Biologic markers determine both the risk and the timing of recurrence in breast cancer (original) (raw)
. Author manuscript; available in PMC: 2015 Feb 11.
Published in final edited form as: Breast Cancer Res Treat. 2011 May 20;129(2):607–616. doi: 10.1007/s10549-011-1564-5
Abstract
Breast cancer has a long natural history. Established and emerging biologic markers address overall risk but not necessarily timing of recurrence. 346 adjuvant naïve breast cancer cases from Guy’s Hospital with 23 years minimum follow-up and archival blocks were recut and reassessed for hormone-receptors (HR), HER2-receptor and grade. Disease-specific survival (DSS) was analyzed by recursive partitioning. To validate insights from this analysis, gene-signatures (proliferative and HR-negative) were evaluated for their ability to predict early versus late metastatic risk in 683 node-negative, adjuvant naïve breast cancers annotated with expression microarray data. Risk partitioning showed that adjuvant naïve node-negative outcome risk was primarily partitioned by tumor receptor status and grade but not tumor size. HR-positive and HER2-negative (HRpos) risk was partitioned by tumor grade; low grade cases have very low early risk but a 20% fall-off in DSS 10 or more years after diagnosis. Higher grade HRpos cases have risk over >20 years. Triple-negative (Tneg) and HER2-positive (HER2pos) cases DSS events occurred primarily within the first 5 years. Among node-positive cases, only low grade conferred late risk, suggesting that proliferative gene signatures that identify proliferation would be important for predicting early but not late recurrence. Using pooled data from four publicly available data sets for node-negative tumors annotated with gene expression and outcome data, we evaluated four prognostic gene signatures: two proliferation-based and two immune function-based. Tumor proliferative capacity predicted early but not late metastatic risk for HRpos cases. The immune function or HRneg specific signatures predicted only early metastatic risk in Tneg and HER2pos cases. Breast cancer prognostic signatures need to inform both risk and timing of metastatic events and may best be applied within subsets. Current signatures predict for outcome risk within 5 years of diagnosis. Predictors of late risk for HR positive disease are needed.
Keywords: Breast cancer, Hormone therapy, Risk partitioning, Risk, Recurrence, Gene signatures
Introduction
Breast cancer is widely recognized as a heterogeneous disease. It is now common in clinical practice to refer to three clinical subgroups (trigroup): HR positive and HER2 negative (HRpos), triple negative (Tneg), and HER2 overexpressing (HER2pos), each with different prognostic characteristics and therapeutic implications. Gene expression profiling has been used to develop new classifiers [1–3] and novel multigene signatures for breast cancer recurrence that improve upon traditional clinical prognostic variables such as nodal status, tumor size, and histologic grade [4, 5].
Due to the overall predominance of HRpos breast cancers and the type of molecular differences that distinguish high from low risk HRpos breast cancers, most of the better known multigene predictors best estimate the recurrence likelihood of HRpos breast cancers [1, 3, 5]. A meta-analysis of various multigene signatures that includes the 70 gene profile [2], MS-14 [6], EMC-76 [7], CSR/wound-response [8], Oncotype Recurrence Score [9], p53 [10], and the genomic grade index [11] have demonstrated that their prognostic values are comparable when evaluated in HRpos breast cancers, presumably due to the fact that proliferation genes within these signatures are a common driving force behind their overall prognostic performance [12, 13]. HRneg breast cancers are usually highly proliferative, and are invariably either classified as high risk or are not the target population of multigene predictors, although novel predictive signatures, not dependent on proliferation gene modules, have been proposed for these aggressive breast cancer subgroups [5, 12, 13].
Breast cancer risk is well known to span 20 years. However, none of the markers we use currently in standard clinical practice, nor the ones recently introduced are used to address the timing of metastatic recurrence.
When to expect a metastatic recurrence of breast cancer is a question of great concern to all patients, regardless of their level of education [14, 15], and information they want at diagnosis and at each follow-up clinic visit. The question is not easily addressed by even the most seasoned oncologists, and women are told the risk of breast cancer persists for many years. Often we project risk estimates based on presumptions about the chance of progression in the absence of adjuvant therapy. However, it is critical to understand the trajectory of breast cancer after surgical excision alone, if we truly want to evaluate prognostic markers, and if we want to be able to properly understand the impact of adjuvant therapy.
Our goal was to develop better models to estimate risk of metastatic recurrence as well as the time dependence of that risk [16]. We hypothesized that the temporal nature of recurrence risk is based on underlying biology of each breast cancer subtype. Given that breast cancer is a mix of heterogeneous subgroups with different outcomes, it can be analytically considered as a mixture model. An ideal way to analyze such data is to use recursive partitioning to identify the most homogeneous subsets mixed within the population. We applied risk partitioning models to a unique dataset from Guy’s Hospital in London, England, with up to 30 years of follow-up, and used these insights to evaluate the time-dependent prognostic value of existing and emerging gene signatures on an independent data set of adjuvant naïve patients.
Methods
Guy’s hospital dataset
The study base for this analysis consists of 561 women from the Guy’s Hospital dataset treated with definitive local regional therapy but without systemic therapy from 1975 to 1982, and followed for a minimum of 23 years. Median follow-up was 27.75 years. Kaplan–Meier analyses of distant recurrence-free survival were developed for patients based on stage (tumor size and nodal status), HR status (ER, PR), HER2 status, age at diagnosis, and tumor grade. For 346 women, tissue blocks were available and new sections were cut to re-evaluate tumor histology and perform immunohistochemistry (ER, PR, HER2). All sections were read and interpreted by a single pathologist. ER and PR status were assessed using the Allred scoring method [17]. HR status was considered positive (Allred score of >2) if either or both ER and PR were positive. HER2 staining was performed using Dako polyclonal A0485. Tumors were histologically graded using the Scarff–Bloom–Richardson (SBR) scoring system [18].
Patient classification by recursive partitioning
The R package “rpart” [19], an open-source implementation of recursive partitioning algorithms (CART) [20], was used to find cut-points and predictor variables that separate individuals into sub-groups by survival patterns. Additional details about Rpart can be found in supplemental methods. The endpoint of interest for this analysis is disease-specific survival (DSS) where death from breast cancer is the event of interest. Variables available for the CART analysis included tumor size, number of lymph nodes involved, SBR grade scores (3–9), HR and HER2 receptor status, age, tri-group status (HR pos vs. triple negative vs. HER2 positive).
Independent assessment of gene signatures and time-dependent metastatic outcome using public breast cancer microarray datasets
An additional independent analysis was performed using data compiled from four pooled publically available data sets, 683 adjuvant-naive, node-negative breast cancer cases (447 ER positive and 236 ER negative), annotated for distant metastasis-free survival (DMFS), were identified [2, 7, 21, 22] (Supplemental Table 1S). Four gene-based signatures, the Celera HRpos metastasis score (MS-14 [6]), the proliferation signature [23], the Immune Response signature (IR [24]), and a newer chemokine-based HRneg/Tneg signature [25] were analyzed for their prognostic value by significant association with either overall DMFS (from time of diagnosis) or delayed DMFS for the subgroup surviving 5 years beyond diagnosis without early metastatic events. Gene signature mapping is described in the supplemental Table 2. Cases were dichotomized into high versus low index groups by the median value. Kaplan–Meier analyses were performed and significance assessed by the log-rank statistic. Kaplan–Meier estimates of distant metastasis free survival may be based on competing events (i.e., death causes other than breast cancer). Unfortunately, deaths from other causes were not available for the public datasets we used so that we were unable to perform a competing risk analysis.
We therefore used Cox regression analysis on the event of interest, distant metastasis, using different gene signatures as predictors.
Results
Table 1 shows the characteristics of the patients in the Guy’s Hospital dataset. The dataset was rich in HRpos patients (76%), with a smaller proportion of Tneg and HER2pos patients (24%). Of the 346 patients that had tumor blocks available, the majority were node-negative (N0), 214 (62%) and T2, 193 (56%) and intermediate grade (SBR 6–7)153 (44%). Of node-negative patients, 33 (15%) were low grade. These features reflect a population of women diagnosed prior to the routine use of screening mammography.
Table 1.
Characteristics of Guy’s Hospital dataset
| Number of patients | Percent | |
|---|---|---|
| Age | ||
| <40 | 26 | 7.5 |
| 40–49 | 84 | 24.3 |
| 50–59 | 108 | 31.2 |
| 60–69 | 76 | 22.0 |
| ≥70 | 52 | 15.0 |
| Nodal status | ||
| 0 nodes | 214 | 61.8 |
| 1–3 nodes | 85 | 24.6 |
| 3+ nodes | 47 | 13.6 |
| Tumor size | ||
| T1 (<2 cm) | 127 | 36.7 |
| T2 (2–5 cm) | 193 | 55.8 |
| T3 (>5 cm) | 26 | 7.5 |
| Grade | ||
| SBR 3–5 (grade 1) | 43 | 12.4 |
| SBR 6–7 (grade 2) | 153 | 44.2 |
| SBR 8–9 (grade 3) | 150 | 43.3 |
| Receptor status | ||
| HER2pos | 47 | 13.6 |
| HRpos/HER2neg | 264 | 76.3 |
| Tneg | 35 | 10.1 |
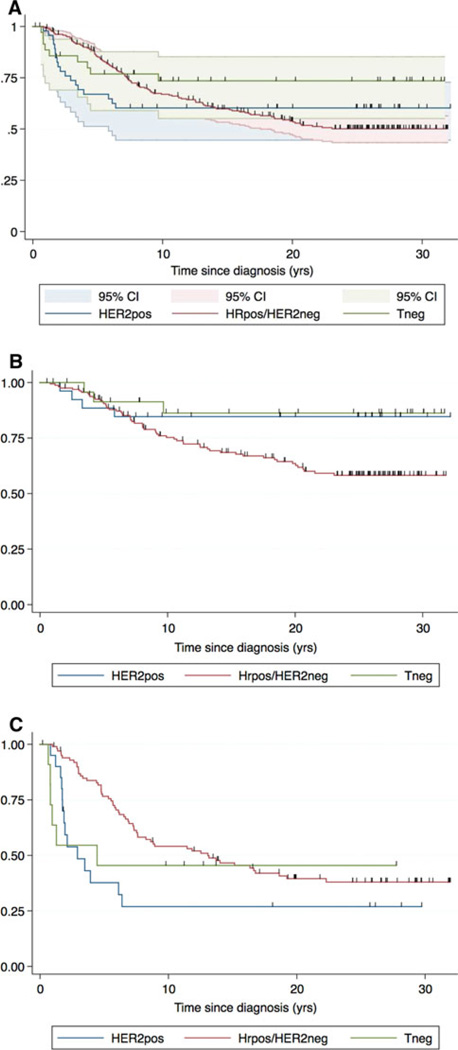
Kaplan–Meier plots of DSS for the entire Guy’s Hospital dataset, divided by trigroup (HRpos, Tneg, and HER2pos) is shown in Fig. 1a with 95% confidence intervals. Figure 1b, c shows the node-negative and positive populations, respectively. The risk of death from HER2pos and Tneg tumors was largely confined to the first 5 years after diagnosis, whereas the risk for women with HRpos tumors continued for 20 years. Kaplan–Meier curves for the HRpos subtype crossed those of HER2pos and Tneg curves at 15 years for the entire population, with fewer than 60% of HRpos cases remaining alive by 20 years (Fig. 1a). The node-negative patients, regardless of subtype, had similar trajectory in the first 5 years. After 5 years, the outcome is significantly better for the Tneg and HER2pos subsets (85%) than for the HRpos subtype, whose risk persists for 20 years with 50% survival. In node-positive women, the HER2pos and Tneg subtypes have worse outcomes early, but over time, HRpos patients fare just as badly.
Fig. 1.
Kaplan–Meier plots for Guy’s Hospital dataset. Kaplan–Meier analyses of recurrence-free survival were developed for 346 Guy’s Hospital patients based on stage (tumor size and nodal status), ER, PR, and HER2 status, age, and grade. Plots for the entire dataset (a), node-negative patients only (b), and node-positive patients only (c) are shown. In each figure, curves are plotted for a trichotomy of classic tumor subtypes: Tneg, HER2pos, and HRpos/HER2neg. For all molecular subsets, Kaplan–Meier plots show an asymptote, suggesting a mixed population of patients with many cured by surgery alone. There were only 20 IHC 2+ cases, and using DAKO A0485, the likelihood of FISH positivity with 2+ IHC staining is 20% [39], thus very few cases (<4) would likely be reclassified
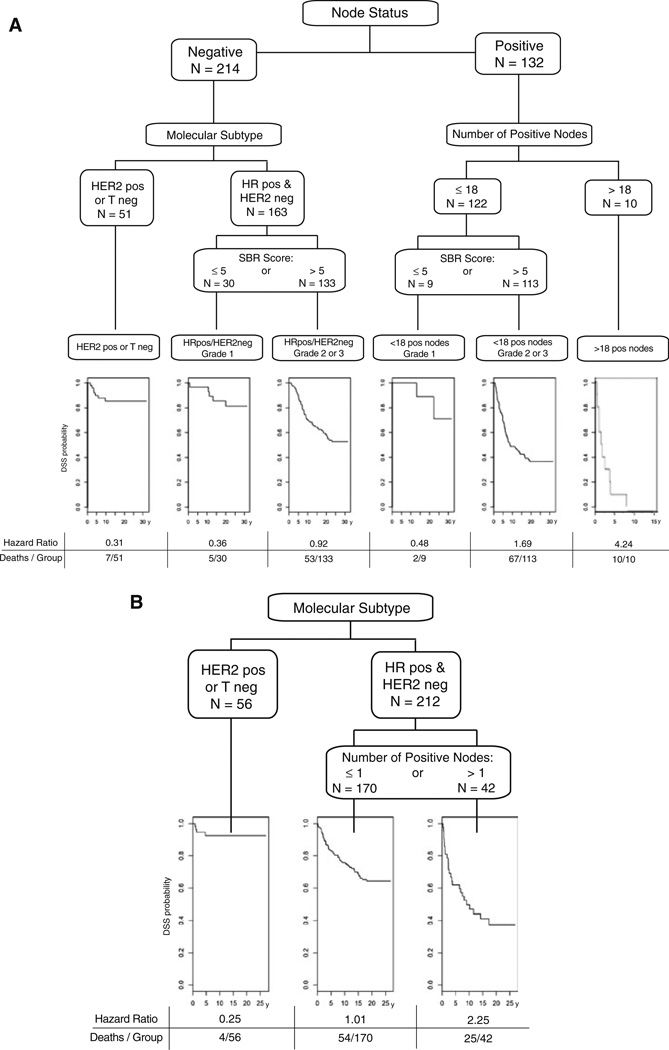
Recursive partitioning analysis of the 346 Guy’s cases at time of diagnosis shows that, using DSS as the outcome, the first and most significant split is node status (Fig. 2a). For the node-negative subgroup, the most important discriminator was HRpos versus Tneg and HER2pos. For the node-negative, HRpos subgroup, SBR ≤ 5 versus not was the discriminating cut point (Fig. 2a). The risk partitioning software itself determined the optimal cut point based on SBR-score, which aligns with the accepted cut-point for low grade. Note that in the node-negative, HRpos, low grade subgroup, almost all of the deaths occurred after 10 years. Tumor size did not serve as a discriminator in determining the risk of recurrence for either node negative or node positive cases.
Fig. 2.
a Results from rpart analysis of Guy’s Hospital dataset for DMFS at diagnosis. Recursive partitioning of patients into subgroups by the R program rpart is shown. The rectangular labels show the factors that drive the splitting of the population in the order of impact. The first split is on node status. For node-negative cases the next split is based on molecular subtype: HER2pos or Tneg versus HRpos/HER2neg. The HRpos/HER2neg are then split by SBR grade ≤5 or >5. For node-positive cases, the first split is for the number of positive nodes (≤18 vs. >18) and for those with ≤18 positive nodes, they are split by SBR Grade ≤5 or >5. The hazard ratio gives the relative risk of dying from breast cancer for that arm of the tree compared to the whole population. For example, the left-uppermost number is 0.31. This means that patients who are node-negative who are HER pos or Tneg are dying from breast cancer at a rate that is 0.31 that of patients in the whole population. The bottom sets of numbers are the number of patients who died from breast cancer within each subgroup and the total number in that subgroup. For the HER2pos or Tneg subgroup, for example, 7 out of 51 died from breast cancer. The DSS curves shown below the final subgroups reflect the timing of the deaths. b Survival time among 5 year survivors. Recursive partitioning of patients who survived 5 years without death by breast cancer by the R program rpart is shown. Trigroup is now the biggest predictor of DSS. In the HRpos group, number of positive nodes (≤1 or >1) determined the splitting pattern. The corresponding Kaplan–Meier curves and hazard ratios are shown below. Note that time = 0 years on the DSS curves is 5 years post-diagnosis
Applying risk partitioning to patients still alive without a cancer event 5 years after diagnosis (Fig. 2b), the dominant factor that separates patients is the trigroup status. HER2-pos or Tneg patients have only a small residual risk, whereas those who are HRpos are split based on having less than 2 positive nodes or not, but for both groups the risk of death continues for an additional 10–15 years.
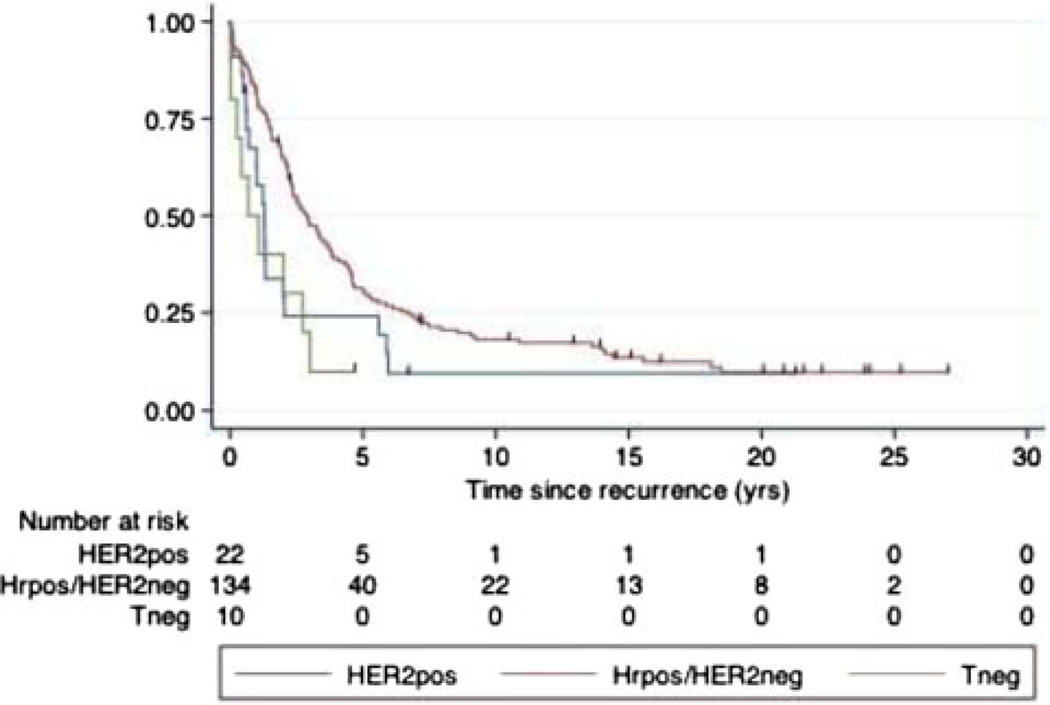
The Guys data are remarkable for having recurrence dates prior to death for 142 of 144 pts that died of breast cancer. Only 2 pts had recurrence noted at time of death. Trigroup status also predicts survival following recurrence. Median survival following a recurrence is 2.9 years (95%CI 2.3–3.7) for HRpos versus 1.2 years (95% CI 0.6–2.0) for Tneg and HER2pos cases (Fig. 3).
Fig. 3.
DSS following recurrence. 142 of the 144 patients who died of breast cancer in the Guy’s dataset have dates of recurrence. The survival following the date of recurrence is shown. For HRpos/HER2neg median survival following a recurrence is 2.9 years (95% CI 2.3–3.7) while for Tneg or HER2pos it is 1.2 years (95% CI 0.6–2.0), P = 0.003. Nearly everyone who recurred eventually died of their disease. The last disease-specific death for HER2 pos, Tneg and HR pos is at 5.9 years, 3 years, and 18.5 years, respectively
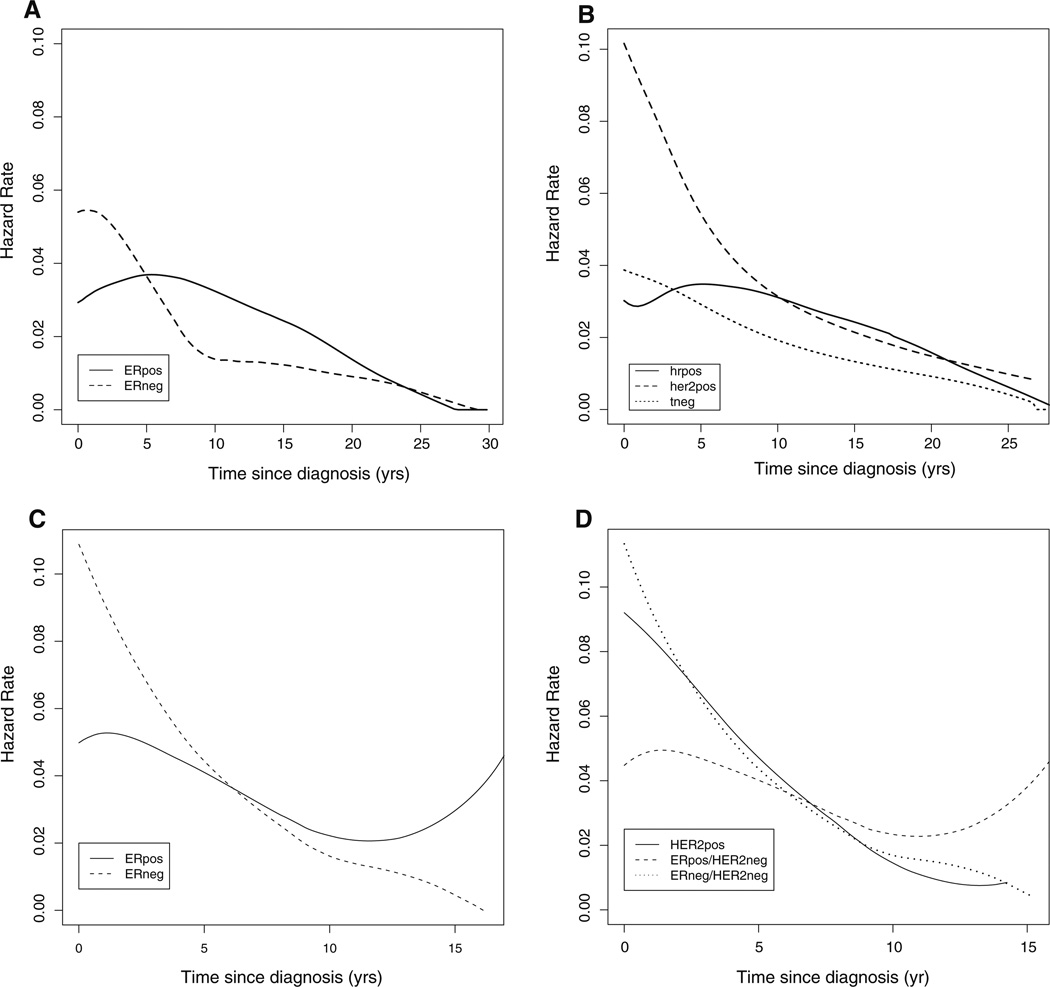
The hazard function for breast cancer specific death for the Guy’s patients is shown by HR status (Fig. 4a) and trigroup status (Fig. 4b). Timing of death from breast cancer was dominated by receptor status. The hazard rates drop substantially after 5 years for HR negative (Fig. 4a) and for Tneg and HER2pos (Fig. 4b) whereas for the HR positive (Fig. 4b) or HRpos (Fig. 4b), the hazards increase at 5 years and remain higher until 20 years.
Fig. 4.
Hazard plots for disease-specific survival by receptor status classification from Guy’s Hospital. a Hazard functions based on HR positivity in the Guy’s dataset are shown. b Hazard functions for HER2pos, HRpos/HER2neg, and Tneg (“trigroup”) patients in the Guy’s dataset are shown. Hazard for the HRpos/HER2neg subgroup declines after peaking at approximately 6 years after diagnosis in contrast to Tneg and HER2pos patients, where hazard declines in the first 5–7 years after diagnosis
The risk partitioning data strongly suggest that the HRpos subtypes with low grade disease or low proliferation are at risk for late recurrence. We therefore wanted to test the ability of expression signatures that predict poor prognosis either on the basis of proliferation, or in the case of the HR negative subtype, immune response (IR), to determine if their prognostic ability was confined to early (within 5 years) or late (after 5 years) death. In order to test this hypothesis, we assembled an independent set of 683 adjuvant naïve, node-negative cases from 4 public databases as described in methods and in Supplemental Table 1. Hazard rates are shown in supplemental Fig. 1.
Four prognostic signatures, relating to two biological processes (proliferation and immune function [26]), were assessed for their prognostic prediction of early or late metastatic events. Cox analysis on the cause-specific hazard, distant metastasis, was used as we were trying to determine whether or not a covariate reflects a “pure” effect regardless of other types of events [27].
Table 2 shows that the proliferative and MS-14 signatures were highly significant in predicting DMFS in the early period (first 5 years) in HRpos/HER2neg tumors, whereas the HRneg/Tneg signature was predictive in the early period for HRneg tumors irrespective of HER2 status. In contrast, the IR signature appears to be significant for predicting events in the first 5 years only in the HRneg/HER2pos cases. Only DMFS was available but is highly predictive of death from breast cancer (Fig. 3). Interestingly, the HRneg/Tneg signature appears to have some predictive ability in HRpos/HER2neg cases; and in particular, a unit increase in HRneg/Tneg signature score is associated with a 2.35-fold higher hazard for late relapses (after 5 years). None of the other signatures evaluated shows significant prognostic value in the late period.
Table 2.
In silico analysis of time dependence of four molecular signatures by marker class
| Signatures | Tumor marker classes | All cases | ||||
|---|---|---|---|---|---|---|
| HR positive | HR negative | |||||
| HER2neg | HER2pos | HER2pos | HER2neg | |||
| Numbers at risk | Year 0 (at diagnosis) | 395 | 52 | 58 | 178 | 683 |
| Year 5 (no prior event) | 286 | 37 | 37 | 118 | 478 | |
| Proliferative* | Prognostic year 0 | <0.0001 | ns | ns | ns | <0.0001 |
| Prognostic year 5+ | ns | ns | ns | ns | ns | |
| HRpos UCSF/Celera | Prognostic year 0 | <0.0001 | ns | ns | ns | <0.0001 |
| Prognostic yr 5+ | ns | 0.154 | ns | ns | ns | |
| HRneg# | Prognostic yr 0 | ns | 0.21 | 0.0008 | 0.0004 | <0.0001 |
| Prognostic year 5+ | 0.013 | ns | ns | ns | 0.03 | |
| Immune# | Prognostic yr 0–5 | ns | ns | 0.07 | ns | ns |
| Prognostic year 5+ | ns | ns | ns | ns | 0.02 |
Discussion
The breast cancer database from Guy’s Hospital in London is unique in enabling the long term assessment of natural history of breast cancer after surgical treatment only. The availability of tissue blocks enabled the uniform reassessment of variables that are considered critical to management today, including grade, and receptor status. Understanding the baseline in the absence of systemic treatment can help to clarify the benefits of adjuvant therapy, and generate hypotheses for timing and appropriate application and refinement of prognostic tools, especially for the low risk subsets.
From the Kaplan–Meier analyses, we observed that, absent systemic therapy, risk for HRpos patients continues for 20 years, whereas the risk for both Tneg and HER2pos patients approaches an asymptote closer to 5 years. This is consistent with other studies that show that HER2pos and Tneg breast cancers recur earlier. Cheang et al. recently compared outcomes of basal tumors and Tneg tumors [28], and show that risk of recurrence rapidly falls off after 5 years and plateaus by 7 years [29]. Survival curves from adjuvant studies in HER2pos patients plateau after 5 years [30] as do those with Tneg tumors [31]. The lack of late risk appears not to be a result of adjuvant treatment, but instead of underlying biology. This information about the time that a woman remains at risk for breast cancer recurrence is critical to patients and can be communicated to women at the time of diagnosis.
The Kaplan–Meier plots show an asymptote well above zero, suggesting a mixed population where many patients are likely to be cured by surgery alone. Recursive partitioning allowed us to look at the conditionally dependent nature of biologic variables and their impact on the shape of the survival curves. Traditional multivariate models assume independence of variables, which may not be a true reflection of breast cancer biology. Risk partitioning enables evaluation of the order with which standard variables impact DSS. The dominant characteristic predicting for DSS is nodal status, followed by classical IHC subtype. For the HRpos tumors only, the dominant characteristic was low grade (SBR score ≤ 5). For the women who have intermediate or high grade, N0, HRpos tumors, risk extends up to 20 years. In the absence of hormonal therapy, the risk of death from breast cancer approaches 50%, a risk much higher than that of the node-negative, HRneg and HER2pos patients. Interestingly, tumor size did not emerge as a predictive factor.
For node-positive cases, grade is more deterministic than molecular subtype, likely reflecting that low grade cancers are almost exclusively HRpos. Again, the risk partitioning model did not select tumor size as a predictor variable for recurrence risk. This is consistent with the Oncotype DX [9] and Mammaprint [2] diagnostic molecular tests, both of which are predictive independent of tumor size. The risk partitioned subsets reflect not only the degree of risk, but also the timing of when that risk occurs. For example, low grade subsets (node-negative or positive), have little or no risk in the first 10 years. Of course, these data do not inform us about whether such late recurrences are controlled by hormonal therapy given at the time of diagnosis.
After 5 years, risk of recurrence is largely confined to the HRpos patients, which is consistent with previous long-term studies [32]. This group faced significant risk of recurrence and death in the absence of systemic therapy. Importantly, the percent that recur in the first 5 years is less than the percent that recur after 5 years. It has been shown that women treated with Tamoxifen continue to recur late, and in randomized trials much of the benefit of adjuvant hormonal therapy is gone after 15 years [33]. However, it is clear from the Guy’s data set, that for some women the risk of recurrence may span the entire 20 year period and for some the risk may be only confined to the later periods, from 10 years after diagnosis to perhaps 20 years. Extended hormone therapy has been shown to reduce the risk of late recurrence [34, 35], but the decision support tools we use today make the assumption that the same relative benefit applies equally to all women with HRpos breast cancer [36]. The RPM analysis suggests this may not be the case. One of the groups at risk may be a subset of patients with small low grade cancers, which is perhaps not the subset where we would expect benefit. It is also possible that some patients might be better served by treatment starting after 5 years, if we could identify those at risk for late recurrence only.
In addition to influencing clinical practice, recursive partitioning models (RPM) applied to datasets with long term follow-up can also provide clinically relevant guidance on how to tailor and develop molecular signatures for prediction and prognosis. To illustrate the potential impact on how to apply signatures to specific breast cancer subsets, we evaluated gene signatures on microarray datasets from a large pooled cohort of node-negative adjuvant naive patients. We show that the proliferative molecular signatures do not predict risk of recurrence 5 or more years after diagnosis. The prognostic value of the immune function-related signatures was also restricted to the first 5 years in HRneg cases. What appears to be needed is the development of signatures capable of predicting metastatic recurrence beyond 5 years, especially for women with HRpos disease. Surprisingly, there may be some late predictive ability of the immune function-related HRneg signatures in HRpos women, but this will need to be validated.
Timing of death is impacted by receptor status, and if HRpos, by grade or proliferative status. For patients that recur, trigroup status also determines the timing of progression to death. The lesson for clinicians and molecular profilers is that there are 2 critical aspects to risk—the magnitude and the timing of risk. Clinicians and drug developers have always focused on what mitigates risk rather than when treatments should be given. We have traditionally thought that treatments must start within 3 months of initial surgical resection to be successful. If clinicians could better predict the timing of metastatic recurrence, such predictions would alter treatment scheduling, duration and post-treatment follow-up. This analysis suggests that, for low grade HRpos patients, there may be a better way to optimize outcomes by using risk profiling to additionally dictate the timing of treatment. Our findings open the door to more creative ways to think about timing, duration, and schedule for HRpos patients, in particular.
An important unmet need in breast cancer management is the prediction of late metastatic risk in HRpos patients. Understanding the time-dependence of risk will allow better ways to develop and validate biomarkers and signatures as well as the tailoring of adjuvant therapy options. For HRneg and HER2pos disease, signatures may be validated with populations that have 5 years of follow-up, since the risk of recurrence drops off sharply by 5 years after diagnosis. For HRpos disease, though, there appears to be two distinct types of signatures needed to offer insight about the benefit of extended adjuvant hormonal therapy: one for early metastatic relapse, and one for late metastatic relapse. The need for an early metastatic relapse signature in HRpos disease is already met by the development of the NKI 70-gene test (Mammaprint, Agendia), 21-gene Recurrence Score (Oncotype DX, Genomic Health), the Celera 14-gene Metastasis Score, the Genomic Grade Index and other proliferation-based signatures. All of these are correlated with the proliferative signature shown in Table 2, which predicts for early but not late recurrence [5]. The 70-gene prognosis signature, for example, has been shown to be highly predictive of recurrence in the first 5 years, but less so after 5 years [37].
The mechanisms for late metastatic relapse in HRpos tumors are not well understood, and it is not clear whether these are intrinsic to the biology of malignant cells in the tumor, host-driven, or a combination of both. Gene signatures that predict early and late recurrence should be able to provide clues to understanding the biology of and to prevent these events.
Limitations to this analysis include the lack of ethnically diverse and relatively small fraction of Tneg and HER2pos breast cancers and small fraction (18%) of HRpos node-negative low grade cases. HER2 status was designated by IHC staining only. There was not sufficient tissue for FISH interrogation of the IHC 2+ cases [38], though few cases (<4) would likely be reclassified. Despite these limitations, our results are in agreement with previous observations concerning proliferative molecular signatures; additionally, they provide the provocative implication that late recurrences in HRpos breast cancers may be linked to altered immune function.
Conclusion
Breast cancer patients demonstrate variability for both risk and timing for developing metastatic disease. To some extent, time dependence can be predicted based on routinely assessed biologic features. These findings should fuel our appetite for evaluating and developing molecular signatures tailored to specific subtypes, and for the timing of metastatic recurrence. Predicting late recurrence in HRpos patients is an unmet need. Improving our ability to identify these groups at the time of diagnosis will improve our ability to plan the short and long-term treatment plans and better evaluate the impact of therapeutic interventions.
Biologic features assessed standardly at diagnosis inform not only risk for but timing of metastatic events. Current prognostic signatures largely predict only early and to improve should focus on evaluation within subsets. In particular predictors of late risk for HR positive disease are needed.
Supplementary Material
Table
Acknowledgments
This work was supported by the Early Detection Research Network (EDRN) initiative of the National Cancer Institute (5 UO1 CA111234); and from the Department of Health via the National Institute for Health Research (NIHR) Comprehensive Biomedical Research Centre award to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust.
Footnotes
Presented in part at the 46th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 4–8, 2010.
Electronic supplementary material The online version of this article (doi:10.1007/s10549-011-1564-5) contains supplementary material, which is available to authorized users.
Conflicts of interest None of the authors declared any conflict of interest.
Contributor Information
Laura J. Esserman, Email: laura.esserman@ucsfmedctr.org, Department of Surgery and Radiology, University of California, San Francisco, CA, USA; Carol Franc Buck Breast Care Center, University of California San Francisco, 1600 Divisadero St, Box 1710, San Francisco, CA 94115, USA.
Dan H. Moore, Department of Biostatistics, University of California, San Francisco, CA, USA
Pamela J. Tsing, Department of Surgery and Radiology, University of California, San Francisco, CA, USA
Philip W. Chu, Department of Surgery and Radiology, University of California, San Francisco, CA, USA
Christina Yau, Buck Institute for Aging, Novato, CA, USA.
Elissa Ozanne, Department of Radiology and Outcomes, Massachusetts General Hospital, Boston, MA, USA.
Robert E. Chung, Department of Demography, University of California, Berkeley, CA, USA
Vickram J. Tandon, Department of Surgery and Radiology, University of California, San Francisco, CA, USA
John W. Park, Department of Medicine, University of California, San Francisco, CA, USA
Frederick L. Baehner, Department of Pathology and Laboratory Medicine, University of California, San Francisco, CA, USA
Stig Kreps, Department of Surgery and Radiology, University of California, San Francisco, CA, USA.
Andrew N. J. Tutt, Breakthrough Breast Cancer Research Unit, Guy’s Hospital, King’s Health Partners AHSC, London, UK
Cheryl E. Gillett, Breast Tissue & Data Bank, Research Oncology, King’s College London, Guy’s Hospital, London, UK
Christopher C. Benz, Buck Institute for Aging, Novato, CA, USA
References
- 1.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001 Sep 11;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van‘t Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002 Jan 31;415(6871):530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 3.Hu Z, Fan C, Oh DS, et al. The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genomics. 2006;7:96. doi: 10.1186/1471-2164-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross JS, Hatzis C, Symmans WF, Pusztai L, Hortobagyi GN. Commercialized multigene predictors of clinical outcome for breast cancer. Oncologist. 2008;13(5):477–493. doi: 10.1634/theoncologist.2007-0248. [DOI] [PubMed] [Google Scholar]
- 5.Pusztai L. Gene expression profiling of breast cancer. Breast Cancer Res. 2009;11(Suppl 3):S11. doi: 10.1186/bcr2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tutt A, Wang A, Rowland C, et al. Risk estimation of distant metastasis in node-negative, estrogen receptor-positive breast cancer patients using an RT-PCR based prognostic expression signature. BMC Cancer. 2008;8:339. doi: 10.1186/1471-2407-8-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Klijn JG, Zhang Y, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005 Feb 19–25;365(9460):671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- 8.Chang HY, Nuyten DS, Sneddon JB, et al. Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proc Natl Acad Sci USA. 2005 Mar 8;102(10):3738–3743. doi: 10.1073/pnas.0409462102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004 Dec 30;351(27):2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 10.Miller LD, Smeds J, George J, et al. An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc Natl Acad Sci USA. 2005 Sep 20;102(38):13550–13555. doi: 10.1073/pnas.0506230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sotiriou C, Wirapati P, Loi S, et al. Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst. 2006 Feb 15;98(4):262–272. doi: 10.1093/jnci/djj052. [DOI] [PubMed] [Google Scholar]
- 12.Wirapati P, Sotiriou C, Kunkel S, et al. Meta-analysis of gene expression profiles in breast cancer: toward a unified understanding of breast cancer subtyping and prognosis signatures. Breast Cancer Res. 2008;10(4):R65. doi: 10.1186/bcr2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desmedt C, Haibe-Kains B, Wirapati P, et al. Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin Cancer Res. 2008 Aug 15;14(16):5158–5165. doi: 10.1158/1078-0432.CCR-07-4756. [DOI] [PubMed] [Google Scholar]
- 14.Lobb EA, Butow PN, Kenny DT, Tattersall MH. Communicating prognosis in early breast cancer: do women understand the language used? Med J Aust. 1999 Sep 20;171(6):290–294. [PubMed] [Google Scholar]
- 15.Lobb EA, Kenny DT, Butow PN, Tattersall MH. Women’s preferences for discussion of prognosis in early breast cancer. Health Expect. 2001;4(1):48–57. doi: 10.1046/j.1369-6513.2001.00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pichon MF, Broet P, Magdelenat H, et al. Prognostic value of steroid receptors after long-term follow-up of 2257 operable breast cancers. Br J Cancer. 1996;73(12):1545–1551. doi: 10.1038/bjc.1996.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17(5):1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 18.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 19.Atkinson EJ, Therneau TM. An introduction to recursive partitioning using the RPART routines. 2000 [Google Scholar]
- 20.Breiman L, Friedman J, Olshen R, Stone C. Classification and regression trees. Belmont, CA: Wadsworth International Group; 1984. [Google Scholar]
- 21.Minn AJ, Gupta GP, Siegel PM, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005 Jul 28;436(7050):518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desmedt C, Piette F, Loi S, et al. Strong time dependence of the 76-gene prognostic signature for node-negative breast cancer patients in the TRANSBIG multicenter independent validation series. Clin Cancer Res. 2007 Jun 1;13(11):3207–3214. doi: 10.1158/1078-0432.CCR-06-2765. [DOI] [PubMed] [Google Scholar]
- 23.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000 Aug 17;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 24.Teschendorff AE, Caldas C. A robust classifier of high predictive value to identify good prognosis patients in ER-negative breast cancer. Breast Cancer Res. 2008;10(4):R73. doi: 10.1186/bcr2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yau C, Esserman L, Moore DH, Waldman F, Sninsky J, Benz CC. A multigene predictor of metastatic outcome in early stage hormone receptor-negative and triple-negative breast cancer. Breast Cancer Res. 2010 Oct 14;12(5):R85. doi: 10.1186/bcr2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reyal F, van Vliet MH, Armstrong NJ, et al. A comprehensive analysis of prognostic signatures reveals the high predictive capacity of the proliferation, immune response and RNA splicing modules in breast cancer. Breast Cancer Res. 2008;10(6):R93. doi: 10.1186/bcr2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pintilie M. Analysing and interpreting competing risk data. Stat Med. 2007 Mar 15;26(6):1360–1367. doi: 10.1002/sim.2655. [DOI] [PubMed] [Google Scholar]
- 28.Cheang MC, Voduc D, Bajdik C, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008 Mar 1;14(5):1368–1376. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 29.Prat A, Parker JS, Karginova O, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010 Sep 2;12(5):R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hobday TJ, Perez EA. Molecularly targeted therapies for breast cancer. Cancer Control. 2005;12(2):73–81. doi: 10.1177/107327480501200202. [DOI] [PubMed] [Google Scholar]
- 31.O’Shaughnessy J, Osborne C, Pippen JE, et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med. 2011 Jan 20;364(3):205–214. doi: 10.1056/NEJMoa1011418. [DOI] [PubMed] [Google Scholar]
- 32.Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14(10):2738–2746. doi: 10.1200/JCO.1996.14.10.2738. [DOI] [PubMed] [Google Scholar]
- 33.EBCTCG. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005 May 14–20;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 34.Goss PE. Letrozole in the extended adjuvant setting: MA.17. Breast Cancer Res Treat. 2007;105(Suppl 1):45–53. doi: 10.1007/s10549-007-9698-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003 Nov 6;349(19):1793–1802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 36.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131(1):18–43. doi: 10.5858/2007-131-18-ASOCCO. [DOI] [PubMed] [Google Scholar]
- 37.Buyse M, Loi S, van’t Veer L, et al. Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J Natl Cancer Inst. 2006 Sep 6;98(17):1183–1192. doi: 10.1093/jnci/djj329. [DOI] [PubMed] [Google Scholar]
- 38.Olivotto IA, Bajdik CD, Ravdin PM, et al. Population-based validation of the prognostic model ADJUVANT! for early breast cancer. J Clin Oncol. 2005 Apr 20;23(12):2716–2725. doi: 10.1200/JCO.2005.06.178. [DOI] [PubMed] [Google Scholar]
- 39.Mrozkowiak A, Olszewski WP, Piascik A, Olszewski WT. HER2 status in breast cancer determined by IHC and FISH: comparison of the results. Pol J Pathol. 2004;55(4):165–171. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table