Whole Exome Sequencing Identifies TTC7A Mutations for Combined Immunodeficiency with Intestinal Atresias (original) (raw)
. Author manuscript; available in PMC: 2014 Sep 1.
Published in final edited form as: J Allergy Clin Immunol. 2013 Jul 4;132(3):656–664.e17. doi: 10.1016/j.jaci.2013.06.013
Abstract
Background
Combined Immunodeficiency with Multiple Intestinal Atresias (CID-MIA) is a rare hereditary disease characterized by intestinal obstructions and profound immune defects.
Objective
We sought to determine the underlying genetic causes of CID-MIA by analyzing the exomic sequence of 5 patients and their healthy direct relatives from 5 unrelated families.
Methods
We performed whole exome sequencing on 5 CID-MIA patients and 10 healthy direct family members belonging to 5 unrelated families with CID-MIA. We also performed targeted Sanger sequencing for the candidate gene TTC7A on 3 additional CID-MIA patients.
Results
Through analysis and comparison of the exomic sequence of the individuals from these 5 families, we identified biallelic damaging mutations in the TTC7A gene, for a total of 7 distinct mutations. Targeted TTC7A gene sequencing in 3 additional unrelated patients with CID-MIA revealed biallelic deleterious mutations in two of them, as well as an aberrant splice product in the third patient. Staining of normal thymus showed that the TTC7A protein is expressed in thymic epithelial cells as well as in thymocytes. Moreover, severe lymphoid depletion was observed in the thymus and peripheral lymphoid tissues from two patients with CID-MIA.
Conclusions
We identified deleterious mutations of the TTC7A gene in 8 unrelated patients with CID-MIA and demonstrated that the TTC7A protein is expressed in the thymus. Our results strongly suggest that TTC7A gene defects cause CID-MIA.
Clinical Implications
Damaging mutations in the gene TTC7A should be scrutinized in patients with CID-MIA. Characterization of the role of this protein in the immune system and intestinal development, as well as in thymic epithelial cells may have important therapeutic implications.
Keywords: Combined Immunodeficiency with Multiple Intestinal Atresias, Tetracopeptide Repeat Domain 7A, Whole Exome Sequencing, Thymus
INTRODUCTION
Hereditary multiple intestinal atresias (MIA, OMIM 243150) is a rare condition characterized by a variable number of atresias that may affect both the small and the large bowel.1, 2 The disease is typically very severe, requiring early surgical intervention. The association of MIA with immunodeficiency was reported for the first time in 19903, and confirmed by several additional studies.4–7 Severe combined immunodeficiency (SCID), leading to increased susceptibility to bacterial and opportunistic infections,4–7 and fatal graft-versus-host disease (GvHD) following transfusion of unirradiated blood products or combined liver and small bowel transplantation have been reported.8, 9
Although most cases of CID-MIA are sporadic, a genetic basis with autosomal recessive inheritance was postulated when 5 French-Canadian cases in 3 sibships with common ancestry were described.10 Recurrence of cases in the same sibship 3, 6, 7, 11, 12 and parental consanguinity5, 11, 13–15 have since been reported in several other families of various descent.
Whole Exome Sequencing (WES) is a powerful tool for studies of hereditary diseases in which obvious gene candidates have been ruled out.16–18 By using WES, we have identified deleterious biallelic mutations in the Tetratricopeptide Repeat Domain 7A (TTC7A) gene in 5 patients from unrelated families with SCID-MIA, belonging to different ethnic groups. We have also identified TTC7A mutations in 3 additional patients, from whom pathological specimens were available. Staining of normal human thymus by immunohistochemistry revealed expression of the TTC7A protein in normal thymus. Severe lymphoid depletion was demonstrated in post-mortem examination of the thymus and peripheral lymphoid tissue of two of the affected patients. Overall, our results strongly indicate that TTC7A mutations are responsible for CID-MIA and interfere with normal thymopoiesis.
METHODS
Human Sample Collection and DNA Extraction
SCID-MIA patients and their direct family members were enrolled in our study upon informed consent under IRB-approved protocol 04-09-113 (Children’s Hospital Boston) and Ethical Committee approval (Spedali Civili Brescia, Italy). Genomic DNA was isolated using the automatic DNA extractor Maxwell 16 (Promega, Madison, WI, USA).
Whole Exome Sequencing and Data Analysis
Whole exome enrichment was performed using Agilent’s SureSelect Human All Exon Kit 50M (Agilent Technologies, Santa Clara, CA, USA) and sequenced with the Illumina HiSeq 2000 sequencer. Sequencing reads were mapped with Burrows-Wheeler Aligner (version 0.6.0)19 and variants were called with the Genome Analysis Toolkit (version 1.3-17-gc62082b).20, 21 Details are described in SUPPLEMENTARY METHODS in Online Repository Materials.
Sanger Sequencing Validation
The chromosomal regions containing the identified TTC7A damaging variants in families F1~F5 were amplified by PCR with the Phusion DNA Polymerase (New England BioLabs, Ipswich, MA, USA) and subjected to Sanger sequencing through ELIM BIOPHARM (http://www.elimbio.com/). A list of sequencing primers used is included in Table E1. Sequencing of genomic DNA corresponding to the coding regions of the TTC7A (ENST00000319190) gene was performed in patient F6-A and his mother, patients F7-A and F8-A, and parents and siblings of patients F2-A and F3-A by direct sequencing after PCR amplification of exons and flanking intronic regions (Primers and conditions available upon request).
RNA Analysis of Human Samples
Total RNA was isolated from Peripheral Blood Mononuclear Cells, fibroblasts and induced pluripotent stem cells (iPSCs) using the RNeasy Mini kit (QIAGEN, Hilden, Germany) and 200 ng of DNase I-treated total RNA was transcribed into first strand cDNA by the GeneAmp RNA PCR kit (Applied Biosystems, Foster City, CA, USA). Analysis of TTC7A and GAPDH (as expression control) expression was performed using RealTime PCR with Assays-on-Demand products and TaqMan Master Mix from Applied Biosystems. Primers for RT-PCR are reported in the SUPPLEMENTARY METHODS in Online Repository Materials.
Immunohistochemistry analysis of TTC7A expression in human tissues
Four micron sections were obtained from formalin-fixed paraffin embedded normal human thymus, mesenteric lymph node biopsy from patient F7-A, and post-mortem paraffin-embedded thymus, ingluinal lymph node and spleen from patient F8-A. Details on immunohistochemistry staining are described in SUPPLEMENTARY METHODS in Online Repository Materials.
Microarray analysis of murine Ttc7 mRNA expression
Microarray analysis of Ttc7 mRNA expression in sorted murine lymphoid cells and in mouse bone marrow thymus were performed as previously described22. Details are provided in SUPPLEMENTARY METHODS in Online Repository Materials.
RESULTS
Clinical and Immunological Features of Patients
We analyzed a total of eight unrelated individuals suffering from CID-MIA. Patient 1 (F1-A) was born to consanguineous parents of Arabic origin and was diagnosed at birth with pyloric and anal atresia. Surgery was complicated by Enterococcus fecalis bacteremia. Immunological investigations at 15 days of life revealed moderate T cell lymphopenia, with marked decrease of CD8+ T cells, decreased in vitro proliferation to phytoemagglutinin (PHA) and severe hypogammaglobulinemia (Fig 1, A and Table I). In spite of supportive treatment, the patient died at 3 months of life during an episode of sepsis due to Klebsiella.
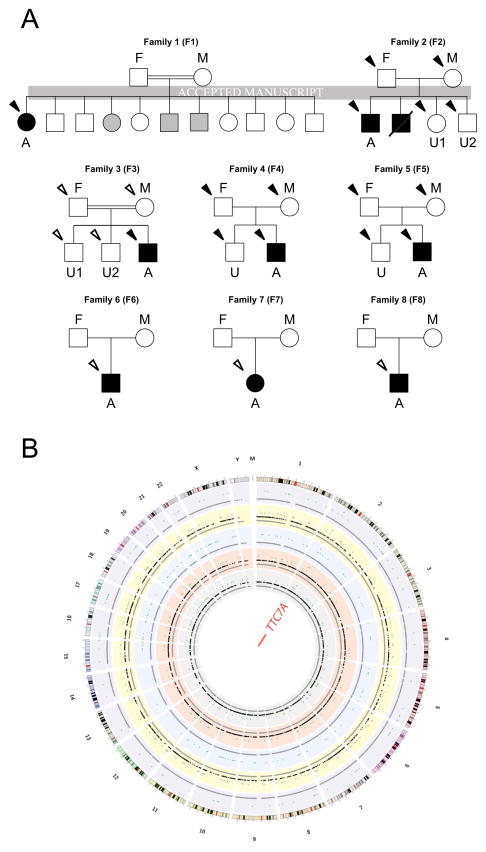
FIG. 1.
Pedigree of CID-MIA families and summary of WES. A, Pedigree of the 8 CID-MIA families. A, Affected Proband; U: Unaffected Offspring(s); F, Father; M, Mother. B, Summary of Whole Exome Sequencing results. Gray dots, all variants; black dots, variants following certain modes of inheritance; green dots, potentially damaging variants; red dots, mutations in TTC7A. Plot made with Circos.41
TABLE I.
Immunological and molecular features of patients with CID-MIA
| Parameter | Pt 1 (15 d) | Pt 2 (4 m) | Pt 3 (10 m) | Pt 4 (2 y) | Pt 5 (2.5 m) | Pt 6 (4m) | Pt 7 (4 m) | Pt 8 (10 m) | Healthy Controls [normal range] |
|---|---|---|---|---|---|---|---|---|---|
| Sample ID | F1-A | F2-A | F3-A | F4-A | F5-A | F6-A | F7-A | F8-A | |
| ALC [cells/μl] | 3290 | 1224 | 620 | 200 | 1220 | 415 | 260 | 1269 | 3400–9000 |
| CD3+ [cells/μl] | 1328 | 338 | 169 | 71 | 74 | 178 | 128 | 824 | 1900–5900 |
| CD4+ [cells/μl] | 1287 | 261 | 135 | 32 | 49 | 124 | 106 | n.d. | 1400–4300 |
| CD8+ [cells/μl] | 23 | 7 | 17 | 49 | 14 | 4 | 2 | n.d. | 500–1700 |
| CD19+ [cells/μl] | 429 | 17 | 2 | 4 | 458 | 79 | 15 | 200 | 571–3860 |
| CD16/56+ [cells/μl] | 97 | 878 | 312 | 30 | 118 | 129 | 55 | 0 | 160–950 |
| RTE (CD4+ 45RA+ 31+) [cellsμl] | 849 | 17 | 35 | 7 | n.d. | n.d. | 14 | n.d. | 800–5800 |
| TRECs [copies/μl] | n.d. | n.d. | n.d. | n.d. | 0–7 | n.d. | n.d. | n.d. | >252 |
| Proliferation to PHA [SI] | 13.3 | n.d.a | 1 | 11.5 | 5.6 | 32 | 1 | n.d. | >67 |
| IgG (mg/dl) | 84 | n.d. | 57 | 242 | <75 | <100 | 180 | 106 | 232–1411 |
| IgA (mg/dl) | <6 | <6 | <6 | 21 | <7 | <6 | <6 | n.d. | 0–83 |
| IgM (mg/dl) | <25 | <25 | <25 | <25 | 5 | <25 | 27 | n.d. | 0–145 |
Patient 2 (F2-A) was born to parents of Serbian origin; one elder brother with a diagnosis of anal atresia had died in the first month of life. The proband underwent surgery at birth because of multiple atresias affecting pylorus, ileum and colon. During hospitalization, he developed two episodes of Staphylococcus sepsis. Laboratory investigation disclosed T and B cell lymphopenia, and undetectable levels of serum IgA and IgM (Fig 1, A and Table I). He died at 4 months of age.
Patient 3 (F3-A) was born to related parents of Bosniak origin. He presented at birth with meconium peritonitis associated with multiple ileal atresias, and underwent several resections. Clinical course was complicated by multiple episodes of sepsis due to Pseudomonas aeruginosa and Candida albicans, and by abdominal abscesses due to Pseudomonas aeruginosa. Laboratory investigations at 10 months of age showed severe T and B cell lymphopenia, absent proliferation to PHA and to anti-CD3, and profound hypogammaglobulinemia (Fig 1, A and Table I). He is alive at 2.8 years of age, receiving total parenteral nutrition.
Patient 4 (F4-A) was born at 35 weeks as part of a fraternal twin gestation. At birth, she was found to have multiple intestinal atresias requiring surgery. She developed multiple episodes of central line, urinary tract, and G-tube site bacterial infections and fungemia. She also developed chicken pox after receiving the varicella vaccine. At 2 years of age, she had extreme lymphopenia, severe impairment of proliferation to PHA, and hypogammaglobulinemia with no protective antibody responses to tetanus, diphtheria, or pneumococcus (Fig 1, A and Table I). She is currently on the small bowel transplant list, receiving parenteral nutrition, IVIG, and Pneumocystis jiroveci prophylaxis with pentamidine.
Patient 5 (F5-A) was born at 37 weeks of gestation to a father of French-Canadian and a mother of mixed European descent. He received multiple surgeries and resections for treatment of intestinal atresias, that were complicated. by E. coli sepsis. During universal statewide newborn screening for SCID at birth23, he was found to have extremely low level of T cell receptor excision circles (TRECs) (17 copies/μl; normal values: ≥252 copies/μl). Immunological investigations disclosed extreme T cell lymphopenia, severe impairment of proliferation to PHA, and agammaglobulinemia (Fig 1, A and Table I). At 3 months of age, he received hematopoietic cell transplantation (HCT) from his sibling who was mismatched at the HLA-A locus in the graft rejection direction only. Conditioning was with serotherapy (anti-thymocyte globulin, ATG) only, and post-transplant course was uncomplicated with no acute or chronic GvHD. He has had multiple resections of atretic intestine post-transplant and continues to be dependent on parenteral nutrition for short gut. He is now 22 months after transplantation, and has attained robust reconstitution of T cell immunity (Table E2).
Patient 6 (F6-A) was born to non-consanguineous Italian parents, and was diagnosed at birth with multiple intestinal atresias requiring surgical interventions. Use of total parenteral nutrition resulted in significant liver toxicity. At 4 months of age, chronic diarrhea and failure to thrive prompted immunological investigations that revealed severe T and B lymphopenia and agammaglobulinemia (Fig 1, A and Table I). Treatment with IVIG and antimicrobial prophylaxis with trimethoprim/sulfamethoxazole were started. At 10 months of age, the patient received hematopoietic stem cell transplantation from a matched unrelated donor. Conditioning included Cyclophosphamide and Thio-Thepa. Hematological reconstitution was achieved at 2 weeks. Clinical course was complicated by acute GvHD affection the skin. Interstitial pneumonia due to Cytomegalovirus led to death at day +55 after transplantation. No data on post-transplant chimerism are available.
Patient 7 (F7-A) was born to unrelated parents of Italian origin. She was diagnosed with pyloric stenosis and underwent pyloroplasty at 3 days of life. In the following weeks, she suffered from multiple episodes of sepsis due to various bacteria (Klebsiella, E. coli, S. aureus) and candida. At 4 months of age, failure to thrive, severe T and B cell lymphopenia, and hypogammaglobulinemia were demonstrated (Fig 1, A and Table I). At the age of 9 months, severe neurodevelopmental delay was present, associated with lack of visual evoked response. The infant was referred elsewhere for possible combined small bowel and hematopoietic cell transplantation.
Patient 8 (F8-A) was the first child born to Italian unrelated parents. The mother was seropositive for HIV and the father was affected by gastric tumor. HIV DNA PCR testing was performed at 2 weeks of age and was negative. Soon after birth, the patient underwent surgery for multiple small bowel atresias. At 7 months of age, he was referred to our institution with failure to thrive dyspnea, and hepatosplenomegaly. Laboratory investigations showed lymphopenia affecting T and NK cells, and hypogammaglobulinemia (Fig 1, A and Table I). During hospitalization, the patient developed disseminated candida infection, and died at 8 months of age.
TTC7A Mutations Were Identified in Affected Probands through WES
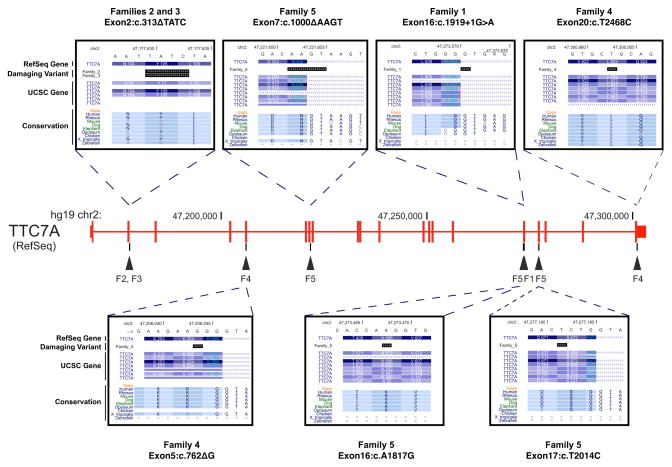
We performed WES on 15 individuals (including the 5 probands) from the 5 core families in our study (families 1, 2, 3, 4 and 5) (Fig 1). Summary of sequencing statistics is listed in Tables E3 and E4. From the identified variants (SUPPLEMENTARY RESULTS, Tables E5 and E6 in Online Repository Materials for details), we screened for variants that followed various potential modes of inheritance for this disease in each of the 5 core families. Of all the potentially damaging variants identified, only those in one gene, Tetratricopeptide Repeat Domain 7A (TTC7A), were found in all the 5 probands of our core families (Fig 2 and Table II). Patient F1-A harbors a homozygous Exon16:c.1919+1G>A mutation, which is predicted to disrupt the invariant splicing donor site (GT) immediately 3′ to Exon 16, and cause read-through into its following intron. Patients F2-A and F3-A both have a homozygous 4 bp deletion (Exon2:c.313ΔTATC), which is predicted to lead to frameshift at codon 105 (p.Y105fs) and Nonsense-Mediated Decay (NMD) of the resulting RNA transcript. Parents and healthy siblings of patients F2-A and F3-A were all found to be heterozygous carriers of this mutation. Patient F4-A inherited a pair of damaging compound heterozygous mutation (Exon5:c.762ΔG on the maternal allele and Exon20:c.T2468C on the paternal allele), with the former predicted to lead to NMD through a frameshift mutation (p.K254fs) and the latter (p.L823P) predicted to be deleterious by both SIFT and PolyPhen-2. Patient F5-A harbored a different combination of compound heterozygous variants [Exon7:c.1000ΔAAGT on the paternal allele, and two deleterious SNVs, Exon16:c.A1817G (p.K606R) and Exon17:c.T2014C (p.S672P) on the maternal allele] which were predicted to abolish the function of both alleles. In each case, the mutations are not found or rare (<1%) in the 1000 genomes project24 and Exome Sequencing Project (ESP6500)25 databases and are located at highly conservative regions of the gene (Fig 2). Four of the 8 deleterious variants were further confirmed by Sanger sequencing in 3 families – F2, F3 and F5 (Fig E1); the other 4 variants were located in regions that were difficult to amplify, since in each case, PCR amplification with designed unique primers resulted in multiple bands in two attempts (data not shown).
FIG. 2.
Schematic representation of the TTC7A gene and position of the mutations identified in CID-MIA patients. Exons are identified by vertical bars. For each site containing the mutations, from top to bottom: hg19 chromosome location, chromosome sequence, RefSeq gene, damaging variant, UCSC gene, Vertebrate Multiz Alignment & Conservation. Information retrieved from the UCSC Genome Browser (http://genome.ucsc.edu/).
TABLE II.
TTC7A mutations identified with WES in the 5 core CID-MIA families
| Families | Chr | Position | Damaging Variant | rsID | 1-SIFT | PolyPhen2 | SIFT-Causes NMD |
|---|---|---|---|---|---|---|---|
| 1 | 2 | 47273571 | Exon16:c.1919+1G>A | - | - | - | - |
| 2 | 2 | 47177629 | Exon2:c.313ΔTATC | - | - | - | YES |
| 3 | 2 | 47177629 | Exon2:c.313ΔTATC | - | - | - | YES |
| 4 | 2 | 47206043 | Exon5:c.762ΔG | - | - | - | YES |
| 4 | 2 | 47300953 | Exon20:c.T2468C | - | 1 | 0.999 | - |
| 5 | 2 | 47221651 | Exon7:c.1000ΔAAGT | - | - | - | YES |
| 5 | 2 | 47273468 | Exon16:c.A1817G | - | 1 | 0.934 | - |
| 5 | 2 | 47277182 | Exon17:c.T2014C | - | 0.99 | 0.984 | - |
| Families | Type | Ref | Alt | A | F | M | U1 | U2 |
|---|---|---|---|---|---|---|---|---|
| 1 | Recessive SDM | G | A | A/A | ||||
| 2 | Recessive FSD | TTATC | T | T/T | TTATC/T | TTATC/T | TTATC/T | TTATC/T |
| 3 | Recessive FSD | TTATC | T | T/T | ||||
| 4 | CH | AG | A | AG/A | AG/AG | AG/A | AG/A | |
| 4 | CH | T | C | T/C | T/C | T/T | T/T | |
| 5 | CH | CAAGT | C | CAAGT/C | CAAGT/C | CAAGT/CAAGT | CAAGT/C | |
| 5 | CH | A | G | A/G | A/A | A/G | A/A | |
| 5 | CH | T | C | T/C | T/T | T/C | T/T |
Subsequently, we retrieved biological specimens from patients F6-A, F7-A and F8-A. Targeted sequencing of the TTC7A gene revealed compound heterozygous mutations in patients F6-A (c.C2033A and c.C2134T, leading to p.S678X and p.Q712X premature terminations, respectively) and F7-A (homozygous for the mutation c.T1196C, leading to a p.L399P amino acid change that is predicted to be deleterious by both SIFT and PolyPhen-2). For patient F8-A, we did not find any damaging variants using the primers designed for TTC7A ORF analysis. We then analyzed the cDNA of the TT7C7A gene split in three portions due to its length. Using a forward primer in Exon 1 and a reverse primer in Exon 4, both an in-frame cDNA product lacking Exons 2 and 3, as well as a normal-sized cDNA product were detected (Fig E2). The genomic mutation causing this aberrant splicing has not been characterized, as introns in the region are between 6,000 and 18,000 bp long.
Altered TTC7A Expression Levels in Patients with CID-MIA
In order to check for RNA expression defects, we analyzed TTC7A expression by qRT-PCR with a TaqMan assay located on the Exon 1-2 boundary in two patients (F3-A and F5-A) for which we have fibroblasts available. Our analysis showed that relative expression of TTC7A in patients F3-A and F5-A, as compared to three healthy unrelated controls, was reduced to 32% and 54%, respectively (Fig E3). We also performed Reverse-Transcription Polymerase Chain Reaction using RNA from Patient F5-A and found that the Exon7:c.1000ΔAAGT mutation indeed led to skipping of Exon 7 (Fig E4). Thus, the TTC7A mutations were shown to affect TTC7A mRNA expression in at least two cases.
Microarray Analysis of Ttc7 Expression
We analyzed expression of Ttc7, the murine orthologue transcript, on hematopoietic and lymphoid tissues and on sorted T and B lymphoid cells with Mouse 430A 2.0 microarrays. As shown in Fig E5, similar relative levels of Ttc7 expression were identified in bone marrow, lymph nodes and in various T and B cell subpopulations; however, higher levels of expression were detected in total thymus.
Expression of the TTC7A Protein in Normal Thymus
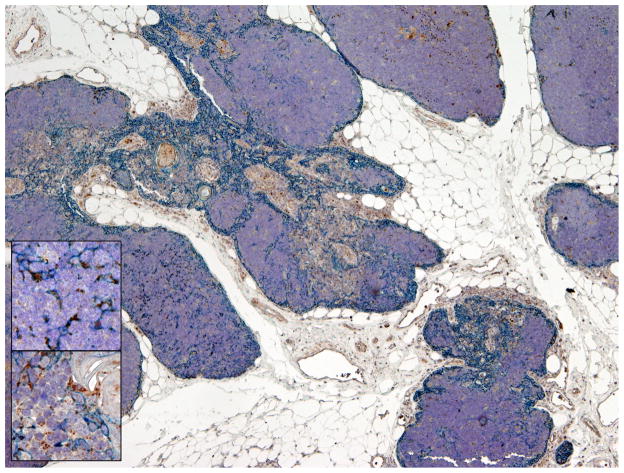
Identification of high levels of Ttc7 expression in murine thymus and, to a lower extent, also in lymphoid cells, prompted us to extend this finding by analyzing TTC7A protein expression in human thymus. Immunostaining for TTC7A in normal human thymus demonstrated reactivity in a subset of cytokeratin-positive cortical and medullary thymic epithelial cells (TECs), with some signal present also in thymocytes (Fig 3). These results suggest that TTC7A may be involved in normal biological function of these cells.
FIG. 3.
Immunostaining for TTC7A protein expression in normal thymus. Double immunostaining for cytokeratin (blue) and TTC7A (brown) shows scattered cytokeratin-positive epithelial cells in the cortex and in the medulla, and a weaker, but discernible signal, in thymocytes (detail of the cortex, upper inset and the medulla, lower inset). Magnification: 4X (inset, 20X).
Severe Lymphoid Depletion in the Thymus and Peripheral Lymphoid Tissues from Patients with CID-MIA
After confirming TTC7A expression in thymus, we further examined the pathologic features of thymus in CID-MIA patients. Post-mortem analysis of the thymus from patient F8-A revealed dysplastic changes, with vague cortico-medullary demarcation, but presence of Hassall’s corpuscles, associated with severe lymphoid depletion (Fig E6, A). Immunostaining for CD3 confirmed the marked reduction of thymocytes (Fig E6, B). A mesenteric lymph node biopsy from the same patient showed marked lymphoid depletion affecting both the cortex and the paracortex, and lack of follicles (Fig E6, C), with markedly reduced number of T and B lymphocytes (Fig E7, A and B). Similarly, marked lymphoid depletion was demonstrated in a mesenteric lymph node biopsy from patient F7-A (Fig E7, C).
DISCUSSION
In this study we have identified deleterious mutations in the TTC7A gene in 8 patients with CID-MIA belonging to unrelated families of distinct ethnic origin, indicating a strong genetic link.
While we were preparing our manuscript, Samuels et al. reported on the occurrence of a homozygous 4-nt deletion (c.1000ΔAAGT) in 5 apparently unrelated French-Canadian patients with MIA, and compound heterozygosity of Exon7:c.1000ΔAAGT + p.L823P (i.e. Exon20:c.T2468C in our report) in one other affected patient.26 Their study cross-validated our findings that TTC7A may be the causal gene for SCID-MIA. The authors had postulated that the common occurrence of the Exon7:c.1000ΔAAGT mutation may reflect a founder effect in French-Canadian patients with MIA. Interestingly, the father of Patient 5 (F5-A) was of French-Canadian origin, and the patient inherited this allele from him. Moreover, homozygosity for another mutation (Exon2:c.313ΔTATC) was identified in patients F2-A and F3-A who are both of Slavian origin, possibly reflecting a founder effect among Slavians.
MIA may occur either isolated or in association with immunodeficiency. Among the patients reported by Samuels et al. with proven TTC7A mutations, only one was shown to suffer from concurrent immunodeficiency, with profound T cell lymphopenia and hypogammaglobulinemia.26 By contrast, all patients included in this study presented with CID-MIA and carry TTC7A mutations, thus suggesting that biallelic mutations in this gene may account for both isolated multiple intestinal atresias and CID-MIA, in affected individuals of different ethnic origin.
The exact incidence of severe immunodeficiency in patients with MIA is not known. The majority of the patients reported in the literature died early in life, before accurate analysis of their immunological status was performed. However, some of the data presented here may offer novel insights. In particular, Patient F5-A from our series had extremely low TREC levels at birth. Retrospective analysis of TREC levels in dried blood spots collected at birth from other patients with MIA (with or without a confirmed diagnosis of associated immunodeficiency) may help assess what is the actual incidence of severe T cell immunodeficiency in this disease.
Immunological studies in our patients have identified similarities, but also some variability. In particular, four of our patients showed profound T cell lymphopenia, consistent with a diagnosis of SCID. However, patient F1-A had only mild T cell lymphopenia. and the majority of his circulating CD4+ cells co-expressed CD45RA and CD31 markers,27 suggesting partially preserved thymic function. On the other hand, profound CD8+ T cell lymphopenia was observed in all patients that were tested, and may represent a more consistent phenotypic marker of impaired cell-mediated immunity and defective thymopoiesis.
Severe hypogammaglobulinemia was a common feature in our series, and has been previously reported.3–6, 8, 28 Interestingly, although all of the patients reported in this series suffered from recurrent and severe infections, the spectrum of infectious episodes differed from what typically observed in SCID, with fewer viral infections and a higher frequency of bloodstream infections due to intestinal microbes. It is possible that this reflects abnormalities of the gut barrier in patients with CID-MIA.
Little is known about expression and function of TTC7A, a member of a large family of proteins containing the tetratricopeptide repeat (TPR) domain, defined by a degenerate consensus sequence of 34 amino acids.29 TPR domain-containing proteins, such as TTC7A, have diverse functions in cell cycle control, protein transport, phosphate turnover, and protein trafficking or secretion, and they can act as chaperones or scaffolding proteins.30 TTC7A is shown to express more abundantly in the thymus, colon and colorectal adenocarcinoma.31 Spontaneous mutations in the murine TTC7A orthologue, Ttc7, have been identified in the flaky skin (fsn) mouse32–37, the hereditary erythroblastic anemia (hea) mouse 30, 38, as well as the Ttc7fsn-Jic mouse.39, 40 These mouse models show anemia, skin abnormalities, and immune dysregulation, but not intestinal atresia, although forestomach epithelial hyperplasia is present in fsn mice. It is plausible that the actual function of the human TTC7A protein and its murine orthologue Ttc7 may have diversified during evolution, although they share 88% amino acid sequence homology (Fig E8).
We have demonstrated abundant expression of TTC7A in a subset of cortical and medullary thymic epithelial cells and in Hassall’s corpuscles, and lower, but clearly discernible expression in thymocytes. Microarray analysis of expression of the murine orthologue, Ttc7, confirmed higher relative levels of expression in the thymus. Postmortem analysis of the thymus from patient F8-A showed severe lymphoid depletion and vague corticomedullary demarcation, with preserved presence of Hassall’s corpuscles. Moreover, severe lymphoid depletion, affecting both T and B cells, was demonstrated in peripheral lymph nodes from patients F7-A and F8-A. Overall, these findings are consistent with the notion that mutations of the TTC7A gene affect immune system development and function and hence cause the combined immunodeficiency associated with MIA. Defining whether the severe immunodeficiency of CID-MIA is intrinsic to lymphoid cells or whether it is mainly due to extra-hematopoietic defects, would have important therapeutic implications. Most patients with CID-MIA have died early in life, and there is very limited experience with attempts to achieve immune reconstitution in this disease. Samuels et al., have reported on an infant with CID-MIA who received an HLA-matched cord blood transplantation at 6.5 months of age, but died at 1 year of age.26 Patient F6-A in our series received HCT from a matched unrelated donor upon conditioning with cyclophosphamide and thio-tepa, but also died early after transplantation. By contrast, patient F5-A in this study received a well-matched HCT without preparative chemotherapy, and has achieved donor T cell engraftment. While the presence of T cells with a naïve phenotype suggests effective de novo thymopoiesis, longer follow-up studies will be needed to confirm efficacy of HCT. Finally, donor-derived, partial immune reconstitution has been observed after combined liver and small bowel transplantation in another infant with CID-MIA.28 Interestingly, in this case, almost all T cells were of donor origin, and were either CD4− CD8− TCRγδ+, or CD3− CD4− CD8αα+, indicative of a possible intestinal intraepithelial origin. While the function of TTC7A has yet to be defined, it may be a key factor to bridge the two processes of both immune system and digestive tract development.
Acknowledgments
Sources of Funding: M.S. is funded by grants from Stanford University and the NIH. L.D.N. is supported by NIH grants 5P01AI076210-04, 1R01AI00887-01, 4U54AI082973-04 (also to S.Y.P.), and the Manton Foundation. Both L.D.N. and W.A.H. are supported by a grant from the Dubai Harvard Foundation for Medical Research. S.G. is supported by Fondazione Nocivelli and University of Brescia. F.F. is supported by MIUR (grant 20104HBZ8E_002). S.-Y.P. is supported by a Translational Investigator Service award from Boston Children’s Hospital. G.I.M.’s research reported in this publication was supported by the National Human Genome Research Institute of the National Institutes of Health under Award Number K99HG007065. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. D.H.P. is a Damon Runyon Fellow supported by the Damon Runyon Cancer Research Foundation (DRG-#2122-22).
We thank the patients and the families for their collaboration.
Abbreviations
CID-MIA
Combined Immunodeficiency with Multiple Intestinal Atresias
HCT
Hematopoietic Cell Transplantation
IHC
Immunohistochemistry
IVIG
Intravenous immunoglobulins
PHA
Phytohemagglutinin
RT-PCR
Reverse-Transcription Polymerase Chain Reaction
TECs
Thymic epithelial cells
TRECs
T cell receptor excision circles
WES
Whole Exome Sequencing
WB
Western Blotting
Footnotes
DATA ACCESSION
Raw sequencing reads can be accessed at the NCBI database of Genotypes and Phenotypes (dbGaP). Accession number will be available before publication of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guttman FM, Braun P, Garance PH, Blanchard H, Collin PP, Dallaire L, et al. Multiple atresias and a new syndrome of hereditary multiple atresias involving the gastrointestinal tract from stomach to rectum. Journal of pediatric surgery. 1973;8:633–40. doi: 10.1016/0022-3468(73)90401-6. [DOI] [PubMed] [Google Scholar]
- 2.Mishalany HG, Der Kaloustian VM. Familial multiple-level intestinal atresias: report of two siblings. The Journal of pediatrics. 1971;79:124–5. doi: 10.1016/s0022-3476(71)80072-0. [DOI] [PubMed] [Google Scholar]
- 3.Moreno LA, Gottrand F, Turck D, Manouvrier-Hanu S, Mazingue F, Morisot C, et al. Severe combined immunodeficiency syndrome associated with autosomal recessive familial multiple gastrointestinal atresias: study of a family. American journal of medical genetics. 1990;37:143–6. doi: 10.1002/ajmg.1320370133. [DOI] [PubMed] [Google Scholar]
- 4.Rothenberg ME, White FV, Chilmonczyk B, Chatila T. A syndrome involving immunodeficiency and multiple intestinal atresias. Immunodeficiency. 1995;5:171–8. [PubMed] [Google Scholar]
- 5.Ali YA, Rahman S, Bhat V, Al Thani S, Ismail A, Bassiouny I. Hereditary multiple intestinal atresia (HMIA) with severe combined immunodeficiency (SCID): a case report of two siblings and review of the literature on MIA, HMIA and HMIA with immunodeficiency over the last 50 years. BMJ case reports. 2011;2011 doi: 10.1136/bcr.05.2010.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore SW, de Jongh G, Bouic P, Brown RA, Kirsten G. Immune deficiency in familial duodenal atresia. Journal of pediatric surgery. 1996;31:1733–5. doi: 10.1016/s0022-3468(96)90066-4. [DOI] [PubMed] [Google Scholar]
- 7.Cole C, Freitas A, Clifton MS, Durham MM. Hereditary multiple intestinal atresias: 2 new cases and review of the literature. Journal of pediatric surgery. 2010;45:E21–4. doi: 10.1016/j.jpedsurg.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Walker MW, Lovell MA, Kelly TE, Golden W, Saulsbury FT. Multiple areas of intestinal atresia associated with immunodeficiency and posttransfusion graft-versus-host disease. The Journal of pediatrics. 1993;123:93–5. doi: 10.1016/s0022-3476(05)81547-1. [DOI] [PubMed] [Google Scholar]
- 9.Reyes J, Todo S, Green M, Yunis E, Schoner D, Kocoshis S, et al. Graft-versus-host disease after liver and small bowel transplantation in a child. Clinical transplantation. 1997;11:345–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Dallaire L, Perreault G. Hereditary multiple intestinal atresia. Birth defects original article series. 1974;10:259–64. [PubMed] [Google Scholar]
- 11.Bilodeau A, Prasil P, Cloutier R, Laframboise R, Meguerditchian AN, Roy G, et al. Hereditary multiple intestinal atresia: thirty years later. Journal of pediatric surgery. 2004;39:726–30. doi: 10.1016/j.jpedsurg.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 12.Arnal-Monreal F, Pombo F, Capdevila-Puerta A. Multiple hereditary gastrointestinal atresias: study of a family. Acta paediatrica Scandinavica. 1983;72:773–7. doi: 10.1111/j.1651-2227.1983.tb09812.x. [DOI] [PubMed] [Google Scholar]
- 13.Gungor N, Balci S, Tanyel FC, Gogus S. Familial intestinal polyatresia syndrome. Clinical genetics. 1995;47:245–7. doi: 10.1111/j.1399-0004.1995.tb04304.x. [DOI] [PubMed] [Google Scholar]
- 14.Gahukamble DB, Adnan AR, Al-Gadi M. Atresias of the gastrointestinal tract in an inbred, previously unstudied population. Pediatric surgery international. 2002;18:40–2. doi: 10.1007/s003830200009. [DOI] [PubMed] [Google Scholar]
- 15.Gahukamble DB, Gahukamble LD. Multiple gastrointestinal atresias in two consecutive siblings. Pediatric surgery international. 2002;18:175–7. doi: 10.1007/s003830000536. [DOI] [PubMed] [Google Scholar]
- 16.Chen R, Snyder M. Systems biology: personalized medicine for the future? Curr Opin Pharmacol. 2012;12:623–8. doi: 10.1016/j.coph.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark MJ, Chen R, Lam HY, Karczewski KJ, Chen R, Euskirchen G, et al. Performance comparison of exome DNA sequencing technologies. Nat Biotechnol. 2011;29:908–14. doi: 10.1038/nbt.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mias G, Snyder M. Personal genomes, quantitative dynamic omics and personalized medicine. Quantitative Biology. 2013;1:71–90. doi: 10.1007/s40484-013-0005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–60. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–8. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green MR, Monti S, Dalla-Favera R, Pasqualucci L, Walsh NC, Schmidt-Supprian M, et al. Signatures of murine B-cell development implicate Yy1 as a regulator of the germinal center-specific program. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2873–8. doi: 10.1073/pnas.1019537108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hale JE, Bonilla FA, Pai SY, Gerstel-Thompson JL, Notarangelo LD, Eaton RB, et al. Identification of an infant with severe combined immunodeficiency by newborn screening. The Journal of allergy and clinical immunology. 2010;126:1073–4. doi: 10.1016/j.jaci.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 24.Genomes Project C, Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Exome Variant Server. NHLBI GO Exome Sequencing Project (ESP) Seattle, WA: [May 21st, 2013 accessed]. (URL: http://evs.gs.washington.edu/EVS/) [Google Scholar]
- 26.Samuels ME, Majewski J, Alirezaie N, Fernandez I, Casals F, Patey N, et al. Exome sequencing identifies mutations in the gene TTC7A in French-Canadian cases with hereditary multiple intestinal atresia. Journal of medical genetics. 2013;50:324–9. doi: 10.1136/jmedgenet-2012-101483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimmig S, Przybylski GK, Schmidt CA, Laurisch K, Mowes B, Radbruch A, et al. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. The Journal of experimental medicine. 2002;195:789–94. doi: 10.1084/jem.20011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilroy RK, Coccia PF, Talmadge JE, Hatcher LI, Pirruccello SJ, Shaw BW, Jr, et al. Donor immune reconstitution after liver-small bowel transplantation for multiple intestinal atresia with immunodeficiency. Blood. 2004;103:1171–4. doi: 10.1182/blood-2003-04-1187. [DOI] [PubMed] [Google Scholar]
- 29.Blatch GL, Lassle M. The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. BioEssays: news and reviews in molecular, cellular and developmental biology. 1999;21:932–9. doi: 10.1002/(SICI)1521-1878(199911)21:11<932::AID-BIES5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 30.White RA, McNulty SG, Nsumu NN, Boydston LA, Brewer BP, Shimizu K. Positional cloning of the Ttc7 gene required for normal iron homeostasis and mutated in hea and fsn anemia mice. Genomics. 2005;85:330–7. doi: 10.1016/j.ygeno.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Wu C, Orozco C, Boyer J, Leglise M, Goodale J, Batalov S, et al. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome biology. 2009;10:R130. doi: 10.1186/gb-2009-10-11-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helms C, Pelsue S, Cao L, Lamb E, Loffredo B, Taillon-Miller P, et al. The Tetratricopeptide repeat domain 7 gene is mutated in flaky skin mice: a model for psoriasis, autoimmunity, and anemia. Experimental biology and medicine. 2005;230:659–67. doi: 10.1177/153537020523000908. [DOI] [PubMed] [Google Scholar]
- 33.Abernethy NJ, Hagan C, Tan PL, Birchall NM, Watson JD. The peripheral lymphoid compartment is disrupted in flaky skin mice. Immunology and cell biology. 2000;78:5–12. doi: 10.1046/j.1440-1711.2000.00866.x. [DOI] [PubMed] [Google Scholar]
- 34.Pelsue SC, Schweitzer PA, Schweitzer IB, Christianson SW, Gott B, Sundberg JP, et al. Lymphadenopathy, elevated serum IgE levels, autoimmunity, and mast cell accumulation in flaky skin mutant mice. European journal of immunology. 1998;28:1379–88. doi: 10.1002/(SICI)1521-4141(199804)28:04<1379::AID-IMMU1379>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 35.Mattsson N, Duzevik EG, Pelsue SC. Expansion of CD22lo B cells in the spleen of autoimmune-prone flaky skin mice. Cellular immunology. 2005;234:124–32. doi: 10.1016/j.cellimm.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Welner R, Swett DJ, Pelsue SC. Age-related loss of bone marrow pre-B- and immature B-lymphocytes in the autoimmune-prone flaky skin mutant mice. Autoimmunity. 2005;38:399–408. doi: 10.1080/08916930500246206. [DOI] [PubMed] [Google Scholar]
- 37.Welner R, Hastings W, Hill BL, Pelsue SC. Hyperactivation and proliferation of lymphocytes from the spleens of flaky skin (fsn) mutant mice. Autoimmunity. 2004;37:227–35. doi: 10.1080/08916930410001666659. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu K, Keino H, Ogasawara N, Esaki K. Hereditary erythroblastic anaemia in the laboratory mouse. Laboratory animals. 1983;17:198–202. doi: 10.1258/002367783781070678. [DOI] [PubMed] [Google Scholar]
- 39.Takabayashi S, Katoh H. A mutant mouse with severe anemia and skin abnormalities controlled by a new allele of the flaky skin (fsn) locus. Experimental animals/Japanese Association for Laboratory Animal Science. 2005;54:339–47. doi: 10.1538/expanim.54.339. [DOI] [PubMed] [Google Scholar]
- 40.Takabayashi S, Iwashita S, Hirashima T, Katoh H. The novel tetratricopeptide repeat domain 7 mutation, Ttc7fsn-Jic, with deletion of the TPR-2B repeat causes severe flaky skin phenotype. Experimental biology and medicine. 2007;232:695–9. [PubMed] [Google Scholar]
- 41.Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–45. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]