Gram Negative Wound Infection in Hospitalised Adult Burn Patients-Systematic Review and Metanalysis- (original) (raw)
Abstract
Background
Gram negative infection is a major determinant of morbidity and survival. Traditional teaching suggests that burn wound infections in different centres are caused by differing sets of causative organisms. This study established whether Gram-negative burn wound isolates associated to clinical wound infection differ between burn centres.
Methods
Studies investigating adult hospitalised patients (2000–2010) were critically appraised and qualified to a levels of evidence hierarchy. The contribution of bacterial pathogen type, and burn centre to the variance in standardised incidence of Gram-negative burn wound infection was analysed using two-way analysis of variance.
Primary Findings
Pseudomonas aeruginosa, Klebsiella pneumoniae, Acinetobacter baumanni, Enterobacter spp., Proteus spp. and Escherichia coli emerged as the commonest Gram-negative burn wound pathogens. Individual pathogens’ incidence did not differ significantly between burn centres (F (4, 20) = 1.1, p = 0.3797; r2 = 9.84).
Interpretation
Gram-negative infections predominate in burn surgery. This study is the first to establish that burn wound infections do not differ significantly between burn centres. It is the first study to report the pathogens responsible for the majority of Gram-negative infections in these patients. Whilst burn wound infection is not exclusive to these bacteria, it is hoped that reporting the presence of this group of common Gram-negative “target organisms” facilitate clinical practice and target research towards a defined clinical demand.
Introduction
1.1 Background and Rationale
Gram-negative infection is a global health concern [1]. Several advances have been registered in the field of intensive care, ventilatory support, skin substitution and fluid balance [2]. However, infection has emerged as a major, often unmitigated complication in burn injury, which incurs significant morbidity, mortality and healthcare cost [3]. Management of acute infection in thermal injury presents unique challenges in terms of clinical diagnosis and rapid institution of effective antimicrobial chemotherapy. Clinical diagnosis is hampered by thermal injury-induced hyperpyrexia, immune suppression, and systemic inflammatory response syndrome [3], [4]. These factors make clinical diagnosis difficult and promote infection [5]. It is, however, well-established that Gram-negative pathogens predominate beyond the early post-burn period [5]. Centres for Disease Control (USA), the British Society of Antimicrobial Chemotherapy, and its European and Asian Counterparts provide extensive documentation regarding aetiological profiles and incidences of the major protagonists in other disease, such as pneumonia and urinary tract infection [6], [7]. This data, in turn, provides aetiological targets for rationalised expedited, targeted antimicrobial prescribing, infection control, and antimicrobial development [8]. Traditional teaching is based on incidence data that is non-standardised and difficult to compare; it maintains that Gram negative burn wound isolates differ between burn centres [9]–[11].
Objective
The purpose of this study is to establish whether the isolates associated with clinical Gram negative burn wound infection differ between burn centres. This study also sought to establish standardised incidence rates for the organisms identified.
Methods
3.1 Aim Construct
The terms of reference in relation to this systematic review and meta-analysis (File S1) in Patient Intervention Comparator and Outcome (PICO) format, are reported in File S2, in conformance to pre-validated criteria [12].
3.2 Literature Search
A combination of National Library of Medicine (NLM) Medical Subject Heading (MeSH) Descriptor Data Browser terms were used to increase search sensitivity [13]. These were used in a first generation electronic search whose results were manually screened for relevance (File S3). The first generation search was performed using the OVID-SP and PUBMED platforms (File S4). The second generation search involved manual back-referencing and Web of Knowledge. Results of the electronic literature search are reported extensively in File S5.
3.3 Inclusion/Exclusion Criteria
This study specifically adhered to the PICO-specified terms of reference as inclusion criteria (File S2). In order to enable data pooling and anlalysis, only primary literature investigating adult hospitalised burn patients only was analysed, and studies including patients with delayed transfer were excluded from this analysis. Primary literature published between 2000 and 2010, in English, studying adult locally-injured hospitalised humans only was included. The limitations of this approach are fully acknowledged, and applicability of the study to other populations is debated in the discussion section.
3.4 Reporting of the Systematic Review and Evidence-based Process
PRISMA guidelines were applied to report the systematic review and evidence-based process. The retrieval process reported heterogeneous methodologies in the primary literature but no randomised controlled trials (RCT’s), requiring these guidelines to be adapted. Systematic review of studies other than RCTs is not new [14]. To ensure comparability and adequate data selection rigorous critical appraisal [15], [16] was applied to determine the quality of the primary studies retrieved, and ensure inclusion of comparable, current, valid and relevant evidence [17]. Pre-validated critical appraisal tools were employed on the primary research to achieve significant depth of appraisal [12], [18]. Two researchers, arbitrated by a third, independently performed critical appraisal. The Oxford Centres for Evidence Based Medicine “levels of evidence” framework was used to provide a framework to reflect the robustness of individual studies [19]. The literature retrieval process is reported in detail (Files S5, S6).
3.5 Operational Definitions and Summary Measures
Primary literature reported various measures of incidence, therefore incidence rates were standardised as number of new cases per 1000 patient-years [20]. As a working definition, an organism was defined as causative if it could be discerned from the study that the organism was isolated from wound in the presence of clinical infection. “New” was defined as the first documentation of an organism, thereby excluding relapse or re-infection. Clinical diagnosis of infection was based on reconciliation of primary literature to the definitions of Greenhalgh et al. [21]. For the purposes of this study, studies reporting on patients whose transfer to the definitive treatment centre was delayed (non-immediate) were excluded.
3.6 Statistical Analysis
Statistical analysis was performed with Graphpad Prism v5 for windows (CA, USA). The contribution of the two independent variables under consideration (bacteria, burn centre) to the variance in standardised incidence of Gram-negative burn wound pathogens was analysed using two-way analysis of variance (ANOVA). Statistical significance was assumed when p<0.05. Statistical analysis was performed blinded, by a qualified statistician (LC).
Results
4.1 Literature Retrieval and Critical Appraisal
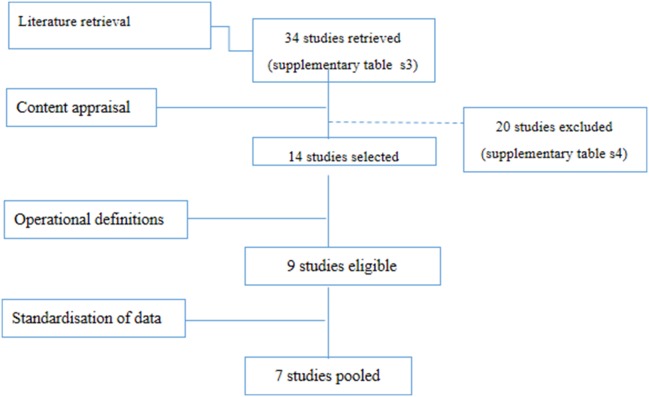
Thirty four studies were retrieved by the initial literature search (Figure 1). Narrative critical appraisal is provided in extensive detail in File S7. Of these studies, 20 did not conform to the inclusion criteria and were excluded (File S6). Fourteen studies were included for critical appraisal (Table 1). A further 5 studies did not conform to operational definitions (section 3.3). “Strength of the evidence” underpinning the remaining studies was evaluated by critical appraisal and is reported in Table 2. Two further studies [22], [23] investigated primary incidence over week 1 post-burn only. Because of this methodological heterogeneity, their results could not be pooled or confidently compared to the rest of the literature. Standardised incidence data from the remaining 7 studies (Table 2) was extracted and pooled (Figure 2). The geographic location of the burn centres from which the data was pooled is reported in Figure 3.
Figure 1. PRISMA-style scheme reporting the literature retrieval and selection strategy arriving to the final 7 studies whose data could be pooled for statistical analysis.
Table 1. Literature Included for Critical Appraisal.
| n | Reference | Design | Aim | Sample |
|---|---|---|---|---|
| 1 | [50] | Retrospective study | “To determine the bacterial profile and antimicrobial susceptibility of the isolates and todescribe the change in trends over the study period.” | 665 |
| 2 | [51] | Retrospective Cohort | “To determine the incidence and cause of nosocomial infections in all patients admitted toour burn intensive care unit (BICU) over a 5-year period” | 76 |
| 3 | [52] | Prospective | Describe a specially designed computer system for the analysis of data, and report theresults from the first 3 years of using the system for routine registration of infection in aconsecutive series of burn patients. | 83 |
| 4 | [9] | Retrospective | “To determine the changing patterns and emerging trends of bacterial isolates and theirantimicrobial susceptibilities” | 759 |
| 5 | [53] | Retrospective cohort | “To analyse the bacterial isolates from the wounds of patients admitted to the BurnsUnit and to determine the sensitivity pattern of the commonly cultured organisms’ | 336 |
| 6 | [11] | Prospective Study | “To investigate the profile of micro-organisms and resistance to antimicrobial agentsin a tertiary referral burn centre” | 113 |
| 7 | [54] | Retrospective Study | “To determine the bacterial profile and antibiotic susceptibility pattern of burnisolates at the Queen Elizabeth Central Hospital (QECH), Blantyre” | 317 |
| 8 | [23] | Prospective | “To determine nosocomial infections in the Tohid Burn Centre in Tehran, Iran” | 582 |
| 9 | [22] | Prospective Clinical Audit | This prospective clinical audit investigated the primary incidence of BWI between theusual burn patients […] and a number of survivors from the Bali bombings duringa 3-month audit. | 64 |
| 10 | [55] | Retrospective Cohort Study | ‘To document burn wound infection and problems faced by the clinicians’ | 71 |
| 11 | [56] | Narrative review | An index case of pseudomonal BWI is reported followed by a narrative review ofincidence mortality, risks and prognosis | N/A |
| 12 | [57] | Narrative Review | A narrative review describing risk two Acinetobacter baumanni outbreaks, and riskfactors [aim not explicitly stated] | 72 |
| 13 | [33] | Case-control arm Retrospective Cohort Arm | “This study was conducted to determine the risk factors for acquisition ofimipenem-resistant Pseudomonas aeruginosa (IRPA) in the burn unit.” | 370 |
| 14 | [6] | Prospective Cohort Study | ‘To determine accurate infection rates, risk factors for infection, and thepercentage of infections.’ | 157 |
Table 2. Incidence of Gram negative organisms causing clinical burn wound infection extracted from eligible primary literature.
| Study centre | Evidence Level | Patients (n) | Study Duration (Years) | BWI Incidence | Gram-ve BWI incidence | P. aeruginosa | A. baumanni | Enterobacter spp. | K. pneumoniae | E. coli | Proteus spp. | Mixed I Infection (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [50] | 4 | 692 | 5 | 192 | 148 | 111 | 13.8 | 7.5 | 7.5 | 5.0 | 6.2 | 13% |
| [52] | 2b | 230 | 3 | 155 | 70 | 39 | 4.3 | 14 | 11.5 | 0 | 6.0 | 12% |
| [51] | 3 | 57 | 5 | 163 | 89 | 31.4 | 38.4 | 21 | 14 | 3.5 | 3.5 | NR |
| [11] | 2b | 113 | 0.5 | 530 | NR | 265* | NR | NR | NR | NR | NR | NR |
| [55] | 4 | 71 | 5 | 253 | 174 | 39.4 | 30 | 30.8 | 67.6 | 11 | 25.2 | 6.1% |
| [9] | 4 | 759 | 5 | 151 | 86.2 | 46 | 13.4 | 30 | 50 | 5.6 | 10.4 | 40%* |
| [6] | 2b | 157 | 1 | 382 | NR | 152 | NR | NR | NR | NR | NR | NR |
Figure 2. Box-whisker plot reporting data dispersion for the standardised incidence of Gram-negative burn wound injury in civilian adult hospitalised patients.
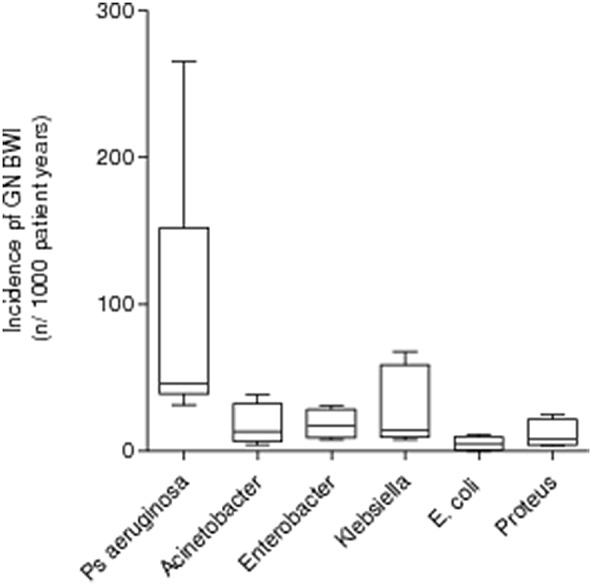
Data shown represents mean±1SD.
Figure 3. Geographic distribution of the centres from which studies were critically appraised.
The study design and sample size are provided in figure 2.
4.2 Data Extraction
Data from clinically and statistically comparable studies was transformed to a standardised incidence rate (n new Gram negative BWI per 1000 patient-years, Table 2). Data-distribution of the standardised incidence rates for each pathogen in the identified Gram-negative BWI profile is represented by a box-whisker plot (Figure 1). Descriptively, all these studies reported a similar set of bacteria consisting of P. aerugionsa, K. pneumoniae, E. coli, Enterobacter spp. and Proteus spp. as the commonest Gram-negative pathogens to be isolated from clinically infected burn wounds in the burn centres studied. Standardised incidences reported in this study report Pseudomonas aeruginosa is the commonest BWI pathogen, but the incidence varied widely and differs significantly from the preceding literature [24].
4.3 Statistical Analyses
The majority of infections, 60.2%±12.5% (mean±1 SD) were Gram-negative. A mean incidence rate of 156 new Gram-negative BWI per 1000 patient-years was calculated. Mixed infection accounted for 10.3±3.7% (mean±1 SD) of infection. Two-way ANOVA reported that identity of the bacterial species (independent variable) was responsible for 47.8% of the total variance (F (5, 20) = 4.13). This was highly statistically significant (p = 0.0098). The same statistical method also reported that Burn Centre accounted for 9.84% of the total variance (F (4, 20) = 5.11), but this effect was not statistically significant (p = 0.3797).
Discussion
This study studied primary literature to establish whether the isolates associated with clinical Gram negative burn wound infection differ between burn centres. Burn wound infection has traditionally been a difficult area for the clinician, and one of the primary reasons is the difficulty in knowing which organisms to target. This study established that organisms causing Gram-negative burn wound infection do not differ significantly between burn centres. Analysis of the standardised data confirm, within this study’s limitations, the principal hypothesis that the organisms causing Gram-negative burn wound infection are similar, regardless of geographic location of the treating centre (p<0.05). This finding presents a significant departure from traditional teaching regarding the behaviour of infected burn wounds (section 1). The lack of standardised data reporting may be one possible explanation for this discord [25]. In contrast, aetiological profiles and incidences of the major protagonists in other disease such as urinary tract infection have long been established, and are constantly reviewed [6], [7]. Establishing the organisms that commonly cause Gram-negative infection in burn wounds may confer to acute burns patients the long-term benefits enjoyed by these other common diseases, such as rationalised, expedited, targeted antimicrobial development, prescribing, infection control, and surveillance of resistance patterns. These findings, integrated with local data regarding susceptibility patters, may facilitate the formulation of “first line” antibiotic treatment strategies, and increase probabilities of therapeutic efficacy. For example, worldwide resistance to commonly used broad spectrum antibiotics such as ceftriaxone is common (from 16.3% E. coli to 64% of Acinetobacter strains studied) [26].
Identifying this set of bacteria may also have implications on defining research strategies in drug redevelopment. Only up to 1.4% of isolates from across the species identified are resistant to colistin, emphasising the usefulness of this antibiotic as a drug of last resort [26]. These observations lend further credence to the strong interest of this venerable class’s redevelopment via semi-synthetic chemistry approaches. The approach to identifying a common set of bacteria responsible for burn wound infection has already borne translational fruit. Recently, an in-depth analysis for commonalities of biochemical and virulence mechanisms involved in the aetiology of infection with these organisms identified that substantial production of bradykinin is common to all these pathogens and leads to enhanced vascular permeability and sequestration of macromolecules. Based on this principle, we recently published evidence of a novel, size-based paradigm for drug targeting in infection [27]; statistically optimised a bioresponsive polymeric payload carrier to achieve this goal [28]; proposed a novel class of macromolecular antimicrobial agents capable of locally triggered enzymatic activation at the infected site, retaining antimicrobial potency to match the conventional clinical equivalent whilst significantly reducing in vivo toxicity [29], [30].
Ample evidence exists to support the notion that morbidity, mortality and quality of life outcomes in burn patients is associated to the organisms identified in this study. For example, wound infection with P. aeruginosa, E. coli, and K. pneumoniae wound is an independent predictor of mortality [3], [31]. These bacteria also promote failure of healing [32] which is of major consequence to the management of extensive burn wounds by serial excision where time to healing is essential and donor sites for grafting come at a premium. Moreover, specific risk factors associated to burn wound infection with these organisms have been identified [33]–[35], and modification of management practices may lower infection rates resulting in improved outcomes. The importance of identifying this set of organisms as the prime perpetrators of Gram-negative burn wound infection, regardless of the treating centre, is therefore apparent.
P. aeruginosa (Figure 2) exhibits an interesting, wider dispersion of dispersion of data compared to the other organisms. A possible explanation lies in the presence of an outlier data set [11] (Table 2), hence the long superior whisker for the P. aeruginosa plot in Figure 2. However ANOVA, is remarkably robust to moderate departures from normality caused by outlier data sets. One possible explanation for this outlier may lie in the susceptibility of the standardised incidence of P. aeruginosa to the overall infection rate reported from the relative burn centre. In fact, the overall infection rate reported from this centre is also high [11].
Such a study presented unique difficulties. Mere presence of organisms on a wound does not imply infection. Histological documentation of infection into viable tissue may secure the diagnosis. However, in practice, few if any centres worldwide have the substantial resources required to fulfil laboratory diagnostic criteria such as routine microbiological tissue histology and electron microscopy on a daily basis. Moreover, definitions of burn wound infection underpinned by these (tissue biopsy and electron microscopy) laboratory investigations are largely considered dated [36], [37]. As a working definition, an organism was defined as causative if it could be discerned from the primary literature that the organism/s was isolated from the wound in the presence of clinical infection. Clinical diagnosis of infection was based on reconciliation of primary literature data to the definitions of Greenhalgh et al. [21]. Studies including re-infection or relapse in their incidence rates were excluded. Whilst the clinical surrogates presented in these definitions may be less specific then exhaustive (but rarely performed) laboratory investigations, the approach presented herein would reduce the potential bias presented by the resources available to the researchers producing the primary literature.
As no RCTs were identified, the conduct of this review was adapted to consider the pooling of data from primary studies with heterogeneous methodologies. Systematic review of studies other than RCTs is well-established in the literature [21]. Therefore, a rigorous critical appraisal process qualified the “strength of the evidence” underpinning the primary data included in the statistical analysis. The critical appraisal process also facilitated the reconciliation of the primary literature to the operational definitions, ensuring comparability of the studies. Such an evidence-based approach is not new [12]. Quality of the evidence provided by the primary literature was limited (Table 2), justifying the use of an evidence-based methodology combined to the systematic review process. Since the burn centres in the primary literature were geographically separate and did not have overlapping catchment areas, it was reasonable to assume that “no interaction” occurred between the two independent variables studied.
The selection of adequate inclusion and exclusion criteria was challenging. A rigorous approach in data retrieval was observed and catalogued to prevent retrieval bias. In order to minimise the possibility data contamination with lurking variables, stringent criteria were applied. Evidence exists that delayed transfer may influence the bacteriological flora burned patients [38]. Studies concur that delayed presentation may alter the pathological flora on a burn wound [3], [39]. Military patients may pass through multiple facilities to the definitive site of treatment, and this delay presented a plausible lurking variable [38], [40]. This data was therefore excluded purely as a necessity to safeguard methodological rigour rather than implying a difference between military and civilian populations. In fact, data from Operation Iraqi Freedom and Operation Enduring Freedom suggests that similar bacterial flora is present in such patients, [38], [40], [41]. Indeed primary studies describing civilian injuries [22], [42] were also excluded for similar reasons.
A five to ten year cut-off for the retrieval of primary data is well-established in evidence-based methodology [12], [43]. Such time-limits for the inclusion of primary data in evidence-based medicine harks back to the main works of Archie Cochrane and DL Sackett [44], [45], affirmed in the Sicily statement for evidence-based research [17] and cited in multiple works since then [46]–[48]. Moreover until the 1980’s burn wounds were treated by the exposure method, with application of topical antimicrobials to the burn wound surface and gradual debridement with immersion hydrotherapy [24]. Thereafter, early burn wound excision and wound closure became the focal point of burn wound management, accompanied by a change from immersion hydrotherapy to showering hydrotherapy, and a consequent decrease in the rate of burn wound infection [24]. It is also well-established that early excision has reduced the incidence of invasive infection, as underscored by key publications immediately preceding our cut-off point for retrieval of primary literature. Pruitt et al. in particular, assert that the change to early excision and grafting significantly changed, both qualitatively and quantitatively, the incidence and identity of organisms causing these infections and their timing [38], [49].
It was assumed that by the year 2000, this method of treatment would have been well-established, coinciding with the cut-off point determined by the evidence-based methodology. However, it is acknowledged that such arbitrary cut-off points may have led to retrieval bias in selection of primary literature.
Conclusion
This study is the first to report that organisms causing clinical Gram-negative burn wound infection do not differ significantly between burn centres. Therefore, these findings establish a “target set” of Gram-negative pathogens for antimicrobial development and timely, effective treatment. P. aerugionsa, K. pneumoniae, E. coli, Enterobacter spp. and Proteus spp. were identified as the commonest Gram-negative pathogens to be isolated from clinically infected burn wounds regardless of the treating centre. It is of course acknowledged that other bacteria may infect the burn wound and it is especially important to monitor emerging infections [51]. However, we hope that finding a “target” set of pathogens may contribute to timely clinical treatment, effective, and clinically oriented antibiotic development. The threat posed by multi-drug resistant pathogens continues to increase, however research and clinical management in this specialist field remain poorly funded, fragmented and oftentimes reported in isolation. The paucity of relevant literature highlighted in this study illustrates the dire necessity for further epidemiological multi-disciplinary collaboration. We augur that the identification of a common aetiological Gram-negative burn wound profile will be a first step in this direction.
Supporting Information
File S1
PRISMA Checklist.
(DOC)
File S2
PICO* framework applied to the study question.
(DOCX)
File S3
Search Terms Used in the Literature Search.
(DOCX)
File S4
Databases Searched Electronically.
(DOCX)
File S5
Results of the Electronic Literature Search.
(DOCX)
File S6
Studies excluded after content analysis.
(DOCX)
File S7
Detailed critical appraisal of the literature.
(DOCX)
Acknowledgments
The authors would like to thank Mr Stephen Honeyman, Mr Ian Malin, and the staff at the Welsh Burns Centre, Swansea, UK for their helpful comments. The authors would also like to thank the St. David’s Medical Foundation, Swansea University, for facilitating research into the conclusions of this study.
Funding Statement
EA and EAA are funded by the Strategic Educational Pathways Scholarship Malta, co-financed by by the European Union – European Social Fund under Operational Programme II. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Individuals employed or contracted by the funders (other than the named authors) played no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, et al. (2009) Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clinical Infectious Diseases 48: 1–12. [DOI] [PubMed] [Google Scholar]
- 2.Herndon DN (2007) Total burn care: Elsevier Health Sciences.
- 3.Church D, Elsayed S, Reid O, Winston B, Lindsay R (2006) Burn wound infections. Clinical Microbiology Reviews 19: 403–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ipaktchi K, Mattar A, Niederbichler AD, Hoesel LM, Vollmannshauser S, et al. (2006) Attenuating burn wound inflammatory signaling reduces systemic inflammation and acute lung injury. Journal of Immunology 177: 8065–8071. [DOI] [PubMed] [Google Scholar]
- 5.Calum H, Moser C, Jensen PO, Christophersen L, Maling DS, et al. (2009) Thermal injury induces impaired function in polymorphonuclear neutrophil granulocytes and reduced control of burn wound infection. Clinical and Experimental Immunology 156: 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wibbenmeyer L, Danks R, Faucher L, Amelon M, Latenser B, et al. (2006) Prospective analysis of nosocomial infection rates, antibiotic use, and patterns of resistance in a burn population. Journal of Burn Care and Research 27: 152–160. [DOI] [PubMed] [Google Scholar]
- 7.British Association of Antimicrobial Chemotherapy (2009) Burn Wound Infection. Web Page.
- 8.Rice LB (2008) Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. Journal of Infectious Diseases 197: 1079–1081. [DOI] [PubMed] [Google Scholar]
- 9.Singh NP, Goyal R, Manchanda V, Das S, Kaur I, et al. (2003) Changing trends in bacteriology of burns in the burns unit, Delhi, India. Burns 29: 129–132. [DOI] [PubMed] [Google Scholar]
- 10.Edwards-Jones V, Greenwood JE (2003) What’s new in burn microbiology? James Laing Memorial Prize Essay 2000. Burns 29: 15–24. [DOI] [PubMed] [Google Scholar]
- 11.Khorasani G, Salehifar E, Eslami G (2008) Profile of microorganisms and antimicrobial resistance at a tertiary care referral burn centre in Iran: emergence of Citrobacter freundii as a common microorganism. Burns 34: 947–952. [DOI] [PubMed] [Google Scholar]
- 12.Straus SE, Richardson WS, Glasziou P, Haynes RB (2005) Evidence-based medicine: how to practice and teach EBM.
- 13.National Library of Medicine (2009) MeSH Browser. Available at: URL: ed.
- 14.Gomez R, Murray CK, Hospenthal DR, Cancio LC, Renz EM, et al. (2009) Causes of mortality by autopsy findings of combat casualties and civilian patients admitted to a burn unit. Journal of the American College of Surgeons 208: 348–354. [DOI] [PubMed] [Google Scholar]
- 15.Mayer D (2004) Essential Evidence Based Medicine. Cambridge: Cambridge University Press.
- 16.Newman M, Roberts T (2002) Critical appraisal I: is the quality of the study good enough for you to use the findings? The Evidence-based Practice Manual for Nurses Edinburgh: Churchill Livingstone: 86–112.
- 17.Dawes M, Summerskill W, Glasziou P, Cartabellotta A, Martin J, et al. (2005) Sicily statement on evidence-based practice. BMC Medical Education 5: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polit D, Beck C, Hungler B (2006) Essentials of Nursing Research, Methods, Appraisal and Utilization. Lippincott Williams & Wilkins, Philadelphia 2: 11–23. [Google Scholar]
- 19.Phillips P, Ball C, Sacket D, Badenoch D, Straus S, et al.. (2001) Oxford Centre for Evidence-based Medicine - Levels of Evidence. Centre For Evidence Based Medicine. Oxford: University of Oxford.
- 20.Lindholm LH, Ibsen H, Dahlöf B, Devereux RB, Beevers G, et al. (2002) Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. The Lancet 359: 1004–1010. [DOI] [PubMed] [Google Scholar]
- 21.Greenhalgh DG, Saffle JR, Holmes JH, Gamelli RL, Palmieri TL, et al. (2007) American Burn Association consensus conference to define sepsis and infection in burns. Journal of Burn Care and Research 28: 776–790. [DOI] [PubMed] [Google Scholar]
- 22.Silla RC, Fong J, Wright J, Wood F (2006) Infection in acute burn wounds following the Bali bombings: a comparative prospective audit. Burns 32: 139–144. [DOI] [PubMed] [Google Scholar]
- 23.Lari AR, Alaghehbandan R (2000) Nosocomial infections in an Iranian burn care center. Burns 26: 737–740. [DOI] [PubMed] [Google Scholar]
- 24.Mayhall CG (2003) The epidemiology of burn wound infections: then and now. Clinical Infectious Diseases 37: 543–550. [DOI] [PubMed] [Google Scholar]
- 25.Azzopardi E (2009) Characterising Aetiology Incidence and Specific Risk factors for Gram negative Burn wound infection. Cardiff University: Cardiff.
- 26.Gales AC, Jones RN, Sader HS (2011) Contemporary activity of colistin and polymyxin B against a worldwide collection of Gram-negative pathogens: results from the SENTRY Antimicrobial Surveillance Program (2006–09). Journal of Antimicrobial Chemotherapy 66: 2070–2074. [DOI] [PubMed] [Google Scholar]
- 27.Azzopardi EA, Ferguson EL, Thomas DW (2013) The enhanced permeability retention effect: a new paradigm for drug targeting in infection. Journal of Antimicrobial Chemotherapy 68: 257–274. [DOI] [PubMed] [Google Scholar]
- 28.Azzopardi EA, Camilleri L, Moseley R, Thomas DW, Ferguson EL (2013) Statistical Characterization of Succinoylated Dextrin Degradation Behavior in Human α-Amylase. Journal of Carbohydrate Chemistry 32: 438–449. [Google Scholar]
- 29.Azzopardi E, Ferguson E, Thomas D (2014) A novel class of bioreponsive nanomedicines for localised reinstatement of bioactivity and specific targeting. The Lancet 383: S9. [Google Scholar]
- 30.Azzopardi EA (2013) Bioresponsive dextrin-colistin conjugates as antimicrobial agents for the treatment of Gram-negative infection: Cardiff University.
- 31.D’Avignon LC, Hogan BK, Murray CK, Loo FL, Hospenthal DR, et al. (2010) Contribution of bacterial and viral infections to attributable mortality in patients with severe burns: an autopsy series. Burns 36: 773–779. [DOI] [PubMed] [Google Scholar]
- 32.Guo S, DiPietro LA (2010) Factors affecting wound healing. Journal of dental research 89: 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozkurt Z, Ertek M, Erol S, Altoparlak U, Akcay MN (2005) The risk factors for acquisition of imipenem-resistant Pseudomonas aeruginosa in the burn unit. Burns 31: 870–873. [DOI] [PubMed] [Google Scholar]
- 34.Simor AE, Lee M, Vearncombe M, Jones-Paul L, Barry C, et al. (2002) An outbreak due to multiresistant Acinetobacter baumannii in a burn unit: risk factors for acquisition and management. Infect Control Hosp Epidemiol 23: 261–267. [DOI] [PubMed] [Google Scholar]
- 35.Polavarapu N, Ogilvie MP, Panthaki ZJ (2008) Microbiology of burn wound infections. J Craniofac Surg 19: 899–902. [DOI] [PubMed] [Google Scholar]
- 36.Peck MD, Weber J, McManus A, Sheridan R, Heimbach D (1998) Surveillance of burn wound infections: a proposal for definitions. Journal of Burn Care and Rehabilitation 19: 386–389. [DOI] [PubMed] [Google Scholar]
- 37.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM (1988) Centres for Disease Control definitions for nosocomial infections, 1988. American Journal of Infection Control 16: 128–140. [DOI] [PubMed] [Google Scholar]
- 38.Keen Iii EF, Robinson BJ, Hospenthal DR, Aldous WK, Wolf SE, et al. (2010) Incidence and bacteriology of burn infections at a military burn center. Burns 36: 461–468. [DOI] [PubMed] [Google Scholar]
- 39.Datubo-Brown D, Kejeh B (1989) Burn injuries in Port Harcourt, Nigeria. Burns 15: 152–154. [DOI] [PubMed] [Google Scholar]
- 40.Keen Iii EF, Robinson BJ, Hospenthal DR, Aldous WK, Wolf SE, et al. (2010) Prevalence of multidrug-resistant organisms recovered at a military burn center. Burns 36: 819–825. [DOI] [PubMed] [Google Scholar]
- 41.Murray CK (2008) Epidemiology of infections associated with combat-related injuries in Iraq and Afghanistan. Journal of Trauma-Injury, Infection, and Critical Care 64: S232–S238. [DOI] [PubMed] [Google Scholar]
- 42.Chim H, Tan BH, Song C (2007) Five-year review of infections in a burn intensive care unit: High incidence of Acinetobacter baumannii in a tropical climate. Burns 33: 1008–1014. [DOI] [PubMed] [Google Scholar]
- 43.Azzopardi EA, McWilliams B, Iyer S, Whitaker IS (2009) Fluid resuscitation in adults with severe burns at risk of secondary abdominal compartment syndrome–an evidence based systematic review. Burns 35: 911–920. [DOI] [PubMed] [Google Scholar]
- 44.Cochrane AL, Fellowship RC (1972) Effectiveness and efficiency: random reflections on health services: Nuffield Provincial Hospitals Trust London.
- 45.Sackett DL, Rosenberg WM, Gray J, Haynes RB, Richardson WS (1996) Evidence based medicine: what it is and what it isn’t. BMJ: British Medical Journal 312: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenberg W, Donald A (1995) Evidence based medicine: an approach to clinical problem solving. British medical journal 310: 1122–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muir Gray J (1997) Evidence-based healthcare: how to make health policy and management decisions. London: Churchill Livingstone: p53.
- 48.Porzsolt F, Ohletz A, Thim A, Gardner D, Ruatti H, et al. (2003) Evidence-based decision making–the six step approach. Evidence Based Medicine 8: 165–166. [PubMed] [Google Scholar]
- 49.Pruitt Jr BA, McManus AT, Kim SH, Goodwin CW (1998) Burn wound infections: current status. World journal of surgery 22: 135–145. [DOI] [PubMed] [Google Scholar]
- 50.Agnihotri N, Gupta V, Joshi RM (2004) Aerobic bacterial isolates from burn wound infections and their antibiograms–a five-year study. Burns 30: 241–243. [DOI] [PubMed] [Google Scholar]
- 51.Chim H, Song C (2007) Aeromonas infection in critically ill burn patients. Burns 33: 756–759. [DOI] [PubMed] [Google Scholar]
- 52.Appelgren P, Bjornhagen V, Bragderyd K, Jonsson CE, Ransjo U (2002) A prospective study of infections in burn patients. Burns 28: 39–46. [DOI] [PubMed] [Google Scholar]
- 53.Kaushik R, Kumar S, Sharma R, Lal P (2001) Bacteriology of burn wounds–the first three years in a new burn unit at the Medical College Chandigarh. Burns 27: 595–597. [DOI] [PubMed] [Google Scholar]
- 54.Komolafe OO, James J, Kalongolera L, Makoka M (2003) Bacteriology of burns at the Queen Elizabeth Central Hospital, Blantyre, Malawi. Burns 29: 235–238. [DOI] [PubMed] [Google Scholar]
- 55.Ozumba UC, Jiburum BC (2000) Bacteriology of burn wounds in Enugu, Nigeria. Burns 26: 178–180. [DOI] [PubMed] [Google Scholar]
- 56.Tredget EE, Shankowsky HA, Rennie R, Burrell RE, Logsetty S (2004) Pseudomonas infections in the thermally injured patient. Burns 30: 3–26. [DOI] [PubMed] [Google Scholar]
- 57.Herruzo R, De La Cruz J, Fernandez-Acenero MJ, Garcia-Caballero J (2004) Two consecutive outbreaks of Acinetobacter baumanii 1-a in a burn Intensive Care Unit for adults. Burns 30: 419–423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1
PRISMA Checklist.
(DOC)
File S2
PICO* framework applied to the study question.
(DOCX)
File S3
Search Terms Used in the Literature Search.
(DOCX)
File S4
Databases Searched Electronically.
(DOCX)
File S5
Results of the Electronic Literature Search.
(DOCX)
File S6
Studies excluded after content analysis.
(DOCX)
File S7
Detailed critical appraisal of the literature.
(DOCX)