Global, regional, and national estimates of pneumonia burden in HIV-infected children in 2010: a meta-analysis and modelling study (original) (raw)
Summary
Background
Globally, pneumonia is a leading cause of mortality and morbidity in children younger than 5 years. Underlying HIV infection is an important risk factor for pneumonia morbidity and mortality in children. There are, however, no global or country level estimates of pneumonia burden in HIV-infected children. We assessed the role of HIV in pneumonia incidence and mortality and estimated the number of pneumonia cases and deaths in HIV-infected children younger than 5 years in 133 high pneumonia-burden countries in 2010.
Methods
We estimated the risk of hospital admission and case fatality rate caused by pneumonia in HIV-infected children compared with HIV-uninfected children from a systematic review of studies published in Medline, Embase, and Global Health between Jan 1, 1980, and Aug 31, 2013. We estimated nationwide pneumonia incidence and mortality with two different models that incorporated several risk factors for paediatric pneumonia hospital admission and mortality (including HIV infection). We then estimated the number of pneumonia episodes and deaths that occurred in HIV-infected children in 2010.
Findings
The odds ratio (OR) for hospital admission for all-cause pneumonia in HIV-infected children compared with HIV-uninfected children was 6·5 (95% CI 5·9–7·2). The risk of death was higher in children with pneumonia and HIV compared with those with pneumonia only (OR 5·9, 95% CI 2·7–12·7). In 2010, 1·4 million pneumonia episodes (uncertainty range [UR] 0·6 million to 3·3 million) and 88 000 pneumonia deaths (UR 47 400–153 000) occurred in HIV-infected children in low-income countries. Of these, 1·2 million pneumonia episodes (UR 0·5 million–2·7 million) and 85 400 deaths (UR 46 000–147 300) were directly attributable to HIV. 1·3 million (90%) pneumonia episodes and 82 400 (93%) pneumonia deaths in HIV-infected children aged younger than 5 years occurred in the WHO African region.
Interpretation
Globally, a small proportion of pneumonia episodes and pneumonia deaths occur in HIV-infected children. However, in the highest HIV-burden countries in sub-Saharan Africa (ie, Swaziland, Lesotho, and Zimbabwe) up to a fifth of all pneumonia cases and 60% of pneumonia deaths occur in HIV-infected children. In these countries, major reductions in child pneumonia mortality can be achieved only if the systemic challenges plaguing the health system (poor coverage of early infant testing for HIV, of antiretroviral drugs in pregnant women and young children, of co-trimoxazole prophylaxis, and of pneumococcal vaccination) can be overcome.
Funding
WHO.
Introduction
Estimated global deaths of children younger than 5 years have fallen from more than 12 million per year to fewer than 7 million per year in the past 20 years.1 However, pneumonia remains the leading cause of death of children outside the neonatal period, accounting for about 0·8 million deaths (uncertainty range [UR] 0·68 million to 0·92 million) in 2013.2 About 120 million episodes (60·8 million to 277·0 million) of childhood pneumonia occurred worldwide in 2010, with more than 14 million cases (10 million to 40 million) of severe pneumonia and 12 million hospital admissions (10 million to 14 million) .3,4 The high incidence of pneumonia globally has been strongly linked to poverty, poor living conditions, poor health services, malnutrition, and HIV infection, especially in Africa and southeast Asia, where the highest incidences and mortalities from pneumonia have been reported.
About 3·2 million children (aged 0–14 years) have HIV infection worldwide, with almost 90% of them in sub-Saharan Africa.5 HIV infection is an important risk factor for pneumonia morbidity and mortality in children.6 Children with HIV are at higher risk of developing severe or very severe pneumonia and of dying from pneumonia than are HIV-uninfected children.7 Additionally, HIV-infected children not receiving antiretroviral treatment have poor response to empirical treatment targeted predominantly against Haemophilus influenzae type b and Streptococcus pneumoniae and need broad-spectrum antibiotics to cover a range of microbial infections.8 As many as 55% of HIV-infected African children die within the first 2 years of life in the absence of antiretroviral treatment,9,10 with pneumonia reported to be the main cause of hospital admissions and deaths in these children.11–13 Severe pneumonia associated with opportunistic infections including Pneumocystis jirovecii pneumonia and cytomegalovirus is common in HIV-infected children.7
The introduction of highly active antiretroviral therapy in the early 2000s reduced morbidity and mortality from pneumonia in HIV-infected children.14 However, the overall incidence of pneumonia in HIV-infected children remains much higher than in the general population.15 HIV-infected children still fare worse on treatment compared with HIV-uninfected children, suggesting the need for a wider range of treatments for opportunistic infections.16 Use of broad-spectrum antibiotics, pneumocystis prophylaxis, and pneumococcal conjugate vaccines is generally low in low-income countries, and coupled with the high costs of health care, has contributed to the high mortality from pneumonia in HIV-infected children.17,18 However, despite a steady global increase in access to antiretroviral therapy in low-income countries, only 34% of eligible children aged 0–14 years received antiretroviral therapy in 22 high priority countries in 2012.19
At present, no estimates exist of the burden of pneumonia in HIV-infected children at global or country levels. We assessed the role of HIV in pneumonia incidence and mortality and estimated the number of pneumonia cases and deaths attributable to HIV in children younger than 5 years in 2010 in individual countries and worldwide.
Methods
Search strategy and selection criteria
We did a systematic review (according to PRISMA guidelines) to identify studies related to pneumonia in children with HIV. We searched three databases: Medline, Embase, and Global Health for studies published between Jan 1, 1980, and Aug 31, 2013 (for search terms, see appendix pp 3–4). We also searched online journals and scanned the reference lists of identified reports.
We included studies of HIV and pneumonia providing numerical estimates of incidence of pneumonia and case fatality rates in HIV-infected or HIV-uninfected children aged younger than 5 years. We excluded studies without numerical estimates, non-human studies, studies without clearly defined study designs and diagnostic criteria for pneumonia and HIV, and review articles. We applied no language restrictions.
Procedures
We defined pneumonia as admission to hospital with cough, difficult breathing, and tachypnoea either confirmed by a physician or a trained care provider. We extracted all data onto a template in Microsoft Excel. We abstracted data systematically by age and various severity categories of all-cause pneumonia, including the number of cases or incidence of pneumonia, in-hospital deaths or case fatality rate from pneumonia, or risk estimates (odds ratio [OR]) and their corresponding 95% CIs for morbidity and mortality in HIV-infected and HIV-uninfected children. The data were extracted by two independent reviewers (DOA and HN) and any disagreements were resolved after discussion. We also contacted authors for additional data when data in published studies were not conducive to inclusion in the meta-analysis.
We did a meta-analysis of risk estimates (OR) for disease incidence and case fatality rate for all-cause pneumonia in HIV-infected compared with HIV-uninfected children (with Stata 13.1) and report pooled estimates and 95% CIs. We used the random effects model (DerSimonian-Laird method) to accommodate heterogeneity across studies included in the analysis.20
We calculated country-level estimates of clinical pneumonia incidence in children aged 0–4 years residing in 133 countries with high pneumonia mortality with an approach broadly similar to that used by the Child Health Epidemiology Reference Group (CHERG).3,21 We calculated the estimates from the effect size (risk ratio) of seven risk factors for clinical pneumonia (low birthweight, lack of exclusive breast feeding, crowding, indoor air pollution, malnutrition, incomplete immunisation at age 1 year, and HIV infection) from three sources: meta-analysis of published reports,22 prevalence of each of these seven risk factors at country level (from the latest Demographic and Health Surveys and Multiple Indicator Cluster Survey), and the incidence of clinical pneumonia in 2010 for low-income countries as a region.3 Data for national prevalence of all seven risk factors were available only for 63 countries. We imputed the missing data with subregional median prevalences. Children were classed as unexposed if they were not exposed to any of the seven risk factors. We assumed that incidences in unexposed children come from a common distribution within a region; that generally, rate ratios for the seven risk factors could be multiplied when two or more risk factors were present; and that risk factors were independently distributed within countries (appendix pp 5–9). Having estimated the country-specific incidence of clinical pneumonia, we calculated the incidence of pneumonia in HIV-infected and HIV-uninfected children, and then the attributable fraction for HIV with the following equation23
Attributable fraction=Ie-I0Ie
where Ie is the incidence of clinical pneumonia in those infected with HIV in each country and I0 is the incidence of clinical pneumonia in those uninfected with HIV in each country. Similarly, we estimated the total number of deaths caused by pneumonia in 2010 for each country, with a single cause model (appendix pp 36–39) and did sensitivity analyses with the CHERG multicause model.24,25 We adjusted our estimates post hoc with national data for the use of H influenzae type b conjugate vaccine and access to antibiotics. Having obtained estimates of the total number of pneumonia deaths for each country, we calculated the number of pneumonia deaths in HIV-infected children, and the proportion of these deaths attributable to HIV (appendix pp 38–39). We then applied the attributable fraction to the pneumonia deaths in children infected with HIV (and the 95% CI) to obtain the number of pneumonia deaths attributable to HIV with an uncertainty range.
Role of the funding source
The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report. HN had full access to all the data in the study and had the final responsibility for the decision to submit for publication.
Results
We identified six studies that reported odds ratio for clinical pneumonia morbidity (including the various sub-categories for severity) in HIV-infected and HIV-uninfected children (table 1, figure 1). Of these studies, only one26 was from outside sub-Saharan Africa. Four15,26–28 were from the period before the introduction of antiretroviral therapy. In the two studies from after the introduction of antiretroviral therapy, the uptake of antiretroviral therapy in the study population was not clearly reported. Only two of the six studies included children in the full age range (2–59 months). Most investigators used modified versions of the WHO Integrated Management of Childhood Illness case definition for clinical pneumonia (table 1).34 All studies used WHO clinical staging for HIV/AIDS,35 the CDC classification system,36 or both. They each involve a rapid HIV screening test and a confirmatory HIV RNA or DNA PCR test to identify HIV-positive children aged younger than 18 months.37
Table 1.
Characteristics of studies analysed
| Study period | Age range | Setting | Design | HIV case definition | Pneumonia case definition | Category | Odds ratio (95% CI) for pneumonia morbidity | Odds ratio for pneumonia mortality*(95% CI) | |
|---|---|---|---|---|---|---|---|---|---|
| USA26 | 1990–94 | 0–12 months | Urban | Hospital-based prospective cohort | HIV status confirmed with PCR serum HIV RNA testing (Amplicor HIV-1 monitor test), CDC classification | Clinical and radiological definition of pneumonia | Clinical pneumonia | 22·1 (6·3–77·7) | .. |
| Nairobi, Kenya27 | 1992–98 | 0–24 months | Mixed | Prospective birth cohort study | HIV DNA PCR testing, WHO classification | Clinical and radiological definition of pneumonia | Clinical pneumonia | 1·3 (0·5–3·5) | .. |
| Harare, Zimbabwe28 | 1997–2001 | 0–11 months | Mixed | Hospital-based prospective cohort enrolled in trial | ELISA for samples collected at ≥18 months, PCR for <18 months, WHO classification | Physician diagnosed acute lower respiratory infection | Clinical pneumonia | 3·0 (1·9–4·8) | .. |
| Soweto, Johannesburg, South Africa29 | 1997–98 | 2–24 months | Urban | Hospital-based prospective cohort | ELISA, based on CDC classification | Pneumonia based on WHO criteria: tachypnoea (>50 breaths per min in children aged 2–12 months and >40 breaths per min in older children), chest wall indrawing, arterial SpO2 <90% on room air | Severe pneumonia | .. | 5·6 (3·1–10·4) |
| Soweto, Johannesburg, South Africa15 | 1997–2001 | 2–59 months | Urban | Hospital-based cohort | ELISA, HIV RNA PCR, CDC criteria for HIV | Admitted to hospital with a study physician diagnosis of pneumonia or bronchiolitis irrespective of clinical or radiographic features (clinical pneumonia); cough with lower chest wall indrawing or feeding difficulties, convulsions, central cyanosis, or encephalopathy, or both (very severe pneumonia); presence of endpoint consolidation (dense fluffy opacity that occupies a portion or whole of the lung that may or may not contain air-bronchograms) or pleural effusion in the lateral pleural space that was spatially associated with the infiltrate on a chest radiograph and confirmed by at least two independent readers or one reader and arbiter with standard WHO training (WHO primary endpoint pneumonia) | Clinical pneumonia; very severe pneumonia; WHO primary endpoint pneumonia | For clinical pneumonia 6·5 (5·9–7·3); for very severe pneumonia 7·9 (7·1–8·9); for WHO primary endpoint pneumonia 14·2 (12·8–15·9) | For clinical pneumonia 14·2 (9·1–22·3) |
| Cape Town, South Africa30 | 1998 | 0–59 months | Rural | Hospital-based prospective study | ELISA, HIV DNA PCR testing, WHO criteria | WHO criteria (tachypnoea or chest wall indrawing) | Clinical pneumonia | .. | 2·5 (1·2–5·5) |
| Manhica, Mozambique31 | 2004–06 | 0–23 months | Rural | Demographic surveillance, prospective study | HIV infection defined according to WHO criteria, by ELISA and HIV RNA PCR | Physician-diagnosed pneumonia with chest wall indrawing (severe pneumonia); presence of endpoint consolidation (dense fluffy opacity that occupies a portion or whole of the lung that may or may not contain air-bronchograms) or pleural effusion in the lateral pleural space that was spatially associated with the infiltrate on a chest radiograph and confirmed by at least two independent readers or one reader and arbiter with standard WHO training (WHO primary endpoint pneumonia) | Severe pneumonia; WHO primary endpoint pneumonia | For severe pneumonia 14·9 (10·9–20·3); for WHO primary endpoint pneumonia 19·7 (12·3–31·4) | For severe pneumonia 12·9 (3·7–45·4) |
| Kampala, Uganda32 | 2005–06 | 2–59 months | Mixed | Hospital-based prospective cohort | HIV defined according to WHO criteria (DNA PCR for <18 months, rapid test for >18 months) | Cough or difficult breathing, tachypnoea and chest wall indrawing | Severe pneumonia | .. | 4·1 (1·7–10·1) |
| Kwazulu-Natal and Limpopo provinces, South Africa33 | 2006–07 | 2–59 months | Rural | Hospital-based cohort | HIV status confirmed with PCR RNA testing, WHO classification | Tachypnoea (>50 breaths per min in children aged 2–11 months, >40 breaths per min in older children; pneumonia); cough with chest indrawing with or without fast breathing (severe pneumonia) | Clinical pneumonia; severe pneumonia | For clinical pneumonia 1·8 (1·0–3·2); for severe pneumonia 7·1 (3·4–14·8) |
Figure 1.
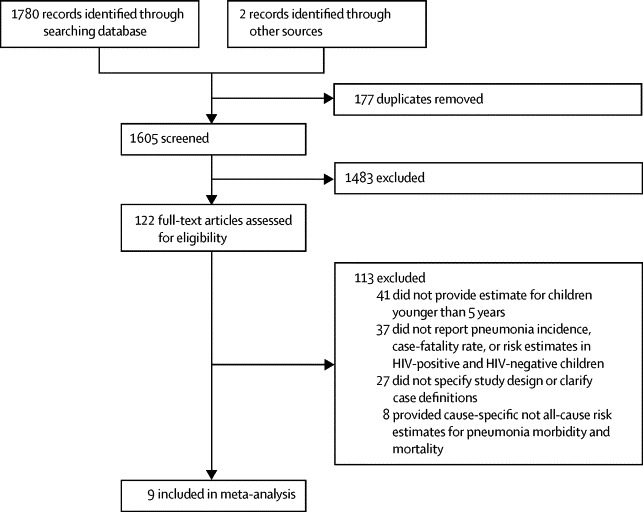
Study selection
The OR for hospital admission for all-cause pneumonia in HIV-infected children (aged 0–59 months) was 6·5 (95% CI 5·9–7·2; appendix pp 56). In a sensitivity analysis including studies that had restricted age ranges, the OR estimates were much the same (appendix pp 57–58).
116·4 million episodes of pneumonia (uncertainty range [UR] 51·4 million to 262·6 million) occurred in 2010 in children younger than 5 years in the 133 high pneumonia-burden countries (appendix pp 11–26). About a quarter (30·7 million) of all pneumonia episodes in low-income countries occurred in the WHO African region. 1·4 million episodes of pneumonia (UR 0·6 million to 3·3 million) occurred in HIV-infected children in low-income countries, of which 1·2 million episodes (UR 0·5 million to 2·7 million) were directly attributable to HIV. In Africa, 1·29 million episodes of pneumonia occurred in HIV-infected children and 1·04 million were attributable to HIV (figure 2). 1·26 million pneumonia episodes in HIV-infected children occurred in the 22 priority countries listed in UNAIDS Global Plan (table 2). The proportion of pneumonia episodes in HIV-infected children varies widely in these countries (median 6%, IQR 3–13). In Swaziland, Lesotho, and Zimbabwe more than 18% of all pneumonia episodes occurred in HIV-infected children and were attributable to HIV.
Figure 2.
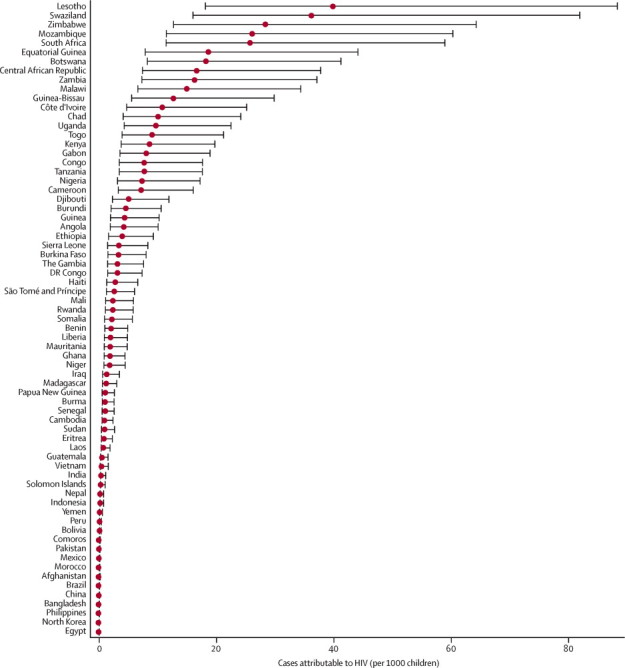
Incidence of pneumonia attributable to HIV in children younger than 5 years in 2010, in 69 Countdown countries
The list excludes Kyrgyzstan, Tajikistan, Turkmenistan, Uzbekistan, and Azerbaijan.
Table 2.
Burden of paediatric pneumonia in HIV-infected children in the 22 priority countries in the UNAIDS Global Plan
| Proportion of all pneumonia episodes in HIV-infected children | Proportion of all pneumonia episodes attributed to HIV | Proportion of all pneumonia deaths in HIV-infected children | Proportion of all pneumonia deaths attributed to HIV | |
|---|---|---|---|---|
| Angola | 2% | 2% | 11% | 11% |
| Botswana | 10% | 8% | 40% | 38% |
| Burundi | 3% | 2% | 13% | 13% |
| Cameroon | 4% | 3% | 20% | 19% |
| Chad | 4% | 3% | 18% | 17% |
| Côte d'Ivoire | 4% | 3% | 19% | 18% |
| D R Congo | 2% | 1% | 9% | 9% |
| Ethiopia | 3% | 2% | 13% | 13% |
| Ghana | 2% | 1% | 9% | 9% |
| India | 0% | 0% | 1% | 1% |
| Kenya | 6% | 5% | 27% | 26% |
| Lesotho | 23% | 19% | 64% | 62% |
| Malawi | 13% | 10% | 46% | 45% |
| Mozambique | 11% | 9% | 41% | 40% |
| Namibia | 13% | 11% | 48% | 46% |
| Nigeria | 4% | 3% | 19% | 18% |
| South Africa | 14% | 12% | 50% | 48% |
| Swaziland | 25% | 20% | 67% | 64% |
| Uganda | 7% | 5% | 29% | 28% |
| Tanzania | 6% | 5% | 27% | 26% |
| Zambia | 13% | 11% | 47% | 45% |
| Zimbabwe | 18% | 15% | 57% | 55% |
Five studies reported in-hospital case fatality rate for HIV-infected and HIV-uninfected children (table 1, figure 1). Of these studies, all but one were from southern sub-Saharan Africa. Studies from after the introduction of antiretroviral therapy31,32 did not report the uptake of antiretroviral therapy. Only three studies involved children for the full age range (2–59 months). Because most deaths caused by pneumonia in HIV-infected children occur in the first year of life (with P jirovecii pneumonia and cytomegalovirus the most common causes), we also included data from studies that included only younger children. The ratio of case fatality rates for pneumonia in HIV-infected and HIV-uninfected children was 5·9 (95% CI 2·7–12·7; appendix pp 59–60).
According to the single cause mortality model, 921 763 (UR 617 085–1 386 843) deaths caused by pneumonia occurred in young children in the post-neonatal age group (1–59 months) in low-income countries in 2010. Of these pneumonia deaths, 88 257 deaths (UR 47 387–152 268) occurred in HIV-infected children and 85 404 (UR 45 853–147 344) were attributable to HIV (appendix pp 11–24,51). These estimates are consistent with those from the CHERG multicause model (appendix pp 52–55).25 82 422 pneumonia deaths (93%) in HIV-infected children aged younger than 5 years occurred in the WHO African region and 79 774 (93%) were attributable to HIV. 78 377 deaths (88%) caused by pneumonia in HIV-infected children occurred in the 22 priority countries listed in UNAIDS Global Plan (table 2). The proportion of pneumonia deaths varied widely in these 22 countries (median 27%, IQR 14–47). In three countries—Swaziland, Lesotho, and Zimbabwe—more than 55% of all the pneumonia deaths could be attributed to HIV.
Discussion
HIV infection is an important risk factor for paediatric pneumonia morbidity and mortality. Our study is the first to estimate the number of pneumonia episodes and deaths in HIV-infected children in low-income countries and provide country-level estimates for pneumonia morbidity and mortality attributable to HIV.
Our estimates of the number of pneumonia episodes and pneumonia deaths are consistent with the CHERG estimates (appendix pp 43–50, 61).3,25 Pneumonia mortality in children aged 0–4 years fell substantially between 2000 and 2010. After adjusting for this decrease, our mortality estimates are consistent with the estimates for pneumococcal pneumonia in HIV-infected children in 2000, which was estimated with an entirely different approach and is therefore not strictly comparable.38 Conventionally, any death of an HIV-infected child has had the cause of death assigned to HIV, and our analysis supports this approach. We have shown that, although only about 1% of childhood pneumonia morbidity and 9% of mortality from childhood pneumonia are attributable to HIV worldwide, in the WHO African region, about 3% of pneumonia episodes and 17% of pneumonia deaths are attributable to HIV.
These estimates have major implications for policy on the management of pneumonia in HIV-infected children, especially with respect to the revised WHO guidelines for antiretroviral therapy and management of community-acquired paediatric pneumonia in HIV-infected children (which entails hospital admission for children with chest wall indrawing).35,39 Although these recommendations seem straightforward to implement at all levels of health systems, including primary care, challenges exist in resource-limited high-burden settings. First, after the implementation of antiretroviral therapy in South Africa, the incidence of pneumococcal pneumonia in HIV-infected children younger than 2 years fell by 42%.40 However, antiretroviral therapy coverage in children remains poor compared with adults. No global estimates are available for antiretroviral therapy coverage in children younger than 5 years. Although antiretroviral therapy coverage for children aged 0–14 years in the 22 priority countries increased in the past decade, it was still disappointingly low in 2011 (29%, 95% CI 26–31).19 Coverage at the national level was highly variable, ranging from about 8% in Chad to more than 95% in Botswana. Coverage for children younger than 5 years is expected to be much lower and cannot be assumed to be the same as in older children, because the median age of initiating antiretroviral therapy is 4 years (Mahy M, UNAIDS, personal communication).
Second, although prevention of mother-to-child transmission of HIV coverage has increased greatly during the past decade, only about half of pregnant women with HIV in Africa received preventive treatment in 2010. Even if coverage was scaled up beyond 95%, a substantial number of new infections would still occur.41 Third, coverage of co-trimoxazole prophylaxis for HIV in 2011 was only 31% (95% CI 26–37), even though opportunistic infections are an important cause of death for HIV-infected children and co-trimoxazole prophylaxis reduces overall infection rates (not only of pneumonia, but also of malaria and sepsis) and has been recommended since 2006 as an essential part of HIV care.19 Fourth, although early infant diagnosis of HIV is being scaled up, only about a third of infants born to HIV-infected mothers in 2012 were tested for HIV in the first 2 months of life and coverage was less than 10% in five of the priority countries (Angola, DR Congo, Malawi, Nigeria, and Chad).19 Implementation of a specific case management package for pneumonia in HIV-infected children is impossible if HIV infection status is unknown.39 Finally, giving pneumococcal vaccine (PCV-9) to HIV-infected children in South Africa reduced all-cause pneumonia by 13%.15 Steenhoff and colleagues42 have reported a 57% reduction in the incidence of physician-diagnosed pneumonia after introduction of antiretroviral therapy and PCV-7 in US children. Coverage of three doses of pneumococcal vaccine is poor, at about 19% worldwide and 21% in the WHO African region (ranging from 12% in Ethiopia to 99% in Malawi) in 2012.43 Moreover, seven of the 22 priority countries are yet to introduce the vaccine.44
Several factors affect our estimates. Although the case definitions used by the various studies in our meta-analysis were broadly similar, they varied substantially in their specific eligibility criteria. Similarly, although we only included studies that used a rapid test for HIV screening followed by confirmation by PCR, there is probably some variation in the sensitivity and specificity of the study-specific test kits. We believe that these differences, along with the precise study designs, would have contributed to heterogeneity in the estimates. In most cases, HIV status was tested only after hospital admission, and is unlikely to have biased the results. The included studies report wide variations in case fatality rate for the two groups—about a five-times difference in HIV-uninfected children and two-times difference in HIV-infected children. This difference could be a result of access to care (in the former), and quality of care and range of pathogens (in the latter). Our systematic review returned only nine studies, most from southern Africa. However, all were of high quality and fulfilled our strict eligibility criteria and reported data for HIV-negative and HIV-positive children. The results of the individual studies that contributed to the analysis are consistent (appendix pp 56–58). The geographical clustering is not surprising given that 21 of the 22 high priority countries are in sub-Saharan Africa and of these, nine (some with the highest burden) are in southern Africa (UNAIDS data13).
Antiretroviral therapy coverage was not reported in the studies, nor was its effect on the risk of all-cause pneumonia in HIV-infected children. Therefore, we could not adjust the estimates for access to antiretroviral therapy, especially in the first few months of life, when it has a major effect on morbidity and mortality.45 This shortcoming could have resulted in some overestimation of the incidence of pneumonia in HIV-infected children. This uncertainty is compounded by the case ascertainment used in the studies to calculate the odds ratio for pneumonia morbidity and mortality in HIV-infected compared with HIV-uninfected children. All the studies were hospital-based, and therefore, these estimates would be affected by health-care seeking behaviour, which is typically poor in low-income countries, resulting in underestimation of the burden. More importantly, we might have underestimated the role of HIV infection in paediatric pneumonia by only considering HIV-infected children in the exposed group. However, HIV exposed but uninfected children (ie, HIV-negative children born to HIV-positive mothers) are at a much higher risk of developing severe pneumonia, treatment failure, and death compared with unexposed children.46 Additionally, our estimates are probably conservative because very young infants are not routinely tested for HIV, even in the priority countries.
The national estimates for pneumonia morbidity and mortality and the proportion of pneumonia cases and deaths attributable to HIV depend on the quality of local data for prevalence of risk factors included in the model. Such data were missing for at least one risk factor in 70 of the 133 countries. Although we imputed the missing data, our sensitivity analyses suggested that our estimates are not sensitive to imputation (appendix pp 27–35). Although our estimates of HIV prevalence in children younger than 5 years take into consideration coverage and efficacy of preventing mother-to-child transmission, prevalence of breastfeeding, and transmission of HIV in breast milk, data were either poor or missing for some countries (especially outside Africa). For the morbidity model, we assumed that all seven risk factors included in the model were distributed independently within countries. This assumption is unlikely to be true because these factors are linked to socioeconomic characteristics such as deprivation. There is likely to be some (unknown) degree of correlation between these risk factors. Similarly, we assumed that the risk ratios for each risk factor were the same across the low-income countries and that the risk ratios combined multiplicatively (with a weak interaction term). Our mortality estimates are restricted to children aged 1–59 months because much of the effect of paediatric HIV on mortality occurs after the neonatal period. Further studies are needed to understand how risk factors cluster in populations, to estimate cause-specific pneumonia burden in HIV-infected children, and to identify appropriate antibiotic treatment regimens for pneumonia in HIV-infected children.
Our estimates have important policy implications. Management of pneumonia in HIV-infected children needs a multipronged integrated approach both from national governments and international agencies. Case ascertainment (of both pneumonia and HIV by PCR virological testing) needs to be strengthened in primary care, and antiretroviral therapy in pregnant women and young children need to be rapidly scaled up, along with co-trimoxazole prophylaxis coverage. Priority countries that have not yet introduced pneumococcal conjugate vaccine should consider doing so, at least for HIV-infected children. Simultaneously, the revised WHO guidelines for management of childhood pneumonia39,47 should be implemented fully if further substantial falls in pneumonia morbidity and mortality are to be achieved in high HIV burden countries.
Acknowledgments
Acknowledgments
We thank WHO Department of Maternal, Newborn, Child and Adolescent Health for financial support for this work. We gratefully acknowledge inputs by Mary Mahy (UNAIDS Joint United Nations Programme on HIV/AIDS) during data analysis and report writing. The findings and conclusions in this report are those of the authors and do not necessarily represent the official views or policies of the WHO.
Declaration of interests
SAM has been a clinical trialist for studies involving vaccines against pneumonia-causing pathogens for GlaxoSmithKline, Pfizer, Sanofi-Aventis, Novartis, and Medimmune. His institution has also received research grants from GlaxoSmithKline, Pfizer, and Novartis. He has been on the speaker's bureau of GlaxoSmithKline, Pfizer, and Sanofi-Aventis and has received travel support and honoraria. He has acted on advisory boards of GlaxoSmithKline, Pfizer, and Novartis. LMM is a staff member of WHO. All other authors declare that they have no competing interests.
Contributors
HN and HC had the idea for the study. DOA did the literature review with oversight from HN. ET, DAM, CR, and HN developed the statistical models, and analysed and interpreted data. HN, DAM, and ET wrote the first draft of the Article. HC, SAM, LMM, and IR interpreted data, contributed to report writing, and critically reviewed the Article. ET and DAM made equal contributions and the ordering of these two authors was determined at random. All authors read and approved the final draft of the Article.
Supplementary Material
Supplementary appendix
References
- 1.United Nations Inter-agency group for Child Mortality Estimation . Levels and Trends in Child Mortality- Report 2013. UNICEF; New York: 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu L, Oza S, Hogan D. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2014 doi: 10.1016/S0140-6736(14)61698-6. http://dx.doi.org/10.1016/S0140-6736(14)61698-6 published online Oct 1. [DOI] [PubMed] [Google Scholar]
- 3.Rudan I, O'Brien KL, Nair H. Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J Glob Health. 2013;3:10401. doi: 10.7189/jogh.03.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nair H, Simoes EA, Rudan I. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet. 2013;381:1380–1390. doi: 10.1016/S0140-6736(12)61901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UNAIDS . Global Report- UNAIDS report on the global AIDS epidemic 2013. UNAIDS; Geneva: 2013. [Google Scholar]
- 6.Jeena P. The role of HIV infection in acute respiratory infections among children in sub-Saharan Africa. Int J Tuberc Lung Dis. 2005;9:708–715. [PubMed] [Google Scholar]
- 7.Zar HJ, Madhi SA. Childhood pneumonia--progress and challenges. S Afr Med J. 2006;96:890–900. [PubMed] [Google Scholar]
- 8.Jeena P, Thea DM, MacLeod WB. Failure of standard antimicrobial therapy in children aged 3-59 months with mild or asymptomatic HIV infection and severe pneumonia. Bull World Health Organ. 2006;84:269–275. doi: 10.2471/blt.04.015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Little K, Thorne C, Luo C. Disease progression in children with vertically-acquired HIV infection in sub-Saharan Africa: reviewing the need for HIV treatment. Curr HIV Res. 2007;5:139–153. doi: 10.2174/157016207780077002. [DOI] [PubMed] [Google Scholar]
- 10.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 11.Zwi KJ, Pettifor JM, Soderlund N. Paediatric hospital admissions at a South African urban regional hospital: the impact of HIV, 1992–1997. Ann Trop Paediatr. 1999;19:135–142. doi: 10.1080/02724939992455. [DOI] [PubMed] [Google Scholar]
- 12.Lucas SB, Peacock CS, Hounnou A. Disease in children infected with HIV in Abidjan, Cote d'Ivoire. BMJ. 1996;312:335–338. doi: 10.1136/bmj.312.7027.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vetter KM, Djomand G, Zadi F. Clinical spectrum of human immunodeficiency virus disease in children in a west African city. Project RETRO-CI. Pediatr Infect Dis J. 1996;15:438–442. doi: 10.1097/00006454-199605000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Lazarous DG, O'Donnell AE. Pulmonary infections in the HIV-infected patient in the era of highly active antiretroviral therapy: an update. Curr Infect Dis Rep. 2007;9:228–232. doi: 10.1007/s11908-007-0036-x. [DOI] [PubMed] [Google Scholar]
- 15.Madhi SA, Kuwanda L, Cutland C, Klugman KP. The impact of a 9-valent pneumococcal conjugate vaccine on the public health burden of pneumonia in HIV-infected and -uninfected children. Clin Infect Dis. 2005;40:1511–1518. doi: 10.1086/429828. [DOI] [PubMed] [Google Scholar]
- 16.Barbosa AN, Souza LR. Occurrence of pneumocystis pneumonia in HIV-infected patients and the interference of the highly active antiretroviral therapy. J Venom Anim Toxins Incl Trop Dis. 2008;14:152–160. [Google Scholar]
- 17.Zar HJ, Madhi SA, Aston SJ, Gordon SB. Pneumonia in low and middle income countries: progress and challenges. Thorax. 2013;68:1052–1056. doi: 10.1136/thoraxjnl-2013-204247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez-Palomo C, Martin-Zamorano M, Benitez E. Pneumonia in HIV-infected patients in the HAART era: incidence, risk, and impact of the pneumococcal vaccination. J Med Virol. 2004;72:517–524. doi: 10.1002/jmv.20045. [DOI] [PubMed] [Google Scholar]
- 19.WHO . Global update on HIV treatment 2013: results, impact and opportunities. World Health Organization; Geneva: 2013. [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008;86:408–416. doi: 10.2471/BLT.07.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson S, Mathews KH, Pulanic D. Risk factors for severe acute lower respiratory infections in children: a systematic review and meta-analysis. Croat Med J. 2013;54:110–121. doi: 10.3325/cmj.2013.54.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd edn. Lippincott Williams & Wilkins; Philadelphia: 2008. [Google Scholar]
- 24.Theodoratou E, Zhang JSF, Kolcic I. Estimating pneumonia deaths of post-neonatal children in countries of low or no death certification in 2008. PLoS ONE. 2011;6:e25095. doi: 10.1371/journal.pone.0025095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu L, Johnson HL, Cousens S. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 26.Kattan M, Platzker A, Mellins RB. Respiratory diseases in the first year of life in children born to HIV-1-infected women. Pediatr Pulmonol. 2001;31:267–276. doi: 10.1002/ppul.1038. [DOI] [PubMed] [Google Scholar]
- 27.Richardson BA, Nduati R, Ngacha DM, Overbaugh J, John-Stewart GC. Acute HIV infection among Kenyan infants. Clin Infect Dis. 2008;46:289–295. doi: 10.1086/524748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koyanagi A, Humphrey JH, Ntozini R. Morbidity among human immunodeficiency virus-exposed but uninfected, human immunodeficiency virus-infected, and human immunodeficiency virus-unexposed infants in Zimbabwe before availability of highly active antiretroviral therapy. Pedriatr Infect Dis J. 2011;30:45–51. doi: 10.1097/INF.0b013e3181ecbf7e. [DOI] [PubMed] [Google Scholar]
- 29.Madhi SA, Petersen K, Madhi A, Khoosal M, Klugman KP. Increased disease burden and antibiotic resistance of bacteria causing severe community-acquired lower respiratory tract infections in human immunodeficiency virus type 1-infected children. Clin Infect Dis. 2000;31:170–176. doi: 10.1086/313925. [DOI] [PubMed] [Google Scholar]
- 30.Zar HJ, Hanslo D, Tannenbaum E. Aetiology and outcome of pneumonia in human immunodeficiency virus-infected children hospitalized in South Africa. Acta Paediatr. 2001;90:119–125. [PubMed] [Google Scholar]
- 31.Roca A, Sigauque B, Quinto L. Estimating the vaccine-preventable burden of hospitalized pneumonia among young Mozambican children. Vaccine. 2010;28:4851–4857. doi: 10.1016/j.vaccine.2010.03.060. [DOI] [PubMed] [Google Scholar]
- 32.Nantanda R, Hildenwall H, Peterson S, Kaddu-Mulindwa D, Kalyesubula I, Tumwine JK. Bacterial aetiology and outcome in children with severe pneumonia in Uganda. Ann Trop Paediatr. 2008;28:253–260. doi: 10.1179/146532808X375404. [DOI] [PubMed] [Google Scholar]
- 33.Horwood C, Butler LM, Vermaak K. Disease profile of children under 5 years attending primary health care clinics in a high HIV prevalence setting in South Africa. Trop Med Int Health. 2011;16:42–52. doi: 10.1111/j.1365-3156.2010.02672.x. [DOI] [PubMed] [Google Scholar]
- 34.Department of Child and Adolescent Health . Handbook: IMCI integrated management of childhood illness. World Health Organization; Geneva: 2005. [Google Scholar]
- 35.WHO . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection – recommendations for a public health approach. World Health Organisation; Geneva: 2013. [PubMed] [Google Scholar]
- 36.Centres for Disease Control and Prevention Revised classification system for human immunodeficiency virus infection in children less than 13 years of age. Morb Mortal Wkly Rep. 1994;43:1–19. [Google Scholar]
- 37.WHO . WHO recommendations on the diagnosis of HIV infection in infants and children. World Health Organization; Geneva: 2010. [PubMed] [Google Scholar]
- 38.O'Brien KL, Wolfson LJ, Watt JP. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 39.WHO . Pocket book of hospital care for children. Second edn. World Health Organization; Geneva: 2013. [PubMed] [Google Scholar]
- 40.Nunes MC, von Gottberg A, de Gouveia L. The impact of antiretroviral treatment on the burden of invasive pneumococcal disease in South African children: a time series analysis. AIDS. 2011;25:453–462. doi: 10.1097/QAD.0b013e328341b7f1. [DOI] [PubMed] [Google Scholar]
- 41.Penazzato M, Bendaud V, Nelson L, Stover J, Mahy M. Estimating future trends in paediatric HIV. AIDS. 2014 doi: 10.1097/QAD.0000000000000481. published online Sept 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steenhoff AP, Josephs JS, Rutstein RM. Incidence of and risk factors for community acquired pneumonia in US HIV-infected children, 2000-2005. AIDS. 2011;25:717–720. doi: 10.1097/QAD.0b013e3283440583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.WHO UNICEF review of national immunization coverage. 1980–2013. http://apps.who.int/immunization_monitoring/globalsummary/wucoveragecountrylist.html (accessed Oct 23, 2014).
- 44.International Vaccine Access Center VIMS Report: Global Vaccine Introduction. http://www.jhsph.edu/research/centers-and-institutes/ivac/vims/IVAC-VIMS-Report-2014-Mar.pdf (accessed Oct 23, 2014).
- 45.Violari A, Cotton MF, Gibb DM. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Izadnegahdar R, Fox MP, Jeena P, Qazi SA, Thea DM. Revisiting pneumonia and exposure status in infants born to HIV-infected mothers. Pediatr Infect Dis J. 2014;33:70–72. doi: 10.1097/INF.0b013e31829f0ade. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.WHO . Caring for newborns and children in the community, adaptation for high HIV or TB settings. Community health worker manual, facilitator notes, chart booklet, referral form. WHO; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary appendix