Sodium-Glucose Linked Transporter 2 (SGLT2) Inhibitors in the Management Of Type-2 Diabetes: A Drug Class Overview (original) (raw)
. 2015 Jul;40(7):451–462.
Abstract
Sodium-glucose linked transporter 2 inhibitors limit the reabsorption of glucose from glomerular filtrate in the kidneys, reducing blood glucose levels in patients with type- 2 diabetes. This article is meant to evaluate this class of medications for clinicians.
Keywords: type-2 diabetes mellitus (T2DM), sodium-glucose linked transporter 2 (SGLT2) inhibitors, canagliflozin, dapagliflozin, empagliflozin
INTRODUCTION
Type-2 diabetes mellitus (T2DM) accounts for more than 90% of all cases of diagnosed diabetes and is expected to become more common because of the growing population of obese individuals in the U.S.1 Diabetes is a complex and chronic disease that affected an estimated 29.1 million Americans (9.3% of the population) in 2012. Of this population, 21.0 million had been diagnosed with diabetes, while 8.1 million were undiagnosed.2
Meeting treatment goals is elusive for many people with diabetes. Data from the National Health and Nutrition Examination Survey for the years 2003 to 2006 showed that only 57% of adults with diagnosed T2DM achieved a hemoglobin A1c (HbA1c) of less than 7%; that 46% had blood pressure levels of less than 130/80 mm Hg; and that 47% had low-density lipoproteincholesterol (LDL-C) levels of less than 100 mg/dL.3 Only 12% of people with T2DM reached all three goals.3
The American Diabetes Association (ADA) recommends that individuals with prediabetes (i.e., impaired glucose tolerance, impaired fasting glucose, or an HbA1c range of 5.7% to 6.4%) undergo lifestyle modifications and receive metformin therapy. These patients should be encouraged to engage in moderate physical activity for at least 150 minutes per week and to lose 7% of their body weight through healthier food choices and an appropriate exercise program. When an individual with prediabetes progresses to diabetes (i.e., HbA1c levels of 6.5% or greater, fasting plasma glucose [FPG] levels of 126 mg/dL or greater, a two-hour 75-g oral glucose tolerance test of 200 mg/dL or greater, or random plasma glucose levels of 200 mg/dL or greater with symptoms), metformin remains the preferred initial therapy.1 If the patient is unable to achieve glycemic targets (AIC of less than 7.0%, preprandial plasma glucose [PG] of 70–130 mg/dL, or postprandial PG of less than 180 mg/dL) with metformin alone, several other drug classes may be tried.
Biguanides are the first-line treatment for patients with T2DM. Other therapeutic options include sulfonylureas, meglitinides, thiazolidinediones, dipeptidyl peptidase-4 (DPP-4) inhibitors, alpha-glucosidase inhibitors, bile acid sequestrants, insulins, glucagon-like peptide-1 (GLP-1) analogs, and sodium-glucose linked transporter 2 (SGLT2) inhibitors.1,4 According to the ADA, a patient-centered approach should be used to guide the choice of pharmacological treatments. Considerations include the drug’s efficacy, cost, potential adverse effects, effects on weight, comorbidities, and risk of hypoglycemia, as well as the patient’s preference.1
Although the idea that the kidneys play a key role in glucose balance is not new, only recently have they been identified as a potential therapeutic target in patients with T2DM. SGLT2 facilitates the reabsorption of glucose from glomerular filtrate. Inhibiting this process promotes glucosuria, thereby reducing blood glucose levels.5,6
This article is intended to familiarize clinicians with the SGLT2 inhibitors, which represent a unique therapeutic approach to patients with T2DM. As indicated above, the action of these drugs in glucose management centers on the vital role of the kidneys in glucose homeostasis. Clinical trials have provided the foundation for clinical recommendations regarding the effects of SGLT2 inhibitors on glucose levels, weight, blood pressure, and lipid levels. Before initiating SGLT2-inhibitor therapy, clinicians should consider the drug’s pharmacokinetic characteristics, potential drug interactions, and adverse-event (AE) profile. During treatment, the patient should be monitored closely.
THE ROLE OF THE KIDNEYS IN GLUCOSE HOMEOSTASIS
The kidneys regulate glucose homeostasis through three different mechanisms: 1) the release of glucose into the circulation via gluconeogenesis; 2) the uptake of glucose from the circulation; and 3) the reabsorption of glucose into the circulation from glomerular filtrate. Of these three mechanisms, the reabsorption of glucose into the circulation appears to be a major contributor to elevated glucose levels in patients with T2DM.7–10
Plasma glucose concentrations are determined by several factors that affect the entry of glucose into, and its removal from, the circulation. In people without diabetes, plasma glucose levels are maintained within a narrow range throughout the day. Minimal plasma concentrations (after moderate fasting or exercise) usually do not go below 54.1 mg/dL, and maximal concentrations (after the ingestion of a meal) usually do not exceed 162.2 mg/dL. The increase and decrease of plasma glucose levels throughout the day are usually due to hormonal and neuronal factors, such as insulin, glucagon, and catecholamines, that regulate the endogenous production of glucose.8
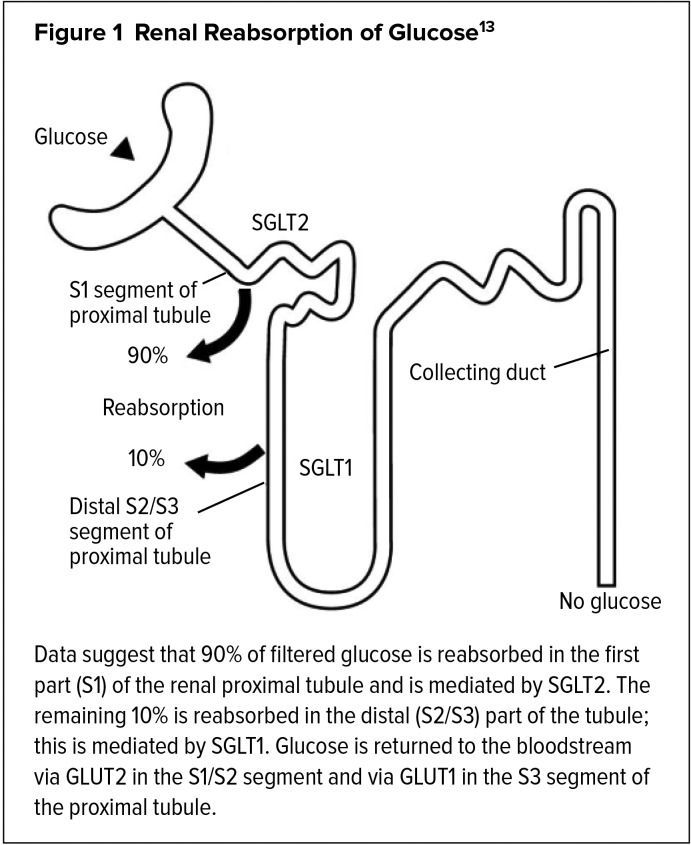
In individuals without diabetes, almost all of the filtered glucose is reabsorbed and returned to the circulation in the proximal tubule of the renal nephron. Glucose is reabsorbed by SGLTs working in tandem with facilitative glucose transporters (GLUTs). Both SGLT2 and GLUT2 are located in the proximal convoluted tubule of the nephron, where they are responsible for 90% of glucose reabsorption. Glucose transporters SGLT1 and GLUT1 are present in the distal convoluted tubules, where they are responsible for the remaining 10% of glucose reabsorption.11,12 The renal reabsorption of glucose by SGLT1 and SGLT2 is shown in Figure 1.13
Figure 1.
Renal Reabsorption of Glucose13
In patients without diabetes, SGLT1 and SGLT2 are able to reabsorb glucose into the circulation until plasma concentrations exceed 180 to 200 mg/dL (the renal threshold), after which glucose starts to appear in the urine (glucosuria).11 However, in patients who are consistently exposed to elevated glucose levels, such as those with T2DM, the kidneys continue to reabsorb glucose, thereby increasing the transport maximum,14 and glucosuria begins to occur at higher-than-normal plasma glucose levels, further contributing to diabetes. Studies of renal cells isolated from the urine of people with diabetes have demonstrated enhanced expression of SGLT2 transporters.15,16
SGLT2 INHIBITORS
The Food and Drug Administration (FDA) has approved three SGLT2 inhibitors as monotherapy for patients with T2DM: canagliflozin (Invokana, Janssen), dapagliflozin (Farxiga, AstraZeneca), and empagliflozin (Jardiance, Boehringer Ingelheim) (Table 1). These agents were approved in March 2013, January 2014, and August 2014, respectively.17–19 Approved fixed-dose combination products include canagliflozin/metformin (Invokamet, Janssen), extended-release dapagliflozin/ metformin (Xigduo XR, AstraZeneca), and empagliflozin/linagliptin (Glyxambi, Boehringer Ingelheim), approved in August 2014, October 2014, and February 2015, respectively.20–22
Table 1.
Dosage and Administration of the SGLT2 Inhibitors
| Dosage and Administration | Canagliflozin35 (Invokana) | Dapagliflozin43 (Farxiga) | Empagliflozin47 (Jardiance) |
|---|---|---|---|
| Strengths and dosage form | 100-mg and 300-mg tablets | 5-mg and 10-mg tablets | 10-mg and 25-mg tablets |
| Recommended starting dose | 100 mg once daily, taken before first meal of the day | 5 mg once daily, taken in morning with or without food | 10 mg once daily, taken in morning with or without food |
| Dose adjustments | Dosage may be increased to 300 mg once daily in patients tolerating 100 mg once daily who have an eGFR of 60 mL/min/1.73 m2 or greater and require additional glycemic control. | Dosage may be increased to 10 mg once daily in patients tolerating 5 mg once daily who require additional glycemic control. | Dosage may be increased to 25 mg once daily in patients tolerating 10 mg once daily who require additional glycemic control. |
| Precautions | Assess renal function before initiating treatment. Do not initiate if eGFR is less than 45 mL/min/1.73 m2. Discontinue if eGFR falls persistently below 45 mL/min/1.73 m2. | Assess renal function before initiating treatment. Do not initiate if eGFR is less than 60 mL/min/1.73 m2. Discontinue if eGFR falls persistently below 60 mL/min/1.73 m2. | Assess renal function before initiating treatment. Do not initiate if eGFR is less than 45 mL/min/1.73 m2. Discontinue if eGFR falls persistently below 45 mL/min/1.73 m2. |
SGLT2 inhibitors are indicated to improve glycemic control in adults with T2DM by reducing the reabsorption of filtered glucose. They can also lower the renal threshold for glucose, thereby increasing urinary glucose excretion.23,24 However, SGLT2 inhibitors block the reabsorption of only about 30% to 50% of the glucose filtered by the kidney.25
SGLT2 inhibitors are not indicated for the treatment of diabetic ketoacidosis. In these patients, clinicians should correct dehydration, hyperglycemia, and electrolyte imbalances; identify comorbid precipitating events; and perform frequent monitoring.26 Furthermore, SGLT2 inhibitors are not indicated for type-1 diabetes. Nevertheless, an exploratory study of dapagliflozin and a proof-of-concept study of empagliflozin have been conducted in this population.27,28
SGLT2 inhibitors provide several clinical benefits in patients with T2DM. For example, by increasing the excretion of glucose and decreasing plasma glucose concentrations, they have the ability to reduce body weight.6,29,30 This effect is the result of reduced body fat secondary to the loss of calories. For each gram of glucose excreted in the urine, 4 calories are also lost.29 A patient will lose 1 pound of body weight when 3,500 calories are lost.31
In addition, insulin resistance or impaired pancreatic beta-cell function do not hinder the effectiveness of SGLT2 inhibitors. The risk of major hypoglycemic events is also low because these agents do not interfere with normal endogenous glucose production in response to hypoglycemia or stimulate insulin release, suggesting that they may preserve the hypoglycemia counter-regulatory response of glucagon-mediated glucose production.32–34
CLINICAL TRIALS
Canagliflozin
Canagliflozin has been evaluated in patients with T2DM in the Canagliflozin Treatment and Trial Analysis (CANTATA) studies as monotherapy; as add-on therapy to metformin, sulfonylureas, metformin plus sulfonylureas, and metformin plus pioglitazone; and in combination with insulin, with or without other antihyperglycemic agents. These randomized, double-blind, placebo- or active-controlled trials examined the change from baseline in glycated HbA1c after 26 or 52 weeks of therapy. Overall, treatment with canagliflozin provided clinically and statistically significant improvements in HbA1c compared with placebo in all of these trials.35 Table 2 summarizes the study findings.
Table 2.
CANTATA Trials of Canagliflozin in Patients With T2DM
| Trial | Study Design | Daily Regimens | Change in HbA1c From Baseline (pp) | Change in FPG From Baseline (mg/dL) | Weight Change From Baseline (%) |
|---|---|---|---|---|---|
| CANTATA-D35 | 26-wk, randomized, double-blind, placebo- and active-controlled trial in 1,284 pts with T2DM inadequately controlled with metformin | CAN 100 mg CAN 300 mg Sitagliptin 100 mg Placebo | CAN 100: −0.79 CAN 300: −0.94 Placebo: −0.17 P < 0.001 for both CAN doses | CAN 100: −27 CAN 300: −38 Placebo: +2 P < 0.001 for both CAN doses | CAN 100: −3.7 CAN 300: −4.2 Placebo: −1.2 P < 0.001 for both CAN doses |
| CANTATA-D239 | 52-wk, randomized, double-blind, active-controlled trial in 755 pts with T2DM inadequately controlled with metformin and a sulfonylurea | CAN 300 mg Sitagliptin 100 mg | CAN 300: −1.03 Sitagliptin 100: −0.66 CAN noninferior to sitagliptin | CAN 300: −30 Sitagliptin 100: −6 P < 0.001 | CAN 300: −2.5 Sitagliptin 100: +0.3 P < 0.001 |
| CANTATA-M36 | 26-wk, randomized, double-blind, placebo-controlled trial in 584 pts with T2DM inadequately controlled with diet and exercise | CAN 100 mg CAN 300 mg Placebo | CAN 100: −0.77 CAN 300: −1.03 Placebo: +0.14 P < 0.001 for both CAN doses | CAN 100: −27 CAN 300: −35 Placebo: +8 P < 0.001 for both CAN doses | CAN 100: −2.8 CAN 300: −3.9 Placebo: −0.6 P < 0.001 for both CAN doses |
| CANTATA-MP38 | 52-wk, randomized, double-blind, placebo- and active-controlled trial in 342 pts with T2DM inadequately controlled with metformin and pioglitazone | CAN 100 mg CAN 300 mg Placebo (first 26 wks) Sitagliptin 100 mg (second 26 wks) | CAN 100: −0.89 CAN 300: −1.03 Placebo: −0.26 P < 0.001 for both CAN doses | CAN 100: −29.4 CAN 300: −35.7 Placebo: +2.5 P < 0.001 for both CAN doses | CAN 100: −2.8 CAN 300: −3.8 Placebo: −0.1 P < 0.001 for both CAN doses (26 wks) |
| CANTATA-MSU35 | 26-wk, randomized, double-blind, placebo-controlled, parallel-group trial in 469 pts with T2DM inadequately controlled with metformin and a sulfonylurea | CAN 100 mg CAN 300 mg Placebo | CAN 100: −0.85 CAN 300: −1.06 Placebo: −0.13 P < 0.001 for both CAN doses | CAN 100: −18 CAN 300: −31 Placebo: +4 P < 0.001 for both CAN doses | CAN 100: −2.1 CAN 300: −2.6 Placebo: −0.7 P < 0.001 for both CAN doses |
| CANTATA-SU40 | 52-wk, double-blind, active-controlled trial in 1,450 pts with T2DM inadequately controlled with metformin | CAN 100 mg CAN 300 mg Glimepiride (maximum dose: 5–6 mg) | CAN 100: −0.82 CAN 300: −0.93 Glimepiride: −0.81 CAN noninferior to glimepiride | CAN 100: −24 CAN 300: −28 Glimepiride: −18 CAN noninferior to glimepiride | CAN 100: −4.2 CAN 300: −4.7 Placebo: +1.0 P < 0.001 for both CAN doses |
CANTATA-M
The phase 3, randomized, double-blind, placebo-controlled CANTATA-M trial was designed to determine the safety and efficacy of canagliflozin 100-mg or 300-mg monotherapy in 584 subjects with T2DM inadequately controlled with diet and exercise. Mean baseline HbA1c values for placebo, canagliflozin 100 mg, and canagliflozin 300 mg were 8.0%, 8.1%, and 8.0%, respectively. After 26 weeks of treatment, baseline HbA1c was reduced by 0.77 percentage points (difference in least squares [LS] mean change, −0.91; 95% confidence interval [CI], −1.1 to −0.70; P < 0.001) and 1.03 percentage points (difference in LS mean change, −1.16; 95% CI, 1.0 to 1.3; P < 0.001) with canagliflozin 100 mg and 300 mg, respectively, compared with an increase of 0.14 percentage points with placebo.36 Canagliflozin 300 mg decreased baseline HbA1c by −1.03 percentage points (difference in LS mean change, −1.16%; 95% CI, −1.3 to −1.0; P < 0.001). The LS mean, a method used to calculate the mean for a group of values, is less sensitive to missing data and theoretically provides a better estimate of a true population mean.37
Moreover, canagliflozin 100 mg and 300 mg provided significantly greater reductions in FPG compared with placebo. At week 26, differences in LS mean changes in FPG were −27 mg/dL and −35 mg/dL for canagliflozin 100 mg and 300 mg, respectively, compared with placebo (P < 0.001 for both doses). Significant dose-related reductions from baseline in body weight were also observed at week 26 with canagliflozin 100 mg (−2.8%) and 300 mg (−3.9%) compared with placebo (−0.6%; P < 0.001 for both doses).36
The rates of urinary tract infections were higher with canagliflozin 100 mg and 300 mg than with placebo, but none led to study discontinuation. AEs related to osmotic diuresis and reduced intravascular volume (i.e., postural dizziness and orthostatic hypotension) were also rare, leading to few discontinuations. Hypoglycemia occurred in 3.6%, 3.0%, and 2.6% of subjects receiving canagliflozin 100 mg, canagliflozin 300 mg, and placebo, respectively, with no reports of severe hypoglycemia.36
CANTATA-MP
Forst and colleagues conducted a 52-week, phase 3, randomized, double-blind, placebo-controlled study to compare canagliflozin (100 mg or 300 mg) with placebo in 342 patients with T2DM that had been inadequately controlled with a combination of metformin and pioglitazone. For the first 26 weeks, the study was placebo-controlled. For the second 26 weeks, the placebo-treated patients were switched to sitagliptin in an active-controlled extension phase. No comparisons were made between canagliflozin and sitagliptin.38
At week 26, canagliflozin 100 mg and 300 mg significantly reduced HbA1c from its baseline value (7.9%) compared with placebo (LS mean percentage-point changes: −0.89, −1.03, and −0.26, respectively). Placebo-subtracted differences in LS mean changes were −0.62 and −0.76 with canagliflozin 100 mg and 300 mg, respectively (P < 0.001 for both doses). The reductions in HbA1c with canagliflozin 100 mg and 300 mg were sustained over 52 weeks of treatment, with LS mean percentage-point changes from baseline of −0.92 and −1.03, respectively.38
Significant improvements from baseline in FPG were observed at week 26 with canagliflozin 100 mg and 300 mg compared with placebo; differences in the LS mean change were −29.4 mg/dL and −35.7 mg/dL versus +2.5 mg/dL, respectively (P < 0.001 for both doses). LS mean changes from baseline in FPG at week 52 were −26.7 mg/dL and −31.5 mg/dL with canagliflozin 100 mg and 300 mg, respectively.38
At week 26, significant dose-related reductions from baseline in body weight were observed with canagliflozin 100 mg and 300 mg compared with placebo; LS mean percent changes compared with placebo were −2.7% and −3.7%, respectively (P < 0.001 for both). LS mean percent changes from baseline in body weight at week 52 were −2.7% and −3.7% with canagliflozin 100 mg and 300 mg, respectively; the mean absolute changes were −5.5 pounds and −7.92 pounds.38 The overall rates of AEs were 69.9%, 76.3%, and 76.5% for canagliflozin 100 mg, canagliflozin 300 mg, and placebo/sitagliptin, respectively. Canagliflozin 100 mg and 300 mg were associated with higher rates of AEs related to osmotic diuresis during 52 weeks of treatment. The proportions of patients with hypoglycemic episodes during 52 weeks of therapy were 4.4%, 6.1%, and 6.1% with canagliflozin 100 mg, canagliflozin 300 mg, and placebo/sitagliptin, respectively. There were no reports of severe hypoglycemia.38
CANTATA-D2
Schernthaner and colleagues compared canagliflozin 300 mg with sitagliptin 100 mg in a phase 3, randomized, double-blind, active-controlled study involving 755 subjects with T2DM that had been inadequately controlled with metformin and a sulfonylurea. After 52 weeks of treatment, canagliflozin 300 mg reduced the baseline HbA1c (8.1%) by −1.03 percentage points (difference in LS means, −0.37; 95% CI, −0.50 to −0.25) compared with a −0.66 reduction with sitagliptin 100 mg, which demonstrated noninferiority to sitagliptin. Significantly greater reductions from baseline in FPG were observed at week 52 with canagliflozin 300 mg compared with sitagliptin 100 mg (−30 mg/dL versus −6 mg/dL, respectively; P < 0.001).39
Treatment with canagliflozin 300 mg also resulted in a significant reduction in body weight at week 52 compared with sitagliptin 100 mg (−2.5% versus +0.3%, respectively; P < 0.001).39
The overall incidence of AEs, serious AEs, and study discontinuations attributable to AEs were similar for canagliflozin 300 mg and sitagliptin 100 mg. Canagliflozin was associated with low rates (less than 2%) of osmotic diuresis-related AEs. The proportions of subjects experiencing at least one hypoglycemic episode were similar between canagliflozin (43%) and sitagliptin (41%).39
CANTATA-SU
Cefalu and colleagues compared canagliflozin (100 mg or 300 mg) with glimepiride (mean maximum dose, 5 mg to 6 mg) in a phase 3, randomized, double-blind, active-controlled trial involving 1,450 patients with T2DM that had been inadequately controlled with metformin. After 52 weeks of treatment, glimepiride reduced the baseline HbA1c (7.8%) by −0.81 percentage points. The LS mean change for canagliflozin 100 mg was −0.82 (difference versus glimepiride, −0.01; 95% CI, −0.11 to −0.09), which indicated its noninferiority to glimepiride. The LS mean change for canagliflozin 300 mg was −0.93 (difference versus glimepiride, −0.12; 95% CI, −0.22 to −0.02), which indicated its superiority to glimepiride. At week 52, subjects in the two canagliflozin groups experienced a greater reduction in FPG compared with those in the glimepiride group. Compared with glimepiride, differences in the LS mean changes were −5.95 mg/dL (95% CI, −10.81 to −1.80) for canagliflozin 100 mg and −9.19 mg/dL (95% CI, −12.61 to −5.41) for canagliflozin 300 mg.40
Both canagliflozin doses significantly reduced the subjects’ body weights at week 52, whereas there was a slight increase in weight with glimepiride (P < 0.0001 for the two canagliflozin doses versus glimepiride).34
Glimepiride was associated with a slightly higher rate of serious AEs compared with canagliflozin 100 mg or 300 mg. AEs of osmotic diuresis were more common with canagliflozin than with glimepiride. The proportions of patients with hypoglycemic episodes were significantly lower with canagliflozin 100 mg and 300 mg compared with glimepiride (P < 0.0001 for both canagliflozin doses).40
CANTATA-D
The CANTATA-D trial was a 52-week, randomized, double-blind, placebo- and active-controlled study to determine the efficacy, safety, and tolerability of canagliflozin compared with placebo and an active control (sitagliptin) in patients with T2DM who were not achieving an adequate response to metformin. A total of 1,284 patients received once-daily canagliflozin (100 mg or 300 mg), sitagliptin (100 mg), or placebo for 26 weeks (period 1). This was followed by another 26 weeks during which the patients treated with canagliflozin or sitagliptin continued treatment, while the patients treated with placebo were switched to active double-blind treatment with sitagliptin 100 mg administered once daily (period 2).41
The trial’s primary outcome measure was the change in HbA1c from baseline to week 26.41 Therefore, the two doses of canagliflozin were compared with placebo at the end of period 1. Mean baseline HbA1c values for placebo, canagliflozin 100 mg, and canagliflozin 300 mg were 7.96%, 7.94%, and 7.95%, respectively. After 26 weeks of treatment, baseline HbA1c was reduced by −0.79 and −0.94 percentage points with canagliflozin 100 mg and 300 mg, respectively, compared with a decrease of −0.17 with placebo. These differences were statistically significant (P < 0.001).35
Treatment with canagliflozin 100 mg and 300 mg also resulted in significant differences from placebo in the changes in FPG (−27 mg/dL and −38 mg/dL versus +2.0 mg/dL, respectively; P < 0.001); in improved two-hour postprandial glucose (−48 mg/dL and −57 mg/dL versus −10 mg/dL; P < 0.001); and in percent weight loss (−3.7% and −4.2% versus −1.2%; P < 0.001).35
CANTATA-MSU
The CANTATA-MSU trial was a randomized, double-blind, placebo-controlled, parallel-group, three-arm study to determine the efficacy, safety, and tolerability of canagliflozin (100 mg and 300 mg) compared with placebo in 469 patients with T2DM who were not achieving an adequate response from antihyperglycemic therapy with a combination of metformin (greater than or equal to 2,000 mg per day, or at least 1,500 mg per day if the higher dose was not tolerated) and a sulfonylurea (maximal or near-maximal dose) to control their diabetes.35,42 The study’s primary outcome measure was the effect of canagliflozin compared with placebo on HbA1c after 26 weeks of treatment.42
At the end of the study, canagliflozin 100 mg and 300 mg resulted in a statistically significant improvement in HbA1c (P < 0.001 for both doses) compared with placebo when added to metformin and a sulfonylurea. Mean baseline HbA1c values for placebo, canagliflozin 100 mg, and canagliflozin 300 mg were 8.12%, 8.13%, and 8.13%, respectively. After 26 weeks of therapy, baseline HbA1c was reduced by −0.85 and −1.06 percentage points with canagliflozin 100 mg and 300 mg, respectively, compared with a decrease of −0.13 with placebo.35
Canagliflozin 100 mg and 300 mg also resulted in greater proportions of patients achieving a significant reduction in FPG compared with placebo (−18 mg/dL and −31 mg/dL versus +4 mg/dL, respectively; P < 0.001) as well as significant reductions in body weight (−2.1% and −2.6% versus −0.7%; P < 0.001) when added to metformin and a sulfonylurea.35
Dapagliflozin
Dapagliflozin has been studied as monotherapy, as add-on therapy to metformin, and as add-on therapy to metformin in comparison with glipizide in patients with T2DM.43 Results from key studies are discussed below.
Dapagliflozin Monotherapy
In a pivotal monotherapy trial, 485 treatment-naïve patients with inadequately controlled T2DM were treated with dapagliflozin 5 mg or 10 mg once daily or placebo. The mean baseline HbA1c levels were 7.8%, 8.0%, and 7.8%, respectively. At week 24, the mean percentage-point reductions in HbA1c were −0.2 for placebo compared with −0.8 and −0.9 for dapagliflozin 5 mg and 10 mg, respectively. The difference between the 10-mg dose of dapagliflozin and placebo was statistically significant (P < 0.0001). Similarly, dapagliflozin 5 mg and 10 mg achieved mean reductions in FPG of −24.1 mg/dL and −28.8 mg/dL compared with a reduction of −4.1 mg/dL with placebo. Again, the difference between dapagliflozin 10 mg and placebo was statistically significant (P < 0.0001).43
Li and colleagues conducted a phase 3, double-blind, parallel-group study to evaluate dapagliflozin 5 mg and 10 mg as monotherapy in 393 treatment-naïve Asian patients with T2DM whose disease was inadequately controlled with diet and exercise. The patients’ mean HbA1c level at baseline was 8.26%. At week 24, the mean percentage-point reductions in HbA1c were −0.29 for placebo compared with −1.04 and −1.11 for dapagliflozin 5 mg and 10 mg, respectively (P < 0.0001 for both doses). Dapagliflozin 5 mg and 10 mg also produced mean reductions from baseline in FPG that were significantly greater than that of placebo (−25.1 mg/dL and −31.6 mg/dL versus an increase of 2.5 mg/dL, respectively; P < 0.0001 for both doses).44 Treatment with dapagliflozin resulted in significant mean reductions from baseline in total weight (−1.64 kg for the 5-mg dose and −2.25 kg for the 10-mg dose; P < 0.0001 for both doses) compared with the mean reduction from baseline observed with placebo (−0.27 kg). The dapagliflozin 5- and 10-mg doses also produced mean reductions from baseline in FPG, which were significantly greater (−25.1 mg/dL, P < 0.0001; and −31.6 mg/dL, P < 0.0001) than that of placebo (+2.5 mg/dL). The reduction from baseline in mean two-hour postprandial glucose was significantly greater with dapagliflozin 5 mg (−46.8 mg/dL; P < 0.0001) and 10 mg (−54.9 mg/dL; P < 0.0001) compared with placebo (−1.1 mg/dL).44
Overall, dapagliflozin was well tolerated in this study. No deaths occurred during the 24-week double-blind period, no major episodes of hypoglycemia were reported, and no patient discontinued the study because of hypoglycemia. Hypoglycemic events were reported in one patient each in the 5- and 10-mg dapagliflozin groups and in two patients in the placebo group.44
Dapagliflozin as Add-On to Metformin
In a phase 3, double-blind, parallel-group, placebo-controlled trial, Bailey et al. randomly assigned one of three doses of dapagliflozin (2.5 mg, n = 135; 5 mg, n = 133; or 10 mg, n = 132) or placebo (n = 134) once daily to 534 T2DM patients with inadequate glycemic control during treatment with daily metformin. After 24 weeks of therapy, HbA1c was significantly reduced in the dapagliflozin groups compared with the placebo group. The mean percentage-point changes from baseline were −0.30 in the placebo group compared with −0.67 (P = 0.0002) for dapagliflozin 2.5 mg; −0.70 (P < 0.0001) for dapagliflozin 5 mg; and −0.84 (P < 0.0001) for dapagliflozin 10 mg. Moreover, mean changes from baseline in FPG were significant in all dapagliflozin groups (−17.84 mg/dL to −23.42 mg/dL) compared with placebo (−5.95 mg/dL).45
At week 24, total weight reductions of 5% or more compared with placebo were observed in 18.1%, 19.5%, and 22.1% of the dapagliflozin 2.5-, 5-, and 10-mg groups, respectively. Symptoms of hypoglycemia occurred in similar proportions of patients in the dapagliflozin (2% to 4%) and placebo groups (3%).45
Dapagliflozin Versus Glipizide as Add-On Therapy
Nauck and colleagues conducted a 52-week, double-blind, active-controlled, noninferiority trial comparing dapagliflozin (maximum dose, 10 mg) with glipizide (maximum dose, 20 mg) in 814 patients with T2DM that was inadequately controlled with metformin monotherapy. At the end of the study, the mean percentage-point change in HbA1c from baseline was identical for dapagliflozin and glipizide (−0.52), indicating that dapagliflozin was noninferior to glipizide.46
Dapagliflozin produced significant mean weight loss (−3.2 kg) compared with the weight gain (+1.2 kg; P < 0.0001) seen with glipizide; significantly increased the proportion of patients achieving a reduction in body weight of 5% or more compared with glipizide (33.3% versus 2.5%, respectively; P < 0.0001); and significantly decreased the proportion of patients experiencing hypoglycemia (3.5% versus 40.8%, respectively; P < 0.0001).46
Empagliflozin
Empagliflozin has been studied as monotherapy and in combination with metformin, a sulfonylurea, pioglitazone, or insulin in patients with T2DM.47 Key results are summarized below.
Empagliflozin Monotherapy
Ferrannini et al. conducted a phase 2b, randomized, double-blind, placebo-controlled study of empagliflozin 5, 10, or 25 mg in patients with T2DM. After 12 weeks of treatment, empagliflozin demonstrated significant dose-dependent percentage-point reductions in HbA1c from baseline (5 mg, −0.4; 10 mg, −0.5; and 25 mg, −0.6) compared with placebo (+0.09) (P < 0.0001 for all empagliflozin doses). Empagliflozin also demonstrated dose-dependent reductions in FPG from base-line (5 mg, −23.24 mg/dL; 10 mg, −29.01 mg/dL; and 25 mg, −30.99 mg/dl; for all doses, P < 0.0001 versus placebo).48
At week 12, mean body weight decreased from baseline in all empagliflozin groups (P < 0.001 versus placebo). The changes from baseline were −3.98 pounds, −5.13 pounds, and −4.47 pounds in the 5-, 10-, and 25-mg empagliflozin groups, respectively.48
The most frequently reported AEs among empagliflozin-treated patients were pollakiuria, thirst, and nasopharyngitis. There were no cases of hypoglycemia.48
Empagliflozin Versus Sitagliptin
A phase 3, randomized, double-blind, placebo-controlled, parallel-group study was conducted to compare empagliflozin 10 mg or 25 mg with sitagliptin 100 mg and placebo in 986 patients with T2DM and insufficient glycemic control. After 24 weeks of treatment, percentage-point changes from baseline in HbA1c were as follows: +0.06 for placebo; −0.65 for sitagliptin 100 mg; −0.66 for empagliflozin 10 mg; and −0.77 for empagliflozin 25 mg (P < 0.0001 for both empagliflozin doses).49
Changes in body weight from baseline were −0.73 pounds for placebo, −0.37 pounds for sitagliptin 100 mg, −4.97 pounds for empagliflozin 10 mg, and −5.46 pounds for empagliflozin 25 mg (P < 0.0001 for both empagliflozin doses).49
The rate of symptomatic hypoglycemic events was less than 1% across all treatment groups.49
Empagliflozin as Add-On to Metformin and Sulfonylurea
Häring and colleagues evaluated empagliflozin 10 mg or 25 mg as an add-on to metformin and a sulfonylurea in a randomized, double-blind, placebo-controlled trial involving 666 patients with T2DM. Reductions in HbA1c after 24 weeks of therapy were significantly greater in the empagliflozin groups than in the placebo group, with adjusted mean percentage-point changes of −0.82 for empagliflozin 10 mg and −0.77 for empagliflozin 25 mg compared with −0.17 for placebo (P < 0.001 for both empagliflozin doses).50
After 24 weeks of treatment, mean changes in FPG from baseline were +5.58 mg/dL for placebo compared with −23.22 mg/dL for both empagliflozin 10 mg and 25 mg (P < 0.001 for both doses). Compared with placebo, there was also a significant reduction in two-hour postprandial glucose with both doses of empagliflozin; the mean changes from baseline were −2.34 mg/dL with placebo compared with −35.64 mg/dL with empagliflozin 10 mg and −36.54 mg/dL with empagliflozin 25 mg (P = 0.003 for both doses).50
Empagliflozin caused a significantly greater reduction in body weight compared with placebo, with mean changes from baseline of −0.89 pounds for placebo compared with −4.75 pounds for empagliflozin 10 mg and −5.26 pounds for empagliflozin 25 mg (P < 0.001 for both doses).50
Confirmed hypoglycemic AEs were reported by more patients in the empagliflozin 10-mg (16.1%) and 25-mg (11.5%) groups than in the placebo group (8.4%). None of these events required medical assistance.50
Empagliflozin as Add-On to Pioglitazone
Kovacs et al. randomly assigned 498 patients with T2DM to receive once-daily empagliflozin 10 mg (n = 165), empagliflozin 25 mg (n = 168), or placebo (n = 165) as an add-on to pioglitazone, with or without metformin, for 24 weeks. At the end of treatment, the mean percentage-point changes from baseline in HbA1c were −0.6 and −0.7 with empagliflozin 10 mg and 25 mg, respectively, compared with −0.1 with placebo (both P < 0.001). FPG decreased with empagliflozin (−16.92 mg/dL for 10 mg and −21.96 mg/dL for 25 mg) and increased with placebo (+6.48 mg/dL; both P < 0.001). Mean changes in body weight were −1.62 kg and −1.47 kg with empagliflozin 10 mg and 25 mg, respectively, compared with +0.34 kg with placebo (both P < 0.001). Confirmed hypoglycemia was reported in 1.2% and 2.4% of patients given empagliflozin and in 1.8% of those given placebo.51
Empagliflozin as Add-On to Insulin
A total of 494 patients with T2DM inadequately controlled on insulin or on insulin in combination with oral drugs participated in a double-blind, placebo-controlled study to evaluate the efficacy of empagliflozin as add-on therapy to insulin over 78 weeks. The patients received empagliflozin (10 mg or 25 mg) or placebo, and insulin could be adjusted as needed.47
Empagliflozin in combination with insulin (with or without metformin and/or a sulfonylurea) provided statistically significant reductions in HbA1c and FPG compared with placebo after 78 weeks of treatment. The mean percentage-point changes from baseline in HbA1c were −0.4 and −0.6 with empagliflozin 10 mg and 25 mg, respectively, compared with −0.1 with placebo (P < 0.0001 for both doses). FPG decreased with empagliflozin (−10.1 mg/dL for 10 mg and −15.2 mg/dL for 25 mg) and increased with placebo (+2.8 mg/dL; P = 0.0049 for 10 mg and P = 0.0052 for 25 mg). Mean changes in body weight were −2.4 kg with both empagliflozin 10 mg and 25 mg compared with +0.7 kg with placebo (P < 0.0001).47
PHARMACOKINETICS
Canagliflozin
The mean absolute oral bioavailability of canagliflozin is approximately 65%. Administration of canagliflozin with a high-fat meal has no effect on the drug’s pharmacokinetics. Canagliflozin can be taken with or without food. The mean steady-state volume of distribution after a single intravenous (IV) infusion in healthy subjects is 119 L, suggesting extensive tissue distribution. Canagliflozin is extensively bound to proteins in plasma (99%), mainly to albumin.35
O-glucuronidation is the major elimination pathway for canagliflozin, which is mainly glucuronidated by the uridine 5’-diphospho glucuronosyltransferases UGT1A9 and UGT2B4 to two inactive O-glucuronide metabolites. Cytochrome P450 CYP3A4-mediated metabolism of canagliflozin is minimal (approximately 7%) in humans. After oral administration in healthy subjects, 41.5%, 7.0%, and 3.2% of the administered radioactive dose was discovered in feces as canagliflozin, as a hydroxylated metabolite, and as an O-glucuronide metabolite, respectively. The mean systemic clearance of canagliflozin is approximately 192 mL/min in healthy subjects after IV administration. The renal clearance of canagliflozin (100 mg and 300 mg) ranged from 1.30 to 1.55 mL/min.35
In a study conducted to determine the pharmacokinetic and pharmacodynamic characteristics of canagliflozin, approximately 1% urinary excretion was observed, which was consistent with previous canagliflozin studies. On further analysis, the researchers concluded that, despite the drug’s low renal excretion rate, sufficient free concentrations of canagliflozin may be present in the tubular lumen to effectively inhibit SGLT2-mediated glucose transport.52
Dapagliflozin
Dapagliflozin is approximately 91% protein-bound. The maximum plasma concentration (Cmax) of oral dapagliflozin is usually achieved within two hours in the fasting state. The Cmax and the area under the curve (AUC) increase dose-proportionately as the dapagliflozin dose is increased in the therapeutic dosing range. The absolute oral bioavailability of dapagliflozin after a single 10-mg dose is 78%. The administration of dapagliflozin with a high-fat meal decreases the drug’s Cmax by up to 50% and prolongs its time to maximum concentration (Tmax) by approximately one hour.43 Since the daily amount of glucose excreted in the urine depends on the AUC of dapagliflozin and not on its peak concentration, changes in the drug’s Cmax have no effect on safety, tolerability, or efficacy; as a result, dapagliflozin may be taken without regard to meals.53
The metabolism of dapagliflozin is primarily mediated by UGT1A9; CYP-mediated metabolism is a minor clearance pathway in humans. Dapagliflozin is primarily metabolized to dapagliflozin 3-O-glucuronide. This inactive metabolite accounted for 61% of a 50-mg dose of [14C]-dapagliflozin. Dapagliflozin and related metabolites are primarily eliminated by the kidneys. After a single 50-mg dose of [14C]-dapagliflozin, 75% and 21% of total radioactivity is excreted in urine and feces, respectively. In addition, less than 2% and approximately 15% of the dose is excreted by these two routes, respectively, as the parent drug. The mean plasma terminal half-life of dapagliflozin is approximately 12.9 hours after a single 10-mg dose.43
Empagliflozin
Peak plasma concentrations of empagliflozin are reached at 1.5 hours after dosing. Systemic exposure of the drug increases in a dose-proportional manner in the therapeutic dosing range. The administration of empagliflozin 25 mg after the intake of a high-fat and high-calorie meal resulted in slightly lower exposure; the AUC decreased by approximately 16% and the Cmax decreased by approximately 37% compared with levels in the fasted state. The observed effect of food on the pharmacokinetics of empagliflozin was not considered clinically relevant, and the drug may be administered with or without food. The apparent steady-state volume of distribution was estimated to be 73.8 L based on a population pharmacokinetic analysis.47
In a pharmacokinetics study of empagliflozin, the researchers found that body weight was the most influential pharmacokinetic covariant. The maximum effect of weight on exposure was ± 30% based on the 2.5th and 97.5th percentiles of observed weights relative to the median observed weight. The authors also found that Asian patients had an approximately 25% greater oral absorption rate. They concluded that no exposure-based dose adjustments were required for the study population based on the covariant effects of sex, race, age, total protein, body weight, and smoking/alcohol history.54
After the administration of an oral [14C]-empagliflozin solution in healthy subjects, plasma protein binding was 86.2%. No major metabolites of empagliflozin were detected in human plasma, and the most abundant metabolites were three glucuronide conjugates (2-O-, 3-O-, and 6-O-glucuronide). Systemic exposure of each metabolite was less than 10% of the total drug-related material. In vitro studies have suggested that the primary route of metabolism of empagliflozin in humans is glucuronidation by UGT2B7, UGT1A3, UGT1A8, and UGT1A9.47
The apparent terminal elimination half-life of empagliflozin was estimated to be 12.4 hours, and the apparent oral clearance was 10.6 L/hour. After the administration of an oral [14C]-empagliflozin solution in healthy subjects, approximately 95.6% of the drug-related radioactivity was eliminated in urine (54.4%) or feces (41.2%). Most of the radioactivity recovered in feces was associated with unchanged parent drug, as was approximately half of the radioactivity excreted in urine.47
DRUG INTERACTIONS
Canagliflozin
The coadministration of rifampin with canagliflozin can decrease the canagliflozin AUC by 51%, which may result in decreased efficacy. If an inducer of UGT enzymes must be used, clinicians should consider increasing the canagliflozin dosage to 300 mg once daily if the patient is currently tolerating canagliflozin 100 mg once daily, has an estimated glomerular filtration rate (eGFR) of 60 mL/min/1.73 m2 or more, and requires additional glycemic control. For patients with an eGFR of less than 60 mL/min/1.73 m2, clinicians should consider other antihyperglycemic therapy. The AUC and mean peak concentration of digoxin (20% and 36%, respectively) increased when this drug was coadministered with canagliflozin 300 mg. Appropriate monitoring is warranted in this situation.35
Since canagliflozin increases the urinary excretion of glucose, urine glucose tests will yield a positive result. Therefore, patients should use an alternative method to measure glycemic control. Although 1,5-AG assays are unreliable for measuring glycemic control while taking canagliflozin, blood glucose monitoring provides an alternative method.35
Dapagliflozin
Like canagliflozin, dapagliflozin increases urinary glucose excretion and will lead to positive urine glucose tests, meaning clinicians should use alternative methods to monitor glycemic control. Monitoring with a 1,5-AG assay is not recommended because this test is unreliable in patients taking SGLT2 inhibitors. As with canagliflozin, blood glucose monitoring is a clinical option.43
Empagliflozin
The coadministration of empagliflozin with diuretics may result in an increased urine volume and frequency of voids, which could enhance the potential for volume depletion.47 Coadministration with insulin or insulin secretagogues may increase the risk for hypoglycemia. Like the other SGLT2 inhibitors, empagliflozin increases urinary glucose excretion, which will lead to positive urine glucose tests. The use of alternative methods (except 1,5-AG assays) is recommended.47
ADVERSE EVENTS ASSOCIATED WITH SGLT2 INHIBITORS
In clinical studies of canagliflozin, dapagliflozin, and empagliflozin, the most common AEs included male and female genital mycotic infections, increased urination, and urinary tract infections.36,39,45 Warnings and precautions for the SGLT2 inhibitors include hypotension, impaired renal function, hypoglycemia when used concomitantly with insulin and insulin secretagogues, genital mycotic infections, and increased LDL-C levels (Table 3). The labeling for canagliflozin includes an additional warning and precaution for hypersensitivity and hyperkalemia when treatment is combined with medications that interfere with potassium excretion. The dapagliflozin label has an additional warning for bladder cancer; consequently, this drug should not be used in patients with active bladder cancer.35,43,55 The empagliflozin label includes a warning for urinary tract infections.47 Additional AEs are summarized in Table 4.
Table 3.
Warnings and Precautions Associated With SGLT2 Inhibitors35,43,47
| Warning/Precaution | Canagliflozin | Dapagliflozin | Empagliflozin |
|---|---|---|---|
| Genital mycotic infections | X | X | X |
| Hypoglycemia when used with insulin or insulin secretagogues | X | X | X |
| Hyperkalemia | X | ||
| Hypersensitivity reactions | X | ||
| Hypotension | X | X | X |
| Impaired renal function | X | X | X |
| Increased LDL-C | X | X | X |
| Urinary tract infections | X |
Table 4.
Selected Adverse Events Associated With SGLT2 Inhibitors in Placebo-Controlled Clinical Trials35,43,47
| % of Patients | ||||||
|---|---|---|---|---|---|---|
| Canagliflozina | Dapagliflozinb | Empagliflozinc | ||||
| Adverse Event | 100 mg (n = 833) | 300 mg (n = 834) | 5 mg (n = 1,145) | 10 mg (n = 1,193) | 10 mg (n = 999) | 25 mg (n = 977) |
| Constipation | 1.8 | 2.3 | 2.2 | 1.9 | — | — |
| Dyslipidemia | — | — | 2.1 | 2.5 | 3.9 | 2.9 |
| Genital mycotic infections | ||||||
| Female | 10.4 | 11.4 | 8.4 | 6.9 | 5.4 | 6.4 |
| Male | 4.2 | 3.7 | 2.8 | 2.7 | 3.1 | 1.6 |
| Increased urination | 5.3 | 4.6 | 2.9 | 3.8 | 3.4 | 3.2 |
| Nausea | 2.2 | 2.3 | 2.8 | 2.5 | 2.3 | 1.1 |
| Urinary tract infection | 5.9 | 4.3 | 5.7 | 4.3 | 9.3 | 7.6 |
SGLT2 inhibitors offer promising options for glucose control, with the ability to reduce body weight and decrease blood pressure. As a class, however, their long-term effects are unknown. Someday they might be the preferred initial therapy in T2DM patients, as metformin is now,1 but they could also have long-term detrimental effects, such as causing or worsening heart failure as pioglitazone and rosiglitazone do.56
The ongoing, randomized, placebo-controlled Canagliflozin Cardiovascular Assessment Study (CANVAS) is investigating the difference in the risk for cardiovascular disease between patients treated with canagliflozin and those given placebo. The study’s secondary objective is to determine the effects of canagliflozin compared with placebo on intermediate efficacy measures, including beta-cell function, the progression of albuminuria, the albumin–creatinine ratio, and the eGFR. The study was also designed to evaluate the short-, medium-, and long-term effects of canagliflozin compared with placebo on HbA1c, FPG, body weight, blood pressure, fasting plasma lipid levels, and adverse effects.57 A recent finding from this study indicated that the addition of canagliflozin to insulin therapy improved glycemic control and reduced body weight.58
The FDA recently warned consumers about SGLT2 inhibitors’ potential to lead to high levels of blood acids called ketones, which may progress to ketoacidosis. This warning is based on reports from the FDA Adverse Event Reporting System (FAERS) database that identified 20 cases of acidosis reported as diabetic ketoacidosis (DKA), ketoacidosis, or ketosis in patients treated with SGLT2 inhibitors from March 2013 to June 6, 2014.59
In all cases, a diagnosis of DKA or ketoacidosis was made by a health care professional, and hospitalization was required to treat the episode. The median time to onset of symptoms following initiation of drug therapy was two weeks (range, one to 175 days). DKA case presentations were atypical in that glucose levels were only mildly elevated at less than 200 mg/dL in some reports, while patients with type-1 diabetes who have DKA typically have glucose levels greater than 250 mg/dL.59
Potential DKA-triggering factors that were identified in some cases included acute illness or recent significant changes such as infection, urosepsis, trauma, reduced caloric or fluid intake, and reduced insulin dose. Patients should watch closely for any signs of ketoacidosis and seek medical attention immediately if they experience symptoms such as difficulty breathing, nausea, vomiting, abdominal pain, confusion, and unusual fatigue or sleepiness.59
The FDA is continuing to investigate this issue and will determine whether changes are needed in the prescribing information for this class of drugs.59
USE OF SGLT2 INHIBITORS IN SPECIAL POPULATIONS
Pregnancy
All three of the FDA-approved SGLT2 inhibitors are classified as pregnancy category C medications. No adequate and well-controlled studies of these drugs have been performed in pregnant women. Based on the results of animal studies, SGLT2 inhibition may affect renal development and maturation. SGLT2 inhibitors should be used in pregnant women only if the potential benefits justify the risk to the fetus.35,43,47
Nursing Mothers
It is not known whether the SGLT2 inhibitors are excreted in human milk. Because of the potential for serious adverse reactions in nursing infants exposed to these medications, a decision should be made as to whether to discontinue nursing or discontinue the SGLT2 inhibitor, taking into account the importance of the drug to the mother.35,43,47
Pediatric Use
The safety and effectiveness of the SGLT2 inhibitors in patients under 18 years of age have not been established.35,43,47 A study to determine the pharmacokinetic and pharmacodynamic characteristics of dapagliflozin in children and adolescents ages 10 to 17 years with T2DM was completed in September 2014. However, no results have been posted on the ClinicalTrials.gov website for this investigation.60 Another study is recruiting participants between 10 and 18 years of age with T2DM to determine the pharmacokinetics, pharmacodynamics, and safety of canagliflozin.61
Geriatric Use
Compared with younger patients, those 65 years of age and older with T2DM had a higher incidence of AEs related to reduced intravascular volume (such as hypotension, postural dizziness, orthostatic hypotension, syncope, and dehydration) during treatment with canagliflozin, particularly with the 300-mg daily dose. In addition, canagliflozin achieved smaller reductions in HbA1c in patients 65 years of age and older compared with younger patients. Trough concentrations increased slightly with age. The risk of hypoglycemia associated with canagliflozin is only marginally higher than that with placebo, but this risk markedly increases when the drug is used in conjunction with insulin or sulfonylureas in the elderly and in patients with chronic kidney disease.35
Bode and colleagues conducted a phase 3, randomized, double-blind study to determine the long-term efficacy and safety of canagliflozin in 624 patients ages 55 to 80 years with T2DM. After 104 weeks of treatment, canagliflozin 100 mg and 300 mg reduced HbA1c (−0.32 and −0.43 percentage points, respectively) compared with placebo (+0.17). Canagliflozin also reduced FPG and body weight. The canagliflozin groups had a higher incidence of urinary tract infections, genital mycotic infections, osmotic diuresis, and volume depletion compared with the placebo group.62
In another study, dapagliflozin was evaluated in patients 65 years of age and older with T2DM. After controlling for the level of renal function (eGFR), treatment efficacy was similar for patients younger than and older than 65 years. In the latter group, however, a higher proportion of patients treated with dapagliflozin experienced AEs related to volume depletion, renal impairment, or renal failure compared with those given placebo.43
In clinical studies, a total of 491 patients treated with empagliflozin were 75 years of age or older. The efficacy of empagliflozin is expected to be reduced in elderly patients with renal impairment. In these older patients, the risks of volume depletion–related AEs were 2.1%, 2.3%, and 4.4% for placebo, empagliflozin 10 mg, and empagliflozin 25 mg, respectively. The risks of urinary tract infections were 10.5%, 15.7%, and 15.1%.47
Renal Impairment
Canagliflozin (100 mg and 300 mg) was evaluated in a study of T2DM patients that included individuals with moderate renal impairment (eGFR, 30 to 59 mL/min/1.73 m2). These patients showed reduced glycemic efficacy and had higher rates of AEs related to reduced intravascular volume and decreased eGFR compared to patients with mild renal impairment or normal renal function (eGFR greater than or equal to 60 mL/min/1.73 m2). In addition, patients treated with canagliflozin 300 mg were more likely to experience increases in potassium.35
In an open-label study, the pharmacokinetic and pharmacodynamic characteristics of canagliflozin were evaluated in 32 patients with T2DM and hepatic or renal impairment. The authors found that the mean Cmax values were 13%, 29%, and 29% higher and that the mean AUC0−∞ values were 17%, 63%, and 50% higher in subjects with mild, moderate, or severe renal impairment, respectively. They also found that urinary glucose excretion decreased as renal function decreased. These results suggested that the pharmacodynamic response to canagliflozin declines with the increasing severity of renal impairment.63
The efficacy and safety of canagliflozin have not been established in T2DM patients with severe renal impairment (eGFR less than 30 mL/min/1.73 m2) or end-stage renal disease (ESRD), or in those undergoing dialysis. Canagliflozin is not expected to be effective in these patients.35
The safety and efficacy of dapagliflozin were evaluated in a study that included patients with moderate renal impairment (eGFR 30 to 59 mL/min/1.73 m2). Compared with placebo-treated patients, dapagliflozin-treated patients in this group did not show improvements in glycemic control; rather, they had more AEs, including renal-related AEs and bone fractures. Therefore, dapagliflozin should not be initiated in patients with moderate renal impairment. Moreover, based on its mechanism of action, dapagliflozin is not expected to be effective in patients with severe renal impairment (eGFR less than 30 mL/min/1.73 m2) or ESRD.43
In a study conducted in Japan, the AUC after a 25-mg dose of empagliflozin increased by 29%, 44%, and 52% in patients with mild (eGFR, 60 to 89 mL/min/1.73 m2), moderate (eGFR, 30 to 59 mL/min/1.73 m2), or severe (eGFR, 15 to 29 mL/min/1.73 m2) renal impairment, respectively, compared to patients with normal renal function.64 The efficacy and safety of empagliflozin have not been established in patients with severe renal impairment or ESRD, or in those receiving dialysis. Empagliflozin is not expected to be effective in these patients.47
Hepatic Impairment
No dosage adjustment is necessary for the currently available SGLT2 inhibitors in patients with mild or moderate hepatic impairment (Child-Pugh score A or B, respectively). In an open-label study that evaluated the pharmacokinetics, pharmacodynamics, and safety of canagliflozin, the authors found that the mean Cmax and AUC0−∞ values differed by less than 11% between patients with normal hepatic function and those with mild or moderate hepatic impairment. This implies that canagliflozin’s pharmacokinetic characteristics were not affected by mild or moderate hepatic impairment.63
The use of canagliflozin in patients with severe hepatic impairment (Child-Pugh score C) is not recommended.35,43 The drug, however, may be used in patients with hepatic impairment.47
In an open-label, parallel-group study investigating the effects of hepatic impairment on the pharmacokinetics, safety, and tolerability of empagliflozin, 36 subjects (24 with hepatic impairment and 12 with normal hepatic function) received a single 50-mg dose of empagliflozin. AEs were reported in three subjects with moderate hepatic impairment, two subjects with severe hepatic impairment, and six subjects with normal hepatic function. The authors concluded that empagliflozin was well tolerated in the setting of hepatic impairment and that drug exposure was less than twofold greater in these patients. Therefore, the authors suggested that no dose adjustments are required in patients with hepatic impairment.65
MONITORING PARAMETERS
Patients treated with any of the SGLT2 inhibitors should have their HbA1c values checked twice yearly if they are meeting their treatment goals. If they are not meeting their goals or if their therapy has been changed, their HbA1c should be checked every three months. In addition, patients should monitor their FPG regularly as directed by their physicians.1
Clinicians should check their patients’ renal function before initiating SGLT2 inhibitor therapy and periodically thereafter. If a patient has an eGFR of less than 60 mL/min/1.73 m2, more-frequent monitoring may be warranted. The volume status should also be monitored before the start of treatment in patients with renal impairment, in patients with low systolic blood pressure, in elderly patients, and in patients receiving diuretics or medications that affect the renin-angiotensinaldosterone system.66
Moreover, physicians should check LDL-C and serum potassium levels periodically after the initiation of treatment. Blood pressure must be monitored during therapy as well.66
Genital mycotic infections are a common adverse effect of SGLT2 inhibitor therapy.66
In light of the recent FDA drug-safety warning regarding SGLT2 inhibitors and the potential elevation of blood acids (ketones), patients should be monitored for symptoms of ketoacidosis. Patients who experience symptoms such as difficulty breathing, nausea, vomiting, abdominal pain, confusion, and unusual fatigue or sleepiness should seek medical attention immediately. If further evaluation confirms a diagnosis of acidosis, including ketoacidosis, in patients with these signs and symptoms, discontinue the SGLT2 inhibitor and take appropriate measures to correct the acidosis and to monitor glucose levels.59
CONCLUSION
The SGLT2 inhibitors canagliflozin, dapagliflozin, and empagliflozin offer an attractive treatment option for patients with T2DM. The ability of these drugs to increase the excretion of glucose by inhibiting SGLT2, the transporter responsible for a major part of glucose reabsorption, allows improved glycemic control in T2DM patients.
Numerous studies have shown that the SGLT2 inhibitors can help patients achieve their glycemic targets while reducing the incidence of hypoglycemia.40,46,48 This is an important benefit for T2DM patients with risk factors for hypoglycemia, such as advanced age, long-term diabetes, high creatinine levels, a lower body mass index, reduced cognitive function, the use of two or more oral glucose-lowering drugs, a history of smoking or microvascular disease, and the need for intense glucose control.67
Footnotes
Disclosure: The authors report no commercial or financial interests in regard to this article.
REFERENCES
- 1.American Diabetes Association: Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention . National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, Georgia: U.S. Department of Health and Human Services; 2014. [Google Scholar]
- 3.Cheung BM, Ong KL, Cherny SS, et al. Diabetes prevalence and therapeutic target achievement in the United States, 1999 to 2006. Am J Med. 2009;122:443–453. doi: 10.1016/j.amjmed.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 4.Garber AL, Abrahamson MJ, Barzilay JI, et al. American Association of Clinical Endocrinologists’ comprehensive diabetes management algorithm 2013 consensus statement. Endocr Pract. 2013;19(suppl 2):1–48. doi: 10.4158/EP13176.CS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valentine V. The role of the kidney and sodium-glucose cotransporter-2 inhibition in diabetes management. Clin Diabetes. 2012;30:151–155. [Google Scholar]
- 6.List JF, Whaley JM. Glucose dynamics and mechanistic implications of SGLT2 inhibitors in animals and humans. Kidney Int. 2011;79(suppl 120):S20–S27. doi: 10.1038/ki.2010.512. [DOI] [PubMed] [Google Scholar]
- 7.Rizza RA, Gerich JE, Haymond MW, et al. Control of blood sugar in insulin-dependent diabetes: comparison of an artificial endocrine pancreas, continuous subcutaneous insulin infusion, and intensified conventional insulin therapy. N Engl J Med. 1980;303:1313–1318. doi: 10.1056/NEJM198012043032301. [DOI] [PubMed] [Google Scholar]
- 8.Gerich JE. Physiology of glucose homeostasis. Diabetes Obes Metab. 2000;2:345–350. doi: 10.1046/j.1463-1326.2000.00085.x. [DOI] [PubMed] [Google Scholar]
- 9.Wahren J, Felig P, Hagenfeldt L. Physical exercise and fuel homeostasis in diabetes mellitus. Diabetologia. 1978;14:213–222. [Google Scholar]
- 10.Consoli A, Kennedy F, Miles J, et al. Determination of Krebs cycle metabolic carbon exchange in vivo and its use to estimate the individual contributions of gluconeogenesis and glycogenolysis to overall glucose output in man. J Clin Invest. 1987;80:1303–1310. doi: 10.1172/JCI113206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao EC, Henry RR. SGLT2 inhibition: a novel strategy for diabetes treatment. Nat Rev Drug Discov. 2010;9:551–559. doi: 10.1038/nrd3180. [DOI] [PubMed] [Google Scholar]
- 12.Vallon V, Platt KA, Cunard R, et al. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol. 2011;22:104–112. doi: 10.1681/ASN.2010030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bays H. Sodium glucose co-transporter type 2 (SGLT2) inhibitors: targeting the kidney to improve glycemic control in diabetes mellitus. Diabetes Ther. 2013;4:195–220. doi: 10.1007/s13300-013-0042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farber SJ, Berger EY, Earle DP. Effect of diabetes and insulin on the maximum capacity of the renal tubules to reabsorb glucose. J Clin Invest. 1951;30:125–129. doi: 10.1172/JCI102424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mogensen CE. Maximum tubular reabsorption capacity for glucose and renal hemodynamics during rapid hypertonic glucose infusion in normal and diabetic subjects. Scand J Clin Lab Invest. 1971;28:101–109. doi: 10.3109/00365517109090668. [DOI] [PubMed] [Google Scholar]
- 16.Rahmoune H, Thompson PW, Ward JM, et al. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes. 2005;54:3427–3434. doi: 10.2337/diabetes.54.12.3427. [DOI] [PubMed] [Google Scholar]
- 17.Johnson & Johnson U.S. FDA approves Invokana (canagliflozin) for the treatment of adults with type 2 diabetes. Mar 29, 2013. Available at: http://www.jnj.com/news/all/us-fda-approves-invokana-canagliflozin-for-the-treatment-of-adults-with-type-2-diabetes. Accessed April 16, 2015.
- 18.Food and Drug Administration. FDA approves Farxiga to treat type 2 diabetes. Jan 8, 2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm380829.htm. Accessed April 16, 2015.
- 19.Food and Drug Administration FDA approves Jardiance to treat type 2 diabetes. Aug 1, 2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm407637.htm. Accessed April 16, 2015.
- 20.Johnson & Johnson U.S. FDA approves Invokamet (canagliflozin/ metformin HCl) for the treatment of adults with type 2 diabetes. Aug 8, 2014. Available at: https://www.jnj.com/news/all/US-FDA-Approves-INVOKAMET-canagliflozin-metformin-HClfor-the-Treatment-of-Adults-with-Type-2-Diabetes. Accessed April 16, 2015.
- 21.AstraZeneca US FDA approves once-daily Xigduo XR tablets for adults with type 2 diabetes. Oct 30, 2014. Available at: http://www.astrazeneca.com/Media/Press-releases/Article/20141030-us-fda-approve-oncedaily-xigduo-xr. Accessed April 16, 2015.
- 22.Boehringer Ingelheim U.S. FDA approves first-in-class Glyxambi (empagliflozin/linagliptin) tablets for adults with type 2 diabetes. Feb 2, 2015. Available at: http://us.boehringer-ingelheim.com/news_events/press_releases/press_release_archive/2015/2-2-2015-us-fda-approves-first-in-class-glyxambi-empagliflozin-linagliptin-tablets-adults-type-2-diabetes.html. Accessed April 16, 2015.
- 23.Stenlöf K, Cefalu WT, Kim KA, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15:372–382. doi: 10.1111/dom.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang Y, Arakawa K, Ueta K, et al. Effect of canagliflozin on renal threshold for glucose, glycemia, and body weight in normal and diabetic animal models. PLoS One. 2012;7:e30555. doi: 10.1371/journal.pone.0030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu JJ, Lee T, DeFronzo RA. Why do SGLT2 inhibitors inhibit only 30–50% of renal glucose reabsorption in humans? Diabetes. 2012;61:2199–2204. doi: 10.2337/db12-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitabchi A, Umpierrez G, Miles J, et al. Hyperglycemic crisis in adult patients with diabetes. Diabetes Care. 2009;32:1335–1343. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henry RR, Rosenstock J, Edelman S, et al. Exploring the potential of the SGLT2 inhibitor dapagliflozin in type 1 diabetes: a randomized, double-blind, placebo-controlled pilot study. Diabetes Care. 2015;38:412–419. doi: 10.2337/dc13-2955. [DOI] [PubMed] [Google Scholar]
- 28.Perkins B, Cherney D, Partridge H, et al. Sodium-glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: results of an 8-week open-label proof-of-concept trial. Diabetes Care. 2014;37:1480–1483. doi: 10.2337/dc13-2338. [DOI] [PubMed] [Google Scholar]
- 29.Bays H, Weinstein R, Law G, et al. Canagliflozin: effects in over-weight and obese subjects without diabetes mellitus. Obesity (Silver Springs) 2014;22:1042–1049. doi: 10.1002/oby.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolinder J, Ljunggren Ö, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97:1020–1031. doi: 10.1210/jc.2011-2260. [DOI] [PubMed] [Google Scholar]
- 31.Mayo Clinic Counting calories: getting back to weight loss basics. Apr 22, 2015. Available at: http://www.mayoclinic.org/healthy-living/weight-loss/in-depth/calories/art-20048065. Accessed April 28, 2015.
- 32.McCrimmon RJ, Evans ML, Jacob RJ, et al. AICAR and phlorizin reverse the hypoglycemia-specific defect in glucagon secretion in the diabetic BB rat. Am J Physiol Endocrinol Metab. 2002;283:E1076–E1083. doi: 10.1152/ajpendo.00195.2002. [DOI] [PubMed] [Google Scholar]
- 33.Han S, Hagan DL, Taylor JR, et al. Dapagliflozin, a selective SGLT2 inhibitor, improves glucose homeostasis in normal and diabetic rats. Diabetes. 2008;57:1723–1729. doi: 10.2337/db07-1472. [DOI] [PubMed] [Google Scholar]
- 34.Rossetti L, Smith D, Shulman GI, et al. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987;79:1510–1515. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Invokana (canagliflozin) prescribing information. Titusville, New Jersey: Janssen Pharmaceuticals, Inc.; Mar, 2015. Available at: http://www.invokana.com/prescribing-information.pdf. Accessed April 15, 2015. [Google Scholar]
- 36.Stenlöf K, Cefalu WT, Kim KA, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15:372–382. doi: 10.1111/dom.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.University of Tennessee Stats Design and Analysis Web Guide Least squares means. Feb, 2014. Available at: http://dawg.utk.edu/glossary/g_least_squares_means.htm. Accessed January 26, 2015.
- 38.Forst T, Guthrie R, Goldenberg R, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab. 2014;16:467–477. doi: 10.1111/dom.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schernthaner G, Gross J, Rosenstock J, et al. Canagloflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea. Diabetes Care. 2013;36:2508–2515. doi: 10.2337/dc12-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cefalu W, Leiter L, Yoon KH, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52-week results from a randomized, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382:941–950. doi: 10.1016/S0140-6736(13)60683-2. [DOI] [PubMed] [Google Scholar]
- 41.ClinicalTrials.gov The CANTATA-D (Canagliflozin Treatment and Trial Analysis)––DPP-4 Inhibitor Comparator Trial. NCT01106677. Jul 25, 2013. Available at: https://clinicaltrials.gov/ct2/show/NCT01106677?term=nct01106677&rank=1. Accessed April 16, 2015.
- 42.ClinicalTrials.gov The CANTATA-MSU Trial (Canagliflozin Treatment and Trial Analysis––Metformin and Sulfonylureas). NCT01106625. Jun 12, 2013. Available at: https://clinicaltrials.gov/ct2/show/NCT01106625. Accessed April 17, 2015.
- 43.Farxiga (dapagliflozin) prescribing information. Wilmington, Delaware: AstraZeneca; Mar, 2015. Available at: http://www.azpicentral.com/farxiga/pi_farxiga.pdf#page=1. Accessed April 15, 2015. [Google Scholar]
- 44.Ji L, Ma J, Li H, et al. Dapagliflozin as monotherapy in drug-naïve Asian patients with type 2 diabetes mellitus: a randomized, blinded, prospective phase III study. Clin Ther. 2014;36:84–100. doi: 10.1016/j.clinthera.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Bailey C, Gross J, Pieters A, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomized, double-blind, placebo-controlled trial. Lancet. 2010;375:2223–2233. doi: 10.1016/S0140-6736(10)60407-2. [DOI] [PubMed] [Google Scholar]
- 46.Nauck M, Del Prato S, Meier J, et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin. Diabetes Care. 2011;34:2015–2022. doi: 10.2337/dc11-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jardiance (empagliflozin) prescribing information. Ridgefield, Connecticut: Boehringer Ingelheim Pharmaceuticals, Inc.; Aug, 2014. Available at: http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing+Information/PIs/Jardiance/jardiance.pdf. Accessed April 15, 2015. [Google Scholar]
- 48.Ferrannini E, Seman L, Seewaldt-Becker E, et al. A phase IIb, randomized, placebo-controlled study of the SGLT2 inhibitor empagliflozin in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15:721–728. doi: 10.1111/dom.12081. [DOI] [PubMed] [Google Scholar]
- 49.ClinicalTrials.gov Efficacy and safety of empagliflozin (BI 10773) versus placebo and sitagliptin over 24 weeks in patients with type 2 diabetes. NCT01177813. May 16, 2014. Available at: https://clinicaltrials.gov/c/show/results/NCT01177813?term=empagliflozin&rank=19§=X4301256#othr. Accessed April 21, 2015.
- 50.Häring HU, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes. Diabetes Care. 2013;36:3396–3404. doi: 10.2337/dc12-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kovacs CS, Seshiah V, Swallow R, et al. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2014;16:147–158. doi: 10.1111/dom.12188. [DOI] [PubMed] [Google Scholar]
- 52.Devineni D, Curtin CR, Polidori D, et al. Pharmacokinetics and pharmacodynamics of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in subjects with type 2 diabetes mellitus. J Clin Pharmacol. 2013;53:601–610. doi: 10.1002/jcph.88. [DOI] [PubMed] [Google Scholar]
- 53.Kasichayanula S, Liu X, Zhang W, et al. Effect of a high-fat meal on the pharmacokinetics of dapagliflozin, a selective SGLT2 inhibitor, in healthy subjects. Diabetes Obes Metab. 2011;13:770–773. doi: 10.1111/j.1463-1326.2011.01397.x. [DOI] [PubMed] [Google Scholar]
- 54.Riggs M, Stabb A, Seman L, et al. Population pharmacokinetics of empagliflozin, a sodium glucose cotransporter 2 inhibitor, in patients with type 2 diabetes. J Clin Pharmacol. 2013;53:1028–1038. doi: 10.1002/jcph.147. [DOI] [PubMed] [Google Scholar]
- 55.Wilding J, Woo V, Soler N, et al. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin. Ann Intern Med. 2012;156:405–415. doi: 10.7326/0003-4819-156-6-201203200-00003. [DOI] [PubMed] [Google Scholar]
- 56.Wooltorton E. Rosiglitazone (Avandia) and pioglitazone (Actos) and heart failure. CMAJ. 2002;166:219. [PMC free article] [PubMed] [Google Scholar]
- 57.Neal B, Perkovic V, de Zeeuw D, et al. Rationale, design, and baseline characteristics of the Canagliflozin Cardiovascular Assessment Study (CANVAS): a randomized placebo-controlled trial. Am Heart J. 2013;166:217–223. doi: 10.1016/j.ahj.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 58.Neal B, Perkovic V, de Zeeuw D, et al. Efficacy and safety of canagliflozin, an inhibitor of sodium glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care. 2015;38:403–411. doi: 10.2337/dc14-1237. [DOI] [PubMed] [Google Scholar]
- 59.Food and Drug Administration FDA Drug Safety Communication: FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. May 15, 2015. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm. Accessed June 4, 2015.
- 60.ClinicalTrials.gov PK study of dapagliflozin in pediatric subjects with T2DM. NCT01525238. Feb 6, 2015. Available at: https://clinicaltrials.gov/ct2/show/NCT01525238. Accessed April 28, 2015.
- 61.ClinicalTrials.gov A study to evaluate the pharmacokinetics, pharmacodynamics, and safety of canagliflozin in older children and adolescents with type 2 diabetes mellitus. NCT02000700. Apr 17, 2015. Available at: https://clinicaltrials.gov/ct2/show/NCT02000700?term=canagliflozin&rank=1. Accessed April 28, 2015.
- 62.Bode B, Stenlöf K, Harris S, et al. Long-term efficacy and safety of canagliflozin over 104 weeks in patients aged 55–80 years with type 2 diabetes. Diabetes Obes Metab. 2015;17:294–303. doi: 10.1111/dom.12428. [DOI] [PubMed] [Google Scholar]
- 63.Devineni D, Curtin CR, Marbury TC, et al. Effect of hepatic or renal impairment on the pharmacokinetics of canagliflozin, a sodium-glucose cotransporter 2 inhibitor. Clin Ther. 2015;37:610–628. doi: 10.1016/j.clinthera.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 64.Sarashina A, Ueki K, Sasaki T, et al. Effects of renal impairment on the pharmacokinetics, pharmacodynamics, and safety of empagliflozin, a sodium glucose cotransporter 2 inhibitor, in Japanese patients with type 2 diabetes mellitus. Clin Ther. 2014;36:1606–1615. doi: 10.1016/j.clinthera.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 65.Macha S, Rose P, Mattheus M, et al. Pharmacokinetics, safety and tolerability of empagliflozin, a sodium glucose cotransporter 2 inhibitor, in patients with hepatic impairment. Diabetes Obes Metab. 2014;16:118–123. doi: 10.1111/dom.12183. [DOI] [PubMed] [Google Scholar]
- 66.Canagliflozin . DrugPoints Summary Micromedex 20. Truven Health Analytics, Inc; Greenwood Village, Colorado: Available at: http://www.micromedexsolutions.com. Accessed January 28, 2015. [Google Scholar]
- 67.Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363:1410–1418. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]