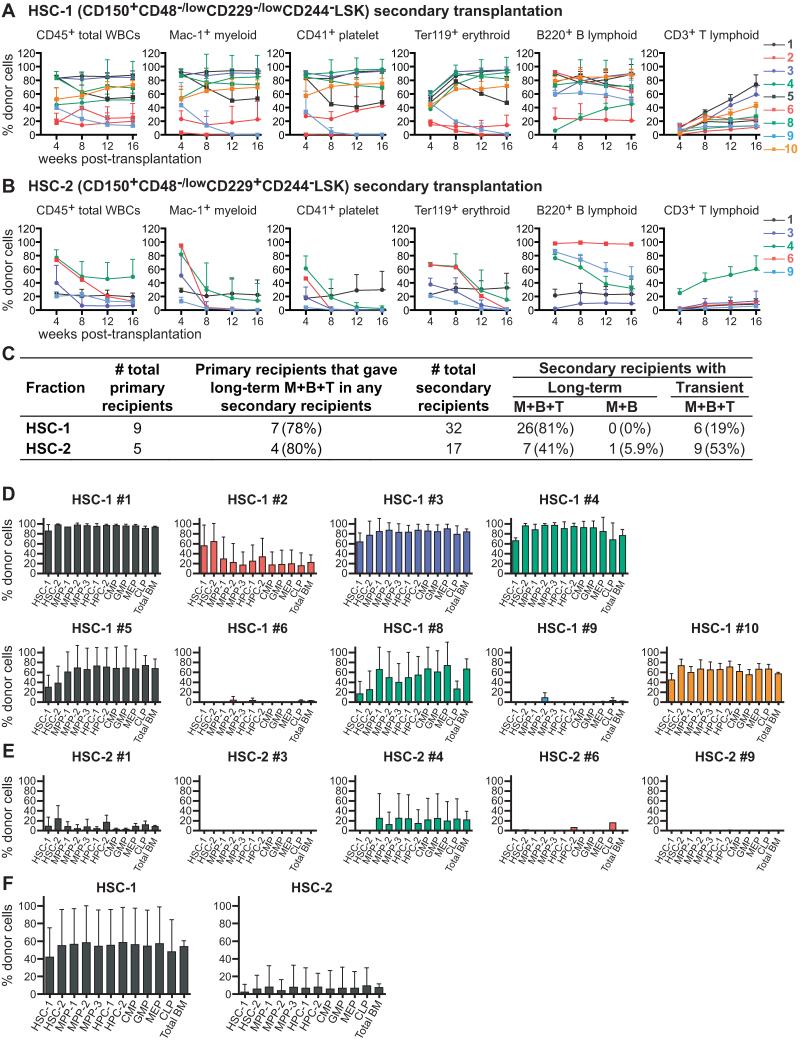
To assess self-renewal potential, five million bone marrow cells from primary recipients that were long-term multilineage reconstituted by donor cells were transplanted into irradiated secondary recipients. Bone marrow from 9 recipients of 5 HSC-1 cells were transplanted into a total of 32 secondary recipients. 26 of these 32 secondary recipients of HSC-1 cells were long-term multilineage reconstituted by donor cells (Figures 4A and 4C). At 17 to 25 weeks after secondary transplantation, most secondary recipients had donor HSC-1, HSC-2, MPP-1, MPP-2, and MPP-3 cells as well as all of the restricted progenitor populations we examined (Figure 4D). HSC-1 cells thus have long-term self-renewal potential and form all of the other stem and progenitor cell populations we examined.
Figure 4. Serial transplantation reveals that HSC-1 cells have more self-renewal potential than HSC-2 cells.
(A and B) Five million bone marrow cells from primary recipients that were long-term reconstituted by 5 HSC-1 (A) or 5 HSC-2 (B) cells were transplanted into secondary recipient mice. The secondary recipient mice were assigned numbers that corresponded to the numbers of the primary recipient mice shown in Figures 3A and 3B. For example, the mean ± S.D. for the frequency of donor blood cells in secondary recipients of cells from primary recipient #1 in Figure 3A is shown as line #1 in Figure 4A. Data are from two independent experiments with 3-4 secondary recipients per primary donor.
(C) Summary of secondary transplantation results from panels A and B.
(D and E) The frequencies of donor-derived hematopoietic stem/progenitor cells in the bone marrow of secondary recipient mice 17-25 weeks after transplantation. As in panels A and B, the secondary recipient mice are numbered according to the primary recipient from which they received cells. Data are mean ± S.D. from 3-4 secondary recipient mice per primary recipient.
(F) The frequencies of donor-derived hematopoietic stem/progenitor cells in all secondary recipients of HSC-1 (mean ± S.D. from the data in Figure 4D, n=32 secondary recipients total) and HSC-2 (mean ± S.D. from the data in Figure 4E, n=17 secondary recipients total) cells.
Five long-term multilineage reconstituted recipients of 5 HSC-2 cells were transplanted into a total of 17 surviving secondary recipients. Eight of these 17 secondary recipients were long-term multilineage reconstituted by donor cells and the other 9 secondary recipients were transiently multilineage reconstituted (Figures 4B and 4C). The secondary recipients of HSC-2 cells that exhibited long-term multilineage reconstitution exhibited declining levels of donor myeloid cells, in contrast to secondary recipients of HSC-1 cells that usually exhibited increasing levels of donor myeloid cells over time (compare Figures 4B and 4A). At 17 to 25 weeks after secondary transplantation, most secondary recipient of HSC-2 cells lacked donor HSC-1, HSC-2, or MPPs (Figure 4E). Secondary recipients of HSC-2 cells exhibited significantly lower levels of donor cell reconstitution than secondary recipients of HSC-1 cells (Figure 4F). HSC-2 cells therefore appear to have more limited self-renewal capacity than HSC-1 cells.
The HSC-2 population can be further subdivided by CD34 expression (Figure S2A). Nine out of 12 recipients of 5 CD34−/low HSC-2 cells were long-term multilineage reconstituted. In contrast, none of the recipients of 5 CD34+ HSC-2 cells were long-term multilineage reconstituted, though they showed transient multilineage or lymphoid-only reconstitution (Figure S4G). These data indicate that CD34+ HSC-2 cells have less HSC activity than CD34−/low HSC-2 cells as expected (Osawa et al., 1996).
We compared the gene expression profiles of HSC-1 and HSC-2 cells with gene sets for megakaryocyte/erythrocyte lineage cells (MkE), granulocyte/macrophage lineage cells (GM), lymphoid lineage cells (Mansson et al., 2007), and fetal liver HSCs (He et al., 2011). The MkE gene set was significantly enriched in HSC-1 cells, whereas GM and lymphoid gene sets were significantly enriched in HSC-2 cells (Figure S4H). Fetal liver HSC-associated genes were significantly enriched in HSC-1 cells (Figure S4H). These results are consistent with our data suggesting that HSC-1 cells are more primitive than HSC-2 cells.
MPP-1 cells include intermediate and transiently reconstituting progenitors
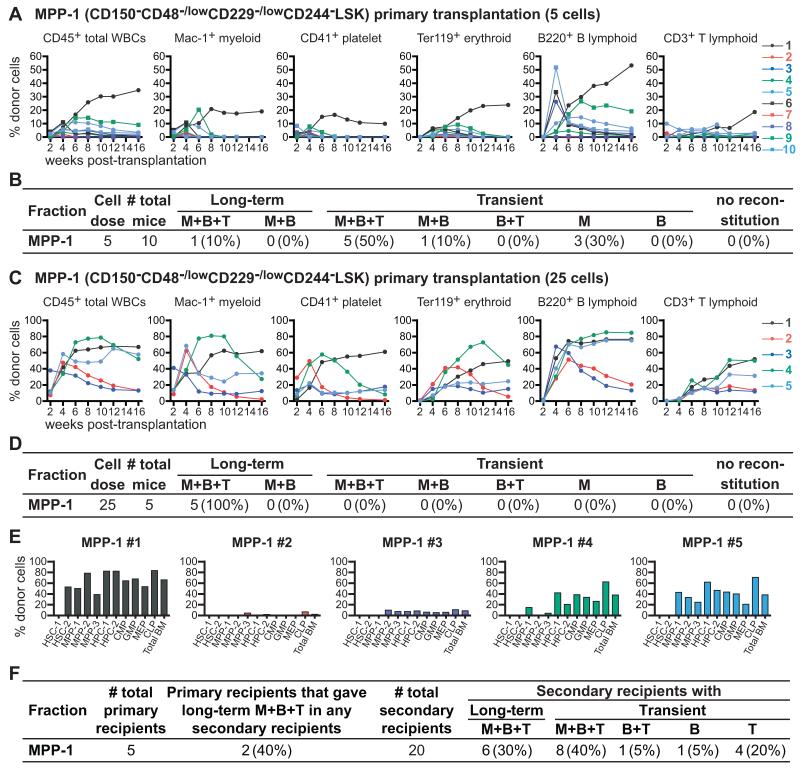
Upon competitive transplantation of 5 MPP-1 cells into irradiated mice only one of 10 recipients was long-term multilineage reconstituted by donor cells (Figures 5A and 5B). Six of 10 recipients were transiently multilineage reconstituted and the remaining recipients were transiently reconstituted only in the myeloid lineage. At a dose of 25 MPP-1 cells, 5 of 5 recipients were long-term multilineage reconstituted (Figures 5C and 5D). However, the levels of myeloid reconstitution in these mice tended to decline over time (Figure 5C), and when we analyzed the bone marrow of these mice 17 weeks after transplantation, none of the recipients had donor-derived HSC-1 cells (Figure 5E). Multiple recipients had donor-derived MPP-1, MPP-2, MPP-3, HPC-1, HPC-2, as well as various restricted progenitor cells.
Figure 5. MPP-1 cells contain a mixture of long-term and transiently reconstituting multipotent progenitors.
(A) Five GFP+CD45.2+ donor cells were transplanted into irradiated CD45.1+ recipient mice along with 200,000 CD45.1+ recipient bone marrow cells. Each line represents the frequency of donor-derived blood cells in a single recipient mouse.
(B) Summary of the primary transplantation results from Figure 5A.
(C) Twenty-five GFP+CD45.2+ donor cells were competitively transplanted into irradiated CD45.1+ recipient mice.
(D) Summary of the results in Figure 5C.
(E) The frequencies of donor-derived hematopoietic stem/progenitor cells in the bone marrow of mice reconstituted by 25 MPP-1 cells, at 17 weeks after transplantation.
(F) Summary of secondary transplantation experiments. Five million bone marrow cells from primary recipients of 25 MPP-1 cells were transplanted into four secondary recipient mice per primary recipient mouse.
To assess self-renewal potential we performed secondary transplants of bone marrow cells from the primary recipients of 25 MPP-1 cells at 17 weeks after transplantation. Two primary recipients gave long-term multilineage reconstitution in the secondary recipients (#1, 5), two gave only transient multilineage reconstitution, and one gave only T lineage reconstitution (Figure 5F). A minority of MPP-1 cells retains the ability to give long-term multilineage reconstitution but these cells appear to have intermediate self-renewal potential and little ability to form HSC-1 or HSC-2 cells. The HSC-2 and MPP-1 populations have successively reduced reconstituting and self-renewal potentials relative to HSC-1 cells.
MPP-2 and MPP-3 are transiently reconstituting multipotent progenitors
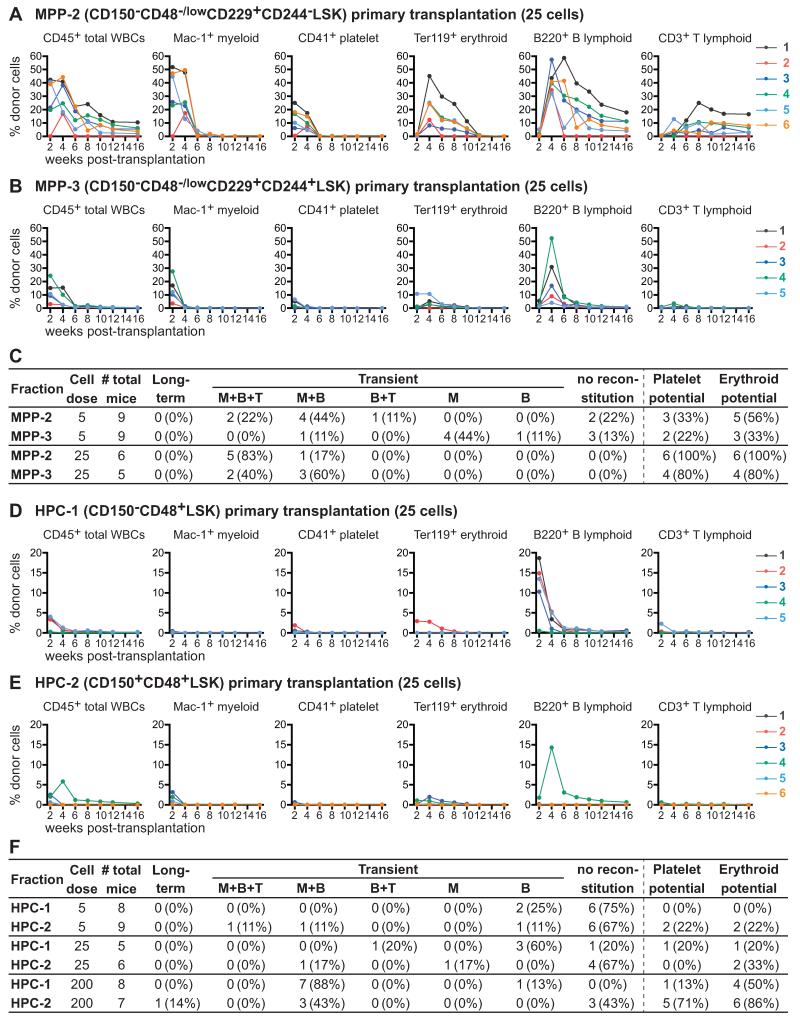
Competitive transplantation of 5 or 25 CD150−CD48−/lowCD229+CD244−LSK MPP-2 cells into irradiated mice yielded transient multilineage reconstitution in nearly all recipients (Figures 6A and 6C). About half of the recipients of 5 MPP-2 cells and all 6 of the recipients of 25 MPP-2 cells were transiently reconstituted by donor erythrocytes and platelets (Figures 6A and 6C). None of the recipients were long-term reconstituted. SLAM family markers can therefore be used to isolate a highly purified population of MPPs, from which only 5 cells can give transient multilineage reconstitution in most recipients, including within the erythroid and platelet lineages.
Figure 6. MPP-2 and MPP-3 cells are transiently reconstituting multipotent progenitors while HPC-1 and HPC-2 cells contain a heterogeneous mix of restricted progenitors.
(A and B) Twenty-five GFP+CD45.2+ MPP-2 (A) or MPP-3 (B) donor cells were transplanted into irradiated CD45.1+ recipient mice along with 200,000 CD45.1+ recipient bone marrow cells. Each line represents the frequency of donor-derived blood cells in a single recipient.
(C) Summary of the reconstitution results from Figure 6A and B.
(D and E) Twenty-five GFP+CD45.2+ HPC-1 (D) or HPC-2 (E) donor cells were transplanted into irradiated CD45.1+ recipient mice along with 200,000 CD45.1+ recipient bone marrow cells. Each line represents the frequency of donor-derived blood cells in a single recipient.
(F) Summary of the reconstitution results from Figure 6D and E. See also Figures S5 and S6.
We also competitively transplanted 5 or 25 CD150−CD48−/lowCD229+CD244+LSK MPP-3 cells into irradiated mice. Recipients of 25 MPP-3 cells were all transiently multilineage reconstituted, though at levels that were significantly (p<0.01 at 4 weeks) lower than in recipients of 25 MPP-2 cells (Figures 6B and 6C). Myeloid chimerism in recipients of 25 MPP-3 cells also appeared to be lost earlier (4-6 weeks after transplantation) than in recipients of 25 MPP-2 cells (6-8 weeks after transplantation). Four of 5 recipients of 25 MPP-3 cells were also transiently reconstituted by platelets and erythrocytes (Figures 6B and 6C). Recipients of 5 MPP-3 cells exhibited transient reconstitution in the myeloid and/or B lineages (Figures 6B and 6C). Two to 3 of the recipients of 5 MPP-3 cells were reconstituted by donor platelets and erythrocytes (Figures 6B and 6C). None of the recipients were long-term reconstituted. MPP-3 cells therefore include multipotent progenitors, though these cells reconstitute more transiently and at lower levels than MPP-2 cells.
HPC-1 and HPC-2 cells contain a mixture of restricted progenitors
Most recipients of 5 HPC-1 cells were not detectably reconstituted by donor cells but two were transiently reconstituted by donor B cells (Figure 6F). Four of 5 recipients of 25 HPC-1 cells were transiently reconstituted by B cells, with or without T cells (Figures 6D, and 6F). Only one of the recipients of 25 HPC-1 cells was transiently reconstituted by platelets and erythrocytes. Seven of 8 recipients of 200 HPC-1 cells were transiently reconstituted by donor myeloid and B cells, 4 of which also had donor erythrocytes but only one of which had platelets (Figure 6F). These data suggest that HPC-1 cells are a heterogeneous population of restricted progenitors that includes early lymphoid progenitors as well as some cells that give very low levels of transient myeloerythroid reconstitution. The kinetics of B lineage reconstitution by HPC-1 cells is earlier than from MPP-3 cells (compare Figure 6D to 6B) suggesting that HPC-1 lymphoid progenitors are more mature than the multipotent MPP-3 cells. HPC-1 cells are therefore the first population from which multilineage reconstitution was not evident from small doses of cells and the only LSK population which rarely formed platelets. Most HPC-1 cells appeared to be restricted progenitors that lacked megakaryocytic potential.
We competitively transplanted 5, 25, or 200 CD150+CD48+LSK HPC-2 cells into irradiated recipient mice. Most recipients of 5 or 25 HPC-2 cells were not detectably reconstituted by donor cells, but most recipients of 200 HPC-2 cells were transiently reconstituted by very low levels of donor erythrocytes and platelets and some were transiently reconstituted by very low levels of donor myeloid and B cells (Figures 6E and 6F). HPC-2 cells appear to contain a heterogeneous collection of restricted progenitors with limited reconstituting potential in vivo.
To better understand the relationship between the SLAM-defined MPPs we studied and previously characterized MPPs we assessed SLAM family marker expression within the CD34−/lowFlt3+LSK, CD34+Flt3−LSK, and CD34+Flt3+LSK populations (Figure S5A). SLAM family markers resolved Flt3+CD34+LSK cells into four distinct subsets of cells in the MPP-2 (8±3% of Flt3+LSK cells), MPP-3 (15±3% of Flt3+LSK cells), HPC-1 (75±5% of Flt3+LSK cells), and HPC-2 (0.7±0.2% of Flt3+LSK cells) populations (Figure S5A). Transplantation of 25 cells from the Flt3+ fractions of these populations into irradiated mice indicated that the Flt3+ MPP-2, MPP-3, and HPC-2 cells each gave rise to platelets and megakaryocytes in vivo while the Flt3+ HPC-1 cells (that represented most of the cells in the CD34+Flt3+LSK population) did not (Figure S6).
The kinetics of reconstitution at early time points in vivo
We analyzed donor chimerism in the myeloid (Mac-1+), erythroid (CD71+), megakaryocyte (CD41+CD61+), or B (B220+) cell lineages in the bone marrow of recipient mice 10 days after transplantation. Each recipient mouse was transplanted with 30 donor HSC-1, HSC-2, MPP-1, MPP-2, MPP-3, HPC-1, or HPC-2 cells without any competitor cells. We detected virtually no donor-derived progeny from HSC-1 cells, consistent with other data indicating that this is a very primitive and quiescent population (Figure S5B). HSC-2 cells gave rise to low levels of donor myeloid, erythroid, megakaryocyte, and B cells, indicating that these cells are more readily activated after transplantation than HSC-1 cells (Figure S5B). MPP-1, MPP-2, and MPP-3 cells all tended to give higher levels of reconstitution in all lineages than HSC-2 cells, indicating that these populations are more mature than HSCs while remaining multipotent and able to form both erythrocytes and megakaryocytes (Figure S5B). HPC-1 cells formed primarily B cells and some myeloid cells, but few, if any, erythroid or megakaryocyte lineage cells. HPC-2 cells formed very low levels of cells in multiple lineages, including myeloid cells, B cells, and megakaryocyte lineage cells (Figure S5B).
Colony forming potential
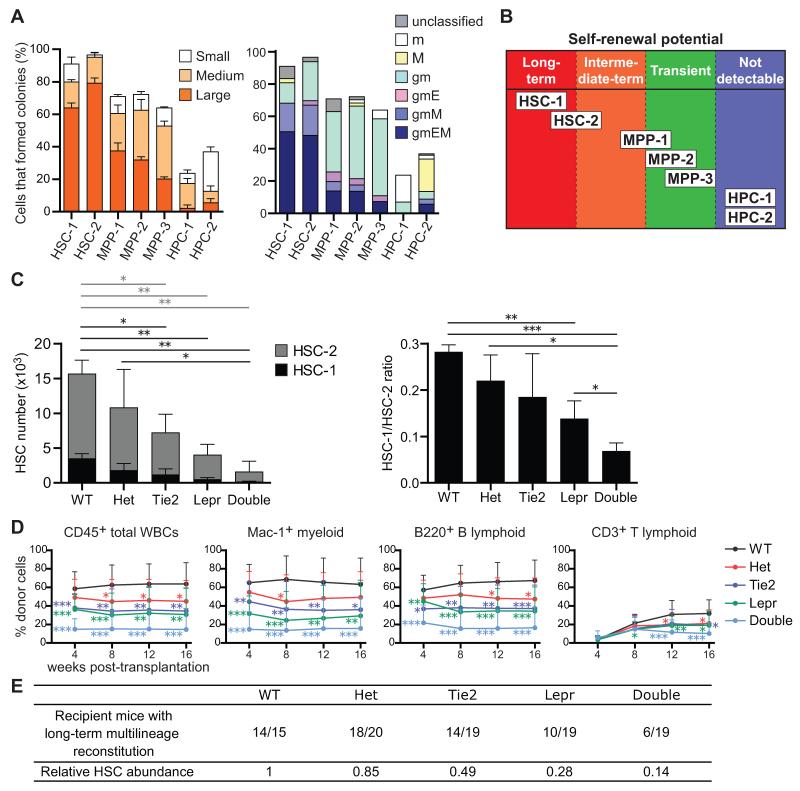
To investigate the clonogenic potential of individual cells in each stem/progenitor cell population without regard to whether they have sufficient reconstituting activity to be detected in vivo, we assessed colony formation in culture. As expected, 91±1.2% and 96±1.2% of HSC-1 and HSC-2 cells formed colonies, usually large mixed myeloid or myeloerythroid/megakaryocyte colonies (Figure 7A). Among MPPs, 64-72% of cells in each population formed colonies, mainly myeloid or mixed myeloerythroid/megakaryocyte colonies (Figure 7A). Thus, most MPP-1, MPP-2, and MPP-3 cells had the potential to form myeloid cells and at least 10-20% of the cells in each population were able to form erythrocytes and/or megakaryocytes.
Figure 7. Quiescent HSC-1 cells are maintained by a perivascular niche in which Scf is expressed by endothelial and perivascular stromal cells.
(A) Colony formation assay. Forty-eight cells from each HSC, MPP, and HPC fraction were sorted, one cell per well. Large (>2 mm in diameter), medium (>1 mm in diameter), and small (<1 mm in diameter) colonies were counted (left panel, mean ± S.D.) and their composition assessed by Giemsa staining after 14 days of culture (right panel, mean ± S.D.). g, granulocyte; m, macrophage; E, erythroblasts; M, megakaryocyte. Data represent 6 independent experiments.
(B) Schematic summarizing the self-renewal potentials of each fraction of hematopoietic stem/progenitor cells distinguished using SLAM family markers.
(C) The number of HSC-1 and HSC-2 cells in femurs and tibias from 5-10 month old adult wild-type (WT), Scf fl/− (Het), Tie2-cre; Scffl/− (Tie2), Lepr-cre; Scffl/− (Lepr), and Tie2-cre; Lepr-cre; Scffl/− (Double) mice (left panel). Statistical significance was separately analyzed for HSC-1 cells (black bars) and HSC-2 cells (gray bars). The ratio of HSC-1 to HSC-2 cells (right panel). Data represent mean ± S.D. from 3 independent experiments.
(D) 300,000 donor bone marrow cells from each genetic background were transplanted into irradiated recipient mice along with 300,000 recipient bone marrow cells. Data represent mean ± S.D. from 4 independent experiments with a total of 15-20 recipient mice per genotype.
(E) Summary of reconstitution results from panel D. Recipient mice were considered long-term multilineage reconstituted if donor myeloid, B, and T cells represented more than 1% of blood cells in each lineage 16 weeks after transplantation. *p<0.05, **p<0.01, ***p<0.001 by student’s _t_-test. See also Figure S7.
Only 24±2.4% of HPC-1 cells and 37±12% of HPC-2 cells formed colonies in culture (Figure 7A). None of the colonies contained erythrocytes or megakaryocytes. When these data are combined with the lymphoid restricted reconstitution patterns observed from HPC-1 cells in vivo (Figures 6D and 6F) the data suggest that HPC-1 cells include a mixture of myeloid-restricted progenitors and lymphoid-restricted progenitors that have largely lost the potential to make erythrocytes or megakaryocytes. Alternatively, a minority of HPC-1 cells may be multipotent progenitors whose ability to reconstitute the myeloid lineage is very limited and cannot be detected from small numbers of HPC-1 cells in vivo. HPC-2 cells formed mainly small megakaryocyte colonies (5-20 cells) as well as smaller numbers of mixed myeloerythroid/megakaryocyte colonies (Figure 7A), consistent with the reconstitution data observed in vivo (Figures 6E and 6F).
Quiescent HSCs are maintained by a perivascular niche
To test whether quiescent HSCs depend upon a perivascular niche we assessed the depletion of HSC-1 and HSC-2 cells from Tie2-Cre; Lepr-Cre; Scffl/− mice. Relative to wild-type controls, both HSC-1 and HSC-2 cells were significantly depleted in Tie2-Cre; Scffl/− mice, in Lepr-Cre; Scffl/− mice, and in Tie2-Cre; Lepr-Cre; Scffl/− mice (Figure 7C). Almost no HSC-1 cells could be found in the bone marrow of Tie2-Cre; Lepr-Cre; Scffl/− mice and the ratio of HSC-1/HSC-2 cells was significantly lower in Tie2-Cre; Lepr-Cre; Scffl/− mice as compared to mice with other genotypes (Figure 7C). MPP-1, MPP-2, and MPP-3 cells, but not HPC-1 cells, were also depleted from the bone marrow of Tie2-Cre; Lepr-Cre; Scffl/− mice (Figure S7A). Most HSCs, including quiescent HSC-1 cells, are therefore maintained by a perivascular niche.
The number of HSC-1 and HSC-2 cells in the spleen of Tie2-Cre; Lepr-Cre; Scffl/− mice was significantly higher than in littermate controls, but most of this increase came from HSC-2 cells (Figure S7B). We did not detect a significant change in the frequency of HSC-1 or HSC-2 cells in the blood of Tie2-Cre; Lepr-Cre; Scffl/− mice (data not shown). Despite the increase in the spleen, the total number of HSC-1 and HSC-2 cells in Tie2-Cre; Lepr-Cre; Scffl/− mice significantly declined relative to controls (Figure S7B). Consistent with this, Tie2-Cre; Lepr-Cre; Scffl/− mice became anemic and white blood cell counts declined upon aging (Figure S7E).
We transplanted 300,000 bone marrow cells from each of these genetic backgrounds along with 300,000 recipient bone marrow cells into irradiated recipient mice. Relative to mice transplanted with wild-type control bone marrow, the levels of donor cell reconstitution were significantly reduced in mice transplanted with bone marrow from Tie2-Cre; Scffl/− mice, Lepr-Cre; Scffl/− mice, or Tie2-Cre; Lepr-Cre; Scffl/− mice (Figure 7D). Fourteen of 15 recipients of wild-type cells and 18 of 20 recipients of Scffl/− control cells were long-term multilineage reconstituted by donor cells. In contrast, only 14 of 19, 10 of 19, and 6 of 19 mice were long-term multilineage reconstituted by donor cells from Tie2-Cre; Scffl/− mice, Lepr-Cre; Scffl/− mice, and Tie2-Cre; Lepr-Cre; Scffl/− mice, respectively (Figure 7E). Using these data we calculated HSC frequencies based on Poisson statistics (Smith et al., 1991). Relative to wild-type littermates, this indicated an approximately 50% decline in HSC frequency in Tie2-Cre; Scffl/− bone marrow, 70% in Lepr-Cre; Scffl/− bone marrow, and 85% in Tie2-Cre; Lepr-Cre; Scffl/− bone marrow (Figure 7E).
Five million bone marrow cells from 3 primary recipients in each treatment were transplanted into 3-4 secondary recipients each. All secondary recipients of Scffl/− bone marrow cells were long-term multilineage reconstituted by donor cell whereas 9 of 10, 5 of 12, and none of 12 recipients were long-term multilineage reconstituted by Tie2-Cre; Scffl/− bone marrow, Lepr-Cre; Scffl/− bone marrow, or Tie2-Cre; Lepr-Cre; Scffl/− bone marrow, respectively (Figure S7D). The failure of Tie2-Cre; Lepr-Cre; Scffl/− bone marrow cells to give long-term multilineage reconstitution of any secondary recipients is consistent with the analysis of HSC-1 and HSC-2 frequencies and confirms that the vast majority of HSCs in the bone marrow, including most quiescent HSCs, depend upon a perivascular niche for their maintenance.
DISCUSSION
The CD150, CD48, CD229, and CD244 SLAM family markers are differentially expressed among LSK cells in a manner that distinguishes functionally distinct subpopulations of HSCs and MPPs (Figures 7B and S7F). Prior use of markers such as CD150 expression level (Morita et al., 2010), CD34 expression (Osawa et al., 1996), Flk2 expression (Christensen and Weissman, 2001; Yang et al., 2005), and α2 integrin expression (Benveniste et al., 2010) distinguished functionally distinct subpopulations of HSCs and MPPs. However, many subsets of HSCs and MPPs remained heterogeneous such that certain subpopulations were largely characterized through retrospective analysis of reconstitution patterns in irradiated mice (Copley et al., 2012). The multiplexing of SLAM family markers distinguishes multiple subsets of HSCs and MPPs that differ in terms of reconstituting potential and other functional properties, allowing each subpopulation to be prospectively isolated to a high degree of purity.
HSC-1 cells were the most quiescent (Figure 2) and gave mainly myeloid-biased reconstitution (Figures 3F, S4E, and S4F). HSC-2 cells divided more frequently and gave mainly lymphoid-biased reconstitution (Figures 2; 3G, S4E and S4F). MPP-1 cells gave transient multilineage reconstitution, including myeloid reconstitution for up to 8 weeks after transplantation (Figures 5A-5D). MPP-1 cells self-renewed and formed all MPP and HPC populations, but never formed HSC-1 cells and almost never formed HSC-2 cells (Figure 5E). MPP-2 cells contained transiently reconstituting multipotent progenitors that reconstituted the myeloid lineage for up to 6 weeks after transplantation (Figures 6A and 6C). MPP-3 cells gave transient multilineage reconstitution, including myeloid reconstitution for up to 4 weeks after transplantation (Figures 6B and 6C). MPP-1, MPP-2, and MPP-3 cells all gave rise to both megakaryocytes/platelets and erythrocytes, in vitro and in vivo, even when only 5 cells from these populations were transplanted into irradiated mice (Figures 5A-5C, 6A-6C, 7A, and S5B).
The ability of Flt3+LSK MPPs to make megakaryocytes and erythrocytes has been debated (Adolfsson et al., 2005; Forsberg et al., 2006; Boyer et al., 2011). One complication is that Flt3+LSK cells are heterogeneous and prior in vivo studies injected 50-10,000 cells into irradiated mice to test developmental potential (Adolfsson et al., 2005; Yang et al., 2005;Forsberg et al., 2006; Luc et al., 2008). The inability to perform a clonal, or near clonal, analysis of developmental potential in vivo made it difficult to take into account the heterogeneity within the population. Our data indicate that SLAM family markers can resolve Flt3+CD34+LSK cells into a hierarchy of at least 4 functionally distinct subpopulations contained within the MPP-2, MPP-3, HPC-1, and HPC-2 populations (Figure S5A). Consistent with prior studies (Adolfsson et al., 2005), most Flt3+LSK cells were contained within the HPC-1 population (Figure S2A and S5A) and the Flt3+ subset of HPC-1 cells formed myeloid and/or B cells but not platelets or erythrocytes (Figures S6D and S6F). What remains uncertain from our data is whether these Flt3+ HPC-1 cells include multipotent cells with both myeloid and lymphoid potential or a mixture of lymphoid-restricted and myeloid-restricted progenitors.
Consistent with other published studies (Forsberg et al., 2006; Boyer et al., 2011) both MPP-2 and MPP-3 cells included Flt3+CD34+LSK cells (Figures S2A and S5A) and the Flt3+ MPP-2 and MPP-3 cells gave transient multilineage reconstitution that included platelets and erythrocytes (Figures S6A-S6C). Our data therefore suggest that there are multiple populations of Flt3+ MPPs that retain erythroid, megakaryocytic, myeloid, and lymphoid potential, though this does not rule out the possibility of an even more mature MPP within the HPC-1 fraction whose myeloid reconstituting activity is difficult to detect in vivo but that nonetheless forms myeloid and lymphoid cells while lacking erythroid and megakaryocytic potential.
Using the new SLAM family markers, we found that quiescent HSC-1 cells are even more dependent upon SCF expression by endothelial cells and perivascular stromal cells than HSC-2 cells (Figures 7C-7E and S7D). Elimination of vascular/perivascular SCF expression in Tie2-Cre; Lepr-Cre; Scffl/− mice eliminated nearly all HSC-1 cells and most HSC-2 cells. Consistent with this, cells that gave long-term multilineage reconstitution in primary and secondary recipient mice were profoundly depleted in the bone marrow of Tie2-Cre; Lepr-Cre; Scffl/− (Figure 7D, E) and these mice developed anemia upon aging (Figure S7E). Most or all quiescent HSCs are maintained in a perivascular niche by factors secreted by endothelial and perivascular stromal cells.
EXPERIMENTAL PROCEDURES
Mice
For reconstitution assays, donor cells were isolated from 8-12-week-old UBC-GFP mice (Schaefer et al., 2001) that were backcrossed at least 6 times onto a C57BL/Ka-CD45.2:Thy1.1 background. Recipient mice were C57BL/Ka-CD45.1:Thy1.2, greater than 8 weeks old, unless otherwise mentioned. For cell surface marker analysis, Ki-67 and PI staining, BrdU incorporation assays, and in vitro colony formation assays, cells were obtained from C57BL/Ka-CD45.2:Thy1.1 mice. Col1A1-H2B-GFP (Foudi et al., 2009) and Rosa26-M2-rtTA(Hochedlinger et al., 2005) transgenic mice were used for H2B-GFP label retention assays. For conditional Scf deletion analysis, we crossed Scffl mice (Ding et al., 2012) with Tie2-Cre (Koni et al., 2001) and Lepr-Cre mice (DeFalco et al., 2001). All mice used in this study were housed in the Unit for Laboratory Animal Medicine at the University of Michigan (UM) or in the Animal Resource Center at the University of Texas Southwestern Medical Center (UTSW). All procedures were approved by the UM Committee on the Use and Care of Animals and by the UTSW Institutional Animal Care and Use Committee.
Flow cytometry
Bone marrow cells were flushed from tibias and femurs using Hank’s Balanced Salt Solution (HBSS, Invitrogen) without calcium or magnesium, supplemented with 1% head-inactivated calf serum (Gibco). When we flushed bones, we drilled a hole in the intact bone using a needle and did not clip off the epiphyses. Bone marrow cells were sometimes obtained by crushing tibias, femurs, pelvic bones, and vertebrae with a mortar and pestle. Both methods gave indistinguishable results. Cells were gently triturated and filtered through a nylon screen (45 μm, Sefar America) to obtain a single-cell suspension. Lineage markers were anti-CD2, anti-CD3ε, anti-CD5, anti-CD8a, anti-B220, anti-Gr-1, and anti-Ter119. Anti-CD41 was included in the lineage cocktail when staining HSCs from young adult mice, but not from older mice (such as in the niche experiments) due to the increase in CD41 expression by HSCs with age. In older mice CD41 was stained separately to exclude CD41high megakaryocyte lineage cells. Bone marrow cells were incubated with biotinylated antibodies against lineage markers, followed by anti-biotin microbeads, and Lineage+ cells were depleted by autoMACS (Miltenyi Biotec). The fraction enriched for Lineage−/low cells was further incubated with phycoetythrin (PE)-Cy7 conjugated anti-CD150 antibody, fluorescein isothiocyanate (FITC)-conjugated anti-CD48, allophycocyanin (APC)-conjugated anti-CD229, PE-conjugated anti-CD244, Alexa Fluor 700 conjugated anti-Sca-1, APC-eFluor 780 conjugated anti-c-Kit, and streptavidin PE-Texas Red. Dead cells were excluded by staining with 4′, 6-diamidino-2-phenylindole (DAPI, Sigma). All antibodies are listed in Supplemental Experimental Procedures.
Supplementary Material
01
HIGHLIGHTS.
- LSK cells can be subdivided into a hierarchy of 7 populations using 4 SLAM markers
- CD229 expression distinguishes functionally distinct subsets of HSCs and MPPs
- CD229− HSCs are mainly myeloid biased and more quiescent than CD229+ HSCs
- Most HSCs, including CD229− quiescent HSCs, are maintained by a perivascular niche
ACKNOWLEDGEMENTS
This work was supported by the National Heart, Lung and Blood Institute (HL097760), the Howard Hughes Medical Institute (HHMI), and the Cancer Prevention and Research Institute of Texas. L.D. was supported by the Helen Hay Whitney Foundation and by HHMI. H.O. was supported by postdoctoral fellowships from the Japanese Society for the Promotion of Science and from the Uehara Memorial Foundation. H.O. performed most experiments. H.O and L.D. performed experiments in Figures 7C-7E, and S7A-S7D. H.O. and S.J.M. designed and interpreted all experiments and wrote the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Balazs AB, Fabian AJ, Esmon CT, Mulligan RC. Endothelial protein C receptor (CD201) explicitly identifies hematopoietic stem cells in murine bone marrow. Blood. 2006;107:2317–2321. doi: 10.1182/blood-2005-06-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerman I, Bhattacharya D, Zandi S, Sigvardsson M, Weissman IL, Bryder D, Rossi DJ. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc. Nat. Acad. Sci. USA. 2010;107:5465–5470. doi: 10.1073/pnas.1000834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste P, Frelin C, Janmohamed S, Barbara M, Herrington R, Hyam D, Iscove NN. Intermediate-term hematopoietic stem cells with extended but time-limited reconstitution potential. Cell Stem Cell. 2010;6:48–58. doi: 10.1016/j.stem.2009.11.014. [DOI] [PubMed] [Google Scholar]
- Boyer SW, Schroeder AV, Smith-Berdan S, Forsberg EC. All hematopoietic cells develop from hematopoietic stem cells through Flk2/Flt3-positive progenitor cells. Cell Stem Cell. 2011;9:64–73. doi: 10.1016/j.stem.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Amer. J Pathol. 2006;169:338–346. doi: 10.2353/ajpath.2006.060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challen GA, Boles NC, Chambers SM, Goodell MA. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-beta1. Cell Stem Cell. 2010;6:265–278. doi: 10.1016/j.stem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc. Nat. Acad. Sci. USA. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copley MR, Beer PA, Eaves CJ. Hematopoietic stem cell heterogeneity takes center stage. Cell Stem Cell. 2012;10:690–697. doi: 10.1016/j.stem.2012.05.006. [DOI] [PubMed] [Google Scholar]
- DeFalco J, Tomishima M, Liu H, Zhao C, Cai X, Marth JD, Enquist L, Friedman JM. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291:2608–2613. doi: 10.1126/science.1056602. [DOI] [PubMed] [Google Scholar]
- Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra B, Kent D, Bowie M, McCaffrey L, Hamilton M, Lyons K, Lee SJ, Brinkman R, Eaves C. Long-term propagation of distinct hematopoietic differentiation programs in vivo. Cell Stem Cell. 2007;1:218–229. doi: 10.1016/j.stem.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Ema H, Sudo K, Seita J, Matsubara A, Morita Y, Osawa M, Takatsu K, Takaki S, Nakauchi H. Quantification of self-renewal capacity in single hematopoietic stem cells from normal and Lnk-deficient mice. Developmental Cell. 2005;8:907–914. doi: 10.1016/j.devcel.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Forsberg EC, Prohaska SS, Katzman S, Heffner GC, Stuart JM, Weissman IL. Differential expression of novel potential regulators in hematopoietic stem cells. PLoS Genet. 2005;1:e28. doi: 10.1371/journal.pgen.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg EC, Serwold T, Kogan S, Weissman IL, Passegue E. New evidence supporting megakaryocyte-erythrocyte potential of flk2/flt3+ multipotent hematopoietic progenitors. Cell. 2006;126:415–426. doi: 10.1016/j.cell.2006.06.037. [DOI] [PubMed] [Google Scholar]
- Foudi A, Hochedlinger K, Van Buren D, Schindler JW, Jaenisch R, Carey V, Hock H. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat. Biotechnol. 2009;27:84–90. doi: 10.1038/nbt.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J. Exp. Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum A, Hsu Y-M, Schuettpelz L, Christopher M, Nagasawa T, Link DC. Cxcl12 production by early mesenchymal progenitors is required for hematopoietic stem cell maintenance. Nature. 2012;495:227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Kim I, Lim MS, Morrison SJ. Sox17 expression confers self-renewal potential and fetal stem cell characteristics upon adult hematopoietic progenitors. Genes & Dev. 2011;25:1613–1627. doi: 10.1101/gad.2052911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochedlinger K, Yamada Y, Beard C, Jaenisch R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121:465–477. doi: 10.1016/j.cell.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Ikuta K, Weissman IL. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc. Nat. Acad. Sci. USA. 1992;89:1502–1506. doi: 10.1073/pnas.89.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent DG, Copley MR, Benz C, Wohrer S, Dykstra BJ, Ma E, Cheyne J, Zhao Y, Bowie MB, Gasparetto M, et al. Prospective isolation and molecular characterization of hematopoietic stem cells with durable self-renewal potential. Blood. 2009;113:6342–6350. doi: 10.1182/blood-2008-12-192054. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, He S, Ashkenazi R, Gentry SN, Teta M, Kushner JA, Jackson TL, Morrison SJ. Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature. 2007;449:238–242. doi: 10.1038/nature06115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM Family Receptors Distinguish Hematopoietic Stem and Progenitor Cells and Reveal Endothelial Niches for Stem Cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Morrison SJ. CD150- cells are transiently reconstituting multipotent progenitors with little or no stem cell activity. Blood. 2008;111:4413–4414. doi: 10.1182/blood-2007-12-129601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, He S, Yilmaz OH, Kiel MJ, Morrison SJ. Enhanced purification of fetal liver hematopoietic stem cells using SLAM family receptors. Blood. 2006;108:737–744. doi: 10.1182/blood-2005-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koni PA, Joshi SK, Temann UA, Olson D, Burkly L, Flavell RA. Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J. Exp. Med. 2001;193:741–754. doi: 10.1084/jem.193.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luc S, Anderson K, Kharazi S, Buza-Vidas N, Boiers C, Jensen CT, Ma Z, Wittmann L, Jacobsen SE. Down-regulation of Mpl marks the transition to lymphoid-primed multipotent progenitors with gradual loss of granulocyte-monocyte potential. Blood. 2008;111:3424–3434. doi: 10.1182/blood-2007-08-108324. [DOI] [PubMed] [Google Scholar]
- Mansson R, Hultquist A, Luc S, Yang L, Anderson K, Kharazi S, Al-Hashmi S, Liuba K, Thoren L, Adolfsson J, et al. Molecular evidence for hierarchical transcriptional lineage priming in fetal and adult stem cells and multipotent progenitors. Immunity. 2007;26:407–419. doi: 10.1016/j.immuni.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y, Ema H, Nakauchi H. Heterogeneity and hierarchy within the most primitive hematopoietic stem cell compartment. J. Exp. Med. 2010;207:1173–1182. doi: 10.1084/jem.20091318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Wandycz AM, Hemmati HD, Wright DE, Weissman IL. Identification of a lineage of multipotent hematopoietic progenitors. Development. 1997;124:1929–1939. doi: 10.1242/dev.124.10.1929. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Muller-Sieburg CE, Cho RH, Thoman M, Adkins B, Sieburg HB. Deterministic regulation of hematopoietic stem cell self-renewal and differentiation. Blood. 2002;100:1302–1309. [PubMed] [Google Scholar]
- Ooi AG, Karsunky H, Majeti R, Butz S, Vestweber D, Ishida T, Quertermous T, Weissman IL, Forsberg EC. The adhesion molecule esam1 is a novel hematopoietic stem cell marker. Stem Cells. 2009;27:653–661. doi: 10.1634/stemcells.2008-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- Papathanasiou P, Attema JL, Karsunky H, Xu J, Smale ST, Weissman IL. Evaluation of the long-term reconstituting subset of hematopoietic stem cells with CD150. Stem Cells. 2009;27:2498–2508. doi: 10.1002/stem.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer BC, Schaefer ML, Kappler JW, Marrack P, Kedl RM. Observation of antigen-dependent CD8+ T-cell/dendritic cell interactions in vivo. Cell. Immunol. 2001;214:110–122. doi: 10.1006/cimm.2001.1895. [DOI] [PubMed] [Google Scholar]
- Smith LG, Weissman IL, Heimfeld S. Clonal analysis of hematopoietic stem-cell differentiation in vivo. Proc. Nat. Acad. Sci. USA. 1991;88:2788–2792. doi: 10.1073/pnas.88.7.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Uchida N, Aguila HL, Fleming WH, Jerabek L, Weissman IL. Rapid and sustained hematopoietic recovery in lethally irradiated mice transplanted with purified Thy-1.1loLin-Sca-1+ hematopoietic stem cells. Blood. 1994;83:3758–3779. [PubMed] [Google Scholar]
- Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- Yang L, Bryder D, Adolfsson J, Nygren J, Mansson R, Sigvardsson M, Jacobsen SE. Identification of Lin(−)Sca1(+)kit(+)CD34(+)Flt3- short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood. 2005;105:2717–2723. doi: 10.1182/blood-2004-06-2159. [DOI] [PubMed] [Google Scholar]
- Yilmaz OH, Kiel MJ, Morrison SJ. SLAM family markers are conserved among hematopoietic stem cells from old and reconstituted mice and markedly increase their purity. Blood. 2006;107:924–930. doi: 10.1182/blood-2005-05-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T, Oritani K, Butz S, Kokame K, Kincade PW, Miyata T, Vestweber D, Kanakura Y. The endothelial antigen ESAM marks primitive hematopoietic progenitors throughout life in mice. Blood. 2009;113:2914–2923. doi: 10.1182/blood-2008-07-167106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01