Hyperlipidemia in Early Adulthood Increases Long-Term Risk of Coronary Heart Disease (original) (raw)
. Author manuscript; available in PMC: 2016 Feb 3.
Abstract
Background
Many young adults with moderate hyperlipidemia do not meet statin treatment criteria under the new AHA/ACC cholesterol guidelines as they focus on 10-year cardiovascular risk. We evaluated the association between years of exposure to hypercholesterolemia in early adulthood and future coronary heart disease (CHD) risk.
Methods and Results
We examined Framingham Offspring Cohort data to identify adults without incident cardiovascular disease to age 55 (n=1478), and explored the association between moderate hyperlipidemia (non-high-density lipoprotein cholesterol [HDL-C] ≥160 mg/dL) duration in early adulthood and subsequent CHD. At median 15-year follow-up, CHD rates were significantly elevated among adults with prolonged hyperlipidemia exposure by age 55: 4.4% for those with no exposure, 8.1% for 1–10 years, and 16.5% for those with 11–20 years exposure (p<0.001); this association persisted after adjustment for other cardiac risk factors including non-HDL-C at age 55 (HR 1.39, 95% CI 1.05–1.85 per decade of hyperlipidemia). Overall, 85% of young adults with prolonged hyperlipidemia would not have been recommended for statin therapy at age 40, under current national guidelines. However, among those not considered statin therapy candidates at age 55, there remained a significant association between cumulative exposure to hyperlipidemia in young adulthood and subsequent CHD risk (adjusted HR 1.67, 95% CI 1.06–2.64).
Conclusions
Cumulative exposure to hyperlipidemia in young adulthood increases subsequent risk of CHD in a dose-dependent fashion. Adults with prolonged exposure to even moderate elevations in non-HDL-C have elevated risk for future CHD and may benefit from more aggressive primary prevention.
Keywords: hyperlipidemia, younger patients, coronary heart disease risk
Hyperlipidemia is a potent risk factor for atherosclerosis and coronary heart disease (CHD) and is present in a substantial proportion of young adults. According to data from the National Health and Nutrition Examination Survey (NHANES), between 11.7% of adults aged 20–39 and 41.2% of adults aged 40–64 had elevated low-density lipoprotein cholesterol (LDL-C) levels, but only 10.6% of adults aged 20–39 and 47.7% of adults age 40–64 with hyperlipidemia were on treatment.1 The newly released American Heart Association (AHA)/American College of Cardiology (ACC) guidelines for treatment of blood cholesterol for the prevention of cardiovascular disease (CVD) recommend statin therapy for all adults with prevalent CVD, LDL-C ≥190 mg/dl, diabetes, or 10-year risk of atherosclerotic CVD ≥7.5%, as assessed by the new Pooled Cohort Equations.2 Although CHD events such as myocardial infarction present suddenly, the advanced extensive complex intramural lesions that lead to plaque rupture develop over decades. Since the natural history of atherosclerosis is prolonged, the risk of clinical events rises exponentially late in life. As a result, the new cholesterol guidelines led to a high number of older adults aged ≥60 years to be recommended for statin therapy, with relatively fewer younger adults meeting statin recommendation tresholds.1
Studies on adults with familial hypercholesterolemia have shown CVD risk is increased early among those with very high LDL-C levels.3 Similarly, adults with extremely low LDL-C levels conferred by genetic polymorphisms have significantly lower than average risk of CVD.4,5 However, the association between prolonged exposure to mild to moderately elevated lipid levels in young adulthood on an individual’s subsequent risk of CHD has not previously been well described.6 Therefore, we used the Framingham Offspring Study to address the impact of duration of hyperlipidemia in young adulthood (ages 35 to 55) and future risk of CHD beyond age 55 years.
Methods
Study Design and Sample
Our study examined data on 5124 individuals from the Offspring Cohort of the Framingham Heart Study recruited between 1971 and 1975.7 In order to identify participants with sufficient observation time to evaluate both the number of years of exposure to hyperlipidemia, as well as the person’s future risk of CHD, participants were eligible for inclusion in this analysis if: 1) they had attended Offspring Cohort examination 4 (1987–1991), 5 (1991–1995), or 6 (1995–1998); 2) were between the ages of 53 and 57 years; and 3) were free of CVD (defined as myocardial infarction, angina, coronary insufficiency, transient ischemic attack, stroke, coronary heart disease death, cardiovascular death, intermittent claudication, or heart failure8) at the time of eligibility assessment. Of exams 4, 5, and 6, the exam closest to age 55 was used as the “baseline” visit. Data from prior exams were used to evaluate the number of years of hyperlipidemia attained by the baseline age. This resulted in a sample of 1478 adults free of CVD at the baseline examination, who were approximately 55 years of age. Participants were then prospectively followed for up to 20 years for the development of CHD (myocardial infarction, angina, coronary insufficiency, coronary heart disease death) events. Median follow-up was 15 years.
Outcomes and Exposures
The primary factor of interest was the number of years of exposure to hyperlipidemia in the 20 years prior to the baseline visit at age 55 (e.g., number of years of elevated non-high-density lipoprotein cholesterol [HDL-C] between ages 35 and 55). Consistent with the lipid measures used in the newest Pooled Cohort Equations, hyperlipidemia in our primary analysis was defined based on non-HDL-C, with levels ≥160 mg/dL considered elevated. This level is equivalent to the 70th percentile of the American population according to NHANES. Since Framingham Offspring examinations occur approximately every 4 years, we interpolated hyperlipidemic status in the years between the examinations. For individuals who developed hyperlipidemia in the time interval between study visits, we assumed that the date of development was midway between the two exams. For individuals with fewer than 20 years of data prior to baseline, we conservatively assumed that the participant was free of hyperlipidemia for the time period without data. For participants with missing data at any follow-up exam, the value from the prior exam was carried forward.
In sensitivity analyses we also examined prior elevation of non-HDL-C as a continuous variable. Each person’s average non-HDL-C over the preceding 20 years was calculated, weighted by the number of years between exams. In addition, LDL-C, rather than non-HDL-C was evaluated using number of years with LDL-C ≥130 mg/dL. Because the Friedewald equation was used to calculate LDL-C, adults with triglycerides over 400 mg/dL at any time were excluded from the LDL-C analysis.
Statistical Analysis
First, using the number of years of hyperlipidemia by age 55, adults were stratified into three groups: 1) those without hyperlipidemia by age 55; 2) those with 1–10 years of hyperlipidemia; and 3) those with 11–20 years of hyperlipidemia. Kaplan-Meier survival curves were generated to evaluate the risk of CHD over the subsequent years, and the log-rank test was used to test the overall survival experience.
Cox proportional hazards regression models were employed to evaluate the relative risk of increasing the number of years with hyperlipidemia on the onset of CHD events by evaluating the association between number of years of exposure to hyperlipidemia at age 55 as a continuous variable between zero and twenty and future risk of CHD. To determine to what extent the association between duration of hyperlipidemia and CHD risk could be attributed to a worse overall health state associated with hyperlipidemia, multivariable analyses were performed adjusting for the following standard non-lipid risk factors: age, sex, systolic blood pressure (SBP), antihypertensive treatment, HDL cholesterol, diabetes, and smoking. Next, to determine if the cumulative exposure to hyperlipidemia was a marker for prevalent hyperlipidemia at age 55 or if the duration of hyperlipidemia was associated with increased CHD risk independent of lipid levels at that age, baseline (age 55) non-HDL-C level was also included in the multivariable analysis. The final multivariable model also included adjustment for lipid-lowering therapy at baseline and over the follow-up period in a time-dependent fashion. Hazard ratios for the association between duration of hyperlipidemia and future CHD risk are presented per 10-year increase.
As secondary analyses, we repeated our primary analysis of association between years of exposure to non-HDL-C ≥160 mg/dL in our sample of young adults who would not be specifically recommended for statin therapy under the 2013 AHA/ACC guidelines. This excluded adults with 10-year CVD risk ≥7.5%, diabetes with LDL-C ≥70 mg/dL, or LDL-C ≥ 190 mg/dL.
Next, we assessed the proportion of adults who would have been recommended for statin therapy at age 40 and age 50 under current guidelines based on diabetes status, LDL-C, and 10-year CVD risk, stratified by years of exposure to hyperlipidemia (zero, 1–10, and 11–20 years of hyperlipidemia). We considered both the ≥7.5% and the ≥5% risk thresholds to determine treatment eligibility per the new guidelines. This analysis was performed to determine the extent to which individuals with prolonged hyperlipidemia would have been identified as treatment candidates by the new guidelines during the period of exposure to hyperlipidemia. Since LDL-C was estimated using Friedewald’s equation and, therefore, unavailable in adults with triglycerides >400 mg/dL, adults with triglycerides >400 mg/dL were considered “statin eligible” in the analysis that evaluated statin recommendations.
In sensitivity analysis, we investigated the robustness of our results to the choice of lipid parameter and choice of threshold. First, the association between years of exposure to LDL-C ≥ 130 mg/dL and future CHD was evaluated using multivariable Cox proportional hazards modeling. Second, to determine whether the results depend on the 160 mg/dL non-HDL-C threshold, the association between the weighted average non-HDL-C over the prior 20 years and future risk of CHD was evaluated using restricted cubic splines. Inflection points in the graph, rounded to the nearest 5 mg/dL non-HDL-C were identified and then used as cut points in a piecewise linear model of prior average non-HDL-C and future CHD risk. Cox proportional hazards modeling was performed evaluating the impact of each 10 mg/dL increase in average prior elevation of non-HDL-C and future risk of CHD, adjusting for the same characteristics at baseline as in the primary analysis (age, sex, SBP, antihypertensive treatment, diabetes, smoking, non-HDL-C, HDL-C, and lipid therapy). Finally, to evaluate the impact of including adults on lipid-lowering therapy at baseline, the primary analyses of the association between years of elevation in non-HDL-C and future CHD risk were repeated excluding adults on lipid-lowering therapy at baseline.
The analysis was approved by the Duke University Institutional Review Board. All Framingham Offspring cohort participants gave informed consent for participation. Statistical analysis was performed using SAS version 9.3.
Results
Study Population
Characteristics of the study sample at baseline are presented in Table 1. A total of 124 individuals in the cohort developed CVD prior to age 55 and were not included in our sample. The final sample included 1478 adults free of CVD at baseline with 0–20 years of hyperlipidemia. Of these, 512 adults did not have hyperlipidemia, 389 adults had 1–10 years of hyperlipidemia, and 577 adults had 11–20 years of hyperlipidemia exposure by baseline age. Individuals with hyperlipidemia at baseline were more likely to be diabetic, male, and smokers, and had higher SBP, body mass index, total cholesterol levels, and lower HDL-C levels than those without hyperlipidemia. Only 85 patients overall (5.8%) were on lipid-lowering treatment at the baseline visit.
Table 1.
Characteristics of Participants Stratified by Years of Hyperlipidemia at Baseline*
| Duration of Hyperlipidemia at Baseline | ||||
|---|---|---|---|---|
| Variable | Total (n=1478) | 0 Years (n=512) | 1–10 Years (n=389) | 11–20 Years (n=577) |
| Age | 55 (54,56) | 55 (54,56) | 55.0 (54,56) | 56.0 (54,56) |
| Female | 791 (53.5%) | 336 (65.6%) | 241 (62.0%) | 214 (37.1%) |
| Smoking | 281 (19.0%) | 89 (17.4%) | 64 (16.5%) | 128 (22.2%) |
| Diabetes | 95 (6.4%) | 19 (3.7%) | 23 (5.9%) | 53 (9.2%) |
| BMI | 27.0 (24.2,30.3) | 25.4 (23.0,28.6) | 26.9 (24.5,30.1) | 28.3 (25.4,31.0) |
| Treatment for cholesterol | 85 (5.8%) | 3 (0.6%) | 10 (2.6%) | 72 (12.5%) |
| Treatment for BP | 282 (19.1%) | 59 (11.5%) | 73 (18.8%) | 150 (26.0%) |
| Systolic BP | 127 (115,137) | 123 (112,134) | 126 (115,136) | 130 (119.0,140.0) |
| Total cholesterol | 209 (184,233) | 182 (166,199) | 215 (199,236) | 230 (207,253) |
| HDL-C cholesterol | 48 (40,60) | 57 (46,70) | 49 (39,60) | 43 (37,51) |
| Non-HDL-C | 158 (132,184) | 125 (109,140) | 165 (148,181) | 183 (163,205) |
Years of Hyperlipidemia and Risk of CHD
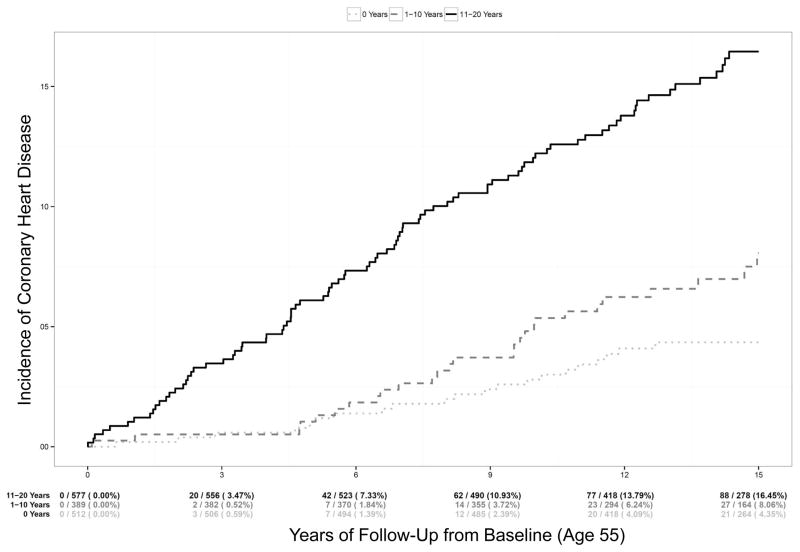
During follow-up, 155 individuals developed new onset CHD, with 136 events by median follow-up of 15 years. Figure 1 presents Kaplan-Meier CHD event rates from baseline up to 15 years of follow-up among patients stratified by the number of years of hyperlipidemia at baseline. A dose-response pattern is seen, with progressively increasing risk of CHD as the number of years of exposure to hyperlipidemia increases (log-rank test p<0.0001). At 15 years, adults with 11–20 years of hyperlipidemia at baseline had an overall CHD risk of 16.5% (95% CI 13.5–19.9%), compared with 8.1% (95% CI 5.5–11.7%) for adults with 1–10 years of hyperlipidemia, and 4.4% (2.9–6.6%) for those without hyperlipidemia at baseline. The unadjusted risk of CHD doubled for every ten years of exposure to hyperlipidemia (Table 2, univariable HR 2.0, 95% CI 1.63–2.45 per decade of hyperlipidemia); this association was attenuated, but remained statistically significant after adjusting for other standard risk factors including sex, age, SBP, antihypertensive therapy, smoking status, HDL-C, and diabetes (adjusted HR 1.49, 95% CI 1.20–1.87 per decade of hyperlipidemia). In addition, the association remained statistically significant after also adjusting for non-HDL-C at baseline (adjusted HR 1.39, 95% CI 1.05–1.85 per decade of hyperlipidemia), suggesting that previous cumulative exposure to hyperlipidemia is associated with increased risk of CHD later in life, independent of the cholesterol level at age 55. This association also remained significant after adjusting for lipid-lowering therapy use at baseline and follow-up.
Figure 1.
Time to Diagnosis of CHD by Number of Years of Hyperlipidemia at Baseline. This figure shows Kaplan-Meier curves of future risk of CHD beginning at age 55 (age range 53–57), stratified by years of hyperlipidemia experienced by age 55. Log-rank p-value <0.0001. *CHD indicates coronary heart disease.
Table 2.
Future Risk of CHD Per Decade of Hyperlipidemia Experienced by Age 55 in Univariable and Multivariable Analyses
| Model | All Adults (n=1514) | Not Recommended for Statin Therapy at Baseline* (n=971) | ||
|---|---|---|---|---|
| HR Per Decade of Hyperlipidemia (95% CI) | p-value | HR Per Decade of Hyperlipidemia (95% CI) | p-value | |
| Univariable: duration of hyperlipidemia | 2.00 (1.63–2.45) | <0.0001 | 1.99 (1.44–2.75) | <0.0001 |
| Duration of hyperlipidemia + standard risk factors at baseline† | 1.49 (1.20 – 1.87) | 0.0004 | 1.77 (1.26 – 2.48) | 0.001 |
| Duration of hyperlipidemia + standard risk factors† + non- HDL-C at baseline | 1.39 (1.05 – 1.85) | 0.022 | 1.67 (1.06 – 2.64) | 0.026 |
| Duration of hyperlipidemia + standard risk factors† + non- HDL-C at baseline + lipid lowering therapy at baseline and follow-up | 1.40 (1.05 – 1.87) | 0.024 | 1.60 (1.00 – 2.56) | 0.048 |
Statin Recommendations Under Current Guidelines
The number of participants who would be specifically targeted for statin therapy according to the statin benefit groups as identified by the new guidelines was calculated (Table 3). Of 577 adults with 11–20 years of hyperlipidemia at the index age of 55, 87 (15.1%) participants would have met criteria for statin therapy at age 40, and 201 (34.8%) would have met criteria at age 50. These numbers were lower among those with 1–10 years of hyperlipidemia at age 55: of 389 adults, 7 (1.8%) would have met criteria for statin therapy at age 40 and 44 (11.3%) would have met criteria at age 50. When we used the lower risk threshold proposed by the guidelines (10-year CVD risk ≥5%) to identify adults eligible for statin therapy, 25.1% of adults with 11–20 years of hyperlipidemia at baseline would have been recommended for statin therapy at age 40, and 51.6% would have been recommended for statin therapy at age 50.
Table 3.
Statin Recommendations at Age 40 and 50 for Adults With and Without Hyperlipidemia at Age 55*
| Variable | Recommended for Statin Therapy, ASCVD Risk ≥7.5% | Recommended for Statin Therapy, ASCVD Risk ≥5% | ||
|---|---|---|---|---|
| Age 40 | Age 50 | Age 40 | Age 50 | |
| 0 years (n=512) | 3 (0.6%) | 16 (3.1%) | 6 (1.2%) | 43 (8.4%) |
| 1–10 years (n=389) | 7 (1.8%) | 44 (11.3%) | 11 (2.8%) | 77 (19.8%) |
| 11–20 years (n=577) | 87 (15.1%) | 201 (34.8%) | 145 (25.1%) | 298 (51.6%) |
When we restricted our analyses to those adults not recommended for statin therapy at age 55 (i.e., 10-year CVD risk below 7.5%, LDL-C <190 mg/dL, and no diabetes with LDL-C ≥ 70 mg/dL; n=971), the association between hyperlipidemia and risk of CHD was preserved; adults with both 1–10 and 11–20 years of hyperlipidemia at baseline had significantly higher rates of CHD compared with adults without hyperlipidemia (Figure 2, p<0.001). In multivariable models adjusting for standard risk factors and non-HDL-C at baseline, each decade of hyperlipidemia at baseline was associated with a 67% increased risk of CHD at follow-up (Table 2, HR 1.67, 95% CI 1.06–2.64, p=0.03).
Figure 2.
Time to Diagnosis of CHD by Number of Years of Hyperlipidemia at Baseline Among Adults Not Recommended for Statin Therapy at Baseline*. This figure shows Kaplan-Meier curves of future risk of CHD stratified by years of hyperlipidemia experienced by age 55 (age range 53–57) among adults not recommended for statin therapy at age 55. Log-rank p-value <0.0001. *Excludes those recommended for statins: ASCVD risk ≥7.5%, LDL-C ≥190, diabetes and LDL-C ≥100. +ASCVD indicates atherosclerotic cardiovascular disease; CHD, coronary heart disease; LDL-C, low-density lipoprotein cholesterol.
Sensitivity Analyses
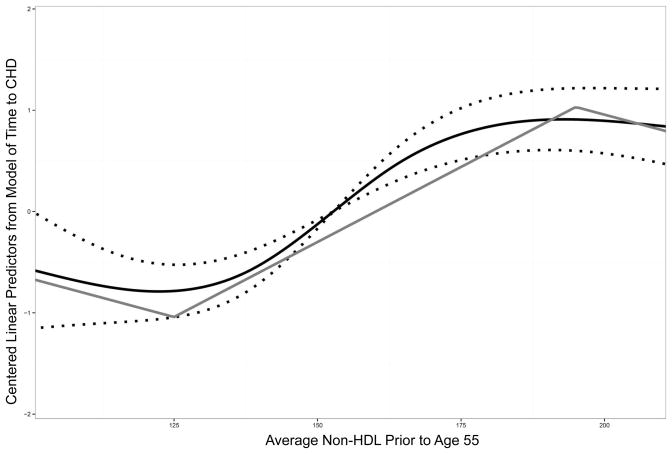
Using an LDL-C level of ≥130 mg/dL rather than non-HDL-C ≥160 mg/dL as the primary exposure yielded similar results (appendix table 1). The results based on average prior non-HDL-C are shown in Figure 3, where they are plotted against future CHD risk approximated using a restricted cubic spline and a piecewise linear model. The effect of weighted non-HDL-C below 125 mg/dl on CHD was non-significant, suggesting that individuals below this cut-point all have similar (lower) risk of CHD. Similarly, the effect of weighted non-HDL-C above 195 mg/dl on CHD was non-significant, suggesting that individuals above this cut-point all have similar (high) risk of CHD. The association between average weighted non-HDL-C between 125–195 mg/dL and CHD was statistically significant. In Cox proportional hazards modeling, every 10 mg/dL increase in average non-HDL-C between 125–195 mg/dL over the preceding 20 years was associated with a 33% increase in future CHD risk (HR per 10 mg/dL increase 1.33, 95% CI 1.23–1.45, p<0.001). After adjusting for standard risk factors including baseline non-HDL-C and lipid therapy at baseline and follow-up, this association remained statistically significant (HR 1.20, 95% CI 1.08–1.35, p=0.001). Finally, excluding adults on lipid-lowering therapy at baseline did not result in substantive changes to the results (appendix table 2).
Figure 3.
Prior Weighted Average Cholesterol and CHD Risk. This figure shows the shapes of the restricted cubic spline and piecewise linear models of average non-HDL cholesterol prior to age 55 and the centered linear predictors from the model of time to coronary heart disease. The black curve shows the restricted cubic spline with 95% confidence intervals (dotted) and grey curve shows the piecewise linear spline with knots at 125 mg/dL and 195 mg/dL. The x-axis is truncated at the 5th and 95th percentiles of prior average non-HDL. *CHD indicates coronary heart disease.
Discussion
In this analysis of adults free of CVD at age 55 in the Framingham Offspring Study, we found that those with the longest prior exposure to moderately elevated non-HDL-C had a nearly fourfold increased rate of CHD at follow-up. Importantly, not only does prevalent hyperlipidemia increase future risk of CHD, but the length of exposure to hyperlipidemia in the fourth and fifth decades of life affects future CHD risk in a dose-responsive manner as the association between exposure to hyperlipidemia in young adulthood and future CHD remained highly significant even after adjustment for non-HDL-C at age 55. This association was preserved in individuals without direct recommendations for statin therapy under the current guidelines. These findings are aligned with the biological understanding of atherosclerosis as a progressive disease due to ongoing vessel injury over time—a substantial part of which is caused by elevated cholesterol levels.6
In addition to the 10-year risk, which was calculated using the Pooled Cohort Equations, the current AHA/ACC cholesterol guideline recommends when making treatment decisions to consider family history, C-reactive protein, coronary artery calcium, ankle brachial index, and lifetime CVD risk. Our data suggests that sustained moderate elevation of lipid levels also confers a substantial risk of future events. Given the potent association between duration of exposure to hyperlipidemia by mid-adulthood and future CHD risk, clinicians should also consider lifestyle intervention or even treatment for adults with prolonged prior exposure to hyperlipidemia. Randomized controlled trial evidence support the clinical benefit of statin therapy for primary prevention in adults in this age group, with a number of primary prevention trials demonstrating that statins initiated in midlife significantly reduce future clinical events.9–11 While the new guidelines identify those patients with a very high lipid level on a single measurement (LDL-C ≥190 mg/dL) as candidates for statin therapy, they do not further differentiate risk in others based on lipoprotein levels. Under current guidelines, only one in six adults in this cohort with prolonged duration of exposure to hyperlipidemia would have been directly recommended for statin therapy at age 40, and one in three at age 50. By design, our analysis cannot answer the question whether early statin intervention in those on the hyperlipidemic trajectory would decrease their future CHD risk. When to initiate treatment in adults with moderately elevated non-HDL-C in early adulthood remains unknown. There are no studies evaluating the long-term effectiveness of statin therapy in adults aged 30–50 with only moderately elevated lipid levels and without other risk factors. Furthermore, initiating statin therapy at younger ages would result in a much longer duration of statin use than has been studied in randomized trials. This lack of knowledge further stresses the need for additional research focused on the safety and efficacy of long-term statin use in early and middle adulthood to reduce cardiovascular disease later in life. This analysis also highlights the fact that risk prediction models focused on a 10-year horizon may underestimate the contribution of prolonged exposure to chronic disease, and the need to continue to evaluate how to best incorporate 30-year or lifetime risk estimates into current prevention guidelines.12, 13
Limitations and Strengths
Our analysis has several limitations. First, we only included adults aged 53–57 who were free of CVD; therefore, the point estimates cannot be extrapolated outside of this age range. Nevertheless, we believe that this analysis demonstrates the long-term impact of hyperlipidemia in young adulthood. Second, our analysis defined hyperlipidemia using a non-HDL-C cutoff of ≥160 mg/dL, which is consistent with how the prior Adult Treatment Panel (ATP) III guidelines defined elevated cholesterol; given that the risk of CHD events expands with increasing levels of non-HDL-C, the use of this or any cut-point may have falsely dichotomized a continuous relationship. However, using a continuous approach which averaged non-HDL-C over the preceding 20 years yielded similar results, showing that the risk associated with exposure to non-HDL-C increased linearly between 125–195 mg/dL. However, the number of individuals with prior average non-HDL-C below 125 mg/dL and above 195 mg/dL is low. Therefore, we cannot definitively determine if the relationship between prior average non-HDL-C and future CHD continues below 125 mg/dL or above 195 mg/dL. Third, this analysis only considered the duration of hyperlipidemia and not the duration of all other risk factors and comorbidities. While the presence of comorbidities was accounted for at age 55, it is likely that the duration of certain comorbidities, such as diabetes and hypertension, may also affect future CHD risk in a similar duration-dependent manner, as demonstrated in this hyperlipidemia analysis.14 Next, our study design excluded 124 individuals with premature CVD before age 55, representing 8% of the initial sample. As a result, some individuals at highest risk, due to hyperlipidemia at a young age, may have been excluded; these exclusions would have led to an underestimation of the association between duration of hyperlipidemia and CHD risk.
Notably, our study also had several strengths. First, our analysis is based on the Framingham Heart Study data collected in an era before widespread statin use, allowing us to evaluate the impact of untreated hyperlipidemia. Not only was the overall rate of lipid-lowering therapy in this group low, but the risk associated with increased duration of hyperlipidemia remained significant even after adjusting for lipid-lowering therapy at baseline and follow-up and excluding adults on lipid lowering therapy at baseline. Second, due to the length of follow-up, the Framingham Offspring data allows for accurate, longer-term assessment of risk factors, as well as follow-up of hard cardiovascular endpoints. Our analysis fully utilizes the consecutive follow-up, as well as risk factor and event ascertainment, during the course of 35 years (20 years of potential exposure and 15 years of follow-up), which is uniquely available in Framingham. Finally, our study design eliminates the possibility that the association seen between duration of hyperlipidemia and risk of coronary events is a result of residual confounding by age, as risk was calculated for all participants starting around age 55.
Conclusions
We conclude that the exposure to hyperlipidemia in the fourth and fifth decades of life is associated with a substantially increased risk of CHD in a dose-responsive fashion, even among adults otherwise predicted to have low risk of CVD. Our findings suggest that adults with longstanding moderate elevations in non-HDL-C should be added to those already identified by the current guidelines as candidates for an informed patient-physician discussion about appropriate lipid management strategies to reduce future risk of heart disease.
Supplementary Material
Clinical Perspective
supplemental material
Acknowledgments
The authors would like to thank Erin Hanley, MS, for her editorial contributions to this manuscript. Ms. Hanley did not receive compensation for her contributions, apart from her employment at the institution where this study was conducted.
Funding Sources: This work was supported internally by the Duke Clinical Research Institute in addition to grant number U19HS021092 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. The Framingham Heart Study is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with Boston University (Contract No. N01-HC-25195). This manuscript has been reviewed by Framingham Heart Study Investigators for Scientific content and consistency of data interpretation with previous Framingham Heart Study publications.
Footnotes
Disclosures: Dr. Navar-Boggan has no relevant conflicts of interest to report. Dr. Peterson has received funding for research grants from Eli Lilly and Janssen Pharmaceuticals, and funding for serving as a consultant/participant on advisory board for Merck, Sanofi-Aventis, Janssen Pharmaceuticals, and Boehringer Ingelheim. Dr. D’Agostino has no relevant conflicts of interest to report. Mr. Neely has no relevant conflicts of interest to report. Dr. Sniderman has no relevant conflicts of interest to report. Dr. Pencina has received funding for serving as a consultant for AbbVie.
References
- 1.Pencina MJ, Navar-Boggan AM, D’Agostino RB, Sr, Williams K, Neely B, Sniderman AD, Peterson ED. Application of new cholesterol guidelines to a population-based sample. N Engl J Med. 2014;370:1422–1431. doi: 10.1056/NEJMoa1315665. [DOI] [PubMed] [Google Scholar]
- 2.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson J, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 3.Hopkins PN, Heiss G, Ellison RC, Province MA, Pankow JS, Eckfeldt JH, Hunt SC. Coronary artery disease risk in familial combined hyperlipidemia and familial hypertriglyceridemia: a case-control comparison from the National Heart, Lung, and Blood Institute Family Heart Study. Circulation. 2003;108:519–523. doi: 10.1161/01.CIR.0000081777.17879.85. [DOI] [PubMed] [Google Scholar]
- 4.Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 5.Ference BA, Yoo W, Alesh I, Mahajan N, Mirowska KK, Mewada A, Kahn J, Afonso L, Williams KA, Sr, Flack JM. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: a Mendelian randomization analysis. J Am Coll Cardiol. 2012;60:2631–2639. doi: 10.1016/j.jacc.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Sniderman AD, Furberg CD. Age as a modifiable risk factor for cardiovascular disease. Lancet. 2008;371:1547–1549. doi: 10.1016/S0140-6736(08)60313-X. [DOI] [PubMed] [Google Scholar]
- 7.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–525. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 8.D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 9.Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AM., Jr Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 10.Barringer TA, 3rd WOSCOPS. West of Scotland Coronary Prevention Group. Lancet. 1997;349:432–433. doi: 10.1016/s0140-6736(05)65059-3. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, Toyota T, Nakaya N, Nishimoto S, Muranaka M, Yamamoto A, Mizuno K, Ohashi Y MEGA Study Group. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368:1155–1163. doi: 10.1016/S0140-6736(06)69472-5. [DOI] [PubMed] [Google Scholar]
- 12.Pencina MJ, D’Agostino RB, Sr, Larson MG, Massaro JM, Vasan RS. Predicting the 30-year risk of cardiovascular disease: the Framingham Heart Study. Circulation. 2009;119:3078–3084. doi: 10.1161/CIRCULATIONAHA.108.816694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berry JD, Liu K, Folsom AR, Lewis CE, Carr JJ, Polak JF, Shea S, Sidney S, O’Leary DH, Chan C, Lloyd-Jones DM. Prevalence and progression of subclinical atherosclerosis in younger adults with low short-term but high lifetime estimated risk for cardiovascular disease: the coronary artery risk development in young adults study and multi-ethnic study of atherosclerosis. Circulation. 2009;119:382–389. doi: 10.1161/CIRCULATIONAHA.108.800235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasan RS, Massaro JM, Wilson PW, Seshadri S, Wolf PA, Levy D, D’Agostino RB. Antecedent blood pressure and risk of cardiovascular disease: the Framingham Heart Study. Circulation. 2002;105:48–53. doi: 10.1161/hc0102.101774. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical Perspective
supplemental material