Flight performance of the largest volant bird (original) (raw)
Significance
A fossil species of pelagornithid bird exhibits the largest known avian wingspan. Pelagornithids are an extinct group of birds known for bony tooth-like beak projections, large size, and highly modified wing bones that raise many questions about their ecology. At 6.4 m, the wingspan of this species was approximately two times that of the living Royal Albatross. Modeling of flight parameters in this species indicates that it was capable of highly efficient gliding and suggests that pelagornithids exploited a long-range marine soaring strategy similar, in some ways, to that of extant albatrosses.
Keywords: Aves, fossil, Oligocene, paleontology, pseudotooth
Abstract
Pelagornithidae is an extinct clade of birds characterized by bizarre tooth-like bony projections of the jaws. Here, the flight capabilities of pelagornithids are explored based on data from a species with the largest reported wingspan among birds. Pelagornis sandersi sp. nov. is represented by a skull and substantial postcranial material. Conservative wingspan estimates (∼6.4 m) exceed theoretical maximums based on extant soaring birds. Modeled flight properties indicate that lift:drag ratios and glide ratios for P. sandersi were near the upper limit observed in extant birds and suggest that pelagornithids were highly efficient gliders, exploiting a long-range soaring ecology.
Flight ability in birds is largely governed by scaling effects, leading to a tradeoff between the benefits of physiological efficiencies and the drawbacks of decreasing power margin (ratio of available muscle power to required mechanical power) as size increases (1–4). Over their ∼150-My history, birds evolved to span at least four orders of magnitude in size (2) and achieve a wide variety of flight styles, such a flap-gliding, soaring, and hovering. Today, the largest directly measured wild individuals of volant birds reach wingspans of ∼3.5 m (Royal Albatross Diomedea exulans) and masses of ∼19 kg (Great Bustard Otis tarda and Kori Bustard Ardeotis kori) (5). In the past, the extinct terrestrial teratorn Argentavis magnificens (6) and the extinct soaring Pelagornithidae (7–10) greatly exceeded these sizes. Given the challenges of flight at large size, there has been much debate over potential upper size limits for different styles of flight in vertebrates (1, 2, 11–13), and the evolution of specialized taxa, like teratorns and pelagornithids, has been tied to environmental factors, such as wind patterns (6, 14–16).
A well-preserved associated skeleton representing the largest known volant bird provides the basis for the present study of flight properties in the remarkable extinct clade Pelagornithidae. Pelagornithidae appeared in the Paleocene (9) and attained a global distribution before going extinct in the Pliocene (8, 17). Skeletal characteristics, including pseudoteeth (spike-like protrusions of the jaw bones), a hinged mandible, and specialized wing bones (7–10, 18, 19), have raised questions about the paleoecology and phylogenetic affinities of Pelagornithidae. Although Pelagornithidae has historically been linked to the Pelecaniformes and Procellariiformes (7, 8, 20), more recent phylogenetic analyses suggest that this extinct clade is the sister taxon to Anseriformes (waterfowl) (9) or Galloanserae (waterfowl and landfowl) (21).
Regardless of their affinities, Pelagornithidae evolved such highly modified skeletal morphologies that it would not be plausible to make meaningful inferences about their flight style and ecology based on extant relatives. Therefore, computer modeling provides the best path toward understanding the flight capabilities of these remarkable birds. Flight 1.25 (2), a program designed to model avian flight under user-specified conditions, is ideally suited to this task, because it models both flapping and gliding flight performance. In this study, Flight 1.25 is applied to infer flight parameters in the largest known pelagornithid species. As with all research on fossil organisms, estimating variables, such as mass and feather length, requires additional extrapolation, and these uncertainties are taken into account with 24 iterative analyses based on combinations of viable mass estimates, aspect ratios, and wingspans as described in Methods.
Systematic Paleontology
Aves Linnaeus, 1758. Pelagornithidae Fürbringer, 1888. Pelagornis Lartet, 1857. P. sandersi sp. nov.
Holotype.
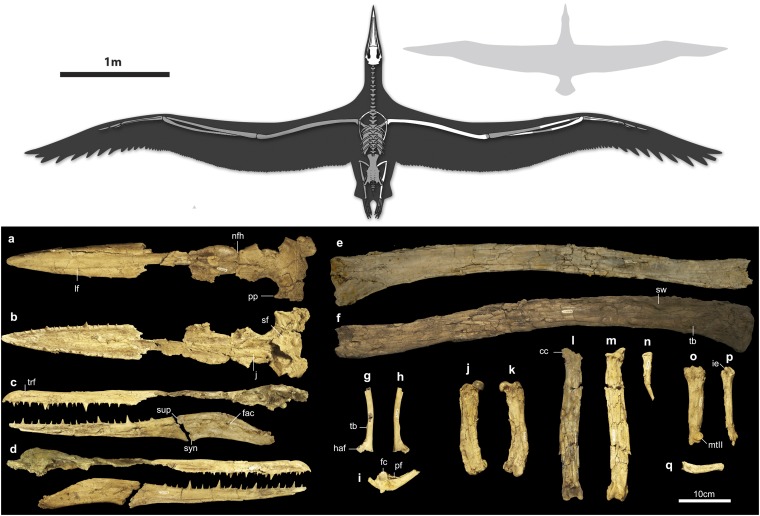
Charleston Museum (ChM) PV4768, cranium and right mandibular ramus, partial furcula, right scapula, right humerus lacking distal end, proximal and distal ends of the right radius, fragments of ulna and carpometacarpus, right femur, tibiotarsus, fibula, tarsometatarsus, and single pedal phalanx (Fig. 1).
Fig. 1.
(Upper) Reconstruction of P. sandersi (elements preserved in the holotype are shown in white) with D. exulans (Royal Albatross; 3-m average wingspan) for scale. (Lower) P. sandersi holotype (ChM PV4768) skull in (a) dorsal, (b) ventral, (c) left lateral (mandible in medial view), and (d) right lateral views (mandible in lateral view). Right humerus in (e) caudal and (f) cranial views. Scapula in (g) lateral and (h) medial views. (i) Partial furcula femur in (j) cranial and (k) caudal views. Tibiotarsus in (l) cranial and (m) caudal views. Fibula in (n) lateral view. Tarsometatarsus in (o) dorsal view (distal portion exposed in the medial view because of deformation) and (p) rotated to show the distal portion in dorsal view. (q) Pedal phalanx. cc, Lateral cnemial crest; fac, fossa aditus canalis neurovascularis; fc, facet; haf, humeral articular facet; ie, intercotylar eminence; j, jugal; lf, lateral furrow; mtII, metatarsal trochlea II; nfh, nasofrontal hinge; pf, pneumatic foramen; pp, paroccipital process; sf, subcondylar fossa; sup, supra-angulare; sw, swelling on crista deltopectoralis; syn, synovial joint; tb, tubercle; trf, transverse furrow.
Etymology.
sandersi honors retired Charleston Museum curator Albert Sanders, collector of the holotype.
Locality and Age.
The holotype was collected from Bed 2 of the Chandler Bridge Formation (22) near Charleston Airport (Charleston, SC). It is late Oligocene (lower Chattian, ~25–28 Ma) in age based on calcareous nannoplankton biostratigraphy (23).
Diagnosis.
P. sandersi exhibits diagnostic characteristics of Pelagornis (10), including rostrum with transverse furrow, tricipital fossa situated on the ventral surface of humerus, and tibiotarsus with medial condyle markedly more anteriorly projected than lateral condyle. P. sandersi can be differentiated from other species of Pelagornis by the slender caudal portion of the mandible [deep and squared in Pelagornis chilensis and Pelagornis (Pseudodontornis) _longirostris_], the more elongate beak, a larger number of mandibular pseudoteeth (31 vs. 20 in P. chilensis), and larger size. P. sandersi can further be differentiated from Pelagornis (Osteodontornis) orri by the presence of two (vs. six) small intervening pseudoteeth between the first two large pseudoteeth in the rostral portion of the upper jaw. It can be differentiated from Pelagornis mauretanicus by the larger size of the caudalmost set of mandibular pseudoteeth, Pelagornis stirtoni by the markedly more robust femur, and Pelagornis (Paleochenoides) mioceanus by lack of a deep sulcus on the fibular trochlea of the femur. SI Appendix has additional comparisons.
Description.
Two low ridges separated by a midline depression mark the skull roof, which has been partially flattened by dorsoventral crushing. A distinct nasofrontal hinge separates the upper jaw from the braincase. A longitudinal furrow extends to the base of the first pseudotooth, which is blunt and subtriangular. The pseudoteeth exhibit a bilaterally symmetrical size progression, with the first two large pseudoteeth separated by two smaller pseudoteeth and the number of intervening small pseudoteeth increasing to three in the caudal part of the jaw. Impressions of the cerebral hemispheres indicate that the sagittal eminence was strongly projected and extended to near the rostral margin of the cerebral hemisphere, which is in contrast to the poorly developed sagittal eminence reported in the Early Eocene pelagornithid Odontopteryx (24). The mandibular ramus is separated into two sections by a complex intraramal joint. Accounting for a small missing portion at the rostral tip, the lower jaw articulated well rostral to the level of the occipital condyle, like in P. longirostris (10). The scapula is remarkably small, like in P. chilensis (10). The furcula shows a shallow facet that may have formed an articulation for the tip of the sternal keel. The gently sigmoid humerus is marked by a massive protuberance on the cranial face (17). The distally displaced deltopectoral crest bears a centrally located swelling. The femur has a deep intercondylar sulcus but lacks a well-defined sulcus on the fibular trochlea. The cnemial crests of the tibiotarsus are short, and the shaft is sigmoidally curved. The tarsometatarsus shows a strongly projected intercondylar eminence, deep infracotylar fossa, and evidence of two hypotarsal canals.
Results
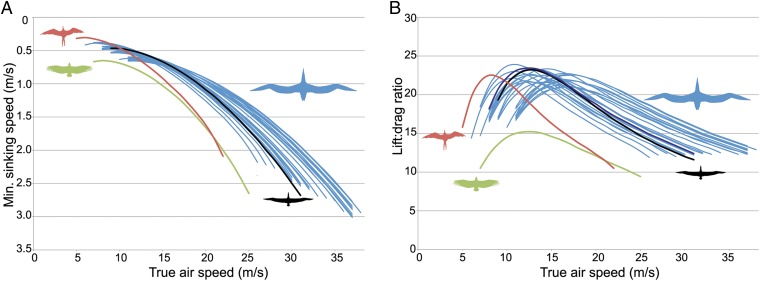
Modeled flight properties calculated in Flight 1.25 (2) indicate that P. sandersi attained high lift:drag ratios in gliding flight and that it was able to glide at fast speeds with low sink rates (Fig. 2). At speeds yielding maximum range (10.6–17.0 m/s), lift:drag ratio is predicted to have reached values of 21.0–23.9. Critically, all 24 permutations support the same general glide polar shape. Among extant soaring birds, the most similar values are observed in albatrosses. Modeled flight properties for condors result in lower lift:drag ratios, whereas both condors and frigatebirds show slower true air speeds at the same sinking speed.
Fig. 2.
(A) Glide polars and (B) lift:drag ratios for P. sandersi modeled in Flight 1.25 (2). Results of 24 analyses using different combinations of mass (21.9–40.1 kg), wingspan (6.06–7.38 m), and aspect ratio (13.0–15.0) estimates in blue; modeled values for a frigatebird (Fregata magnificens; red), albatross (Diomedea chrysostoma; black), and vulture (Coragyps atratus; green) from the Wings database in Flight 1.25 are shown for comparison. Individual analyses are presented in SI Appendix.
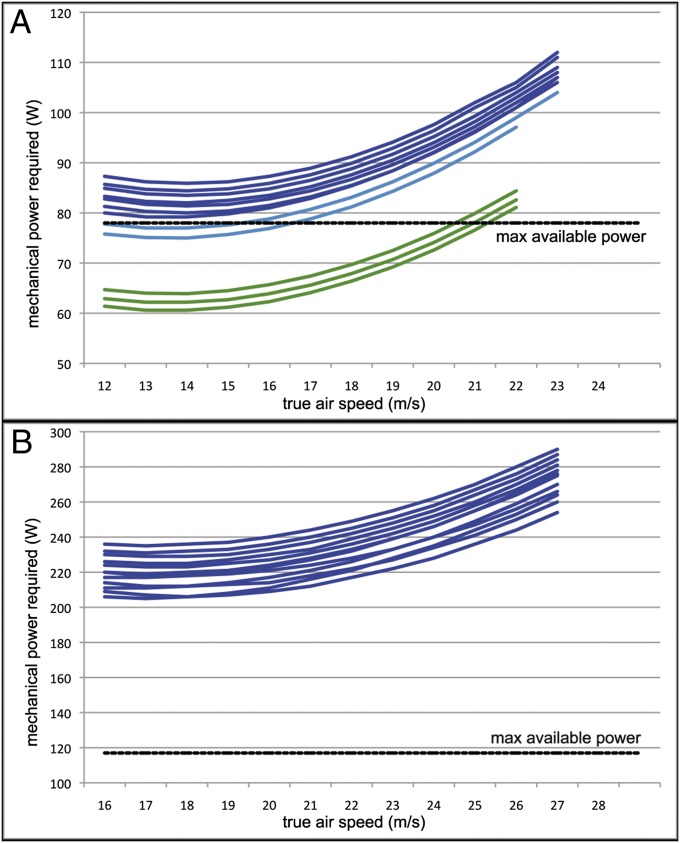
Estimating gliding performance does not require knowledge of flight muscle energy output or flapping style. Power curve calculations require estimating these additional parameters and thus, entail more uncertainty. Modeled power curves (Fig. 3) do not unambiguously resolve whether P. sandersi was capable of horizontal flapping flight under aerobic power. In the results of analyses using the lowest body mass estimates and the highest wingspan estimates for P. sandersi, most of the power curve falls under the level of the maximum power available from aerobic metabolism (Fig. 3, green lines), consistent with horizontal flapping flight capabilities at a range of speeds. However, analyses using higher mass estimates and lower wingspan estimates result in power curves that fall completely above the maximum power available from aerobic metabolism (Fig. 3, dark blue lines). It should be noted that the latter results do not require that P. sandersi was incapable of launching and soaring, because power can also be obtained from air currents and renewed by dynamic soaring. Intervals of flapping flight may also have been possible using anaerobic power. Physiological scaling suggests that anaerobic flight muscle fraction should be high for a bird with the 21.9–40.1 kg estimated mass of P. sandersi (25). Thus, the powered flight results are best interpreted as supporting a low power margin, but these results do not necessarily rule out intervals of powered horizontal flight or standing takeoff (Table S1). Full results of all analyses are provided in SI Appendix.
Fig. 3.
Power curves for P. sandersi calculated in Flight 1.25 using (A) the 21.9-kg mass estimate and (B) the 40.1-kg mass estimate with maximum estimated aerobic power for comparison. A and B each display the results of 12 iterative analyses with alternate combinations of wingspan and aspect ratio estimates. In A, horizontal flight under solely aerobic power is supported by iterations with 6.4-m wingspan and 14.0–15.0 aspect ratio (light blue) as well as all iterations with 7.38-m wingspan (green). All other runs did not support horizontal flight capabilities under solely aerobic power. Individual results for all 24 runs are provided in SI Appendix.
Discussion
P. sandersi attained the largest known avian wingspan (∼6.4 m), which raises questions about the paleoecology of giant pelagornithids. As size increases, power required for flight increases more rapidly than power available for flight (2). Thus, aerodynamic theory suggests an upper limit of 12–16 kg for aerobically powered, continuous flapping flight (1, 2, 13). However, even soaring birds must retain some ability to flap to survive in variable wind environments (13). Otherwise, grounding could prove fatal. Flapping frequency provides another theoretical limit for flight. Flapping frequency scales to body mass in such a way that predicts that albatross-like birds would be unable to stay aloft at sizes above 5.1-m wingspan (13). P. sandersi surpassed theoretical mass limits for flapping flight and wingspan limits for soaring flight based on previous models, but the extremely elongated wings and reduced hind limbs indicate it was a volant bird. Such large size was aerodynamically viable, because P. sandersi was proportionally much longer winged than an albatross: at two times the wingspan but only two to four times the mass, the fossil species departed greatly from geometric similarity with the largest albatrosses. Analyses presented here indicate that this species was capable of efficient gliding flight. Regardless of whether horizontal flapping flight was possible under aerobic power, a running takeoff can aid launch. Once airborne, birds can also obtain and renew power from external forces, such as air currents (2, 12, 16).
Larger sizes lead to greater soaring efficiency. In particular, longer wings reduce the relative size of wingtip vortices, reducing drag and thereby, improving glide ratio (4). Modeled flight properties indicate that P. sandersi attained high lift:drag ratios in gliding flight and was able to glide at fast speeds with low sink rates (Fig. 2). By virtue of their remarkably long wings, pelagornithids could achieve a similar minimum sink rate to frigatebirds at optimal glide speed, despite having higher inferred wing loading. In turn, higher wing loading results in faster gliding speeds. Overall, the modeled parameters, which are robust to uncertainty in wingspan, aspect ratio, and mass estimates, suggest that P. sandersi was able to maintain an expansive range to exploit feeding patches.
However, large size also conveys disadvantages, hindering movement on the ground or sea surface and increasing vulnerability to bone breakage under high-stress conditions. When encountering unfavorable winds, Wandering Albatrosses alight on the ocean surface (26), using a running launch to take off again and flapping flight to climb. Extant frigatebirds, which have wingspans of ∼3 m and highly reduced hind limbs, are incapable of ocean landings (27). Extreme modification of the proximal end of the humerus (8) and reduction of the hind limb make it debatable whether P. sandersi was capable of taking off from the ocean surface. If P. sandersi was unable to launch from the ocean surface, plunge diving and surface dabbling feeding strategies would be ruled out. Prey captured near the surface while in flight were potential food sources for P. sandersi, which is in keeping with the proposed gripping or trapping function of the pseudoteeth (8, 18). Kleptoparasitism and nest raiding may also have been options, because P. sandersi would have greatly exceeded the size of contemporary Sulidae and Procellariiformes from the Chandler Bridge Formation (SI Appendix).
Extant birds exploit various strategies to survive in marine environments. Wandering Albatrosses (D. exulans) use a tendinous shoulder lock to hold the wing in a horizontal position (28) and dynamic soaring near the boundary layer above waves to achieve metabolically efficient flight. Frigatebirds pursue a different strategy, taking advantage of ascending air currents to maintain high altitudes and occasionally descending to the surface to exploit patchily distributed feeding opportunities (29). Although it remains unknown whether P. sandersi evolved a tendinous strut similar to albatrosses, estimated wing loading (51.2–160.3 N/m2) and modeled flight properties (Fig. 2) support a more albatross-like than frigatebird-like flight style. The global distribution of pelagornithid fossils is also inconsistent with a reliance on ascending air currents, which restricts extant frigatebirds to the tropics.
Our understanding of the ecology of pelagornithids is only starting to develop. Data reported here suggest that they were remarkably efficient fliers, which together with their global distribution across all seven continents (8–10, 30) and long temporal range, makes the cause of their ultimate extinction all of the more mysterious.
Methods
Size Constraints.
Skeletal wing length was estimated at 2.463 m (per wing) based on intact elements (Table 1) and the proportions in P. chilensis. Skeletal proportions of the wing show little variation within Pelagornis as far as can be established from reasonably complete skeletons. The 2.463-m value is conservative given that this wing length is 115% that of P. chilensis, whereas directly comparable element lengths range from 115% (femur) to 126% (skull) of those in P. chilensis. As preserved, the humerus measures 810 mm in length, and complete length is estimated to be 940 mm, given that no portion of the musculus brachialis fossa or dorsal supracondylar process is preserved. This estimate agrees with the other measurable dimensions of the humerus: proximal width measures 92.5 mm, and total length ranges from 10.0 to 11.5 times proximal width in complete humeri of other giant Pelagornis specimens (10, 17). Width is, thus, consistent with a length of 925–1,064 mm.
Table 1.
Measurements (in millimeters) from Pelagornis sandersi holotype (ChM PV4768)
| Measurement | Value |
|---|---|
| Skull length | 569.0 |
| Humerus preserved length | 810.0 |
| Humerus estimated complete length | 940.0 |
| Humerus proximal width | 92.5 |
| Femur length | 176.8 |
| Femur proximal width | 40.9 |
| Tibiotarsus length | 280.0 |
| Tibiotarsus distal width | 38.0 |
| Tarsometatarsus length | >150.0 |
| Wingspan (feathers estimated from scaling P. orri feathers) | 6,276–6,436 |
| Wingspan (feathers estimated from skeleton:feather regressions) | 6,058–6,382 |
| Wingspan (feathers estimated from extant Procellariiformes) | 7,380 |
Body width (distance between glenoid fossae) was estimated at 287.5 mm and scaled from the 250-mm body width of P. chilensis, which is based on the intact furcula of that species (10). This value is consistent with the observation that body width scales isometrically in closely related birds (31). This value results in body width accounting for 3.9–4.7% of total wingspan (depending on method used to estimate primary feather length), consistent with the observation that body width in birds accounts for 4.12% of total wingspan on average (31). Thus, total skeletal wingspan is estimated at 5.214 m.
Primary feathers extend the length of the wing beyond that of the articulated skeleton in all birds, and skeletal wing to primary feather length proportions vary greatly among clades (32). Given this uncertainty, three methods for estimating primary feather length were used. The only direct evidence for feather proportions in Pelagornithidae comes from a specimen of P. orri that preserves flight feathers in association with a nearly complete skeleton (7). The longest intact feather from that specimen measures 400 mm, but it is uncertain whether this feather corresponds to the first primary, which is typically the longest feather and contributes directly to maximum wingspan. Scaling up this 400-mm feather length based on the relative length of the wing bones would yield a first primary length of 550–630 mm for P. sandersi (the range is because of differences in wing bone proportions in P. orri and P. chilensis). Because the isolated P. orri feathers are proportionally short given wing skeleton length and therefore, likely to represent shorter feathers from a more proximal region of the primary series (7), 550 mm can be considered a minimum estimate. Regressions calculated from the wing proportions of extant birds were used as a second set of estimates. Three recently published equations (32) were applied: one equation based on a regression ignoring phylogenetic relationships and two equations using independent contrasts based on different global phylogenies for Aves. Applying these regressions to P. sandersi results in estimates of first primary length ranging from 441 to 603 mm. A third estimate was obtained by extrapolating from the proportions in large Procellariiformes. Spread wing specimens including all skeletal elements are rare in museum collections (32). The humerus is typically removed or truncated from spread wings, making it difficult to obtain precise measurements of the skeletal length and wing length of a single individual. One suitable spread wing with a completely intact bony wing was identified from the giant petrel Macronectes giganteus (National Museum of Natural History, Smithsonian Institution: USNM 631190). In this individual, the first primary accounted for 30.6% of overall wing length. If proportions were similar in P. sandersi, it would indicate a primary length of 1.083 m. Although this method provides the highest estimate, it is worth noting that the feather:skeleton length proportions from the procellariiform are lower than in measured vultures [e.g., feathers accounted for >33% of wing length in Cathartes aura (USNM 561353), and the spread wing in this specimen was truncated, indicating that the true value was higher].
These data, thus, support a total wingspan of 6.06–7.38 m for P. sandersi depending on feather estimate method. For comparison, the most complete wing element of the teratorn A. magnificens is a humerus estimated at 570 mm in length. No fossil feathers are known for teratorns, but scaling based on the relative feather length in the extant Gymnogyps californicus (California Condor) results in total wingspan estimates of 5.70–6.07 m (15), whereas applying the three regressions (32) results in wingspan estimates of 5.09–5.57 m. Past estimates of 7 m or more (15, 16) are based on extrapolation from mass estimates rather than wing elements, but these estimates require leading primary lengths of ∼1.5 m, which contrasts sharply with both ratios seen in extant terrestrial soaring birds and the observation that primary length exhibits negative allometry with increasing body size (32).
Mass estimation for extinct birds has relied primarily on the circumference of the weight-bearing bones of the hind limb. Two regressions (33) for estimating the mass of birds based on a large sample of extant taxa were applied:
logm=(2.411×logCf)−0.065 andlogm=(2.424×logCt)+0.076,
where m is mass (grams), Cf is the least femur circumference (millimeters), and Ct is the least tibiotarsus circumference (millimeters).
Because of partial flattening, it is not possible to directly measure the circumference of the femur or tibiotarsus in P. sandersi. However, the proportions of the preserved elements are closely similar to those in P. chilensis. Isometric scaling based on length differences results in estimated circumferences of the femur and tibiotarsus of 67.2 and 73.7 mm, respectively. These values are consistent with measurements around the intact but partially flattened bones. Applying the regression from femur circumference results in a mass estimate of 21.9 kg while applying the regression from tibiotarsus circumference results in a mass estimate of 40.1 kg. A recently published regression from femur circumference (34) based on a larger dataset yielded a mass estimate of 21.8 kg (<1% difference from the value used in the analyses).
Flight Modeling.
The software package Flight 1.25 (2) was used to model flight parameters for P. sandersi. This program requires user specification of environmental and wing-shape variables. Given the uncertainty inherent to estimating size parameters in fossil taxa, 24 iterations of the analyses were conducted with different combinations of mass estimates (21.9 and 40.1 kg), wingspan estimates (6.06, 6.13, 6.4, and 7.38 m), and aspect ratios (13.0, 14.0, and 15.0). Wingspan reduction was set to the linear option in all analyses, which reflects the observation that gliding birds do not adjust their wings for minimum drag (2). All inputs not explicitly mentioned above were left at default settings.
For all calculations, air density was set using the International Standard Atmosphere values for sea level and 15 °C, yielding a density of 1.225 kg/m3. Although conditions encountered by birds will vary with weather, this density provides a baseline for comparisons. Results show little sensitivity to assumptions about temperature or flight altitude; for example, doubling temperature or increasing altitude to 1,000 m does not shift any of the estimated parameters in SI Appendix, Table S2 by more than 5%. Gravity varies slightly across latitudes because of the centripetal force caused by Earth’s rotation and the fact that our planet is not a perfect sphere. Gravity at sea level at 30°S latitude (the approximate paleolatitude of the fossil locality) was determined using table 2.1 in ref. 2. This reference point provides a value of 9.793 m/s2 for the analyses.
For the power curve analyses, maximum continuous metabolic output was estimated at 78–117 W for P. sandersi based on the observation that power available scales according to the equation _P_available = 10(mass)2/3 in vertebrates (35). Power required was estimated in Flight 1.25. Given the lack of direct data on pelagornithid musculature or flight style, values falling within the range of modern birds were selected. Flapping flight style with a flight muscle fraction of 0.17 was specified, which is the default value in Flight 1.25 and based on the observation that flight muscle mass averages 17% of overall mass across almost all birds (36). A conversion efficiency of 0.23 was used along with a basal metabolism rate calculated with the equation basal metabolism rate = 3.79(mass)0.723 and a respiration factor of 1.1, all of which represent default values for fuel to muscle work conversion efficiency based on empirical work (2).
Supplementary Material
Supporting Information
Acknowledgments
I thank James Malcom for reporting the discovery and assisting in collection, Al Sanders for leading the collection and providing helpful discussion, Carl Borick, Grahame Long, and Jennifer McCormick for collections access and hospitality at the Charleston Museum, Storrs Olson for arranging initial preparation of the specimen, Liz Bradford for creating the line artwork, Michelle Sclafani and Alyssa Stubbs for assisting with measurements, Helen James and Chris Milensky for access to spread wings, and David Rubilar-Rodgers for sharing images of P. chilensis. Comments by Michael Habib and three anonymous reviewers substantially improved this manuscript. This work was supported by National Science Foundation Award DEB: 0949899 and National Evolutionary Synthesis Center Grant NSF EF-0905606.
Footnotes
Conflict of interest statement: Editor P.E.O. served as a committee member for the dissertation of D.T.K. in 2007.
*This Direct Submission article had a prearranged editor.
References
- 1.Pennycuick CJ. Mechanics of flight. In: Farner DS, King JR, editors. Avian Biology. New York: Academic; 1975. pp. 1–75. [Google Scholar]
- 2.Pennycuick CJ. Modelling the Flying Bird. Amsterdam: Academic; 2008. [Google Scholar]
- 3.Raynor JM. Form and function in avian flight. Curr Ornithol. 1988;5:1–66. [Google Scholar]
- 4.Lindhe Norberg UM. Structure, form, and function of flight in engineering and the living world. J Morphol. 2002;252(1):52–81. doi: 10.1002/jmor.10013. [DOI] [PubMed] [Google Scholar]
- 5.Alonso JC, et al. The most extreme sexual size dimorphism among birds: Allometry, selection, and early juvenile development in the Great Bustard (Otis tarda) Auk. 2009;126(3):657–665. [Google Scholar]
- 6.Campbell KE, Jr, Tonni EP. A new genus of teratorn from the Huayquerian of Argentina (Aves: Teratornithidae) Nat Hist Mus Los Angeles Co Contrib Sci. 1980;330:59–68. [Google Scholar]
- 7.Howard H. A gigantic 'toothed' marine bird from the Miocene of California. Santa Barbara Mus Nat Hist Depart Geol Bull. 1957;1:1–23. [Google Scholar]
- 8.Olson SL. The fossil record of birds. In: Farner DS, King JR, Parkes KC, editors. Avian Biology. Vol 8. New York: Academic; 1985. pp. 79–238. [Google Scholar]
- 9.Bourdon E. Osteological evidence for sister group relationship between pseudo-toothed birds (Aves: Odontopterygiformes) and waterfowls (Anseriformes) Naturwissenschaften. 2005;92(12):586–591. doi: 10.1007/s00114-005-0047-0. [DOI] [PubMed] [Google Scholar]
- 10.Mayr G, Rubilar-Rogers D. Osteology of a new giant bony-toothed bird from the Miocene of Chile, with a revision of the taxonomy of Neogene Pelagornithidae. J Vertebr Paleontol. 2010;30(5):1313–1330. [Google Scholar]
- 11.Habib M. Constraining the air giants: Limits on size in flying animals as an example of constraint-based biomechanical theories of form. Biol Theory. 2013;8(3):245–252. [Google Scholar]
- 12.Witton MP, Habib MB. On the size and flight diversity of giant pterosaurs, the use of birds as pterosaur analogues and comments on pterosaur flightlessness. PLoS ONE. 2010;5(11):e13982. doi: 10.1371/journal.pone.0013982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato K, et al. Scaling of soaring seabirds and implications for flight abilities of giant pterosaurs. PLoS ONE. 2009;4(4):e5400. doi: 10.1371/journal.pone.0005400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell KE, Tonni EP. Size and locomotion in teratorns (Aves: Teratornithidae) Auk. 1983;100(2):390–403. [Google Scholar]
- 15.Chatterjee S, Templin RJ, Campbell KE., Jr The aerodynamics of Argentavis, the world’s largest flying bird from the Miocene of Argentina. Proc Natl Acad Sci USA. 2007;104(30):12398–12403. doi: 10.1073/pnas.0702040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vizcaíno SF, Fariña RA. On the flight capabilities and distribution of the giant Miocene bird Argentavis magnificens (Teratornithidae) Lethaia. 1999;32(4):271–278. [Google Scholar]
- 17.Boessenecker RW, Smith NA. Latest Pacific basin record of a bony-toothed bird (Aves, Pelagornithidae) from the Pliocene Purisima Formation of California, U.S.A. J Vertebr Paleontol. 2011;31(3):652–657. [Google Scholar]
- 18.Zusi RL, Warheit KI. On the evolution of intraramal mandibular joints in pseudodontorns (Aves: Odontopterygia) Nat Hist Mus Los Angeles Co Sci Ser. 1992;36:351–361. [Google Scholar]
- 19.Louchart A, et al. Structure and growth pattern of pseudoteeth in Pelagornis mauretanicus (Aves, Odontopterygiformes, Pelagornithidae) PLoS ONE. 2013;8(11):e80372. doi: 10.1371/journal.pone.0080372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison CJO, Walker CA. A review of the bony-toothed birds (Odontopterygiformes): With descriptions of some new species. Tertiary Res Special Paper. 1976;2:1–62. [Google Scholar]
- 21.Mayr G. Cenozoic mystery birds – on the phylogenetic affinities of bony-toothed birds (Pelagornithidae) Zool Scr. 2011;40(5):448–467. [Google Scholar]
- 22.Sanders AE, Weems RE, Lemon EM., Jr Chandler Bridge Formation: A new stratigraphic unit in the lower coastal plain of South Carolina. US Geol Survey Bull. 1982;1529(H):105–124. [Google Scholar]
- 23.Edwards LE, et al. US Geological Survey Open-File Report 00-049-B. US Geological Society; 2000. Supplement to the preliminary stratigraphic database for subsurface sediments of Dorchester County, South Carolina. [Google Scholar]
- 24.Milner A, Walsh S. Avian brain evolution: New data from Palaeogene birds (Lower Eocene) from England. Zool J Linn Soc. 2009;155(1):198–219. [Google Scholar]
- 25.Marden JH. From damselflies to pterosaurs: How burst and sustainable flight performance scale with size. Am J Physiol. 1994;266(4 Pt 2):R1077–R1084. doi: 10.1152/ajpregu.1994.266.4.R1077. [DOI] [PubMed] [Google Scholar]
- 26.Jouventin P, Weimerskirch H. Satellite tracking of wandering albatrosses. Nature. 1990;343(6260):746–748. [Google Scholar]
- 27.Prince PA, Morgan RA. Diet and feeding ecology of Procellariiformes. In: Croxall JP, editor. Seabirds: Feeding Ecology and Role in Marine Ecosystems. Cambridge, United Kingdom: Cambridge Univ Press; 1987. pp. 135–172. [Google Scholar]
- 28.Pennycuick CJ. The flight of petrels and albatrosses (Procellariiformes), observed in South Georgia and its vicinity. Philos Trans R Soc Lond B Biol Sci. 1982;300(1098):75–106. [Google Scholar]
- 29.Weimerskirch H, Chastel O, Barbraud C, Tostain O. Flight performance: Frigatebirds ride high on thermals. Nature. 2003;421(6921):333–334. doi: 10.1038/421333a. [DOI] [PubMed] [Google Scholar]
- 30.Fitzgerald EMG, Park T, Worthy TH. First giant bony-toothed bird (Pelagornithidae) from Australia. J Vertebr Paleontol. 2012;32(4):971–974. [Google Scholar]
- 31.Nudds RL, Rayner JMV. Scaling of body frontal area and body width in birds. J Morphol. 2006;267(3):341–346. doi: 10.1002/jmor.10409. [DOI] [PubMed] [Google Scholar]
- 32.Nudds RL, Kaiser GW, Dyke GJ. Scaling of avian primary feather length. PLoS ONE. 2011;6(2):e15665. doi: 10.1371/journal.pone.0015665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell KE, Jr, Marcus L. The relationship of hindlimb bone dimensions to body weight in birds. Nat Hist Mus Los Angeles Co Sci Ser. 1992;36:395–412. [Google Scholar]
- 34.Field DJ, Lynner C, Brown C, Darroch SA. Skeletal correlates for body mass estimation in modern and fossil flying birds. PLoS ONE. 2013;8(11):e82000. doi: 10.1371/journal.pone.0082000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Templin RJ. The spectrum of animal flight: Insects to pterosaurs. Prog Aerosp Sci. 2000;36:393–436. [Google Scholar]
- 36.Schmidt-Nielson K. Scaling: Why Is Animal Size So Important? Cambridge, United Kingdom: Cambridge Univ Press; 1986. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information