Reaction-based small-molecule fluorescent probes for chemoselective bioimaging (original) (raw)
. Author manuscript; available in PMC: 2014 Jul 14.
Published in final edited form as: Nat Chem. 2012 Dec;4(12):973–984. doi: 10.1038/nchem.1500
Abstract
The dynamic chemical diversity of elements, ions and molecules that form the basis of life offers both a challenge and an opportunity for study. Small-molecule fluorescent probes can make use of selective, bioorthogonal chemistries to report on specific analytes in cells and in more complex biological specimens. These probes offer powerful reagents to interrogate the physiology and pathology of reactive chemical species in their native environments with minimal perturbation to living systems. This Review presents a survey of tools and tactics for using such probes to detect biologically important chemical analytes. We highlight design criteria for effective chemical tools for use in biological applications as well as gaps for future exploration.
The natural world is a complex collection of elements, ions and molecules that continuously drive a web of interacting chemical reactions. Living organisms and their environments sense each other through a diverse array of reactions spanning electron transfer, acid–base chemistry and metal–ligand substitutions, as well as stoichiometric and catalytic transformations combining these elementary chemical steps. A molecular-level understanding of these processes presents not only a grand challenge, but also a unique opportunity and motivation for chemists to create new ways to study biological systems in their native contexts. As a step towards this ultimate goal, molecular imaging offers a powerful approach to interrogate intact living samples in real time with spatial resolution by combining synergistic advances in synthetic probe design and biological imaging instrumentation. Fluorescence methods are particularly useful owing to (i) the widespread use of confocal, two-photon and epifluorescence light microscopy and (ii) the development of new functional fluorescent reagents that can monitor intra- and extracellular events with high chemoselectivity.
All chemical species undergo dynamic molecular transformations and/or changes in their local environment within the biological milieu; thus, an emerging bioinspired strategy for fluorescence-based molecular imaging is to sort and identify species of interest within this complex mixture by exploiting differences in molecular reactivity, rather than traditional lock-and-key molecular recognition and binding (Fig. 1a–c). This reaction-based approach to sensing can, in principle, provide specificity that is bioorthogonal to (that is, does not interfere with) the endogenous chemical reactivity of cells, tissues and organisms.
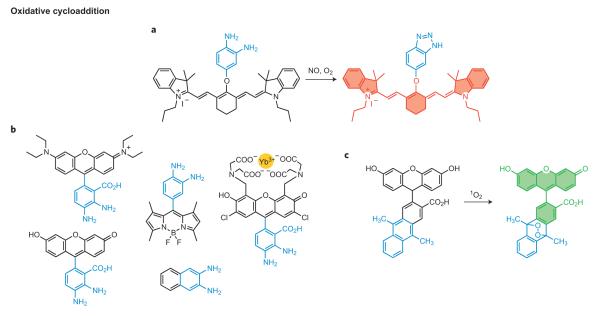
Figure 1. Overview of organic and metal-mediated reaction-based strategies for the chemoselective bioimaging of small-molecule and metal ion analytes in biological systems.
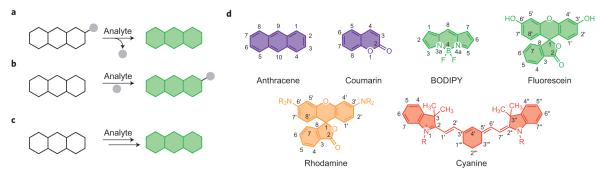
Representative approaches to turn-on or ratiometric fluorescence detection by a, bond-cleavage reactions, b, organic addition and/or metal–ligand substitution reactions, and c, tandem reaction cascades to unmask a fluorogenic scaffold. The green ‘polyaromatic’ shape represents a generic fluorophore. d, A representative set of common dyes that can be transformed into fluorescent probes for chemoselective bioimaging, sorted by structure and emission colour.
In this Review, we will summarize progress in the development of reaction-based fluorescent probes for chemoselective bioimaging in living systems. We will specifically focus on synthetic small-molecule indicators and biologically relevant analytes. Rather than assembling a comprehensive list of fluorescent probes, our purpose is to provide an overview of the tools and tactics currently available for detecting chemical species in biological environments using this approach; we note other excellent reviews on related topics1–5. A key theme is that the discovery or recognition of even a single type of robust and selective chemical reaction that operates under biological conditions can be widely applied. We will begin by describing the fundamental rules and principles that need to be considered during probe development. We will then highlight a selection of recent advances in fluorophores for detecting biologically important small molecules and metal ions, organized by the type of reaction strategy employed (for example organic versus metal-mediated). We will close with a discussion of current and future challenges in this field, with particular emphasis on unmet needs in functional bioimaging probes.
Design principles
Effective reaction-based fluorescent probes for bioimaging applications must meet stringent requirements. Most importantly, a useful probe should respond selectively to its intended target in a complex biological system that contains a host of competing analytes. This ideal case is often complicated or perturbed by interfering species that may have similar reactivity, closely related or even identical functional groups, and/or higher working concentrations. Biocompatibility is another critical consideration. For a reaction to be a suitable trigger for detection and imaging applications, it must proceed with reasonable kinetics in water under biological constraints of physiological pH, high salt content and large excess of reactive nucleophilic thiols such as glutathione (GSH) and cysteine (Cys). One must pay particularly close attention to such reaction conditions when the target analyte is present at low concentrations or has a short chemical lifetime in a given biological specimen. Finally, a useful reaction-based probe should be fully bioorthogonal: it must not interfere with endogenous cellular and tissue processes, and must generate products that are inert and non-toxic to living systems. Thus the key chemical challenge for reaction-based bioimaging is to identify suitable reactions that meet the needs of chemoselectivity and bioorthogonality. Success in this approach requires not only a working knowledge of fundamental organic, organometallic and inorganic reactivity but also an understanding of the intrinsic reactivity of the target analyte in its biological setting.
In addition to the reaction trigger, selecting a suitable fluorophore platform is essential (Fig. 1d). High optical brightness is important to reduce the amount of probe needed for bioimaging experiments, which minimizes the potential for interference from endogenous cellular analytes and reactions. A turn-on emission increase or a shift in excitation/emission profiles is preferred over a turn-off quenching response. A turn-on response gives a bright signal against a dark background, which maximizes spatial resolution. Likewise, a shift in excitation/emission maxima can be used for ratiometric imaging, which allows for internal calibration of reacted and unreacted probe to minimize artefacts that may arise from variations in light intensity, sample thickness and heterogeneity, and dye distribution. An appropriate fluorophore scaffold should also be non-toxic and have excitation and emission profiles in the visible or near-infrared region, or be suitable for two-photon excitation, in order to minimize sample damage or interference from autofluorescence. Finally, the balance between hydrophobicity and hydrophilicity should be considered in the context of membrane permeability, cellular retention and water solubility. Ideally, an indicator must allow monitoring of specific intra- and/or extracellular regions.
Organic reaction-based approaches
Organic transformations that exploit a small molecule as a selective reagent or catalyst can form the basis for reaction-based bioimaging schemes. We highlight a subset of representative examples for detection of biologically important signalling and stress molecules, focusing on reactive oxygen, nitrogen and sulfur species owing to their small size and transient nature. These design principles can be applied to many other analytes. The discussion is organized by the type of reactivity (for example addition or cleavage; oxidative or reductive) as well as the small-molecule target of interest.
Oxidative cycloaddition reactions
Cycloadditions offer a broadly useful set of potential reactions for chemoselective bioimaging, as formation of new heterocycles conjugated to a fluorescent scaffold can readily alter its optical properties. In seminal early work in this vein, Nagano and co-workers discovered that aromatic vicinal diamines can react with nitric oxide (NO) in the presence of O2 to form the corresponding triazole compounds6. This transformation inspired the development of the diaminofluoresceins (DAFs), the first fluorescent probes for assaying NO production in living cells6. DAFs are fluorescent but only weakly so because of photoinduced electron transfer (PET) quenching from the electron-rich amino substituents; however, conversion to the triazole functionality results in a turn-on emission because the extent of PET quenching is smaller for this less-electron-donating functionality.
From this single starting point has come a host of NO indicators with enhanced capabilities, including higher sensitivity and photostability, as well as an expanded colour palette (Fig. 2a,b)6–11. In the figures we have highlighted the organic moiety that undergoes the chemical transformation in blue, and the fluorophores are outlined and shaded in colours corresponding to the emission wavelength. Included are the diaminorhodamines (DARs)8 and diamino probes on BODIPY9 and cyanine10 scaffolds. DAFs, DARs and their congeners have been applied to study NO signalling and stress events in a variety of models, including cells, tissue slices and even whole organisms, revealing insights into the roles of NO signalling and stress in cardiovascular function and disease, cancer, immune response, neurotransmission and fertilization. In a more general sense, the diamino switch provides a lasting example of the versatility of a single reaction in interrogating specific biochemical processes. Shear, Anslyn and co-workers recently developed a related probe, NO550, that proceeds through electrophilic substitution of an NO-derived _N_-nitrosoaniline intermediate12.
Figure 2. Representative oxidative cycloaddition reactions for fluorescence bioimaging.
a, Transformation of a vicinal diamine to a triazole moiety mediated by nitric oxide (NO) under aerobic conditions. b, A family of reaction-based dyes that all operate using the same general reaction-based switch as in a. c, Conversion of an anthracene derivative to the corresponding endoperoxide by singlet oxygen cycloaddition.
In another clever example of reaction-based sensing, Nagano and co-workers applied the well-known cycloaddition reaction between singlet oxygen (1O2) and anthracene to form an endoperoxide to prepare DPAX10 and DMAX (Fig. 2b)11, two selective probes for 1O2. In the absence of 1O2, the anthracene moiety serves as a PET quencher of the xanthene fluorophore, but conversion to the endoperoxide product on oxidative cycloaddition perturbs PET and results in a >50-fold fluorescence turn-on response11. More importantly, this work represents one of the first examples of fluorescent probes that have been rationally designed through computational calculations11. Based on this general reaction, Yuan and co-workers have developed several anthracene-derivatized lanthanide complexes for luminescent 1O2 detection, one of which can image 1O2 generation in live HeLa cells13.
Oxidative cleavage reactions
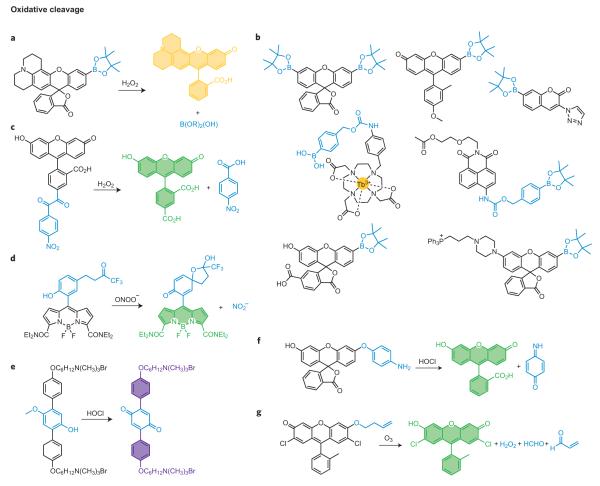
Another versatile strategy for reaction-based fluorescent detection of small molecules is to use a profluorophore approach, where reaction of a small molecule selectively releases a cage on a non- or weakly fluorescent pro-fluorophore to produce the parent dye. Several examples of this approach have proved fruitful for bioimaging of reactive small-molecule signalling and stress agents. Our laboratory has focused on applying the hydrogen peroxide-mediated conversion of arylboronates to phenols for H2O2 detection14. Unlike many reactive oxygen species (ROS) that operate via one-electron transfer or electrophilic oxidation pathways, H2O2 can be a potent nucleophile, especially when deprotonated. As such, the hydroperoxide anion is proposed to attack the boron centre to form a transient tetra-substituted intermediate, and subsequent C-to-O boronate migration will generate a labile borate species that hydrolyses to the corresponding phenol. Based on this strategy, we developed Peroxyfluor-1 (PF1), the first fluorescent probe for selective imaging of H2O2 in biological systems15. Installation of boronic ester groups at the 3′and 6′ positions of a fluorescein scaffold forces the dye to adopt a closed non-fluorescent spirolactone configuration. Hydrogen-peroxide-mediated deprotection generates the open fluorescent fluorescein dye, resulting in a >1,000-fold fluorescent enhancement with high selectivity for H2O2 over a variety of ROS and RNS, including superoxide, nitric oxide, hydroxyl radical and lipid peroxides. The boronate deprotection trigger is generally applicable to a variety of fluorescent scaffolds, and these congeners have expanded the chemical toolbox of selective H2O2 reporters to other excitation/emission colour palettes16–20 as well as ratiometric21, targetable22,23, trappable24,25 and in vivo bioluminescent versions26 (Fig. 3a,b)15,17,19,21,22,25,27,28. Moreover, our laboratory has applied these new chemical tools to discover that specific aquaporin channels can regulate membrane H2O2 entry and intracellular signal transduction24, as well as that the mammalian brain requires endogenous, regulated H2O2 production derived from NADPH oxidase 2 (Nox) for normal growth and proliferation of neural stem cells and subsequent neurogenesis24. We have also defined the physiology of H2O2 signalling for growth, migration, differentiation and phagocytic responses in a variety of cancer cell22, immune cell19 and primary neuron models17. Finally, the boronate switch has also been used to prepare H2O2-responsive prochelators29,30, pro-drugs31,32, polymers33,34 and mass spectrometry probes35. As with the diamino switch for NO detection, the H2O2-sensitive boronate cage provides a second general example that illustrates the potential power of a single robust chemical reaction to improve our biological knowledge. In the boronate case, sensing and imaging strategies have been expanded to include therapeutic and materials applications, pointing a path forward for other robust reaction-based switches.
Figure 3. Representative oxidative cleavage reactions for small-molecule detection.
a, Oxidative cleavage of a boronic ester to a phenol triggered by hydrogen peroxide (H2O2). b, A family of reaction-based dyes that all operate using the same general reaction-based switch as in part a. c, Dicarbonyl fragmentation assisted by H2O2 oxidation. d, Trifluoroketone oxidation and elimination by peroxynitrite. e, Oxidation and cleavage of a _p_-methoxyphenol by hypochlorous acid (HOCl). f, Oxidative _O_-dearylation processes mediated by highly reactive oxygen species such as HOCl. g, Ozonolysis of olefins with β-elimination.
Dicarbonyl cleavage reactions offer another attractive strategy for selective H2O2 detection. Nagano and co-workers recently developed NBzF, a H2O2 probe based on a benzil fragmentation reaction (Fig. 3c)36. Specifically, benzil reacts with H2O2 to form benzoic anhydride via a Baeyer–Villiger type mechanism and generates two equivalents of benzoic acid in a subsequent hydrolysis step. The NBzF pro-fluorophore is weakly fluorescent owing to efficient donor PET from the fluorophore to the lowest unoccupied molecular orbital of the benzil moiety, but H2O2-induced cleavage releases 4-nitrobenzoic acid and the fluorescent 5-carboxyfluorescein product with a 150-fold fluorescent enhancement. Moreover, NBzF can be used to visualize endogenous H2O2 bursts generated by macrophages and cancer cells. In parallel efforts to this work, our laboratory exploited the H2O2-mediated oxidation of ketoacids to carboxylic acids to develop a H2O2-responsive hyperpolarized 13C MRI contrast agent37. Specifically, 13C-labelled benzoylformic acid (13C-BFA) reacts selectively with H2O2 over other ROS to generate the corresponding 13C-benzoic acid. This process can be monitored in a ratiometric mode by using dynamic nuclear polarization techniques.
Peroxynitrite (ONOO−) is a potent reactive nitrogen species (RNS) generated through the spontaneous reaction between NO and superoxide (O2−). In an innovative study38, Yang and co-workers reported that ONOO− can selectively oxidize a trifluoroketone functionality to form a labile dioxirane intermediate, which can then decompose and oxidize a proximal anisole ring to the corresponding dienone functionality with concomitant release of an alcohol (Fig. 3d)39. HKGreen-1 was developed based on this reaction design and can image changes in ONOO− levels in primary cultured neuronal cells38. Next-generation versions give improved turn-on responses and can visualize endogenous ONOO− fluxes produced upon immune stimulation39,40.
Hypochlorous acid (HOCl) is an important highly reactive oxygen species (hROS) in biology, particularly in the immune system, where it is generated via myeloperoxidase activity. Yang and co-workers have reported that the HOCl-mediated oxidation and cleavage of a _p_-methoxyphenol can serve as a selective trigger for detecting this hROS (Fig. 3e)41, and a related oxidation reaction of a spirothioether can also be used for HOCl sensing42. Oxidative _O_-dearylations have also been used by Nagano and co-workers for monitoring hROS, with preference for peroxynitrite, hydroxyl radical, and hypochlorite over other ROS and RNS (Fig. 3f)43, and Hilderbrand and co-workers reported a near-IR SNAFL platform that can visualize HOCl generated during immune response44. Related selenoether oxidations have also been reported by Han and co-workers to show some specificity for ONOO− detection45.
Finally, ozonolysis is a reaction that oxidatively fragments an olefin into two carbonyl products and has been elegantly used by Koide and co-workers to create a probe for chemoselective ozone sensing46. Specifically, ozonolysis of a pendant homoallyl ether moiety on a fluorescent platform with ozone forms an aldehyde intermediate that can undergo subsequent β-elimination to release acrolein and the free fluorescent dye (Fig. 3g)46. The reaction is compatible with environmental and biological samples and shows no interference from competing ROS or biological oxidants.
Reductive cleavage reactions
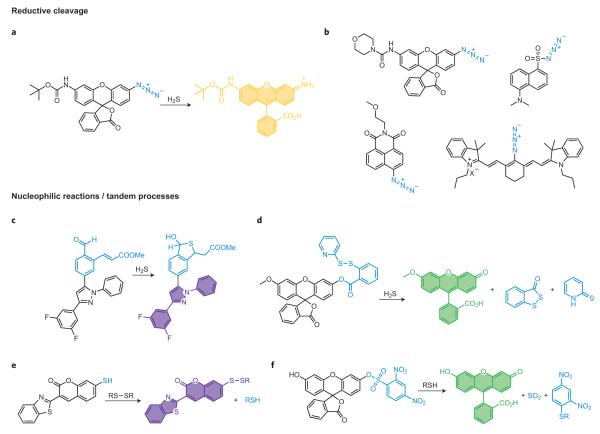
In addition to oxidative processes, reductive transformations can also be used for reaction-based small-molecule detection. A prime example is the hydrogen-sulfide-mediated reductive cleavage of azides to amines as a means of monitoring H2S (ref. 47). This reaction is particularly amenable to bioimaging applications, as azides are widely valued for their inert chemical behaviour in biological specimens ranging from extracellular fluids to cells, tissues and various in vivo animal models, where they can be used as partners for bioorthogonal coupling reactions (for example copper and copper-free click chemistry41, Staudinger–Bertozzi ligations42). Our laboratory has used the selective conversion of azides to amines for H2S detection by coupling this bioorthogonal functionality to aromatic dye platforms for tuning to biologically relevant redox potentials (Fig. 4a,b)47–50. Based on this approach we developed Sulfidefluors 1 and 2 (SF1 and SF2), initial members of a new family of fluorescent H2S indicators with high selectivity for this reactive sulfur species (RSS) over biologically relevant thiols, including GSH and Cys, as well as a host of competing ROS and RNS. SF1 and SF2 are the first fluorescent probes capable of imaging H2S in living cells, and recent efforts have expanded this general reaction-based method for visualization of endogenous H2S fluxes generated in a variety of biological models. Concomitant with our efforts, Wang and co-workers showed that sulfonyl azides could be rapidly reduced to the corresponding sulfonamides by H2S in aqueous buffer and in biological fluids48. The generality of the azide reduction concept for reaction-based H2S sensing has been further illustrated by notable reports using cyanine49 and naphthalimide50 dye platforms, as well as a genetically encoded fluorescent H2S reporter51. Because of their demonstrated bioorthogonal nature, azide switches offer much promise as tools for interrogating and/or manipulating H2S biology.
Figure 4. Representative reductive cleavage, nucleophilic reactions and tandem processes for detection of small molecules.
a, Selective reduction of aromatic azides to anilines by hydrogen sulfide (H2S). b, A family of reaction-based dyes that all operate using the same general reaction-based switch as in part a. c, Detection of H2S by a tandem Michael addition. d, Dual nucleophilic addition strategy for H2S visualization. e, Thiol-mediated cleavage of electron-poor aryl sulfonates. f, Reversible sensing of thiols by disulfide–thiol exchange chemistry.
Nucleophilic reactions and tandem processes
Lewis acid–base chemistry can also provide a suite of potential reactions for small-molecule sensing. The rapid expansion of H2S probes provides prime examples of this approach. In this context, He, Zhao and co-workers recently created an exquisite strategy for imaging H2S in living cells based on a tandem Michael addition reaction52. Hydrogen sulfide is a potent nucleophile that can react reversibly with aldehydes to form hemithioacetals. If an electrophilic α,β-unsaturated acrylate methyl ester functional group is positioned in proximity to the aldehyde, the trapped thiol can subsequently undergo an intramolecular Michael addition to form a stable thioacetal product (Fig. 4c)52. This tandem reaction is highly selective for H2S over other biologically competing thiols such as GSH or Cys, which can attack the aldehyde but not perform the subsequent Michael addition reaction. A BODIPY derivative from this family can image elevations in H2S levels in cells as well as enzymatically generated H2S bursts in vitro52, and the highly tunable nature of this reaction offers the prospect that even more reactive versions can be devised. A related but unique design strategy reported by Xian and co-workers also astutely recognizes that H2S can potentially undergo two nucleophilic reactions, using pendant disulfide and ester groups as the two electrophilic sites for reaction with H2S (Fig. 4d)53. This reaction can be used to monitor changes in H2S levels in plasma and cells, and sets the stage for next-generation analogues54.
In a separate line of research, nucleophilic aromatic substitutions are routinely used to functionalize aromatic compounds, and such transformations have been recently adapted for fluorometric thiol detection. In particular, the 2,4-dinitrobenzenesulfonyl functionality is commonly used as a protecting group for alcohols and amines and can be released by nucleophilic thiol reactivity (Fig. 4e)55. Maeda and co-workers have elegantly used this concept to monitor a wide range of biologically relevant thiols55, as well as related probes for superoxide56. Using the same trigger, Wang’s laboratory developed a selective thiophenol sensor based on a 4-amino-7-nitro-2,1,3-benzoxadiazole platform, where the dye is non-fluorescent when the 4 position is capped with an electron-withdrawing 2,4-dinitrobenzenesulfonamide group but on reaction with a thiol cleaves to give the electron-rich and fluorescent amine57. In addition, by performing assays at pH 7.3, they exploited the large difference in the pKa values between thiophenols and aliphatic thiols (6.5 compared with 8.5) to enhance the selectivity for thiophenols57. Hilderbrand and co-workers expanded this approach to red-fluorescent platforms to achieve high signal-to-noise responses (>120-fold)58. A few final selected examples for nucleophilic thiol detection are from Chmielewski’s laboratory where they detect GSH by a thiol-induced disulfide cleavage to release Rhodamine 110 (ref. 59) and from Murthy’s laboratory where a thiol-coumarin derivative is used for reversible redox sensing (Fig. 4f)60.
Metal-mediated reaction-based fluorescent probes
Metal-mediated reactivity offers a powerful and complementary approach to purely organic transformations for reaction-based detection of small-molecule biological analytes. The reversible nature of metal–ligand bonds for supramolecular host–guest chemistry, as well as the greater geometric and redox flexibility endowed by metals compared with their organic counterparts, affords a wide array of opportunities to tune thermal and photochemical reactivity of fluorescent dyes. In this section we highlight a selection of metal-mediated strategies for reaction-based bioimaging organized by the type of reactivity used as a detection switch (for example metal–ligand substitutions resulting in fluorophore displacement, metal-directed redox cleavage or addition reactions). Again, these examples are meant to provide a flavour of activity in the field rather than a comprehensive list.
Fluorophore displacement by metal–ligand substitution
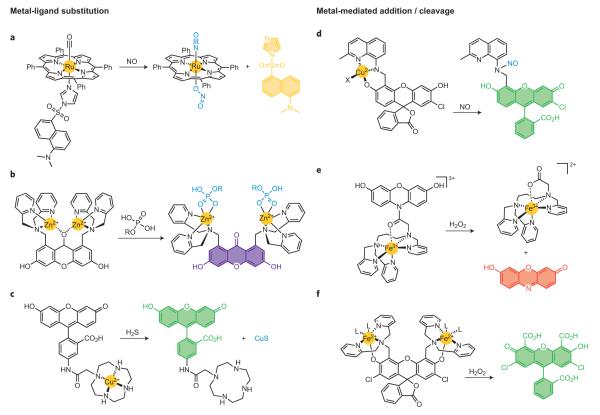
Metal ion centres are often potent quenchers of fluorescent excited states owing to their unpaired electrons or partially filled orbitals, as well as heavy-atom effects that can turn off emission by electron- or energy-transfer quenching pathways. As such, metal–ligand substitutions offer a versatile approach to tune fluorescence properties and small-molecule responses of pendant dyes. Classic examples of metal–dye substitutions to achieve turn-on fluorescent sensing schemes in abiotic systems include Anslyn’s61 and Fabbrizzi’s62 work on dye displacement strategies at metal-coordinated dye scaffolds. In the context of reactive biological small-molecule detection, Lippard’s laboratory and others have pursued a number of platforms to detect NO directly by displacement of ligand-fluorophores from quenching, chelated metal centres (Fig. 5a)63. This general approach relies on quenching of pendant fluorophores by coordination to a paramagnetic and/or heavy-metal centre, where NO can selectively displace and release the fluorophore by metal–ligand substitution with a concomitant emission turn-on response. This strategy has been applied to a broad spectrum of coordination chemistry motifs; examples include iron cyclam64, cobalt aminotroponimine65, ruthenium porphyrin63 and dirhodium tetracarboxylate66 platforms.
Figure 5. Representative metal–ligand substitution and metal-mediated redox addition/cleavage reactions for small-molecule detection.
a, Direct NO detection by displacement of quenched dye–ligand conjugates from metal complexes such as ruthenium tetraphenylporphyrin. b, Phosphate detection through coordination to dizinc cores with dye displacement and/or hydrolysis. c, Displacement of a dye-quenching metal centre induced by H2S with concomitant precipitation of the metal sulfide. d, Metal-mediated reductive _N_-nitrosylation of a metal–dye complex for NO detection. e,f, Iron-mediated oxidation and cleavage from pendant dyes induced by H2O2.
An alternative yet mechanistically related approach is to displace a metal quencher from a chelating fluorescent platform. Canary’s metal exchange strategy67 and Anslyn’s coupled displacement/catalysis method for Cu(II) monitoring68 are representative pioneering examples. Hamachi and co-workers have pursued an approach of metal–ligand dye displacement for detection of biologically relevant phosphates69,70. Specifically, his laboratory has synthesized a variety of Zn(II)-coordinated di-2-picolylamine (DPA) derivatives of acridine and xanthone dyes, where the fluorophore xanthone carbonyl oxygen or acridine nitrogen atoms can coordinate with the bimetallic Zn(II) centres and quench fluorescence (Fig. 5b)69. On binding of a phosphorylated substrate such as ATP to the Zn(II)–DPA moiety, the pendant fluorophore is displaced from the metal first-coordination spheres to generate an emission turn-on response.
Finally, Chang and co-workers71 and Nagano have exploited the metal displacement strategy for H2S detection using the classic gravimetric precipitation of CuS from Cu(II) complexes (Fig. 5c)72. HSpi-1 is a cyclen-functionalized fluorescein dye, and Cu(II) binding to the fluorophore–ligand conjugate quenches its emission72. Addition of micromolar levels of H2S triggers displacement of bound metal to precipitate CuS and release the free dye, resulting in roughly a 50-fold enhancement. Copper(II)-bound HSpi-1 exhibits excellent selectivity for H2S over other many other biologically relevant thiols such as GSH, as well as a range of ROS and RNS, and can be used to detect elevations in sulfide levels in living cells.
Metal-mediated redox addition or cleavage reactions
In addition to fluorophore displacement strategies that involve simple Lewis acid–base metal–ligand substitution chemistry, metal-induced redox processes also provide a wealth of opportunities for small-molecule bioimaging. Perhaps nowhere is this concept better illustrated than by the reductive _N_-nitrosylation and release of metal quenchers from fluorophore scaffolds, which has emerged, through pioneering work by Ford73, Lippard74 and others, as a versatile and general method for direct NO detection. Ford used reductive nitrosylation of cyclam-tethered lumophores to release quenching copper centres as a means for NO monitoring in solution73. In separate work, Lippard and co-workers synthesized CuFL, the first metal-based probe for direct and selective detection of NO in aqueous solution and in living cells74. The probe CuFL consists of a copper-bound aminoquinoline-fluorescein conjugate where the paramagnetic Cu(II) centre quenches dye fluorescence. On direct reaction with NO, a metal-mediated reductive nitrosylation reaction releases the bound metal ion, with a concomitant increase in emission. Further mechanistic studies directly identify the secondary amine as the site of NO modification and show that this mechanism gives high selectivity for NO over a variety of RNS and ROS analytes (Fig. 5d)74. A variety of copper-based fluorescent NO probes have been prepared and illustrate the generality of this reaction-based approach, including trappable congeners that are suitable for long-term time-course imaging75 and expansion of the colour palette for potential multicolour applications76. More importantly, CuFL and its next-generation analogues have been used to image endogenous NO production directly in biological models ranging from bacteria to macrophages to olfactory bulb slices74–76.
Oxidative reactivity at metal centres can also provide a path to chemoselective bioimaging applications. We highlight two recent examples77,78 of non-haem iron-peroxide reactivity for chemoselective monitoring of H2O2, inspired by the haem-mediated horseradish peroxidase–Amplex red (_N_-acetyl-3,7-dihydroxyphenoxazine) assay that has been used extensively to indirectly quantify the activity of various H2O2-producing enzymes. Hitomi and co-workers recently developed the H2O2 probe MBFh1 by linking an Fe(III)-polypyridine complex to a non-fluorescent 3,7-dihydroxyphenoxazine amide (Fig. 5e)77. Reaction between H2O2 and MBFh1 leads to iron-mediated oxidation, cleavage and release of a fluorescent resorufin dye. Nam’s laboratory has elegantly exploited a classic zinc-sensor motif for metal-based H2O2 detection78. Formation of the bis-iron complex of Zinpyr-1 (ZP1) turns-off its basal fluorescence. Addition of H2O2 triggers iron-induced oxidative cleavage of the dipicolylamine ligands on the ZP1 scaffold to produce the corresponding bis-carboxylate fluorescein with concomitant release of iron ions (Fig. 5f)78. This probe shows selectivity for H2O2 over many ROS and can be used to track changes in H2O2 localized to lysosomes in living cells.
Reaction-based probes for metal ion detection
In a departure from traditional fluorescent metal sensors that rely solely on molecular recognition and binding79,80, the intrinsic and distinct reactivity profiles of metal ions can provide an alternative approach to their detection. In this context, we will highlight select examples that exploit the Lewis acidity, organometallic reactivity and small-molecule activation modes of metal ions for chemoselective fluorescent probe design, focusing our discussion largely on indicators that have been successfully used in biological applications.
Lewis acid hydrolysis and related non-redox reactions
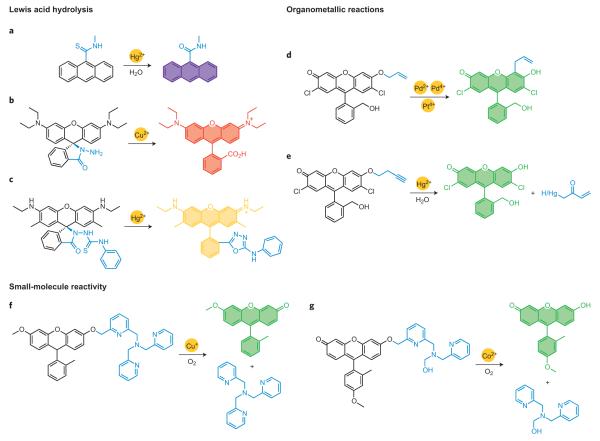
The inherent Lewis acidity of metal ions can be exploited to accelerate hydrolysis and related non-redox reactions. When coupled to hard– soft preferences for formation and cleavage of metal–ligand bonds, a variety of strategies can emerge for chemoselective turn-on or ratiometric detection. Two classic abiotic examples by Czarnik and colleagues have inspired a host of subsequent elegant chemical designs for imaging metal ion pools in biological fluids, cells, tissues and whole organisms. One reaction-based system establishes that the strong thiophilic nature of Hg(II) can selectively enhance the activation and desulfurization/hydrolysis of thiocarbonyl compounds, such as the conversion of thioamides to carboxylates (Fig. 6a)81. The second study, on the Cu(II)-catalysed hydrolysis of hydrazines to carboxylates with concomitant ring-opening of a non-fluorescent spirorhodamine to its fluorescent open form, highlights this platform as a general and versatile dye sensing scaffold (Fig. 6b)2,82.
Figure 6. Representative Lewis acid hydrolysis, organometallic and small-molecule activation reactions for specific metal ion detection.
a, Mercury-mediated desulfurization/hydroylsis of thiocarbonyl compounds. b, Copper-catalysed hydrolysis of hydrazines with spirodye ring-opening. c, Mercury-catalysed cycloaddition of thiourea substrates. d, Claisen rearrangement reactions for palladium and platinum detection. e, Oxymercuration of terminal alkynes. f,g, Aerobic copper- or cobalt-mediated C–O bond cleavage.
Against this backdrop, the mercury-promoted cycloaddition of thiourea substrates has emerged as a general reaction-based strategy for detection of this toxic heavy metal. Tian’s laboratory has explored Hg(II)-facilitated guanylations that have allowed turn-on detection of methylmercury species83. Tae, Shin and coworkers have used various spirorhodamine platforms for monitoring mercury levels in cells as well as in zebrafish, with high signal-to-noise responses (Fig. 6c)84. Moreover, Qian’s laboratory has expanded this approach to ratiometric fluorescence resonance energy transfer (FRET) cassettes85, and Kim and co-workers have reported related analogues for detection of mercury levels in blood plasma86. Other desulfurizations have also been adapted to create selective and sensitive mercury chemodosimeters. For instance, Ma87 and Zheng/Xu88 simultaneously reported Rhodamine B thiolactones for selective Hg(II) detection, where the closed and non-fluorescent spirorhodamine forms react with Hg(II) ions to yield HgS and the corresponding open and fluorescent parent rhodamine dyes. The conversion of thioacetals to carbonyls has also been applied to monitoring biological and environmental mercury. Kim and co-workers described a thioacetal coumarin for use in blood plasma assays89, and Ahn’s laboratory recently reported a unique two-photon probe for ratiometric mercury detection in cells90.
Finally, several copper- and silver-promoted hydrolysis reactions have been reported for fluorescent metal ion detection in aqueous media, with potential utility in biological samples. Pyridine-based ester cleavage strategies reported by Kramer91 and Mokhir92 and their colleagues have shown copper selectivity in metal competition assays, and esterase-insensitive versions may be applicable for cellular use. Ahn’s laboratory has introduced an inventive halide activation strategy for Ag(I) visualization and has applied this method to detect antimicrobial silver nanoparticles93.
Organometallic reaction approaches to monitoring metal ions
Organometallic reactions represent another family of metal-mediated processes that can be used for metal ion detection. In this context, the high yield, reaction rate and biological compatibility of copper-accelerated alkyne click (CuAAC) chemistry offers an ideal reaction-based mechanism for detection of this essential biological metal. An early example from Fahrni’s laboratory recognized that CuAAC on 7-substituted coumarin dyes could convert a weakly fluorescent coumarin bearing an electron-poor alkyne substituent into a more fluorescent congener with an electron-rich triazole at the same position94. Moreover, other triazole dyes generated by means of CuAAC reactions have been used to label newly synthesized proteins, glycans, lipids and DNA through a turn-on fluorogenic response95. A variation of this strategy has been used by Viguier and Hulme, who exploited a CuAAC reaction to initiate a turn-on in sensitized lanthanide luminescence by coupling an organic dansyl sensitizer to a lanthanide complex96.
Koide and co-workers have elegantly established that Tsuji–Trost97and Claisen98 chemistry of metal–allyl complexes offers another versatile approach to metal ion sensing, particularly for palladium and platinum species used in pharmaceutical catalysis and therapeutics. The Tsuji–Trost probes operate through hydrolysis of an allyl ether by an electrophilic metal–allyl intermediate, whereas other conditions favour aromatic Claisen rearrangements (Fig. 6d)97,98. Adjustments in pH, ligand additives and other experimental conditions can promote specificity for palladium or platinum as well as oxidation state selectivity97. Ahn’s laboratory has devised related _O_-propargylated fluorescein derivatives that can monitor palladium in a variety of oxidation states (for example Pd(0), Pd(II), Pd(IV)) and can be applied for bioimaging of palladium in zebrafish99, and Zhang and co-workers have prepared a ratiometric naphthalimide version100.
Koide’s laboratory has also developed fluorescent probes for Hg(II) based on the oxymercuration of alkynes101 and alkenes102, where the phenolic hydroxyl group of a fluorescent dye is caged with a terminal butyne unit or a vinyl ether moiety. The Hg(II)-catalysed hydration and oxidation of the alkyne or alkene to the corresponding ketone triggers facile β-elimination to yield the latent dye (Fig. 6e)101. These probes have been applied in assays to detect mercury in fish, dental and environmental samples. Ahn’s laboratory has reported a related aryl vinyl ether probe for assaying mercury in fish103, and Lin and co-workers have used alkynes with thioamide directing groups to achieve mercury imaging in live cells104.
Metal ion detection through small-molecule reactivity
A third type of reaction-based strategy for metal ion detection is to achieve specificity through metal-mediated activation of small molecules that are naturally abundant in biological systems. Oxygen-facilitated reactions offer a prime example of this approach, as only a limited subset of biologically relevant metal ions (for example Cu(I), Mn(II), Fe(II), Co(II)) are responsible for the majority of O2-mediated redox chemistry in living systems. In an elegant study, Taki and co-workers105 prepared and characterized the Cu(I)-responsive fluorescent probe FluTPA2. In the absence of Cu(I), FluTPA2 is inert to oxygen and weakly fluorescent owing to reduction of the xanthenone ring and alkylation of its phenolic oxygen. Aerobic C–O bond cleavage promoted by Cu(I) followed by air oxidation releases the free Tokyo Green dye with a >100-fold turn-on response (Fig. 6f)105. FluTPA2 shows high selectivity for copper ions over a range of biologically relevant metal ion competitors. Our laboratory has used an analogous approach to provide the first selective fluorescence probe for cobalt in aqueous solution and in living cells106. Cobalt Probe 1 (CP1) features a Tokyo Green scaffold alkylated with a N3O ligand designed to quench fluorescence and serve as a receptor for Co(II). Binding of Co(II) in the presence of air triggers oxidative C–O bond cleavage and a roughly 18-fold turn-on emission increase (Fig. 6g)106. We note that the dual requirement for metal binding and O2 reactivity confers high selectivity for Co(II) over a range of alkali and alkaline earth metal ions as well as transition metal ions. Indeed, this reaction-based strategy results in a final product that does not bind a metal centre, making it generally applicable to detection of other redox-active and/or paramagnetic metal ions that are potent quenchers. Most importantly, both FluTPA2 and CP1 are capable of imaging changes in Cu(I) and Co(II) ion pools, respectively, in living cells, illustrating the biocompatibility of this reaction-based approach105,106.
Concluding remarks and future prospects
The reaction-based approach to selective molecular imaging in biological systems is a rapidly growing field that can enhance the study of life processes with precise chemical detail. Fundamental organic, organometallic and inorganic reactivity principles, combined with an eye towards biological discovery, have given rise to a variety of new chemical tools for probing the complex roles of small molecules and metal ions in physiology and pathology. Indeed, the utility of these indicators in biological fluids, cells, tissues and even whole organisms, from bacteria and yeast to multicellular plants and animals, showcases the breadth and potential of this interface between chemistry and biology. Using but a small set of examples, we have highlighted how reaction-based bioimaging can elucidate the contributions of transient small-molecule signal or stress agents such as nitric oxide, hydrogen peroxide, hypochlorous acid and hydrogen sulfide in basic cell proliferation, differentiation and migration pathways underlying immune response, cardiovascular function, neurotransmission and neurogenesis (Fig. 7). We have also surveyed molecular probes that can diagnose toxic levels and distributions of metal ions and map normal resting labile metal pools (Fig. 7)10,44,52,75,84.
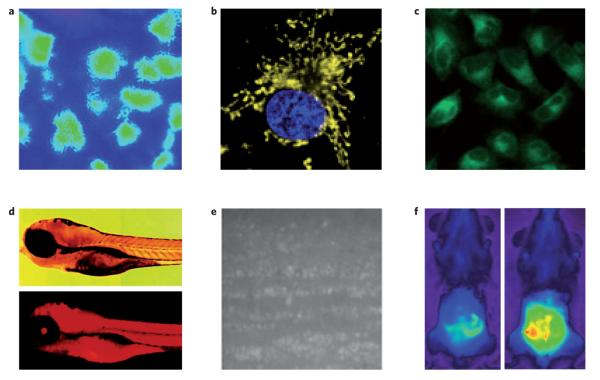
Figure 7. Representative bioimaging applications with reaction-based small-molecule fluorescent probes for highly reactive species and metal ions.
a, Levels of NO in a rat kidney visualized with DAC-P. b, Mitochondrial-localized H2O2 fluxes in cancer cells imaged by MitoPY1. c, Detection of H2S in mammalian cells with SFP-2. d, Visualizing accumulation of mercury pools in zebrafish with a spirorhodamine-based probe. e, Production of NO in olfactory bulb slices on depolarization detected by the metal–dye probe Cu2(FL2E). f, Visualization of myeloperoxidase-derived HOCl in a mouse model for peritonitis with SNAPF. Figures reproduced with permission from: a, ref. 10 © 2005 ACS; c, ref. 52 © 2011 NPG; d, ref. 84 © 2006 ACS; e, ref. 75 © 2010 PNAS; f, ref. 44 © 2007 Elsevier; b was kindly provided by B. C. Dickinson.
A host of enticing opportunities awaits. The greatest challenge is the discovery and identification of selective, bioorthogonal reactions that form the basis for new detection schemes75. Many elegant chemical strategies for fluorescence detection continue to be reported and are essential for building the foundation for molecular imaging applications. To achieve ultimate biological and biomedical impact, however, a greater emphasis is needed upfront on developing reactions that are compatible with living systems (for example aqueous solution and physiological pH, high ionic strength, millimolar levels of GSH and other nucleophiles). We highlight two classic reviews by de Silva and colleagues107 and by Czarnik1 that presage real-world applications of chemosensors that can operate in aqueous environments.
Equally important in terms of new reaction design and development is fundamental biology; the ultimate impact of chemical tools will be in the discovery and understanding of new biological processes. Here, both the choice of an important biological problem and the development of chemical tools to address such questions are essential for pushing the boundaries of the field. Indeed, chemistry can change the way we do biology. For example, the study of calcium, a ubiquitous metal signal in cells, has been revolutionized by the invention of small-molecule fluorescent indicators, chelators and caged compounds, most notably by Tsien’s laboratory108. In more recent work, reaction-based approaches are inherently attractive for distinguishing transient, reactive small molecules such as NO, H2O2 and H2S, as they are constantly being produced, trafficked and consumed or transformed in biological environments. Researchers should continue to apply their expanding toolbox to disentangle the spatial and temporal effects that lead these Janus-faced molecules to trigger physiological and/or pathological responses. Likewise, essential transition metals such as iron, copper and zinc cannot be created or destroyed, and the body must actively control their accumulation, trafficking and efflux. Probes that can non-invasively monitor dynamic metal pools should shed light on homeostatic processes at the cellular and whole organism level. Finally, indicators for toxic analytes such as mercury are most usefully applied to devise new diagnostics and treatments for heavy-metal accumulation, or ultimately to contribute to our understanding of mercury poisoning.
In addition to these considerations, most of the probes described in this Review operate with high selectivity and sensitivity but are based on irreversible, stoichiometric reactions109. New detection schemes that incorporate reversible yet chemoselective reactions, as well as catalytic processes where signals can be amplified, offer challenging but important future directions for exploration. They could improve signal-to-noise responses and dynamic imaging significantly, but much work remains to be done. Other opportunities in the field of bioimaging include the targeted delivery and retention of reaction-based indicators. For subcellular localization the task is more tractable as there are a number of existing approaches to label fluorophores site-specifically; however, the more difficult tasks of delivering fluorescent probes to specific tissues and organs, as well as devising ways to localize indicators to diseased growths like tumours and plaques, await further study.
Finally, our Review has focused on fluorescence imaging probes, but we note that reaction-based approaches are applicable to a variety of imaging modalities, including MRI, PET/SPECT, bioluminescence and ultrasound/photoacoustic detection. Many of these offer the ability to probe into animals and even humans, allowing the translation of this reaction-based approach to preclinical and clinical diagnostics and to related applications. Taken together, the creativity of chemists to invent, study and apply molecular reactivity offers unlimited possibilities to visualize and understand the world around us, and it indicates an exciting future for reaction-based bioimaging.
Acknowledgements
We thank the NIH (GM 79465), the Packard Foundation, Amgen, Astra Zeneca and Novartis for funding our laboratory’s work on bioimaging. C.J.C. is an Investigator with the Howard Hughes Medical Institute. J.C. thanks the Human Frontiers Science Program for a postdoctoral fellowship and S.C.D. thanks Novartis for a graduate fellowship. We thank L. Lavis for sharing a figure template.
Footnotes
Additional information Reprints and permissions information is available online at http://www.nature.com/ reprints.
Competing financial interests The authors declare no competing financial interests.
References
- 1.Czarnik AW. Chemical communication in water using fluorescent chemosensors. Acc. Chem. Res. 1994;27:302–308. [Google Scholar]
- 2.Kim HN, Lee MH, Kim HJ, Kim JS, Yoon J. A new trend in rhodamine-based chemosensors: application of spirolactam ring-opening to sensing ions. Chem. Soc. Rev. 2008;37:1465–1472. doi: 10.1039/b802497a. [DOI] [PubMed] [Google Scholar]
- 3.Cho DG, Sessler JL. Modern reaction-based indicator systems. Chem. Soc. Rev. 2009;38:1647–1662. doi: 10.1039/b804436h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jun ME, Roy B, Ahn KH. ‘Turn-on’ fluorescent sensing with ‘reactive’ probes. Chem. Commun. 2011;47:7583–7601. doi: 10.1039/c1cc00014d. [DOI] [PubMed] [Google Scholar]
- 5.Du J, Hu M, Fan J, Peng X. Fluorescent chemodosimeters using ‘mild’ chemical events for the detection of small anions and cations in biological and environmental media. Chem. Soc. Rev. 2012;41:4511–4535. doi: 10.1039/c2cs00004k. [DOI] [PubMed] [Google Scholar]
- 6.Nagano T, et al. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal. Chem. 1998;70:2446–2453. doi: 10.1021/ac9801723. [DOI] [PubMed] [Google Scholar]
- 7.Nagano T, Takizawa H, Hirobe M. Reactions of nitric oxide with amines in the presence of dioxygen. Tetrahedron Lett. 1995;36:8239–8242. [Google Scholar]
- 8.Kojima H, et al. Bioimaging of nitric oxide with fluorescent indicators based on the rhodamine chromophore. Anal. Chem. 2001;73:1967–1973. doi: 10.1021/ac001136i. [DOI] [PubMed] [Google Scholar]
- 9.Nagano T, Gabe Y, Urano Y, Kikuchi K, Kojima H. Highly sensitive fluorescence probes for nitric oxide based on boron dipyrromethene chromophore-rational design of potentially useful bioimaging fluorescence probe. J. Am. Chem. Soc. 2004;126:3357–3367. doi: 10.1021/ja037944j. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki E, et al. Highly sensitive near-infrared fluorescent probes for nitric oxide and their application to isolated organs. J. Am. Chem. Soc. 2005;127:3684–3685. doi: 10.1021/ja042967z. [DOI] [PubMed] [Google Scholar]
- 11.Terai T, Urano Y, Izumi S, Kojima H, Nagano T. A practical strategy to create near-infrared luminescent probes: conversion from fluorescein-based sensors. Chem. Commun. 2012;48:2840–2842. doi: 10.1039/c2cc16553h. [DOI] [PubMed] [Google Scholar]
- 12.Yang YJ, et al. A highly selective low-background fluorescent imaging agent for nitric oxide. J. Am. Chem. Soc. 2010;132:13114–13116. doi: 10.1021/ja1040013. [DOI] [PubMed] [Google Scholar]
- 13.Song B, Wang GL, Tan MQ, Yuan JL. A europium(III) complex as an efficient singlet oxygen luminescence probe. J. Am. Chem. Soc. 2006;128:13442–13450. doi: 10.1021/ja062990f. [DOI] [PubMed] [Google Scholar]
- 14.Lippert AR, De Bittner GCV, Chang CJ. Boronate oxidation as a bioorthogonal reaction approach for studying the chemistry of hydrogen peroxide in living systems. Acc. Chem. Res. 2011;44:793–804. doi: 10.1021/ar200126t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang MCY, Pralle A, Isacoff EY, Chang CJ. A selective, cell-permeable optical probe for hydrogen peroxide in living cells. J. Am. Chem. Soc. 2004;126:15392–15393. doi: 10.1021/ja0441716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lo LC, Chu CY. Development of highly selective and sensitive probes for hydrogen peroxide. Chem. Commun. 2003:2728–2729. doi: 10.1039/b309393j. [DOI] [PubMed] [Google Scholar]
- 17.Miller EW, Tulyathan O, Isacoff EY, Chang CJ. Molecular imaging of hydrogen peroxide produced for cell signaling. Nature Chem. Biol. 2007;3:349–349. doi: 10.1038/nchembio871. [DOI] [PubMed] [Google Scholar]
- 18.Du LP, Li MY, Zheng SL, Wang BH. Rational design of a fluorescent hydrogen peroxide probe based on the umbelliferone fluorophore. Tetrahedron Lett. 2008;49:3045–3048. doi: 10.1016/j.tetlet.2008.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dickinson BC, Huynh C, Chang CJ. A palette of fluorescent probes with varying emission colors for imaging hydrogen peroxide signaling in living cells. J. Am. Chem. Soc. 2010;132:5906–5915. doi: 10.1021/ja1014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karton-Lifshin N, et al. A unique paradigm for a turn-on near-infrared cyanine-based probe: noninvasive intravital optical imaging of hydrogen peroxide. J. Am. Chem. Soc. 2011;133:10960–10965. doi: 10.1021/ja203145v. [DOI] [PubMed] [Google Scholar]
- 21.Srikun D, Miller EW, Dornaille DW, Chang CJ. An ICT-based approach to ratiometric fluorescence imaging of hydrogen peroxide produced in living cells. J. Am. Chem. Soc. 2008;130:4596–4597. doi: 10.1021/ja711480f. [DOI] [PubMed] [Google Scholar]
- 22.Dickinson BC, Chang CJ. A targetable fluorescent probe for imaging hydrogen peroxide in the mitochondria of living cells. J. Am. Chem. Soc. 2008;130:9638–9639. doi: 10.1021/ja802355u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srikun D, Albers AE, Nam CI, Iavaron AT, Chang CJ. Organelletargetable fluorescent probes for imaging hydrogen peroxide in living cells via SNAP-tag protein labeling. J. Am. Chem. Soc. 2010;132:4455–4465. doi: 10.1021/ja100117u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller EW, Dickinson BC, Chang CJ. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl Acad. Sci. USA. 2010;107:15681–15686. doi: 10.1073/pnas.1005776107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dickinson BC, Peltier J, Stone D, Schaffer DV, Chang CJ. Nox2 redox signaling maintains essential cell populations in the brain. Nature Chem. Biol. 2011;7:106–112. doi: 10.1038/nchembio.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van de Bittner GC, Dubikovskaya EA, Bertozzi CR, Chang CJ. In vivo imaging of hydrogen peroxide production in a murine tumor model with a chemoselective bioluminescent reporter. Proc. Natl Acad. Sci. USA. 2010;107:21316–21321. doi: 10.1073/pnas.1012864107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lippert AR, Gschneidtner T, Chang CJ. Lanthanide-based luminescent probes for selective time-gated detection of hydrogen peroxide in water and in living cells. Chem. Commun. 2010;46:7510–7512. doi: 10.1039/c0cc01560a. [DOI] [PubMed] [Google Scholar]
- 28.Du LPY, Ni NTY, Li MY, Wang BH. A fluorescent hydrogen peroxide probe based on a ‘click’ modified coumarin fluorophore. Tetrahedron Lett. 2010;51:1152–1154. doi: 10.1016/j.tetlet.2009.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charkoudian LK, Pham DM, Franz KJ. A pro-chelator triggered by hydrogen peroxide inhibits iron-promoted hydroxyl radical formation. J. Am. Chem. Soc. 2006;128:12424–12425. doi: 10.1021/ja064806w. [DOI] [PubMed] [Google Scholar]
- 30.Wei Y, Guo M. Hydrogen peroxide triggered prochelator activation, subsequent metal chelation, and attenuation of the fenton reaction. Angew. Chem. Int. Ed. 2007;46:4722–4725. doi: 10.1002/anie.200604859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jourden JLM, Cohen SM. Hydrogen peroxide activated matrix metalloproteinase inhibitors: a prodrug approach. Angew. Chem. Int. Ed. 2010;49:6795–6797. doi: 10.1002/anie.201003819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuang YY, Baakrishnan K, Gandhi V, Peng XH. Hydrogen peroxide inducible DNA cross-linking agents: targeted anticancer prodrugs. J. Am. Chem. Soc. 2011;133:19278–19281. doi: 10.1021/ja2073824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sella E, Shabat D. Self-immolative dendritic probe for direct detection of triacetone triperoxide. Chem. Commun. 2008:5701–5703. doi: 10.1039/b814855d. [DOI] [PubMed] [Google Scholar]
- 34.Broaders KE, Grandhe S, Frechet JMJ. A biocompatible oxidation-triggered carrier polymer with potential in therapeutics. J. Am. Chem. Soc. 2011;133:756–758. doi: 10.1021/ja110468v. [DOI] [PubMed] [Google Scholar]
- 35.Cocheme HM, et al. Measurement of H2O2 within living Drosophila during aging using a ratiometric mass spectrometry probe targeted to the mitochondrial matrix. Cell Metab. 2011;13:340–350. doi: 10.1016/j.cmet.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abo M, et al. Development of a highly sensitive fluorescence probe for hydrogen peroxide. J. Am. Chem. Soc. 2011;133:10629–10637. doi: 10.1021/ja203521e. [DOI] [PubMed] [Google Scholar]
- 37.Lippert AR, Keshari KR, Kurhanewicz J, Chang CJ. A hydrogen peroxide-responsive hyperpolarized 13C MRI contrast agent. J. Am. Chem. Soc. 2011;133:3776–3779. doi: 10.1021/ja111589a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang D, Wang HL, Sun ZN, Chung NW, Shen JG. A highly selective fluorescent probe for the detection and imaging of peroxynitrite in living cells. J. Am. Chem. Soc. 2006;128:6004–6005. doi: 10.1021/ja0603756. [DOI] [PubMed] [Google Scholar]
- 39.Sun ZN, et al. BODIPY-based fluorescent probe for peroxynitrite detection and imaging in living cells. Org. Lett. 2009;11:1887–1890. doi: 10.1021/ol900279z. [DOI] [PubMed] [Google Scholar]
- 40.Peng T, Yang D. HKGreen-3: a rhodol-based fluorescent probe for peroxynitrite. Org. Lett. 2010;12:4932–4935. doi: 10.1021/ol102182j. [DOI] [PubMed] [Google Scholar]
- 41.Zhang WJ, Guo C, Liu LH, Qin JG, Yang CL. Naked-eye visible and fluorometric dual-signaling chemodosimeter for hypochlorous acid based on water-soluble p-methoxyphenol derivative. Org. Biomol. Chem. 2011;9:5560–5563. doi: 10.1039/c1ob05550j. [DOI] [PubMed] [Google Scholar]
- 42.Koide Y, Urano Y, Hanaoka K, Terai T, Nagano T. Development of an Si-rhodamine-based far-red to near-infrared fluorescence probe selective for hypochlorous acid and its applications for biological imaging. J. Am. Chem. Soc. 2011;133:5680–5682. doi: 10.1021/ja111470n. [DOI] [PubMed] [Google Scholar]
- 43.Setsukinai K, Urano Y, Kakinuma K, Majima HJ, Nagano T. Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. J. Biol. Chem. 2003;278:3170–3175. doi: 10.1074/jbc.M209264200. [DOI] [PubMed] [Google Scholar]
- 44.Shepherd J, et al. A fluorescent probe for the detection of myeloperoxidase activity in atherosclerosis-associated macrophages. Chem. Biol. 2007;14:1221–1231. doi: 10.1016/j.chembiol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu FBA, et al. A near-IR reversible fluorescent probe modulated by selenium for monitoring peroxynitrite and imaging in living cells. J. Am. Chem. Soc. 2011;133:11030–11033. doi: 10.1021/ja202582x. [DOI] [PubMed] [Google Scholar]
- 46.Garner AL, et al. Specific fluorogenic probes for ozone in biological and atmospheric samples. Nature Chem. 2009;1:316–321. doi: 10.1038/nchem.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lippert AR, New EJ, Chang CJ. Reaction-based fluorescent probes for selective imaging of hydrogen sulfide in living cells. J. Am. Chem. Soc. 2011;133:10078–10080. doi: 10.1021/ja203661j. [DOI] [PubMed] [Google Scholar]
- 48.Peng HJ, et al. A fluorescent probe for fast and quantitative detection of hydrogen sulfide in blood. Angew. Chem. Int. Ed. 2011;50:9672–9675. doi: 10.1002/anie.201104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu FBA, et al. An ICT-based strategy to a colorimetric and ratiometric fluorescence probe for hydrogen sulfide in living cells. Chem. Commun. 2012;48:2852–2854. doi: 10.1039/c2cc17658k. [DOI] [PubMed] [Google Scholar]
- 50.Montoya LA, Pluth MD. Selective turn-on fluorescent probes for imaging hydrogen sulfide in living cells. Chem. Commun. 2012;48:4767–4769. doi: 10.1039/c2cc30730h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen S, Chen ZJ, Ren W, Ai HW. Reaction-based genetically encoded fluorescent hydrogen sulfide sensors. J. Am. Chem. Soc. 2012;134:9589–9592. doi: 10.1021/ja303261d. [DOI] [PubMed] [Google Scholar]
- 52.Qian Y, et al. Selective fluorescent probes for live-cell monitoring of sulphide. Nature Commun. 2011;2:495. doi: 10.1038/ncomms1506. [DOI] [PubMed] [Google Scholar]
- 53.Liu CR, et al. Capture and visualization of hydrogen sulfide by a fluorescent probe. Angew. Chem. Int. Ed. 2011;50:10327–10329. doi: 10.1002/anie.201104305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu C, et al. Reaction based fluorescent probes for hydrogen sulfide. Org. Lett. 2012;14:2184–2187. doi: 10.1021/ol3008183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maeda H, et al. 2,4-Dinitrobenzenesulfonyl fluoresceins as fluorescent alternatives to Ellman’s reagent in thiol-quantification enzyme assays. Angew. Chem. Int. Ed. 2005;44:2922–2925. doi: 10.1002/anie.200500114. [DOI] [PubMed] [Google Scholar]
- 56.Maeda H, et al. A design of fluorescent probes for superoxide based on a nonredox mechanism. J. Am. Chem. Soc. 2005;127:68–69. doi: 10.1021/ja047018k. [DOI] [PubMed] [Google Scholar]
- 57.Jiang W, Fu QQ, Fan HY, Ho J, Wang W. A highly selective fluorescent probe for thiophenols. Angew. Chem. Int. Ed. 2007;46:8445–8448. doi: 10.1002/anie.200702271. [DOI] [PubMed] [Google Scholar]
- 58.Bouffard J, Kim Y, Swager TM, Weissleder R, Hilderbrand SA. A highly selective fluorescent probe for thiol bioimaging. Org. Lett. 2008;10:37–40. doi: 10.1021/ol702539v. [DOI] [PubMed] [Google Scholar]
- 59.Pires MM, Chmielewski J. Fluorescence imaging of cellular glutathione using a latent rhodamine. Org. Lett. 2008;10:837–840. doi: 10.1021/ol702769n. [DOI] [PubMed] [Google Scholar]
- 60.Reddie KG, et al. Fluorescent coumarin thiols measure biological redox couples. Org. Lett. 2012;14:680–683. doi: 10.1021/ol203105c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nguyen BT, Anslyn EV. Indicator-displacement assays. Coord. Chem. Rev. 2006;250:3118–3127. [Google Scholar]
- 62.Fabbrizzi L, Licchelli M, Pallavicini P, Sacchi D, Taglietti A. Sensing of transition metals through fluorescence quenching or enhancement—a review. Analyst. 1996;121:1763–1768. [Google Scholar]
- 63.Lim MH, Lippard SJ. Fluorescence-based nitric oxide detection by ruthenium porphyrin fluorophore complexes. Inorg. Chem. 2004;43:6366–6370. doi: 10.1021/ic035418n. [DOI] [PubMed] [Google Scholar]
- 64.Katayama Y, Takahashi S, Maeda M. Design, synthesis and characterization of a novel fluorescent probe for nitric oxide (nitrogen monoxide) Anal. Chim. Acta. 1998;365:159–167. [Google Scholar]
- 65.Franz KJ, Singh N, Lippard SJ. Metal-based NO sensing by selective ligand dissociation. Angew. Chem. Int. Ed. 2000;39:2120–2122. doi: 10.1002/1521-3773(20000616)39:12<2120::aid-anie2120>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 66.Hilderbrand SA, Lim MH, Lippard SJ. Dirhodium tetracarboxylate scaffolds as reversible fluorescence-based nitric oxide sensors. J. Am. Chem. Soc. 2004;126:4972–4978. doi: 10.1021/ja038471j. [DOI] [PubMed] [Google Scholar]
- 67.Royzen M, Dai ZH, Canary JW. Ratiometric displacement approach to Cu(II) sensing by fluorescence. J. Am. Chem. Soc. 2005;127:1612–1613. doi: 10.1021/ja0431051. [DOI] [PubMed] [Google Scholar]
- 68.Wu QY, Anslyn EV. Catalytic signal amplification using a Heck reaction. an example in the fluorescence sensing of Cu(II) J. Am. Chem. Soc. 2004;126:14682–14683. doi: 10.1021/ja0401038. [DOI] [PubMed] [Google Scholar]
- 69.Ojida A, et al. Bis(Dpa-Zn-II) appended xanthone: excitation ratiometric chemosensor for phosphate anions. Angew. Chem. Int. Ed. 2006;45:5518–5521. doi: 10.1002/anie.200601315. [DOI] [PubMed] [Google Scholar]
- 70.Ojida A, et al. Design of dual-emission chemosensors for ratiometric detection of ATP derivatives. Chem. Asian J. 2006;1:555–563. doi: 10.1002/asia.200600137. [DOI] [PubMed] [Google Scholar]
- 71.Choi MG, Cha S, Lee H, Jeon HL, Chang SK. Sulfide-selective chemosignaling by a Cu2+ complex of dipicolylamine appended fluorescein. Chem. Commun. 2009:7390–7392. doi: 10.1039/b916476f. [DOI] [PubMed] [Google Scholar]
- 72.Sasakura K, et al. Development of a highly selective fluorescence probe for hydrogen sulfide. J. Am. Chem. Soc. 2011;133:18003–18005. doi: 10.1021/ja207851s. [DOI] [PubMed] [Google Scholar]
- 73.Tsuge K, DeRosa F, Lim MD, Ford PC. Intramolecular reductive nitrosylation: reaction of nitric oxide and a copper(II) complex of a cyclam derivative with pendant luminescent chromophores. J. Am. Chem. Soc. 2004;126:6564–6565. doi: 10.1021/ja049444b. [DOI] [PubMed] [Google Scholar]
- 74.Lim MH, Xu D, Lippard SJ. Visualization of nitric oxide in living cells by a copper-based fluorescent probe. Nature Chem. Biol. 2006;2:375–380. doi: 10.1038/nchembio794. [DOI] [PubMed] [Google Scholar]
- 75.McQuade LE, et al. Visualization of nitric oxide production in the mouse main olfactory bulb by a cell-trappable copper(II) fluorescent probe. Proc. Natl Acad. Sci. USA. 2010;107:8525–8530. doi: 10.1073/pnas.0914794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pluth MD, Chan MR, McQuade LE, Lippard SJ. Seminaphthofluorescein-based fluorescent probes for imaging nitric oxide in live cells. Inorg. Chem. 2011;50:9385–9392. doi: 10.1021/ic200986v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hitomi Y, Takeyasu T, Funabiki T, Kodera M. Detection of enzymatically generated hydrogen peroxide by metal-based fluorescent probe. Anal. Chem. 2011;83:9213–9216. doi: 10.1021/ac202534g. [DOI] [PubMed] [Google Scholar]
- 78.Song D, et al. A fluorescence turn-on H2O2 probe exhibits lysosome-localized fluorescence signals. Chem. Commun. 2012;48:5449–5451. doi: 10.1039/c2cc31632c. [DOI] [PubMed] [Google Scholar]
- 79.Domaille DW, Que EL, Chang CJ. Synthetic fluorescent sensors for studying the cell biology of metals. Nature Chem. Biol. 2008;4:168–175. doi: 10.1038/nchembio.69. [DOI] [PubMed] [Google Scholar]
- 80.Que EL, Domaille DW, Chang CJ. Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem. Rev. 2008;108:1517–1549. doi: 10.1021/cr078203u. [DOI] [PubMed] [Google Scholar]
- 81.Chae MY, Czarnik AW. Fluorometric chemodosimetry. Mercury(II) and silver(I) indication in water via enhanced fluorescence signaling. J. Am. Chem. Soc. 1992;114:9704–9705. [Google Scholar]
- 82.Dujols V, Ford F, Czarnik AW. A long-wavelength fluorescent chemodosimeter selective for Cu(II) ion in water. J. Am. Chem. Soc. 1997;119:7386–7387. [Google Scholar]
- 83.Guo Z, Zhu WH, Zhu MM, Wu XM, Tian H. Near-infrared cell-permeable Hg2+-selective ratiometric fluorescent chemodosimeters and fast indicator paper for MeHg+ based on tricarbocyanines. Chem. Eur. J. 2010;16:14424–14432. doi: 10.1002/chem.201001769. [DOI] [PubMed] [Google Scholar]
- 84.Ko SK, Yang YK, Tae J, Shin I. In vivo monitoring of mercury ions using a rhodamine-based molecular probe. J. Am. Chem. Soc. 2006;128:14150–14155. doi: 10.1021/ja065114a. [DOI] [PubMed] [Google Scholar]
- 85.Zhang XL, Xiao Y, Qian XH. A ratiometric fluorescent probe based on FRET for imaging Hg2+ ions in living cells. Angew. Chem. Int. Ed. 2008;47:8025–8029. doi: 10.1002/anie.200803246. [DOI] [PubMed] [Google Scholar]
- 86.Lee MH, Lee SW, Kim SH, Kang C, Kim JS. Nanomolar Hg(II) detection using Nile blue chemodosimeter in biological media. Org. Lett. 2009;11:2101–2104. doi: 10.1021/ol900542y. [DOI] [PubMed] [Google Scholar]
- 87.Shi W, Ma H. Rhodamine B thiolactone: a simple chemosensor for Hg2+ in aqueous media. Chem. Commun. 2008:1856–1858. doi: 10.1039/b717718f. [DOI] [PubMed] [Google Scholar]
- 88.Zhan X-Q, Qian Z-H, Zheng H, Su B-Y, Lan Z, Xu J-G. Rhodamine thiospirolactone. highly selective and sensitive reversible sensing of Hg(II) Chem. Commun. 2008:1859–1861. doi: 10.1039/b719473k. [DOI] [PubMed] [Google Scholar]
- 89.Kim JH, et al. Fluorescent coumarinyldithiane as a selective chemodosimeter for mercury(II) ion in aqueous solution. Tetrahedron Lett. 2009;50:5958–5961. [Google Scholar]
- 90.Rao AS, et al. Reaction-based two-photon probes for mercury ions: fluorescence imaging with dual optical windows. Org. Lett. 2012;14:2598–2601. doi: 10.1021/ol3009057. [DOI] [PubMed] [Google Scholar]
- 91.Kierat RM, Kramer R. A fluorogenic and chromogenic probe that detects the esterase activity of trace copper(II) Bioorg. Med. Chem. Lett. 2005;15:4824–4827. doi: 10.1016/j.bmcl.2005.07.042. [DOI] [PubMed] [Google Scholar]
- 92.Kovacs J, Mokhir A. Catalytic hydrolysis of esters of 2-hydroxypyridine derivatives for Cu2+ detection. Inorg. Chem. 2008;47:1880–1882. doi: 10.1021/ic7022242. [DOI] [PubMed] [Google Scholar]
- 93.Chatterjee A, et al. Selective fluorogenic and chromogenic probe for detection of silver ions and silver nanoparticles in aqueous media. J. Am. Chem. Soc. 2009;131:2040–2041. doi: 10.1021/ja807230c. [DOI] [PubMed] [Google Scholar]
- 94.Zhou Z, Fahrni CJ. A fluorogenic probe for the copper(I)-catalyzed azide-alkyne ligation reaction: modulation of the fluorescence emission via 3(n,π*)-1(π,π*) inversion. J. Am. Chem. Soc. 2004;126:8862–8863. doi: 10.1021/ja049684r. [DOI] [PubMed] [Google Scholar]
- 95.Le Droumaguet C, Wang C, Wang Q. Fluorogenic click reaction. Chem. Soc. Rev. 2010;39:1233–1239. doi: 10.1039/b901975h. [DOI] [PubMed] [Google Scholar]
- 96.Viguier RFH, Hulme AN. A sensitized europium complex generated by micromolar concentrations of copper(I): toward the detection of copper(I) in biology. J. Am. Chem. Soc. 2006;128:11370–11371. doi: 10.1021/ja064232v. [DOI] [PubMed] [Google Scholar]
- 97.Garner AL, Koide K. Studies of a fluorogenic probe for palladium and platinum leading to a palladium-specific detection method. Chem. Commun. 2009:86–88. doi: 10.1039/b814197e. [DOI] [PubMed] [Google Scholar]
- 98.Garner AL, Koide K. Oxidation state-specific fluorescent method for palladium(II) and platinum(IV) based on the catalyzed aromatic Claisen rearrangement. J. Am. Chem. Soc. 2008;130:16472–16473. doi: 10.1021/ja8065539. [DOI] [PubMed] [Google Scholar]
- 99.Santra M, Ko SK, Shin I, Ahn KH. Fluorescent detection of palladium species with an O-propargylated fluorescein. Chem. Commun. 2010;46:3964–3966. doi: 10.1039/c001922d. [DOI] [PubMed] [Google Scholar]
- 100.Zhu BC, et al. A 4-hydroxynaphthalimide-derived ratiometric fluorescent chemodosimeter for imaging palladium in living cells. Chem. Commun. 2011;47:8656–8658. doi: 10.1039/c1cc13215f. [DOI] [PubMed] [Google Scholar]
- 101.Song FL, Watanabe S, Floreancig PE, Koide K. Oxidation-resistant fluorogenic probe for mercury based on alkyne oxymercuration. J. Am. Chem. Soc. 2008;130:16460–16461. doi: 10.1021/ja805678r. [DOI] [PubMed] [Google Scholar]
- 102.Ando S, Koide K. Development and applications of fluorogenic probes for mercury(II) based on vinyl ether oxymercuration. J. Am. Chem. Soc. 2011;133:2556–2566. doi: 10.1021/ja108028m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Santra M, et al. A chemodosimeter approach to fluorescent sensing and imaging of inorganic and methylmercury species. Chem. Commun. 2009:2115–2117. doi: 10.1039/b900380k. [DOI] [PubMed] [Google Scholar]
- 104.Lin WY, Cao XW, Ding YD, Yuan L, Long LL. A highly selective and sensitive fluorescent probe for Hg2+ imaging in live cells based on a rhodamine-thioamide–alkyne scaffold. Chem. Commun. 2010;46:3529–3531. doi: 10.1039/b927373e. [DOI] [PubMed] [Google Scholar]
- 105.Taki M, Iyoshi S, Ojida A, Hamachi I, Yamamoto Y. Development of highly sensitive fluorescent probes for detection of intracellular copper(I) in living systems. J. Am. Chem. Soc. 2010;132:5938–5939. doi: 10.1021/ja100714p. [DOI] [PubMed] [Google Scholar]
- 106.Au-Yeung HY, New EJ, Chang CJ. A selective reaction-based fluorescent probe for detecting cobalt in living cells. Chem. Commun. 2012;48:5268–5270. doi: 10.1039/c2cc31681a. [DOI] [PubMed] [Google Scholar]
- 107.De Silva AP, et al. Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 1997;97:1515–1566. doi: 10.1021/cr960386p. [DOI] [PubMed] [Google Scholar]
- 108.Palmer AE, Tsien RY. Measuring calcium signaling using genetically targetable fluorescent indicators. Nature Protoc. 2006;1:1057–1065. doi: 10.1038/nprot.2006.172. [DOI] [PubMed] [Google Scholar]
- 109.Yang Y, Zhao Q, Feng W, Li F. Luminescent chemodosimeters for bioimaging. Chem. Rev. 2012 doi: 10.1021/cr2004103. http://dx.doi.org/10.1021/cr2004103. [DOI] [PubMed] [Google Scholar]