Large Cell Neuroendocrine Carcinoma of the Lung: Clinico-Pathologic Features, Treatment, and Outcomes (original) (raw)
. Author manuscript; available in PMC: 2017 Sep 1.
Published in final edited form as: Clin Lung Cancer. 2016 Jan 21;17(5):e121–e129. doi: 10.1016/j.cllc.2016.01.003
Abstract
Clinical features and management of large cell neuroendocrine lung carcinoma of the lung are poorly described. We report a series of 49 patients with stage IV large cell neuroendocrine lung carcinomas; 47% had brain metastases. In 17 patients with molecular testing, 24% of tumors harbored KRAS mutations. Response to platinum/etoposide in these patients was poorer than historic data for small-cell lung cancer, however prognosis was similar.
Background
Large cell neuroendocrine carcinoma (LCNEC) accounts for approximately 3% of lung cancers. Pathologic classification and optimal therapies are debated. We report the clinicopathologic features, treatment and survival of a series of patients with stage IV LCNEC.
Materials and Methods
Cases of pathologically-confirmed stage IV LCNEC evaluated at Memorial Sloan Kettering Cancer Center from 2006 to 2013 were identified. We collected demographic, treatment, and survival data. Available radiology was evaluated by Response Evaluation Criteria In Solid Tumors (RECIST) 1.1 criteria.
Results
Forty-nine patients with stage IV LCNEC were identified. The median age was 64 years, 63% of patients were male, and 88% were smokers. Twenty-three patients (n = 23/49; 47%) had brain metastases, 17 at diagnosis and 6 during the disease course. Seventeen LCNEC patients (35%) had molecular testing, of which 24% had KRAS mutations (n = 4/17). Treatment data for first-line metastatic disease was available on 37 patients: 70% (n = 26) received platinum/etoposide and 30% (n = 11) received other regimens. RECIST was completed on 23 patients with available imaging; objective response rate was 37% (95% confidence interval, 16%–62%) with platinum/etoposide, while those treated with other first-line regimens did not achieve a response. Median overall survival was 10.2 months (95% confidence interval, 8.6–16.4 months) for the entire cohort.
Conclusion
Patients with stage IV LCNEC have a high incidence of brain metastases. KRAS mutations are common. Patients with stage IV LCNEC do not respond as well to platinum/etoposide compared with historic data for extensive stage small-cell lung cancer; however, the prognosis is similar. Prospective studies are needed to define optimum therapy for stage IV LCNEC.
Keywords: Brain metastases, KRAS mutation, Platinum-etoposide chemotherapy, Small cell lung carcinoma, Stage IV
Introduction
Large cell neuroendocrine carcinomas (LCNECs) are a rare subset of lung cancers, accounting for 15% of neuroendocrine tumors and 3% of all lung cancers.1–3 Travis et al first described these in 1991, as poorly differentiated high-grade carcinomas, with tumor cytologic features that are morphologically distinct from small cell lung cancer (SCLC), but that retain demonstrable neuroendocrine differentiation by light microscopy and immunohistochemistry or electron microscopy.1 They are classified on the spectrum of neuroendocrine neoplasms as a high-grade malignancy together with SCLC, and are distinct from other lung neuroendocrine neoplasms, including low-grade typical carcinoids (TC) and intermediate grade atypical carcinoids (AC). LCNEC can harbor components of adenocarcinoma, squamous cell carcinoma, giant cell carcinoma, or spindle cell carcinoma; such tumors are termed combined LCNEC. Adenocarcinoma is the most common histologic type found combined with LCNEC.4,5 When SCLC is combined with LCNEC, the tumor is both clinically and morphologically viewed as a SCLC. While the previous 2004 World Health Organization (WHO) classification categorized LCNEC as a variant of large cell carcinomas,6,7 in the current 2015 WHO classification, these tumors are no longer classified under large cell carcinoma, but in a group of neuroendocrine neoplasms with SCLC, TC, and AC.8
There are limited published data regarding the natural history, clinical course, and treatment of patients with advanced LCNEC. The most appropriate chemotherapy regimen for patients with advanced LCNEC has not been defined, and those that have been evaluated are summarized in Table 19–14. The National Cancer Control Network (NCCN) recommends treatment according to non—small-cell lung cancer (NSCLC) guidelines; however, LCNECs are frequently treated with the same chemotherapeutic regimens used for SCLC, given that they are both high-grade neuroendocrine neoplasms.9–11,15,16 While response rates of approximately 50% and up to 80% have been demonstrated in prospective studies utilizing platinum/etoposide in patients with extensive stage SCLC (ES-SCLC),12–14,17–19 current reports suggest that LCNEC are substantially less chemo-responsive to this regimen.9,10,15,16 We are unaware of any randomized studies investigating how patients with advanced LCNECs should be optimally treated.
Table 1.
Published Clinical Studies of Treatment and Survival in Advanced Large Cell Neuroendocrine Carcinomas of the Lung
| Study | No. of Patients | Stage IV Disease (N) | De Novo Stage IVa (N) | Recurrent Stage IVb (N) | ORR, All Chemo-Therapy (N) | First-Line Plt/E (N) | ORR to Plt/E (N) | First-Line (Other) | ORR to Other (N) | Median PFS (mos) | Median OS (mos) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Retrospective | |||||||||||
| Doddoli et al9 | 21 | 3 | 3 | 0 | NR | NR | NR | NR | NR | NR | 54 |
| Yamazaki et al10 | 20 | 11 | 6 | 5 | 50% (10/21) | 9 | 56% (5/9) | Cis/Vind/Mito = 6 | 45% (5/11) | 3.4 | 7.9 |
| Cis/Vind = 4 | |||||||||||
| Cis alone = 1 | |||||||||||
| Rossi et al11 | 83 | 27 | 0 | 27 | 29% (8/27) | 12 | 50% (6/12) | Cis/Gem = 10 | 12% (2/17) | NR | 51 (Plt/E) |
| Carbo/Taxol = 3 | 21 (Other) | ||||||||||
| Gem = 2 | |||||||||||
| Sun et al12 | 45 | 45 | NR | NR | 56% (25/45) | 11 | 73% (8/11) | Carbo/E = 10 | 50 (17/34) | 6.1 (Plt/E) | 11.1 |
| Plt/Gem = 11 | 4.9 (Other) | ||||||||||
| Plt/Pem = 3 | |||||||||||
| Plt/Taxol = 6 | |||||||||||
| Cis/Vin = 1 | |||||||||||
| Vin/Gem = 1 | |||||||||||
| Gefitinib = 1 | |||||||||||
| Erlotnib = 1 | |||||||||||
| Prospective | |||||||||||
| Niho et al13 | 30 | 30 | NR | NR | 46.7% | N/A | N/A | Cis/Irino | 47% | 5.8 | 12.6 |
| Le Treut et al14 | 29 | 29 | NR | NR | 34% | 29 | 34% | N/A | N/A | 5.2 | 7.7 |
Patients with LCNEC have a poor prognosis. The median survival of patients with early stage LCNECs is estimated to be between 19 and 40 months, with 5-year survival between 21% and 62%, based on retrospective studies of between 5 and 144 patients.4,9,15,20–37 Reported stage-for-stage 5-year survival rates in LCNEC are: stage I (33%–62%), stage II (18%–75%), stage III (8%–45%), and 0% in patients with stage IV disease.15,25,35–37 Clinicopathologic factors that correlate with poor survival in published studies include advanced tumor stage, tumor size (greater than 3 cm), and male gender.15,23
Little is known about the inherent biology and optimal management strategies for patients with advanced LCNEC. Herein we describe the clinicopathologic features, response to first-line systemic therapy and survival data including time to progression (TTP), and overall survival (OS) for patients with stage IV LCNEC from a single institution.
Materials and Methods
After Institutional Review Board approval was obtained, we performed a retrospective review of patients diagnosed with stage IV LCNECs evaluated at Memorial Sloan Kettering Cancer Center between 2006 and 2013. These patients were identified by a search of an institutional electronic database. Clinicopathologic, treatment, and survival information for these patients were collected. Available tissue was subjected to pathology re-review to confirm the diagnosis of LCNEC according to the 2015 WHO classification,8 which includes presence of neuroendocrine morphology (organoid nesting, palisading, trabeculae, and/or rosettes), high mitotic rate, defined as > 10 mitoses per 10 high-power fields (HPF) and typically necrosis, and immunohistochemical expression of at least one neuroendocrine marker—synaptophysin, chromogranin-A, CD56/NCAM (neural cell adhesion molecule). In small biopsy and cytology samples, if a tumor’s surface area available for evaluation was less than 10 HPF, mitotic count was performed for the maximal number of available HPFs. LCNEC was distinguished from SCLC based on distinctive non—small-cell cytomorphological features, including the presence of prominent nucleoli and/or vesicular chromatin and/or abundant cytoplasm. Representative morphologic and immunohistochemical findings in LCNEC are illustrated in Figure 1.
Figure 1. Microscopic Images of a Representative Case of LCNEC. (A) Low-Power and (B) High-Power Images of H&E-Stained Sections Demonstrating Classic LCNEC Morphology, Including Neuroendocrine Architecture (Nesting With Peripheral Palisading and Rosettes), High Mitotic Rate and Necrosis, and Non—Small-Cell Cytologic Features (Abundant Cytoplasm, Prominent Nucleoli). Immunohistochemistry for Synaptophysin (C) and CD56 (D) Shows Diffuse Labeling, Supporting Neuroendocrine Differentiation.
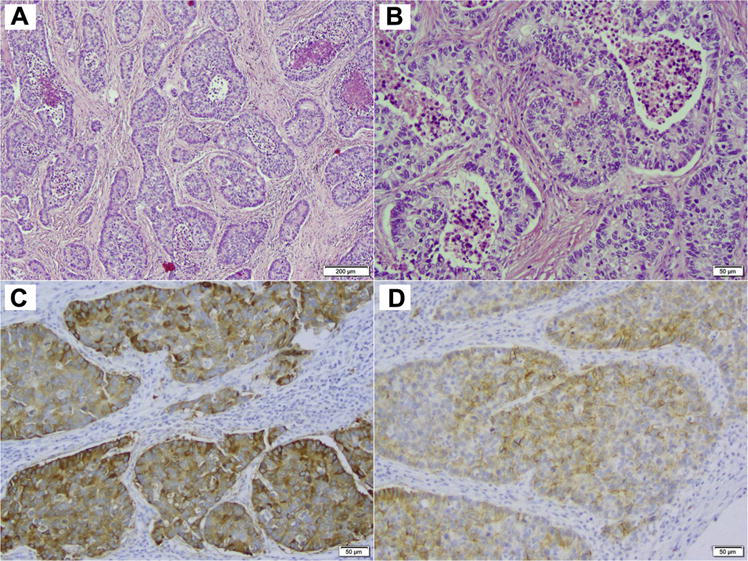
Abbreviations: H&E = hematoxylin and eosin; LCNEC = large cell neuroendocrine carcinoma.
Patients with stage IV disease were sub-classified into ‘de novo stage IV’ to describe those individuals who had metastatic disease at initial presentation, and ‘recurrent stage IV’ to denote those individuals who received definitive therapy for stage I/II/III disease at initial diagnosis, and later developed progression. Patients with oligometastatic disease at presentation who received definitive therapy were reported separately.
When an adequate tissue sample was available, molecular testing was performed by polymerase chain reaction-based fragment length analysis, mass spectrometry genotyping (Sequenom), and Sanger sequencing.38,39 For all patients with available diagnostic imaging, formal retrospective Response Evaluation Criteria In Solid Tumors (RECIST) 1.1 assessment was performed.40 Objective response rate (ORR; complete response + partial response) for platinum/etoposide versus other regimens was calculated with exact 95% confidence intervals (CI).
TTP and OS were calculated using the Kaplan-Meier method. TTP analyses included patients who had at least one repeat diagnostic imaging following the start of first-line treatment and excluded patients who received treatment outside of the institution. TTP was defined as the time from the date of diagnosis of radiologic or pathologically confirmed stage IV disease, until the date of radiologic progression on first-line therapy. Patients who did not experience radiologic progression were censored at the stop date of first-line treatment. OS was defined as the time from the date of diagnosis of radiologic or pathologically-confirmed stage IV disease, until the date of death from any cause. Patients with oligometastatic disease at initial disease presentation were removed from analyses of survival and response to treatment. Patients who were still alive were censored at the date of last follow-up. The log-rank test was used for subgroup comparisons of TPP and OS. For TPP and OS comparisons of first-line treatment regimens, the endpoints were defined from start date of first-line treatment or date of stage IV diagnosis plus 4 weeks, if the start date of first-line treatment was unknown. All statistical tests were 2-sided and used a 5% significance level (P < .05). Statistical analyses were performed using R (version 3.0.1; R Development Core Team) with the “survival” and “survcomp” packages.
Results
Clinicopathologic Features
Forty-nine patients with pathologically confirmed stage IV pure LCNEC were identified. No patients with adenocarcinoma, squamous carcinoma, or SCLC components were included in this study. The diagnosis was obtained from the following sample types: lobectomy (n = 8; 16%), lung wedge resection (n = 8; 16%), excisional biopsy of a metastatic site (n = 6; 12%), core biopsy (n = 7; 14%), lymph node biopsy (n = 10; 20%), transbronchial biopsy (n = 6; 12%), and fine-needle aspirate (n = 4; 8%). Clinical features of the patients are summarized in Table 2. A large number of patients in this dataset had brain metastases (n = 23/49; 47%): 35% had brain metastases at diagnosis (n = 17/49), and 12% (n = 6/49) developed brain metastases later in the course of disease. Seven patients (n = 7/49; 14%) presented with an acutely symptomatic brain lesion, diagnosed by brain metastectomy or biopsy, followed either by focal or whole-brain radiation therapy.
Table 2.
Clinical Characteristics of Patients With Stage IV Large Cell Neuroendocrine Carcinomas of the Lung (N = 49)
| Factor | N (%) |
|---|---|
| Age | |
| Median | 64 years |
| ≤64 | 26 (53) |
| >64 | 23 (47) |
| Gender | |
| Male | 31 (63) |
| Female | 18 (27) |
| Smoking history | |
| Never | 6 (12) |
| Former | 38 (78) |
| Current | 5 (10) |
| Karnofsky Performance Status | |
| <80 | 12 (24) |
| ≥80 | 37 (76) |
| Lactate dehydrogenase | |
| ≤250 | 12 (24) |
| >250 | 13 (27) |
| Brain metastases | |
| Yesa | 23 (47) |
| No | 26 (53) |
| Liver metastases | |
| Yes | 15 (31) |
| No | 34 (69) |
| Bone metastases | |
| Yes | 13 (27) |
| No | 36 (73) |
| Stage IV disease | |
| De novob | 32 (65) |
| Recurrentc | 13 (27) |
| Oligometastatic diseased | 4 (8) |
Thirty-two patients (n = 32/49; 65%) had de novo stage IV LCNEC, 13 patients had recurrent LCNEC after initial stage I/II/II disease, and 4 patients had oligometastatic disease. Of the 4 patients with oligometastatic disease, 3 patients had stage IA LCNEC and between 1 and 3 isolated brain metastases, and 1 patient had 3 separate discrete LCNEC lesions, 1 in each lung and 1 on the scalp. All 4 patients were treated definitively and exhibited prolonged disease-free and overall survival.
Treatment Data
Data for first-line treatment of advanced disease was available for 37 of the 49 patients in this series. Of the 12 patients that did not receive therapy: 4 declined therapy, 4 did not have adequate functional status to receive systemic therapy, and 4 patients had definitive therapy for oligometastatic disease. Twenty-six patients (70%; n = 26/37) were treated with first-line platinum/etoposide (ie, either with carboplatin or cisplatin). Eleven patients received other first-line regimens, which included: taxane (n = 1), platinum/taxane (n = 3)/bevacizumab (n = 1), pemetrexed (n = 1)/+platinum (n = 1), temozolomide (n = 2), platinum/everolimus clinical trial (n = 1), cyclophosphamide/doxorubicin/etoposide (n = 1). The median number of lines of treatment received was 2 (range, 1–6).
Molecular Analysis
Seventeen patients had tumor tissue samples that underwent molecular testing at initial diagnosis. Four patients’ tumors (24%) harbored KRAS mutations (2 G12D; 1 G12C; 1 Q61H), and none of the samples were found to contain an EGFR mutation or ALK rearrangement. All patients in this dataset had pure LCNEC, and therefore, none of these patients had an adenocarcinoma component.
Response to Therapy
ORR was calculated in 23 patients with available radiologic imaging, suitable for assessment by RECIST 1.1 (Table 3). The ORR for evaluable patients who received systemic therapy was 30% (95% CI, 13%–53%). Radiologically evaluable patients who received first-line platinum/etoposide (n = 19) demonstrated an ORR of 37% (95% CI, 16%–62%). None of the 4 radiologically evaluable patients who received other regimens in first line achieved a response to therapy (ORR, 0%; 95% CI, 0%–60%). The sample size was too small to assess statistical significance.
Table 3.
Response to First-Line Systemic Therapy by RECIST 1.1 in Patients With Stage IV Large Cell Neuroendocrine Carcinomas of the Lung (N = 23)
| RECIST Response | Plt/Etoposide (n = 19) | Other Regimens (n = 4)a |
|---|---|---|
| PR | 7 | 0 |
| SD | 11 | 3 |
| PD | 1 | 1 |
Perioperative Therapy in Patients With Recurrent LCNEC
Thirteen patients originally had early stage disease, and later developed recurrent LCNEC (Table 4). Initially, these patients were diagnosed with stage I (n = 4), stage II (n = 2), stage III (n = 7) disease, respectively, and were treated definitively. Six of these patients received perioperative therapy: adjuvant platinum/etoposide after surgery (n = 5: 1, stage 1; 1, stage II; 3, stage III), and neoadjuvant cisplatin/pemetrexed/radiation followed by surgery and adjuvant pemetrexed (n = 1, stage III). The median time from the original diagnosis of localized disease to recurrent disease was 13.3 months (range, 4.1–50.1 months). Patients who received perioperative therapy had a non-significant numeric improved median duration of survival from the time of diagnosis of recurrent metastatic disease, compared with those who did not (19.4 months vs. 11.8 months). Patients initially diagnosed with stage I disease (n = 4) had a median survival of 6.9 months, compared with those with stage II/III disease (n = 9), where the median survival was not reached. Due to small patient numbers, these analyses were not assessable for statistical significance. The 4 patients with oligometastatic disease who were treated definitively had a prolonged time to progression and overall survival of between 6.0 to 64.0 and 9.0 to 64.9 months, respectively.
Table 4.
Treatment and Survival Data for Recurrent Large Cell Neuroendocrine Carcinomas of the Lung (n = 13)
| Factor | N | Median OSa (months) (95% CI) | P Value |
|---|---|---|---|
| Entire cohort | 13 | 11.8 (9.0-NA) | |
| Stage at initial diagnosis | |||
| I | 4 | 6.9 (2.9-NA) | .001 |
| II/III | 9 | NR | |
| Peri-operative chemotherapy | |||
| Yes | 6 | 19.4 (5.0-NA) | 0.61 |
| No | 7 | 11.8 (8.8-NA) |
Survival Data
TTP analysis was performed on patients with available treatment data, and who had at least 1 radiologic assessment following start of first-line treatment (n = 30). We arrived at 30 patients for the TTP analysis as of the original 49 patients; 9 did not receive systemic therapy, 5 received therapy outside Memorial Sloan Kettering Cancer Center, and 1 had no repeat radiologic imaging. Additionally, the 4 patients with oligometastatic disease were not included in this analysis. The median TTP for patients with de novo stage IV disease was 6.4 months (95% CI, 5.1–9.7 months; n = 21) compared with 7.1 months (95% CI, 3.3 to not available [NA]; n = 9) for those with recurrent disease (P = .37; Figure 2A). Median TTP for patients who received first-line chemotherapy with platinum/etoposide (n = 20) was 4.6 months (95% CI, 3.9–11.1 months) compared with 4.7 months (95% CI, 3.3-NA) in patients who received other regimens (n = 10; P = .89; Figure 2B).
Figure 2. (A) Time to Progression for Patients With De Novo Stage LCNECs at Diagnosis (n = 21) Compared With Those With Recurrent, Metastatic Disease After Having Initially Presented With Stage I/II/III Disease (n = 9). (B) Time to Progression for Patients Who Received First-Line Chemotherapy Platinum/Etoposide (n = 20), Versus Those Who Received an Alternative Regimen (n = 10). (C) Overall Survival for Patients With De Novo Stage IV LCNECs at Diagnosis (n = 32) Compared With Those With Recurrent, Metastatic Disease After Having Initially Presented With Stage I/II/III Disease (n = 13). (D) Overall Survival for Patients Who Received First-Line Chemotherapy With Platinum/Etoposide (n = 26), Versus Those Who Received an Alternative Regimen (n = 11).
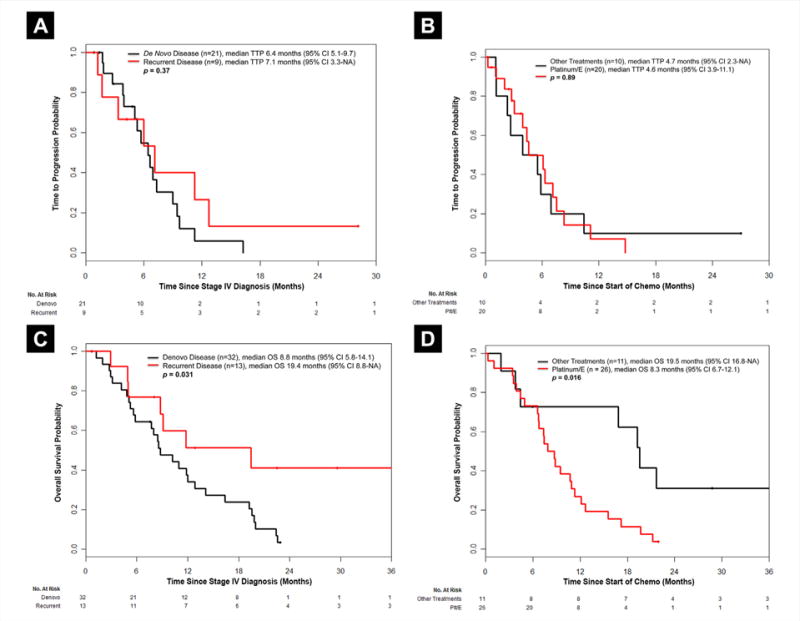
Abbreviations: E = etoposide; LCNEC = large cell neuroendocrine carcinoma; OS = overall survival; Plt/E = platinum/etoposide; TTP = time to progression.
Median OS was 10.2 months (95% CI, 8.5–16.4 months) for 45 patients, as the 4 oligometastatic cases were excluded from this analysis. Patients with de novo stage IV disease had a median OS of 8.8 months (n = 32), compared with those with recurrent stage IV disease who had a median OS of 19.4 months (n = 13; P = .031; Figure 2C). Patients who received first-line chemotherapy with platinum/etoposide (n = 26) had poorer OS compared with those who received other regimens (n = 11; median OS, 8.3 months vs. 19.5 months; P = .016; Figure 2D). No clinical factors were significantly associated with OS.
Discussion
We report a large series of patients with stage IV LCNECs of the lung, focusing on clinicopathologic factors including the incidence of brain metastases, presence of oncogenic driver mutations, response to first-line systemic therapy, and survival outcomes.
The clinicopathologic features of the patients in this study were consistent with the known demographics in this disease. A large number of patients in this series had brain metastases, with 35% of stage IV LCNEC patients having intracranial lesions at diagnosis (n = 17/49) and 12% (n = 6/49) developing them later in their disease. The clinical course of SCLC patients is similar, with brain metastases detected in up to 18% of patients at diagnosis,41 and the probability of developing such lesions ranging from 50% to 80% in patients who survive 2 years, according to actuarial analyses.42 The large number of LCNEC patients who develop brain metastases raises the question of the potential role for prophylactic cranial irradiation in this disease, similar to SCLC.
This study demonstrates that a subset of LCNECs have KRAS mutations, confirming the findings of several previous studies. In aseriesof 41 “histologically-pure” LCNECs, which are those tumors that do not contain other histologic components, EGFR and KRAS were evaluated by Sequenom, and 7 samples were found to harbor KRAS mutations.43 EGFR/KRAS/DDR2/BRAF sequencing was performed in a second series of 11 patients with pure LCNEC,44 and one KRAS mutation was noted.44 Importantly, SCLC tumors do not harbor KRAS mutations.43,45,46 Furthermore, genomic studies have demonstrated that subsets of LCNECs may harbor other alterations, seen in both adenocarcinomas and even squamous cell lung carcinomas.47 Large prospective diagnostic molecular analyses on LCNECs are imperative and may further help to identify tumor-specific vulnerabilities with therapeutic implications.
The response to chemotherapy in this disease is poor when compared with that seen in ES SCLC, but similar to that of patients with advanced NSCLC.48 We observed an ORR to platinum/etoposide of 37% (95% CI, 16%–62%), which is consistent with the results of 2 prospective phase II studies in LCNEC that report response rates of 38% to platinum/etoposide16 and 55% to cisplatin/irinotecan,11 respectively. By contrast, published studies report ORRs to platinum/etoposide for ES SCLC that range from 46%–78%.12–14,17–19 Several retrospective studies in LCNEC separated chemotherapy regimens into NSCLC-type and SCLC-type. They demonstrate ORRs to regimens other than platinum/etoposide of 12% to 50% and report a difference in survival, favoring SCLC-type chemotherapy.10,15 However, there is considerable overlap between regimens used for NSCLC and SCLC, which may limit the value of these types of analyses. Our study demonstrates no response in the 5 patients that received ‘other regimens’; however, 3 cases achieved stable disease (0%; 95% CI, 0%–60%). Notably, 4 of the 5 patients who received ‘other regimens’ were radiologically evaluated by RECIST 1.1, and 1 case was assessed by retrospective review of radiology reports. This study cannot determine the optimal chemotherapy regimen for these patients, and highlights that prospective clinical trials are needed to determine if platinum/ etoposide versus other regimens would lead to improved outcomes.
Receipt of perioperative chemotherapy in patients who received definitive therapy for initial stage I/II/III LCNEC was associated with a numerical improvement in OS from the time of recurrent metastatic disease when compared with those with de novo LCNEC in this study. Patients with recurrent LCNEC may have a different inherent biology than those diagnosed with de novo metastatic disease. Stage I LCNEC patients in this series had poor outcomes, which may indicate a need for perioperative therapy in these patients (n = 1/5 received perioperative therapy, vs. n = 5/9 patients with stage II/III disease); however, small numbers in our study limit our interpretation of the outcomes of these patients. Importantly, perioperative chemotherapy in these patients should be considered, as suggested elsewhere.23,24,35,36,49–51
Patients with stage IV LCNEC have poor survival, as seen with all lung cancers. The median OS of the entire cohort was 10.2 months, which is within the reported range in advanced LCNEC.9–11,15,16,34 Patients who received first-line platinum/etoposide demonstrated similar median TTP, yet poorer median OS than those who received ‘other’ regimens (4.6 months vs. 4.7 months; P = .89 and 8.3 months vs. 19.5 months; P = .016, respectively). The factors that may have contributed to the poorer OS in patients receiving first-line platinum/etoposide in the current study are unclear, though underlying comorbidities, difficulty in tolerating chemotherapy, a high incidence of brain metastases, or older age are considerations.
Conclusions
This study is a large dataset reporting clinicopathologic factors, response to therapy, and survival outcomes in patients with stage IV LCNECs, which are a rare group of thoracic malignancies. Our study demonstrates that a large proportion of patients have brain metastases, and a subset of patients with LCNEC harbor KRAS mutations. Our data suggest that LCNECs may not respond to platinum/etoposide as robustly as that seen in reported trials in ES SCLC. Given the retrospective analysis with limited patient numbers, specific insight into which type of regimen is preferable for advanced stage LCNEC could not be determined. Patients with advanced LCNEC have a dismal prognosis that is comparable to patients with other metastatic lung cancers without targetable driver mutations. Prospective randomized studies in this disease should be prioritized, as well as diagnostic mutational pathology evaluation. Identifying potential oncogenic drivers and genomic alterations relevant in LCNEC biology will allow for the development of clinical studies tailored to molecular subgroups, a strategy that has contributed to substantial successes in NSCLC.
Clinical Practice Points
- The clinicopathologic features and optimum management for patients with advanced large cell neuroendocrine carcinomas (LCNEC) of the lung are not well-known. Our data suggest that patients with advanced LCNEC have a unique biology.
- In this analysis, patients with stage IV LCNEC have a high incidence of brain metastases suggesting that those with metastatic LCNEC may benefit from routine brain-directed surveillance during their course of disease and prophylactic therapy similar to our practice in SCLC.
- In addition, a subset of LCNEC tumors harbor KRAS mutations. Molecular analysis should be considered in the assessment of LCNEC patients.
- We demonstrate that patients with stage IV LCNEC have a poorer response to first-line platinum/etoposide when compared with historic data from patients with ES SCLC; yet, the prognosis for these patients is similar to those with ES SCLC.
- Our results support that dedicated prospective clinical studies in advanced LCNEC are warranted to elucidate the optimum management in this disease.
Acknowledgments
This work was supported, in part, by the National Cancer Institute of the National Institutes of Health under award number P30 CA008748 (recipient is Craig Thompson).
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
References
- 1.Travis WD, Linnoila RI, Tsokos MG, et al. Neuroendocrine tumors of the lung with proposed criteria for large-cell neuroendocrine carcinoma. An ultrastructural, immunohistochemical, and flow cytometric study of 35 cases. Am J Surg Pathol. 1991;15:529–53. doi: 10.1097/00000478-199106000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med. 2010;134:1628–38. doi: 10.5858/2009-0583-RAR.1. [DOI] [PubMed] [Google Scholar]
- 3.Fasano M, Della Corte CM, Papaccio F, Ciardiello F, Morgillo F. Pulmonary large-cell neuroendocrine carcinoma: from epidemiology to therapy. J Thorac Oncol. 2015;10:1133–41. doi: 10.1097/JTO.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Travis WD, Rush W, Flieder DB, et al. Survival analysis of 200 pulmonary neuroendocrine tumors with clarification of criteria for atypical carcinoid and its separation from typical carcinoid. Am J Surg Pathol. 1998;22:934–44. doi: 10.1097/00000478-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Travis WD, Brambilla E, Muller-Hermelink HK, et al. Pathology and Genetics: Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC; 2004. [Google Scholar]
- 6.Travis WD. The 2015 WHO classification of lung tumors. Pathologe. 2014;35(Suppl 2):188. doi: 10.1007/s00292-014-1974-3. [DOI] [PubMed] [Google Scholar]
- 7.Travis WD, Krug LM, Rusch V, et al. Large Cell Neuroendocrine Carcinoma Textbook of Uncommon Cancer. Chichester, England: John Wiley & Sons; 2006. pp. 298–306. [Google Scholar]
- 8.Travis WD, Bambilla E, Burke AP, Marx A, Nicholson A, editors. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Geneva: WHO Press; 2015. International Agency for Research on Cancer. [DOI] [PubMed] [Google Scholar]
- 9.Yamazaki S, Sekine I, Matsuno Y, et al. Clinical responses of large cell neuroendocrine carcinoma of the lung to cisplatin-based chemotherapy. Lung Cancer. 2005;49:217–23. doi: 10.1016/j.lungcan.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Sun JM, Ahn MJ, Ahn JS, et al. Chemotherapy for pulmonary large cell neuro-endocrine carcinoma: similar to that for small cell lung cancer or non-small cell lung cancer? Lung Cancer. 2012;77:365–70. doi: 10.1016/j.lungcan.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Niho S, Kenmotsu H, Sekine I, et al. Combination chemotherapy with irinotecan and cisplatin for large-cell neuroendocrine carcinoma of the lung: a multicenter phase II study. J Thorac Oncol. 2013;8:980–4. doi: 10.1097/JTO.0b013e31828f6989. [DOI] [PubMed] [Google Scholar]
- 12.Lara PN, Jr, Natale R, Crowley J, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol. 2009;27:2530–5. doi: 10.1200/JCO.2008.20.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85–91. doi: 10.1056/NEJMoa003034. [DOI] [PubMed] [Google Scholar]
- 14.Fukuoka M, Furuse K, Saijo N, et al. Randomized trial of cyclophosphamide, doxorubicin, and vincristine versus cisplatin and etoposide versus alternation of these regimens in small-cell lung cancer. J Natl Cancer Inst. 1991;83:855–61. doi: 10.1093/jnci/83.12.855. [DOI] [PubMed] [Google Scholar]
- 15.Rossi G, Cavazza A, Marchioni A, et al. Role of chemotherapy and the receptor tyrosine kinases KIT, PDGFRalpha, PDGFRbeta, and Met in large-cell neuroendocrine carcinoma of the lung. J Clin Oncol. 2005;23:8774–85. doi: 10.1200/JCO.2005.02.8233. [DOI] [PubMed] [Google Scholar]
- 16.Le Treut J, Sault MC, Lena H, et al. Multicentre phase II study of cisplatin-etoposide chemotherapy for advanced large-cell neuroendocrine lung carcinoma: the GFPC 0302 study. Ann Oncol. 2013;24:1548–52. doi: 10.1093/annonc/mdt009. [DOI] [PubMed] [Google Scholar]
- 17.Roth BJ, Johnson DH, Einhorn LH, et al. Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small-cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. J Clin Oncol. 1992;10:282–91. doi: 10.1200/JCO.1992.10.2.282. [DOI] [PubMed] [Google Scholar]
- 18.Zatloukal P, Cardenal F, Szczesna A, et al. A multicenter international randomized phase III study comparing cisplatin in combination with irinotecan or etoposide in previously untreated small-cell lung cancer patients with extensive disease. Ann Oncol. 2010;21:1810–6. doi: 10.1093/annonc/mdq036. [DOI] [PubMed] [Google Scholar]
- 19.Schmittel A, Sebastian M, Fischer von Weikersthal L, et al. A German multicenter, randomized phase III trial comparing irinotecan-carboplatin with etoposide-carboplatin as first-line therapy for extensive-disease small-cell lung cancer. Ann Oncol. 2011;22:1798–804. doi: 10.1093/annonc/mdq652. [DOI] [PubMed] [Google Scholar]
- 20.Wick MR, Berg LC, Hertz MI. Large cell carcinoma of the lung with neuroendocrine differentiation. A comparison with large cell “undifferentiated” pulmonary tumors. Am J Clin Pathol. 1992;97:796–805. doi: 10.1093/ajcp/97.6.796. [DOI] [PubMed] [Google Scholar]
- 21.Dresler CM, Ritter JH, Patterson GA, Ross E, Bailey MS, Wick MR. Clinical-pathologic analysis of 40 patients with large cell neuroendocrine carcinoma of the lung. Ann Thorac Surg. 1997;63:180–5. doi: 10.1016/s0003-4975(96)01058-2. [DOI] [PubMed] [Google Scholar]
- 22.Rusch VW, Klimstra DS, Venkatraman ES. Molecular markers help characterize neuroendocrine lung tumors. Ann Thorac Surg. 1996;62:798–809. doi: 10.1016/s0003-4975(96)00435-3. discussion: 809–10. [DOI] [PubMed] [Google Scholar]
- 23.Sarkaria IS, Iyoda A, Roh MS, et al. Neoadjuvant and adjuvant chemotherapy in resected pulmonary large cell neuroendocrine carcinomas: a single institution experience. Ann Thorac Surg. 2011;92:1180–6. doi: 10.1016/j.athoracsur.2011.05.027. discussion: 1186–7. [DOI] [PubMed] [Google Scholar]
- 24.Iyoda A, Hiroshima K, Moriya Y, et al. Postoperative recurrence and the role of adjuvant chemotherapy in patients with pulmonary large-cell neuroendocrine carcinoma. J Thorac Cardiovasc Surg. 2009;138:446–53. doi: 10.1016/j.jtcvs.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 25.Takei H, Asamura H, Maeshima A, et al. Large cell neuroendocrine carcinoma of the lung: a clinicopathologic study of eighty-seven cases. J Thorac Cardiovasc Surg. 2002;124:285–92. doi: 10.1067/mtc.2002.122523. [DOI] [PubMed] [Google Scholar]
- 26.Jiang SX, Kameya T, Shoji M, Dobashi Y, Shinada J, Yoshimura H. Large cell neuroendocrine carcinoma of the lung: a histologic and immunohistochemical study of 22 cases. Am J Surg Pathol. 1998;22:526–37. doi: 10.1097/00000478-199805000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Yuste M, Matilla JM, Alvarez-Gago T, et al. Prognostic factors in neuroendocrine lung tumors: a Spanish Multicenter Study. Spanish Multicenter Study of Neuroendocrine Tumors of the Lung of the Spanish Society of Pneumonology and Thoracic Surgery (EMETNE-SEPAR) Ann Thorac Surg. 2000;70:258–63. doi: 10.1016/s0003-4975(00)01369-2. [DOI] [PubMed] [Google Scholar]
- 28.Jung KJ, Lee KS, Han J, et al. Large cell neuroendocrine carcinoma of the lung: clinical, CT, and pathologic findings in 11 patients. J Thorac Imaging. 2001;16:156–62. doi: 10.1097/00005382-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Mazieres J, Daste G, Molinier L, et al. Large cell neuroendocrine carcinoma of the lung: pathological study and clinical outcome of 18 resected cases. Lung Cancer. 2002;37:287–92. doi: 10.1016/s0169-5002(02)00099-5. [DOI] [PubMed] [Google Scholar]
- 30.Hage R, Seldenrijk K, deBruin P, vanSwieten H, vandenBosch J. Pulmonarylarge-cell neuroendocrine carcinoma (LCNEC) Eur J Cardiothorac Surg. 2003;23:457–60. doi: 10.1016/s1010-7940(02)00837-0. [DOI] [PubMed] [Google Scholar]
- 31.Paci M, Cavazza A, Annessi V, et al. Large cell neuroendocrine carcinoma of the lung: a 10-year clinicopathologic retrospective study. Ann Thorac Surg. 2004;77:1163–7. doi: 10.1016/j.athoracsur.2003.09.070. [DOI] [PubMed] [Google Scholar]
- 32.Battafarano RJ, Fernandez FG, Ritter J, et al. Large cell neuroendocrine carcinoma: an aggressive form of non-small cell lung cancer. J Thorac Cardiovasc Surg. 2005;130:166–72. doi: 10.1016/j.jtcvs.2005.02.064. [DOI] [PubMed] [Google Scholar]
- 33.Zacharias J, Nicholson AG, Ladas GP, Goldstraw P. Large cell neuroendocrine carcinoma and large cell carcinomas with neuroendocrine morphology of the lung: prognosis after complete resection and systematic nodal dissection. Ann Thorac Surg. 2003;75:348–52. doi: 10.1016/s0003-4975(02)04118-8. [DOI] [PubMed] [Google Scholar]
- 34.Doddoli C, Barlesi F, Chetaille B, et al. Large cell neuroendocrine carcinoma of the lung: an aggressive disease potentially treatable with surgery. Ann Thorac Surg. 2004;77:1168–72. doi: 10.1016/j.athoracsur.2003.09.049. [DOI] [PubMed] [Google Scholar]
- 35.Iyoda A, Hiroshima K, Toyozaki T, et al. Adjuvant chemotherapy for large cell carcinoma with neuroendocrine features. Cancer. 2001;92:1108–12. doi: 10.1002/1097-0142(20010901)92:5<1108::aid-cncr1427>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 36.Veronesi G, Morandi U, Alloisio M, et al. Large cell neuroendocrine carcinoma of the lung: aretrospective analysis of 144 surgical cases. Lung Cancer. 2006;53:111–5. doi: 10.1016/j.lungcan.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Asamura H, Kameya T, Matsuno Y, et al. Neuroendocrine neoplasms of the lung: a prognostic spectrum. J Clin Oncol. 2006;24:70–6. doi: 10.1200/JCO.2005.04.1202. [DOI] [PubMed] [Google Scholar]
- 38.Pan Q, Pao W, Ladanyi M. Rapid polymerase chain reaction-based detection of epidermal growth factor receptor gene mutations in lung adenocarcinomas. J Mol Diagn. 2005;7:396–403. doi: 10.1016/S1525-1578(10)60569-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li AR, Chitale D, Riely GJ, et al. EGFR mutations in lung adenocarcinomas: clinical testing experience and relationship to EGFR gene copy number and immunohistochemical expression. J Mol Diagn. 2008;10:242–8. doi: 10.2353/jmoldx.2008.070178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 41.Seute T, Leffers P, ten Velde GP, Twijnstra A. Neurologic disorders in 432 consecutive patients with small cell lung carcinoma. Cancer. 2004;100:801–6. doi: 10.1002/cncr.20043. [DOI] [PubMed] [Google Scholar]
- 42.Komaki R, Cox JD, Whitson W. Risk of brain metastasis from small cell carcinoma of the lung related to length of survival and prophylactic irradiation. Cancer Treat Rep. 1981;65:811–4. [PubMed] [Google Scholar]
- 43.Rekhtman N, Marchetti A, Lau C, et al. Analysis of EGFR and KRAS Mutations in Small Cell Carcinoma and Large Cell Neuroendocrine Carcinoma of Lung. IASLC 14th World Conference on Lung Cancer; Amsterdam, The Netherlands. 2011. [Google Scholar]
- 44.Yashima H, Shimizu K, Araki T, et al. Assessment of DDR2, BRAF, EGFR and KRAS mutations as therapeutic targets in non-adenocarcinoma lung cancer patients. Mol Clin Oncol. 2014;2:714–8. doi: 10.3892/mco.2014.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rudin CM, Durinck S, Stawiski EW, et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet. 2012;44:1111–6. doi: 10.1038/ng.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peifer M, Fernandez-Cuesta L, Sos ML, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet. 2012;44:1104–10. doi: 10.1038/ng.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rekhtman N, Pietanza MC, Hellmann MD, et al. Next-generation sequencing of pulmonary large cell neuroendocrine carcinoma reveals small cell carcinoma-like and non-small cell carcinoma-like subsets. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-2946. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Belani CP, Lee JS, Socinski MA, et al. Randomized phase III trial comparing cisplatin-etoposide to carboplatin-paclitaxel in advanced or metastatic non-small cell lung cancer. Ann Oncol. 2005;16:1069–75. doi: 10.1093/annonc/mdi216. [DOI] [PubMed] [Google Scholar]
- 49.Saji H, Tsuboi M, Matsubayashi J, et al. Clinical response of large cell neuroendocrine carcinoma of the lung to perioperative adjuvant chemotherapy. Anticancer Drugs. 2010;21:89–93. doi: 10.1097/CAD.0b013e328330fd79. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka Y, Ogawa H, Uchino K, et al. Immunohistochemical studies of pulmonary large cell neuroendocrine carcinoma: a possible association between staining patterns with neuroendocrine markers and tumor response to chemotherapy. J Thorac Cardiovasc Surg. 2013;145:839–46. doi: 10.1016/j.jtcvs.2012.03.036. [DOI] [PubMed] [Google Scholar]
- 51.Iyoda A, Hiroshima K, Moriya Y, et al. Prospective study of adjuvant chemotherapy for pulmonary large cell neuroendocrine carcinoma. Ann Thorac Surg. 2006;82:1802–7. doi: 10.1016/j.athoracsur.2006.05.109. [DOI] [PubMed] [Google Scholar]