The Ciliary Inner Dynein Arm, I1 dynein, is assembled in the Cytoplasm and Transported by IFT before Axonemal Docking (original) (raw)
. Author manuscript; available in PMC: 2015 Oct 30.
Published in final edited form as: Cytoskeleton (Hoboken). 2014 Oct 30;71(10):573–586. doi: 10.1002/cm.21192
Abstract
To determine mechanisms of assembly of ciliary dyneins, we focused on the Chlamydomonas inner dynein arm, I1 dynein, also known as dynein f. I1 dynein assembles in the cytoplasm as a 20S complex similar to the 20S I1 dynein complex isolated from the axoneme. The intermediate chain subunit, IC140 (IDA7), and heavy chains (IDA1, IDA2) are required for 20S I1 dynein preassembly in the cytoplasm. Unlike I1 dynein derived from the axoneme, the cytoplasmic 20S I1 complex will not rebind I1-deficient axonemes in vitro. To test the hypothesis that I1 dynein is transported to the distal tip of the cilia for assembly in the axoneme, we performed cytoplasmic complementation in dikaryons formed between wild-type and I1 dynein mutant cells. Rescue of I1 dynein assembly in mutant cilia occurred first at the distal tip and then proceeded toward the proximal axoneme. Notably, in contrast to other combinations, I1 dynein assembly was significantly delayed in dikaryons formed between ida7 and ida3. Furthermore, rescue of I1 dynein assembly required new protein synthesis in the ida7 x ida3 dikaryons. Based on additional observations, we postulate that IDA3 is required for 20S I1 dynein transport. Cytoplasmic complementation in dikaryons using the conditional kinesin-2 mutant, fla10-1 revealed that transport of I1 dynein is dependent on kinesin-2 activity. Thus, I1 dynein complex assembly depends upon IFT for transport to the ciliary distal tip prior to docking in the axoneme.
Keywords: Cilia, flagella, axonemes, Chlamydomonas dikaryon zygotes, I1 dynein
Introduction
Motile cilia/flagella play critical roles in vertebrate development and normal function of organs in the adult (Drummond 2012; Koefoed et al. 2014; Satir and Christensen 2007; Wallingford 2012). Ciliary motility is driven by several different axonemal dynein motors, which are organized into the outer dynein arms (ODAs) and inner dynein arms (IDAs) (Gokhale et al. 2009; King and Kamiya 2009; Porter and Sale 2000) in most organisms. The axonemal dyneins are large multi-subunit complexes, and mutations in genes that encode dynein subunits, or components required for the assembly, transport or docking of the axonemal dyneins, result in a wide range of developmental defects and diseases in the adult (Horani et al. 2014; Zariwala et al. 2007). For example, defective assembly of the dyneins can result in primary cilia dyskinesia (PCD) due to a failure in normal ciliary motility (Knowles et al. 2013b; Zariwala et al. 2011). However, we are just beginning to understand the molecular mechanisms by which dyneins, and other large axonemal complexes, are assembled and targeted in the cilium.
The best-studied axonemal dynein is the ODA, a large complex of at least 16 subunits in Chlamydomonas axonemes including three heavy chains (Hom et al. 2011; King and Kamiya 2009). The ODA complex assembles in a step-wise manner beginning with preassembly in the cytoplasm (“preassembly” is defined as cytoplasmic assembly of complexes destined for the axoneme) (Ahmed and Mitchell 2005; Fowkes and Mitchell 1998) followed by transport to and within the ciliary compartment by the intraflagellar transport (IFT) machinery (Ahmed et al. 2008; Hou et al. 2007). The preassembly of the ODA also requires a number of additional, conserved proteins, which when defective result in a failure in ODA assembly in the axoneme and in many cases PCD (Austin-Tse et al. 2013; Horani et al. 2013; Horani et al. 2012; Hornef et al. 2006; Knowles et al. 2013a; Knowles et al. 2013b; Kobayashi and Takeda 2012; Loges et al. 2009; Loges et al. 2008; Mitchison et al. 2012; Moore et al. 2013; Omran et al. 2008; Onoufriadis et al. 2014; Panizzi et al. 2012; Zariwala et al. 2006). For transport, the ODA16 gene is thought to encode an adapter required for interaction between the preassembled ODA and IFT, also mediated by IFT46 (Ahmed et al. 2008; Hou et al. 2007). Moreover, assembly of the ODA in the axoneme requires additional proteins for docking on the outer doublet microtubule (Casey et al. 2003; Dean and Mitchell 2013; Ide et al. 2013; Onoufriadis et al. 2013; Owa et al. 2014; Panizzi et al. 2012; Wirschell et al. 2004). However, despite these advances, little is known about the role that non-dynein cytoplasmic factors play in the preassembly of the ODA and we do not understand the mechanism of loading and unloading of the ODA from IFT.
Compared to the ODAs, which are relatively homogeneous in structure and composition, repeating every 24 nm, the IDAs are heterogeneous, organized into seven dynein structures (dyneins a, b, c, d, e, f/I1 and g), each distinct in composition and each targeted to a unique position in the axonemal 96 nm repeat (Bui et al. 2012; Goodenough and Heuser 1985; King and Kamiya 2009). Based on phenotypic analysis of Chlamydomonas mutants, the IDAs appear to each contribute to different parameters and regulation of axonemal motility (Brokaw and Kamiya 1987; Kamiya 1991; Kamiya et al. 1991; Kubo et al. 2012; Porter et al. 1992; VanderWaal et al. 2011; Yagi et al. 2005). The mutants also reveal that failure in assembly of one IDA type results in a gap in inner arm structure that repeats every 96 nm (Bui et al. 2012; Heuser et al. 2012; Piperno et al. 1990; Porter et al. 1992). This result indicated that each IDA is targeted to a unique position on the outer doublet microtubule and assembles independent of the other IDAs. Consistent with this interpretation, in vitro reconstitution of IDAs with axonemes lacking a specific subset of inner arm structures also demonstrated that each IDA type is targeted to a unique position in the axonemal 96 nm repeat (Smith and Sale 1992; Yamamoto et al. 2006). Recent evidence also indicates that preassembly of IDAs requires additional non-dynein cytoplasmic proteins (Tarkar et al. 2013; Yamamoto et al. 2010) and the IFT complex B protein TTC26/DYF13 (Ishikawa et al. 2014). However, in contrast to the ODA, little is known about the assembly, transport and targeted docking of the IDAs.
To address questions of assembly of the IDAs, we focused on the I1 dynein, also known as dynein f (Kagami and Kamiya 1992; Piperno et al. 1990; Wirschell et al. 2007). I1 dynein is a complex located at the proximal most position in the 96 nm repeat, and unlike the other IDAs, which are single headed-dyneins each containing a single heavy chain, I1 dynein is a two headed dynein containing two distinct heavy chains, 1α and 1β (Myster et al. 1997; Myster et al. 1999; Perrone et al. 2000) that are distinct in function (Kotani et al. 2007; Toba et al. 2011). In addition to the 1α and 1β heavy chains, I1 dynein is composed of three intermediate chains IC140, IC138 and IC97 (Bower et al. 2009; Hendrickson et al. 2004; Perrone et al. 1998; Porter et al. 1992; VanderWaal et al. 2011; Wirschell et al. 2009; Yang and Sale 1998), five different light chains, TcTex1, TcTex 2b, LC7a, LC7b and LC8 (Bowman et al. 1999; DiBella et al. 2001; DiBella et al. 2004a; DiBella et al. 2004b; Harrison et al. 1998; Wirschell et al. 2009) and FAP120 (Ikeda et al. 2009). When isolated from the axoneme, I1 dynein sediments as a 20S complex (Piperno et al. 1990). While the molecular composition of I1 dynein is known, several questions remain unanswered. Where is the 20S I1 complex assembled? Is I1 dynein transported by IFT for assembly in the axoneme? How is I1 dynein localized and docked on the outer doublet microtubule?
In this study, we take advantage of Chlamydomonas wild-type and select mutant cells defective in I1 dynein assembly and test the hypotheses that the I1 complex preassembles in the cytoplasm and is then transported by IFT for docking in the axoneme (Fig. 1). We show that I1 dynein preassembles as a 20S complex in the cytoplasm, that preassembly of the 20S complex requires the heavy chains and IC140 and that the 20S complex is transported to the distal tip of the cilium for docking in the axoneme. We further show that transport requires IFT and predict that the I1 dynein – IFT interaction also requires the gene product encoded by IDA3.
Figure 1. Schematic model depicting steps leading to I1 dynein assembly in the axoneme.
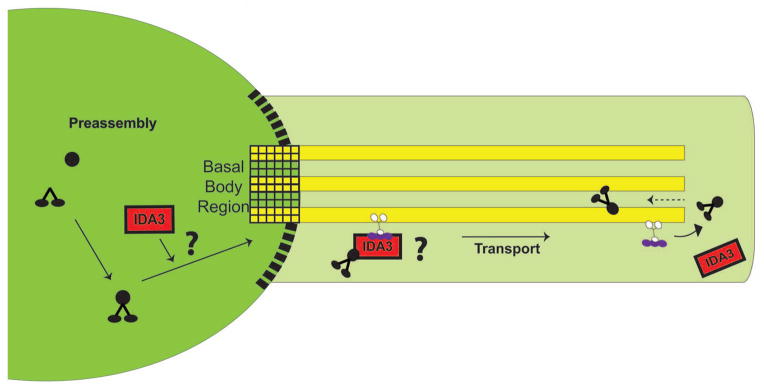
We tested the hypotheses that I1 dynein preassembles in the cytoplasm and that the preassembled I1 complex is transported to the distal tip of the cilium by IFT. Based on the characterization of the ida3 mutant in this paper, we hypothesize that IDA3, a cytoplasmic protein is required for the entry or transport of I1 dynein. IDA3 could be a modifier required for I1 dynein transport or an adapter linking I1 dynein to IFT (both possibilities indicated by a “?”). The postulated barrier between the cytoplasm and the ciliary compartment is shown as black boxes.
Results
I1 dynein preassembles as a 20S complex in the cytoplasm
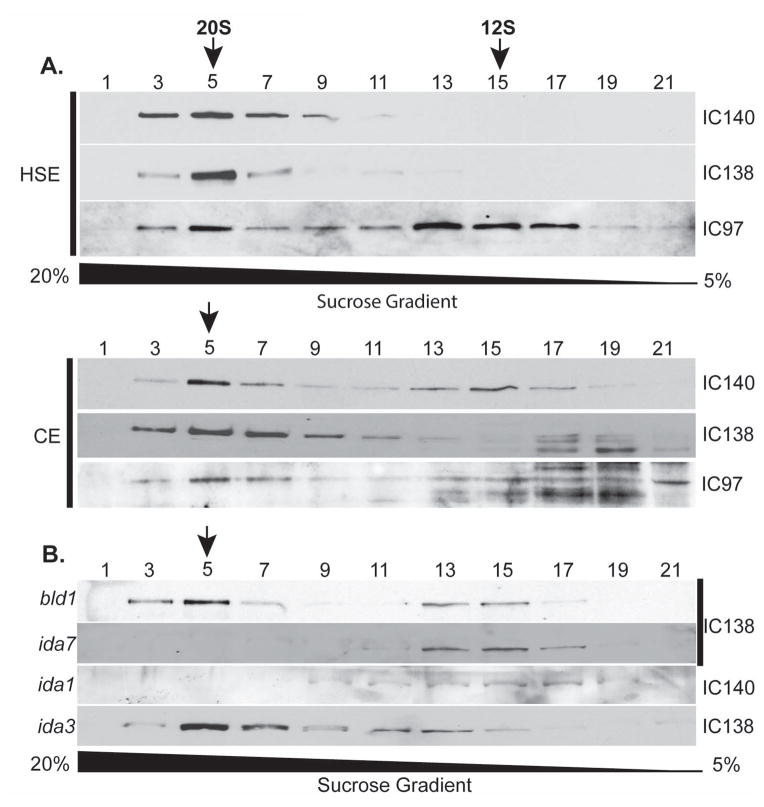
Axonemal I1 dynein is a multisubunit complex that, when fully assembled, sediments at 20S (Piperno et al. 1990; Porter et al. 1992; Smith and Sale 1991). To test the hypothesis that I1 dynein preassembles as a 20S complex in the cytoplasm prior to transport to the cilia and axoneme, we fractionated cytoplasmic extracts from wild-type cells by velocity sedimentation on sucrose gradients (Fig. 2A). Similar to the axonemal I1 dynein, cytoplasmic I1 dynein sediments at 20S, suggesting the complex is fully assembled in the cell body (Fig. 2). Axonemal and cytoplasmic fractions were probed with antibodies to various I1 subunits to confirm assembly. The 20S complex from the axoneme and cytoplasm contains both heavy chains (HC1α and HC1β) (data not shown) and three intermediate chains (IC138, IC140 and IC97) (Fig. 2A). Immunoblots of the cytoplasmic 20S I1 dynein also revealed the presence of the light chains TcTex1, TcTex 2b, LC7a, LC7b and LC8 in the complex (data not shown).
Figure 2. I1 dynein assembles in the cytoplasm as a 20S complex.
(A) Immunoblots of fractions from velocity sedimentation of axonemal high salt extracts (HSE) and cytoplasmic extracts (CE) were analyzed. The I1 dynein subunits IC140, IC138 and IC97 co-sediment at fraction 5 (arrows) in both axonemal high salt extracts and cytoplasmic extracts from wild-type cells. (B) Cytoplasmic extracts derived from bld1, ida1, ida3 and ida7 were fractionated by velocity sedimentation and analyzed by immunoblots using IC138 or IC140 as a marker of I1 dynein. Cytoplasmic I1 dynein from bld1 and ida3 cosediment with wild-type 20S I1 complex. The 20S I1 complex is missing in the I1 dynein heavy chain mutant ida1 and ida2 (data not shown) and in the IC140-null mutant ida7.
Given that cytoplasmic extracts contain a 12S radial spoke precursor complex and an intact 20S radial spoke that has been shown to be recycled from the ciliary axoneme (Diener et al. 2011; Qin et al. 2004), it is possible that the 20S I1 complex observed in the cytoplasm is also a recycled axonemal product. To test this idea, cytoplasmic extracts from the aflagellate mutant bld1 (Brazelton et al. 2001), which lacks cilia and recycled axonemal components, were fractionated and examined for the presence of the 20S I1 complex. The sedimentation profile from bld1 extracts shows that the 20S I1 complex forms as in wild-type extracts (Fig. 2B) indicating that the cytoplasmic 20S I1 complex is formed in the cytoplasm before transport to the cilium.
The assembly of I1 dynein in the axoneme is dependent on the heavy chains (Myster et al. 1997; Myster et al. 1999; Perrone et al. 2000) and the intermediate chain IC140 (Perrone et al. 1998). We hypothesized that HC1α, HC1β and IC140 are also required for the cytoplasmic preassembly of the 20S I1 dynein complex. To test this, we examined I1 dynein assembly in cytoplasmic extract fractions from pf9-3/ida1 (HC1α-null), ida2-6 (HC1β-null) and ida7 (IC140-null) (Myster et al. 1997; Myster et al. 1999; Perrone et al. 2000; Perrone et al. 1998). As predicted, the 20S I1 dynein complex failed to assemble in cytoplasmic extracts from ida1, ida2 (data not shown) and ida7 (lower panel, Fig. 2B). Therefore, both the heavy chains and IC140 are required for 20S complex preassembly (Fig. 2B).
The cytoplasmic 20S I1 dynein complex preassembles in ida3 but is defective in entry to the ciliary compartment
In the ida3 mutant, I1 dynein fails to assemble in the axoneme (Kamiya et al. 1991). The IDA3 gene is not yet identified, however the ida3 mutant has proven to be informative. We reasoned that failure in assembly in the axoneme is a consequence of defective 20S I1 complex assembly in the cytoplasm. To test this, we compared wild-type and ida3 cytoplasmic fractions for the presence of the 20S I1 complex. Immunoblot analysis indicated that I1 dynein from ida3 cytoplasmic extracts forms a 20S complex that co-sediments with the I1 complex from wild-type extracts (Fig. 2B). Moreover, the 20S I1 complex in the ida3 cytoplasm has the same heavy and intermediate chain composition as in wild type (data not shown). Thus, the ida3 mutant is not defective in preassembly of the 20S I1 complex.
We next tested the hypothesis that ida3 is defective in I1 docking in the axoneme. We applied the in vitro reconstitution assay, which takes advantage of I1 dynein’s ability to rebind to I1 depleted axonemes at precise locations along the axoneme in vitro (Smith and Sale 1992). Varying amounts of I1 dynein isolated from oda2 axonemes (Fig. 3, HSE) or cytoplasm (Fig. 3, cyt) were combined with a fixed amount of ida3 axonemes and ida7 control axonemes in the presence of ATP (Toba et al. 2011; Yamamoto et al. 2006). Immunoblot analysis with the I1 intermediate chain IC140 indicated that I1 dynein from oda2 axonemal extracts (Fig. 3, HSE) is capable of rebinding equally to ida3 and ida7 mutant axonemes (Fig. 3, P). In both cases, I1 bound the axonemes to saturation, indicating that I1 is binding to specific sites along the axoneme. This result demonstrated that ida3 axonemes are able to support I1 docking in the axoneme, and IDA3 is not likely an axonemal docking component.
Figure 3. The ida3 mutant is not defective in docking of I1 dynein to the axonemes in vitro.
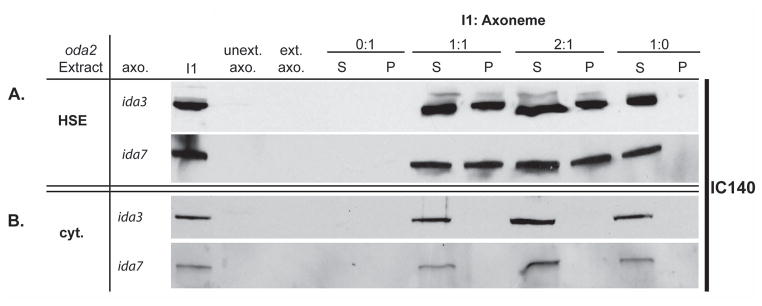
(A) High salt extracts (HSE) containing I1 dynein from oda2 axonemes were reconstituted onto salt-extracted I1-deficient ida3 and ida7 axonemes in the presence of 1mM ATP. Varying ratios of axonemal I1 (HSE) to extracted axonemes (I1: Axonemes) were combined in an ATP-containing buffer, incubated on ice for 30 min and probed with IC140 as a marker for I1 dynein in immunoblots analysis. Each mixture was centrifuged to separate the supernatant (S = unbound I1 dynein) and pellet (P = bound I1 dynein) fractions. Axonemal I1 (HSE) reconstituted onto both ida3 and ida7 axonemes results in I1 dynein binding to the axonemes to saturation (P = pellet/bound I1). (B) I1 dynein-containing cytoplasmic extracts (cyt.) from oda2 were reconstituted onto ida3 and ida7 axonemes in the presence of ATP. Cytoplasmic I1 dynein (cyt.) does not bind to the axoneme as indicated by I1 presence in the supernatant (S) only.
We then used in vitro reconstitution to test the hypothesis that 20S cytoplasmic I1 dynein assembles in a form competent to rebind axoneme. Cytoplasmic extracts from oda2 (containing wild-type 20S I1 dynein) were combined with a fixed amount of ida3 or ida7 axonemes in the presence of ATP. In contrast to isolated axonemal I1 dynein (Fig. 3A), cytoplasmic I1 dynein, from either oda2 or ida3 cytoplasm, failed to reconstitute with either ida3 or ida7 axonemes (Fig. 3B). The same results were obtained using the antibody to IC138 to assess in vitro reconstitution. Thus, the preassembled 20S I1 dynein, despite assembling as a 20S complex, is not competent to bind the axoneme.
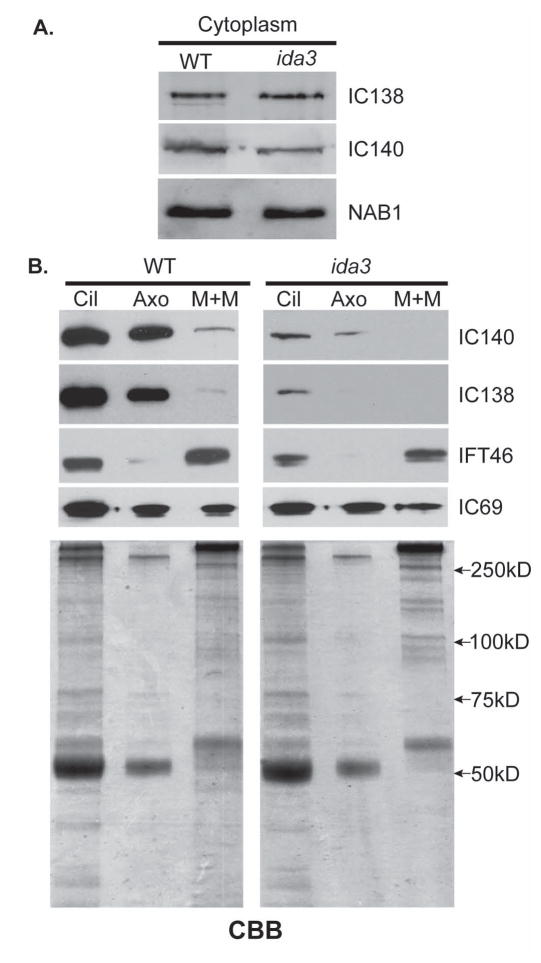
Since ida3 is neither deficient in the preassembly of 20S I1 dynein (Fig. 2B) nor in in vitro docking of axonemal I1 dynein (Fig. 3A), we tested whether I1 dynein is present in the ciliary compartment in ida3. We first confirmed that the preassembled 20S I1 complexes were present at a similar concentration in ida3 and wild-type extracts (data not shown). We examined the membrane plus matrix (M+M) fractions, the ciliary fraction in which IFT complexes and their cargoes are typically present (Cole et al. 1998; Piperno and Mead 1997; Qin et al. 2004). Detergent (NP-40) generated wild-type and ida3 M+M fractions were loaded at five times the relative volume of the cilia and axoneme fractions, to detect I1 dynein in the M+M. The fractions were then analyzed by immunoblots for the presence of I1 dynein (Fig. 4). As expected, I1 dynein was significantly reduced in ida3 cilia and axonemes compared to wild type. In addition, I1 dynein is present in the wild-type M+M fraction (Fig. 4, top left panels). In contrast, at the same protein concentration, I1 dynein is missing in M+M fraction from ida3 (Fig. 4, top right panels). The same results were obtained using a freeze-thaw method to generate the M+M fraction (data not shown) (Behal and Cole 2013). Thus, compared to wild-type, the 20S I1 complex does not appear to enter the ciliary compartment in ida3 mutant cells.
Figure 4. The 20S I1 dynein assembled in the ida3 mutant cytoplasm is defective in entry to the ciliary compartment.
(A) Immunoblots comparing I1 intermediate chains, IC138 and IC140, in wild-type and ida3 cytoplasmic extracts. NAB1 was used a marker and loading control (Mussgnug et al. 2005) (B) Immunoblots of ciliary (Cil), axonemal (Axo) and membrane + matrix (M+M) fractions were analyzed using antibodies to the IC138 and IC140 subunits of I1 dynein. For detection of axonemal subunits (IC69, IC140, IC138), the M+M was loaded at five times the relative amount of cilia and axonemes. Analysis showed a significant reduction in ciliary and axonemal I1 dynein in ida3 compared to wild-type. In addition, M+M fractions show that I1 dynein is absent in ida3 compared to wild-type indicating inefficient entry into the ciliary compartment. The M+M samples from WT and ida3 were also examined by immunoblots at twice the protein load (data not shown), and I1 dynein subunits were never present in ida3. The outer arm component, IC69, is present in equivalent amounts in WT and ida3 M+M and serves as a control for IFT cargo. The IC69 immunoblot also validates that the defect in ida3 is specific to I1 dynein. IFT46 serves as a positive control for M+M fractionation. CBB = Coomassie Brilliant Blue loading control.
I1 dynein is transported to the distal tip of the cilium prior to docking onto the axoneme
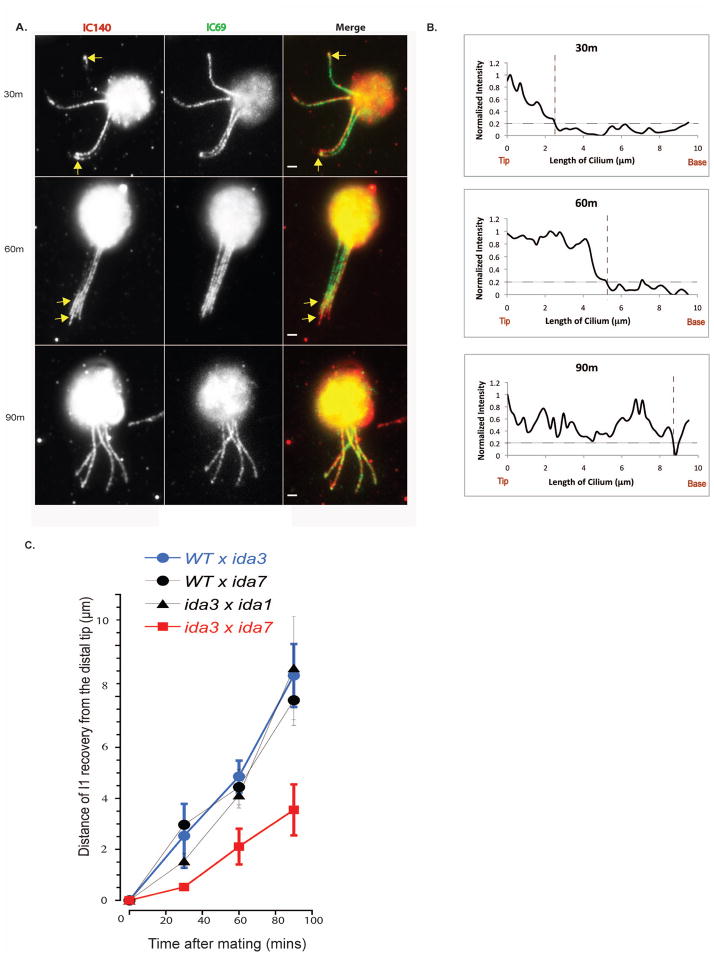
Since IFT typically transports cargo to the distal tip of the cilium prior to docking onto the axoneme (Wren et al. 2013), we performed dikaryon rescue experiments to determine the pattern of I1 dynein assembly (Bower et al. 2013; Johnson and Rosenbaum 1992; Piperno et al. 1996); reviewed in (Dutcher 2014). Wild-type and I1-deficient (ida1, ida3 and ida7) cells were mated to allow for cytoplasmic complementation in temporary dikaryons. Resulting dikaryons were fixed at 30, 60, and 90 minutes for immunofluorescence with an antibody to IC140 as a marker for I1 dynein (Fig. 5A). By 30 minutes, I1 dynein assembly began at the distal tip of the ida7 axoneme and proceeded toward the proximal end (Fig. 5A, yellow arrows). I1 dynein assembled along the entire length of the axoneme in less than 90 minutes (Fig. 5A, bottom panels). We estimated the rate of I1 dynein assembly along the axoneme from tip to base by generating fluorescence intensity profiles of individual cilia undergoing I1 dynein rescued-assembly at each time point (Fig. 5B). The length of I1 dynein assembly along the axoneme was measured from the distal tip to a point defined when the normalized fluorescence intensity dropped below 0.2 AU (Fig. 5B, dashed lines; see (Alford et al. 2013). Quantification of I1 staining from the tip revealed that assembly occurred progressively starting at the distal tip and proceeding to proximal end of the axoneme (Fig. 5C, blue line). Therefore, I1 dynein is transported to the tip of the cilium before incorporation into the axoneme.
Figure 5. Dikaryon rescue of I1 dynein assembly occurs from the distal tip.
(A) Immunofluorescence of IC140 in ida7 x wild-type (WT) dikaryons. Cytoplasmic complementation in WT and I1-deficient dikaryons results in the rescue of I1 dynein assembly (arrows) from the distal tip to base in the ida7 mutant cilia. Scale bar = 2μm. (B) Quantification of I1 dynein staining along the cilia of I1 mutants at various time points post mating. Normalized fluorescence intensity is plotted against the distance of rescue from the tip for one rescuing mutant cilium of the WT x ida7 dikaryon shown in part A. The length of I1 assembly at the ciliary tip (vertical dotted line) increased progressively over time: 2.5 μm at 30m, 5.25 μm at 60m, and 8.75 μm at 90m. (C) Comparison of dikaryon rescue rates of I1 assembly in various mutant combinations. The length of IC140 staining at the distal end of the cilium is plotted versus time. WT x ida3 (blue circles, n = 83) and WT x ida7 (black circles, n = 29) dikaryons demonstrate progressive rescue of axonemal I1 dynein assembly with complete recovery at 90m. Dikaryons between ida3 and ida1 show this same pattern of rescue along all four cilia (black triangles, n = 59). Rescue of axonemal I1 dynein assembly is delayed in ida3 x ida7 dikaryons (red squares, n = 30), but occurs from the distal tip like other dikaryon combinations.
We also tested whether cytoplasmic complementation could rescue the assembly of I1 dynein in ida3 axonemes. Dikaryons between wild-type and ida3 illustrated rescue in the same tip to base assembly pattern compared to wild-type x ida7 dikaryons (Fig. 5C, compare black circle and triangle line). Similar to wild-type x ida7 and wild-type x ida3 dikaryons, rescue of axonemal I1 dynein assembly was seen in ida3 x ida1 dikaryons (Fig. 5C, black diamond line). In addition, in ida3 x ida1 and ida3 x ida7 dikaryons, the rescue of I1 dynein assembly was synchronous in all four cilia. However, in contrast to other combinations, the rate of I1 axonemal assembly is delayed in ida3 x ida7 dikaryons relative to other dikaryon combinations examined (Fig. 5C, red line).
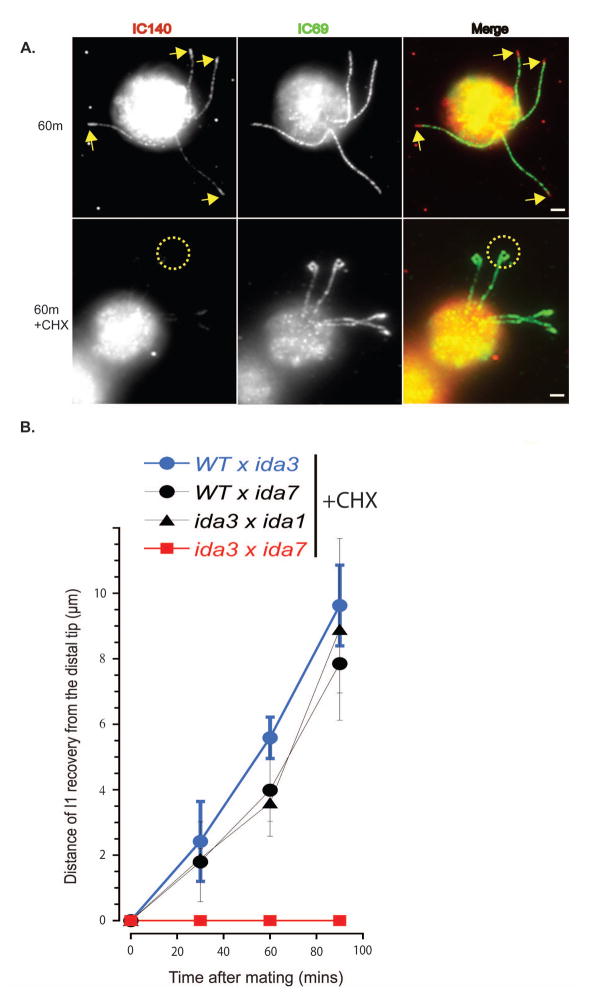
We hypothesized that the delay in rescued assembly of I1 dynein in the ida7 x ida3 dikaryons was due to the requirement of new protein synthesis. To test this, we repeated dikaryon rescue in the presence of the protein synthesis inhibitor cyclohexamide (Fig. 6). In wild-type x ida3, wild-type x ida7, and ida3 x ida1 dikaryons, rescued axonemal I1 dynein assembly occurred at the same rate and in the same tip to base assembly pattern irrespective of the presence (Fig. 6A, lower panel and Fig. 6B) or absence (Fig. 6A, upper panel, Fig. 6B) of cyclohexamide. In contrast to other dikaryon combinations tested in the presence of cycloheximide, rescue of I1 dynein assembly was blocked in ida3 x ida7 dikaryons (Fig. 6A, yellow circles; 6B, red line and boxes). Thus, the delay in rescue of I1 dynein assembly is due to a requirement for new protein synthesis in the ida3 x ida7 dikaryon. One interpretation of these results is that IC140 and IDA3 form a complex that requires the presence of one or both proteins for complex formation and stability (see Discussion).
Figure 6. Dikaryon rescue of axonemal I1 assembly requires protein synthesis of IC140 and/or IDA3.
(A) Immunofluorescence of IC140 in ida3 x ida7 dikaryons 60 minutes after mixing gametes. Rescue of axonemal I1 dynein assembly is seen at the distal end as in Figure 5A (arrows, top panel). In contrast, in the presence of the protein synthesis inhibitor, cyclohexamide (CHX), the recovery of I1 dynein is not seen in the cilia (dotted circle, bottom panel). Scale bar = 2μm. (B) Quantification of IC140 staining from the distal tip of I1-deficient cilia as in Figure 5B in the presence of CHX. Axonemal I1 dynein assembly occurs progressively from the distal tip to base in WT x ida3 (blue circles, n = 25), wild-type x ida7 (black circles, n = 25) and ida3 x ida1 (black triangles, n = 22) dikaryons. In contrast I1 dynein is not assembled on the axoneme in ida3 x ida7 dikaryons (red squares, n = 29).
Transport of I1 dynein requires the IFT anterograde motor, kinesin-2
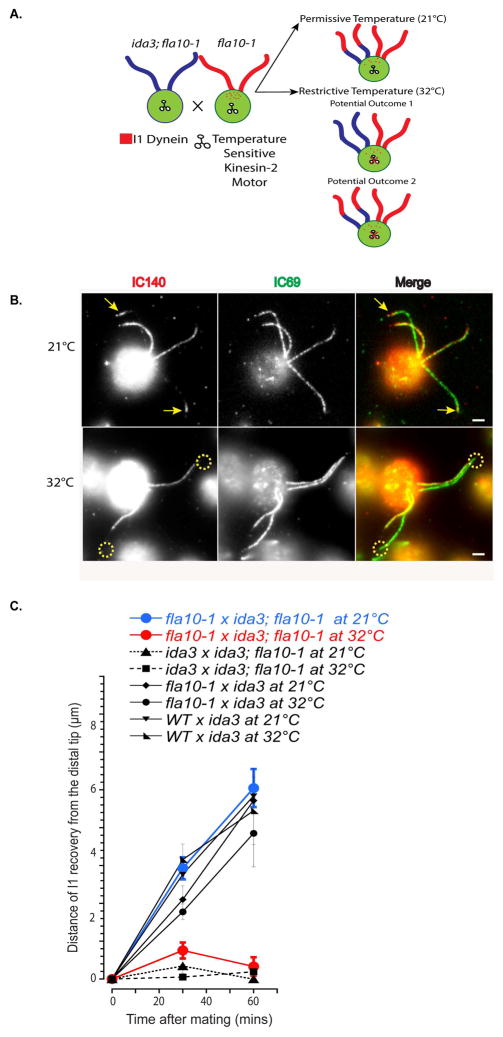
Based on our observations that I1 dynein is transported to the distal tip before docking to the axoneme in dikaryons, and recent evidence showing axonemal components such as the DRC are transported by IFT (Wren et al. 2013), we proposed that I1 transport to the distal tip is mediated by IFT. To test this idea, we took advantage of the conditional mutant, fla10-1, a temperature-sensitive allele of the KHP1 locus encoding the heterotrimeric kinesin-2 motor required for anterograde IFT (Kozminski et al. 1995; Walther et al. 1994). An ida3; fla10-1 double mutant was generated to perform cytoplasmic complementation in dikaryons. The design of the experiment and potential outcomes are illustrated in Fig. 7A (Here we use a semicolon to separate each unlinked mutation in the double mutants - www.cell.com/trends/genetics/pdf/S0168-9525(98)80010-3.pdf).
Figure 7. Transport of I1 to the tip of the cilium requires kinesin-2.
(A) Diagram of dikaryon rescue experimental design using the temperature sensitive mutant, fla10-1. (B) Immunofluorescence of IC140 in fla10-1 x ida3; fla10-1 dikaryons 60 minutes after mixing gametes. At the permissive temperature (21°C), recovery of axonemal I1 dynein assembly occurs from the distal end of ida3 cilia (arrows). At the restrictive temperature (32°C), I1 dynein assembly on the axoneme is not seen in ida3; fla10-1 cilia (dotted circles). Scale bar = 2μm. (C) Quantification of IC140 staining at the distal end of I1-deficient cilia at permissive (21°C) and restrictive (32°C) temperatures. Axonemal I1 dynein assembly occurs progressively from the distal tip to base in wild-type x ida3 (n = 19) and fla10-1 x ida3 (black diamonds and black circles, n = 39) dikaryons at both permissive and restrictive temperatures. While I1 assembly at the distal tip is seen at the permissive temperature in fla10-1 x ida3; fla10-1 dikaryons (blue circles, n = 52), I1 assembly at the tip does not occur at restrictive temperature (red circles, n = 19). As a control, ida3; fla10-1 x ida3 dikaryons were tested for a lack of I1 axonemal assembly at both temperatures (black dotted triangles and squares, n = 46).
We analyzed fla10-1 x ida3; fla10-1 dikaryons at permissive (21°C) and restrictive (32°C) temperatures for rescue of axonemal I1 dynein assembly from the ciliary tip. Dikaryons were fixed at two time-points post-mixing for immunofluorescence with the IC140 antibody. As described before (Pan and Snell 2002; Piperno et al. 1996), the number of zygotes formed at restrictive temperature (32°C) was reduced as compared to permissive temperature (21°C). However, sufficient numbers of fla10-1 x ida3; fla10-1 dikaryons were formed for analysis. At permissive temperature (21°C), I1 assembly was observed at the distal tip of ida3; fla10-1 mutant cilia in the dikaryon (Fig. 7B, arrows) and progressed towards the proximal base end of the axoneme (Fig. 7C, blue line). Complete rescue of I1 assembly along the length of ida3; fla10-1 cilia was seen after 90 minutes (data not shown). In contrast, at restrictive temperature (32°C) rescue of I1 assembly did not occur in the ida3; fla10-1 mutant cilia (Fig. 7B, dashed circles; Fig. 7B, red line). This result provides strong evidence that I1 dynein transport to the distal tip of the cilium requires functional kinesin-2 and IFT.
To control for secondary effects of temperature variation on dikaryon rescue, fla10-1 x ida3 and wild-type x ida3 dikaryons were also analyzed by immunofluorescence for rescued I1 assembly at permissive and restrictive temperatures. As expected, we observed rescue of I1 dynein in the dikaryons formed between fla10-1 x ida3 and WT x ida3, at both temperatures. Rescue of I1 assembly occurred at comparable rates from tip to base, indicating that increase in temperature had no effect on the function of the wild type proteins, FLA10 and IDA3 (Fig. 7C). As an additional essential control, ida3 x ida3; fla10-1 dikaryons (lacking IDA3) were analyzed since rescue is not expected at either temperature. Consistent with this prediction, ida3 x ida3; fla10-1 dikaryons do not rescue I1 assembly in any of the four ciliary axonemes at either temperature (Fig. 7C, black dashed lines). Together, these results indicate that that kinesin-2/IFT is required for I1 transport to the distal tip of the axonemes.
Discussion
Our goal was to define the mechanisms of assembly of ciliary I1 dynein. The primary conclusions from the data presented here include: the 20S I1 complex is preassembled in the cytoplasm; assembly of the cytoplasmic 20S I1 complex requires the heavy chains and intermediate chain IC140 and is not a recycled product of the axoneme; the 20S I1 is transported by IFT to the distal tip of the cilium before docking in the axoneme. In addition, as indicated in the model (Fig. 1), IFT and IDA3 are required for transport of I1 dynein. Other evidence also indicates transport of I1 dynein is facilitated by the TTC26/DYF13/IFT56 subunit of the IFT B complex (Ishikawa et al. 2014), which may be functionally analogous to the IFT46 subunit required for ODA transport (Hou et al. 2007).
One of the surprising observations in this study includes the failure of the 20S I1 from the cytoplasm to reconstitute I1-deficient axonemes in the presence of ATP (Fig. 3B). This result is in contrast to reconstitution using the 20S I1 dynein derived from the axoneme: I1 dynein derived from the axoneme specifically restores I1 dynein to I1 dynein-depleted axonemes (Fig. 3A and (Smith and Sale 1992; Yamamoto et al. 2006). One possibility is that the cytoplasmic 20S I1 complex lacks one of the light chains required for docking in the axoneme. While the light chains have been detected in the cytoplasmic 20S complex, we cannot definitively assign these light chains to the I1 dynein complex: the same light chains are shared with the outer dynein arms, radial spokes and the cytoplasmic dynein (King and Kamiya 2009). Thus, refined purification of the cytoplasmic I1 dynein (e.g. (Toba et al. 2011) is required to test this idea and to definitively determine the light chain composition of the cytoplasmic complex.
It is equally possible that the I1 complex must be modified before docking onto the axoneme. For example, before docking on the axoneme, the radial spokes undergo additional assembly and modification at the ciliary distal tip involving protein assembly and altered phosphorylation (Diener et al. 2011; Gupta et al. 2012; Yang et al. 2005). Similarly, I1 dynein may require further modification at the distal tip prior to docking to the axoneme. Future studies will be directed towards understanding how these large preassembled complexes acquire microtubule binding competency in the axoneme.
The ida3 mutant is defective in entry and/or transport of the I1 dynein complex
The ida3 mutant was isolated in a screen to identify mutations in assembly or function of the inner dynein arms (Kamiya et al. 1991). We confirmed that the ida3 mutant is defective in I1 dynein assembly in the axoneme (Fig. 4) and that the ida3 mutation maps to chromosome 3 (our unpublished data). While we have not yet identified the IDA3 gene, the ida3 mutant has proven to be very informative with regard to I1 dynein assembly. A key observation is that the 20S I1 complex preassembles in the ida3 cytoplasm as in wild-type (Fig. 2B). The simplest interpretation of this result is that IDA3 is not a factor required for I1 dynein preassembly. Another possibility is that IDA3 encodes an axonemal protein required for docking I1 dynein, analogous to ODA-DC proteins (Casey et al. 2003; Dean and Mitchell 2013; Ide et al. 2013; Owa et al. 2014; Takada et al. 2002; Wirschell et al. 2004). However, our in vitro reconstitution analysis does not support this model and indicates that the ida3 axoneme is not defective in I1 dynein docking.
Based on our observation that I1 dynein preassembles in the ida3 mutant cytoplasm but not in the axoneme, the simplest model is that IDA3 is required for transport of I1 dynein into the ciliary compartment (Fig. 1). Consistent with this model, the I1 dynein complex is preassembled in the cytoplasmic compartment (Fig. 2B), but I1 dynein does not appear in the M+M fraction of ida3 cilia (Fig. 4). As illustrated in Figure 1, IDA3 may be an adapter that selectively links I1 dynein to IFT for entry and transport within the ciliary compartment. The adapter model is founded on studies of ODA16 as an adapter that mediates interaction between the ODA and IFT46 (Ahmed et al. 2008; Hou et al. 2007). Additionally, new evidence indicates IFT, and associated axonemal cargoes (such as the radial spokes), are carried with membrane vesicles destined to the cilium (Wood and Rosenbaum 2014). Furthermore, based on studies of N-DRC (nexin-dynein regulatory complex) transport (Wren et al. 2013), IFT is required for both entry into and transport within the ciliary compartment. An alternative to the adapter model is that the IDA3 protein is a modifier, such as a protein kinase, that is required for selective control of interaction between the I1 complex and IFT and entry into the cilium. Definitive tests of these ideas require the identification of IDA3 and characterization of the IDA3 protein.
I1 dynein is transported to the distal tip by IFT
Cilia assemble by addition of tubulin and protein complexes at the distal end of the growing axoneme (Johnson and Rosenbaum 1992; Witman 1975). Although not necessarily identical to de novo ciliary assembly, analysis of rescued assembly of I1 dynein in the cilia of Chlamydomonas quadraflagellate dikaryons has been a particularly powerful approach demonstrating many proteins are transported to the distal tip of the cilium for incorporation in the axoneme (Bower et al. 2013; Johnson and Rosenbaum 1992; Piperno et al. 1996; Wren et al. 2013); reviewed in (Dutcher 2014). Consistently, analysis of dikaryons, generated using at least one I1 dynein assembly mutant such as ida1, ida3 or ida7, revealed that the I1 dynein complex is transported to the ciliary distal tip before incorporation in the axoneme (Fig. 5). Furthermore, quantification of assembly as a function of time demonstrated that assembly begins at the distal axoneme and then proceeds progressively toward the proximal axoneme until rescue is complete (Fig. 5C). With the exception of one dikaryon combination, ida3 x ida7, which displayed a delayed rescue of I1 dynein assembly, the relative rate of tip to base rescue was uniform across several different dikaryon combinations (Fig. 5C, wild-type x ida3; wild-type x ida7; ida1 x ida3). Furthermore, in our assay the rate of rescue of I1 dynein was synchronous for both cilia in dikaryons made from complementary I1 dynein assembly mutants (e.g. ida1 x ida3; ida1 x ida7).
Our assay also resolved a delay in rescue in I1 dynein assembly in the ida3 x ida7 dikaryons (Fig. 5C, red boxes). We determined that the delay in I1 dynein assembly in the axonemes from the ida3 x ida7 dikaryon combination is due to a requirement of new protein synthesis (Fig. 6). In contrast, new protein synthesis was not required for I1 dynein assembly in axonemes from the ida3 x ida1 dikaryon (Fig. 6B). One interpretation of these results is that IC140, the gene product of IDA7, and the IDA3 protein form a complex, and that this interaction cannot be complemented in the ida3 x ida7 dikaryon without new protein synthesis. This hypothesis may be consistent with a model in which IDA3 interacts with IC140 as an adapter required for I1 dynein transport into the ciliary compartment (Fig. 1).
Upon entry into the cilium, the 20S I1 dynein is transported to the ciliary tip by IFT. This conclusion is based on the failure of rescue of I1 dynein assembly upon inactivation of the IFT anterograde motor kinesin-2 at restrictive temperature (see Methods and (Kozminski et al. 1995; Piperno et al. 1996). Additionally, failure of I1 dynein rescue in the ida3 and _ida3; fla10_-1 dikaryons (Fig. 7C) revealed that a functional copy of IDA3 is essential for I1 assembly in the axoneme. Once I1 is unloaded from the transport complex, I1 dynein may diffuse towards the base (dashed arrow, Fig. 1), similar to DRC4, followed by incorporation into the axoneme at unoccupied I1 dynein docking sites (Wren et al. 2013). This result also indicates that the majority of the I1 dynein complex remains associated with IFT until reaching the distal tip where unloading of cargo presumably occurs. This interpretation is consistent with the live cell imaging study of Wren et al., (Wren et al. 2013) in which the majority of the N-DRC is transported as a cargo to the distal tip of the axoneme prior to release and diffusion. However, diffusion of I1 dynein, prior to docking in the axoneme, cannot be assessed by the assays we employed in this paper. Live cell imaging will be required for further tests of how I1 dynein enters the ciliary compartment and to determine the sites of loading, rate of assembly and diffusion after unloading from IFT.
Materials and Methods
Cell Strains and Culture
Chlamydomonas strains used in this study were wild-type (CC-125, CC-124, CC-620 and CC-621), ida3 (CC-2668, CC-2669), ida7 (CC-3921 5b10; ida7-1), fla10 (CC-1919, CC-4617), ida3; fla10-1 (this study), ida1 (CC-2664) and ida2 (CC-2666). All strains except for ida3; fla10-1 were obtained from the Chlamydomonas Resource Center (University of Minnesota, St. Paul, MN). Cells were grown in tris-acetate-phosphate (TAP) medium with aeration on a 14:10h light/dark cycle. Dikaryons between wild type and I1-deficient cells were generated by mixing gametes produced by differentiating each cell type for 4 hours in M-N medium (Harris 1989). The ida3; fla10-1 double mutant was generated by crossing _fla10_-1 (−) and ida3 (+) followed by screening progeny based on the ida3 motility and ciliary resorption at 32°C. PCR and Sanger sequencing confirmed the selected candidates for the fla10-1 mutation (Vashishtha et al. 1996).
Preparation of Axonemes and Membrane + Matrix
Cells were grown to mid-log phase and deciliated by treating the cells with 25mM dibucaine (Witman 1986) followed by centrifugation to separate the cilia and cell bodies. To prepare axonemes, the isolated cilia were demembranated by 1% Nonidet P-40 (Darmstadt, Germany) in HMDE + 25mM NaCl (10mM Hepes, 5mM MgSO4, 1mM DTT, 0.5mM EGTA, pH 7.4) and the axonemal pellet was resuspended/dissolved in HMDE + NaCl (10mM Hepes, 5mM MgSO4, 1mM DTT, 0.5mM EDTA, 25mM NaCl and protease inhibitors, pH 7.4) and fixed with Laemmli sample buffer at a concentration of 1mg/mL. The membrane+matrix (M+M) fraction was obtained by demembranating the pelleted cilia with 1% Nonidet-P40 in HMDE + 25mM NaCl buffer and fixed with Laemmli sample buffer for immunoblotting. For detection of axonemal subunits (IC69, IC140, IC138), the M+M was loaded at five times the relative volume of cilia and axonemes.
Preparation and Fractionation of Axonemal and Cytoplasmic Extracts
Axonemal Extracts: Wild type and I1-deficient axonemes were isolated as described above. Axonemes were then treated with HMDE + 0.6M NaCl for 30 minutes to solubilize the dyneins. The extracted dyneins in the high-salt extract (HSE) were dialyzed against HMDE + 25mM NaCl for 2 × 30mins. Cytoplasmic Extracts from wild type and I1-deficient whole cells were prepared at using the method described in (Alford et al. 2013). Cells were lysed by mechanical agitation by glass beads at room temperature. The axonemal HSE and cytoplasmic extracts were fractionated by velocity sedimentation on 5–20% sucrose gradients as described in Alford et al. 2013 or utilized for reconstitution experiments as described below.
In vitro Reconstitution
Varying amounts of extracts isolated as described above were mixed with a fixed amount of extracted I1-dynein deficient axonemes in the presence of 1mM ATP. The final volumes of all the reconstitutions were equalized with buffer. The reconstitution mixtures were separated by centrifugation into supernatant (S, unbound I1) and pellet (P, bound I1) fractions. The axonemal pellets were resuspended to the original volume and fixed for SDS-PAGE.
Antibodies and Immunoblots
SDS-PAGE and immunoblotting were performed using standard procedures. Primary antibodies used in this study include the following: mouse monoclonal against IC69 (clone 1869A hybridoma, Sigma, St. Louis, MO); rabbit polyclonal antibodies against IC140 (Yang and Sale 1998), IC138 (Hendrickson et al. 2004), IC97 (Wirschell et al. 2009) and IFT46 clone 17600 (Hou et al. 2007). The secondary antibodies (goat anti-mouse or rabbit) used in immunoblots were purchased from BioRad (Hercules, CA). The secondary anti rabbit or goat conjugated with Alex Fluor 488 or 555 were purchased from Invitrogen (Eugene, OR).
Immunofluorescence microscopy and quantification
For immunofluorescence of whole cells, cells were allowed to adhere for 5 min to poly-L-lysine coated cover slips, excess cells were wicked off, and the cover slips were submerged in −20°C methanol for 5 min. The cover slips were then air-dried, rehydrated with 1X PBS, blocked for 30 min at room temperature with blocking solution (6% fish skin gelatin, 1% BSA, and 0.05% Tween-20 in PBS, pH 7.0), incubated with primary antibodies (4°C overnight), washed 3X with blocking solution, washed 3X, incubated with secondary antibodies (Alexa Fluor–conjugated IgG; 1:1000; Invitrogen, Eugene, OR) for 30 min at RT, washed 3x with PBS, then mounted with ProLong Antifade Gold (Invitrogen, Eugene, OR). Images were captured using a BX60 wide field microscope (Olympus, Tokyo, Japan) under a 60x objective, and with a digital camera (Orca-ER, Hamamatsu, Bridgewater, NJ) and Slidebook software (Intelligent Imaging Innovations, Denver, CO). Quantifying I1 dynein assembly from the tip of mutant cilia in dikaryons was performed as described in Alford et al., (Alford et al. 2013). All the image analysis was performed using Image J (FIJI, National Institutes of Health, Bethesda, MD). For each combination of dikaryons (Ex. WT x ida3, ida3 x ida7), the fluorescence signal (Arbitrary units, AU) starting from the tip of the rescuing cilia was measured as function of distance (μm). The fluorescence intensity values corresponding to every pixel along the length were normalized to the brightest intensity value in the data set and plotted against the length of the cilium (μm) as illustrated in the line scan (Figure 5B). A threshold value of 0.2 was established, and distance at which the normalized fluorescence intensity value dropped and stayed below 0.2 for at least three consecutive data points was recorded as the absolute distance at which the IC140 staining from the tip ended (μm). The progressive rescue of I1 dynein assembly from the tip as a function of time after mixing the gametes was plotted using Kaliedograph (Reading, PA). The standard error and p-values were calculated in MS Excel (Redmond, WA).
Dikaryon rescue using the fla10-1 mutants was performed as described (Piperno et al. 1996). Briefly both gametes, ida3; fla10-1 and fla10-1 were preincubated at the restrictive temperature, 32°C, for at least 38 minutes prior to mixing in order to inactivate the kinesin. Secondly, the dikaryons involving fla10-1 and ida3; fla10-1 double mutant gametes could be analyzed for I1 assembly for a maximum time of 60 minutes after mixing, after which dikaryons began to lose their cilia due to resorption.
Acknowledgments
We are grateful to A. Mattheyses, M. Powers, V. Faundez, L. A. Fox (Emory University) and Tal Kramer (Harvard University) for many helpful discussions. This work was supported by a pilot grant to W.S.S. from the Department of Pediatrics and the Pediatric Research Center, Children’s Hospital of Atlanta (CHOA), the Emory University School of Medicine Integrated Cellular Imaging Microscopy Core and grants from the NIH (S.K.D. GM-032843; W.S.S GM-051173). R.V. and E.L.H. were supported by predoctoral fellowships from American Heart Association. R.Y. was supported by the Uehara Memorial Foundation Postdoctoral Fellowship and the Japan Society for the Promotion of Science Postdoctoral Fellowship for Research Abroad. L.M.A. was supported by a training grant from the NIH (Emory University FIRST Postdoctoral Career Development Award K12 GM000608).
References
- Ahmed NT, Gao C, Lucker BF, Cole DG, Mitchell DR. ODA16 aids axonemal outer row dynein assembly through an interaction with the intraflagellar transport machinery. J Cell Biol. 2008;183(2):313–22. doi: 10.1083/jcb.200802025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed NT, Mitchell DR. ODA16p, a Chlamydomonas flagellar protein needed for dynein assembly. Mol Biol Cell. 2005;16(10):5004–12. doi: 10.1091/mbc.E05-07-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford LM, Mattheyses AL, Hunter EL, Lin H, Dutcher SK, Sale WS. The Chlamydomonas mutant pf27 reveals novel features of ciliary radial spoke assembly. Cytoskeleton (Hoboken) 2013 doi: 10.1002/cm.21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin-Tse C, Halbritter J, Zariwala MA, Gilberti RM, Gee HY, Hellman N, Pathak N, Liu Y, Panizzi JR, Patel-King RS, et al. Zebrafish Ciliopathy Screen Plus Human Mutational Analysis Identifies C21orf59 and CCDC65 Defects as Causing Primary Ciliary Dyskinesia. Am J Hum Genet. 2013;93(4):672–86. doi: 10.1016/j.ajhg.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behal RH, Cole DG. Analysis of interactions between intraflagellar transport proteins. Methods Enzymol. 2013;524:171–94. doi: 10.1016/B978-0-12-397945-2.00010-X. [DOI] [PubMed] [Google Scholar]
- Bower R, Tritschler D, Vanderwaal K, Perrone CA, Mueller J, Fox L, Sale WS, Porter ME. The N-DRC forms a conserved biochemical complex that maintains outer doublet alignment and limits microtubule sliding in motile axonemes. Mol Biol Cell. 2013;24(8):1134–52. doi: 10.1091/mbc.E12-11-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower R, VanderWaal K, O’Toole E, Fox L, Perrone C, Mueller J, Wirschell M, Kamiya R, Sale WS, Porter ME. IC138 defines a subdomain at the base of the I1 dynein that regulates microtubule sliding and flagellar motility. Mol Biol Cell. 2009;20(13):3055–63. doi: 10.1091/mbc.E09-04-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman AB, Patel-King RS, Benashski SE, McCaffery JM, Goldstein LS, King SM. Drosophila roadblock and Chlamydomonas LC7: a conserved family of dynein-associated proteins involved in axonal transport, flagellar motility, and mitosis. J Cell Biol. 1999;146(1):165–80. [PMC free article] [PubMed] [Google Scholar]
- Brazelton WJ, Amundsen CD, Silflow CD, Lefebvre PA. The bld1 mutation identifies the Chlamydomonas osm-6 homolog as a gene required for flagellar assembly. Curr Biol. 2001;11(20):1591–4. doi: 10.1016/s0960-9822(01)00485-7. [DOI] [PubMed] [Google Scholar]
- Brokaw CJ, Kamiya R. Bending patterns of Chlamydomonas flagella: IV. Mutants with defects in inner and outer dynein arms indicate differences in dynein arm function. Cell Motil Cytoskeleton. 1987;8(1):68–75. doi: 10.1002/cm.970080110. [DOI] [PubMed] [Google Scholar]
- Bui KH, Yagi T, Yamamoto R, Kamiya R, Ishikawa T. Polarity and asymmetry in the arrangement of dynein and related structures in the Chlamydomonas axoneme. J Cell Biol. 2012;198(5):913–25. doi: 10.1083/jcb.201201120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey DM, Inaba K, Pazour GJ, Takada S, Wakabayashi K, Wilkerson CG, Kamiya R, Witman GB. DC3, the 21-kDa subunit of the outer dynein arm-docking complex (ODA-DC), is a novel EF-hand protein important for assembly of both the outer arm and the ODA-DC. Mol Biol Cell. 2003;14(9):3650–63. doi: 10.1091/mbc.E03-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol. 1998;141(4):993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean AB, Mitchell DR. Chlamydomonas ODA10 is a conserved axonemal protein that plays a unique role in outer dynein arm assembly. Mol Biol Cell. 2013;24(23):3689–96. doi: 10.1091/mbc.E13-06-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBella LM, Benashski SE, Tedford HW, Harrison A, Patel-King RS, King SM. The Tctex1/Tctex2 class of dynein light chains. Dimerization, differential expression, and interaction with the LC8 protein family. J Biol Chem. 2001;276(17):14366–73. doi: 10.1074/jbc.M011456200. [DOI] [PubMed] [Google Scholar]
- DiBella LM, Sakato M, Patel-King RS, Pazour GJ, King SM. The LC7 Light Chains of Chlamydomonas Flagellar Dyneins Interact with Components Required for Both Motor Assembly and Regulation. Mol Biol Cell. 2004a doi: 10.1091/mbc.E04-06-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBella LM, Smith EF, Patel-King RS, Wakabayashi K, King SM. A novel Tctex2-related light chain is required for stability of inner dynein arm I1 and motor function in the Chlamydomonas flagellum. J Biol Chem. 2004b;279(20):21666–76. doi: 10.1074/jbc.M313540200. [DOI] [PubMed] [Google Scholar]
- Diener DR, Yang P, Geimer S, Cole DG, Sale WS, Rosenbaum JL. Sequential assembly of flagellar radial spokes. Cytoskeleton (Hoboken) 2011;68(7):389–400. doi: 10.1002/cm.20520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond IA. Cilia functions in development. Curr Opin Cell Biol. 2012;24(1):24–30. doi: 10.1016/j.ceb.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutcher SK. The awesome power of dikaryons for studying flagella and basal bodies in Chlamydomonas reinhardtii. Cytoskeleton (Hoboken) 2014;71(2):79–94. doi: 10.1002/cm.21157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowkes ME, Mitchell DR. The role of preassembled cytoplasmic complexes in assembly of flagellar dynein subunits. Mol Biol Cell. 1998;9(9):2337–47. doi: 10.1091/mbc.9.9.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhale A, Wirschell M, Sale WS. Regulation of dynein-driven microtubule sliding by the axonemal protein kinase CK1 in Chlamydomonas flagella. J Cell Biol. 2009;186(6):817–24. doi: 10.1083/jcb.200906168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough UW, Heuser JE. Outer and inner dynein arms of cilia and flagella. Cell. 1985;41(2):341–2. doi: 10.1016/s0092-8674(85)80003-9. [DOI] [PubMed] [Google Scholar]
- Gupta A, Diener DR, Sivadas P, Rosenbaum JL, Yang P. The versatile molecular complex component LC8 promotes several distinct steps of flagellar assembly. J Cell Biol. 2012;198(1):115–26. doi: 10.1083/jcb.201111041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EH. The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use. San Diego: Academic Press; 1989. p. 780. [DOI] [PubMed] [Google Scholar]
- Harrison A, Olds-Clarke P, King SM. Identification of the t complex-encoded cytoplasmic dynein light chain tctex1 in inner arm I1 supports the involvement of flagellar dyneins in meiotic drive. J Cell Biol. 1998;140(5):1137–47. doi: 10.1083/jcb.140.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson TW, Perrone CA, Griffin P, Wuichet K, Mueller J, Yang P, Porter ME, Sale WS. IC138 is a WD-repeat Dynein Intermediate Chain Required for Light Chain Assembly and Regulation of Flagellar Bending. Mol Biol Cell. 2004;12:5431–5442. doi: 10.1091/mbc.E04-08-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser T, Barber CF, Lin J, Krell J, Rebesco M, Porter ME, Nicastro D. Cryoelectron tomography reveals doublet-specific structures and unique interactions in the I1 dynein. Proc Natl Acad Sci U S A. 2012;109(30):E2067–76. doi: 10.1073/pnas.1120690109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hom EF, Witman GB, Harris EH, Dutcher SK, Kamiya R, Mitchell DR, Pazour GJ, Porter ME, Sale WS, Wirschell M, et al. A unified taxonomy for ciliary dyneins. Cytoskeleton (Hoboken) 2011;68(10):555–65. doi: 10.1002/cm.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horani A, Brody SL, Ferkol TW. Picking up speed: advances in the genetics of primary ciliary dyskinesia. Pediatr Res. 2014;75(1–2):158–64. doi: 10.1038/pr.2013.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horani A, Brody SL, Ferkol TW, Shoseyov D, Wasserman MG, Ta-shma A, Wilson KS, Bayly PV, Amirav I, Cohen-Cymberknoh M, et al. CCDC65 mutation causes primary ciliary dyskinesia with normal ultrastructure and hyperkinetic cilia. PLoS One. 2013;8(8):e72299. doi: 10.1371/journal.pone.0072299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horani A, Druley TE, Zariwala MA, Patel AC, Levinson BT, Van Arendonk LG, Thornton KC, Giacalone JC, Albee AJ, Wilson KS, et al. Whole-exome capture and sequencing identifies HEATR2 mutation as a cause of primary ciliary dyskinesia. Am J Hum Genet. 2012;91(4):685–93. doi: 10.1016/j.ajhg.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornef N, Olbrich H, Horvath J, Zariwala MA, Fliegauf M, Loges NT, Wildhaber J, Noone PG, Kennedy M, Antonarakis SE, et al. DNAH5 mutations are a common cause of primary ciliary dyskinesia with outer dynein arm defects. Am J Respir Crit Care Med. 2006;174(2):120–6. doi: 10.1164/rccm.200601-084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Qin H, Follit JA, Pazour GJ, Rosenbaum JL, Witman GB. Functional analysis of an individual IFT protein: IFT46 is required for transport of outer dynein arms into flagella. J Cell Biol. 2007;176(5):653–65. doi: 10.1083/jcb.200608041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide T, Owa M, King SM, Kamiya R, Wakabayashi K. Protein-protein interactions between intermediate chains and the docking complex of Chlamydomonas flagellar outer arm dynein. FEBS Lett. 2013;587(14):2143–9. doi: 10.1016/j.febslet.2013.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Yamamoto R, Wirschell M, Yagi T, Bower R, Porter ME, Sale WS, Kamiya R. A novel ankyrin-repeat protein interacts with the regulatory proteins of inner arm dynein f (I1) of Chlamydomonas reinhardtii. Cell Motil Cytoskeleton. 2009;66(8):448–56. doi: 10.1002/cm.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Ide T, Yagi T, Jiang X, Hirono M, Sasaki H, Yanagisawa H, Wemmer KA, Stainier DY, Qin H, et al. TTC26/DYF13 is an intraflagellar transport protein required for transport of motility-related proteins into flagella. Elife. 2014;3:e01566. doi: 10.7554/eLife.01566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Rosenbaum JL. Polarity of flagellar assembly in Chlamydomonas. J Cell Biol. 1992;119(6):1605–11. doi: 10.1083/jcb.119.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagami O, Kamiya R. Translocation and Rotation of Microtubules Caused by Multiple Species of Chlamydomonas Inner-Arm Dynein. Journal of Cell Science. 1992;103:653–664. [Google Scholar]
- Kamiya R. Selection of Chlamydomonas dynein mutants. Methods Enzymol. 1991;196:348–55. doi: 10.1016/0076-6879(91)96031-l. [DOI] [PubMed] [Google Scholar]
- Kamiya R, Kurimoto E, Muto E. Two types of Chlamydomonas flagellar mutants missing different components of inner-arm dynein. J Cell Biol. 1991;112(3):441–7. doi: 10.1083/jcb.112.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SM, Kamiya R. Axonemal Dyneins: Assembly, Structure, and Force Generation. In: Witman GB, editor. The Chlamydomonas Sourcebook: Cell Motility and Behavior. 2. Oxford: Academic Press; 2009. pp. 131–208. [Google Scholar]
- Knowles MR, Leigh MW, Ostrowski LE, Huang L, Carson JL, Hazucha MJ, Yin W, Berg JS, Davis SD, Dell SD, et al. Exome sequencing identifies mutations in CCDC114 as a cause of primary ciliary dyskinesia. Am J Hum Genet. 2013a;92(1):99–106. doi: 10.1016/j.ajhg.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles MR, Ostrowski LE, Loges NT, Hurd T, Leigh MW, Huang L, Wolf WE, Carson JL, Hazucha MJ, Yin W, et al. Mutations in SPAG1 cause primary ciliary dyskinesia associated with defective outer and inner dynein arms. Am J Hum Genet. 2013b;93(4):711–20. doi: 10.1016/j.ajhg.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi D, Takeda H. Ciliary motility: the components and cytoplasmic preassembly mechanisms of the axonemal dyneins. Differentiation. 2012;83(2):S23–9. doi: 10.1016/j.diff.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Koefoed K, Veland IR, Pedersen LB, Larsen LA, Christensen ST. Cilia and coordination of signaling networks during heart development. Organogenesis. 2014;10(1):108–25. doi: 10.4161/org.27483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani N, Sakakibara H, Burgess SA, Kojima H, Oiwa K. Mechanical properties of inner-arm dynein-f (dynein I1) studied with in vitro motility assays. Biophys J. 2007;93(3):886–94. doi: 10.1529/biophysj.106.101964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KG, Beech PL, Rosenbaum JL. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J Cell Biol. 1995;131(6 Pt 1):1517–27. doi: 10.1083/jcb.131.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T, Yagi T, Kamiya R. Tubulin polyglutamylation regulates flagellar motility by controlling a specific inner-arm dynein that interacts with the dynein regulatory complex. Cytoskeleton (Hoboken) 2012;69(12):1059–68. doi: 10.1002/cm.21075. [DOI] [PubMed] [Google Scholar]
- Loges NT, Olbrich H, Becker-Heck A, Haffner K, Heer A, Reinhard C, Schmidts M, Kispert A, Zariwala MA, Leigh MW, et al. Deletions and point mutations of LRRC50 cause primary ciliary dyskinesia due to dynein arm defects. Am J Hum Genet. 2009;85(6):883–9. doi: 10.1016/j.ajhg.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loges NT, Olbrich H, Fenske L, Mussaffi H, Horvath J, Fliegauf M, Kuhl H, Baktai G, Peterffy E, Chodhari R, et al. DNAI2 mutations cause primary ciliary dyskinesia with defects in the outer dynein arm. Am J Hum Genet. 2008;83(5):547–58. doi: 10.1016/j.ajhg.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison HM, Schmidts M, Loges NT, Freshour J, Dritsoula A, Hirst RA, O’Callaghan C, Blau H, Al Dabbagh M, Olbrich H, et al. Mutations in axonemal dynein assembly factor DNAAF3 cause primary ciliary dyskinesia. Nat Genet. 2012;44(4):381–9. S1–2. doi: 10.1038/ng.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJ, Onoufriadis A, Shoemark A, Simpson MA, zur Lage PI, de Castro SC, Bartoloni L, Gallone G, Petridi S, Woollard WJ, et al. Mutations in ZMYND10, a gene essential for proper axonemal assembly of inner and outer dynein arms in humans and flies, cause primary ciliary dyskinesia. Am J Hum Genet. 2013;93(2):346–56. doi: 10.1016/j.ajhg.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussgnug JH, Wobbe L, Elles I, Claus C, Hamilton M, Fink A, Kahmann U, Kapazoglou A, Mullineaux CW, Hippler M, et al. NAB1 is an RNA binding protein involved in the light-regulated differential expression of the light-harvesting antenna of Chlamydomonas reinhardtii. Plant Cell. 2005;17(12):3409–21. doi: 10.1105/tpc.105.035774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myster SH, Knott JA, O’Toole E, Porter ME. The Chlamydomonas Dhc1 gene encodes a dynein heavy chain subunit required for assembly of the I1 inner arm complex. Mol Biol Cell. 1997;8(4):607–20. doi: 10.1091/mbc.8.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myster SH, Knott JA, Wysocki KM, O’Toole E, Porter ME. Domains in the 1alpha dynein heavy chain required for inner arm assembly and flagellar motility in Chlamydomonas. J Cell Biol. 1999;146(4):801–18. doi: 10.1083/jcb.146.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omran H, Kobayashi D, Olbrich H, Tsukahara T, Loges NT, Hagiwara H, Zhang Q, Leblond G, O’Toole E, Hara C, et al. Ktu/PF13 is required for cytoplasmic pre-assembly of axonemal dyneins. Nature. 2008;456(7222):611–6. doi: 10.1038/nature07471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoufriadis A, Paff T, Antony D, Shoemark A, Micha D, Kuyt B, Schmidts M, Petridi S, Dankert-Roelse JE, Haarman EG, et al. Splice-site mutations in the axonemal outer dynein arm docking complex gene CCDC114 cause primary ciliary dyskinesia. Am J Hum Genet. 2013;92(1):88–98. doi: 10.1016/j.ajhg.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoufriadis A, Shoemark A, Munye MM, James CT, Schmidts M, Patel M, Rosser EM, Bacchelli C, Beales PL, Scambler PJ, et al. Combined exome and whole-genome sequencing identifies mutations in ARMC4 as a cause of primary ciliary dyskinesia with defects in the outer dynein arm. J Med Genet. 2014;51(1):61–7. doi: 10.1136/jmedgenet-2013-101938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owa M, Furuta A, Usukura J, Arisaka F, King SM, Witman GB, Kamiya R, Wakabayashi K. Cooperative binding of the outer arm-docking complex underlies the regular arrangement of outer arm dynein in the axoneme. Proc Natl Acad Sci U S A. 2014;111(26):9461–6. doi: 10.1073/pnas.1403101111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Snell WJ. Kinesin-II is required for flagellar sensory transduction during fertilization in Chlamydomonas. Mol Biol Cell. 2002;13(4):1417–26. doi: 10.1091/mbc.01-11-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzi JR, Becker-Heck A, Castleman VH, Al-Mutairi DA, Liu Y, Loges NT, Pathak N, Austin-Tse C, Sheridan E, Schmidts M, et al. CCDC103 mutations cause primary ciliary dyskinesia by disrupting assembly of ciliary dynein arms. Nat Genet. 2012;44(6):714–9. doi: 10.1038/ng.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone CA, Myster SH, Bower R, O’Toole ET, Porter ME. Insights into the structural organization of the I1 inner arm dynein from a domain analysis of the 1beta dynein heavy chain. Mol Biol Cell. 2000;11(7):2297–313. doi: 10.1091/mbc.11.7.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone CA, Yang P, O’Toole E, Sale WS, Porter ME. The Chlamydomonas IDA7 locus encodes a 140-kDa dynein intermediate chain required to assemble the I1 inner arm complex. Mol Biol Cell. 1998;9(12):3351–65. doi: 10.1091/mbc.9.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G, Mead K. Transport of a novel complex in the cytoplasmic matrix of Chlamydomonas flagella. Proc Natl Acad Sci U S A. 1997;94(9):4457–62. doi: 10.1073/pnas.94.9.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G, Mead K, Henderson S. Inner dynein arms but not outer dynein arms require the activity of kinesin homologue protein KHP1(FLA10) to reach the distal part of flagella in Chlamydomonas. J Cell Biol. 1996;133(2):371–9. doi: 10.1083/jcb.133.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G, Ramanis Z, Smith EF, Sale WS. Three distinct inner dynein arms in Chlamydomonas flagella: molecular composition and location in the axoneme. J Cell Biol. 1990;110(2):379–89. doi: 10.1083/jcb.110.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ME, Power J, Dutcher SK. Extragenic suppressors of paralyzed flagellar mutations in Chlamydomonas reinhardtii identify loci that alter the inner dynein arms. J Cell Biol. 1992;118(5):1163–76. doi: 10.1083/jcb.118.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ME, Sale WS. The 9 + 2 axoneme anchors multiple inner arm dyneins and a network of kinases and phosphatases that control motility. J Cell Biol. 2000;151(5):F37–42. doi: 10.1083/jcb.151.5.f37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Diener DR, Geimer S, Cole DG, Rosenbaum JL. Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body. J Cell Biol. 2004;164(2):255–66. doi: 10.1083/jcb.200308132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- Smith EF, Sale WS. Microtubule binding and translocation by inner dynein arm subtype I1. Cell Motil Cytoskeleton. 1991;18(4):258–68. doi: 10.1002/cm.970180403. [DOI] [PubMed] [Google Scholar]
- Smith EF, Sale WS. Structural and functional reconstitution of inner dynein arms in Chlamydomonas flagellar axonemes. J Cell Biol. 1992;117(3):573–81. doi: 10.1083/jcb.117.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada S, Wilkerson CG, Wakabayashi K, Kamiya R, Witman GB. The outer dynein arm-docking complex: composition and characterization of a subunit (oda1) necessary for outer arm assembly. Mol Biol Cell. 2002;13(3):1015–29. doi: 10.1091/mbc.01-04-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkar A, Loges NT, Slagle CE, Francis R, Dougherty GW, Tamayo JV, Shook B, Cantino M, Schwartz D, Jahnke C, et al. DYX1C1 is required for axonemal dynein assembly and ciliary motility. Nat Genet. 2013;45(9):995–1003. doi: 10.1038/ng.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toba S, Fox LA, Sakakibara H, Porter ME, Oiwa K, Sale WS. Distinct roles of 1alpha and 1beta heavy chains of the inner arm dynein I1 of Chlamydomonas flagella. Mol Biol Cell. 2011;22(3):342–53. doi: 10.1091/mbc.E10-10-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWaal KE, Yamamoto R, Wakabayashi K, Fox L, Kamiya R, Dutcher SK, Bayly PV, Sale WS, Porter ME. bop5 Mutations reveal new roles for the IC138 phosphoprotein in the regulation of flagellar motility and asymmetric waveforms. Mol Biol Cell. 2011;22(16):2862–74. doi: 10.1091/mbc.E11-03-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashishtha M, Walther Z, Hall JL. The kinesin-homologous protein encoded by the Chlamydomonas FLA10 gene is associated with basal bodies and centrioles. J Cell Sci. 1996;109 (Pt 3):541–9. doi: 10.1242/jcs.109.3.541. [DOI] [PubMed] [Google Scholar]
- Wallingford JB. Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Annu Rev Cell Dev Biol. 2012;28:627–53. doi: 10.1146/annurev-cellbio-092910-154208. [DOI] [PubMed] [Google Scholar]
- Walther Z, Vashishtha M, Hall JL. The Chlamydomonas FLA10 gene encodes a novel kinesin-homologous protein. J Cell Biol. 1994;126(1):175–88. doi: 10.1083/jcb.126.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirschell M, Hendrickson T, Sale WS. Keeping an eye on I1: I1 dynein as a model for flagellar dynein assembly and regulation. Cell Motil Cytoskeleton. 2007;64(8):569–579. doi: 10.1002/cm.20211. [DOI] [PubMed] [Google Scholar]
- Wirschell M, Pazour G, Yoda A, Hirono M, Kamiya R, Witman GB. Oda5p, a novel axonemal protein required for assembly of the outer dynein arm and an associated adenylate kinase. Mol Biol Cell. 2004;15(6):2729–41. doi: 10.1091/mbc.E03-11-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirschell M, Yang C, Yang P, Fox L, Yanagisawa HA, Kamiya R, Witman GB, Porter ME, Sale WS. IC97 Is a Novel Intermediate Chain of I1 Dynein That Interacts with Tubulin and Regulates Interdoublet Sliding. Mol Biol Cell. 2009;20(13):3044–54. doi: 10.1091/mbc.E09-04-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witman GB. The site of in vivo assembly of flagellar microtubules. Ann N Y Acad Sci. 1975;253:178–91. doi: 10.1111/j.1749-6632.1975.tb19199.x. [DOI] [PubMed] [Google Scholar]
- Witman GB. Isolation of Chlamydomonas flagella and flagellar axonemes. Methods Enzymol. 1986;134:280–90. doi: 10.1016/0076-6879(86)34096-5. [DOI] [PubMed] [Google Scholar]
- Wood CR, Rosenbaum JL. Proteins of the ciliary axoneme are found on cytoplasmic membrane vesicles during growth of cilia. Curr Biol. 2014;24(10):1114–20. doi: 10.1016/j.cub.2014.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren KN, Craft JM, Tritschler D, Schauer A, Patel DK, Smith EF, Porter ME, Kner P, Lechtreck KF. A differential cargo-loading model of ciliary length regulation by IFT. Curr Biol. 2013;23(24):2463–71. doi: 10.1016/j.cub.2013.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T, Minoura I, Fujiwara A, Saito R, Yasunaga T, Hirono M, Kamiya R. An axonemal dynein particularly important for flagellar movement at high viscosity. Implications from a new Chlamydomonas mutant deficient in the dynein heavy chain gene DHC9. J Biol Chem. 2005;280(50):41412–20. doi: 10.1074/jbc.M509072200. [DOI] [PubMed] [Google Scholar]
- Yamamoto R, Hirono M, Kamiya R. Discrete PIH proteins function in the cytoplasmic preassembly of different subsets of axonemal dyneins. J Cell Biol. 2010;190(1):65–71. doi: 10.1083/jcb.201002081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto R, Yagi T, Kamiya R. Functional binding of inner-arm dyneins with demembranated flagella of Chlamydomonas mutants. Cell Motil Cytoskeleton. 2006;63(5):258–65. doi: 10.1002/cm.20121. [DOI] [PubMed] [Google Scholar]
- Yang C, Compton MM, Yang P. Dimeric novel HSP40 is incorporated into the radial spoke complex during the assembly process in flagella. Mol Biol Cell. 2005;16(2):637–48. doi: 10.1091/mbc.E04-09-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Sale WS. The Mr 140,000 intermediate chain of Chlamydomonas flagellar inner arm dynein is a WD-repeat protein implicated in dynein arm anchoring. Mol Biol Cell. 1998;9(12):3335–49. doi: 10.1091/mbc.9.12.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zariwala MA, Knowles MR, Omran H. Genetic defects in ciliary structure and function. Annu Rev Physiol. 2007;69:423–50. doi: 10.1146/annurev.physiol.69.040705.141301. [DOI] [PubMed] [Google Scholar]
- Zariwala MA, Leigh MW, Ceppa F, Kennedy MP, Noone PG, Carson JL, Hazucha MJ, Lori A, Horvath J, Olbrich H, et al. Mutations of DNAI1 in primary ciliary dyskinesia: evidence of founder effect in a common mutation. Am J Respir Crit Care Med. 2006;174(8):858–66. doi: 10.1164/rccm.200603-370OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zariwala MA, Omran H, Ferkol TW. The emerging genetics of primary ciliary dyskinesia. Proc Am Thorac Soc. 2011;8(5):430–3. doi: 10.1513/pats.201103-023SD. [DOI] [PMC free article] [PubMed] [Google Scholar]