Variable β-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment (original) (raw)
Abstract
Invasion and dissemination of well-differentiated carcinomas are often associated with loss of epithelial differentiation and gain of mesenchyme-like capabilities of the tumor cells at the invasive front. However, when comparing central areas of primary colorectal carcinomas and corresponding metastases, we again found the same differentiated epithelial growth patterns. These characteristic phenotypic changes were associated with distinct expression patterns of β-catenin, the main oncogenic protein in colorectal carcinomas, and E-cadherin. Nuclear β-catenin was found in dedifferentiated mesenchyme-like tumor cells at the invasive front, but strikingly, as in central areas of the primary tumors, was localized to the membrane and cytoplasm in polarized epithelial tumor cells in the metastases. This expression pattern was accompanied by changes in E-cadherin expression and proliferative activity. On the basis of these data, we postulate that an important driving force for progression of well-differentiated colorectal carcinomas is the specific environment, initiating two transient phenotypic transition processes by modulating intracellular β-catenin distribution in tumor cells.
The hallmarks of malignant transformation are the capabilities of invasion and metastasis. For these processes to proceed, tumor cells must be able to detach, migrate, gain access to blood or lymphatic vessels, and disseminate in the body (1). These traits are normally not associated with differentiated epithelial cells. However, well-differentiated adenocarcinomas, like many common colon carcinomas, grow in tubular structures and retain an epithelial phenotype; nevertheless, they can metastasize. We postulated that loss of an epithelial and gain of a dedifferentiated mesenchyme-like phenotype of the tumor cells at the invasive front enable these differentiated tumors to develop invasive and metastatic growth characteristics (2).
Determinants of an epithelial phenotype are homophilic cell adhesions and polarity. Both are mediated by cell surface expression of E-cadherin (3). Loss of functional E-cadherin and acquisition of other marker proteins like fibronectin or vimentin can indicate a loss of the epithelial phenotype and a switch toward a more mesenchymal dedifferentiated phenotype (4, 5). β-Catenin is bound to membrane-associated E-cadherin and essential for its correct positioning and function (3). Loss-of-function mutations in the adenomatosis polyposis coli (APC) tumor suppressor gene are the initial genetic alteration in most colon cancers and lead to a cytoplasmic and subsequent nuclear accumulation of β-catenin (6, 7). Alternatively, in some cases, cellular accumulation of β-catenin is acquired because of stabilizing mutations in the β-catenin gene (7). Functioning in the nucleus together with DNA-binding proteins of the T cell factor (TCF) family, β-catenin becomes a transcriptional activator and thus assumes the role of a main oncoprotein in colorectal carcinogenesis (8). Not only target genes like c-myc (9) and cyclin D1 (10) but also genes necessary for invasive growth, like matrilysin (11, 12), fibronectin (13), CD44 (14), and uPAR (15), are activated by nuclear β-catenin. Thus the distinct intracellular distribution of β-catenin has a strong impact on the phenotype and behavior of the tumor cell. We have previously described a nuclear accumulation of β-catenin in dedifferentiated mesenchyme-like tumor cells found at the invasive front a membranous and cytoplasmic expression in central organized areas of colorectal adenocarcinomas, indicating localized regulation of biochemical and morphological attributes of tumor cells (2, 16).
The linear model of tumor progression assumes that the mesenchyme-like capabilities necessary for metastasis formation—including dissociation, migration, and dissemination—are acquired and fixed by genetic alterations of the tumor cells as late steps in carcinogenesis (17). However, a common observation by pathologists is that metastases of colorectal adenocarcinomas often completely resemble the primary tumor and exhibit organized epithelial and tubular structures, despite the obvious phenotypic switch at the invasive front. Such observations suggest that once metastasis has occurred, tumor cells are able to regain the epithelial phenotype of their primary tumors. Consequently, dedifferentiated phenotypes, which such cells demonstrated during the processes of invasion and dissemination, may not be fixed irrevocably by genetic alterations in the genomes of these cells.
To find evidence for ongoing phenotypic transition processes and their potential regulatory forces during metastasis formation, we analyzed the expression patterns of β-catenin and E-cadherin in the primary tumors, invasive fronts, and lymph node metastases of well-differentiated colorectal adenocarcinomas and linked them to growth patterns and proliferative activity.
Materials and Methods
Tissue Specimens.
Formalin-fixed paraffin-embedded tissues from patients who underwent surgery without additional treatment were retrieved from the archive of the Institute for Pathology, University of Erlangen–Nürnberg. Only well-differentiated colorectal adenocarcinomas with lymph node metastases were included, irrespective of other criteria. Poorly differentiated or anaplastic carcinomas, showing no clearly epithelial phenotype in the primary tumor, were excluded from this study.
Immunohistochemistry.
CK18, β-catenin, E-cadherin, and Ki-67 were stained as described (2). In addition, a rabbit anti-β-catenin antiserum (1:750, Sigma) and a mouse monoclonal antibody against vimentin (clone V9, Dako) were used.
Genetic Analysis of Tumors.
Microdissection of normal and tumor cells from formalin-fixed paraffin-embedded tissues, DNA isolation, and sequencing of exon 3 of the β-catenin genes were done as previously described (18). Loss of one APC gene allele was determined by loss of heterozygosity analysis by using microsatellite markers (D5S346, D5S107, D5S489, and D5S656). Primer sequences used were 5′-AGC AGA TAA GAC AGT ATT ACT AGT T and 5′-ACT CAC TCT AGT GAT AAA TCG CG (D5S346), 5′-GGC ATC AAC TTG AAC AGC AT and 5′-GAT CCA CTT TAA CCC AAA TAC (D5S107), 5′-CAT AAT AAA CTG ATG TTG ACA CAC and 5′-GCT AAG AAA ATA CGA CAA CTA AAT G (D5S656), and 5′-ACC AGA CTT GTA TAT GTG TGT GT and 5′-TTC ACT TGT TGA TGG GCT (D5S489). PCR conditions were 35 cycles (20 sec 94°C, 20 sec 55°C, 30 sec 72°C); reaction conditions were 5 μl of isolated DNA, 2.5 mM MgCl2 in 25 μl of total volume. For sequence analysis of the mutation cluster region of the APC gene, we established a protocol of three overlapping nested PCRs encompassing codons 1275 and 1569 (S. Ruckert and A.J., unpublished work).
Confocal Laser Scanning Microscopy.
Subclones of SW480 and LS174T colon carcinoma cells (American Type Culture Collection**,** Rockville, MD) were grown at low or high density on glass coverslips in 24-well culture plates in DMEM/10% FCS. Specific staining was performed directly in the culture plates for 2 h after fixation with 2% paraformaldehyde. After washing, slides were incubated for 60 min with Cy3-coupled goat anti-mouse antiserum (red) (Jackson ImmunoResearch) and Alexa Flour 488-coupled goat anti-rabbit antiserum (green) (Molecular Probes). Confocal laser scanning microscopy was performed by using a Bio-Rad 1000 microscope.
Spheroid Invasion Assay.
Spheroid invasion assay was performed as described (19). Fibroblasts were derived from adult skin of a healthy donor. Tumor lines were SW480 and LS174T colon carcinoma and BT-474 breast carcinoma cells (American Type Culture Collection).
Results
Defining the Growth Patterns of Well-Differentiated Colorectal Carcinomas and Their Metastases.
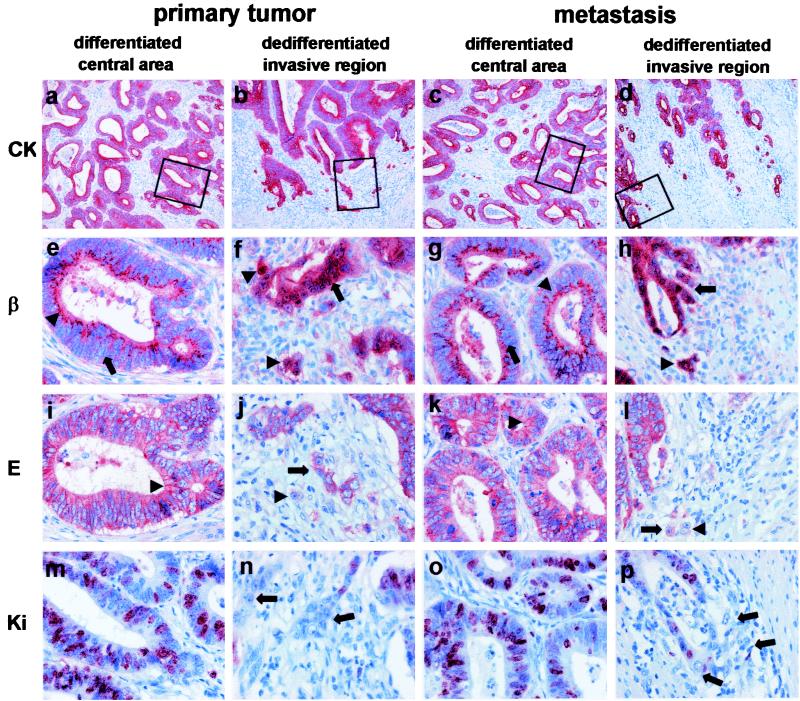
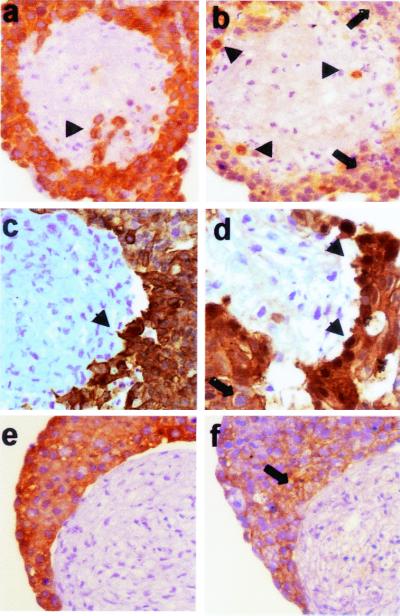
A comparison of the growth patterns was performed on cytokeratin 18 (CK 18)-stained sections of 72 cases. Strikingly, there was no difference in the grade of differentiation in the primary tumors and their corresponding lymph node metastases. Both showed an identical epithelial growth pattern with polarized tumor cells forming clear tubular structures in the central tumor areas (Fig. 1 a and c). At the invasive margin of both the primary tumor and the metastases, the tumor cells acquired a dedifferentiated phenotype, characterized by an opening of the tubules and detachment of small cell clusters, finally leading to isolated tumor cells (Fig. 1 b and d). These isolated cells lost their polar orientation, showed a fibroblastoid morphology, and sometimes weakly expressed vimentin (Figs. 2 and 3). Moreover, we previously described that these cells can express fibronectin (2), indicating a loss of epithelial and gain of more mesenchyme-like capabilities of tumor cells at the invasive front. Despite the loss of the epithelial phenotype at the invasive front, the epithelial differentiation state, characterized by polarized epithelial tumor cells performing tubuloneogenesis, was seen once again in the lymph node metastases (Fig. 1c). Hence the loss of epithelial morphology by the tumor cells appeared to be reversible, because they regained this phenotype after establishing themselves at distant sites.
Figure 1.
Correlation of growth and the expression patterns of β-catenin, E-cadherin, and Ki-67 in well-differentiated colorectal adenocarcinomas. Shown are central areas (first column) and invasive front (second column) of the primary tumor and central areas (third column) and invasive front (fourth column) of the corresponding metastasis. Stainings are for CK-18 (first row), β-catenin (second row), E-cadherin (third row), and Ki-67 (fourth row). Boxes indicate magnified regions in stained serial sections. Specific staining is red and nuclear counterstaining is blue [×100 (a_–_d) and ×400 (e_–_p)]. CK 18 stainings show a differentiated growth pattern with tubular structures in the centers of primary tumor (a) and metastasis (c) and loss of tubular growth and tumor cell dissemination in the corresponding invasive fronts (b and d). Tumor cells are clearly polarized in the differentiated central areas in both primary tumor and metastasis, and β-catenin is localized distinctly in the apical cytoplasm and membrane of the tumor cells (arrowheads) (e and g). Note that β-catenin is not detectable in the nuclei (arrows). In contrast, tubules at the invasive front break up, and tumor cells lose their polar orientation and dissociate (arrows) (f and h). This morphological change is accompanied by nuclear accumulation of β-catenin (arrows and arrowheads). Correspondingly, tumor cells in differentiated areas of the primary tumor express membranous E-cadherin (arrowheads) (i), which is also expressed in central areas of the metastasis (k). Disseminating tumor cells at the invasive fronts with nuclear β-catenin either completely lost E-cadherin (arrowheads) or showed a cytoplasmic expression (arrows) (j and l). A high Ki-67 ratio indicating strong proliferation is found only in differentiated tubular areas of primary tumors and metastases retaining an epithelial phenotype (m and o). Disseminating, dedifferentiated tumor cells with mesenchymal phenotype did not express Ki-67 (arrows) (n and p).
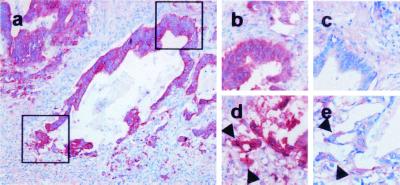
Figure 2.
Weak expression of vimentin in dedifferentiated tumor cells. CK 18 staining of a neoplastic tubulus in the invasive region of a colorectal carcinoma [×100 (a)]. Magnified region of the differentiated (upper square) or dedifferentiated area (lower square) stained in serial section against β-catenin (b and d) or vimentin (c and e). Note that dedifferentiated tumor cells with nuclear β-catenin (d, arrowheads) express vimentin weakly (e, arrowheads) but more strongly than cells in the more differentiated area (c).
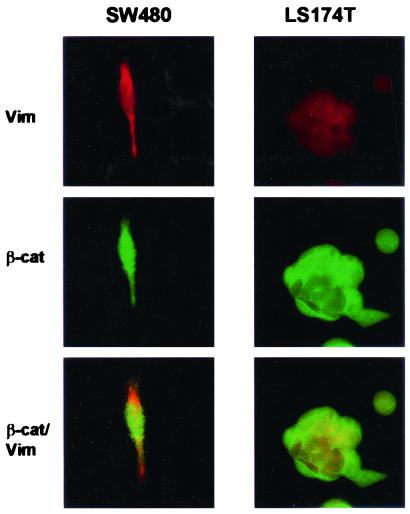
Figure 3.
Vimentin expression in SW480 and LS174T colon carcinoma cells. Confocal microscopy for expression of vimentin and β-catenin, indicating strong expression of vimentin in SW480 cells and weaker expression in LS174T cells, is shown.
Expression Patterns of β-Catenin and E-Cadherin in Primary Tumors and Metastases.
Next, we analyzed whether these histopathologic changes were accompanied by alterations in the expression patterns of the critical molecules β-catenin and E-cadherin. We found no nuclear β-catenin in the well-differentiated central tumor areas but a distinct membranous and cytoplasmic staining of the apical (luminal) third of the tumor cell (Fig. 1e). This pattern was indistinguishable from that of normal colonic epithelium (not shown). In contrast, at the invasive front, the expression of β-catenin changed toward a strong nuclear staining accompanied occasionally by diffuse cytoplasmic expression (Fig. 1f). This expression pattern persisted in disseminated isolated tumor cells at the metastatic site (not shown). Unexpectedly, the membranous and cytoplasmic expression pattern seen the primary tumor was also present in the central areas of the metastases (Fig. 1g). Thus, we saw an identical lack of nuclear β-catenin expression in the central areas of primary tumors and their metastases (compare Fig. 1 e and g) and strong nuclear β-catenin expression at the invasive fronts of both primary tumors and metastases (compare Fig. 1 f and h). These changes in the β-catenin localization were detected in 64 of the 72 (89%) analyzed well-differentiated colon carcinomas.
Retention of membranous E-cadherin expression was found in the central areas of the primary tumors in 60% of the analyzed cases (Fig. 1i). In all of these cases, however, tumor cells at the invasive front, expressing nuclear β-catenin, had lost their membranous E-cadherin (Fig. 1j). Some cells showed cytoplasmic E-cadherin, whereas others showed no E-cadherin at all. This staining pattern could also be demonstrated for the invasive front of the metastases (Fig. 1l). However, for E-cadherin positive primary tumors, membranous E-cadherin expression was detectable again in the central areas of the derived metastases (compare Fig. 1 i and k). Thus, the characteristic phenotypical and morphological changes in the primary tumors and their invasive fronts and metastases described above are paralleled by distinct changes in the intracellular distribution patterns of β-catenin and E-cadherin.
To determine whether APC gene mutations are absolutely necessary for the observed intratumorous switches in the phenotype and expression patterns of β-catenin and E-cadherin, we analyzed the APC and β-catenin gene status. By loss of heterozygosity analysis of microdissected tumor areas, we found a deletion of one APC-allele in 11 of 21 analyzed cases. In four of these cases, the exact mutation in the remaining APC allele could be determined by sequencing (truncating mutations in codons 1328, 1356, 1365, and 1512). Two cases had no detectable APC gene alteration but did have a stabilizing mutation in β-catenin gene codon 45. The rest of the analyzed cases (8 of 21) were not informative for APC. Thus, although most cases had inactivating mutations in the APC gene, the described abilities of these tumors do not necessarily depend on genetic alterations in this gene, because two cases showed oncogenic β-catenin gene mutations.
Proliferative Activity in Primary Tumors and Metastases.
To correlate proliferation with the above-defined growth patterns and expressions of β-catenin and E-cadherin, we used Ki-67 as proliferation marker. High numbers of Ki-67-expressing cells were found in the central areas of the primary tumors and metastases with epithelial growth pattern and membranous expression of E-cadherin and β-catenin (Fig. 1 m and o). However, tumor cells in dissociating tubules and isolated tumor cells at the invasive fronts lost Ki-67 expression in all investigated cases (Fig. 1 n and p). Thus dedifferentiation with acquisition of mesenchyme-like abilities during tumor cell dissemination is associated with a shutdown of proliferative activity. Moreover, only disseminated tumor cells showing an epithelial phenotype seem to proliferate again. Hence the loss of epithelial phenotype by tumor cells was accompanied by a loss of proliferative capacity.
Regulated Expression of Nuclear β-Catenin in Colon Cancer Cells.
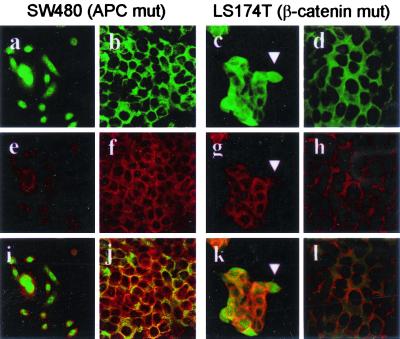
Our observations suggest that phenotypic transitions and heterogenous expression patterns of β-catenin within a tumor mass, which are occurring at later stages of tumor progression, can be explained only by additional factors that further modulate the consequences arising after genetic alterations in the APC, or more rarely in the β-catenin, gene. To prove this, we analyzed colon carcinomas cells with either a truncating APC mutation at codon 1338 and a complete loss of the second allele (SW480) or a stabilizing β-catenin mutation at codon 45 (LS174T) (20). At low density, SW480 cells grow in a fibroblastoid phenotype with protruding lamellipodia, resembling the mesenchyme-like growth of colon carcinoma cells at the invasive front. Thereby, SW480 cells strongly express nuclear β-catenin and perinuclear cytoplasmic E-cadherin (Fig. 4 a, e, and i). With increasing density after 3–4 days in culture, SW480 cells switched to an epithelial phenotype, associated with a translocation of β-catenin from the nucleus to the cytoplasm and membrane and a membranous colocalization of E-cadherin (Fig. 4 b, f, and j). Similar, albeit weaker, transitional behavior was detected with β-catenin-mutant line LS174T. Despite a more pronounced epithelial phenotype, which was found very soon within 24 h in a subconfluent state, LS174T cells at the outer rim of cell cluster often showed nuclear β-catenin, cytoplasmic E-cadherin, and a mesenchyme-like phenotype (Fig. 4 c, g, and k). These data show that existing APC or β-catenin gene mutations do not force the tumor cells into a single phenotypic state but instead enable mutant cells to assume at least two phenotypic configurations similar to what is observed in the analyzed tumors.
Figure 4.
Switch in the expression patterns of β-catenin and E-cadherin in colon carcinoma cell lines. SW480 and LS174T cells are grown at low or high density. Confocal laser scanning microscopy showing staining for β-catenin (green, a–d), E-cadherin (red, e–h) and combination of both (i–f, yellow staining indicates a colocalization of both proteins). At low density, SW480 cells grow fibroblastoid with protruding lammelipodia. Note that β-catenin is nuclear and weakly cytoplasmic (a), E-cadherin shows a granular perinuclear distribution (e), and both proteins do not colocalize (i). With increasing density, SW480 cells acquire a more epithelial growth pattern. β-Catenin translocates from the nucleus to the cytoplasm and membrane (b) and E-cadherin from perinuclear region to the membrane and submembranous cytoplasm (f). Yellow staining (j) indicates a colocalization of β-catenin and E-cadherin at the membrane of the tumor cells. LS174T shows similar features, albeit more rapidly changing towards an epithelial phenotype. However, at low confluency, tumor cells at the rims of clusters show a more mesenchyme-like phenotype (arrowhead) with nuclear β-catenin (c) and cytoplasmic E-cadherin (g), which do not colocalize (k).
To further mimic the situation in colon cancer, we performed an in vitro invasion assay by cocultivating SW480 and LS174T cells with fibroblast spheroids. Most SW480 cells clustered around the fibroblast spheroids lacked nuclear β-catenin. However, some tumor cells in close proximity to the fibroblasts and those infiltrating the spheroids showed a nuclear accumulation of β-catenin (Fig. 5 a and b). LS174T was less infiltrative but showed a similar distribution of nuclear β-catenin in cells surrounding the fibroblasts (Fig. 5 c and d). A noninfiltrating control cell line expressed only membranous β-catenin (Fig. 5 e and f). These results support our findings in colorectal carcinomas, according to which the invasive phenotype is correlated with nuclear translocation of β-catenin. Moreover, the cellular model shows that nuclear β-catenin accumulation in colorectal cancer is not a consequence of mutated APC alone but can be modulated by external signals.
Figure 5.
Nuclear accumulation of β-catenin in SW480 cells infiltrating fibroblast spheroids. CK-18 (a, c, and e) and β-catenin (b, d, and f) staining of invasive SW480 cells (a and b), less invasive LS174T cells (c and d), and the noninvasive control cell line BT-474 (e and f). Specific staining is brown, and nuclear counter staining is blue. CK 18 staining marks tumor cells surrounding or infiltrating (arrowhead) the fibroblast spheroid (a and c). Note that infiltrating tumor cells accumulate nuclear β-catenin (arrowheads), whereas most surrounding clustered tumor cells lack nuclear β-catenin (arrows) (b and d). The control tumor line expressed not nuclear but only membranous β-catenin (arrow) (f).
Discussion
We analyzed the growth, proliferative activity, and expression patterns of β-catenin and E-cadherin in well-differentiated colorectal carcinomas and their metastases. Genetic analyses verified loss-of-function mutations in the APC gene in most cases and more rarely stabilizing mutations in the β-catenin gene. These tumors have an epithelial growth pattern characterized by adherent polarized tumor cells forming tubular structures. Only at the invasive front do tumor cells dissociate from tubules and gain the ability to disseminate by a process of dedifferentiation, resembling an epithelial–mesenchymal transition. However, a differentiated growth pattern with tubuloneogenesis is again found in derived metastases. These characteristic phenotypes and growth patterns are accompanied by a nuclear accumulation of β-catenin and a loss of plasma membrane-associated E-cadherin in cells found at the invasive fronts and the disseminated cells at the metastatic site. Expression of cytoplasmic and membranous β-catenin and E-cadherin is seen once again in the central differentiated areas of the metastases. Moreover, tumor cells with a dedifferentiated mesenchyme-like phenotype at the invasive front have lost the proliferative activity that is strongest in the differentiated areas of the primary tumor and metastases.
On the basis of these observations, we propose a model for the progression of well-differentiated tumors, which includes two phenotypic transition processes: an epithelial–mesenchymal transition-like dedifferentiation at the invasive front, favoring detachment, migration, and dissemination, and a subsequent redifferentiation with regain of epithelial capabilities. This regain of epithelial characteristics might be necessary for tumor cell proliferation at metastatic sites, because dedifferentiation of disseminating tumor cells appears to include an arrest in proliferative activity.
Nonetheless, the present data are consistent with this scheme but do not prove it, because direct functional proof of the postulated transition processes is still lacking. Thus, it is formally possible that metastatic tumor cells never lose their epithelial phenotype and that only invasive cells acquire a mesenchymal phenotype. However, we think that this is less likely, because in such a scenario, tumor dissociation and metastasis formation would have to be considered as separate processes, requiring separate capabilities, but there is evidence that conditions supporting invasion also support metastasis formation. For instance, highly invasive colon carcinomas, characterized by a loss of epithelial growth patterns at the invasive fronts, also exert an increased rate of metastases (21).
If a dedifferentiation is followed by a redifferentiation in the metastases, these transition processes must be transient and thus cannot be explained by additional genetic alterations. An important consequence of the proposed model, therefore, is that mechanisms of invasive and metastatic growth for well-differentiated carcinomas are predominantly controlled by the tumor environment. We suggest that specific signals from the tumor environment regulate both transition processes and are the main driving force of invasive and metastatic growth of differentiated colorectal carcinomas. Thus progression of these tumors would be enabled by ongoing perpetuating transition processes. In contrast, anaplastic tumors already may have lost epithelial qualities by acquisition of additional genetic alterations and often directly show a predominant disseminated growth pattern, making additional transient transition processes unnecessary. This feature is clearly visible for diffuse types of gastric cancers, which harbor inactivating E-cadherin mutations in a high percentage (22). Thus, we postulate two principal mechanisms of tumor progression, which are of course overlapping: an “environmental” type, dominating in well-differentiated colon carcinomas, and a “genetic” type, used in less differentiated or anaplastic carcinomas. We therefore would suggest extending the multistep carcinogenesis model, on the basis of a stepwise acquisition of genetic alterations, by including an active role of the specific tumor environment in colorectal tumor progression.
There are various lines of evidence showing that nuclear β-catenin is involved in tumor progression (23). Increasing numbers of target genes are found that are directly involved in tumor invasion and metastasis, e. g., matrilysin (11, 12), CD44 (14), fibronectin (13), and uPAR (15). We described a nuclear accumulation of β-catenin at the invasive front and in isolated tumor cells, whereas its localization in central tumor areas often was like that in normal colon epithelium (2, 16). Unexpectedly, we found membranous and cytoplasmic β-catenin and membranous E-cadherin also in growing metastases. Thus, the postulated two phenotypic transitions are clearly accompanied by distinct changes in the β-catenin expression pattern. An intermediate nuclear overexpression of β-catenin would have two determining consequences: loss of E-cadherin-mediated cell–cell adhesion, and polarity and activation of genes necessary for invasion and dissociation. Therefore, the outstanding question is what regulates nuclear translocation of β-catenin in the tumors. Recently, normal APC was shown to have a nuclear export function for β-catenin, which is lost in most colorectal carcinomas (24, 25). Indeed, nuclear APC can be found in most colon carcinomas and in colon carcinoma cell lines (our unpublished data). However, our observations indicate that the initial APC gene mutations may be the basis in most cases, but cannot be the only explanation, for nuclear accumulation of β-catenin, because β-catenin localization is heterogenous in the analyzed APC-mutant tumors and in the APC-mutant line SW480. Moreover, two analyzed cases with these features had β-catenin mutations. A recent publication showing there is an additional cofactor-independent nuclear transport of β-catenin (26) could explain these data and our observations with lines SW480 and LS174T. Colon cancer cells with functional APC (LS174T) could more efficiently export nuclear β-catenin than tumor cells with mutated APC (SW480), which, however, still could use an APC-independent export mechanism regulated by the environment. By using a spheroid invasion model, we could further show that infiltrating SW480 cells increase nuclear β-catenin, whereas most surrounding SW480 cells lack detectable nuclear β-catenin. Thus, this cell line mimics what we see in colorectal carcinomas. Tumors are increasingly considered complex tissues, composed of tumor cells, normal stromal cells, and extracellular matrix, all interacting together (27). We therefore postulate that specific signals from the tumor microenvironment, e.g., by stromal cells, the extracellular matrix, or cytokines, which were already shown to have strong influence on the malignant phenotype of breast cancer (28), also regulate intracellular β-catenin distribution and subsequently the behavior of colon tumor cells. For instance, trefoil factors are described to induce nuclear translocation of β-catenin in colon carcinoma cells (29). A possible direct influence of the extracellular matrix was demonstrated by showing that activation of integrin linked kinase leads to transcriptional repression of the E-cadherin gene and nuclear accumulation of β-catenin (30). Alternatively, the specific microenvironment may alter promoter methylation of relevant genes. This methylation was demonstrated for the E-cadherin promoter, which is hypermethylated at the invasive front of breast cancers, leading to a transient loss of E-cadherin expression (31).
We recently postulated that by acquiring APC gene mutations, tumor cells reach a morphogenetic competence similar to embryonic cells during gastrulation (2). A tumor cell accumulating nuclear β-catenin might, therefore, stay in a less differentiated state and acquire a competence for an efficient adaptation to different environmental milieus through the indicated transition processes. A change of milieu with loss of nuclear β-catenin in the central areas of primary tumor and metastasis could then allow the observed epithelial redifferentiation on the basis of a retained self-organizing morphogenetic competence of the tumor cells. Recent results by Mariadason et al. (32) and Naishiro et al. (33), demonstrating that suppression of T cell factor/β-catenin signaling in colon cancer cell lines leads to epithelial differentiation, fit well with this suggestion on the basis of our observations.
One apparent drawback of our model involves the postulated regaining of the epithelial growth pattern and a retranslocation of β-catenin from the nucleus to the cytoplasm and membrane, representing a second transition step during metastatic growth. Why do disseminated dedifferentiated tumor cells undergo such an epithelial redifferentiation? We found a strong reduction of proliferative activity in dissociating tumor cells expressing high amounts of nuclear β-catenin. Our previous results showed that a correlated expression of nuclear β-catenin and c-myc was not associated with the proliferating fraction of colon adenomas (34). Our findings are supported by previous observations in colorectal cancer research, according to which tumor cells at the luminal side and in central areas proliferated more strongly than at the invasive front (35). Obviously, in well-differentiated tumors, a loss of epithelial capabilities is coupled with a shutdown of proliferation in the same dedifferentiation program. Accordingly, to expand metastatic growth, disseminated mesenchyme-like tumor cells of well-differentiated carcinomas must regain their epithelial function.
Taken together, we think growth and progression of well-differentiated colorectal carcinomas is still a highly regulated morphogenetic process separated in different morphogenetic areas and suggest that (i) two switch processes are necessary for metastasis formation, both an initial dedifferentiation and a subsequent epithelial redifferentiation; and (ii) these transitions must be transient and ongoing, indicating that the tumor cells are still highly susceptible to their environment. Thus, we propose that the main driving force for invasion and metastasis formation in these tumors is not only additional genetic alterations but also exogenetic signals and therefore would like to extend the multistep carcinogenesis model by an active role of the specific tumor environment. Regulated nuclear translocation of β-catenin at the invasive front by such signals might allow the phenotypic transition steps in colorectal carcinomas on the basis of genetic alterations in the APC or β-catenin gene. A direct functional proof of this model, which specifies the role of the microenvironment in these processes, would also yield insights into new types of tumor therapy.
Acknowledgments
We are grateful to R. Weinberg for encouraging comments and critical reading of the manuscript and thank G. Mayr and M. Bienz for helpful discussions. For support in photographic work, we thank K. Amann, R. Hallmann, and T. Koschek and for expert technical assistance, U. Suchy, C. Knoll, C. Egerer-Sieber, and G. Herbig. This work was supported by a grant from the Wilhelm-Sander-Stiftung (no. 99.065.1) to T.B., A.J., and T.K.
Abbreviations
APC
adenomatosis polyposis coli
CK 18
cytokeratin 18
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Woodhouse E C, Chuaqui R F, Liotta L A. Cancer. 1997;80:1529–1537. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1529::aid-cncr2>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- 2.Kirchner T, Brabletz T. Am J Pathol. 2000;157:1113–1121. doi: 10.1016/s0002-9440(10)64626-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barth A I, Nathke I S, Nelson W J. Curr Opin Cell Biol. 1997;9:683–690. doi: 10.1016/s0955-0674(97)80122-6. [DOI] [PubMed] [Google Scholar]
- 4.Perl A K, Wilgenbus P, Dahl U, Semb H, Christofori G. Nature (London) 1998;392:190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 5.Hirohashi S. Am J Pathol. 1998;153:333–339. doi: 10.1016/S0002-9440(10)65575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korinek V, Barker N, Morin P J, van Wichen D, de Weger R, Kinzler K W, Vogelstein B, Clevers H. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 7.Morin P J, Sparks A B, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler K W. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 8.Peifer M. Science. 1997;275:1752–1753. doi: 10.1126/science.275.5307.1752. [DOI] [PubMed] [Google Scholar]
- 9.He T C, Sparks A B, Rago C, Hermeking H, Zawel L, da Costa L T, Morin P J, Vogelstein B, Kinzler K W. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 10.Tetsu O, McCormick F. Nature (London) 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 11.Brabletz T, Jung A, Dag S, Hlubek F, Kirchner T. Am J Pathol. 1999;155:1033–1038. doi: 10.1016/s0002-9440(10)65204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crawford H C, Fingleton B M, Rudolph-Owen L A, Heppner Goss K J, Rubinfeld B, Polakis P, Matrisian L M. Oncogene. 1999;18:2883–2891. doi: 10.1038/sj.onc.1202627. [DOI] [PubMed] [Google Scholar]
- 13.Gradl D, Kuhl M, Wedlich D. Mol Cell Biol. 1999;19:5576–5587. doi: 10.1128/mcb.19.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wielenga V J, Smits R, Korinek V, Smit L, Kielman M, Fodde R, Clevers H, Pals S T. Am J Pathol. 1999;154:515–523. doi: 10.1016/S0002-9440(10)65297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mann B, Gelos M, Siedow A, Hanski M L, Gratchev A, Ilyas M, Bodmer W F, Moyer M P, Riecken E O, Buhr H J, Hanski C. Proc Natl Acad Sci USA. 1999;96:1603–1608. doi: 10.1073/pnas.96.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brabletz T, Jung A, Hermann K, Gunther K, Hohenberger W, Kirchner T. Pathol Res Pract. 1998;194:701–704. doi: 10.1016/s0344-0338(98)80129-5. [DOI] [PubMed] [Google Scholar]
- 17.Liotta L A, Stetler-Stevenson W G. Cancer Res. 1991;51:5054s–5059s. [PubMed] [Google Scholar]
- 18.Gunther K, Brabletz T, Kraus C, Dworak O, Reymond M A, Jung A, Hohenberger W, Kirchner T, Kockerling F, Ballhausen W G. Dis Colon Rectum. 1998;41:1256–1261. doi: 10.1007/BF02258226. [DOI] [PubMed] [Google Scholar]
- 19.Kunz-Schughart L A. Cell Biol Int. 1999;23:157–161. doi: 10.1006/cbir.1999.0384. [DOI] [PubMed] [Google Scholar]
- 20.Rowan A J, Lamlum H, Ilyas M, Wheeler J, Straub J, Papadopoulou A, Bicknell D, Bodmer W F, Tomlinson I P. Proc Natl Acad Sci USA. 2000;97:3352–3357. doi: 10.1073/pnas.97.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ono M, Sakamoto M, Ino Y, Moriya Y, Sugihara K, Muto T, Hirohashi S. Cancer. 1996;78:1179–1186. doi: 10.1002/(SICI)1097-0142(19960915)78:6<1179::AID-CNCR3>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 22.Becker K F, Atkinson M J, Reich U, Becker I, Nekarda H, Siewert J R, Hofler H. Cancer Res. 1994;54:3845–3852. [PubMed] [Google Scholar]
- 23.Reichert M, Muller T, Hunziker W. J Biol Chem. 2000;275:9492–9500. doi: 10.1074/jbc.275.13.9492. [DOI] [PubMed] [Google Scholar]
- 24.Rosin-Arbesfeld R, Townsley F, Bienz M. Nature (London) 2000;406:1009–1012. doi: 10.1038/35023016. [DOI] [PubMed] [Google Scholar]
- 25.Henderson B R. Nat Cell Biol. 2000;2:653–660. doi: 10.1038/35023605. [DOI] [PubMed] [Google Scholar]
- 26.Wiechens N, Fagotto F. Curr Biol. 2001;11:18–27. doi: 10.1016/s0960-9822(00)00045-2. [DOI] [PubMed] [Google Scholar]
- 27.Hanahan D, Weinberg R A. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 28.Bissell M J, Weaver V M, Lelievre S A, Wang F, Petersen O W, Schmeichel K L. Cancer Res. 1999;59:1757s–1763s. [PubMed] [Google Scholar]
- 29.Efstathiou J A, Noda M, Rowan A, Dixon C, Chinery R, Jawhari A, Hattori T, Wright N A, Bodmer W F, Pignatelli M. Proc Natl Acad Sci USA. 1998;95:3122–3127. doi: 10.1073/pnas.95.6.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan C, Costello P, Sanghera J, Dominguez D, Baulida J, de Herreros A G, Dedhar S. Oncogene. 2001;20:133–140. doi: 10.1038/sj.onc.1204052. [DOI] [PubMed] [Google Scholar]
- 31.Graff J R, Gabrielson E, Fujii H, Baylin S B, Herman J G. J Biol Chem. 2000;275:2727–2732. doi: 10.1074/jbc.275.4.2727. [DOI] [PubMed] [Google Scholar]
- 32.Mariadason J M, Bordonaro M, Aslam F, Shi L, Kuraguchi M, Velcich A, Augenlicht L H. Cancer Res. 2001;61:3465–3471. [PubMed] [Google Scholar]
- 33.Naishiro Y, Yamada T, Takaoka A S, Hayashi R, Hasegawa F, Imai K, Hirohashi S. Cancer Res. 2001;61:2751–2758. [PubMed] [Google Scholar]
- 34.Brabletz T, Herrmann K, Jung A, Faller G, Kirchner T. Am J Pathol. 2000;156:865–870. doi: 10.1016/s0002-9440(10)64955-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmqvist R, Rutegard J N, Bozoky B, Landberg G, Stenling R. Am J Pathol. 2000;157:1947–1953. doi: 10.1016/S0002-9440(10)64833-X. [DOI] [PMC free article] [PubMed] [Google Scholar]