Guillain-Barré Syndrome outbreak caused by ZIKA virus infection in French Polynesia (original) (raw)
. Author manuscript; available in PMC: 2017 May 25.
Abstract
Background
From October 2013 to April 2014, French Polynesia experienced the largest Zika virus (ZIKV) outbreak ever described at that time. During the same period, an increase in Guillain-Barré syndrome (GBS) was reported, suggesting a possible association between ZIKV and GBS.
Patients and Methods
A case-control study was performed to identify the role of ZIKV and dengue virus (DENV) infection in developing GBS. Cases were GBS patients diagnosed at the Centre Hospitalier de Polynésie Française during the outbreak period. Controls were age-, gender-, and residence-matched patients who presented at the hospital with a non-febrile illness (Control group 1 [CTR1]; n=98), and age-matched patients with acute ZIKV disease and no neurological symptoms (Control group 2 [CTR2]; n=70). Virological investigations included RT-PCR for ZIKV, and both microsphere immunofluorescent and seroneutralization assays for ZIKV and DENV. Anti-glycolipid reactivity was studied in GBS patients using both ELISA and combinatorial microarrays.
Results
Forty-two patients were diagnosed with GBS during the study period. Ninety-eight percent of GBS patients had anti-ZIKV IgM or IgG, and all had neutralizing antibodies against ZIKV compared to 55.7% with neutralizing antibodies in the CTR1 group (P<0.0001). Ninety-three percent of GBS patients had ZIKV IgM and 88% had experienced a transient illness in median six days before the onset of neurological symptoms, suggesting recent ZIKV infection. GBS patients had electrophysiological findings compatible with the acute motor axonal neuropathy (AMAN) type, and had rapid evolution of disease (median duration of the installation and plateau phases was 6 and 4 days, respectively). Twelve (29%) patients required respiratory assistance. No patients died. Anti-glycolipid antibody activity, notably against GA1, was found in 13 (31%) patients by ELISA and 19/41 (46%) by glycoarray at admission. The typical AMAN-associated anti-ganglioside antibodies were rarely present. There was no significant difference in past dengue history between GBS patients and the two control groups.
Conclusion
This is the first study providing evidence for ZIKV infection causing GBS. As ZIKV is spreading rapidly across the Americas, at risk countries need to prepare for adequate intensive care beds capacity for managing GBS patients.
Background
Zika virus (ZIKV) is an arthropod-borne virus (arbovirus) in the genus Flavivirus, family Flaviviridae.1 ZIKV was first isolated from a Rhesus monkey in 1947 in the Zika forest of Uganda.2 The first human infection was reported in Nigeria in 1954.3 Like dengue (DENV) and chikungunya (CHIKV) viruses, ZIKV adapted from an ancestral transmission cycle involving non-human primates and a broad spectrum of canopy dwelling mosquito species as vectors to an urban / periurban cycle involving humans as reservoirs and widely distributed Aedes (Stegomyia) mosquitoes as vectors.4
From the 1950s, ZIKV was only reported as circulating sporadically in Africa and South-East Asia.5 In 2007, ZIKV was isolated for the first time in the Pacific, on the Micronesian island of Yap.6 From October 2013 to April 2014, French Polynesia experienced the largest Zika outbreak ever reported at that time.7 It was estimated that more than 32,000 patients consulted for suspected ZIKV infection, with a weekly incidence peaking on week 9 of the outbreak.8 From 2014, ZIKV spread to other Pacific islands, notably Easter island (Chile). In March 2015, Brazil reported autochthonous transmission of ZIKV,9 and an outbreak was declared 6 months later.10 As of February 1, 2016, ZIKV had emerged in 25 countries and territories in South/Central America, with alarming reports of microcephaly cases among neonates in Brazil.11
Previously to the French Polynesian outbreak, ZIKV infection used to be described as a mild febrile illness with clinical symptoms including maculopapular rash, joints and muscles pain, headache and non-purulent conjunctivitis.6 Between November 2013 and February 2014 in French Polynesia, 42 patients presented at hospital with Guillain-Barré syndrome (GBS), an autoimmune disease causing acute or subacute flaccid paralysis, contrasting with reports of 5, 10, 3 and 3 in 2009, 2010, 2011, and 2012, respectively.12 Other arboviral diseases like West Nile, Japanese Encephalitis, chikungunya and dengue had already been reported to sometimes cause GBS,13–16 but only during the outbreak in French Polynesia was this severe neurological complication first described associated with ZIKV infection.17 The temporal coincidence between the peaks in incidence of ZIKV and GBS cases, and also the concurrent circulation of DENV serotypes 1 and 318 suggested possible causal relationship between the three events. Using two control series, we addressed the hypothesis that ZIKV infection with or without DENV concurrent or sequential infection may be a risk factor for the development of GBS.
Methods
Study design and participants
A case-control study was performed to identify the role of ZIKV and dengue virus (DENV) infection in developing GBS. Cases were GBS patients diagnosed at the Centre Hospitalier de Polynésie Française in Papeete, Tahiti, during the outbreak period. As a routine, all patients with suspicion of GBS in French Polynesia are referred to the CHPF for diagnosis confirmation. All of the patients included in this study were diagnosed as developing a GBS by neurologists or staff in intensive care units according to international criteria.19 Clinical and demographic data were collected from medical records obtained during patients’ hospitalisation. The data recorded for all patients included: patient’s age, gender, island of residence, medical history and co-morbidities, clinical signs and symptoms, illness duration and severity. Electrophysiological assessment was performed for all patients using standard electromyography [EMG] techniques including motor nerve conduction studies of the median nerve (recording of the abductor pollicis brevis), the ulnar nerve (recording of the abductor digiti minimi) and the peroneal nerve (recording of the extensor digitorum brevis), as well as sensory nerve conduction studies in radial and sural nerves.
To estimate the proportion of ZIKV infections in the general population, to be further compared with the GBS series, a first control group (CTR1, n=98) was recruited among patients hospitalised or consulting for non-febrile illness at the CHPF. Patients from the CTR1 group were matched for age (±10 years), gender and island of residence with patients in the GBS group. Each patient in CTR1 group had a blood sample taken ±7 days from the admission date of the matching GBS case.
To investigate a possible role of past DENV infection(s) in developing a GBS in ZIKV infected patients, a second control group (CTR2, n=70) was recruited among age-matched (± 10 years) patients with RT-PCR-confirmed ZIKV infection, but who did not develop any neurological complication.
The epidemic curve of ZIKV in French Polynesia was obtained by extrapolating data from a sentinel network of clinicians who have been reporting the number of suspected ZIKV cases on a weekly basis from October 2013 until April 2014 to the Bureau de Veille Sanitaire – Direction de la Santé de Polynésie Française.
Zika and dengue virus infection diagnosis
For the GBS group, a first blood sample was collected at hospital admission and one to three additional blood samples were collected three weeks, two and/or three months later. For the CTR1 group, the blood sample was collected within a 7-day period from the indexed GBS case for 59 (60.2%) patients, and with a median (IQR) period of 13 (9-16) days for the remaining controls.
Diagnosis of ZIKV acute infection in patients from GBS and CTR2 groups was performed using a ZIKV specific RT-PCR protocol adapted from Lanciotti et al.20 Serum was considered positive for ZIKV if the two distinct genomic regions targeted by the RT-PCR were amplified.
Detection of IgM against ZIKV and DENV in blood samples from patients in GBS and CTR1 groups was performed using indirect immunofluorescent assay (IFA) on Vero cells (African Green Monkey kidney cells) infected with either ZIKV[PF13-251013-18] or DENV[D1-Hawaii 1944].
Detection of IgG against ZIKV and each of the four DENV serotypes was performed on blood samples from patients in GBS, CTR1 and CTR2 groups using a recombinant-antigen based microsphere immunoassay (MIA) adapted from Beck et al.21 (see details in Supplementary material).
Detection of neutralizing antibodies against ZIKV and each of the four DENV serotypes was performed for patients in the GBS and CTR1 groups using a microseroneutralization assay performed on Vero cells inoculated with serial dilutions of each serum previously incubated with titrated ZIKV[PF13-251013-18] or DENV serotype 1 to 4 strains that were isolated during previous outbreaks in French Polynesia (see details in Supplementary material).
Immunochemical reaction with glycolipids
The sera from both GBS patients (n=42 at admission, n=31 at 3 months) and healthy blood donors (collected prior to April 2013; n=20) were tested by ELISA (Bühlmann-Gangliocombi®) for IgG/IgM reactivity to the glycolipids GM1, GA1, GM2, GD1a, GD1b and GQ1b at 1:100 dilution. As per kit instructions, results were considered as positive, equivocal, and negative when showing >50%, 30-50%, and <30% binding, respectively. Sera (n=41 at admission, n=27 at three months) were also tested by a combinatorial microarray screening method based on a refinement and miniaturization of previous published combinatorial glycoarray assay22 (see details in Supplementary material).
Immunosuppression test for exploration of molecular mimicry mechanisms
The sera from six patients showing high reactivity towards GA1 were tested against ZIKV viral proteins by Western blot (see details in Supplementary material). Molecular mimicry was evaluated using the method by Neil.23 (see details in Supplementary material).
Sample size
The primary objectives of this study were to determine the association between GBS and ZIKV infection in French Polynesia and to determine whether possible co-infection or pre-existing immunity to dengue (and a specific DENV serotype) appear to facilitate the development of GBS. With two controls per case, and on the assumption that 70% of GBS patients and 40% of controls reported a recent ZIKV infection, the statistical power to detect a difference between the GBS group and the control group was calculated to be 86%.
Statistical analysis
The risk of developing GBS per ZIKV infection was calculated by dividing the total estimated number of GBS cases reported in French Polynesia (n=42) by the total number of people infected by ZIKV during the epidemic period. This latter number was calculated by multiplying the attack rate (66%) estimated during a post epidemic population-based serological survey24 by the total population of French Polynesia (268 270 inhabitants; 2012 census). The association between ZIKV positive serology, DENV positive serology, and GBS was analysed using exact conditional logistic regression. As the humoral response elicited by acute ZIKV infection may trigger production of anti-DENV IgG related to past DENV infections, we adjusted the odds-ratio (OR) describing the association between anti-DENV IgG and GBS for the presence of anti-ZIKV IgG. All ORs are given with their 95% confidence intervals. Motor nerve conduction parameters values were compared to reference values using one-sample t tests, and 1st week values were compared to 4th month values using Wilcoxon matched pairs signed rank sum tests. First week values of the 19 patients with electrophysiological measurements at 4th month were compared to those of the 18 patients without follow-up using a Mann Whitney test. Data were collected using EpiData 3.1 software and all statistical analyses were performed using STATA 14, StataCorp LP.
Ethical considerations
The study protocol was approved by the Comité d’Ethique de la Polynésie française (N°69/CEPF 2014), and all patients provided informed consent for their participation in the study.
Role of the funding source
The funders had no role in the design of the study, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study, except for the results of the combinatorial microarray (HSK & HW), and had final responsibility for the decision to submit the publication.
Results
Epidemiological dynamics of the outbreak
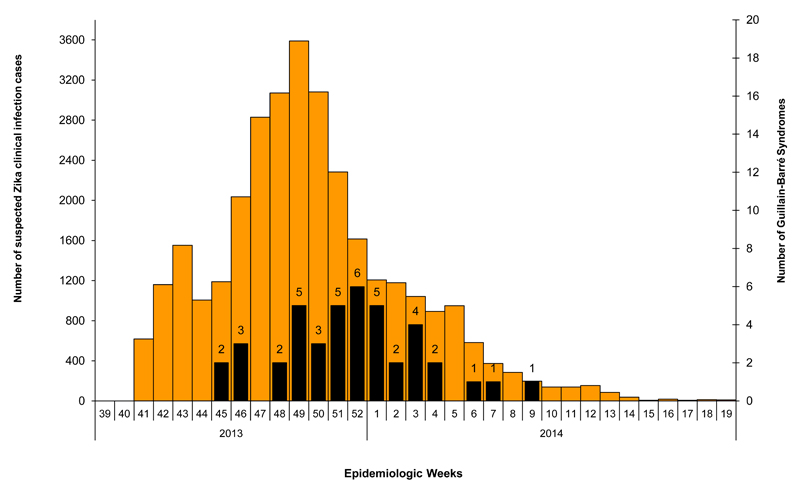
Cases of ZIKV infection were reported weekly from October 2013 until March 2014 (See Figure 1). The first GBS case was reported on week 5 of the outbreak, while the peaks of the Zika epidemic and GBS cases were reached on week 9 and 12, respectively. In total, 42 cases of GBS were recorded during the ZIKV outbreak. Based on a 66% attack rate of ZIKV infection in the general population, the risk of GBS was estimated at 0.24 per 1000 ZIKV infections.
Figure 1.
Epidemic curve of ZIKV virus suspected cases and Guillain-Barré syndromes in French Polynesia 2013-2014. ZIKV cases are shown in orange and GBS cases in black
Patients characteristics
The median (IQR) age of GBS patients was 42 (36-56) years, 31 (74%) were males and 38 (90%) were born in French Polynesia.
The clinical characteristics of the patients in the GBS group are shown in Table 1. Most (88%) had a recent history of viral syndrome, in median 6 days prior to the onset of neurological manifestations. Rash (81%), arthralgia (74%), fever (58%) were the most commonly reported symptoms.
Table 1.
Clinical characteristics of patients with GBS (n=42) in French Polynesia 2013-2014
| n (%) or median | IQR | |
|---|---|---|
| Age (years) | 42 | 36-56 |
| Male sex | 31 (74) | |
| Obesity | 11 (26) | |
| Smoking (n=40) | 12 (30) | |
| High Blood Pressure | 7 (17) | |
| Heart disease | 3 (7) | |
| Previous viral syndrome | 37 (88) | |
| Conjunctivitis (n=31) | 15 (48) | |
| Rash (n=36) | 29 (81) | |
| Fever (n=31) | 18 (58) | |
| Arthalgia (n=31) | 23 (74) | |
| Edema of the limbs (n=29) | 9 (31) | |
| Time between reported viral syndrome and onset of neurological symptoms (days) (n=37) | 6 | 4-10 |
| Time between onset of neurological symptoms and admission (days) | 4.5 | 2-8 |
| Symptoms at admission | ||
| Muscle weakness | 31 (74) | |
| Symmetric muscle weakness | 27 (64) | |
| Muscle weakness limited to lower limbs | 18 (43) | |
| Incapacity to walk (n=41) | 18 (43) | |
| Areflexia or decreased reflexes | 26 (62) | |
| Facial palsy | 27 (64) | |
| Bilateral facial palsy | 14 (33) | |
| Unilateral facial palsy | 13 (31) | |
| Trouble swallowing | 10 (24) | |
| Paresthesia | 35 (83) | |
| Time between onset of neurological symptoms and peak of illness (days) | 6 | 4-9 |
| Time between admission and peak of illness (days) | 1 | 0-2 |
| Symptoms at nadir | ||
| Muscle weakness | 36 (86) | |
| Symmetric muscle weakness | 33 (79) | |
| Muscle weakness limited to lower limbs | 17 (40) | |
| Incapacity to walk | 26 (62) | |
| Areflexia or decreased reflexes | 20 (48) | |
| Facial palsy | 33 (79) | |
| Bilateral facial palsy | 25 (60) | |
| Unilateral facial palsy | 8 (19) | |
| Trouble swallowing | 19 (45) | |
| Trouble breathing | 14 (33) | |
| Duration of plateau phase of illness (days) | 4 | 3-10 |
| Treatment | ||
| Intravenous immune globulins | 42 (100) | |
| Plasmapheresis | 1 (2) | |
| Patients admitted to intensive care | 16 (38) | |
| Trouble swallowing | 12 (29) | |
| Respiratory assistance | 12 (29) | |
| Duration of hospitalisation (days) | 11 | 7-20 |
| Duration of hospitalisation for patients admitted to intensive care (days) | 51 | 16-70 |
| Lumbar puncture results | ||
| Proteins (mg/dL) | 1.47 | 0.92-2.21 |
| Increased CSF protein concentration (cut-off : 0.52 mg/dL) | 39 (93) | |
| Cells (/mm3) | 4 | 1-7 |
The main characteristics of the GBS were the rapid progression to nadir (median of 6 days between the onset of neurological symptoms to the nadir), and the short plateau phase (median of 4 days). Clinical presentation at admission was manifested by generalised weakness (74%), with incapacity to walk (44%). Facial palsy was common (64%). Ninety three percent of the patients had increased (> 0.52 mg/dL) protein concentration in the cerebrospinal fluid obtained by lumbar puncture. Sixteen (38%) patients were admitted to intensive care unit, and 12 (29%) required respiratory assistance. All GBS cases received treatment by immunoglobulins, and one had plasmapheresis. The median duration of hospitalisation was 11 days for all patients, and 51 days for the 16 patients who were admitted into intensive care. No patients died. Three months after discharge, 24 (43%) patients were able to walk without assistance.
ZIKV and DENV infection diagnosis
Acute ZIKV infection, as confirmed by a positive RT-PCR result, was observed for all patients in the CTR2 group, but for none of the 41 patients tested in the GBS group (Table 2A); thus corroborating clinical observations, notably the absence of fever, suggesting that the patients in the GBS group were no longer viremic at admission.
Table 2A. Results of molecular and immunological analyses of GBS and control patients in French Polynesia 2013-2014.
Detection of Zika RNA (RT-PCR), Zika & Dengue IgM (IFA), Zika IgG (MIA) and neutralizing antibodies (Neut).
| Zika | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| vRNA | IgM | IgG | IgM/IgG | Neut. | IgM Zika/ IgM Dengue n (%) | ||||||||
| n (%) | n (%) | n (%) | +/+ | +/- | -/+ | -/- | ZIKV+ n (%) | n (%) | +/+ | +/- | -/+ | -/- | |
| GBS (N=42a) | 0 (0) | 39 (92.9) | 29 (69.0) | 27 | 12 | 2 | 1 | 41 (97.6) | 42 (100) | 8 (19.1) | 31 (73.8) | 0 (0) | 3 (7.1) |
| CTR1 (N=98) | ND | 17 (17.3) | 25 (25.5) | 7 | 10 | 18 | 63 | 35 (35.7) | 54 (55.7) | 6 (6.1) | 11 (11.2) | 8 (8.2) | 73 (74.5) |
| CTR2 (N=70) | 70 (100) | ND | 5 (7.1) | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
Recent infection by ZIKV was supported by the detection of anti-ZIKV IgM antibodies in 92.9% and 17.3% of the patients in GBS and CTR1 groups, respectively (Table 2A). As possible cross-reactivity between anti-DENV and anti-ZIKV IgM responses had previously been described, IFA was performed using the two viruses. In the GBS group, 73.8% of the patients had IgM against ZIKV but not against DENV. All 19.1% patients with anti-DENV IgM also had IgM against ZIKV, suggesting that the anti-DENV IgM response could result from cross-reactivity.
When combining the results of both anti-ZIKV IgM and IgG, previous occurrence of a ZIKV infection was suggested for 97.6% of the patients in the GBS group and 35.7% in the CTR1 group (OR [95% CI] = 59.7 [10.4 - +∞]; P<0.0001; Table 2C). Moreover, a neutralizing response against ZIKV was observed for 100% of the patients in the GBS group and 55.7% in the CTR1 group (OR [95% CI] = 34.1 [5.8 - +∞]; P<0.0001).
Table 2C. Results of molecular and immunological analyses of GBS and control patients in French Polynesia 2013-2014.
ZIKV and DENV serological patterns associated with GBS
| GBSa (n=42) | CTR1 (n=98) | OR [95% CI] | ORa [95% CI] | CTR2 (n=70) | OR [95% CI] | ORa [95% CI] | |
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | |||||
| ZIKV IgM and/or IgG positivity | 41 (97.6) | 35 (35.7) | 59.7 [10.4 - +∞] | ||||
| Positive ZIKV seroneutralization | 42 (100.0) | 54 (55.7) | 34.1 [5.8 - +∞] | ||||
| DENV IgG positivity | 40 (95.2) | 87 (88.8) | 2.0 [0.4 - 19.9] | 1.0 [0.2 - 11.5] | 58 (82.9) | 6.0 [0.8 - 269.5] | 4.0 [0.5-184.7] |
The interpretation of anti-DENV IgM is difficult due to possible cross-reactivity with anti-ZIKV IgM. Still, there was no indication of increased recent infection with DENV among GBS patients when compared to the CTR1 group (Table 2A; P>0.05). Past history of dengue was common among GBS patients (95.2% on the last sample available, away from the immunological boost associated with recent ZIKV infection). It was non significantly different from that of the CTR1 group (88.8%; OR [95% CI] = 2.0 [0.4 – 19.9]; P = 0.62) and the CTR2 group (82.9%; OR [95% CI] = 6.04 [0.81 – 269.5]; P = 0.10) (Table 2C and Suppl. Table 1). These non significant differences were further attenuated after stratifying by the presence of anti-ZIKV IgG, suggesting that the humoral response elicited by ZIKV infection also triggered production of anti-DENV IgG (Table 2B & 2C). This is corroborated by the examination of ZIKV and DENV IgG responses in the blood samples serially collected from the GBS patients. Indeed, the number of patients with anti-ZIKV IgG increased from the earliest to the intermediate and then to the latest sample, while the reverse occurred for anti-DENV IgG (Suppl. Table 1 & Suppl. Table 2). A possible explanation would be that an anamnestic anti-DENV IgG response in GBS patients might have been transiently boosted by the ZIKV infection.
Table 2B. Results of molecular and immunological analyses of GBS and control patients in French Polynesia 2013-2014.
Dengue IgG (MIA) and neutralizing responses (Neut)
| IgG Dengue n (%) | Dengue Neut. n (%) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GBSa | CTR1 | CTR2 | ||||||||||||||||||
| IgG Z-b | IgG Z+b | Total | IgG Z- | IgG Z+ | Total | IgG Z- | IgG Z+ | Total | GBS | |||||||||||
| ≥ 2 serotypes | 11 | (84.6) | 25 | (86.2) | 36 | (85.7) | 42 | (57.5) | 23 | (92.0) | 65 | (66.3) | 42 | (64.6) | 4 | (80.0) | 46 | (65.8) | 41 | (100) |
| 1 serotype | 0 | - | 4 | (13.8) | 4 | (9.5) | 20 | (27.4) | 2 | (8.0) | 22 | (22.5) | 11 | (16.9) | 1 | (20.0) | 12 | (17.1) | 0 | - |
| No infection | 2 | (15.4) | 0 | - | 2 | (4.8) | 11 | (15.1) | 0 | - | 11 | (11.2) | 12 | (18.5) | 0 | - | 12 | (17.1) | 0 | - |
| 13 | 29 | 42 | 73 | 25 | 98 | 65 | 5 | 70 | 41 |
Serological tests for Campylobacter jejuni (n=41), HIV (n=42), cytomegalovirus (n=32), Epstein-Barr virus (n=32) and herpes simplex virus type 1 and 2 (n=8) were negative.
Neurophysiological assessment
A total of 37 patients underwent electrophysiological examination during the first week after GBS onset (Table 3). Motor nerve conduction study showed the same pattern in all tested nerves, with prolonged distal latencies (P<0.0001) and marked reduction of the distal compound muscle action potential (CMAP) amplitude (P<0.0001), indicative of severe conduction alteration in the distal nerve segments. By contrast, there was no significant conduction slowing or block in intermediate motor nerve segments (throughout forearm and legs). Amplitude and conduction velocity of sensitive potentials were not significantly altered in radial and sural nerves.
Table 3.
Evolution of motor nerve conduction parameters (mean values) after GBS onset.
| Median | Ulnar | Fibular | |||||||
|---|---|---|---|---|---|---|---|---|---|
| DML (ms) | Ampli (mV) | MCV (m/s) | DML (ms) | Ampli (mV) | MCV (m/s) | DML (ms) | Ampli (mV) | MCV (m/s) | |
| N < 3.7 | N > 6.0 | N > 48 | N < 3.0 | N > 6.0 | N > 48 | N < 5.0 | N > 3.0 | N > 42 | |
| 1st week n = 37 | 12.4a | 2.9a | 47 | 5.8a | 3.9a | 52 | 9.9a | 2.4c | 41 |
| 1st week subgroup n = 19 | 14.1a | 2.4a | 45 | 6.1a | 3.7b | 49 | 10.1c | 2.1c | 39 |
| 4th month n = 19 | 7.1c | 6.0c | 47 | 4.4c | 5.6c | 46 | 6.0c | 3.8c | 46 |
A second nerve conduction study was performed four months later including 19 GBS patients for whom a baseline assessment was available (there was no difference in baseline values of the 19 patients with follow-up compared the 18 without follow-up). By comparison to the first study, results showed a clear improvement of the distal conduction abnormalities (P<0.001), with reduction of the prolonged distal latencies and near normalization of CMAP amplitudes (Table 3 and Suppl. Figure 1). Altogether, these findings are suggestive of an acute motor axonal neuropathy (AMAN).
Detection of reactivity towards glycolipids by ELISA
By ELISA, at admission, sera from 13 (31%) GBS patients showed a positive reactivity (% of binding >50%) against different glycolipids (Table 4 and Suppl. Table 2). Ten (24%) had an equivocal percentage of binding (between 30 and 50%). Among these 23 patients, 17 had a reactivity directed toward glycolipid GA1 (8 positive, 9 equivocal) and it was either isolated or shared with other glycolipids. At three months, the proportion of reactive sera had slightly increased (48%). Controls were negative (n=20). These results were heterogeneous and low intensity for 50% of them.
Table 4.
Positive (>50%) reactivity to glycolipids in sera of GBS patients (n=42) and controls (n=20) in French Polynesia 2013-2014
| GBS at onset(n=42)n (%) | GBS at 3 months(n=31)n (%) | Controlsa(n=20)n (%) | |
|---|---|---|---|
| Glycolipid | |||
| GM1 | 0 (0) | 8 (26) | 0 (0) |
| GA1 | 8 (19) | 10 (32) | 0 (0) |
| GM2 | 2 (5) | 1 (3) | 0 (0) |
| GD1a | 5 (12) | 9 (29) | 0 (0) |
| GD1b | 3 (7) | 9 (29) | 0 (0) |
| GQ1b | 0 (0) | 0 (0) | 0 (0) |
| Any | 13 (31) | 15 (48) | 0 (0) |
Test of reactivity of GBS patient’s sera with ZIKV viral proteins by Western blot
Western blot was used to test the reactivity against ZIKV viral proteins in the serum from 6 GBS patients, 4 with high reactivity against GA1 (patients 6, 11, 20 and 29 of Suppl. Table 2), and 2 patients with no reactivity against GA1 (patients 13 and 27 of Suppl. Table 2). All sera showed intense reactivity with viral proteins regardless of their reactivity towards GA1 (Suppl. Figure 2).
Determination of specific interaction to GA1 by serum absorption with GA1
The reactivity of serum n°20 towards ZIKV proteins was not inhibited even at the highest GA1 amount (600 µg; Suppl. Figure 3). We further tested serum n°6 and did not observe any competition with a GA1 amount of 300 µg (data not shown).
Detection of reactivity towards glycolipids by combinatorial microarray
Combinatorial microarrays were used for screening glycolipid complexes as antigens. The majority of serum samples tested was negative or had low level binding to some single and or heteromeric glycolipid complex. Notably, antibodies against GA1:sulphatide complex were frequently observed (19/41; 46.3%) in patient sera, with intermediate binding intensities, above the threshold of positivity (p=0.001). In addition, a significant number of patient sera had antibodies raised against GA1 in complex with cholesterol and/or phosphatidylserine, although most were of low binding intensity (see Supplementary material and Suppl. Figure 4).
Discussion
This is the first study to evaluate the role of ZIKV infection in a large number of GBS patients diagnosed during a ZIKV outbreak. The serological investigations conducted on the blood samples from the 42 patients who developed a GBS during the French Polynesian ZIKV outbreak confirm that all these patients have experienced ZIKV infection. Moreover, the presence of IgM (92.9%) and the information that most (88%) patients reported a transient viral syndrome compatible with ZIKV disease in median 6 days prior to the onset of neurological symptoms, suggested a recent ZIKV infection. GBS patients were no longer viremic for ZIKV at the time of admission, consistent with previous data showing that ZIKV viremia rarely exceeds five days after disease onset.25 However, detection of virus in urine by RT-PCR may be a valuable alternative.26 As DENV 1 and 3 serotypes were co-circulating at the time of the ZIKV epidemic,18 we investigated whether DENV infection might have contributed to the occurrence of GBS. Analysis of dengue serology (IFA, MIA, seroneutralization) did not support recent DENV infection. Most (95.2%) of the GBS patients had pre-existing dengue immunity, but this did not appear as being significantly different from the control groups.
GBS is an acute, immune-mediated polyradiculoneuropathy typically arising after minor viral and bacterial infections. Motor function is usually affected, beginning distally and progressing proximally over up to a 4-week period.27 Patients suffer from generalised weakness, areflexia and varying degree of sensory disturbances and involvement of cranial nerves.28 The risk of GBS increases with age and men are more commonly affected than women.29 The pathophysiology is incompletely understood, but is known to mostly occur 2-8 weeks after an infection. GBS is the leading cause of non-traumatic paralysis, with a global incidence of 1-4 per 100,000 persons-years. The range of infections reported to have preceded GBS include upper respiratory infections, notably influenza and pseudo-influenza, digestive tract infections, notably Campylobacter jejuni, as well as cytomegalovirus and Epstein-Barr virus infections.30–32 The incidence rate of GBS cases during the French Polynesian outbreak was estimated at 0.24 per 1,000 ZIKV infections, at the lower range of the 0.25 to 0.65 per 1,000 observed following C. jejuni infections.33 It is unlikely that GBS cases were missed during the study period, since routine procedures for systematic confirmation of GBS diagnosis pre-existed the ZIKV epidemic, and all cases were systematically referred to the CPHF for diagnosis confirmation. While it is unknown whether attack rates of ZIKV epidemics will be as high in Latin America affected regions as compared to Pacific islands (73% in Micronesia6 and 66% in French Polynesia24), high number of GBS cases may be expected in the coming months as the result of this association. The results of our study support that ZIKV should be added to the list of infectious pathogens susceptible to cause GBS.
GBS patients in the present series had electrophysiological findings compatible with the AMAN type. EMG assessments performed during the first week of the disease showed marked distal motor nerve conduction alterations, which explain the neuromuscular weakness. Prolonged distal latencies and reduced distal CMAP amplitude may have been as first interpreted as demyelinating conduction slowing and block, leading to classify the GBS pattern as acute inflammatory demyelinating neuropathy (AIDP) with possible axonal degeneration. However, the disappearance of the distal motor conduction alterations during the follow-up in a subset of patients, without development of abnormal temporal dispersion or conduction slowing in intermediate nerve segments, was consistent with “reversible conduction failure” already described in AMAN.34,35 In GBS patients, such nodal/paranodal dysfunctions would be rather strictly localized in distal motor nerve endings.36 The clinical outcome of these ZIKV GBS patients was generally favourable, despite a rapid onset and short plateau phase, as may be seen in other patient groups suffering from the AMAN type of GBS.37 Three months after discharge, 43% of the patients were able to walk without assistance.
Among the molecular mechanisms contributing to the pathogenesis of GBS, a broad range of anti-glycolipid IgG antibodies, notably directed to gangliosides, has been previously described, particularly in axonal variants of the disease.38,39 Results in this current study, using both ELISA and combinatorial microarray techniques found less than 50% of sera at admission with a significant autoimmune response against glycolipids, including gangliosides and/or glycolipid complexes (see supplementary material). This low detection rate for the AMAN clinical subtype, may be a reflection of the unique nature of the preceding infection and study population, in contrast with more typical post-Campylobacter GBS/AMAN clinical cohorts. These findings suggest that there may be autoantibodies in this post-ZIKV GBS cohort that cannot be fully identified by current methods. Moreover, complementary analysis of sera with reactivity against GA1 did not reveal any competition between GA1 and ZIKV proteins, thus suggesting the lack of antigenic mimicry between ZIKV antigens and GA1 in these GBS patients and casting doubt on the relevance of the anti-GA1 antibodies to neuropathy pathogenesis. The disease may not be anti-glycolipid antibody mediated, rather be mediated by other autoantibody specificities or unknown neurotoxic factors. Alternatively viral neurotoxicity may contribute a more direct but as yet unexplained role.
Because almost all of the GBS patients were of Polynesian origin and as distribution of HLA alleles had been previously described as being involved in certain forms of GBS,40 a possible role of ethnicity in triggering GBS was hypothesized. However, the high incidence of GBS recently reported in Brazil, El Salvador and Columbia during local ZIKV outbreaks11,41 suggests that, whenever involved, such host factors may not be specific to the ethnic groups living in French Polynesia.
In conclusion, this is the first study to document a large series of patients who developed a GBS following ZIKV infection, a virus that previously used to be considered as causing only mild disease. Most (88%) of the GBS patients reported symptomatic ZIKV infection that preceded by a median of 6 days the occurrence of neurological symptoms. All GBS were of the AMAN type, characterised by distal motor nerve involvement, the absence of typical patterns and levels of anti-glycolipid antibodies, and faster recovery than usually observed in typical GBS. As ZIKV is spreading rapidly across the Americas, at risk countries need to prepare for adequate intensive care beds capacity for managing GBS patients.
Supplementary Material
Suppl. Figure 1
Changes in motor conduction study in one of the patients. Stimulation of right ulnar nerve at wrist and elbow, recording of the abductor digiti minimi. A : 5 days after paralysis onset : CMAP amplitude : 1.5 mV, distal motor latency (DML) : 4.1 ms, motor conduction velocity (MCV) : 50 m/s. Calibration 2 mV/div., 5 ms/div. B : 3 months later : CMAP amplitude : 8.1 mV, DML : 3.4 ms, MCV : 49 m/s. Calibration 5 mV/div., 5 ms/div.
Suppl. Figure 2
Test of reactivity of ZIKV positive patients’ sera towards ZIKV viral proteins by Western Blot
Lanes 1 and 2: non heated and heated control cell extracts; lanes 3 and 4: non heated and heated ZIKV positive cell extracts.
A: Coomassie stained SDS PAGE
B: Western Blot revealed by using anti-ZIKV 4G2 monoclonal antibody
Sera 6, 11,20, 29: GA1+ patients
Sera 13, 27: GA1- patients
Suppl. Figure 3
Competition experiments using GA1
Patient’s serum was incubated in presence of various amounts of GA1: 100 µg (B); 300 µg (C), 600 µg (D). A: control without GA1.
Suppl. Figure 4
Combinatorial microarray heatmaps
Each patient and healthy control serum was screened against 78 single and heteromeric glycolipid targets on a microarray assay. For ease of comparison, IgG (A) and IgM (B) data was visually displayed as heat maps, in which the rainbow scale was used to assign a colour to each interaction, which indicated the intensity of the antibody binding for that target.
Suppl. Table 1
Suppl. Table 2
Suppl. Table 3
Supplementary material
Acknowledgments
We are grateful to Dr Maite Aubry, ILM, for implementing the seroneutralization assay, and to Maria van Kerkhove and Rebecca Grant for critically reviewing the manuscript. The study received funding from the French Government's Investissement d'Avenir Programme (Labex Integrative Biology of Emerging Infectious Diseases, IBEID, grant n°ANR-10-LABX-62-IBEID) and the European Union Seventh Framework Programme [FP7/2007-2013] under Grant Agreement n°278433-PREDEMICS. The work of SKH and HJW was supported by the Wellcome Trust (092805).
Footnotes
Contributors
VMC-L, AB, VC, HPM, DM, AF, JN and FG conceived and designed the study; VMC-L, SL, CR, JV, AT, JCM and PD developed, performed and interpreted the virological analyses; VC, HJW, SKH, LM, and JN developed, performed and interpreted the immunological analyses; SM, LB, PL and FG provided care to the patients and designed the clinical report forms; ALV, CD, AB and HPM designed the case report forms and collected the epidemiological data; FG and EF performed the electrophysiological assessments; AB, TD, HPM, and AF performed the statistical analyses; VMC-L, AB, VC, HJW, EF, AF and JN wrote the first version of the manuscript. All authors critically reviewed and approved the final version of the manuscript.
References
- 1.Gubler DJ, Kuno G, Markoff L. Flaviviruses. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, et al., editors. Fields virology. 5th ed. Vol. 34. Philadelphia, PA: Lippincott Williams & Wilkins Publishers; 2007. pp. 1155–1227. [Google Scholar]
- 2.Dick GWA, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–20. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 3.Macnamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg. 1954;48:139–45. doi: 10.1016/0035-9203(54)90006-1. [DOI] [PubMed] [Google Scholar]
- 4.Musso D, Cao-Lormeau VM, Gubler DJ. Zika virus: following the path of dengue and chikungunya? Lancet. 2015;386:243–4. doi: 10.1016/S0140-6736(15)61273-9. [DOI] [PubMed] [Google Scholar]
- 5.Faye O, Freire CCM, Iamarino A, et al. Molecular Evolution of Zika Virus during Its Emergence in the 20th Century. PLoS Negl Trop Dis. 2014;8 doi: 10.1371/journal.pntd.0002636. published online Jan 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duffy MR, Chen TH, Hancock, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–43. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 7.Cao-Lormeau VM, Roche C, Teissier A, et al. Zika virus, French Polynesia, South Pacific. Emerg Infect Dis. 2013;20:1085–86. doi: 10.3201/eid2006.140138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mallet HP, Vial AL, Musso D. Bilan de l’épidémie à virus Zika en Polynésie Française 2013-2014. [Last accessed Feb 6, 2016];Bulletin d’information sanitaires, épidémiologiques et statistiques. http://www.hygiene-publique.gov.pf/IMG/pdf/no13_-_mai_2015_-_zika.pdf. [Google Scholar]
- 9.Zanluca C, de Melo VC, Mosimann AL, dos Santos GI, dos Santos CN, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz. 2015 doi: 10.1590/0074-02760150192. published online June 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dyer O. Zika virus spreads across Americas as concerns mount over birth defects. BMJ. 2015;351 doi: 10.1136/bmj.h6983. published online Dec 23. [DOI] [PubMed] [Google Scholar]
- 11.Samarasekera U, Triunfol M. Concern over Zika virus grips the world. Special Report. The Lancet. 2016;387:521–24. doi: 10.1016/S0140-6736(16)00257-9. [DOI] [PubMed] [Google Scholar]
- 12.European Centre for Disease Prevention and Control. Rapid Risk Assessment: Zika virus infection outbreak, French Polynesia. Stockholm: ECDC; 2014. Feb 13, [Google Scholar]
- 13.Leis AA, Stokic DS. Neuromuscular manifestations of West Nile virus infection. Front Neurol. 2012;3:37. doi: 10.3389/fneur.2012.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravi V, Taly AB, Shankar SK, et al. Association of Japanese encephalitis virus infection with Guillain-Barre syndrome in endemic areas of south India. Acta Neurol Scand. 1994;90:67–72. doi: 10.1111/j.1600-0404.1994.tb02681.x. [DOI] [PubMed] [Google Scholar]
- 15.Lebrun G, Chadda K, Reboux AH, Martinet O, Gaüzère BA. Guillain-Barré syndrome after chikungunya infection. Emerg Infect Dis. 2009;15:495–6. doi: 10.3201/eid1503.071482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solomon T, Dung NM, Vaughn DW, et al. Neurological manifestations of dengue infection. The Lancet. 2000;355:1053–59. doi: 10.1016/S0140-6736(00)02036-5. [DOI] [PubMed] [Google Scholar]
- 17.Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain-Barré syndrome – case report, French Polynesia, December 2013. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.ES2014.19.9.20720. pii=20720. [DOI] [PubMed] [Google Scholar]
- 18.Roth A, Mercier A, Lepers C, et al. Concurrent outbreaks of dengue, chikungunya and Zika virus infections - an unprecedented epidemic wave of mosquito-borne viruses in the Pacific 2012-2014. Euro Surveill. 2014;19:2–9. doi: 10.2807/1560-7917.es2014.19.41.20929. [DOI] [PubMed] [Google Scholar]
- 19.Fokke C, van den Berg B, Drenthen J, Walgaard C, van Doorn PA, Jacobs BC. Diagnosis of Guillain-Barré syndrome and validation of Brighton criteria. Brain. 2014;137:33–43. doi: 10.1093/brain/awt285. [DOI] [PubMed] [Google Scholar]
- 20.Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14:1232–39. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beck C, Desprès P, Paulous S, et al. A High-Performance Multiplex Immunoassay for Serodiagnosis of Flavivirus-Associated Neurological Diseases in Horses. Biomed Res Int. 2015 doi: 10.1155/2015/678084. published online May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rinaldi S, Brennan KM, Willison HJ. Combinatorial glycoarray Methods. Mol Biol. 2012;808:413–423. doi: 10.1007/978-1-61779-373-8_28. [DOI] [PubMed] [Google Scholar]
- 23.Neil J, Choumet V, Le Coupanec A, et al. Guillain-Barre syndrome: first description of a snake envenomation aetiology. J Neuroimmunol. 2012;242:72–77. doi: 10.1016/j.jneuroim.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Aubry M, Teissier A, Roche C, et al. Serosurvey of dengue, Zika and other mosquito-borne viruses in French Polynesia. 64th Annual Meeting of the American Society of Tropical Medicine and Hygiene; 26-29 Oct; Philadelphia, USA. 2015. [Google Scholar]
- 25.Musso D, Roche C, Nhan TX, Robin E, Teissier A, Cao-Lormeau VM. Detection of Zika virus in saliva. J Clin Virol. 2015;68:53–5. doi: 10.1016/j.jcv.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 26.Gourinat AC, O'Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. Detection of Zika virus in urine. Emerg Infect Dis. 2015;21:84–6. doi: 10.3201/eid2101.140894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van den Berg B, Walgaard C, Drenthen J, Fokke C, Jacobs BC, van Doorn PA. Guillain-Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol. 2014;10:469–482. doi: 10.1038/nrneurol.2014.121. [DOI] [PubMed] [Google Scholar]
- 28.Wim Ang C, Jacobs BC, Laman JD. The Guillain-Barré syndrome; a true case of molecular mimicry. Trends in Immunology. 2004;25:261–66. doi: 10.1016/j.it.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 29.McGrogan A, Madle GC, Seaman HE, de Vries CS. The epidemiology of Guillain-Barré syndrome worldwide. A systematic literature review. Neuroepidemiology. 2009;32:150–163. doi: 10.1159/000184748. [DOI] [PubMed] [Google Scholar]
- 30.Tam CC, O’Brien SJ, Petersen I, Islam A, Hayward A, Rodrigues LC. Guillain-Barré syndrome and preceding infection with campylobacter, influenza and Epstein-Barr virus in the general practice research database. PloS One. 2007;2 doi: 10.1371/journal.pone.0000344. published online Apr 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehmann HC, Hartung H-P, Kieseier BC, Hughes RAC. Guillain-Barré syndrome after exposure to influenza virus. Lancet Infect Dis. 2010;10:643–651. doi: 10.1016/S1473-3099(10)70140-7. [DOI] [PubMed] [Google Scholar]
- 32.Grimaldi-Bensouda L, Alpérovitch A, Besson G, et al. Guillain-Barre syndrome, influenza like illnesses, and influenza vaccination during seasons with and without circulating A/H1N1 viruses. Am J Epidemiol. 2011;174:326–335. doi: 10.1093/aje/kwr072. [DOI] [PubMed] [Google Scholar]
- 33.Yuki N, Harthung HP. Guillain-Barré Syndrome. N Engl J Med. 2012;366:2294–304. doi: 10.1056/NEJMra1114525. [DOI] [PubMed] [Google Scholar]
- 34.Kokubun N, Shahrizaila N, Koga M, Hirata K, Yuki N. The demyelination neurophysiological criteria can be misleading in Campylobacter jejuni-related Guillain-Barre syndrome. Clin Neurophysiol. 2013;124:1671–79. doi: 10.1016/j.clinph.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Uncini A, Kuwabara S. Electrodiagnostic criteria for Guillain-Barre syndrome: a critical revision and the need for an update. Clin Neurophysiol. 2012;123:1487–95. doi: 10.1016/j.clinph.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 36.Ho TW, Hsieh ST, Nachamkin I, et al. Motor nerve terminal degeneration provides a potential mechanism for rapid recovery in acute motor axonal neuropathy after Campylobacter infection. Neurology. 1997;48:717–24. doi: 10.1212/wnl.48.3.717. [DOI] [PubMed] [Google Scholar]
- 37.Hiraga A, Mori M, Ogawara K, Hattori T, Kuwabar D. Differences in patterns of progression in demyelinating and axonal Guillain-Barré syndromes. Neurology. 2003;61:471–4. doi: 10.1212/01.wnl.0000081231.08914.a1. [DOI] [PubMed] [Google Scholar]
- 38.Rinaldi S, Willison HJ. Ganglioside antibodies and neuropathies. Curr Opin Neurol. 2008;21:540–546. doi: 10.1097/WCO.0b013e32830b84b7. [DOI] [PubMed] [Google Scholar]
- 39.Willison HJ. Gangliosides as targets for autoimmune injury to the nervous system. J Neurochem. 2007;103(Suppl 1):143–149. doi: 10.1111/j.1471-4159.2007.04718.x. [DOI] [PubMed] [Google Scholar]
- 40.Monos DS, Papaioakim M, Ho TW, Li CY, McKhann GM. Differential distribution of HLA alleles in two forms of Guillain-Barre syndrome. J Infect Dis. 1997;176(Suppl 2):S180–182. doi: 10.1086/513786. [DOI] [PubMed] [Google Scholar]
- 41.Cardoso CW, Paploski IAD, Kikuti M, et al. Outbreak of acute exanthematous illness associated with Zika, chikungunya, and dengue viruses, Salvador, Brazil [letter] Emerg Infect Dis. 2015 doi: 10.3201/eid2112.151167. published online Dec 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Figure 1
Changes in motor conduction study in one of the patients. Stimulation of right ulnar nerve at wrist and elbow, recording of the abductor digiti minimi. A : 5 days after paralysis onset : CMAP amplitude : 1.5 mV, distal motor latency (DML) : 4.1 ms, motor conduction velocity (MCV) : 50 m/s. Calibration 2 mV/div., 5 ms/div. B : 3 months later : CMAP amplitude : 8.1 mV, DML : 3.4 ms, MCV : 49 m/s. Calibration 5 mV/div., 5 ms/div.
Suppl. Figure 2
Test of reactivity of ZIKV positive patients’ sera towards ZIKV viral proteins by Western Blot
Lanes 1 and 2: non heated and heated control cell extracts; lanes 3 and 4: non heated and heated ZIKV positive cell extracts.
A: Coomassie stained SDS PAGE
B: Western Blot revealed by using anti-ZIKV 4G2 monoclonal antibody
Sera 6, 11,20, 29: GA1+ patients
Sera 13, 27: GA1- patients
Suppl. Figure 3
Competition experiments using GA1
Patient’s serum was incubated in presence of various amounts of GA1: 100 µg (B); 300 µg (C), 600 µg (D). A: control without GA1.
Suppl. Figure 4
Combinatorial microarray heatmaps
Each patient and healthy control serum was screened against 78 single and heteromeric glycolipid targets on a microarray assay. For ease of comparison, IgG (A) and IgM (B) data was visually displayed as heat maps, in which the rainbow scale was used to assign a colour to each interaction, which indicated the intensity of the antibody binding for that target.
Suppl. Table 1
Suppl. Table 2
Suppl. Table 3
Supplementary material