Triiodothyronine Promotes Cardiac Differentiation and Maturation of Embryonic Stem Cells via the Classical Genomic Pathway (original) (raw)
Abstract
Embryonic stem cells (ESCs) can differentiate into functional cardiomyocytes and thus represent a promising cell source for cardiac regenerative therapy. Nevertheless, the therapeutic application of ESC-derived cardiomyocytes is limited by the low efficacy of the current protocol for cardiac differentiation and their immature phenotypes. Although thyroid hormone is essential for normal cardiac development and function, its role in the cardiac differentiation of ESCs, as well as the maturation of ESC-derived cardiomyocytes, remains unclear. In this study, we examined the cardiac differentiation of murine ESCs in the presence of T3 for 7 d using flow cytometry, RT-PCR, cellular electrophysiology study, and confocal calcium imaging. Compared with control conditions, T3 supplementation increased the number of ESC-derived cardiomyocytes and was accompanied by up-regulation of a panel of cardiac markers, including Nkx2.5, myosin light chain-2V, as well as α- and β-myosin heavy chain. More importantly, electrophysiological study revealed that ESC-derived cardiomyocytes exhibited more adult-like phenotypes after T3 supplementation based on action potential characteristics. They also exhibited more adult-like calcium homeostasis properties. These phenotypic changes were associated with up-regulation of sarco(endo)plasmic reticulum calcium ATPase-2a and ryanodine receptor-2 expression. In addition, the classical (genomic) pathway was shown to be involved in T3-induced cardiac differentiation of ESCs. Our results show that T3 supplementation promotes cardiac differentiation of ESCs and enhances maturation of electrophysiological, as well as calcium homeostasis, properties of ESC-derived cardiomyocytes.
Triiodothyronine promotes cardiac differentiation of embryonic stem cells. More importantly, the resultant cardiomyocytes exhibit more mature phenotypes, potentially facilitating host-graft integration and minimizing arrhythmogenesis.
Embryonic stem cells (ESCs) are pluripotent and can differentiate into virtually all cell types of the three embryonic germ layers including cardiomyocytes. They thus represent an unlimited ex vivo cell source for cardiac regenerative therapy (1, 2, 3, 4). Spontaneous differentiation of ESCs toward cardiac lineage is nonetheless inefficient, and it is difficult to obtain sufficient cardiomyocytes for cellular replacement therapy (1, 5, 6). More importantly, ESC-derived cardiomyocytes display immature phenotypes relative to their adult counterparts (1): ESC-derived cardiomyocytes appear to have immature electrophysiological properties as well as underdeveloped Ca2+ handling machinery (2, 3, 4). Such immaturity not only results in ineffective contractile force generation but may also create arrhythmogenic substrates, raising potential safety concerns for ESC-based cardiac therapy (1).
Thyroid hormone exerts diverse effects on the cardiovascular system. During ontogenesis, thyroid hormone is essential for normal cardiac development (5, 6, 7). In the perinatal period, it regulates the isoform switching of several myocardial proteins including myosin heavy chain (MHC) and titin (8, 9) from the fetal to the adult type. It also prevents expression of the fetal gene in neonatal cardiomyocytes to enhance normal cardiac maturation (8, 10). In adult cardiomyocytes, thyroid hormone plays an important role in normal cardiac physiology (11, 12) and the development of cardiac hypertrophy (13, 14, 15, 16). The effects of thyroid hormone on cellular processes of fetal and adult cardiomyocytes appear to be cell type specific and involve multiple regulatory mechanisms. It therefore remains unclear whether thyroid hormone can be exploited to promote cardiac differentiation of ESCs as well as to drive the maturation of ESC-derived cardiomyocytes. In this study, we demonstrated that thyroid hormone enhanced the cardiac differentiation of ESCs and facilitated the maturation of the electrophysiological and calcium homeostasis properties of ESC-derived cardiomyocytes via the classical genomic pathway.
Results
Thyroid hormone-enhanced cardiac differentiation of ESCs
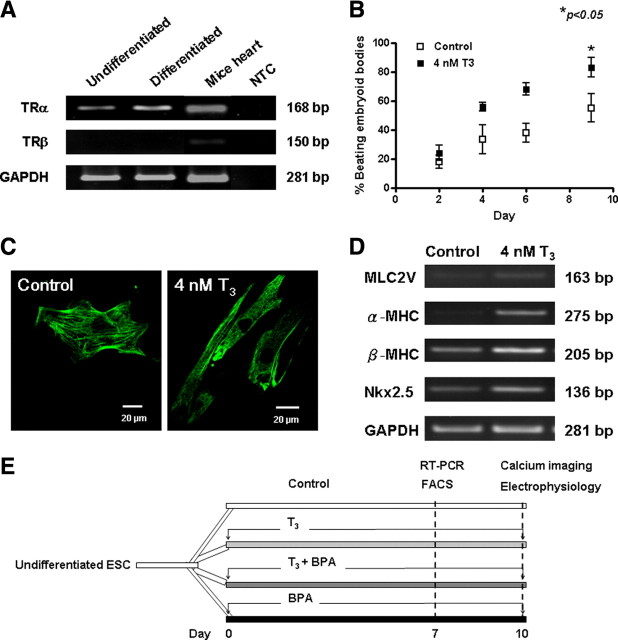
First, we determined the expression of thyroid receptor α (TRα) and thyroid receptor β (TRβ) in undifferentiated and differentiated ESCs using RT-PCR. The mRNA level of TRα was robustly expressed in undifferentiated ESCs and increased further during differentiation (Fig. 1A). In contrast, TRβ expression was not detected in undifferentiated or differentiated ESCs. Cardiac differentiation in vitro was determined as the appearance of spontaneous beating embryoid bodies (EBs) under the microscope. Spontaneous beating outgrowths were observed in both control (17.8 ± 4.3%, n = 3) and T3-treated EBs (24.0 ± 5.3%, n = 3) as early as d 2 (P > 0.05). Nonetheless, T3 supplementation resulted in a significantly higher percentage of spontaneous beating outgrowths of EBs compared with control on d 9 (83 ± 6.9% vs. 55 ± 9.7%; P < 0.05) (Fig. 1B).
Fig. 1.
T3-enhanced cardiac differentiation. A, Gene expression of thyroid receptor (TR) isoforms in undifferentiated and differentiated ESCs with adult mouse heart and no template control (NTC) as positive and negative control, respectively. B, Percentage of beating clusters. C, Detection of a typical cardiac-specific protein, troponin-T, in cardiomyocytes derived from differentiated mESCs under control environment (left) and in the presence of T3 (right) at d 7. Striations were commonly observed in T3 group. The cells were stained with antitroponin-T antibody (green). D, Cardiac maker gene expressions of differentiating mESCs as revealed by semiquantitative RT-PCR. E, Schematic diagram of T3 treatment of mESCs upon differentiation and subsequent functional analysis. FACS, Fluorescence-activated cell sorting.
Figure 1C shows the immunocytochemical pattern of a cardiac-specific protein, troponin-T, in ESC-derived cardiomyocytes. Morphological assessment revealed that cardiomyocytes derived in the control group were round and of shorter length. Cardiomyocytes derived from T3-treated EBs were needle like with long tapering ends, resembling the morphology of adult murine ventricular cardiomyocytes (17).
The effect of T3 on cardiac marker gene expression was then examined using RT-PCR. The expression of a panel of cardiac marker genes including Nkx2.5, α-MHC, β-MHC, and myosin light chain (MLC)-2v were up-regulated by T3 treatment (Fig. 1D). Of note, the expression of α-MHC, the adult isoform of MHC, was markedly up-regulated in T3-treated cardiomyocytes.
T3-induced cardiac differentiation of ESCs through classical pathway
Counting spontaneously beating embryoid bodies is a very crude method that does not take account of the beating area. Thus, to further study the underlying signaling pathway involving T3-induced cardiac differentiation, flow cytometry was used to accurately quantify the number of troponin-T-positive cardiomyocytes after differentiation. Consistent with the results of beating outgrowths and RT-PCR, the percentage of troponin-T-positive cells was significantly higher in the T3 group (5.38 ± 0.91%, n = 3) than the control group (3.13 ± 0.21%; n = 3; P < 0.05) (Fig. 2, A–C).
Fig. 2.
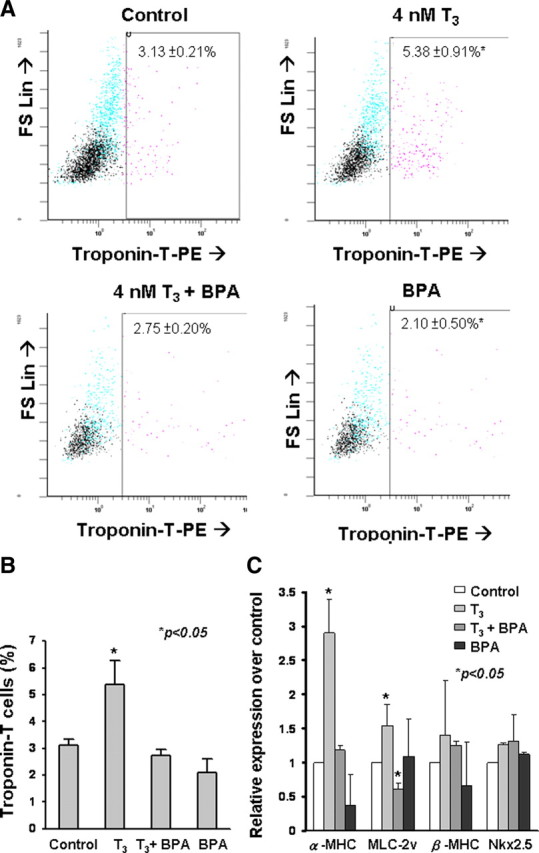
Relative number of mESC-derived cardiomyocytes (troponin-T-positive cells) on d 7 as determined by flow cytometry. A, Dot plots of troponin-T-positive cells of control, T3 (4 nmol/liter), T3 (4 nmol/liter) in combination with BPA (5.3 μmol/liter), and BPA (5.3 μmol/liter) as revealed by fluorescence-activated cell sorting. B, The data were summarized in histogram. Data were obtained from triplicate experiments (n = 3; *, P < 0.05), calculated as the percentage of troponin-positive cells, and compared with unpaired t test. C, Cardiac maker α-MHC, MLC2V, β-MHC, and Nkx2.5 gene expression of differentiating mESCs as revealed by quantitative RT-PCR (Abbreviations: MLC2V: MLC2 ventricular transcripts; Nkx2.5, NK2 transcription factor-related locus). Data were obtained from triplicate experiments (n = 3; *, P < 0.05), calculated as fold of gene expression of control (2-ΔΔCt), and analyzed with unpaired t test.
As binding of T3 to its nuclear receptors activates thyroid hormone responsive elements in the promoter regions of target genes by the classical (genomic) pathway (18), we determined whether this pathway was involved in T3-induced cardiac differentiation. Bisphenol A (BPA), a potent thyroid nuclear receptor antagonist, when coadministrated with T3, significantly reduced the percentage of T3-induced troponin-T-positive cells to control levels (2.75 ± 0.20%, n = 3) (Fig. 2B). Furthermore, after administration of BPA alone, the percentage of troponin-T-positive cells was also similar to the control group (2.10 ± 0.50%, n = 3) (Fig. 2B).
Quantitative RT-PCR revealed that T3 supplementation was associated with significant up-regulation of late (mature) cardiac marker genes: α-MHC and MLC-2v, but not early (immature) markers: β-MHC or Nkx2.5 for cardiac differentiation in ESC-derived cardiomyocytes compared with those in the control group (Fig. 2C). This enhancement of cardiac gene expression after T3 supplementation was abolished by coadministration of T3 and BPA. The administration of BPA alone did not result in any statistically significant change in cardiac gene expression (Fig. 2C). This suggests that the effects of T3 on cardiac differentiation of ESCs involve the classical genomic pathway. Because thyroid hormone is also known to modulate cellular function through other nongenomic signaling pathways including Akt- and MAPK-signaling cascades (ERK1/2, JNK, and p38 MAPK) (9, 14, 19), we also compared the effect of LY294002 and U0126, which inhibit Akt- and MAPK-signal cascades, respectively, on the modulation of T3-induced cardiac differentiation of ESCs. The administration of LY294002 or U0126 had no impact on T3-induced cardiac differentiation, suggesting the lack of involvement of these cascades in the process (see supplemental data published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org).
Maturation of electrophysiological properties by thyroid hormones
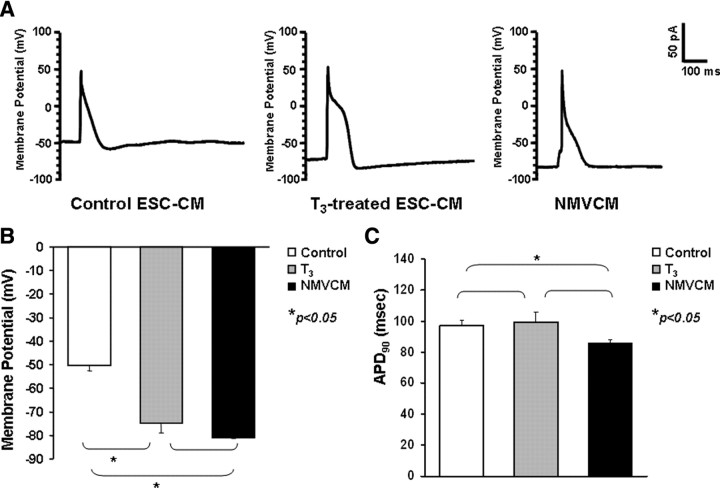
The electrophysiological properties of ESC-derived cardiomyocytes were characterized by a whole-cell patch clamp experiment to determine whether maturation was influenced by T3-induced cardiac differentiation. Figure 3A depicts representative single action potential waveforms recorded upon electrical stimulation of control, T3-treated ESC-derived cardiomyocytes and neonatal mouse ventricular cardiomyocytes. Compared with the control group (−50.27 ± 2.22 mV), T3-treated ESC-derived cardiomyocytes displayed a more negative resting membrane potential than the control ESC-derived cardiomyocytes (−74.72 ± 4.12 mV; P < 0.05), resembling that of neonatal mouse ventricular cardiomyocytes (−81.00 ± 0.30 mV; _P_ > 0.05) (Fig. 3B). There was no statistically significant difference in the action potential duration at 90% repolarization (APD90) between T3-treated ESC-derived cardiomyocytes (99.4 ± 6.7 msec) and neonatal mouse ventricular cardiomyocytes (86.0 ± 2.2 msec; P < 0.05; Fig. 3C). Despite this, the APD90 of control ESC-derived cardiomyocytes (97.0 ± 3.4 msec) was significantly longer than that of neonatal mouse ventricular cardiomyocytes (P < 0.05, Fig. 3C).
Fig. 3.
Action potential morphology and characteristic. A, Representative action potential waveforms elicited upon electrical stimulation of control and T3-treated ESC-derived cardiomyocytes (ESC-CM) and neonatal mouse ventricular cardiomyoyctes (NMVCM). Membrane potential (panel B) and action potential (panel C) duration at 90% repolarization (APD90). Unpaired t test was performed between T3-treated and control group (n = 3; *, P < 0.05). ms, Millisecond.
Maturation of calcium-handling properties by thyroid hormones
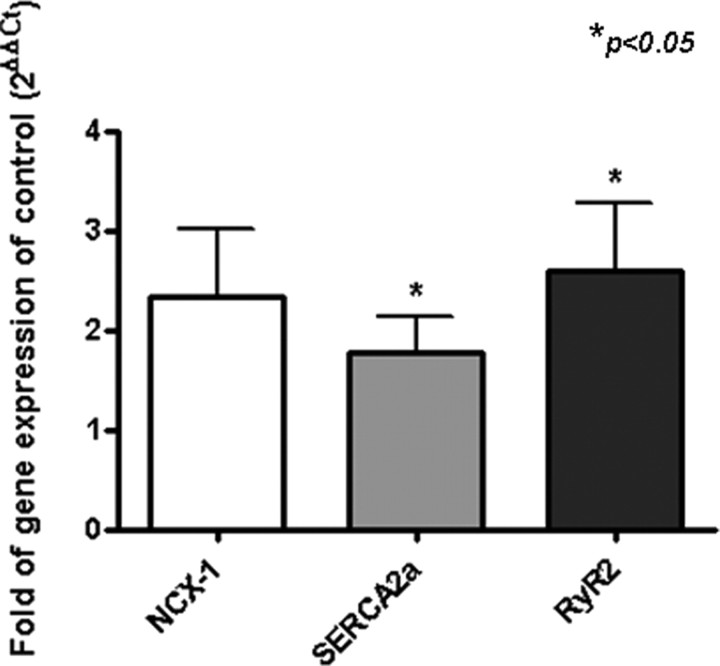
In addition to electrophysiological characteristics, we studied calcium homeostasis, the process underlying excitation-contraction coupling, of ESC-derived cardiomyocytes. First, the expression of two key calcium-handling proteins, sarco/endoplasmic reticulum Ca2+-ATPase (SERCA)-2a and ryanodine receptor (RyR)-2, were significantly up-regulated in T3-treated ESC-derived cardiomyocytes compared with the control group (n = 3 in each group; P < 0.05) (Fig. 4). The functional consequence of the up-regulation of these calcium-handling protein genes upon T3-induced cardiac differentiation was then characterized by measuring spontaneous calcium oscillations in single cardiomyocytes with confocal laser microscopy.
Fig. 4.
Gene expression of calcium-handling proteins NCX-1, SERCA2a, and RyR2 on SR. Data were obtained from three separate qPCR experiments (*, P < 0.05), calculated as fold of gene expression of control (2-ΔΔCt), and analyzed with unpaired t test. NCX-1, Na/Ca exchanger; SERCA-2a: sarcoplasmic reticulum Ca2+ ATPase.
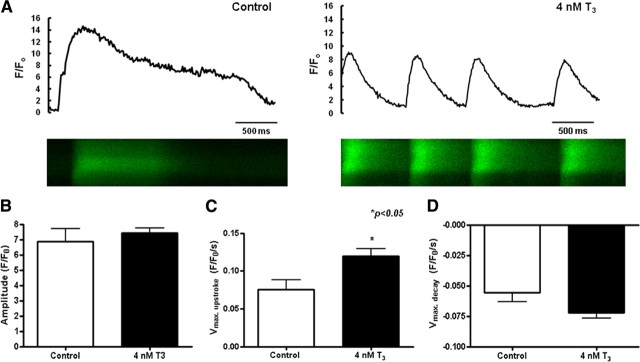
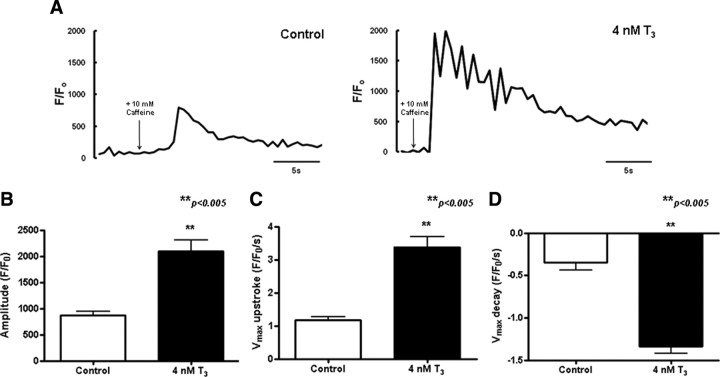
Consistent with the up-regulation of the calcium-handling proteins, T3-treated ESC-derived cardiomyocytes exhibited more mature calcium-handling properties than controls (Fig. 5). Specifically, T3-treated ESC-derived cardiomyocytes generated a significantly larger maximal upstroke velocity (P < 0.05; n =7) (Fig. 5C) and a higher maximal decay velocity (Fig. 5D). To further determine whether T3 treatment is associated with increased internal sarco/endoplasmic reticulum (SR) calcium store, caffeine (10 mm), a ryanodine receptor 2 (RyR2) opener, was used to release SR calcium and thus estimate SR calcium content. The peak amplitude of caffeine-induced calcium transients of T3-treated ESC-derived cardiomyocytes (2098 ± 208.30 F/F0; n = 5) was significantly larger than that of the control (875 ± 66.03 F/F0; n = 5; P < 0.05) (Fig. 6B). Likewise, the maximal upstroke velocity (3.38 ± 0.32 vs. 1.17 ± 0.10 F/F0/sec; n = 5; P < 0.05) (Fig. 6C) and maximal decay velocity (−1.34 ± 0.08 vs. −0.34 ± 0.09 F/F0/sec; n = 5; P < 0.05)(Fig. 6D) were also significantly higher in T3-treated ESC-derived cardiomyocytes. These findings indicate a substantial increase in SR calcium content conferred by T3 supplementation in ESC-derived cardiomyocytes.
Fig. 5.
Representative tracings of spontaneous rhythmic calcium transients in cardiomyocytes derived from control and 7-d T3-treated ESC-derived cardiomyocytes (panel A). Amplitude (panel B), maximal upstroke velocity (Vmax upstroke) (panel C), and maximal decay velocity (Vmax decay) (panel D) of calcium transients in mESC-derived cardiomyocytes. Unpaired t test was performed between T3-treated and control groups (n = 7; *, P < 0.05). ms, Millisecond.
Fig. 6.
Representative tracings of caffeine-induced calcium release in cardiomyocytes derived from control and 7-d T3-treated embryoid bodies (panel A). Amplitude (panel B), maximal upstroke velocity (Vmax upstroke) (panel C), and maximal decay velocity (Vmax decay) (panel D) of calcium transients in the mESC-derived cardiomyocytes (n = 5). Unpaired t test was performed between T3-treated and control group (**, P < 0.005). s, Second.
Discussion
Main findings
To our knowledge, this is the first systematic study to evaluate the effects of thyroid hormone on cardiac differentiation of ESCs. Our results demonstrate that T3 promotes cardiogenesis and myofibrillogenesis of ESCs and enables maturation of the calcium-handling properties of cardiomyocytes derived from ESCs. The main findings are the following: 1) T3-treated EBs show an increase in differentiation into cardiomyocytes (in terms of the percentage of beating outgrowths and troponin-positive cardiomyocytes); 2) T3 supplementation during cardiac differentiation from ESCs increases gene expression of cardiogenesis (Nkx2.5) and myofibrillogenesis (MLC-2v, α- and β-MHC); 3) T3 enhances cardiogenesis of ESCs through the classical pathway but not the Akt- and MAPK-signaling cascades; 4) electrophysiological study revealed that T3 supplementation led to a more mature cardiac phenotype in ESC-derived cardiomyocytes; and 5) T3 enhances the expression of calcium-handling proteins (RyR2 and SERCA-2a) with corresponding maturation of calcium-handling properties in ESC-derived cardiomyocytes.
Cardiac differentiation
Thyroid hormone has an important role in cardiac development and cardiovascular physiology. The close link between the thyroid and the heart begins in the fetus. Although the circulating level of T3 is relative low at this time (20), its level increases almost 2000-fold during the first postnatal weeks (21), coinciding with the time of rapid organ maturation. Thyroid hormone regulates cellular function through various signaling pathways including 1) the classical genomic pathway, 2) MAPK system, and 3) inositol triphosphate/Akt-signaling pathway (16). The activation of these pathways, nonetheless, appears to be cell type specific. For instance, at a molecular level, T3 forms complexes with TRα and TRβ, which in turn bind to thyroid hormone-responsive elements in the promoter regions of target genes (classical genomic pathway). In adult cardiomyocytes, these thyroid hormone receptor complexes activate genes including myocardial contractile proteins: α-MHC (22, 23, 24, 25, 26), and calcium-handling proteins (24, 27, 28): SERCA-2a and phospholamban (29). Contrary to this, the titin isoform transition is regulated primarily via the phosphatidylinositol 3-kinase/Akt pathway in the embryonic heart (9). Nevertheless, the effects of T3 on the cardiac differentiation of ESCs and the maturation of ESC-derived cardiomyocytes remain unknown. Our data indicate that T3 supplementation during spontaneous cardiac differentiation of ESCs results in an increased number of cardiomyocytes and is associated with increased expression of early cardiac-specific transcription factors (Nkx2.5) as well as cardiac-specific markers (MLC2v, α- and β-MHC). The T3-induced cardiac differentiation of ESCs is mediated, at least in part, via the activation of the classical genomic pathway, as evidenced by its abolition after application of BPA, a specific blocker for the classical pathway, which displaced T3 from the thyroid hormone receptor.
Maturation of ESC-derived cardiomyocytes
In adult cardiomyocytes, a relatively small calcium influx enters cells through L-type calcium channels during phase 2 of action potentials and, in turn, triggers a large calcium release from the internal calcium store, SR, through ryanodine receptors (30). This process, known as calcium-induced calcium release, is the primary mechanism that links electrical excitation and mechanical contraction in cardiomyocytes. During diastole, calcium is actively removed from the cytosol, mainly through SERCA-2a back into SR and NCX-1 out of cells (22). Previous studies have demonstrated that thyroid hormone significantly alters expression of SERCA-2a, and thus the calcium handling properties in cultured adult cardiomyocytes (31). ESC-derived cardiomyocytes are known to exhibit immature calcium dynamics such as small cytosolic calcium transient amplitudes, slow rise and decay kinetics, and reduced calcium content of SR (4, 32, 33). From a physiological point of view, impaired calcium storage, release, and removal determine a poor calcium cycle, thereby adversely affecting excitation-contraction coupling (23). This is partly related to the underdeveloped SR and partly to the developmental expression profiles of calcium -handling proteins in ESC-derived cardiomyocytes (4, 32). In the present study, T3 supplementation favorably altered the calcium-handling properties of ESC-derived cardiomyocytes including larger calcium transients and faster rate of rise and decay of calcium transients. In addition, these cardiomyocytes also appeared to have a larger internal store of calcium as evidenced by the larger amplitude of caffeine-mediated calcium release. These phenotypic changes were associated with up-regulation of calcium-handling proteins (RyR2, NCX-1, and SERCA-2a). In addition, ESC-derived cardiomyocytes with T3 treatment exhibited more mature electrophysiological phenotypes with more negative resting membrane potential, maximal diastolic potential, and a much longer action potential duration, resembling those of adult ventricular cardiomyocytes (17). In fact, the electrophysiological data may also explain the more mature calcium-handling properties. Specifically, the prolonged action potential duration, in particular the phase 2 plateau, allowed longer duration for calcium influx and provided a more negative resting membrane potential, generating a greater electrochemical gradient for calcium influx.
Clinical implications
The findings of this study have potential clinical implications. Although ESC-derived cardiomyocytes hold promise for cardiac regeneration, the current protocols for cardiac differentiation of ESCs are inefficient; thus it is difficult to obtain sufficient cardiomyocytes for clinical therapy. Moreover, immature calcium-handling and electrophysiological properties of ESC-derived cardiomyocytes can result in poor functional integration, at best, or lethal arrhythmia, at worst, after cellular transplantation, and thus further limit their potential therapeutic applications (2, 12, 13, 14). To yield sufficient mature cardiomyocyte phenotypes, in vitro manipulation of ESC-derived cardiomyocytes is required. Our data suggest that thyroid hormone can be exploited not only to enhance cardiac differentiation but also to promote maturation of ESC-derived cardiomyocytes ex vivo for therapeutic use.
Materials and Methods
Murine ESC culture
A murine ESC-line D3 (CRL-1934, American Type Culture Collection, Manassas, VA) was used in this study and cultured as previously described (7, 8). Briefly, undifferentiated ESCs were cultured on an irradiation-inactivated monolayer of mouse embryonic fibroblast feeders in DMEM (GIBCO, Karlsruhe, Germany), supplemented with 15% fetal bovine serum (GIBCO), 0.1 mmol/liter mercaptoethanol (Sigma-Aldrich, St. Louis, MO), nonessential amino acids (stock solution diluted 1:100; Hyclone Laboratories, Logan, UT) and 1000 U/ml of recombinant murine leukemia-inhibitory factors (Chemicon, Hofheim, Germany).
Cardiac differentiation
Differentiation of ESCs was induced through formation of embryoid bodies (EB) (∼800 ESCs in 20 μl of culture medium in the absence of leukemia-inhibitory factors) (7). To study the effects of thyroid hormone on cardiac differentiation, EBs were formed in medium supplemented with 4 nmol/liter T3 (Sigma-Aldrich, St. Louis, MO) from d 0 to d 10. In a separate set of experiments, BPA (5.3 μmol/liter), a specific inhibitor of the classical genomic pathway of T3 action (9), was coadministered with T3 and/or administrated alone from d 0 to d 10 (Fig. 1E). The percentage of ESC-derived cardiomyocytes was quantified using fluorescence-activated cell sorting analysis on d 7 of murine (m)ESC differentiation. Briefly, 10-cm dishes of EBs were dissociated to single cells with collagenase B (1 mg/ml). Cells were permeabilized by Cytofix/Cytoperm permeabilization kit (BD Biosciences, San Diego, CA), and stained with murine monoclonal anti-Troponin T (dilution 1:100; NeoMarker, Fremont, CA) followed by secondary antibody, antimouse IgG H+L-PE (dilution 1:100; Beckman Coulter, Fullerton, CA). Analysis was performed using the Beckman Coulter FC500 flow cytometer in which 10,000 events were counted. The primary antibody, IgG1, was used as an isotypic control to determine background signal.
The beating outgrowths were microsurgically dissected from D3 mESC-derived EBs using a glass knife at d 10, followed by incubation in collagenase B (1 mg/ml) at 37 C for 30 min with occasional dispersion by pipetting up and down. The isolated cells were incubated at room temperature for 1 h in Kraftbrühe solution containing (mm) 85 KCl, 30 K2HPO4, 5 MgSO4, 1 EGTA, 2 Na2-ATP, 5 pyruvic acid, 5 creatine, 20 taurine, and 20 d-glucose. Cells were subsequently plated on a 0.1% gelatin-coated glass coverslip in 24-well culture plates with corresponding differentiation medium. Calcium imaging and patch clamping from isolated cells or cell clusters were performed after 2 d while some of the cells were fixed in 4% paraformaldehyde for immunocytological staining at 4 C.
RT-PCR
Total RNA from 7-d-old EBs was extracted with Trizol reagent (Invitrogen, Life Technologies, Carlsbad, CA). Reverse transcription was then performed using 1 μg of total RNA in a final volume of 20 μl, using QuantiTect reverse transcription kit (QIAGEN, Hilden, Germany, http://www1.qiagen.com) according to the manufacturer’s instructions. Details of a panel of cardiac-specific genes are summarized in Table 1. The PCR products were analyzed using 1% agarose gel electrophoresis and visualized with ethidium bromide staining. GAPDH served as an internal control. Quantitative PCR analysis was performed with a real-time PCR Detector (Opticon 2 DNA Engine, MJ Research, Waltham, MN) using the iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA). For amplification, after initial holds for 5 min at 95 C, 50 cycles of 95 C for 15 sec followed by 55 C for 30 sec and 72 C for 30 sec, melt curve analysis was performed. The relative quantification of PCR products was performed according to the 2−ΔΔCt method, using mouse GAPDH as an internal control. Where ΔΔCt = [(Cttarget gene − Ct GAPDH) control group – (Cttarget gene − CtGAPDH) T3 group]].
Table 1.
PCR primers and PCR conditions
| Gene | Accession no. | Forward/reverse (5′→3′) | Annealing temperature (°C) | Product (bp) | Cycles | Ref. |
|---|---|---|---|---|---|---|
| TR-A | NM_178060 | 5′-GTGACACCCTGACCCTGAGT-3′ | 57 | 168 | 30 | Designed by primer3 |
| 5′-TTGACATTAGCAGCACAGCC-3′ | ||||||
| TR-B | NM_009380 | 5′-GAAGACAGTCCGGCTCTCAG-3′ | 57 | 150 | 30 | Designed by primer3 |
| 5′-GGCCATGTCCAAGTCAGAGT-3′ | ||||||
| Nkx2.5 | NM_008700 | 5′-GCTACAAGTGCAAGCGACAG-3′ | 60 | 184 | 30 | Designed by primer3 |
| 5′-GGGTAGGCGTTGTAGCCATA-3′ | ||||||
| GATA 4 | NM_008092 | 5′-TCTCCCAGGAACATCAAAACC-3′ | 60 | 125 | 30 | Designed by primer3 |
| 5′-GTGTGAAGGGGTGAAAAGG-3′ | ||||||
| GATA 6 | NM_010258.3 | 5′-CAAAAGCTTGCTCCGGTAAC-3′ | 60 | 116 | 30 | Designed by primer3 |
| 5′-TGAGGTGGTCGCTTGTGTAG-3′ | ||||||
| MLC2V | NM_010861 | 5′-GACCCAGATCCAGGAGTTCA-3′ | 60 | 163 | 30 | Designed by primer3 |
| 5′-AATTGGACCTGGAGCCTCTT-3′ | ||||||
| α-MHC | GI 191623 | 5′-GATGCCCAGATGGCTGACTT-3′ | 57 | 275 | 30 | (36 ) |
| 5′-GGTCAGCATGGCCATGTCCT-3′ | ||||||
| β-MHC | NM_080728.2 | 5′-GCCAACACCAACCTGTCCAAGTTC-3′ | 64 | 203 | 30 | (37 ) |
| 5′-TGCAAAGGCTCCAGGTCTGAGGGC-3′ | ||||||
| RyR2 | NM_023868.2 | 5′-TGAGTTCCTGCTGTCCTGTG-3′ | 60 | 181 | 30 | Designed by primer3 |
| 5′-CTCTGCCAACTCCAAGAAGG-3′ | ||||||
| NCX-1 | NM_011406 | 5′-TGTGTTTACGTGGTCCCTGA-3′ | 60 | 138 | 30 | Designed by primer3 |
| 5′-CTCCACAACTCCAGGAGAGC-3′ | ||||||
| SERCA-2a | NM_001110140 | 5′-AAGCTATGGGAGTGGTGGTG-3′ | 60 | 138 | 30 | Designed by primer3 |
| 5′-GCAATGCAAATGAGGGAGAT-3′ | ||||||
| GAPDH | NM_011406 | 5′-ACATCAAGAAGGTGGTGAAGCAGG-3′ | 60 | 157 | 30 | Designed by primer3 |
| 5′-CTCTTGCTCTCAGATCCTTGCTGG-3′ |
Electrophysiological study
For electrophysiological characterization of ESC-derived cardiomyocytes (d 10), the standard whole-cell patch-clamp recording was performed to record the action potential phenotypes (HEKA Instruments Inc., Southboro, MA) (34). Patch pipettes were prepared from 1.5-mm thin-walled borosilicate glass tubes using a Sutter micropipette puller P-97 and had typical resistances of 3–5 mΩ when filled with an internal solution containing (mm): 110 K+ aspartate, 20 KCl, 1 MgCl2, 0.1 Na-GTP, 5 Mg-ATP, 5 Na2-phosphocreatine, 5 EGTA, 10 HEPES, and pH adjusted to 7.3 with KOH. The external Tyrode’s bath solution consisted of (mm): 140 NaCl, 5 KCl, 1 MgCl2, 0.4 KH2PO4, 1.8 CaCl2, 10 glucose, 5 HEPES, and pH adjusted to 7.4 with NaOH. Spontaneous electrical activity was measured whereas the ESC-derived cardiomyocytes were left passive without current input. For electrically silent ESC-derived cardiomyocytes, a stimulation of 0.1–1 nA for 5 msec was given to elicit an action potential. Twenty consecutive action potentials from spontaneously firing ESC-derived cardiomyocytes were recorded to ensure stable waveforms for analysis. Data were corrected for the liquid junction potentials of +20.3 mV.
Confocal calcium imaging
To assess calcium-handling properties, ESC-derived cardiomyocytes (day 10) were loaded with 5 μm Fluo-3AM (Sigma) for 45 min at 37 C in Tyrode solution. Calcium transients of single ESC-derived cardiomyocytes were recorded with a confocal imaging system (Olympus Fluoview System version 4.2 FV300 TIEMPO) mounted on an upright Olympus microscope (IX71) with temporal resolution of the line scan at 274 frames per sec (2000 scans per 7.3 sec) (35). Signals were quantified as the background-subtracted fluorescence intensity changes normalized to the background-subtracted baseline fluorescence using Image J.
Statistical analysis
Continuous variables are expressed as mean ± sd. Statistical comparisons were performed using Student’s t test. Calculations were performed with SPSS (version 14.0). A P value <0.05 was considered statistically significant.
Footnotes
Disclosure summary: The authors have nothing to disclose.
First Published Online July 28, 2010
1
Y.-K.L. and K.-M.N. contributed equally to this work.
Abbreviations: BPA, Bisphenol A; EB, embryoid bodies; ESC, embryonic stem cell; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MHC, myosin heavy chain; MLC, myosin light chain; RyR2, ryanodine receptor 2; SERCA, sarco/endoplasmic reticulum Ca2+-ATPase; SR, sarco/endoplasmic reticulum; TR, thyroid receptor.
References
- 1.Siu CW, Moore JC, Li RA2007. Human embryonic stem cell-derived cardiomyocytes for heart therapies. Cardiovasc Hematol Disord Drug Targets 7:145–152 [DOI] [PubMed] [Google Scholar]
- 2.Au KW, Liao SY, Lee YK, Lai WH, Ng KM, Chan YC, Yip MC, Ho CY, Wu EX, Li RA, Siu CW, Tse HF2009. Effects of iron oxide nanoparticles on cardiac differentiation of embryonic stem cells. Biochem Biophys Res Commun 379:898–903 [DOI] [PubMed] [Google Scholar]
- 3.Fu JD, Li J, Tweedie D, Yu HM, Chen L, Wang R, Riordon DR, Brugh SA, Wang SQ, Boheler KR, Yang HT2006. Crucial role of the sarcoplasmic reticulum in the developmental regulation of Ca2+ transients and contraction in cardiomyocytes derived from embryonic stem cells. FASEB J 20:181–183 [DOI] [PubMed] [Google Scholar]
- 4.Lieu DK, Liu J, Siu CW, McNerney GP, Tse HF, Abu-Khalil A, Huser TR, Li RA2009. Absence of transverse tubules contributes to non-uniform Ca2+ wavefronts in mouse and human embryonic stem cell-derived cardiomyocytes. Stem Cells Dev 18:1493–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canavan JP, Holt J, Goldspink DF1994. The influence of thyroid hormones on the growth of the atria and ventricles of the heart in immature rats. J Endocrinol 142:171–179 [DOI] [PubMed] [Google Scholar]
- 6.Cluzeaut F, Maurer-Schultze B1986. Proliferation of cardiomyocytes and interstitial cells in the cardiac muscle of the mouse during pre- and postnatal development. Cell Tissue Kinetics 19:267–274 [DOI] [PubMed] [Google Scholar]
- 7.Mayhew TM, Pharaoh A, Austin A, Fagan DG1997. Stereological estimates of nuclear number in human ventricular cardiomyocytes before and after birth obtained using physical disectors. J Anat 191:107–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein I, Ojamaa K2001. Thyroid hormone and the cardiovascular system. N Engl J Med 344:501–509 [DOI] [PubMed] [Google Scholar]
- 9.Krüger M, Sachse C, Zimmermann WH, Eschenhagen T, Klede S, Linke WA2008. Thyroid hormone regulates developmental titin isoform transitions via the phosphatidylinositol-3-kinase/AKT pathway. Circ Res 102:439–447 [DOI] [PubMed] [Google Scholar]
- 10.Dillmann WH2002. Cellular action of thyroid hormone on the heart. Thyroid 12:447–452 [DOI] [PubMed] [Google Scholar]
- 11.Klein I1990. Thyroid hormone and the cardiovascular system. Am J Med 88:631–637 [DOI] [PubMed] [Google Scholar]
- 12.Polikar R, Burger AG, Scherrer U, Nicod P1993. The thyroid and the heart. Circulation 87:1435–1441 [DOI] [PubMed] [Google Scholar]
- 13.Dillmann W2009. Cardiac hypertrophy and thyroid hormone signaling. Heart Failure Rev 15:125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinugawa K, Jeong MY, Bristow MR, Long CS2005. Thyroid hormone induces cardiac myocyte hypertrophy in a thyroid hormone receptor α1-specific manner that requires TAK1 and p38 mitogen-activated protein kinase. Mol Endocrinol 19:1618–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinugawa K, Yonekura K, Ribeiro RC, Eto Y, Aoyagi T, Baxter JD, Camacho SA, Bristow MR, Long CS, Simpson PC2001. Regulation of thyroid hormone receptor isoforms in physiological and pathological cardiac hypertrophy. Circ Res 89:591–598 [DOI] [PubMed] [Google Scholar]
- 16.Ojamaa K2009. Signaling mechanisms in thyroid hormone-induced cardiac hypertrophy. Vasc Pharmacol 52:113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maltsev VA, Wobus AM, Rohwedel J, Bader M, Hescheler J1994. Cardiomyocytes differentiated in vitro from embryonic stem cells developmentally express cardiac-specific genes and ionic currents. Circ Res 75:233–244 [DOI] [PubMed] [Google Scholar]
- 18.Harvey CB, Williams GR2002. Mechanism of thyroid hormone action. Thyroid 12:441–446 [DOI] [PubMed] [Google Scholar]
- 19.Pantos C, Xinaris C, Mourouzis I, Malliopoulou V, Kardami E, Cokkinos DV2007. Thyroid hormone changes cardiomyocyte shape and geometry via ERK signaling pathway: potential therapeutic implications in reversing cardiac remodeling? Mol Cell Biochem 297:65–72 [DOI] [PubMed] [Google Scholar]
- 20.Morreale de Escobar G, Calvo R, Escobar del Rey F, Obregón MJ1994. Thyroid hormones in tissues from fetal and adult rats. Endocrinology 134:2410–2415 [DOI] [PubMed] [Google Scholar]
- 21.Gauthier K, Chassande O, Plateroti M, Roux JP, Legrand C, Pain B, Rousset B, Weiss R, Trouillas J, Samarut J1999. Different functions for the thyroid hormone receptors TRalpha and TRbeta in the control of thyroid hormone production and post-natal development. EMBO J 18:623–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kessler-Icekson G, Schlesinger H, Leger JJ, Leger J, Braverman Y, Binah O1988. Ventricular myosin of the shrew Crocidura russula, correlation with contractile properties. J Mol Cell Cardiol 20:1069–1073 [DOI] [PubMed] [Google Scholar]
- 23.Rohrer D, Dillmann WH1988. Thyroid hormone markedly increases the mRNA coding for sarcoplasmic reticulum Ca2+-ATPase in the rat heart. J Biol Chem 263:6941–6944 [PubMed] [Google Scholar]
- 24.Dillmann WH1990. Biochemical basis of thyroid hormone action in the heart. Am J Med 88:626–630 [DOI] [PubMed] [Google Scholar]
- 25.Horowitz B, Hensley CB, Quintero M, Azuma KK, Putnam D, McDonough AA1990. Differential regulation of Na, K-ATPase α 1, α 2, and β subunit mRNA and protein levels by thyroid hormone. J Biol Chem 265:14308–14314 [PubMed] [Google Scholar]
- 26.Muller A, Zuidwijk MJ, Simonides WS, van Hardeveld C1997. Modulation of SERCA2 expression by thyroid hormone and norepinephrine in cardiocytes: role of contractility. Am J Physiol Heart Circ Physiol 272:H1876–H1885 [DOI] [PubMed]
- 27.Oppenheimer JH1981. The molecular basis of thyroid hormone action: scattered pieces of jigsaw puzzle. Prog Clin Biol Res 74:45–55 [PubMed] [Google Scholar]
- 28.Shenoy R, Klein I, Ojamaa K2001. Differential regulation of SR calcium transporters by thyroid hormone in rat atria and ventricles. Am J Physiol Heart Circ Physiol 281:H1690–H1696 [DOI] [PubMed]
- 29.Tavi P, Sjögren M, Lunde PK, Zhang SJ, Abbate F, Vennström B, Westerblad H2005. Impaired Ca2+ handling and contraction in cardiomyocytes from mice with a dominant negative thyroid hormone receptor α1. J Mol Cell Cardiol 38:655–663 [DOI] [PubMed] [Google Scholar]
- 30.Bers DM, Despa S2006. Cardiac myocytes Ca2+ and Na+ regulation in normal and failing hearts. J Pharmacol Sci 100:315–322 [DOI] [PubMed] [Google Scholar]
- 31.Forini F, Paolicchi A, Pizzorusso T, Ratto GM, Saviozzi M, Vanini V, Iervasi G2001. 3,5,3′-Triiodothyronine deprivation affects phenotype and intracellular [Ca2+]i of human cardiomyocytes in culture. Cardiovasc Res 51:322–330 [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Fu JD, Siu CW, Li RA2007. Functional sarcoplasmic reticulum for calcium handling of human embryonic stem cell-derived cardiomyocytes: insights for driven maturation. Stem Cells 25:3038–3044 [DOI] [PubMed] [Google Scholar]
- 33.Liu J, Lieu DK, Siu CW, Fu JD, Tse HF, Li RA2009. Facilitated maturation of Ca2+ handling properties of human embryonic stem cell-derived cardiomyocytes by calsequestrin expression. Am J Physiol 297:C152–C159 [DOI] [PMC free article] [PubMed]
- 34.Chan YC, Siu CW, Lau YM, Lau CP, Li RA, Tse HF2009. Synergistic effects of inward rectifier (IK1) and pacemaker (If) currents on the induction of bioengineered cardiac automaticity. J Cardiovasc Electrophysiol 20:1048–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ng KM, Lee YK, Chan YC, Lai WH, Fung ML, Li RA, Siu CW, Tse HF2010. Exogenous expression of HIF-1 α promotes cardiac differentiation of embryonic stem cells. J Mol Cell Cardiol 48:1129–1137 [DOI] [PubMed] [Google Scholar]
- 36.Mauritz C, Schwanke K, Reppel M, Neef S, Katsirntaki K, Maier LS, Nguemo F, Menke S, Haustein M, Hescheler J, Hasenfuss G, Martin U2008. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation 118:507–517 [DOI] [PubMed] [Google Scholar]
- 37.Wobus AM, Guan K, Yang HT, Boheler KR2002. Embryonic stem cells as a model to study cardiac, skeletal muscle, and vascular smooth muscle cell differentiation. Methods Mol Biol 185:127–156 [DOI] [PubMed] [Google Scholar]