Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9 (original) (raw)
Abstract
PCSK9 encodes proprotein convertase subtilisin/kexin type 9a (PCSK9), a member of the proteinase K subfamily of subtilases. Missense mutations in PCSK9 cause an autosomal dominant form of hypercholesterolemia in humans, likely due to a gain-of-function mechanism because overexpression of either WT or mutant PCSK9 reduces hepatic LDL receptor protein (LDLR) in mice. Here, we show that livers of knockout mice lacking PCSK9 manifest increased LDLR protein but not mRNA. Increased LDLR protein led to increased clearance of circulating lipoproteins and decreased plasma cholesterol levels (46 mg/dl in _Pcsk9_–/– mice versus 96 mg/dl in WT mice). Statins, a class of drugs that inhibit cholesterol synthesis, increase expression of sterol regulatory element-binding protein-2 (SREBP-2), a transcription factor that activates both the Ldlr and Pcsk9 genes. Statin administration to _Pcsk9_–/– mice produced an exaggerated increase in LDLRs in liver and enhanced LDL clearance from plasma. These data demonstrate that PCSK9 regulates the amount of LDLR protein in liver and suggest that inhibitors of PCSK9 may act synergistically with statins to enhance LDLRs and reduce plasma cholesterol.
Keywords: low-density lipoprotein receptor, lipoproteins, proteinase, sterol regulatory element-binding protein
The activity of the low-density lipoprotein receptor (LDLR) in liver is the major determinant of plasma LDL cholesterol concentrations (1, 2). Transcription of the LDLR is regulated by sterol regulatory element-binding protein-2 (SREBP-2), one of three SREBP family members that regulate the expression of many enzymes involved in cholesterol and fatty acid synthesis (3, 4). When hepatocellular sterols are low, SREBP-2 is activated, which restores cholesterol to normal levels by simultaneously activating enzymes required for de novo cholesterol synthesis and by increasing cholesterol uptake from the plasma through enhanced expression of the LDLR (3).
Recent studies suggest that hepatic LDLRs also may be post-transcriptionally regulated by proprotein convertase subtilisin/kexin type 9a (PCSK9) (5–7). PCSK9 belongs to the proteinase K subfamily of subtilases, which are proteinases synthesized as soluble zymogens that subsequently undergo autocatalytic cleavage to active enzymes (8). Pcsk9 was identified as an SREBP-regulated gene in liver by using oligonucleotide arrays hybridized with RNA from livers of mice that either overexpressed or lacked SREBPs (4, 9). Pcsk9 was regulated in a manner similar to other SREBP-responsive genes involved in lipid homeostasis, suggesting that PCSK9 might also participate in lipid metabolism.
This suggestion was confirmed by the finding that missense mutations in PCSK9 are associated with an autosomal dominant form of hypercholesterolemia (10–12). The clinical phenotype of these subjects is indistinguishable from two other autosomal dominant forms of hypercholesterolemia, both of which are caused by defective receptor-mediated clearance of LDL: (i) familial hyper-cholesterolemia, which is caused by mutations in the LDLR; and (ii) familial defective apolipoprotein B (apoB), caused by mutations in the ligand for the LDLR (13). This similarity raised the possibility that PCSK9 somehow lowers the amount or activity of LDLRs in liver.
This hypothesis was supported by the finding that overexpression of mutant forms of PCSK9 in mice significantly reduced LDLR protein in liver and raised plasma LDL (5–7). Overexpression of WT PCSK9 reduced hepatic LDLRs to a similar extent as expression of PCSK9 mutant forms (7). These studies suggested that PCSK9 might function normally to reduce LDLR expression levels in liver. If this hypothesis is correct, then the elimination of PCSK9 through targeted disruption of its gene should lead to an increase in LDLRs and a decrease in plasma LDL. To test this hypothesis, we deleted Pcsk9 in mice and characterized the effects on cholesterol metabolism.
Materials and Methods
DNA manipulations were performed by using standard molecular biology techniques (14). Cholesterol and triglyceride concentrations in plasma and liver were measured as described (15). Plasma lipoprotein fractions were separated by FPLC gel filtration by using a Superose 6 column. Measurements of cholesterol concentrations eluted from the FPLC fractions and Coomassie staining of plasma lipoproteins were performed as described (16). Protein concentrations were determined by using the BCA Protein Assay Reagent (Pierce). Other reagents were obtained from Sigma–Aldrich.
Construction of Targeting Vector for Disruption of Pcsk9. Mouse Pcsk9 was disrupted by using a gene-replacement vector that deleted the 3′ half of exon 2 through intron 4. Details of the gene-targeting vector construction are available upon request.
ES Cell Culture for Disruption of Pcsk9. Passage 11 SM-1 ES cells were electroporated with the Pcsk9 targeting vector as described (17). Recombined clones were identified by PCR using primers P1 (5′-GCT TCT GAG GCG GAA AGA ACC AGC-3′) from the 5′ coding region of the neo gene and P2 (5′-TCA TCA TCC AAT GGG TGG GCC TGA AG-3′) from the promoter of Pcsk9 located outside of the targeting vector. The targeted allele produced a 1.1-kb PCR product. Targeted clones were confirmed by Southern blot analysis using a 0.35-kb DNA probe from the Pcsk9 promoter region (Fig. 8, which is published as supporting information on the PNAS web site).
Generation of Pcsk9 Knockout Mice. Two targeted ES clones with a disrupted Pcsk9 allele were injected separately into C57BL/6J blastocysts, yielding chimeric males whose coat color (agouti) indicated a contribution of ES cells from 75% to 100%. Three chimeric males derived from each clone subsequently produced offspring that harbored the disrupted Pcsk9 allele. Mice carrying the disrupted allele were identified by Southern blotting or by PCR (Fig. 8).
Mice were housed in colony cages and maintained on a 12-h light/12-h dark cycle, fed Teklad Mouse/Rat Diet 7002 from Harlan Teklad Premier Laboratory Diets, and killed at the end of the dark cycle. All animal experiments were performed with the approval of the Institutional Animal Care and Research Advisory Committee at the University of Texas Southwestern Medical Center.
Real-Time RT-PCR. Total RNA was prepared from mouse livers by using an RNA STAT-60 kit (Tel-Test, Friendswood, TX). The primers for real-time PCR and details of PCR conditions were as described (17–19).
Antibodies and Immunoblot Analysis. The murine PCSK9 amino acid sequence was analyzed by using protean software (DNASTAR, Madison, WI), and two segments (amino acids 163–188 and amino acids 220–240) predicted to be immunogenic were synthesized by the Protein Chemistry Technology Center at the University of Texas Southwestern Medical Center. Peptides were conjugated to KLH by using the Imject Maleimide Activated mcKLH Kit (Pierce), and rabbits were immunized with a mixture of the peptides (20 μg each) as described (15). IgG fractions from preimmune and immune sera were purified by using the Immunopure (A/G) IgG purification kit (Pierce). The resulting antibody detected two proteins corresponding to proprotein (≈76 kDa) and cleaved (≈62 kDa) forms of PCSK9. Polyclonal antibodies directed against the mouse LDLR, LDL receptor-related protein (LRP), receptor-associated protein (RAP), autosomal recessive hypercholesterolemia (ARH), SREBP-1, and SREBP-2 were as described (7).
For immunoblot analysis, membranes (105 × g pellet) and nuclear extracts were prepared from mouse liver as described (15, 19). For whole-cell lysates, equal portions of liver were homogenized in buffer A (20 mM Tris·HCl, pH7.4/0.25 M sucrose/50 mM KCl/2 mM MgCl2/1 mM sodium EDTA/supplemented with 1 mM phenylmethanesulfonyl fluoride/1 mM 1,10-phenanthroline/50 μg/ml leupeptin/1 μg/ml pepstatin A/0.5 μg/ml aprotinin/25 μg/ml _N-_acetylleucylleucylnorleucinal). The homogenate was centrifuged at 103 × g for 15 s to pellet cellular debris. Protein concentrations were measured in the supernatant, and equal aliquots of protein were mixed with an equal volume of buffer B (62.5 mM Tris·HCl, pH 6.8/8 M urea/15% SDS (wt/vol)/10% glycerol (vol/vol)/100 mM DTT) and heated for 5 min at 95°C.
Samples were subjected to SDS/PAGE as described (15, 19). Immunoblot analyses were performed by using either a horseradish peroxidase-conjugated anti-rabbit secondary antibody from donkey (Amersham Pharmacia Biosciences) and SuperSignal West Pico Chemiluminescent Substrate System (Pierce), or a 125I-labeled secondary anti-rabbit antibody from donkey (Amersham Pharmacia Biosciences) (15).
Immunohistochemistry. Indirect immunofluorescence using antibodies against LDLR in livers of WT, _Ldlr_–/–, and _Pcsk9_–/– mice was carried out as described (20).
Plasma Clearance of [125I]LDL. Mouse LDL (density 1.019–1.063 g/ml) was prepared from the pooled plasma of 70 _Ldlr_–/– mice by sequential ultracentrifugation and radiolabeled with sodium 125I (16). Clearance of 125I-labeled apoB (apoB48 plus apoB100) from plasma of WT and _Pcsk9_–/– mice was measured at the indicated times by isopropanol precipitation (16, 17).
ApoB Secretion from Primary Hepatocytes. Hepatocytes were isolated as described (7) and cultured in Methionine/Cysteine-free DMEM (Sigma) supplemented with 5% lipoprotein-deficient newborn calf serum, 4 mM l-glutamine, 100 units/ml penicillin G sodium, and 100 μg/ml streptomycin sulfate (medium A) for 2 h. The hepatocytes were pulse-labeled with 250 μCi/ml (1 Ci = 37 GBq) [35S]Methionine/Cysteine (Redivue PRO-MIX [35S], Amersham Pharmacia Biosciences) in medium A without serum for 30 min. Thereafter, hepatocytes were washed twice with PBS, and the medium was changed to DMEM supplemented with 10 mM l-methionine, 10 mM l-cysteine, 5% lipoprotein-deficient newborn calf serum, 100 units/ml penicillin G sodium, and 100 μg/ml streptomycin sulfate. The cells were incubated for the indicated times, after which the medium and cells were harvested as described (7).
ApoB48 and apoB100 were immunoprecipitated and separated on a 4% SDS/PAGE gel (7). The gels were dried and exposed to a PhosphorImager plate, and the resulting signals were quantified by using a PhosphorImager Molecular Dynamics Storm 820 system (Amersham Pharmacia Biosciences). ApoB secreted from the hepatocytes was calculated as the percentage of apoB initially synthesized during the pulse-labeling period.
Diet Studies. Mice were fed ad libitum a cereal-based powdered diet (Teklad Mouse/Rat Diet 7001) or the powdered diet supplemented with 0.2% (wt/wt) lovastatin (Merck & Co.) for 7 days.
Results
The strategy used to disrupt Pcsk9 in mice is shown in Fig. 8. A targeting vector containing the neomycin-resistance gene was used to replace the C-terminal half of exon 2, exon 3, and exon 4. Northern blotting and immunoblot analysis confirmed that this deletion eliminated detectable PCSK9 mRNA and protein (Fig. 8).
Matings between Pcsk9+/– mice produced offspring in the expected Mendelian 1:2:1 ratio. _Pcsk9_–/– mice were normal in appearance. Body and liver weights were not significantly different from littermate WT mice (Table 1). No significant differences were found when the hepatic cholesterol and triglyceride concentrations were compared with WT littermates. Plasma cholesterol levels in the _Pcsk9_–/– mice were 48% lower than those of WT mice, despite similar levels of plasma triglycerides.
Table 1. Phenotypic comparison of wild-type and _Pcsk9_–/– mice.
| Parameter | WT | _Pcsk9_-/- |
|---|---|---|
| No. of mice | 4 | 4 |
| Body weight, g | 25.5 ± 0.6 | 30.0 ± 1.6 |
| Liver cholesterol, mg/g | 2.20 ± 0.16 | 2.00 ± 0.02 |
| Liver TG, mg/g | 9.2 ± 0.6 | 7.2 ± 0.7 |
| Plasma cholesterol, mg/dl | 95.7 ± 9.4 | 46.3 ± 1.9* |
| Plasma TG, mg/dl | 70.0 ± 11 | 85.8 ± 7.5 |
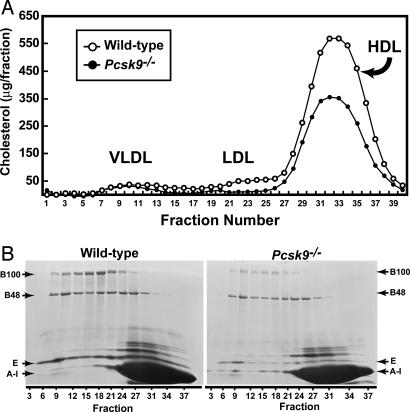
Lipoproteins from pooled plasma of 16 WT and 16 _Pcsk9_–/– male mice were isolated by ultracentrifugation and fractionated by FPLC (Fig. 1_A_). Normal mouse plasma contains very little apoB-containing lipoproteins [very low-density lipoprotein (VLDL) or LDL]; the majority of circulating cholesterol in mice is associated with high-density lipoprotein (HDL). In plasma from _Pcsk9_–/– mice, the level of LDL cholesterol was even further reduced so that it was nearly undetectable. The level of HDL cholesterol was reduced by ≈30%. Mouse HDL contains apoE, which allows it to bind to LDLRs. Fig. 1_B_ shows the protein composition of the lipoprotein fractions resolved by FPLC. As expected from the reduction in LDL cholesterol, the plasma level of apoB100 was clearly lower in the _Pcsk9_–/– mice and the level of apoB48 was also diminished. There also was a marked reduction in the apoE content of VLDL and HDL fractions.
Fig. 1.
FPLC profiles and SDS/PAGE of plasma apolipoproteins from WT and _Pcsk9_–/– mice. (A) Plasma from 16 WT and 16 _Pcsk9_–/– male mice was pooled (6.5 ml for each genotype) and subjected to ultracentrifugation at d = 1.215 g/ml. The lipoprotein fractions were separated by FPLC gel filtration, and the cholesterol content of each fraction was measured (16). (B) SDS/PAGE of plasma apolipoproteins from WT and _Pcsk9_–/– mice. Equal aliquots (0.5 ml) from three consecutive FPLC fractions were pooled and delipidated, and apoproteins were precipitated (16). Apoproteins were subjected to 3–15% gradient SDS/PAGE and stained with Coomassie blue. The positions of migration of apoB100, apoB48, apoE, and apoAI are indicated.
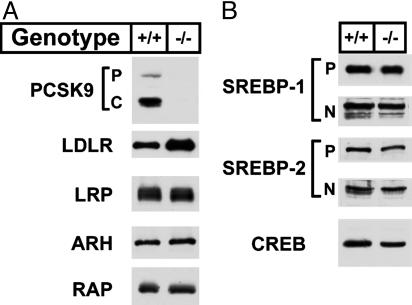
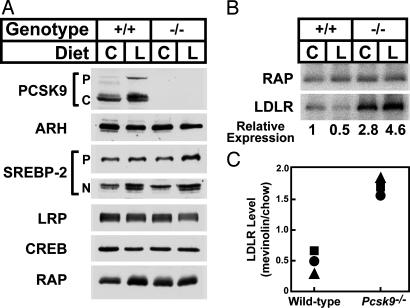
The reduction in apoE- and apoB-containing lipoproteins suggested that less VLDL was secreted from liver or else these particles were cleared faster from the circulation in the _Pcsk9_–/– mice. The major route of clearance of apoE- and apoB-containing lipoproteins is by means of LDLR-mediated endocytosis in the liver (2). Fig. 2 shows the levels of LDLR and other proteins in pooled livers from four _Pcsk9_–/– and four WT littermate controls as determined by immunoblotting. As expected, no PCSK9 protein was detected in the livers of _Pcsk9_–/– mice when a polyclonal antibody directed against the mouse protein was used for immunoblotting (Fig. 2_A_). The level of hepatic LDLR protein was ≈2.8-fold higher in the _Pcsk9_–/– mice than in the WT mice as determined by densitometric scanning of the autoradiogram. No changes were found in the amount of LRP, a member of the LDLR family (21), and ARH, an adaptor protein involved in hepatic LDLR internalization (20), or in SREBP-1 or SREBP-2, the transcriptional regulators of the Ldlr and lipid biosynthetic enzymes (Fig. 2_B_). Similarly, no changes in protein levels were measured for the VLDL receptor, another member of the LDLR family, or scavenger receptor class B type I (SR-BI), a receptor involved in HDL clearance (data not shown).
Fig. 2.
Levels of proteins in livers of WT and _Pcsk9_–/– mice. Livers from mice described in Table 1 were pooled, and aliquots of membrane protein (40 μg), whole cell lysate (30 μg), or nuclear protein (30 μg) were subjected to SDS/PAGE (7). (A) Immunoblot analyses of PCSK9, ARH (whole cell lysate), LDLR, LRP, and RAP (membrane fraction). P and C for PCSK9 denote the proprotein and cleaved forms of PCSK9, respectively. (B) Immunoblot analysis of SREBP-1, SREBP-2 (membrane and nuclear fractions), and cAMP response element binding protein (CREB) (nuclear fraction). P and N denote the precursor and cleaved nuclear forms of SREBP-1 and SREBP-2. Similar results were obtained in four independent experiments.
To determine whether the increase in LDLR was secondary to an increase in the level of mRNA encoding the LDLR, we measured the level by quantitative RT-PCR. We found no changes in the mRNAs encoding the LDLR or several other proteins involved in cholesterol and fatty acid biosynthesis (see Table 3, which is published as supporting information on the PNAS web site).
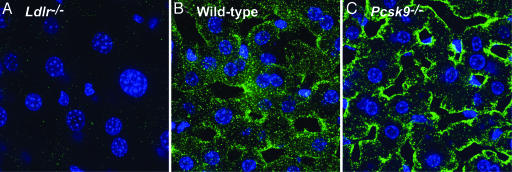
Indirect immunofluorescence confocal microscopy was used to confirm the increase in LDLRs on the surface of the hepatocytes from _Pcsk9_–/– mice (Fig. 3). The specificity of the polyclonal anti-LDLR antibody was confirmed by the absence of staining in a liver of an _Ldlr_–/– mouse (Fig. 3_A_) (22). A marked increase in staining for the LDLR was observed in hepatocytes from _Pcsk9_–/– mice compared with those from WT mice (compare Fig. 3 B and C).
Fig. 3.
Indirect immunofluorescence in liver using antibodies against LDLR. Frozen sections of liver from _Ldlr_–/– (A), WT (B), and _Pcsk9_–/– (C) mice were incubated with a polyclonal antibody against the LDLR. Bound IgG was detected with 20 μg/ml Alexa Fluor 488-labeled goat anti-rabbit IgG (20).
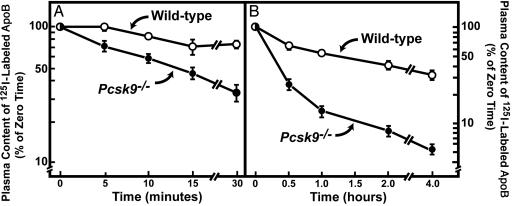
To measure the rate of LDL clearance from plasma, we isolated LDL from the blood of _Ldlr_–/– mice and labeled its apoB with 125I. Radiolabeled LDL was injected into two different groups of WT and _Pcsk9_–/– mice (six mice in each group). The first group was used to measure the rate of disappearance of 125I-labeled LDL during the first 30 min after injection (Fig. 4_A_), and the second group was used to measure the rate of LDL clearance at longer time points (Fig. 4_B_). The time required for clearance of ≈50% of the 125I-LDL was 12 min in _Pcsk9_–/– mice and >60 min in WT mice. We attribute this 5-fold increase in 125I-LDL clearance to the ≈3-fold increase in hepatic LDLRs in _Pcsk9_–/– mice.
Fig. 4.
Plasma clearance of 125I-labeled mouse LDL in WT and _Pcsk9_–/– mice. (A) Six male mice (10–12 weeks of age) of the indicated genotype were injected i.v. with 125I-labeled LDL (30 μg of protein, 294 cpm/ng apoB protein). Blood was obtained at 30 s (time 0) and at 5, 10, 15, and 30 min for quantification of plasma content of 125I-labeled total apoB (17). (B) Six male mice (10–12 weeks of age) of the indicated genotype were injected i.v. with the same 125I-labeled LDL used in A. Blood was obtained at 30 s (time 0) and at 0.5, 1, 2, and 4 h for quantification of plasma content of 125I-labeled total apoB. Data are plotted as the percentage of zero time value. Each value represents mean ± SEM of six mice.
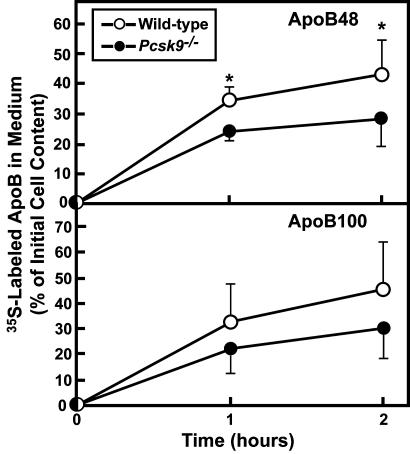
We next determined whether the absence of Pcsk9 altered the rate of apoB secretion from primary hepatocytes derived from WT and _Pcsk9_–/– mice. For this purpose, freshly isolated hepatocytes were incubated with [35S]methionine/cysteine. After a 30-min labeling period, the medium was switched to medium containing cold methionine/cysteine. ApoB100 and apoB48 were immunoprecipitated from cells and medium after 1 or 2 h, and the amount of labeled apoB was quantified by SDS/PAGE autoradiography. The deletion of Pcsk9 was associated with a slight reduction in the secretion of apoB48 (Fig. 5). ApoB100 secretion was not significantly different in hepatocytes from WT or _Pcsk9_–/– mice. Whether this reduction is due to a presecretory mechanism that degrades apoB or to recapture of secreted apoB owing to higher levels of LDLR protein expression in hepatocytes from _Pcsk9_–/– mice could not be determined in these experiments.
Fig. 5.
Rates of apoB secretion by primary hepatocytes from WT mice and _Pcsk9_–/– mice. Hepatocytes were prepared from mice of the indicated genotype, and apoB48 and apoB100 were immunoprecipitated and separated by SDS/PAGE gel electrophoresis as described under Materials and Methods. The data are expressed as the apoB content in the medium as a percentage of the 35S-labeled apoB in the cells at zero time. Each value is mean ± SEM of duplicate incubations from eight WT and eight _Pcsk9_–/– mice. *, Statistical difference of P < 0.05 (Student's t test).
The SREBP-mediated up-regulation of PCSK9 creates a potential problem for therapy with 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase inhibitors like lovastatin and other statins. These inhibitors block cholesterol synthesis and thereby cause an increase in cleaved SREBP-2. Nuclear SREBP-2 enhances the transcription of the LDLR gene, leading to increased LDLRs and a fall in plasma LDL. The elevated nuclear SREBP-2 also increases the mRNA for PCSK9, and this result would lead to a reduction in LDLR protein. If this scenario is correct, lovastatin should increase LDLRs to a greater extent in _Pcsk9_–/– mice than in WT mice.
To test this hypothesis, WT and knockout mice were fed normal chow or chow supplemented with 0.2% lovastatin. Table 2 contains pooled data from three independent experiments. Addition of 0.2% lovastatin to chow did not significantly reduce the mean plasma cholesterol in WT mice (71 mg/dl versus 81 mg/dl). As observed previously, the _Pcsk9_–/– mice maintained on chow had a plasma cholesterol level that was significantly lower than WT mice (51 mg/dl versus 81 mg/dl). Addition of lovastatin to the diet of knockout mice resulted in the plasma cholesterol level falling to 41 mg/dl; this value was significantly less than those measured in _Pcsk9_–/– mice fed chow. Plasma triglycerides were significantly reduced in mice from both genotypes fed lovastatin.
Table 2. Phenotypic comparison of wild-type and _Pcsk9_-/- mice fed 0.2% lovastatin.
| WT | Pcsk9 | |||
|---|---|---|---|---|
| Parameter | Chow | 0.2% lov. | Chow | 0.2% lov. |
| No. of mice | 12 | 12 | 12 | 12 |
| Body weight, g | 24.4 ± 1.0 | 26.4 ± 1.2 | 25.8 ± 1.0 | 24.7 ± 0.7 |
| Plasma cholesterol, mg/dl | 80.9 ± 5.1 | 71.1 ± 3.0 | 51.4 ± 2.3* | 40.8 ± 1.8*,** |
| Plasma TG, mg/dl | 94.4 ± 8.7 | 64.5 ± 4.6* | 100 ± 5.9 | 66.2 ± 4.2*,** |
Analysis of hepatic gene expression revealed that feeding the lovastatin-containing diet to WT and _Pcsk9_–/– mice resulted in increases in SREBP-2 mRNA levels and a corresponding increase in all measured SREBP target gene mRNA levels, including those encoding 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase, PCSK9, and LDLR (Table 4, which is published as supporting information on the PNAS web site). The fold-increase of cholesterol biosynthetic gene mRNAs in livers of lovastatin-treated _Pcsk9_–/– mice was similar to that measured in WT mice fed the same diet.
Lovastatin administration to WT mice led to an increase in PCSK9 protein in liver (Fig. 6_A_). Lovastatin also resulted in the predicted increase in nuclear SREBP-2 protein levels in WT and _Pcsk9_–/– mice. To more accurately quantify the changes in levels of LDLR and RAP, a 125I-labeled secondary antibody was used to generate the data shown in Fig. 6_B_. LDLR protein levels in livers of WT mice fed lovastatin were consistently lower than chow-fed mice despite the measured increase in LDLR mRNA expression. The expression of the LDLR was 2.8-fold higher in livers of chow-fed _Pcsk9_–/– mice compared with the WT mice and was further increased after administration of lovastatin to a level 4.6-fold higher than chow-fed WT mice. Fig. 6_C_ shows the relative amount of LDLR protein in livers of WT and _Pcsk9_–/– mice administered lovastatin from three independent experiments. In all experiments, lovastatin administration resulted in a significantly greater increase in LDLR expression in the absence of PCSK9.
Fig. 6.
Levels of proteins in livers of WT and _Pcsk9_–/– mice fed chow (C) or chow supplemented with 0.2% lovastatin (L). Livers from four male mice in the groups of Table 2 were pooled, and aliquots of membrane protein (40 μg), whole cell lysate (30 μg), or nuclear protein (30 μg) were subjected to SDS/PAGE. (A) Immunoblot analysis of PCSK9, ARH (whole cell lysate), SREBP-2 (membrane and nuclear fractions), cAMP response element binding protein (CREB) (nuclear fraction), LRP, and RAP (membrane fraction). P and C for PCSK9 denote the proprotein and cleaved forms of PCSK9. For SREBP-2, P and N denote the precursor and cleaved nuclear forms. (B) Immunoblot analyses of LDLR and RAP. A 125I-labeled secondary anti-rabbit antibody from donkey was used for the LDLR and RAP to quantify the expression of the LDLR by using a PhosphorImager. The relative expression of the LDLR protein is normalized to the amount of LDLR expressed in livers of WT mice fed chow. (C) Relative amount of hepatic LDLR protein in WT and _Pcsk9_–/– mice fed 0.2% lovastatin versus chow. Each symbol represents an independent experiment with four mice per group.
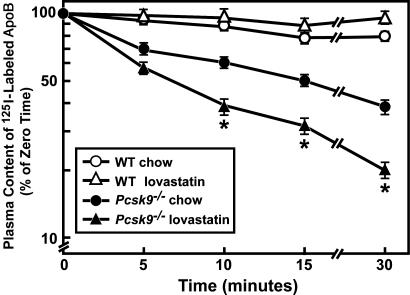
To determine whether lovastatin increased LDL clearance, radiolabeled LDL was injected into WT and _Pcsk9_–/– mice fed chow or lovastatin (eight mice per group) (Fig. 7). Lovastatin administration to WT mice did not significantly alter LDL clearance from the plasma after only 30 min. _Pcsk9_–/– mice again displayed a marked increase in the rate of LDL clearance from plasma compared with that of WT mice. Lovastatin administration to _Pcsk9_–/– mice resulted in an additional ≈2-fold increase in the rate of labeled LDL clearance from the plasma compared with chow-fed _Pcsk9_–/– mice. This change was similar in magnitude to the change measured in LDLR protein levels in chow versus lovastatin-fed _Pcsk9_–/–mice (Fig. 6_B_).
Fig. 7.
Plasma clearance of 125I-labeled mouse LDL in WT and _Pcsk9_–/– mice fed chow or lovastatin. Eight male mice (10–12 weeks of age) of the indicated genotype were fed chow or chow supplemented with 0.2% lovastatin for 7 days. Plasma clearance of 125I-labeled total apoB was determined as described in the legend of Fig. 4. *, Statistical difference of P < 0.05 (Student's t test) between _Pcsk9_–/– mice fed chow or lovastatin.
Discussion
The current study demonstrates that the inactivation of Pcsk9 reduces plasma cholesterol levels primarily by increasing LDLR protein expression in liver and accelerating the clearance of circulating cholesterol. These results, together with the previous observation that overexpression of WT PCSK9 in mice leads to elevated plasma LDL cholesterol levels by reducing the LDLR protein in liver (7), indicate that one function of PCSK9 is to negatively regulate LDLR protein levels. No changes were detected in SREBP-2 protein or in the mRNAs of genes encoding cholesterol synthesis enzymes in livers of _Pcsk9_–/– mice, which suggests that the reduction in plasma cholesterol levels was not due to decreased cholesterol synthesis. A small reduction in apoB48 secretion was detected in primary hepatocytes derived from _Pcsk9_–/– mice; however, the marked increase in plasma LDL clearance measured in the _Pcsk9_–/– mice suggests that the primary mechanism responsible for lower plasma cholesterol levels is increased LDLR expression.
3-Hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase inhibitors function by reducing cellular cholesterol levels, which activates SREBP-2, leading to the transcriptional activation of the LDLR. Inasmuch as SREBP-2 also activates Pcsk9, the increase in PCSK9 expression may normally attenuate the increase in LDLR expression in animals administered statins. The data of Fig. 6 are consistent with this interpretation. The administration of lovastatin to WT mice increased nuclear SREBP-2 protein and the mRNAs of both the LDLR and PCSK9 (Table 4). Despite the transcriptional activation of the Ldlr, total hepatic LDLR protein was slightly lower than WT mice fed chow, and the plasma cholesterol levels were not statistically reduced (Table 2 and Fig. 6). One reason for the apparent paradoxical response seems to be the simultaneous induction of PCSK9, which posttranscriptionally reduces LDLR protein levels. This interpretation is supported by the findings that _Pcsk9_–/– mice administered lovastatin had higher LDLR protein levels, increased LDL clearance from plasma, and lower plasma cholesterol levels than _Pcsk9_–/– mice fed chow. These results suggest that inhibitors of PCSK9 may have beneficial effects on plasma cholesterol levels, especially when combined with statins.
Our findings in mice are consistent with those of Ness et al. (23), who previously showed that statins administered to rats increased the LDLR mRNA levels but not LDLR protein levels in liver. The lack of an increase in LDLR protein was attributed to increased LDLR degradation in these studies. It is likely that a statin-induced increase in PCSK9 expression also was responsible for their results. Lovastatin administration did reduce plasma triglycerides in WT mice in the absence of a detectable increase in LDLR protein or LDL clearance from plasma. A reduction in VLDL production by statins probably accounts for this observation.
Studies investigating the role of PCSK9 in regulating apoB secretion in liver have resulted in conflicting results. Two studies showed that apoB secretion is unaltered by PCSK9 overexpression (5, 7), whereas another reported that PCSK9 overexpression increases apoB secretion from the livers of mice (6). In the absence of PCSK9, it seems that there is reduced apoB48 secretion from mouse primary hepatocytes. The increase in LDLR protein expression resulting from the deletion of Pcsk9 could explain this observation by means of the mechanism described by Twisk et al. (24), who previously demonstrated that the LDLR protein can mediate posttranslational presecretory degradation of apoB in hepatocytes. A second possibility is that increased LDLR expression on the cell surface mediates immediate VLDL recapture, thus reducing total apoB secretion into the medium. Additional studies will be required to clarify the operative mechanism.
The current study confirms that the LDLR is regulated both at the transcriptional and posttranscriptional levels. SREBP-2 transcriptionally activates Pcsk9 and Ldlr and thus promotes both the synthesis and destruction of the LDLR. The biological reason why SREBP-2 up-regulates both Ldlr and Pcsk9 still cannot be easily reconciled with the current information. However, it is possible that the simultaneous transcriptional activation of Pcsk9 and Ldlr by SREBP-2 may provide a posttranscriptional mechanism to degrade the LDLR and shorten the protein's half-life, which could protect the cell from excessive LDL uptake and cholesterol accumulation (7).
Several important questions regarding the function of PCSK9 remain unanswered. First, the substrate(s) of PCSK9 has not been defined, and whether this protease directly cleaves the LDLR, or acts indirectly by destroying another protein, remains to be determined. Second, although recent studies indicate that PCSK9 mediates the degradation of the LDLR in a post-ER compartment (25), it is not known whether this degradation occurs intracellularly or after the LDLR reaches the cell surface. Third, the importance of PCSK9-mediated regulation of LDLR expression in tissues other than liver remains to be determined. PCSK9 is expressed at low levels in all tissues tested (8). The liver normally accounts for 65–70% of all LDL-cholesterol clearance in mice (2). Most tissues other than liver and the adrenal gland have low LDLR activity and presumably low LDLR protein levels (2). In experiments not shown, levels of LDLR protein in the adrenal gland were higher in _Pcsk9_–/– mice, suggesting that PCSK9 also may regulate the expression of LDLR in extrahepatic tissues. Further delineation of each tissue's contribution to plasma LDL-cholesterol clearance in _Pcsk9_–/– mice will require more quantitative LDL uptake studies.
The results reported here are consistent with the findings of Cohen et al. (26), who found that individuals heterozygous for a nonsense mutation in PCSK9 had a 40% reduction in LDL cholesterol levels. Individuals with loss-of-function mutations in PCSK9 were heterozygous for the mutant allele. Heterozygous Pcsk9+/– mice had a phenotype that was intermediate between homozygous _Pcsk9_–/– mice and WT mice in terms of plasma cholesterol concentrations and LDL clearance, which is consistent with a gene-dosage effect. The phenotypes observed in humans with mutations in PCSK9, combined with results of the current studies in mice, suggest that variations in the levels of PCSK9 significantly affect plasma cholesterol levels and that inhibitors of PCSK9 may be useful for the treatment of hypercholesterolemia.
Supplementary Material
Supporting Information
Acknowledgments
We thank Jonathan Cohen, Helen H. Hobbs, David W. Russell, Michael S. Brown, and Joseph L. Goldstein for critical reading of the manuscript. Tuyet Dang, Scott Clark, Amy Cox, Richard Gibson, Anh Pho, and Judy Sanchez provided excellent technical assistance. This work was supported by grants from the Perot Family Foundation and by National Institutes of Health Grants HL-20948 and HL-38049. D.E.C. is supported by the University of Texas Southwestern Physician Scientist Training Program.
Author contributions: S.R., D.E.C., R.G., N.N.A., Y.B., Y.-A.M., and J.D.H. designed research; S.R., D.E.C., R.G., N.N.A., Y.B., Y.-A.M., and J.D.H. performed research; S.R., Y.-A.M., and J.D.H. analyzed data; Y.K.H. and R.E.H. contributed new reagents/analytic tools; and S.R. and J.D.H. wrote the paper.
Abbreviations: ARH, autosomal recessive hypercholesterolemia; Apo, apolipoprotein; LDLR, low-density lipoprotein receptor; LRP, LDLR-related protein; PCSK9, proprotein convertase subtilisin/kexin type 9a; SREBP, sterol regulatory element-binding protein; RAP, receptor-associated protein; VLDL, very low-density lipoprotein; HDL, high-density lipoprotein.
References
- 1.Goldstein, J. L. & Brown, M. S. (1977) Annu. Rev. Biochem. 46**,** 897–930. [DOI] [PubMed] [Google Scholar]
- 2.Dietschy, J. M., Turley, S. D. & Spady, D. K. (1993) J. Lipid Res. 34**,** 1637–1659. [PubMed] [Google Scholar]
- 3.Horton, J. D., Goldstein, J. L. & Brown, M. S. (2002) J. Clin. Invest. 109**,** 1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horton, J. D., Shah, N. A., Warrington, J. A., Anderson, N. N., Park, S. W., Brown, M. S. & Goldstein, J. L. (2003) Proc. Natl. Acad. Sci. USA 100**,** 12027–12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maxwell, K. N. & Breslow, J. L. (2004) Proc. Natl. Acad. Sci. USA 101**,** 7100–7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benjannet, S., Rhainds, D., Essalmani, R., Mayne, J., Wickham, L., Jin, W., Asselin, M. C., Hamelin, J., Varret, M., Allard, D., et al. (2004) J. Biol. Chem. 279**,** 48865–48875. [DOI] [PubMed] [Google Scholar]
- 7.Park, S. W., Moon, Y.-A. & Horton, J. D. (2004) J. Biol. Chem. 279**,** 50630–50638. [DOI] [PubMed] [Google Scholar]
- 8.Seidah, N. G., Benjannet, S., Wickham, L., Marcinkiewicz, J., Jasmin, S. B., Stifani, S., Basak, A., Prat, A. & Chretien, M. (2003) Proc. Natl. Acad. Sci. USA 100**,** 928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maxwell, K. N., Soccio, R. E., Duncan, E. M., Sehayek, E. & Breslow, J. L. (2003) J. Lipid Res. 44**,** 2109–2119. [DOI] [PubMed] [Google Scholar]
- 10.Abifadel, M., Varret, M., Rabès, J.-P., Ouguerram, K., Devillers, M., Cruaud, C., Benjannet, S., Wickham, L., Erlich, D., Villéger, L., et al. (2003) Nat. Genet. 34**,** 154–156. [DOI] [PubMed] [Google Scholar]
- 11.Leren, T. P. (2004) Clin. Genet. 65**,** 419–422. [DOI] [PubMed] [Google Scholar]
- 12.Timms, K. M., Wagner, S., Samuels, M. E., Forbey, K., Goldfine, H., Jammulapati, S., Skolnick, M. H., Hopkins, P. N., Hunt, S. C. & Shattuck, D. M. (2004) Hum. Genet. 114**,** 349–353. [DOI] [PubMed] [Google Scholar]
- 13.Rader, D. J., Cohen, J. & Hobbs, H. H. (2003) J. Clin. Invest. 111**,** 1795–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sambrook, J. & Russell, D. W. (2001) Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY).
- 15.Shimano, H., Horton, J. D., Hammer, R. E., Shimomura, I., Brown, M. S. & Goldstein, J. L. (1996) J. Clin. Invest. 98**,** 1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horton, J. D., Shimano, H., Hamilton, R. L., Brown, M. S. & Goldstein, J. L. (1999) J. Clin. Invest. 103**,** 1067–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang, J., Goldstein, J. L., Hammer, R. E., Moon, Y.-A., Brown, M. S. & Horton, J. D. (2001) Proc. Natl. Acad. Sci. USA 98**,** 13607–13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang, G., Yang, J., Horton, J. D., Hammer, R. E., Goldstein, J. L. & Brown, M. S. (2002) J. Biol. Chem. 277**,** 9520–9528. [DOI] [PubMed] [Google Scholar]
- 19.Engelking, L. J., Kuriyama, H., Hammer, R. E., Horton, J. D., Brown, M. S., Goldstein, J. L. & Liang, G. (2004) J. Clin. Invest. 113**,** 1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones, C., Hammer, R. E., Li, W.-P., Cohen, J. C., Hobbs, H. H. & Herz, J. (2003) J. Biol. Chem. 278**,** 29024–29030. [DOI] [PubMed] [Google Scholar]
- 21.Herz, J. (2001) Neuron 29**,** 571–581. [DOI] [PubMed] [Google Scholar]
- 22.Ishibashi, S., Brown, M. S., Goldstein, J. L., Gerard, R. D., Hammer, R. E. & Herz, J. (1993) J. Clin. Invest. 92**,** 883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ness, G. C., Zhao, Z. & Lopez, D. (1996) Arch. Biochem. Biophys. 325**,** 242–248. [DOI] [PubMed] [Google Scholar]
- 24.Twisk, J., Gillian-Daniel, D. L., Tebon, A., Wang, L., Barrett, P. H. & Attie, A. D. (2000) J. Clin. Invest. 105**,** 521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maxwell, K. N., Fisher, E. A. & Breslow, J. L. (2005) Proc. Natl. Acad. Sci. USA 102**,** 2069–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen, J., Pertsemlidis, A., Kotowski, I. K., Graham, R., Garcia, C. K. & Hobbs, H. H. (2005) Nat. Genet. 37**,** 161–165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information