Disrupting spinal noradrenergic activation delays recovery of acute incision induced hypersensitivity and increases spinal glial activation in the rat (original) (raw)
. Author manuscript; available in PMC: 2017 Feb 1.
Abstract
Clinical studies suggest that descending inhibitory controls from the brainstem are important for speeding recovery from pain following surgery. We examined the effects of destroying spinally projecting noradrenergic neurons via intrathecally administered antibody to dopamine β-hydroxylase conjugated to saporin (DβH-saporin) on recovery in an acute incisional pain model. Mechanical and thermal paw withdrawal thresholds and non-evoked spontaneous guarding scores were tested for several weeks postoperatively and analyzed using mixed effects growth curve modeling. DβH-saporin treatment resulted in a significant prolongation in the duration of mechanical and to a lesser degree thermal hypersensitivity in the ipsilateral paw of incised rats but did not increase the duration of spontaneous guarding. DβH-saporin treatment was also associated with increased microglial and astrocyte activation in the ipsilateral spinal cord 21 days post-incision compared to IgG-saporin treated controls. Chronic intrathecal administration of the α2 adrenergic receptor antagonist atipamezole (50-200 μg/day) produced similar effects. These data suggest that spinally projecting noradrenergic pathways and spinal α2 adrenergic receptor activation are important for speeding recovery from hypersensitivity following surgical incision possibly by reducing spinal glial activation. Interventions that augment the noradrenergic system may be important to speed recovery from pain after surgery.
Perspective
Endogenous descending spinal noradrenergic activation promotes resolution of incision induced hypersensitivity and inhibits spinal microglial and astrocyte activation in part through α2 adrenergic receptors.
Keywords: Descending inhibition, postoperative pain, chronification, glial plasticity, growth curve
Introduction
The majority of patients who undergo surgery experience acute pain that resolves as the wound heals. However, 5-15% of patients following common surgeries exhibit very slow recovery and experience clinically meaningful pain that persists for months to years impacting physical function and reducing their quality of life. The mechanisms responsible for the speed of recovery in pain after injury are not clear, however dysregulation of supraspinal processing, impaired descending inhibitory pathways, altered spinal pain processing, and primary sensory neuron plasticity have been hypothesized to contribute pain chronicity. Clinical studies have measured individual endogenous analgesia triggered by a painful stimulus using conditioned pain modulation (CPM) and show that a weak CPM response preoperatively correlates with a greater incidence of persistence of pain for months or years following thoracotomy 65 and abdominal surgery61. We recently demonstrated that descending spinal noradrenergic activity is an important component of CPM and also important to speed of resolution of mechanical hypersensitivity following partial nerve injury in rats44. However, this model may not be predictive of recovery from surgery that does not involve injury to major peripheral nerves, as chronic pain can develop in patients in the absence of obvious signs of nerve injury. Additionally, mechanistic differences have been observed between acute incisional and neuropathic pain models including differences in mechanisms of spinal sensitization (non-NMDA vs NMDA dependent) 46 and spinal α2 adrenergic receptor signaling including greater reliance of α2 mediated analgesic effects on cholinergic mechanisms following nerve injury 37. In the current study, we measure the impact of blocking or disrupting descending noradrenergic input on resolution of evoked and spontaneous pain related behaviors in an incision model that lacks injury to major peripheral nerves and typically resolves within days.
The spinal cord receives extensive neuronal input from noradrenergic brainstem nuclei including A5, A6 (the locus coeruleus), and A7. The neurotransmitter norepinephrine is released into the spinal cord following noxious peripheral stimulation 57 and has an important role in suppressing nociceptive transmission in acute, inflammatory and neuropathic pain models 41; however its contribution to resolution of acute incisional pain has not been examined. The analgesic action of norepinephrine is primarily due to presynaptic inhibition of glutamate release via α2-adrenoceptors on primary afferent terminals 24, 29, 49, 55 and postsynaptic activation of α1 and α2-adrenoceptors on inhibitory and excitatory interneurons, respectively 4. Additionally, α2 adrenergic receptor agonists and antidepressants (e.g. norepinephrine reuptake inhibitors) have been reported to inhibit spinal cord glial activation after surgery in preclinical pain model as a potential mechanism of speeded recovery and anti-hypersensitivity.15, 53
We hypothesize that disrupting spinal noradrenergic fibers or blocking spinal α2 adrenergic receptor activation would delay resolution of postoperative evoked and ongoing pain and enhance spinal glial activation following surgical incision. We used two complimentary approaches to test this hypothesis. In one, we disrupted descending spinal noradrenergic fibers using a toxin that selectively ablated this population of neurons and evaluated the significance of descending noradrenergic fiber loss in the surgical recovery of mechanical and thermal hypersensitivity and guarding behavior. In the other, we chronically blocked α2 adrenergic receptor signaling by continuous spinal administrations of the α2 adrenergic receptor antagonist, atipamezole prior to incision and for several days postoperatively. Since our primary interest was the effect of these manipulations on speed of recovery from postoperative hypersensitivity, we utilized growth curve analysis of daily postoperative pain behaviors as the primary outcome measure. We also examined changes in glial cells that are thought to contribute to the generation and maintenance of hypersensitivity.
Methods
Animals
A total of 109 male Sprague–Dawley rats (Harlan Industries, Indianapolis, IN), weighing 180–250 g, were used for experiments. All studies conformed to the Wake Forest University Guidelines on the ethical use of animals, and studies were performed under Animal Care and Use Committee (Winston-Salem, North Carolina) approval. Animals were housed under a 12-hour light–dark cycle, with food and water ad libitum. Rats were randomly assigned to surgery or treatment groups, however no formal methods or software was used to make group assignments.
Drugs
For ablative studies, anti-DβH-saporin and control immunoglobulin G (IgG) -saporin were obtained from Advanced Targeting Systems, San Diego, CA and injected intrathecally at a dose of 5 μg in 10μl of sterile saline via percutaneous lumbar puncture at the L5-L6 interspace two weeks prior to incision surgery. Successful puncture of the dura was confirmed by the presence of a tail flick. Previous studies demonstrate that spinal anti- DβH-saporin at this dose reduces nearly all spinally projecting noradrenergic neurons including those originating from the locus coeruelus (A6), A5 and A7 cell groups. Supraspinally, there is a reduction of DβH immunoreactive fibers in the cerebral cortex and thalamic nuclei, however noradrenergic fibers in the paraventricular hypothalamic nuclei and amygdala are largely preserved 18, 22. Previous studies have demonstrated that dopaminergic and serotonergic innervation are not altered by lumbar intrathecal injection of DβH-saporin at this dose.22 For pharmacological studies, atipamezole (a selective α2 adrenergic receptor (AR) antagonist; Tocris Bioscience, Bristol, United Kingdom) was delivered by an implanted mini-osmotic pump (Alzet®, Durect Co., Cupertino, CA, USA) connected to an intrathecal catheter. The pump contained atipamezole in different concentrations in order to deliver four doses: 0, 50, 100, and 200μg/day 0.5μl/h up to 14 days. Three days after the pump implantation, the incision surgery was conducted.
Plantar incision surgery
Plantar incision was performed as previously described 7. In brief, rats were anesthetized with 2-3% of isoflurane. The plantar aspect of the left hind paw was prepared in a sterile manner with a 10% povidone-iodine solution. A 1-cm midline incision was made using a No. 11 surgical blade starting 0.5 cm from the proximal edge of the heel. The plantaris muscle was elevated with curved forceps and incised longitudinally. The model includes surgical incision of the skin, muscle, and fascia of the rat hind paw. The wound was closed with two 5.0 nylon mattress sutures, and covered with triple antibiotic ointment. Sham surgery for plantar incision consisted of all perioperative procedures including inhalational isoflurane without incision of the skin.
Intrathecal catheterization
For studies involving chronic spinal drug delivery rats were implanted with intrathecal catheters as previously described 43, 63 and drug was delivered via mini-osmotic pumps. Briefly, a. small incision was made at the back of the neck and a puncture was made in the atlanto-occipital membrane of the cisterna magnum. A polyethylene catheter (external diameter: 0.23 mm, internal volume: 6 μL; ReCathCo LLC, Allison Park, PA, USA) was inserted for 7.5 cm caudal so that the tip reached the lumbar enlargement of the spinal cord, and then it was secured on the fascia of paravertebral muscle and the tip of the catheter was internalized and the incision skin incision was enclosed with sutures. Seven days after intrathecal catheterization (three days prior to incision) a small incision was made at the base of the neck and mini-osmotic pumps (Model 2002, ALZET Osmotic Pumps, Cupertino, CA, USA) were attached to the catheter and implanted for continual drug infusion.
Behavioral analysis
For all behavioral analysis, individuals who conducted assays were blinded to the group allocation. Because the incision was on the plantar aspects of the paw the examiner could not be blinded to surgery. Paw withdrawal thresholds to mechanical stimuli were assessed using von Frey filaments (Stoelting Co., Wood Dale, IL, USA) using the up-down statistical method 8, 42. In brief, filaments were applied to the hindpaw medial to the incision to the bending point for 6 second and a brisk paw withdrawal was considered a positive response. Thermal withdrawal latency was assessed using a Paw Thermal Stimulator System (UARDG-Mk3C; UARD Group, University of California San Diego, La Jolla, CA, USA) similar to previous studies 43, 45. Thermal testing was performed after the rat had been placed in a clear plastic box on a glass surface maintained at 30°C. A calibrated radiant heat source was focused on the hind paw, and the latency to withdrawal was recorded, using a 20-s maximum exposure to avoid tissue injury. Withdrawal latency was measured two times to thermal stimulation of the medial aspects of the hindpaw adjacent to the incision. The two observations were averaged for each animal. We assessed guarding behavior as a measure of non-evoked pain or pain at rest 7. Rats were placed on an elevated mesh (grid 8×8 mm) under a clear plastic cage (30×30×15 cm). The incised and non-incised paws were viewed. Both paws of each animal were closely observed during a 1 min period repeated every 5 minutes for 1 hour. A score of 0, 1, or 2 was given depending on the position in which each paw was found during the majority of the 1-min scoring period. Full weight-bearing of the paw (score =0) was present if the wound was blanched or distorted by the mesh. If a paw was completely off the mesh without any touch, a score of 2 was given. If the area of the wound touched the mesh gently without any blanching or distorting, a 1 was recorded. The cumulative guarding score was the sum of the 12 scores (Range: 0-24) obtained during the 1 h session for each paw. Pain related behaviors were assessed prior to intrathecal drug treatment, prior to incision (D0), and the first two days after incision, then every 2 days until 20 days after incision.
Tissue preparation and immunohistochemistry
Rats were anesthetized with sodium pentobarbital (intraperitoneal injection; 100 mg/kg), the thorax was opened, and 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by fixative (4% paraformaldehyde in 0.1 M PBS, pH 7.4) was perfused through the left ventricle with a peristaltic pump (20 ml/min). The spinal cord were removed, immersed in fixative for 12 h at 4°C, the immersed in 30% sucrose at 4°C for cryoprotection until ready to be section ed. Transverse spinal cord sections (40 μm) were cut using a cryostat (Leica CM3050S, Leica Biosystems GmbH, Wetzlar, Germany). Sections were blocked with 3% normal donkey serum in 0.3% Triton X-100 for 1 h at room temperature and incubated overnight at 4°C with pri mary antibodies: ionizing calcium binding adapter molecule 1 (IBA1; 1:2 000, rabbit anti-rat; Wako chemicals, Richmond, VA, USA), a microglial marker; glial fibrillary acidic protein (GFAP; 1:1 000, rabbit ant-rat; Dako Inc., Carpinteria, CA, USA), a marker for astrocytes; and DβH (1:500, mouse anti-rat; Millipore, Billerica, MA, USA) for noradrenergic neurons. Negative controls were obtained for each antibody by omitting the primary antibody. After incubation with primary antibodies, sections were washed three times for 10-15 min each in PBS. Subsequently, sections were incubated for 2-3 h at room temperature with Cy3- and biotin-conjugated secondary antibody (1:500; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA). After washing three times, streptavidin-Cy2 was used for 1 h at room temperature and followed with another three 10-min washes. Finally, the sections were mounted on Superfrost™ Plus slides, and then run them through 70%, 95%, 100% ethanol, and xylene for 2 min each, and cover slipped with DPX mounting media (Fisher Scientific, Pittsburgh, PA).
Image analysis
Images of the spinal cord were captured on a Nikon E600 epifluorescence microscope fitted with a CCD digital camera using a 10× objective at a resolution of 1 600 × 1 200 pixels or a Zeiss LSM510 laser scanning confocal system. Quantification for each immunostaining was performed in 5 randomly selected sections from each animal. Image analysis software (Image J; NIH Image, National Institutes of Health, Bethesda, MD, USA) was used to quantify immunofluorescence. As in our previous study 18 the upper and lower threshold optical densities were adjusted to match positive immunoreactivity for each antibody. The image thresholds were determined and applied uniformly to all sections. A fixed area (250×250 μm2) was positioned in the dorsal medial and central third of the spinal cord dorsal horn (corresponding to the topography of the central projections of sensory neurons that innervate the incised region of the hindpaw) 11, 56 and the number of pixels within the threshold value was quantified. Data are expressed as number of pixels in the area. To limit variability in immunohistochemical measurements, all groups of rats were processed on the same day and the same threshold value was applied to all images for a given antibody. The individuals quantifying the images were blind to the treatment.
Data analysis
The primary outcome measure was speed of recovery from hypersensitivity. Longitudinal measures of mechanical withdrawal threshold, thermal latency and spontaneous guarding values were modeled using growth curve analysis as previous described 3. Linear, quadratic and ln(time) models were employed to model individual change over time and the quadratic model was chosen using the Bayesian Information Criterion (BIC) . This analysis gives rise to intercept (initial pain at time 0), slope (linear rate of change in pain measure), and quadratic term (acceleration and deceleration over time reflecting inflections in change during recovery) parameters. The intercept, slope and quadratic term estimates can vary across individual rats (random effects) and as a function of treatment group or condition (fixed effects) allowing us to examine the ability of interventions to impair or improve several aspects of postoperative pain trajectory. Bonferroni correction was made for the within group models (comparisons within incision or sham groups separately) considering p<.025 to be statistically significant. Group averaged trajectories are presented with 95% confidence limits and analyzed using SAS® v9.4 software (SAS Institute Inc., Cary, NC, USA). Mechanical withdrawal thresholds and thermal withdrawal latencies were also analyzed using a two-way repeated measures analysis of variance (RM-ANOVA) where group and treatment were considered as independent variables in the model. Bonferroni post hoc correction was made for multiple comparisons. Longitudinal guarding scores were also analyzed using Friedman repeated analysis of variance on ranks followed by Dunnett's test for within group comparisons and Kruskal Wallis one way analysis of variance on ranks followed by Tukey'stest for within timepoint group comparisons.
Immunohistochemical data was analyzed using one or two-way ANOVA followed by the Bonferroni post hoc correction for multiple comparisons among different groups. Immunohistochemical data are presented as mean ± SEM and analyzed using Sigma Plot (Version 12.0; Systat Inc., San Jose, CA). All reported P values are two-tailed and a P value of less than 0.05 is accepted for statistical significance. For immunohistochemical and behavioral analysis, we did not define a priori a minimum biologically important difference and determine group size based on a formal power analysis or simulations. Rather, we used group sizes typical for these kind of experiments 3, 44 For ablative studies a group size of 6 was planned, rats were excluded if DβH-saporin treatment failed to ablate greater than 95% of spinal noradrenergic fibers based on DβH-immunoreactivity No rats were excluded due to incomplete ablation. For pharmacological studies a group size of 14 was planned, rats were excluded if they exhibited neurological complications from intrathecal catheterization. A total of three rats had to be excluded resulting in the following group sizes: vehicle (n=13) and atipamezole 50μg/day (n=14), 100μg/day (n=12) 200μg/day (n=14). For the DβH-saporin study, spinal cord sections from all the animals from the 4 treatment groups (n=6 per group) were processed simultaneously. For the chronic atipamezole study, rats from each dose including the vehicle treatment group were randomly selected (n=5 per group) and processed simultaneously.
Results
Depletion of descending spinal noradrenergic fibers prior to incision delays recovery from mechanical hypersensitivity
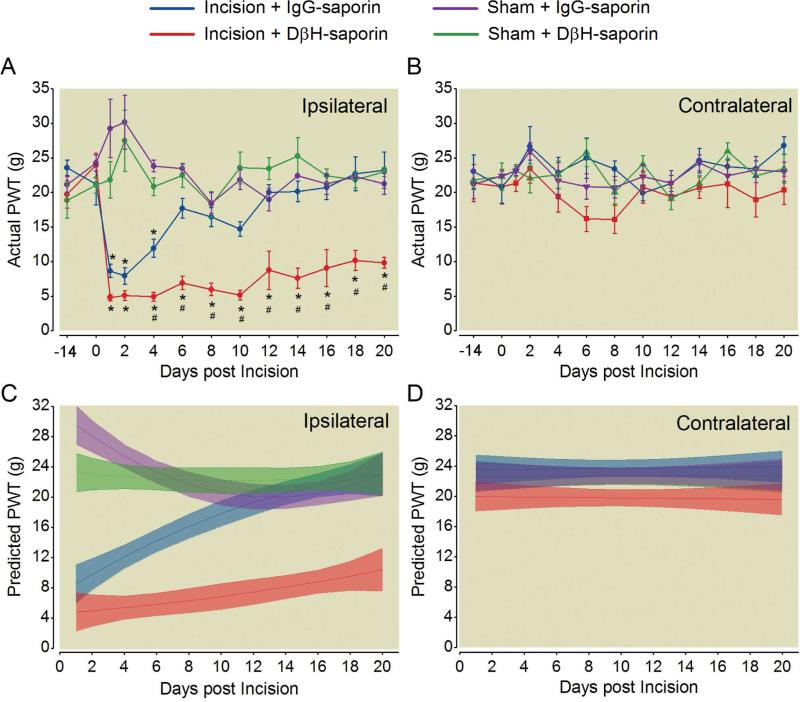
Fourteen days following spinal administration of DβH-saporin or control IgG-saporin, mechanical withdrawal thresholds in the ipsilateral (Fig 1A) or contralateral paw (Fig. 1B) were not significantly different between treatment groups prior to surgery. Following plantar incision, DβH-saporin treated incision rats had a greater mechanical hypersensitivity compared to IgG-saporin treated incision rats in the ipsilateral (Fig.1A, Day 4 through Day 21) but not the contralateral paw (Fig 1B). Mechanical withdrawal thresholds were not significantly different between groups throughout the time course of the study in rats that underwent sham procedure (Fig 1A, B).
Figure 1.
Spinal depletion of noradrenergic fibers prior to plantar incision delays resolution of ipsilateral mechanical hypersensitivity. Rats received intrathecal treatment with dopamine β-hydroxylase (DβH)-saporin or control immunoglobulin G (IgG)-saporin 14 days before plantar incision or sham procedure and were assessed for mechanical hypersensitivity with von Frey filaments in the ipsilateral (A) and contralateral (B) hindpaw. Data is expressed as Mean ± SEM. Two-way repeated-measures ANOVA with Bonferroni multiple comparisons. # P < 0.05 for within time point comparison to Incision + IgG-saporin value; * P < 0.001 for within treatment group comparisons to pre-incision (D0) baseline value. Modeled group trajectories of postoperative mechanical withdrawal thresholds in the ipsilateral (C) and contralateral (D) paw of treated rats. Group averaged trajectories depict the mean fit for all the animals within each treatment group (n = 6 per group) with 95% CIs indicated by shading.
Modeled postsurgical mechanical withdrawal thresholds in incision rats administered DβH-saporin had similar predicted intercepts in the ipsilateral paw compared to incision rats administered IgG-saporin (P=0.0643) but significantly different trajectories with a smaller slope indicating slower recovery (slope: P=0.0001; quadratic: P=0.05) with non-overlapping 95% CIs from 2 to at least 21 days postoperatively (Fig. 1C). The trajectories were not significantly different in the contralateral paw between DβH-saporin or IgG-saporin treated incised rats (Fig. 1D). Sham rats administered DβH-saporin had a significantly lower predicted intercept (P=0.0176) compared to sham rats administered IgG saporin, but this effect was small and transient, with groups exhibiting non-overlapping 95% CIs only for two days postoperatively. When modeling both incision and sham cohorts from the same treatment group simultaneously, we show that the duration of ipsilateral mechanical hypersensitivity was 8 days in IgG-saporin treated incision rats compared to at least 21 days in DβH-saporin treated incision rats based on non-overlapping 95% CIs of modeled trajectories (Fig. 1C).
Depletion of descending spinal noradrenergic fibers prior to incision delays recovery from thermal hypersensitivity
Fourteen days following spinal administration of DβH-saporin or control IgG-saporin, thermal withdrawal latencies in the ipsilateral (Fig 2A) or contralateral paw (Fig. 2B) were not significantly different between treatment groups prior to surgery. Following plantar incision, DβH-saporin treated incision rats had a greater thermal hypersensitivity compared to IgG-saporin treated incision rats in the ipsilateral (Fig.2A, Day 8 and 10) but not the contralateral paw (Fig 2B). Thermal withdrawal latencies were not significantly different between groups throughout the time course of the study in rats that underwent sham procedure (Fig 2A, B).
Figure 2.
Spinal depletion of noradrenergic fibers prior to plantar incision delays resolution of ipsilateral thermal hypersensitivity. Rats received intrathecal treatment with dopamine β-hydroxylase (DβH)-saporin or control immunoglobulin G (IgG)-saporin 14 days before plantar incision or sham procedure and were assessed for thermal response latency with a radiant heat device in the ipsilateral (A) and contralateral (B) hindpaw. Data is expressed as Mean ± SEM. Two-way repeated-measures ANOVA with Bonferroni multiple comparisons. # P < 0.001 for within time point comparison to Incision + IgG-saporin value,* P < 0.003 for within treatment group comparison to pre-incision (D0) baseline value. Modeled group trajectories of postoperative thermal withdrawal latencies in the ipsilateral (C) and contralateral (D) paw of treated rats. Group averaged trajectories depict the mean fit for all the animals within each treatment group (n = 6 per group) with 95% CIs indicated by shading.
Modeled withdrawal latencies in incision rats administered DβH-saporin showed similar predicted intercepts in the ipsilateral paw compared to incision rats administered IgG-saporin (p=0.928), but less rapid recovery based on a smaller slope and smaller acceleration rate (slope: P=0.001; quadratic: P=0.0002) with non-overlapping 95% CIs from 4 to 14 days postoperatively (Fig. 2C). No significant thermal hypersensitivity developed in the contralateral paw of incised rats (Fig. 2D) or sham rats treated with DβH-saporin or IgG saporin (intercept: P=0.56; slope: P=0.751; quadratic: P=0.578). When modeling both incision and sham cohorts from the same treatment group simultaneously, we show that the duration of thermal hypersensitivity was increased from 6 days in IgG-saporin treated incision rats to 12 days in DβH-saporin treated incision rats (Fig. 2C).
Depletion of descending spinal noradrenergic fibers prior to incision does not delay resolution of spontaneous guarding following incision
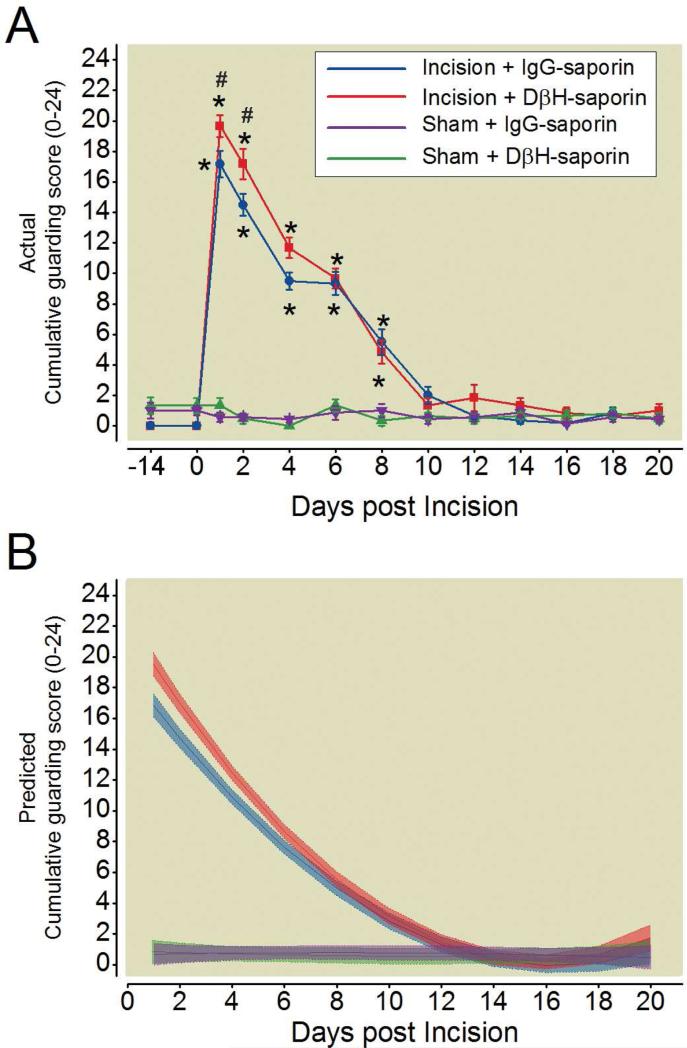
In a separate group of rats, we assessed the impact of DβH-saporin treatment on spontaneous guarding, a non-evoked measure of pain. The magnitude of guarding behavior was slightly but significantly increased in DβH-saporin treated incision rats compared to IgG-saporin treated incision rats at early postoperative time points (Fig. 3A, Day 1 and 2), however the duration of guarding behavior was similar between treatment groups resolving by day 8 post-incision (Fig 3A). No spontaneous guarding behavior was present in sham rats from either treatment group (Fig. 3A).
Figure 3.
Spinal depletion of noradrenergic fibers prior to plantar incision does not alter duration of spontaneous guarding behavior. Rats received intrathecal treatment with dopamine β-hydroxylase (DβH)-saporin or control immunoglobulin G (IgG)-saporin 14 days before plantar incision or sham procedure and were assessed for spontaneous guarding behavior in the ipsilateral hindpaw (A). Data is expressed as Mean ± SEM. Kruskal-Wallis ANOVA on ranks followed by Tukey test . # P < 0.05 for within time point comparison to Incision + IgG-saporin value. Friedman repeated measures ANOVA on ranks followed by Dunnett test, * P < 0.05 for within treatment group comparison to pre-incision (D0) baseline value. Modeled group trajectories of cumulative guarding scores in the ipsilateral (B) paw of treated rats. Group averaged trajectories depict the mean fit for all the animals within each treatment group (n = 6 per group) with 95% CIs indicated by shading.
Modeled guarding scores in incision rats administered DβH-saporin showed slightly higher predicted intercepts in the ipsilateral paw compared to incision rats administered IgG-saporin (P=0.0035) resulting in a more negative slope (slope: P=0.02); however, the duration of spontaneous guarding was similar between DβH-saporin and IgG-saporin treated incision rats persisting for 10 days in both groups (Fig. 3C). No guarding behavior was present in sham rats from either treatment group (intercept: P=0.39; slope: P=0.34; quadratic: P=0.32).
Depletion of descending spinal noradrenergic fibers increases post-incision spinal microglia and astrocyte activation
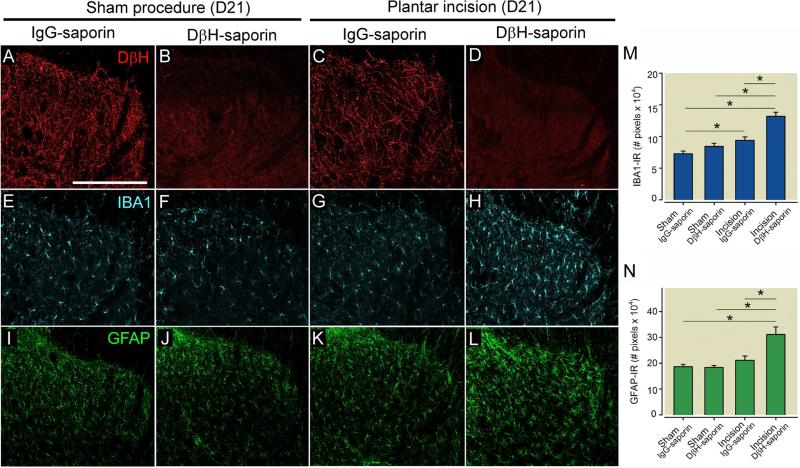
We assessed spinal cord noradrenergic fiber density in treated rats using DβH immunohistochemistry (Fig 4A-D). Spinal noradrenergic fiber density did not differ between IgG-saporin treated sham and incision rats, with a typical pattern throughout the dorsal horn (Fig. 4A,C) but DβH-saporin treatment depleted nearly all noradrenergic fibers in sham and incision rats (Fig. 4B,D). No animals had to be excluded due to incomplete depletion. We then examined the effects of incision surgery and spinal noradrenergic fiber depletion on spinal microglial and astrocyte activation in the ipsilateral spinal cord at day 21 following incision surgery. IBA1-immunoreactivity (IR) was significantly increased in DβH-saporin treated incision rats compared to IgG-saporin treated incision rats and compared to sham operated rats from both treatment groups (Fig. 4E, F,G,H, M). IBA1-immunoreactivity (IR) was significantly increased in the spinal cord of IgG treated incision rats compared to IgG treated sham rats (Fig 4M). GFAP-IR was significantly increased in DβH-saporin treated incision rats compared to IgG-saporin treated incision rats and sham operated rats from both treatment groups (Fig. 4I-L, N).
Figure 4.
Spinal depletion of noradrenergic fibers increases microglial and astrocyte activation following plantar incision in rats. Rats were administered DβH-saporin (i.t., 5μg) to deplete spinal noradrenergic fibers or control IgG saporin 14 days prior to surgery. Representative images of ipsilateral dorsal medial spinal cord of rats 21 days following plantar incision or sham procedure labeled with antibodies against dopamine β hydroxylase (DβH, red, A-D) for noradrenergic fibers, ionized calcium binding adaptor molecule 1 (IBA1,blue, E-H) for microglia and glial fibrillary acid protein (GFAP, green, I-L) for astrocytes. Note loss of noradrenergic fibers in spinal cord of DβH-saporin treated rats accompanied by increased IBA1-IR and GFAP-IR in rats following plantar incision but not sham procedure. Immunofluorescence levels of IBA1 (M) and GFAP (N) in the dorsal medial spinal cord were quantified. Data is presented as mean ± SEM. n = 6 per group. Groups were analysis by Two-way ANOVA, IBA1: p (Surgery × Treatment) = 0.015, GFAP: p (Surgery × Treatment) = 0.005, Bonferroni Multiple Comparison Test * p < 0.001. Scale bar in A = 250 μms.
Perioperative blockade of spinal α2 adrenergic receptor signaling prolongs mechanical hypersensitivity
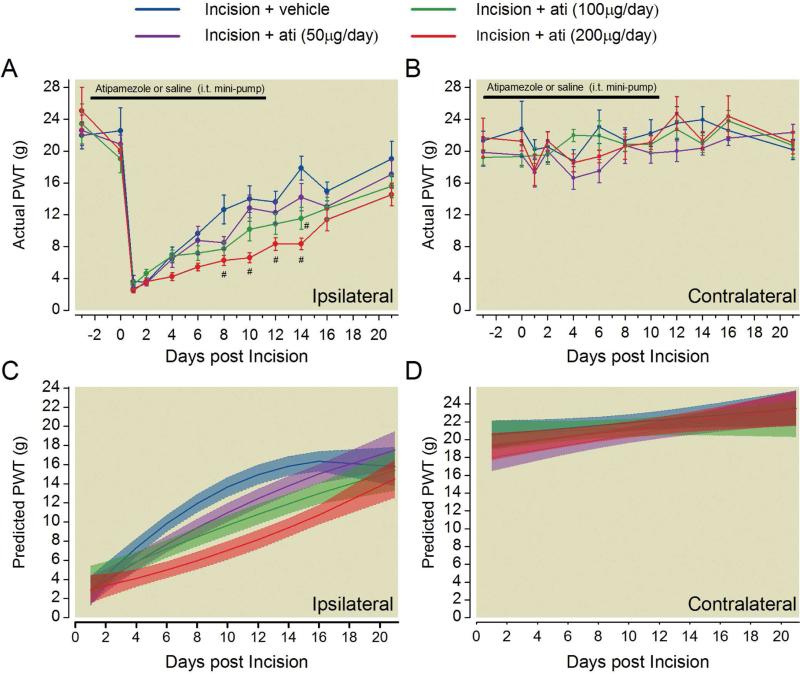
Rats were administered chronic spinal infusion of atipamezole in 50, 100, and 200 μg/day doses beginning 4 days prior to until 10 days after surgery. The baseline mechanical withdrawal thresholds after 3 days of drug treatment and prior to surgery was not significantly different between treatment groups in the ipsilateral (Fig. 5A) or contralateral paw (Fig 5B). Based on two-way RM ANOVA, ipsilateral mechanical paw withdrawal thresholds were significantly different from vehicle treated rats from postoperative days 8 through 14 (P <0.001) for the highest atipamezole dose (200 μg/day) whereas the intermediate dose (100 μg/day) was only significantly different on postoperative day 14 (P <0.001).Contralateral paw withdrawal thresholds were not significantly different at any postoperative time point (Fig. 5B)
Figure 5.
Blockade of spinal α2 adrenergic receptors delays the resolution of mechanical hypersensitivity after plantar incision. Rats were chronically treated with varying doses of the α2 adrenergic receptor antagonist atipamezole or saline beginning three days prior to until eleven days after plantar incision. Mechanical withdrawal thresholds in the ipsilateral (A) and contralateral (B) hindpaw of treated rats prior to and following incision surgery. Data is expressed as Mean ± SEM. Two-way repeated-measures ANOVA with Bonferroni multiple comparisons. # P < 0.05 for within time point comparison to Incision + vehicle treated value. Modeled group trajectories of postoperative mechanical withdrawal thresholds in the ipsilateral (C) and contralateral (D) paw. The highest dose of atipamezole (200μg/day, red) had significantly lower slope of recovery evident as non-overlapping 95% confidence intervals compared to vehicle treated rats (blue) from 4 to 18 days postoperatively. Group averaged trajectories depict the mean fit for all the animals within each treatment group (n=12-14 per group) with 95% CIs indicated by shading.
Modeled mechanical withdrawal thresholds in ipsilateral paw of atipamezole-treated incision rats showed less rapid recovery compared to vehicle treated incised rats, and this effect was dependent on atipamezole dose (Fig. 5C, Table 1). The group modeled trajectory of the highest dose of atipamezole had non-overlapping 95% confidence intervals compared to vehicle treated rats from 4 to 18 days postoperatively. The predicted intercepts were not significantly different between groups (Table 1). Modeled contralateral paw withdrawal thresholds produced similar trajectories in atipamezole and vehicle treated rats (Fig. 5D).
Table 1.
Growth curve modeling of ipsilateral mechanical withdrawal thresholds in rats administered chronic spinal atipamezole or vehicle
| Predictor | Parameter | Estimate | (Lower Bound, Upper Bound) | p |
|---|---|---|---|---|
| Entire population | Intercept | 2.8022 | (1.381, 4.223) | <0.0001 |
| Entire population | Slope | 1.661 | (1.336, 1.985) | <0.0001 |
| Entire population | Quadratic | −0.0506 | (−.0658, −0.0353) | <0.0001 |
| Group | Intercept | |||
| Ati (200μg/day) | 0.1934 | (−1.780, 2.167) | 0.845 | |
| Ati (100μg/day) | 1.037 | (−1.0143, 3.0883) | 0.315 | |
| Ati (50μg/day) | 0.0297 | (−2.3358, 0.8086) | 0.976 | |
| Vehicle | REF | |||
| Group × Time | Slope | |||
| Ati (200μg/day) | −1.319 | (−1.769, −0.868) | <0.0001 | |
| Ati (100μg/day) | −0.959 | (−1.427, −0.491) | <0.0001 | |
| Ati (50μg/day) | −0.613 | (−1.064, −0.162) | 0.0078 | |
| Vehicle | REF | |||
| Group × Time2 | Quadratic | |||
| Ati (200μg/day) | 0.0622 | (0.0224, 0.0664) | <0.0001 | |
| Ati (100μg/day) | 0.0444 | (0.0411, 0.0834) | <0.0001 | |
| Ati (50μg/day) | 0.0349 | (0.0137, 0.0561) | 0.0013 | |
| Vehicle | REF |
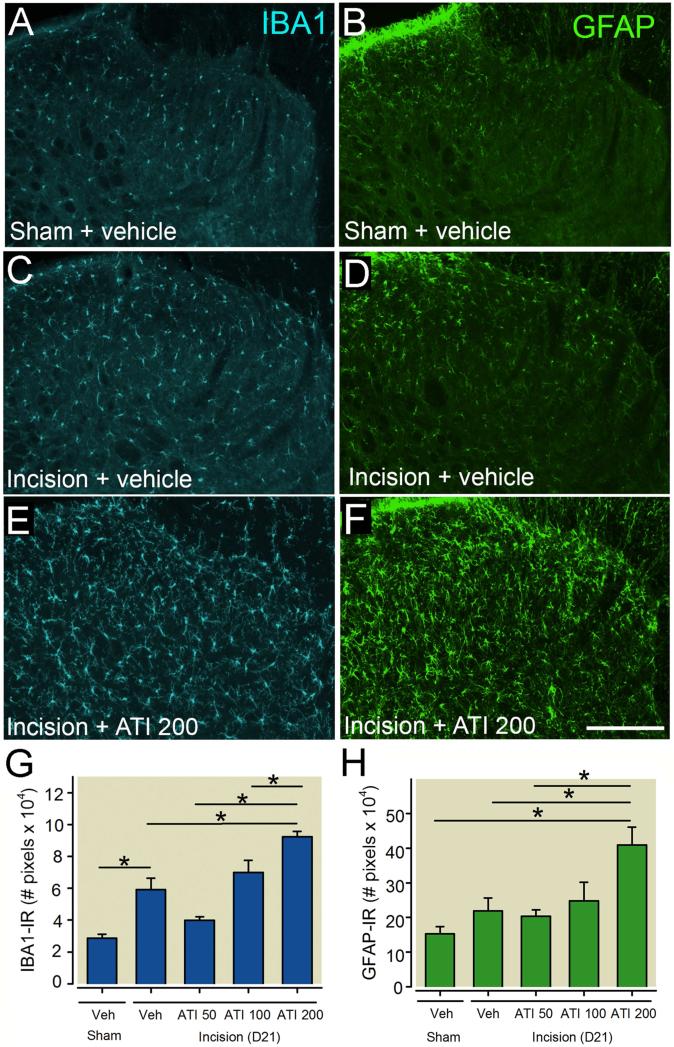
Blockade of spinal α2 adrenergic receptor signaling increased spinal microglia and astrocyte activation following incision
The same rats used for behavior were sacrificed at 21 days post incision to examine if α2 adrenergic receptor blockade had long lasting effects on spinal microglial and astrocyte activation in the spinal cord ipsilateral to incision (Fig.6A-H). Incision rats that received the highest dose of atipamezole (200μg/day) had significantly greater IBA1-IR compared to all treatment groups (Fig. 6A,C,E,G). IBA1-IR was significantly increased in the spinal cord of vehicle treated incision rats compared to vehicle treated sham rats (Fig. 6G). Incision rats that received the highest does of atipamezole (200μg/day) had significantly greater GFAP-IR compared to vehicle, 50μg/day treated incision rats and sham rats but not 100μg/day incision rats (Fig. 6B,D,F,H).
Figure 6.
Blockade of spinal α2 adrenergic receptor increases microglial and astrocyte activation in the ipsilateral spinal cord 21 days following plantar incision in rats. Representative images of ionized calcium binding adaptor molecule 1 (IBA1, blue, A,C,E) for microglia and glial fibrillary acidic protein (GFAP, green, B,D,F) for astrocytes in rats without surgery (A,B) or in rats with plantar incision administered chronic intrathecal vehicle solution (C,D) or atipamezole (200μg/day, E, F) beginning three days prior to until eleven days following plantar incision. Immunofluorescence levels of IBA1 (G) and GFAP (H) in the dorsal medial spinal cord. Data are presented as mean ± SEM. n = 5 per group. Groups were analysis by one-way ANOVA, IBA1: p (Treatment) = 0.002, GFAP: p (Treatment) <0.001, Bonferroni Multiple Comparison Test * P < 0.05. Scale bar in A = 250 μms.
Discussion
In the present study, we provide evidence that spinal noradrenergic signaling hastens resolution of hypersensitivity following surgical incision, as depletion of spinal noradrenergic fibers with DβH-saporin slowed the resolution of hypersensitivity but not spontaneous guarding after incision. Similarly, chronic blockade of spinal α2 adrenergic receptors delayed recovery of mechanical hypersensitivity, evident as a slower predicted recovery based on growth curve analysis. Both approaches to disrupt spinal noradrenergic signaling increased microglial and astrocyte activation following incision. These data suggest that spinal noradrenergic transmission and α2 adrenergic receptor activation is critical to the normal resolution of incisional hypersensitivity and glial plasticity.
Role of descending noradrenergic fibers and signaling in resolution of postoperative incisional pain behaviors
The current study adds to an emerging role of descending noradrenergic neurotransmission in postoperative recovery as we show for the first time a contribution of descending spinal noradrenergic activation to recovery of hypersensitivity following acute plantar incision. Previous studies reported increased noradrenergic tone following incision evident as increased spinal norepinephrine content one day 25 and increased basal spinal norepinephrine release for at least three days 58 following plantar incision. Spinal administration of the norepinephrine reuptake inhibitors reboxetine, milnacipran, or Xen2174 attenuated mechanical hypersensitivity following plantar incision37, 39 and was reversed by spinal administration of the α2 adrenergic receptor antagonist idazoxan37. When given prior to incision, Xen2174 had lasting effects reducing mechanical hypersensitivity for several days37. These and the current study support an important role for increased endogenous noradrenergic activity in the resolution of post-incisional mechanical and thermal hypersensitivity.
Chronic blockade of α2 adrenergic receptors in the spinal cord with atipamezole markedly delayed, but did not prevent the recovery of incision-induced mechanically hypersensitivity based on a positive slope of recovery. These results suggest that α2 adrenergic receptor activation is important for recovery similar to results in rodent models of nerve injury 19, 20, however additional noradrenergic receptor subtypes or other mechanisms may also be important for promoting resolution. For example, chronic β2 receptor agonist administration reduces allodynia in patients and rodents with chronic neuropathic pain or injury 9, 64 and history of chronic β2 adrenergic receptor agonist treatment at the time of surgery predicts a lower incidence of chronic neuropathic pain following thoracotomy 54. Alternatively, alterations in other endogenous spinal inhibitory systems may play a role in promoting resolution of incisional hypersensitivity including endocannabinoids 1, oxytocin 17, opioids 10, and serotonin 60.
An important observation in the current study was lack of contralateral sensitization in rats with incision. Contralateral hypersensitivity following unilateral injury has been reported in rodent inflammatory 16 and neuropathic pain models 40. Following partial nerve injury, contralateral sensitization may be suppressed by descending noradrenergic tone as spinal α2 antagonists 20 or ablation of noradrenergic fibers with DβH-saporin 44 unmasks or exacerbates contralateral hypersensitivity. In the current study, contralateral hypersensitivity was not present in rats with plantar incision nor was it unmasked in rats treated preoperatively with DβH-saporin or chronic atipamezole suggesting that contralateral sensitization does not occur in the plantar incision model.
We also predicted that disrupting spinal noradrenergic activity would increase the duration of ongoing or non-evoked pain based on the known inhibitory effects of the spinal α2 adrenergic receptor agonist clonidine on ongoing postsurgical 13, 28, 31 and chronic pain 14, 48 in humans. We observed a slight increase in the magnitude of spontaneous guarding for two days in DβH-saporin treated incision rats, but no significant difference in the duration of guarding behavior. Guarding behavior in the plantar incision model coincides temporally with increased spontaneous activity of spinal cord neurons and Aδ and C fiber afferents that innervate deep tissue around the incision 6, 47, 62. The lack of profound increase in the duration of guarding suggests that descending noradrenergic system may be insufficient to suppress aberrant afferent barrage originating from the site of incision. However recent studies using operant based assays have provided evidence for ongoing pain in preclinical pain models in the absence of overt guarding 26, 35. Rats with peripheral nerve injury exhibit conditioned place preference to spinal clonidine 26 and the norepinephrine reuptake inhibitor reboxetine 19 while sham operated rats do not. Additionally, nerve injured but not sham operated rats self administer spinal clonidine 30 suggesting that nerve injury produces a tonic aversive state. Future studies, will need to determine if these more operant based measures of ongoing pain are present at later time points in incision rats with disruption of spinal noradrenergic activity.
Significance of enhanced microglial and astrocyte activation in resolution of incision induced hypersensitivity
Spinal microglial and astrocyte activation have been well documented following plantar incision in the rat 21, 38, 42 occurring as early as one day following surgery and resolving within seven days 50, 59. This time course correlates with the typical resolution of mechanical hypersensitivity in this model. In the current study, we observed increased IBA1-IR and GFAP-IR in the medial aspects of the ipsilateral spinal cord dorsal horn as late as 21 days after plantar incision in rats with ablated spinal noradrenergic fibers or following chronic spinal infusion of atipamezole. We previously observed similar results at early 18 and late time points 44 after partial nerve injury. These data suggest that reduced spinal noradrenergic tone slows or prevents resolution of neuroinflammation in the presence of peripheral tissue injury due to incision or nerve injury. The increased glial activation may result from enhanced release of excitatory neurotransmitters (e.g. glutamate, substance P) due to reduced presynaptic inhibition of α2A containing primary afferents 5, 24 or possibly direct effects via α2A or β2 ARs expressed on spinal glial cells 32-34. Consistent with our findings, exogenous α2 adrenergic receptor agonists 15 and the norepinephrine reuptake inhibitor tramadol 53 alleviate nerve injury induced mechanical hypersensitivity and reduce spinal astrocyte activation and these effects are prevented by spinal α2 adrenergic receptor blockade 53. A role for spinal neuroimmune interactions in persistent hypersensitivity after incision and inflammation has been proposed. Therapies that target microglial 42, 59 and astrocyte signaling 51 reduce mechanical hypersensitivity following incision and sequestration of spinal CCL2 44 and p38 MAPK inhibition 61 provide sustained alleviation of mechanical hypersensitivity in rats with incisional injury. Future studies are needed to determine if there is a causal link between the enhanced glial activation and the delayed resolution of hypersensitivity observed in this study. A limitation of the current study was confining our IHC analysis to the ipsilateral spinal cord which was based on the lack of contralateral hypersensitivity in DβH-saporin or atipamezole treated incision rats. Additionally, we did not examine the impact of chronic infusion of atipamezole in sham rats although we confirmed previously published results demonstrating no changes in microglial or astrocyte activation in non-injured rats following DβH-saporin treatment 22. A more precise examination of the spatiotemporal alterations in spinal glial activation following blockade of spinal noradrenergic activation is warranted in future studies.
Therapeutic strategies to speed recovery from pain after surgery
The current study demonstrates that reducing spinal noradrenergic signaling slows recovery of mechanical hypersensitivity, and we speculate that enhancing spinal noradrenergic signaling might speed recovery of pain after surgery, particularly in patients with impaired descending noradrenergic inhibition. For example, increased stimulation of spinal α2-ARs by intrathecal injection of clonidine significantly reduces the area of mechanical hypersensitivity around an incision 48 and 72 hours after abdominal surgery and results in a lower incidence of pain six months later 12, 27. A relationship between peri-incisional hypersensitivity and slow recovery from pain has also been observed following breast augmentation surgery as persistent hyperesthesia even in the absence of spontaneous pain at 6 weeks predicted the likelihood of persistent pain 1 year 52 and 4 years after surgery23. Surprisingly, few studies have examined the impact of antidepressants, many of which engage descending inhibitory systems including serotonin, norepinephrine or dual reuptake inhibitors, on duration of pain presence after surgery. In a double blinded placebo controlled trial of patients having breast cancer surgery the serotonin and norepinephrine reuptake inhibitor venlafaxine reduced the incidence of pain six months after mastectomy 2. The majority of these early studies did not stratify patients based on their preoperative CPM or presumed risk. In chronic pain patients, less efficient CPM in patients with chronic diabetic neuropathy predicted analgesic efficacy of duloxetine66 and tapentodol36, however similar studies involving relatively pain free patients prior to surgery have not been conducted. Collectively, these studies suggest that targeting therapies that augment spinal noradrenergic signaling to patients with reduced CPM may decrease the duration and severity of pain in those most at risk. Drugs that augment this system or engage multiple descending inhibitory systems may be strategies that speed recovery from acute postsurgical pain that warrant further investigation.
Highlights.
- Mixed growth curve analysis is used to model pain related behaviors following plantar incision in the rat
- Spinal noradrenergic signaling hastens resolution of hypersensitivity but not guarding behavior following incision
- Blockade of spinal noradrenergic signaling increases spinal glial activation after incision
Acknowledgments
Disclosures and research funding
This work was supported from NIH grant GM099863 awarded to C.M. Peters and NIH grant GM48085 awarded to J.C. Eisenach.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alkaitis MS, Solorzano C, Landry RP, Piomelli D, DeLeo JA, Romero-Sandoval EA. Evidence for a role of endocannabinoids, astrocytes and p38 phosphorylation in the resolution of postoperative pain. PloS one. 2010;5:e10891. doi: 10.1371/journal.pone.0010891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amr YM, Yousef AA. Evaluation of efficacy of the perioperative administration of Venlafaxine or gabapentin on acute and chronic postmastectomy pain. The Clinical Journal of Pain. 2010;26:381–385. doi: 10.1097/AJP.0b013e3181cb406e. [DOI] [PubMed] [Google Scholar]
- 3.Aschenbrenner CA, Houle TT, Gutierrez S, Eisenach JC. Modeling Individual Recovery after Peripheral Nerve Injury in Rats and the Effects of Parturition. Anesthesiology. 2014 doi: 10.1097/ALN.0000000000000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baba H, Goldstein PA, Okamoto M, Kohno T, Ataka T, Yoshimura M, Shimoji K. Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 2): effects on somatodendritic sites of GABAergic neurons. Anesthesiology. 2000;92:485–492. doi: 10.1097/00000542-200002000-00031. [DOI] [PubMed] [Google Scholar]
- 5.Bourgoin S, Pohl M, Mauborgne A, Benoliel JJ, Collin E, Hamon M, Cesselin F. Monoaminergic control of the release of calcitonin gene-related peptide- and substance P-like materials from rat spinal cord slices. Neuropharmacology. 1993;32:633–640. doi: 10.1016/0028-3908(93)90076-f. [DOI] [PubMed] [Google Scholar]
- 6.Brennan TJ. Pathophysiology of postoperative pain. Pain. 2011;152:S33–40. doi: 10.1016/j.pain.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 8.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of neuroscience methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 9.Cok OY, Eker HE, Yalcin I, Barrot M, Aribogan A. Is there a place for beta-mimetics in clinical management of neuropathic pain? Salbutamol therapy in six cases. Anesthesiology. 2010;112:1276–1279. doi: 10.1097/ALN.0b013e3181d40399. [DOI] [PubMed] [Google Scholar]
- 10.Corder G, Doolen S, Donahue RR, Winter MK, Jutras BL, He Y, Hu X, Wieskopf JS, Mogil JS, Storm DR, Wang ZJ, McCarson KE, Taylor BK. Constitutive mu-opioid receptor activity leads to long-term endogenous analgesia and dependence. Science (New York, N.Y. 2013;341:1394–1399. doi: 10.1126/science.1239403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corder G, Siegel A, Intondi AB, Zhang X, Zadina JE, Taylor BK. A novel method to quantify histochemical changes throughout the mediolateral axis of the substantia gelatinosa after spared nerve injury: characterization with TRPV1 and substance P. J Pain. 2010;11:388–398. doi: 10.1016/j.jpain.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Kock M, Lavand'homme P, Waterloos H. The short-lasting analgesia and long-term antihyperalgesic effect of intrathecal clonidine in patients undergoing colonic surgery. Anesthesia and analgesia. 2005;101:566–572. doi: 10.1213/01.ANE.0000157121.71808.04. table of contents. [DOI] [PubMed] [Google Scholar]
- 13.Eisenach JC, Lysak SZ, Viscomi CM. Epidural clonidine analgesia following surgery: phase I. Anesthesiology. 1989;71:640–646. doi: 10.1097/00000542-198911000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Eisenach JC, Rauck RL, Buzzanell C, Lysak SZ. Epidural clonidine analgesia for intractable cancer pain: phase I. Anesthesiology. 1989;71:647–652. doi: 10.1097/00000542-198911000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Feng X, Zhang F, Dong R, Li W, Liu J, Zhao X, Xue Q, Yu B, Xu J. Intrathecal administration of clonidine attenuates spinal neuroimmune activation in a rat model of neuropathic pain with existing hyperalgesia. European journal of pharmacology. 2009;614:38–43. doi: 10.1016/j.ejphar.2009.04.044. [DOI] [PubMed] [Google Scholar]
- 16.Gao YJ, Xu ZZ, Liu YC, Wen YR, Decosterd I, Ji RR. The c-Jun N-terminal kinase 1 (JNK1) in spinal astrocytes is required for the maintenance of bilateral mechanical allodynia under a persistent inflammatory pain condition. Pain. 2010;148:309–319. doi: 10.1016/j.pain.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutierrez S, Liu B, Hayashida K, Houle TT, Eisenach JC. Reversal of peripheral nerve injury-induced hypersensitivity in the postpartum period: role of spinal oxytocin. Anesthesiology. 2013;118:152–159. doi: 10.1097/ALN.0b013e318278cd21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashida K, Peters CM, Gutierrez S, Eisenach JC. Depletion of endogenous noradrenaline does not prevent spinal cord plasticity following peripheral nerve injury. J Pain. 2012;13:49–57. doi: 10.1016/j.jpain.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes S, Hickey L, Donaldson LF, Lumb BM, Pickering AE. Intrathecal reboxetine suppresses evoked and ongoing neuropathic pain behaviours by restoring spinal noradrenergic inhibitory tone. Pain. 2015;156:328–334. doi: 10.1097/01.j.pain.0000460313.73358.31. [DOI] [PubMed] [Google Scholar]
- 20.Hughes SW, Hickey L, Hulse RP, Lumb BM, Pickering AE. Endogenous analgesic action of the pontospinal noradrenergic system spatially restricts and temporally delays the progression of neuropathic pain following tibial nerve injury. Pain. 2013;154:1680–1690. doi: 10.1016/j.pain.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito N, Obata H, Saito S. Spinal microglial expression and mechanical hypersensitivity in a postoperative pain model: comparison with a neuropathic pain model. Anesthesiology. 2009;111:640–648. doi: 10.1097/ALN.0b013e3181b05f42. [DOI] [PubMed] [Google Scholar]
- 22.Jasmin L, Boudah A, Ohara PT. Long-term effects of decreased noradrenergic central nervous system innervation on pain behavior and opioid antinociception. The Journal of comparative neurology. 2003;460:38–55. doi: 10.1002/cne.10633. [DOI] [PubMed] [Google Scholar]
- 23.Kaasa T, Romundstad L, Roald H, Skolleborg K, Stubhaug A. Hyperesthesia one year after breast augmentation surgery increases the odds for persisting pain at four years. Scandinavian Journal of Pain. 2010;1:75–81. doi: 10.1016/j.sjpain.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Kawasaki Y, Kumamoto E, Furue H, Yoshimura M. Alpha 2 adrenoceptor-mediated presynaptic inhibition of primary afferent glutamatergic transmission in rat substantia gelatinosa neurons. Anesthesiology. 2003;98:682–689. doi: 10.1097/00000542-200303000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Kimura M, Obata H, Saito S. Antihypersensitivity effects of tramadol hydrochloride in a rat model of postoperative pain. Anesthesia and analgesia. 2012;115:443–449. doi: 10.1213/ANE.0b013e31825683c3. [DOI] [PubMed] [Google Scholar]
- 26.King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nature neuroscience. 2009;12:1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavand'homme P, De Kock M. The use of intraoperative epidural or spinal analgesia modulates postoperative hyperalgesia and reduces residual pain after major abdominal surgery. Acta anaesthesiologica Belgica. 2006;57:373–379. [PubMed] [Google Scholar]
- 28.Lavand'homme PM, Roelants F, Waterloos H, Collet V, De Kock MF. An evaluation of the postoperative antihyperalgesic and analgesic effects of intrathecal clonidine administered during elective cesarean delivery. Anesthesia and analgesia. 2008;107:948–955. doi: 10.1213/ane.0b013e31817f1595. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Eisenach JC. alpha2A-adrenoceptor stimulation reduces capsaicin-induced glutamate release from spinal cord synaptosomes. The Journal of pharmacology and experimental therapeutics. 2001;299:939–944. [PubMed] [Google Scholar]
- 30.Martin TJ, Kim SA, Eisenach JC. Clonidine maintains intrathecal self-administration in rats following spinal nerve ligation. Pain. 2006;125:257–263. doi: 10.1016/j.pain.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 31.Mendez R, Eisenach JC, Kashtan K. Epidural clonidine analgesia after cesarean section. Anesthesiology. 1990;73:848–852. doi: 10.1097/00000542-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Mori K, Ozaki E, Zhang B, Yang L, Yokoyama A, Takeda I, Maeda N, Sakanaka M, Tanaka J. Effects of norepinephrine on rat cultured microglial cells that express alpha1, alpha2, beta1 and beta2 adrenergic receptors. Neuropharmacology. 2002;43:1026–1034. doi: 10.1016/s0028-3908(02)00211-3. [DOI] [PubMed] [Google Scholar]
- 33.Morioka N, Abe H, Araki R, Matsumoto N, Zhang FF, Nakamura Y, Hisaoka-Nakashima K, Nakata Y. A beta1/2 adrenergic receptor-sensitive intracellular signaling pathway modulates CCL2 production in cultured spinal astrocytes. Journal of cellular physiology. 2014;229:323–332. doi: 10.1002/jcp.24452. [DOI] [PubMed] [Google Scholar]
- 34.Morioka N, Tanabe H, Inoue A, Dohi T, Nakata Y. Noradrenaline reduces the ATP-stimulated phosphorylation of p38 MAP kinase via beta-adrenergic receptors-cAMP-protein kinase A-dependent mechanism in cultured rat spinal microglia. Neurochemistry international. 2009;55:226–234. doi: 10.1016/j.neuint.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Navratilova E, Xie JY, King T, Porreca F. Evaluation of reward from pain relief. Annals of the New York Academy of Sciences. 2013;1282:1–11. doi: 10.1111/nyas.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niesters M, Proto PL, Aarts L, Sarton EY, Drewes AM, Dahan A. Tapentadol potentiates descending pain inhibition in chronic pain patients with diabetic polyneuropathy. British journal of anaesthesia. 2014;113:148–156. doi: 10.1093/bja/aeu056. [DOI] [PubMed] [Google Scholar]
- 37.Obata H, Conklin D, Eisenach JC. Spinal noradrenaline transporter inhibition by reboxetine and Xen2174 reduces tactile hypersensitivity after surgery in rats. Pain. 2005;113:271–276. doi: 10.1016/j.pain.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 38.Obata H, Eisenach JC, Hussain H, Bynum T, Vincler M. Spinal glial activation contributes to postoperative mechanical hypersensitivity in the rat. J Pain. 2006;7:816–822. doi: 10.1016/j.jpain.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Obata H, Kimura M, Nakajima K, Tobe M, Nishikawa K, Saito S. Monoamine-dependent, opioid-independent antihypersensitivity effects of intrathecally administered milnacipran, a serotonin noradrenaline reuptake inhibitor, in a postoperative pain model in rats. The Journal of pharmacology and experimental therapeutics. 2010;334:1059–1065. doi: 10.1124/jpet.110.168336. [DOI] [PubMed] [Google Scholar]
- 40.Obata H, Sakurazawa S, Kimura M, Saito S. Activation of astrocytes in the spinal cord contributes to the development of bilateral allodynia after peripheral nerve injury in rats. Brain research. 2011;1363:72–80. doi: 10.1016/j.brainres.2010.09.105. [DOI] [PubMed] [Google Scholar]
- 41.Pertovaara A. The noradrenergic pain regulation system: a potential target for pain therapy. European journal of pharmacology. 2013;716:2–7. doi: 10.1016/j.ejphar.2013.01.067. [DOI] [PubMed] [Google Scholar]
- 42.Peters CM, Eisenach JC. Contribution of the chemokine (C-C motif) ligand 2 (CCL2) to mechanical hypersensitivity after surgical incision in rats. Anesthesiology. 2010;112:1250–1258. doi: 10.1097/ALN.0b013e3181d3d978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters CM, Hayashida K, Ewan EE, Nakajima K, Obata H, Xu Q, Yaksh TL, Eisenach JC. Lack of analgesic efficacy of spinal ondansetron on thermal and mechanical hypersensitivity following spinal nerve ligation in the rat. Brain research. 2010;1352:83–93. doi: 10.1016/j.brainres.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peters CM, Hayashida KI, Suto T, Houle TT, Aschenbrenner CA, Martin TJ, Eisenach JC. Individual Differences in Acute Pain-induced Endogenous Analgesia Predict Time to Resolution of Postoperative Pain in the Rat. Anesthesiology. 2015 doi: 10.1097/ALN.0000000000000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peters CM, Ririe D, Houle TT, Aschenbrenner CA, Eisenach JC. Nociceptor-selective peripheral nerve block induces delayed mechanical hypersensitivity and neurotoxicity in rats. Anesthesiology. 2014;120:976–986. doi: 10.1097/ALN.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 46.Pogatzki-Zahn EM, Zahn PK, Brennan TJ. Postoperative pain--clinical implications of basic research. Best practice & research. 2007;21:3–13. doi: 10.1016/j.bpa.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Pogatzki EM, Gebhart GF, Brennan TJ. Characterization of Adelta- and C-fibers innervating the plantar rat hindpaw one day after an incision. Journal of neurophysiology. 2002;87:721–731. doi: 10.1152/jn.00208.2001. [DOI] [PubMed] [Google Scholar]
- 48.Rauck RL, North J, Eisenach JC. Intrathecal clonidine and adenosine: effects on pain and sensory processing in patients with chronic regional pain syndrome. Pain. 2015;156:88–95. doi: 10.1016/j.pain.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 49.Riedl MS, Schnell SA, Overland AC, Chabot-Dore AJ, Taylor AM, Ribeiro-da-Silva A, Elde RP, Wilcox GL, Stone LS. Coexpression of alpha 2A-adrenergic and delta-opioid receptors in substance P-containing terminals in rat dorsal horn. The Journal of comparative neurology. 2009;513:385–398. doi: 10.1002/cne.21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romero-Sandoval A, Chai N, Nutile-McMenemy N, Deleo JA. A comparison of spinal Iba1 and GFAP expression in rodent models of acute and chronic pain. Brain research. 2008;1219:116–126. doi: 10.1016/j.brainres.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romero-Sandoval A, Eisenach JC. Spinal cannabinoid receptor type 2 activation reduces hypersensitivity and spinal cord glial activation after paw incision. Anesthesiology. 2007;106:787–794. doi: 10.1097/01.anes.0000264765.33673.6c. [DOI] [PubMed] [Google Scholar]
- 52.Romundstad L, Breivik H, Roald H, Skolleborg K, Romundstad PR, Stubhaug A. Chronic pain and sensory changes after augmentation mammoplasty: long term effects of preincisional administration of methylprednisolone. Pain. 2006;124:92–99. doi: 10.1016/j.pain.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 53.Sakakiyama M, Maeda S, Isami K, Asakura K, So K, Shirakawa H, Nakagawa T, Kaneko S. Preventive and alleviative effect of tramadol on neuropathic pain in rats: roles of alpha(2)-adrenoceptors and spinal astrocytes. Journal of pharmacological sciences. 2014;124:244–257. doi: 10.1254/jphs.13223fp. [DOI] [PubMed] [Google Scholar]
- 54.Salvat E, Schweitzer B, Massard G, Meyer N, de Blay F, Muller A, Barrot M. Effects of beta agonists on post-thoracotomy pain incidence. European journal of pain (London, England) 2015 doi: 10.1002/ejp.673. [DOI] [PubMed] [Google Scholar]
- 55.Stone LS, Broberger C, Vulchanova L, Wilcox GL, Hokfelt T, Riedl MS, Elde R. Differential distribution of alpha2A and alpha2C adrenergic receptor immunoreactivity in the rat spinal cord. J Neurosci. 1998;18:5928–5937. doi: 10.1523/JNEUROSCI.18-15-05928.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Swett JE, Woolf CJ. The somatotopic organization of primary afferent terminals in the superficial laminae of the dorsal horn of the rat spinal cord. The Journal of comparative neurology. 1985;231:66–77. doi: 10.1002/cne.902310106. [DOI] [PubMed] [Google Scholar]
- 57.Tyce GM, Yaksh TL. Monoamine release from cat spinal cord by somatic stimuli: an intrinsic modulatory system. The Journal of physiology. 1981;314:513–529. doi: 10.1113/jphysiol.1981.sp013722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y, Feng C, Wu Z, Wu A, Yue Y. Activity of the descending noradrenergic pathway after surgery in rats. Acta anaesthesiologica Scandinavica. 2008;52:1336–1341. doi: 10.1111/j.1399-6576.2008.01778.x. [DOI] [PubMed] [Google Scholar]
- 59.Wen YR, Suter MR, Ji RR, Yeh GC, Wu YS, Wang KC, Kohno T, Sun WZ, Wang CC. Activation of p38 mitogen-activated protein kinase in spinal microglia contributes to incision-induced mechanical allodynia. Anesthesiology. 2009;110:155–165. doi: 10.1097/ALN.0b013e318190bc16. [DOI] [PubMed] [Google Scholar]
- 60.Wijnvoord N, Albuquerque B, Haussler A, Myrczek T, Popp L, Tegeder I. Inter-strain differences of serotonergic inhibitory pain control in inbred mice. Molecular pain. 2010;6:70. doi: 10.1186/1744-8069-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilder-Smith OH, Schreyer T, Scheffer GJ, Arendt-Nielsen L. Patients with chronic pain after abdominal surgery show less preoperative endogenous pain inhibition and more postoperative hyperalgesia: a pilot study. Journal of pain & palliative care pharmacotherapy. 2011;24:119–128. doi: 10.3109/15360281003706069. [DOI] [PubMed] [Google Scholar]
- 62.Xu J, Richebe P, Brennan TJ. Separate groups of dorsal horn neurons transmit spontaneous activity and mechanosensitivity one day after plantar incision. European journal of pain (London, England) 2008 doi: 10.1016/j.ejpain.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 63.Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiology & behavior. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- 64.Yalcin I, Tessier LH, Petit-Demouliere N, Waltisperger E, Hein L, Freund-Mercier MJ, Barrot M. Chronic treatment with agonists of beta(2)-adrenergic receptors in neuropathic pain. Experimental neurology. 2010;221:115–121. doi: 10.1016/j.expneurol.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 65.Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best LA, Granot M. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain. 2008;138:22–28. doi: 10.1016/j.pain.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 66.Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M, Granovsky Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain. 2012;153:1193–1198. doi: 10.1016/j.pain.2012.02.021. [DOI] [PubMed] [Google Scholar]