Cardiovascular Outcomes Trials in Type 2 Diabetes: Where Do We Go From Here? Reflections From a Diabetes Care Editors’ Expert Forum (original) (raw)
Abstract
In December 2008, the U.S. Food and Drug Administration issued guidance to the pharmaceutical industry setting new expectations for the development of antidiabetes drugs for type 2 diabetes. This guidance expanded the scope and cost of research necessary for approval of such drugs by mandating long-term cardiovascular outcomes trials (CVOTs) for safety. Since 2008, 9 CVOTs have been reported, 13 are under way, and 4 have been terminated. Reassuringly, each of the completed trials demonstrated the noninferiority of their respective drugs to placebo for their primary cardiovascular (CV) composite end point. Notably, four additionally provided evidence of CV benefit in the form of significant decreases in the primary CV composite end point, two suggested reductions in CV death, and three suggested reductions in all-cause mortality. Although these trials have yielded much valuable information, whether that information justifies the investment of time and resources is controversial. In June 2016, a Diabetes Care Editors’ Expert Forum convened to review the processes and challenges of CVOTs, discuss the benefits and limitations of their current designs, and weigh the merits of modifications that might improve the efficiency and clinical value of future trials. Discussion and analysis continued with the CVOT trial results released in June 2017 at the American Diabetes Association’s Scientific Sessions and in September 2017 at the European Association for the Study of Diabetes scientific meeting. This article summarizes the discussion and findings to date.
INTRODUCTION
In December 2008, the U.S. Food and Drug Administration (FDA) issued guidance to the pharmaceutical industry setting out new expectations for the development of antidiabetes drugs for type 2 diabetes (1). This guidance focused specifically on cardiovascular (CV) safety, largely in recognition of the excess burden of cardiovascular disease (CVD) in type 2 diabetes (2). The FDA was responding to prevailing concerns about the potential for increased CVD risk associated with certain antidiabetes drugs, notably the thiazolidinedione (TZD) rosiglitazone (3,4). The guidance—and subsequent similar requirements from the European Medicines Agency (5)—effectively expanded the scope and cost of research necessary to secure approval of new antidiabetes drugs by mandating long-term safety trials.
In the 9 years since the guidance was issued, 9 long-term, prospective CV outcomes trials (CVOTs) have been completed, 13 are under way, and 4 were started but terminated early. Collectively, these studies have involved more than 190,000 participants. The nine completed trials examined three dipeptidyl peptidase 4 (DPP-4) inhibitors (6–8), four glucagon-like peptide 1 (GLP-1) receptor agonists (9–12), and two sodium–glucose cotransporter 2 (SGLT2) inhibitors (13,14). Each demonstrated the noninferiority of their respective drugs to placebo in their major adverse cardiac event (MACE) primary composite end point. Four additionally provided evidence of CV benefit in the form of significant decreases in the MACE primary composite end point (10,11,13,14), two found reductions in CV death (10,13), and three showed reductions in all-cause mortality (10,12,14)—although the statistical robustness of findings for these secondary end points in some cases may be controversial.
The completed CVOTs have provided much valuable information, and because numerous antidiabetes drugs have been developed and tested in the past decade, it appears that the FDA mandate has not discouraged pharmaceutical companies from pursuing approval for potentially successful drugs. Still, questions remain regarding whether the information obtained through CVOTs designed according to the FDA mandate justifies the time and resources needed, especially in light of the neutral results of many of the studies. In June 2016, a Diabetes Care Editors’ Expert Forum convened and began to review the processes and challenges of CVOTs, discuss the benefits and drawbacks of their current designs, and weigh the merits of potential modifications that might improve the efficiency and clinical value of future trials. This process continued through September 2017 to include additional CVOT data reported at that time. This article summarizes the proceedings of the Expert Forum and CVOT findings to date.
PRE-GUIDANCE RESEARCH AND CONTROVERSY
Recent data indicate that, because of advances in research and clinical care, morbidity and mortality have decreased significantly in both type 1 and type 2 diabetes. However, individuals with diabetes still have greater CV risk than those without diabetes (15). Thus, additional strategies to reduce this risk continue to be evaluated.
Well before 2008, diabetes was known to more than double the risk for CV events and was considered by some a coronary artery disease equivalent (16). Although several major clinical trials of more versus less stringent glycemic control initially failed to demonstrate that intensive glucose lowering significantly reduces CV risk (17–23), meta-analyses indicated a modestly reduced risk of nonfatal myocardial infarction (MI) (24,25). In addition, long-term noninterventional follow-up of the DCCT (Diabetes Control and Complications Trial) (26,27) in type 1 diabetes and the UKPDS (UK Prospective Diabetes Study) (28) and VADT (Veterans Affairs Diabetes Trial) (29) in type 2 diabetes suggested an eventual “legacy” CV benefit of initially tighter glycemic control (Table 1) (17–22,26–30), but one that was not as apparent as the effect on microvascular complications. However, increased CV mortality in patients assigned to a more intensive glucose-lowering strategy in the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial (20,30) and no reduction in CV mortality risk in the VADT (22,29) tempered enthusiasm for stringent glucose lowering, particularly in older patients at high CV risk.
Table 1.
Early major trials evaluating the effects of intensive glycemic control of diabetes
| Study | Diabetes type | CV composite | MI | CV mortality | All-cause mortality | ||||
|---|---|---|---|---|---|---|---|---|---|
| DCCT/EDIC (17,26,27) | Type 1 | ↔ | ↓ | — | — | — | — | ↔ | ↓ |
| UKPDS | Type 2 | ||||||||
| Main randomization (SU or insulin vs. conventional therapy) (18,28) | — | — | ↔ | ↓ | — | — | ↔ | ↓ | |
| Additional randomization of overweight patients (metformin vs. SU vs. conventional therapy) (19,28) | — | — | ↓* | ↓* | — | — | ↓* | ↓* | |
| ACCORD (20,30) | Type 2 | ↔ | ↔ | ↓ | ↔ | ↑ | ↑ | ↑ | ↔ |
| ADVANCE (21) | Type 2 | ↔† | ↔ | ↔ | ↔ | ||||
| VADT (22,29) | Type 2 | ↔ | ↓ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
Less well understood was the CV safety profile of available antidiabetes drugs. Before the FDA issued its 2008 guidance, approval decisions for such medications focused largely on short-term glycemic efficacy (i.e., A1C reduction), along with safety data from 6- to 12-month phase 2 and 3 randomized clinical trials. These trials typically enrolled younger cohorts with relatively recent onset of diabetes and low CV risk. Indeed, individuals with established CVD were usually excluded. Thus, these studies had low CV event rates, and the events that did occur were neither prespecified as end points nor independently adjudicated. As a result, estimates of the CV safety of these drugs were inconsistent and unreliable (31,32).
Concerns about this gap in assessment of CV safety grew after two highly controversial meta-analyses of CV risk (3,33) were published in the mid-2000s—the first on muraglitazar, an investigational dual peroxisome proliferator–activated receptor (PPAR)-α and -γ agonist, and the second on the FDA-approved TZD (and PPAR-γ agonist) rosiglitazone. The muraglitazar analysis showed more than twice the incidence of CV complications, including congestive heart failure (HF) and death, with the drug compared with standard therapy (33). Although this drug had received a vote for approval from the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee (34), these findings effectively ended its development. The meta-analysis of rosiglitazone, which had already been marketed for a decade, suggested a statistically significant 43% increased risk of MI and a statistically nonsignificant 64% increased risk of CV death versus comparators (3). In the wake of these publications and the heated controversy they ignited, the FDA issued its CV safety guidance.
After a subsequent study specifically designed to test the CV safety of rosiglitazone did not confirm the earlier findings (35,36), the FDA lifted its prescription restrictions for rosiglitazone (37). Even so, use of rosiglitazone remains negligible. Trials of another TZD, pioglitazone, have shown CV benefit in high-risk patients. The PROactive (Prospective Pioglitazone Clinical Trial in Macrovascular Events) study (38) found that pioglitazone reduced the secondary composite end point of all-cause mortality, nonfatal MI, and stroke in patients with type 2 diabetes who were at high risk for macrovascular events. The more recent IRIS (Insulin Resistance Intervention After Stroke) trial (39,40) found that, in insulin-resistant patients (many with prediabetes but none with diabetes) with previous ischemic stroke or transient ischemic attack, treatment with this drug significantly reduced fatal and nonfatal MI and stroke.
REVIEW OF THE GUIDANCE
The FDA, following its usual process, cast its expectations as recommendations rather than requirements, but also noted that they were “for immediate implementation to ensure that relevant issues related to minimizing cardiovascular risk are considered in ongoing drug development programs” (1). The guidance outlined a detailed process for evaluating the CV safety of type 2 diabetes drugs in development (Table 2) (1,41).
Table 2.
Summary of the 2008 FDA guidance on CVOTs (1)
| To adequately evaluate the CV safety of type 2 diabetes drugs in development, future development programs should include: |
|---|
| • Phase 2 and 3 trials that include patients at higher risk for CV events, are of sufficient size and duration to enable enough CV events to allow for a meaningful evaluation of CV risk, and are designed to facilitate later meta-analysis; the CV events should include CV mortality, MI, and stroke and can also include hospitalization for ACS, urgent revascularization procedures, and other end points such as HF hospitalization* |
| • Independent adjudication of CV events |
| • Meta-analysis of the phase 2 and 3 trials to be conducted at the end of the research program, following a protocol developed in advance that prespecifies the end points to be assessed and the statistical methods to be employed |
| • Analysis of premarketing data comparing the CV events occurring with the agent to those occurring with the control group and demonstrating that the upper limit of a two-sided 95% CI of the estimated risk ratio is <1.8; if this cannot be done through the meta-analysis described above, it should be accomplished in a separate, large CV safety trial |
| • For agents whose 95% CI upper limit falls between 1.3 and 1.8 in premarketing analysis, completion of a postmarketing trial or continuation of a premarketing trial after approval may be needed to conclusively show that the upper limit of the two-sided 95% CI is <1.3 with a “reassuring” point estimate of overall CV risk |
It was suggested that separate CVOTs would be necessary only if meta-analyses could not rule out an unacceptable level of risk. However, the number of CV events accrued during a typical phase 2 or 3 development program is usually insufficient to provide high statistical confidence. Thus, as noted in a review by Regier et al. (42), every new antidiabetes drug approved since 2008 has had a dedicated CVOT to evaluate whether the upper limit of a two-sided 95% CI of the estimated hazard ratio (HR) is <1.8, the requirement necessary to gain initial approval. Although the FDA’s guidance specifically excluded insulin from its CV safety evaluation, noting that its necessity as a lifesaving therapy for type 1 diabetes makes such evaluation impractical, some newer insulin formulations have been studied in CVOT trials based on findings from meta-analysis of phase 2 and 3 studies (43).
SUMMARY OF CVOTS TO DATE
Trial Designs
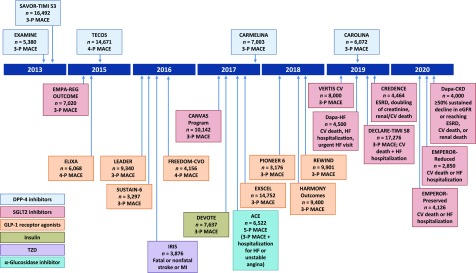
Figure 1 displays a timeline of completed and ongoing diabetes CVOTs, as well as several related trials that assessed CV outcomes but were not initiated as a direct result of the 2008 FDA guidance (6–14,39,44–58). These trials were mainly designed to rule out unacceptable CV risk, but some were powered to estimate superiority after noninferiority was demonstrated. They typically have studied populations in which some or all patients had advanced atherosclerotic CV risk or established CVD to ensure accrual of sufficient events in a timely manner. Some had active run-in periods to enhance adherence to the study drug. Their participants have a long duration of diabetes (mean 7.1–16.4 years), with baseline mean A1C ranging from 7.2 to 8.7%. The characteristics of the completed CVOTs and their primary findings are summarized in Table 3 (6–14,59,60). Ongoing CVOTs are summarized in Supplementary Table 1 (44–56), and Supplementary Table 2 summarizes additional trials that assessed CV outcomes but were not initiated as a direct result of the 2008 FDA guidance (39,57,58,61).
Figure 1.
Completed and ongoing CVOTs (6–14,39,44–58). 3-P, 3-point; 4-P, 4-point; 5-P, 5-point. DECLARE-TIMI 58, Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events; ESRD, end-stage renal disease; HARMONY Outcomes, Effect of Albiglutide, When Added to Standard Blood Glucose Lowering Therapies, on Major Cardiovascular Events in Subjects With Type 2 Diabetes Mellitus; PIONEER 6, A Trial Investigating the Cardiovascular Safety of Oral Semaglutide in Subjects With Type 2 Diabetes; REWIND, Researching Cardiovascular Events With a Weekly Incretin in Diabetes; VERTIS CV, Cardiovascular Outcomes Following Ertugliflozin Treatment in Type 2 Diabetes Mellitus Participants With Vascular Disease.
Table 3.
CVOTs completed after issuance of the FDA 2008 guidance
| DPP-4 inhibitors | GLP-1 receptor agonists | SGLT2 inhibitors | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SAVOR-TIMI 53 (6) (n = 16,492) | EXAMINE (7,59) (n = 5,380) | TECOS (8) (n = 14,671) | ELIXA (9) (n = 6,068) | LEADER (10) (n = 9,340) | SUSTAIN-6 (11)* (n = 3,297) | EXSCEL (12) (n = 14,752) | EMPA-REG OUTCOME (13,60) (n = 7,020) | CANVAS Program (14) (n = 10,142) | |
| Intervention | Saxagliptin/placebo | Alogliptin/placebo | Sitagliptin/placebo | Lixisenatide/placebo | Liraglutide/ placebo | Semaglutide/placebo | Exenatide QW/placebo | Empagliflozin/placebo | Canagliflozin/ placebo |
| Main inclusion criteria | Type 2 diabetes and history of or multiple risk factors for CVD | Type 2 diabetes and ACS within15–90 days before randomization | Type 2 diabetes and preexisting CVD | Type 2 diabetes and an acute coronary event within 180 days before screening | Type 2 diabetes and preexisting CVD, kidney disease, or HF at ≥50 years of age or ≥1 CV risk factor at ≥60 years of age | Type 2 diabetes and preexisting CVD, HF, or CKD at ≥50 years of age or ≥1 CV risk factor at ≥60 years of age | Type 2 diabetes with or without preexisting CVD | Type 2 diabetes and preexisting CVD, with BMI ≤45 kg/m2 and eGFR ≥30 mL/min/1.73 m2 | Type 2 diabetes and preexisting CVD at ≥30 years of age or ≥2 CV risk factors at ≥50 years of age |
| A1C inclusion criterion (%) | ≥6.5 | 6.5–11.0 | 6.5–8.0 | 5.5–11.0 | ≥7.0 | ≥7.0 | 6.5–10.0 | 7.0–10.0 | 7.0–10.5 |
| Age (years)† | 65.1 | 61.0 | 65.4 | 60.3 | 64.3 | 64.6 | 62 | 63.1 | 63.3 |
| BMI (kg/m2)† | 31.1 | 28.7 | 30.2 | 30.2 | 32.5 | 32.8 | 31.8 | 30.7 | 32 |
| Diabetes duration (years)† | 10.3 | 7.1 | 11.6 | 9.3 | 12.8 | 13.9 | 12 | 57% >10 | 13.5 |
| Events planned/observed (n) | 1,040/1,222 | 650/621 | 1,300/1,690 | 844/805 | ≥611/1,302 | ≥122/254 | 1,360/1,744 | 691/772 | 688/1,011 |
| Median follow-up (years) | 2.1 | 1.5 | 3.0 | 2.1 | 3.8 | 2.1 | 3.2 | 3.1 | 2.4 |
| Statin use (%) | 78 | 91 | 80 | 93 | 72 | 73 | 74 | 77 | 75 |
| Prior CVD/CHF (%) | 78/13 | 100/28 | 74/18 | 100/22 | 81/18 | 60/24 | 73.1/16.2 | 99/10 | 65.6/14.4 |
| A1C/A1C change (%)‡ | 8.0/–0.3 | 8.0/–0.3 | 7.2/–0.3 | 7.7/–0.3 | 8.7/–0.4 | 8.7/–0.7 or –1.0§ | 8.0/–0.53 | 8.1/–0.3‖ | 8.2/–0.58 |
| Year started/reported | 2010/2013 | 2009/2013 | 2008/2015 | 2010/2015 | 2010/2016 | 2013/2016 | 2010/2017 | 2010/2015 | 2009/2017 |
| Primary outcome¶ | 3-point MACE | 3-point MACE | 4-point MACE | 4-point MACE | 3-point MACE | 3-point MACE | 3-point MACE | 3-point MACE | 3-point MACE |
| 1.00 (0.89–1.12) | 0.96 (95% UL ≤1.16) | 0.98 (0.89–1.08) | 1.02 (0.89–1.17) | 0.87 (0.78–0.97) | 0.74 (0.58–0.95) | 0.91 (0.83–1.00) | 0.86 (0.74–0.99) | 0.86 (0.75–0.97)# | |
| Key secondary outcome¶ | Expanded MACE | 4-point MACE | 3-point MACE | Expanded MACE | Expanded MACE | Expanded MACE | Individual components of MACE (see below) | 4-point MACE | All-cause and CV mortality (see below) |
| 1.02 (0.94–1.11) | 0.95 (95% UL ≤1.14) | 0.99 (0.89–1.10) | 1.00 (0.90–1.11) | 0.88 (0.81–0.96) | 0.74 (0.62–0.89) | 0.89 (0.78–1.01) | |||
| CV death¶ | 1.03 (0.87–1.22) | 0.85 (0.66–1.10) | 1.03 (0.89–1.19) | 0.98 (0.78–1.22) | 0.78 (0.66–0.93) | 0.98 (0.65–1.48) | 0.88 (0.76–1.02) | 0.62 (0.49–0.77) | 0.96 (0.77–1.18)** |
| 0.87 (0.72–1.06)# | |||||||||
| MI¶†† | 0.95 (0.80–1.12) | 1.08 (0.88–1.33) | 0.95 (0.81–1.11) | 1.03 (0.87–1.22) | 0.86 (0.73–1.00) | 0.74 (0.51–1.08) | 0.97 (0.85–1.10) | 0.87 (0.70–1.09) | 0.89 (0.73–1.09)# |
| Stroke¶†† | 1.11 (0.88–1.39) | 0.91 (0.55–1.50) | 0.97 (0.79–1.19) | 1.12 (0.79–1.58) | 0.86 (0.71–1.06) | 0.61 (0.38–0.99) | 0.85 (0.70–1.03) | 1.18 (0.89–1.56) | 0.87 (0.69–1.09)# |
| HF hospitalization¶ | 1.27 (1.07–1.51) | 1.19 (0.90–1.58) | 1.00 (0.83–1.20) | 0.96 (0.75–1.23) | 0.87 (0.73–1.05) | 1.11 (0.77–1.61) | 0.94 (0.78–1.13) | 0.65 (0.50–0.85) | 0.67 (0.52–0.87)# |
| Unstable angina hospitalization¶ | 1.19 (0.89–1.60) | 0.90 (0.60–1.37) | 0.90 (0.70–1.16) | 1.11 (0.47–2.62) | 0.98 (0.76–1.26) | 0.82 (0.47–1.44) | 1.05 (0.94–1.18) | 0.99 (0.74–1.34) | — |
| All-cause mortality¶ | 1.11 (0.96–1.27) | 0.88 (0.71–1.09) | 1.01 (0.90–1.14) | 0.94 (0.78–1.13) | 0.85 (0.74–0.97) | 1.05 (0.74–1.50) | 0.86 (0.77–0.97) | 0.68 (0.57–0.82) | 0.87 (0.74–1.01)** |
| 0.90 (0.76–1.07)# | |||||||||
| Worsening nephropathy¶‡‡ | 1.08 (0.88–1.32) | — | — | — | 0.78 (0.67–0.92) | 0.64 (0.46–0.88) | — | 0.61 (0.53–0.70) | 0.60 (0.47–0.77)# |
Most of the CVOTs were designed as phase 4 event-driven trials requiring >600 primary end point events to rule out with statistical confidence an HR with an upper confidence limit ≥1.3 for a 3-point MACE (CV death, nonfatal MI, and nonfatal stroke). In three trials (TECOS [Trial Evaluating Cardiovascular Outcomes with Sitagliptin] [8], ELIXA [Evaluation of Lixisenatide in Acute Coronary Syndrome] [9]), and FREEDOM-CVO [A Study to Evaluate Cardiovascular Outcomes in Patients With Type 2 Diabetes Treated With ITCA 650] [53]) a 4-point MACE, adding hospitalization for unstable angina to the components of the 3-point MACE, is the primary end point. In the ACE (Acarbose Cardiovascular Evaluation) trial (58) of the α-glucosidase inhibitor acarbose, conducted in China in patients with coronary heart disease and impaired glucose tolerance (IGT), the primary end point is a 5-point MACE, adding hospitalization for unstable angina or HF to the components of the 3-point MACE. Additionally, most analysis plans permitted testing for superiority to placebo if the noninferiority HR threshold of <1.3 were achieved, providing an opportunity to show potential CV benefit.
All of the CVOTs in populations with diabetes were designed to promote “glycemic equipoise” between the treatment groups to minimize the potentially confounding effect of differences in glycemic control. Accordingly, treatment intensification with other oral antidiabetes drugs or insulin was more prevalent in the control groups. Even so, modest between-group differences in A1C changes were observed at the end of most trials, with higher values in the placebo arms.
All primary results were analyzed in the intention-to-treat (ITT) (as randomized) or modified ITT (as treated with at least one dose) population, with evaluations in the on-treatment or per-protocol populations reported as sensitivity analyses. Most study patients were receiving near-optimal CV risk management at baseline, as shown by a high proportion of patients receiving antihypertensive, lipid-lowering, and antiplatelet medications. Differences in study populations, baseline patient characteristics, and designs make it difficult to compare results among these trials.
Effects on Cardiometabolic Risk Factors
The impact on cardiometabolic risk factors with DPP-4 inhibitors was minimal except for small reductions in A1C of 0.3% at study end (6–8).
The EMPA-REG OUTCOME (BI 10773 [Empagliflozin] Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) trial of the SGLT2 inhibitor empagliflozin showed a small but statistically significant 0.3% A1C reduction at study end, similar reductions in systolic (4–5 mmHg) and diastolic (2 mmHg) blood pressure and weight (∼2 kg), and minimal increases in LDL and HDL cholesterol, with no changes observed in heart rate (13). In the CANVAS (Canagliflozin Cardiovascular Assessment Study) Program (14), there were statistically significant reductions in mean A1C updated over time (0.58%), systolic (3.93 mmHg) and diastolic (1.39 mmHg) blood pressure, and weight (1.6 kg) and increases in LDL and HDL cholesterol (4.68 and 2.05 mg/dL, respectively) with canagliflozin treatment (14).
In the ELIXA trial of the GLP-1 receptor agonist lixisenatide (9), there were statistically significant reductions in A1C at study end (0.3%), systolic blood pressure (0.8 mmHg), and weight (0.7 kg), with a slightly increased heart rate (0.4 bpm). In the LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) trial (10), treatment with liraglutide was associated with a small but statistically significant increase in heart rate (3.0 bpm), with reductions in mean updated A1C (0.4%), systolic blood pressure (1.2 mmHg), and weight (2.3 kg) and a slight increase in diastolic blood pressure (0.6 mmHg). Similar results were found in SUSTAIN-6 (Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes) (11) with 0.5 and 1.0 mg doses of semaglutide; heart rate increased by 2.0 and 2.5 bpm, respectively, with robust reductions in A1C at study end (0.7 and 1.0%, respectively), systolic blood pressure (1.3 and 2.6 mmHg, respectively), and weight (2.9 and 4.3 kg, respectively) and no difference in diastolic blood pressure. Finally, in the EXSCEL (Exenatide Study of Cardiovascular Event Lowering) trial (12) with once-weekly exenatide, mean updated A1C was 0.53% lower, weight was 1.3 kg lower, and systolic blood pressure was 1.6 mmHg lower, but heart rate was 2.5 bpm higher.
Overall, the impact of treatment interventions on cardiometabolic risk factors was small, and the resulting effects of these changes on CV outcomes remain unclear.
CV Outcomes
DPP-4 Inhibitors
Five trials enrolling 49,618 patients have been designed to evaluate the CV safety of DPP-4 inhibitors. Three have reported outcome results (6–8), and two are ongoing (44,45). One additional trial, OMNEON (A Study to Assess Cardiovascular Outcomes Following Treatment With Omarigliptin [MK-3102] in Participants With Type 2 Diabetes Mellitus [MK-3102-018]) (62), evaluating the investigational once-weekly omarigliptin in 4,202 patients with type 2 diabetes and CVD, was terminated as a business decision (63). Although all three completed trials (6–8) met the primary objective of excluding an HR upper limit ≥1.3, none were associated with any suggestion of CV benefit. Likewise, at its termination after a median follow-up of 96 weeks, the OMNEON trial reported an HR of 1.00 (95% CI 0.77–1.29) for its primary 3-point MACE end point (62).
The SAVOR-TIMI 53 (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction) trial (6) with saxagliptin suggested an increased risk for incident HF (HR 1.27 [95% CI 1.07–1.51], P = 0.007 [6]), with some support provided by a trend noted in the EXAMINE (Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care) trial with alogliptin (HR 1.19 [95% CI 0.90–1.58], P = 0.220 [7,59]), earning a warning by the FDA, especially in patients with underlying heart and kidney disease (64).
CAROLINA (Cardiovascular Outcome Study of Linagliptin Versus Glimepiride in Patients With Type 2 Diabetes) (44) was designed to establish noninferiority of linagliptin relative to the sulfonylurea glimepiride, thus including an active comparator. However, because the CV safety of glimepiride has not been established, a placebo-controlled trial (CARMELINA [Cardiovascular and Renal Microvascular Outcome Study With Linagliptin in Patients With Type 2 Diabetes Mellitus] [45]) is also being conducted to establish the CV and renal safety of linagliptin by including an enriched population with CV risk and evidence of renal compromise. These trials may help to answer the question of CV safety with sulfonylureas, which has been lingering for nearly 50 years (65).
Overall, the DPP-4 inhibitors have established MACE safety.
SGLT2 Inhibitors
The CV and renal safety of SGLT2 inhibitors is being evaluated in nine trials enrolling 62,378 patients. Of these, two—EMPA-REG OUTCOME (13) and the CANVAS Program (14)—have been completed, and each reported positive CV and renal outcomes. The other seven trials are scheduled for completion within the next 2–3 years (46–52).
In four SGLT2 inhibitor trials, a composite 3-point MACE is the primary end point; in two, a composite renal outcome is the primary end point; and in three, a composite of HF outcomes and CV death is the primary end point in people with established HF. Although primarily renal studies, CANVAS-R (CANVAS-Renal) (66) (data from which were included in the combined report on the overall CANVAS Program [14]) and the ongoing CREDENCE (Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy) trial (46) also prospectively collected/are collecting adjudicated CV outcomes, as required by the FDA mandate. Dapa-CKD (A Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients With Chronic Kidney Disease) (50) will also evaluate CV death or HF hospitalization in addition to the primary composite renal outcome. Plans for a dedicated study to evaluate empagliflozin on the progression of CKD were recently announced (67). Although primarily HF studies, Dapa-HF (Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients With Chronic Heart Failure [49]) and EMPEROR-Reduced (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure with Reduced Ejection Fraction [51]), both of which are in HF patients with reduced ejection fraction, and EMPEROR-Preserved (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction [52]), in HF patients with preserved ejection fraction, will also prospectively evaluate adjudicated renal outcomes.
EMPA-REG OUTCOME (13) was an event-driven trial in patients at high CV risk randomly treated with 10 or 25 mg of empagliflozin or placebo, with a median observation time of 3.1 years. The primary hypothesis was noninferiority for the primary 3-point MACE end point with empagliflozin (pooled doses of 10 and 25 mg) versus placebo, with an HR upper limit of <1.3. The primary end point was significantly reduced with empagliflozin treatment (HR 0.86 [95% CI 0.74–0.99], P = 0.038). This reduction was driven by a significant decrease in CV death (HR 0.62 [95% CI 0.49–0.77], P < 0.001), with numerical, but not statistically significant, differences in nonfatal MI (favoring empagliflozin) and nonfatal stroke (favoring placebo). There was no difference in the key secondary end point of 3-point MACE plus hospitalization for unstable angina (HR 0.89 [95% CI 0.78–1.01], P = 0.08). Significant reductions in prespecified end points of all-cause mortality (32%) and HF hospitalization (35%) were also observed. Superiority in terms of CV death was large and clinically important (38% risk reduction), statistically robust (P < 0.001), based on a large number of events (n = 309), occurring early (within 3 months), consistently seen with both doses across subgroups, and established across sensitivity analyses that accounted for silent MIs (which were excluded from event adjudication), nonassessable deaths, and missing data. There was an increased rate of treatment-emergent genital infection but no increase in other adverse events, including diabetic ketoacidosis, bone fractures, venous thromboembolism, hypoglycemic events, amputations, and acute kidney injury. Interestingly, reductions in CV outcomes appeared to be unexplained by empagliflozin’s glucose-lowering effect or by its effects on blood pressure, weight, or uric acid, suggesting that other mechanisms activated by SGLT2 inhibition may contribute to this drug’s cardioprotective effects (68–70).
Based on this favorable benefit-risk profile, the FDA approved a new indication for empagliflozin on 2 December 2016 “to reduce the risk of cardiovascular death in adult patients with type 2 diabetes mellitus and cardiovascular disease” (71), a claim that represented a clinical breakthrough in treating patients with type 2 diabetes. It was also a regulatory breakthrough because CV death was a secondary outcome but, in view of the positive results of the primary outcome and the robustness of the CV death data, this indication was approved. On 15 December 2016, the American Diabetes Association (ADA) endorsed this recommendation in its Standards of Medical Care in Diabetes—2017 (72). Although EMPA-REG OUTCOME was the first trial to demonstrate the CV superiority of an antidiabetes drug, more research is needed to elucidate the underlying mechanisms, confirm whether the CV benefits are a class effect, and determine whether empagliflozin conveys a CV benefit to patients without established CVD or diabetes.
The CANVAS Program was originally designed in two phases to establish the CV protective effects of canagliflozin in high-risk patients with type 2 diabetes and established CVD or risk factors for CVD (73). The first phase was included in the meta-analysis presented to the FDA for regulatory approval (74), which ruled out an HR upper limit ≥1.8. However, these results were publicly disclosed at an advisory committee meeting in 2013 after partial (group-level) unblinding due to an observed dose-dependent increase in LDL cholesterol. The FDA felt that the lipid data necessitated public disclosure, and with it, the CV event data were also disclosed, which had the potential to compromise the postmarketing phase of the trial to fulfill the requirement of ruling out an HR upper limit ≥1.3. Because of these extenuating circumstances, the FDA accepted an integrated analysis plan, whereby data from extended follow-up of the CANVAS first phase before and after the data disclosure (20 November 2012) would be combined with new data from CANVAS-R (66) to address CV safety as assessed by exclusion of a postmarketing risk ratio of 1.3. A sequential hypothesis testing plan was used, whereby a truncated integrated data set of pooled data from CANVAS after 20 November 2012 plus CANVAS-R was prespecified as the principal data set for analysis for superiority of all-cause mortality and CV death (75).
The primary 3-point MACE end point was significantly reduced with canagliflozin treatment (HR 0.86 [95% CI 0.75–0.97], P = 0.02), thereby meeting the criterion for noninferiority using the 1.3 risk margin (14). It is debatable, from a rigorous statistical perspective, whether an inference of superiority is justified because it was apparently not prespecified in the testing sequence. Because the all-cause mortality difference was not statistically significant in the truncated integrated data set (HR 0.90 [95% CI 0.76–1.07], P = 0.24), given the statistical rules of the prespecified hierarchical testing strategy, all subsequent results, including the favorable effects on HF hospitalization, progression of albuminuria, and worsening nephropathy, were deemed exploratory. The point estimate for nonfatal or total stroke was favorable for canagliflozin.
There was an increased rate of treatment-emergent genital infection as expected, but no increase in venous thromboembolism or hypoglycemic events. Diabetic ketoacidosis was very low but slightly numerically increased with canagliflozin, and there was a nearly twofold increase in risk for lower-leg amputation (6.3 and 3.4 events/1,000 patient-years for canagliflozin and placebo, respectively) and a 26% relative increase in risk of bone fracture with canagliflozin treatment. The underlying mechanisms for these findings remain unclear. There are some potential biological explanations for bone fractures, but none for the small but significant and unexpected increase in lower-leg amputations. Neither bone fracture nor lower-limb amputation has been documented with the other SGLT2 inhibitors, so additional evaluation is need to clarify whether this should be viewed as a class effect.
GLP-1 Receptor Agonists
The CV safety of GLP-1 receptor agonists has been assessed in eight trials enrolling 60,090 patients, of which four have reported outcomes (9–12). A fifth trial has been completed but not reported (53), and the other three are scheduled for completion within the next 1–2 years (54–56).
The first reported, ELIXA (9), which was conducted in patients with a history of recent acute coronary syndrome (ACS), was CV neutral, confirming the noninferiority of lixisenatide with respect to a 4-point MACE but showing no beneficial effect on any CV outcome.
The second trial, LEADER (10), demonstrated the CV noninferiority, as well as the statistical superiority, of once-daily treatment with liraglutide. The reduction in 3-point MACE (HR 0.87 [95% CI 0.78–0.97]) with liraglutide was driven by a significant reduction in CV death (HR 0.78 [95% CI 0.66–0.93]) with numerical, but not statistically significant, differences in nonfatal MI and nonfatal stroke favoring liraglutide. The P value for the 3-point MACE met superiority in all sensitivity analyses. All-cause mortality, but not HF hospitalization, was also significantly reduced with liraglutide. An interesting observation is the delayed separation of the Kaplan-Meier curves (indicating time to benefit) in LEADER (>12 months for CV death and >18 months for all-cause deaths and HF hospitalization) (10), suggesting that the CV benefit may be conveyed via an impact on atherosclerotic disease, which contrasts with the earlier time to benefit (<3 months) in the EMPA-REG OUTCOME trial (13). On 25 August 2017, based on the results of the LEADER trial, the FDA approved a new indication for liraglutide “to reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes mellitus and established cardiovascular disease” (76).
SUSTAIN-6 confirmed the noninferiority of once-weekly treatment with 0.5 or 1 mg of the long-acting semaglutide, which is currently under FDA review (11). This phase 3 trial was designed to rule out an HR upper limit ≥1.8 and achieved statistical superiority (HR 0.74 [95% CI 0.58–0.95], P < 0.001), but superiority analysis was not prespecified. The favorable effect on the 3-point MACE was accompanied by a significant decrease in nonfatal stroke (HR 0.61 [95% CI 0.38–0.99], P = 0.04) and a nonsignificant decrease in nonfatal MI (HR 0.74 [95% CI 0.51–1.08], P = 0.12), with no trend for CV death or all-cause mortality. The risk reduction for the primary outcome was seen despite an increase in pulse rate, an effect seen in all CVOTs in this drug class to date. On 18 October 2017, the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee voted in favor of approval for semaglutide (77).
The fourth trial, EXSCEL (12), was performed in a usual-care setting among patients with type 2 diabetes with or without previous CVD. It confirmed the noninferiority, but not superiority, of once-weekly treatment with 2 mg of the long-acting extended-release exenatide (HR 0.91 [95% CI 0.83–1.00], P = 0.06). The risk of death from any cause was 6.9% in the exenatide group and 7.9% in the placebo group (HR 0.86 [95% CI, 0.77–0.97]). This difference was considered only exploratory on the basis of the hierarchical testing plan. The rates of CV death, fatal or nonfatal MI, fatal or nonfatal stroke, HF hospitalization, and ACS hospitalization; the incidences of acute pancreatitis, pancreatic cancer, and medullary thyroid carcinoma; and serious adverse events including severe hypoglycemia did not differ significantly between the two treatment groups.
Of interest, the treatment persistence with weekly exenatide was low, with 43% drug discontinuation. The pragmatic nature of the study design, with visits every 6 months and limited study support, may explain the low treatment adherence and persistence. It is remarkable that despite this limited drug exposure and a heterogeneous population of whom 27% had no history of CVD, the 3-point MACE reduction of 9% came so close to reaching statistical significance, with HRs of almost all measured parameters in the direction of benefit.
Although full results have not yet been published, the phase 3 FREEDOM-CVO trial, which evaluated continuous delivery of exenatide and was designed for safety purposes to accrue a limited number of CV events, reported in a press release (53) that the primary objective of achieving an HR upper limit <1.8 had been met, and a new drug application has been submitted to the FDA (78). Treatment persistence could not have been an issue in this trial given the continuous delivery of exenatide via a subcutaneously implanted mini-osmotic pump. However, the limited number of participants prevents any definitive conclusions beyond meeting the HR requirement for regulatory approval.
The heterogeneity in CV outcomes (i.e., null effects with lixisenatide and exenatide and favorable effects with liraglutide and semaglutide) might be mainly the result of differences in study populations, trial designs, and treatment persistence. However, it could also reflect differences in pharmacokinetic and pharmacodynamic properties (i.e., short-acting lixisenatide is a once-daily prandial drug that acts primarily on postprandial glucose, whereas liraglutide and semaglutide are longer-acting drugs that act mainly on fasting glucose with a carryover effect on postprandial glucose) or other trial or drug differences, including in structural similarity to human GLP-1. What is clear so far is that drugs in this class, if tolerated, are safe, and, if affordable, should be considered more often for type 2 diabetes management.
Other Drugs
PPAR Agonists.
Two trials to determine the CV effects of aleglitazar, a dual activator of PPAR-α and -γ, were started but terminated early due to safety concerns and futility for efficacy. The AleCardio trial (79) enrolled 7,226 patients with a recent ACS event. At its termination after a median follow-up of 104 weeks, several CV and non-CV safety concerns were identified, including increased risks of HF and bone fractures, decreased estimated glomerular filtration rate (eGFR), and evidence of increased risk of gastrointestinal bleeding, which was unanticipated. The ALEPREVENT trial (80) enrolled 1,999 patients with prediabetes or type 2 diabetes and established stable CVD. At its termination after a brief treatment period (58 ± 38 days), aleglitazar was associated with increased incidences of hypoglycemia and muscular events.
As previously mentioned, the IRIS trial of the TZD pioglitazone (39,40) reported a significant 24% reduction in its composite end point of fatal or nonfatal stroke or MI compared with placebo after 4.8 years of follow-up in 3,876 insulin-resistant subjects with a recent ischemic stroke or transient ischemic attack but without diabetes. No between-group difference in all-cause mortality was found.
TOSCA.IT (Thiazolidinediones or Sulfonylureas Cardiovascular Accidents Intervention Trial) (81), a trial comparing the CV safety of pioglitazone to a sulfonylurea in 3,028 patients, was terminated on the basis of a futility analysis after a median follow-up of 57 months. A total of 213 events were adjudicated and included in the CV analysis. The primary composite end point (nonfatal MI, nonfatal stroke, all-cause death, and urgent coronary revascularization) showed no significant difference between pioglitazone and sulfonylurea treatment (HR 0.96 [95% CI 0.74–1.26], P = 0.790). Pioglitazone was associated with a reduced risk of severe hypoglycemia (<0.2 vs. 2.0%, P < 0.0001) and moderate hypoglycemia (10 vs. 32%, P < 0.001). Despite the fact that pioglitazone showed evidence of CV benefit in both the PROactive (38) and IRIS (39,40) trials, this study failed to replicate the treatment benefit, albeit in a lower-risk population. It should be noted that TOSCA.IT was ultimately underpowered, with a substantial percentage of patients not taking their assigned medication because of concerns that emerged during the trial about a possible link between pioglitazone and bladder cancer. A longer and larger active-controlled study (e.g., CAROLINA [44]) should shed more light on the relative CV effects of sulfonylureas.
Insulin.
Although undertaken 5 years before the 2008 FDA guidance and thus outside of the primary focus of this article, the ORIGIN Trial (Outcome Reduction with an Initial Glargine Intervention) (82,83), established the neutral CV effects of insulin glargine compared with standard glycemic care in 12,537 participants with CV risk factors plus impaired fasting glucose, IGT, or type 2 diabetes who were followed for a median of 6.2 years.
More recently, DEVOTE (A Trial Comparing Cardiovascular Safety of Insulin Degludec Versus Insulin Glargine in Patients With Type 2 Diabetes at High Risk of Cardiovascular Events) (57), involving 7,637 patients with established CVD and a mean diabetes duration of 16 years, confirmed the CV safety of insulin degludec compared with insulin glargine (HR 0.91 [95% CI 0.78–1.06], P = 0.21). This study was conducted after the FDA required a preapproval degludec CVOT based on the results of its phase 2 and 3 meta-analysis (43). Degludec was statistically superior with regard to hypoglycemia risk, with a lower rate of both severe and nocturnal severe hypoglycemia (by 40 and 53%, respectively; P < 0.001 for both comparisons). These findings corresponded to absolute incidence rates for severe hypoglycemia of 4.9 and 6.6% and for severe nocturnal hypoglycemia of 1.0 and 1.9% for degludec and glargine, respectively. Interestingly, despite the differences in severe hypoglycemia, no differences in CV mortality were found.
α-Glucosidase Inhibitors.
The ACE trial of acarbose (58) in 6,522 Chinese patients with coronary heart disease and IGT showed no relative risk reduction for the primary outcome of 5-point MACE (3-point MACE plus hospitalization for unstable angina or HF) (HR 0.98 [95% CI 0.86–1.11], P = 0.73) or for any secondary end points compared with placebo. Although acarbose treatment did not reduce CV risk, a modest reduction in incident type 2 diabetes was observed (HR 0.82 [95% CI 0.71–0.94], P = 0.005) over a median follow-up of 5 years with a dose of 50 mg three times daily.
Although ORIGIN, IRIS, DEVOTE, and ACE were not initiated as a direct result of the 2008 FDA guidance and OMNEON, AleCardio, ALEPREVENT, and TOSCA.IT were terminated early, these trials nonetheless contributed important insights to our overall understanding of the CV effects of pharmacotherapies for type 2 diabetes.
Benefits and Challenges of the Current CVOT Design
Benefits
Trials have shown that insulins glargine and degludec, sitagliptin, alogliptin, saxagliptin, lixisenatide, and once-weekly exenatide (6–9,12,57,82,83) have neutral effects on MACE outcomes, thereby supporting the use of these drugs when needed to improve glycemic control with the goal of limiting microvascular complications without increasing CV risk. Well-designed and adequately powered CVOTs for other antidiabetes drugs have also provided valuable information beyond their primary safety purpose. The evidence for CV benefit from empagliflozin, canagliflozin, liraglutide, and possibly semaglutide versus placebo (10,11,13,14), supplemented by data from other studies, already has prompted reconsideration of treatment guidelines (70,84) and is likely to alter clinical practice and reduce CV events and deaths among people similar to those enrolled in these trials.
Other insights from these trials have derived from secondary end points, which are in some cases less statistically robust but nonetheless of potential importance.
Notably, recent CVOTs have focused attention on the pressing problem of HF, which affects older people with diabetes more frequently than MI (85). Empagliflozin caused an early, large, and somewhat unexpected reduction in hospitalization for or death from HF (HR 0.61 [95% CI 0.47–0.79], P < 0.001) (86). Canagliflozin also had favorable effects on HF, although perhaps of lesser magnitude (14). Less certain, but also of interest, is the incidental observation of increased HF with saxagliptin and a consistent nonsignificant trend with alogliptin, despite no effect on other CV end points (6,59). If this harm is confirmed, it will draw attention to previously unsuspected effects of and differences among DPP-4 inhibitors, which block cleavage of many circulating peptides and thus may have various downstream effects (either benign or harmful) other than reducing clearance and increasing blood levels of GLP-1.
Another clinically important benefit of these trials has been assessment of the effect of the tested drugs on kidney disease, itself a CV risk factor. For example, empagliflozin (HR 0.61 [95% CI 0.53–0.70], P < 0.001) (60), canagliflozin (HR 0.60 [95% CI, 0.47–0.77], P < 0.0001) (14), liraglutide (HR 0.78 [95% CI 0.67–0.92], P = 0.003) (10), and semaglutide (HR 0.64 [95% CI 0.46–0.88], P = 0.005) (11) all reduced progression of renal disease more than would be expected from the improvements in glucose and blood pressure provided by these drugs. Importantly, the renal benefits of the SGLT2 inhibitors extended to actual deterioration of renal function as denoted by a doubling of serum creatinine or major reduction in eGFR, whereas those of the GLP-1 receptor agonists appear to be mainly on the progression to macroalbuminuria. However, semaglutide appears to have a deleterious effect of diabetic retinopathy, possibly related to rapid and dramatic improvement in glycemic control in patients with preexisting retinopathy (11). A lesser trend for adverse eye outcomes with liraglutide requires further assessment (10).
Together, these findings have energized the medical community. Many CV experts appear to have revised their previous skepticism about the potential for CV benefits from diabetes-specific therapies. Diabetes researchers are exploring mechanisms that may explain the clinical effects first noted in these trials. The findings with empagliflozin and canagliflozin, which alter renal clearance of sodium as well as glucose and thus cause hemodynamic changes, have led to reconsideration of abnormalities in salt and water balance that are intrinsic to diabetes, an area long inactive in physiological research (87). Another provocative proposal is that injured myocardial and renal tissues may benefit from access to the energy supplied by modestly higher circulating ketone levels during treatment with SGLT2 inhibitors (68,69). These hypotheses open new areas of investigation for both clinical and basic research.
Challenges
Aside from obvious questions about competing priorities for allocation of financial, clinical, and professional resources and, in some cases, delays in regulatory approval, the currently advised structure for CVOTs has several practical limitations (Table 4). The most serious of these is the lack of generalizability of findings to the entire population of people with diabetes.
Table 4.
Limitations of current CVOT structure and opportunities for improvement
| Current limitations | |
|---|---|
| Lack of generalizability | Current CVOTs include participants who are at high risk for a CV event or death and thus are not representative of the larger population. |
| Short timeline for assessing potential benefits | CV benefit may not become apparent until long after initiation of treatment. Current CVOTs do not assess outcomes occurring >5 years after the onset of treatment. |
| Short timeline for assessing potential harm | CVOTs lasting <5 years are not likely to detect risks that may become apparent only after years of treatment. This may be especially concerning for agents with complex mechanisms of action. |
| Placebo-controlled design | Nearly all CVOTs to date have tested one drug against placebo, with both groups attempting to attain comparable glycemic control using regimens that also include other medications. Problems with this design include 1) comparable glycemic control generally has not been achieved and the lower A1Cs found with the study drug may contribute to CV benefits, 2) some drugs used in the comparison groups may themselves have an adverse effect on CV events, and 3) the CV benefits found with four agents to date make the future use of placebo ethically challenging. |
| Opportunities for improvement | |
| Lower-risk, more diverse populations | Primary intervention trials in lower-risk populations could determine whether diabetes medications offer CV protection for those who do not yet have CVD. This would require larger and/or longer studies but would yield valuable information with regard to CVD prevention. |
| Longer-term follow-up | Trial designs that prespecify longer-term follow-up could better identify longer-term safety issues and late beneficial effects, produce better cost-effectiveness data, and improve understanding of changing treatment requirements over time. Such a design would require new consent procedures to permit lifelong follow-up, strategies to increase therapy adherence and persistence, expanded use of EMRs, and innovative statistical approaches to permit serial reporting of key clinical outcomes over time. |
| Active comparators | Using an active comparator instead of placebo could address the drawbacks of placebo-controlled trials but will require sufficient knowledge of the CV impact of the comparator to avoid confounding interpretation of the results. Although challenging, this may become feasible as understanding of the CV safety of newer agents increases. Across-trials consistency in enrollment criteria and the capturing of baseline patient characteristics will facilitate such efforts. |
| Innovative designs | Adoption of factorial or adaptive designs, superiority trials, trials embedded within health care systems or networks, and/or employment of “big data” to dissect the effects of new diabetes medications may provide practical opportunities for further investigation. |
| Standardized definitions | Standard definitions of important safety and microvascular outcomes would facilitate better comparisons among agents. Collaborative efforts should be made to standardize definitions of high-priority safety and microvascular outcomes, akin to those established for CV outcomes. |
| Modification of end points and analyses | Incorporating weighted composite end points that include estimation of the severity of events, as well as multiple events in the same patient, may yield more nuanced findings in future studies. The design of trials for new agents should be informed by data from previous CVOTs within the same drug class. Important secondary outcomes with robust statistical findings that are biologically plausible and supportable by external evidence should be independently considered even if primary composite outcomes are not achieved. Such approaches would require buy-in from regulatory bodies and mechanisms to ensure equity for developers of first-in-class interventions and/or those who willingly adopt more complex trial designs. How best to incorporate predefined safety concerns into primary analyses should also be considered. |
| Establishment of biorepositories | Future trials should obtain informed consent to store participants’ biological samples in case unexpected results warrant further investigation. Such biorepositories could increase opportunities to investigate various mechanisms contributing to CV events and key subgroups and will become increasingly important for subsequent biomarker, gut microbiota, and genomic analyses to facilitate precision medicine opportunities. |
| Enhanced efficiency and cost-sharing options | Conducting a CVOT for each new diabetes drug is cumbersome and expensive. Strategies to enhance trial efficiency, such as collecting CV outcomes data from trials designed for other purposes, should be strongly considered, as should new models for cost-sharing among pharmaceutical, governmental, and other organizations. |
| Involvement of patients and advocacy organizations | Involving patients and their advocates in designing future trials will help to ensure that patients’ views and wishes are taken into account and that patient-related outcome measures are fully integrated. Such efforts would likely increase patient buy-in and help to minimize discontinuation, improve treatment adherence and persistence, and avoid missing data. |
As currently performed, randomized controlled trials (RCTs) require participants to have certain inclusion characteristics. They must be able to provide informed consent, agree to attend the study site for an extended period of time, and be physically able to do so. To answer questions of safety and efficacy, they must be at high risk to experience the outcome in question (in this case, a CV event or death) in order to provide an adequate number of events in an acceptable period of time. They cannot be entirely representative of the general population, and therefore the findings of such trials can be extrapolated to a wider population only with considerable caution. As an example, the EMPA-REG OUTCOME trial provides reasonable confidence that people with type 2 diabetes and a history of previous CVD are likely to benefit from empagliflozin therapy in the same manner as did the patients in the trial itself (13). However, there is less certainty that empagliflozin will similarly improve outcomes for individuals with diabetes and no history of CVD or for those with CVD but without diabetes. In the CANVAS Program, one-third of participants had only CV risk factors and no evidence of CVD. Perhaps the less impressive but still positive outcomes with canagliflozin in this research program may be related to this population subset that might require longer drug exposure in a longer-term trial (14). Notably, a subgroup analysis of the LEADER trial suggested the hypothesis that liraglutide effectively lowered CV risk only in people with established CVD (HR 0.83 [95% CI 0.74–0.93] compared with HR 1.20 [95% CI 0.86–1.67] in those without CVD, with a significant unadjusted interaction P value of 0.04) (10). Due to the large number of statistical tests that were performed for subgroups, the possibility that this was a chance observation cannot be excluded. Nonetheless, the FDA-approved indication for liraglutide for CV risk reduction was restricted only to those with established CVD.
In addition, the present CVOT design has serious limitations related to the natural histories of diabetes and CVD. Because the concerns of regulatory agencies, pharmaceutical companies, health systems, and in many cases, individuals with diabetes have relatively short timelines, current CVOTs address only short-term outcomes—not those occurring >5 years after the onset of treatment. However, several lines of evidence argue for the importance of longer-term follow-up.
Experience from the UKPDS and DCCT/EDIC (Epidemiology of Diabetes Interventions and Complications) cohorts, which were treated early in the course of diabetes, shows that important medical complications of diabetes, including blindness, renal failure, amputation, CV death, and others, do not usually appear in the first 5 years. Rather, each has a characteristic and much longer development timeline (26–28). In the case of CVD, significant numbers of CV events in the UKPDS and DCCT/EDIC did not accumulate until after >10 years of follow-up. In part because of the slow accrual of events, the apparent CV benefits of early randomization to intensive glycemic control were not observed until long after initiation of treatment. The CV benefits likewise occurred long after the change in A1C difference disappeared after cessation of randomized treatment, leading to the concept of a “legacy effect,” for which there is no proven explanation. These long-term benefits were shown through observational and possibly incomplete data within which residual confounding cannot be excluded, so they cannot confidently be attributed only to glycemic control. Nevertheless, a mechanism plausibly underlying the legacy effect is a reduction of glucose-related changes of vascular tissue structure, occurring during intensive glycemic therapy but persisting thereafter, that may have slowed the early progression of atherosclerosis and led to a much later reduction in overt CV events. Whether any nonglycemic effect of treatments in these trials contributed to this benefit cannot be determined.
This view is consistent with the findings of other large trials of short-term glucose-lowering strategies using similar treatments that were applied later in the natural history of type 2 diabetes. In ACCORD (20,88) and VADT (22,29), there were only modest reductions in some secondary CV end points in patients with a long duration of diabetes and high CV risk, but in ACCORD, there was an unexplained early increase in mortality. Specifically, very intensive glucose-lowering treatment goals achieved mainly with the use of metformin, sulfonylureas, TZDs, and intensive insulin regimens in these trials were unable to diminish the risk of events in patients who already had advanced CV pathology.
The failure of targeting very intensive glucose lowering in ACCORD and VADT highlights the benefits of treatment with empagliflozin, canagliflozin, liraglutide, and semaglutide in their respective CVOTs. Favorable CV effects with these drugs presumably resulted from effects beyond simply lowering glucose in patients with advanced diabetes, except perhaps in the trial testing semaglutide, during which a pronounced A1C reduction occurred in the group receiving active therapy. Whether the beneficial effects of these drugs would be similar or different if they were initiated closer to the time of diabetes diagnosis and earlier in the course of CVD development remains to be seen.
The current CVOT design also provides only limited objective information about harmful outcomes that occur >5 years after an intervention. Whether the new antidiabetes drugs currently being evaluated have risks that may appear only after years of therapy is unknown, but CVOTs with a duration of <5 years are not likely to detect them. Concerns may be greater for therapeutic drugs with complex mechanisms of action that are not currently well understood, including TZDs, DPP-4 inhibitors, and (despite their strong short-term protective effects) SGLT2 inhibitors.
CVOTs OF THE FUTURE: OPPORTUNITIES FOR IMPROVEMENT
As previously noted, the completed CVOTs have yielded some important information about both safety concerns and potential medical benefits. Regarding safety, the increased risk of HF hospitalization with saxagliptin in SAVOR-TIMI 53 (6) and a nonsignificant trend with aloglipitin in EXAMINE (7,59) resulted in the addition of new safety warnings to the labels of those medications (64). Given these safety signals for HF, which were unexpected from the registration trials, it is likely that studies of individual antidiabetes drugs will continue to be required.
Further, four of the completed trials (LEADER, SUSTAIN-6, EMPA-REG OUTCOME, and the CANVAS Program) reported clinically relevant relative risk reductions of MACE outcomes in patients with high CV risk (Table 5) (10,11,13,14,60). Although it is inherently inappropriate to compare results among trials because they are conducted in different populations, with different protocols, and at different sites, the numbers needed to treat (NNTs) to prevent a MACE occurrence in EMPA-REG OUTCOME (63 over 3.1 years), LEADER (53 over 3.8 years), and SUSTAIN-6 (44 over 2 years) were similar to those observed for widely recommended therapies to prevent CVD such as lipid lowering with statins, antihypertensive therapy, and aspirin (89). These three trials, together with similar but more controversial findings in the CANVAS Program, support an early and evolving case for the use of these antihyperglycemic therapies in patients with type 2 diabetes and evidence of clinical CVD who are >50 years of age.
Table 5.
Risk reduction in four completed trials showing evidence of CV benefit
| LEADER (10) | SUSTAIN-6 (11) | EMPA-REG OUTCOME (13,60) | CANVAS Program (14) | |
|---|---|---|---|---|
| Subjects (n) | 9,340 | 3,297 | 7,020 | 10,142 |
| Mean age (years) | 64.3 | 64.6 | 63.1 | 63.3 |
| Diabetes duration (years)* | 12.8 | 13.9 | 57% >10 | 13.5 |
| Mean baseline A1C (%) | 8.7 | 8.7 | 8.1 | 8.2 |
| Mean placebo-corrected A1C difference (%)† | −0.4 | −0.7 (0.5 mg dose) | −0.24 (10 mg dose) | −0.58 |
| −1.0 (1.0 mg dose) | −0.36 (25 mg dose) | |||
| Median follow-up duration (years) | 3.8 | 2.1 | 3.1 | 2.4 |
| 3-point MACE RRR (%) | 13 | 26 | 14 | 14 |
| 3-point MACE ARR (%) | 1.9 | 2.3 | 1.6 | —‡ |
| CV death RRR (%) | 22 | 2 | 38 | 4§; 13‖ |
| Nonfatal MI RRR (%) | 12 | 26 | 13 | 15 |
| Nonfatal stroke RRR (%) | 11 | 39 | +24 | 10 |
| All-cause mortality RRR (%) | 15 | +5 | 32 | 13§; 10‖ |
| HF hospitalization RRR (%) | 13 | +11 | 35 | 33 |
| Worsening nephropathy RRR (%)¶ | 22 | 36 | 39 | 40 |
Although the trials conducted thus far appear to both satisfy the FDA guidance recommendations and provide additional useful information, there are clearly opportunities for improvement (Table 4). The next generation of diabetes trials should be smarter, simpler, and innovatively designed to make more efficient use of resources and produce more generalizable results while still addressing safety issues. The design and conduct of future trials should be standardized enough to permit reliable between-trial comparisons but also nimble enough to explore unanticipated safety concerns that may arise. In pursuing these goals, some modifications to the current CVOT noninferiority trial design, outlined below, are worthy of consideration.
Lower-Risk and More Diverse Populations
Incorporation of recent trial findings into diabetes care guidelines has been limited to date, in part because the populations studied were selected for high CV risk. Although this practice is consistent with FDA recommendations and essential to demonstrate safety, it remains unclear whether results seen in high-risk patients are translatable to patients with a shorter duration of diabetes or without established CV complications.
Enrollment of very high-risk subjects also may hamper the ability of trials to detect a benefit from drug therapy. As examples, the ELIXA trial of lixisenatide (9) and the EXAMINE trial of alogliptin (7) exclusively recruited patients with a recent hospitalization for an acute coronary event. Such patients are easy to identify based on hospital records and are likely to have a high number of future MACE events. Thus, such trials may be completed more quickly than those enrolling patients with more stable CVD. However, this strategy may fail to detect a beneficial effect of the intervention studied because the CVD is too advanced, the drug exposure is too short, and/or the early events may be less amenable to the intervention.
The best way to demonstrate benefit might be to select lower-risk patients who have not yet manifested CVD to find out whether their likelihood of developing CVD can be reduced. To achieve a statistically significant number of MACE events, this will require larger and/or longer studies but would yield valuable information about CVD prevention.
Longer-Term Follow-Up
Trial designs that prespecify longer-term follow-up may be desirable to identify any longer-term safety issues and beneficial effects that are either slowly evolving or resulting from a legacy effect of earlier treatment. Toward that end, innovative consent procedures that permit lifelong follow-up should be explored, as should strategies to increase patient adherence to and persistence with assigned therapy. The ability to capture medication adherence and relevant clinical and laboratory data will be crucial and should be facilitated by use of more sophisticated electronic medical records (EMRs).
Statistical approaches would need to be developed (and approved by regulatory bodies) to permit robust assessment of key clinical outcomes over an extended timescale. For example, a trial could provide definitive early information on the degree of glucose lowering achieved (e.g., at 1 year) and also be powered to assess the impact of an intervention on a primary clinical outcome at a later time (e.g., at 3 years). Continued follow-up thereafter could examine recurrent or less frequent clinical outcomes, maximize collection of safety data, and better assess the durability of the glucose-lowering effect.
Even a few such trials could yield a rich harvest of clinically relevant information to better inform clinical guidelines and therapeutic choices for patients and produce more robust cost-effectiveness data for health care providers. Longer trials would also provide a better understanding of the evolution of treatment requirements over time, especially if participants are randomized to specific therapeutic sequences.
Active Comparators
Another difficulty in interpreting and applying the results of most CVOTs completed to date concerns comparing the drug in question to placebo, with both groups attempting to attain comparable glycemic control using regimens that include other medications. There are several problems with this design. First, in general, comparable glycemic control is not achieved, with the study drug group usually having lower A1C values. Such differences might contribute to any CV benefits seen. Second, some drugs used more frequently in the comparison groups may themselves have CV effects. Third, moving forward, it may not, for ethical reasons, be possible to restrict background use of drugs that have recently been shown to have beneficial effects.
However, using an active comparator as a control will require sufficient knowledge of the CV impact of the comparator to avoid confounding interpretation of the results. Although challenging, this may become feasible as our understanding of the CV safety of newer antidiabetes drugs increases. Future trials may successfully assess the impact of a new drug compared with a previously tested drug if the newer trial enrolls a patient population and adopts a design that is similar to that with which the comparator drug was studied. Combinations of drugs known to be cardioprotective may also need to be tested to explore whether the CV benefits are compounded. Between-trial consistency in study design, enrollment criteria, and ascertainment of clinical data will facilitate these efforts.
Innovative Designs
Creative trial designs likely will be needed to fully address current concerns. In addition to traditional RCTs, factorial or adaptive designs, superiority trials, trials embedded within health care systems or networks, and/or employment of “big data” to dissect the effects of new diabetes medications may provide practical opportunities for further investigation. Factorial designs would increase efficiency by allowing simultaneous testing of multiple interventions, and superiority trials would put greater emphasis on potential benefits of therapy and likely make recruitment easier by increasing the value proposition for participants. Pragmatic designs that embed studies within health care systems’ EMR platforms would provide a natural conduit for conducting long-term posttrial observational follow-up studies. However, lessons from EXSCEL (12) must be considered to avoid having low treatment persistence in such designs.
Embedding RCTs into routine clinical care would also enable the multidimensional collection of additional clinical outcomes relevant to diabetes that are not collected routinely in current trials because of cost and complexity. Such outcomes include rates of cancer, bone fractures, depression, and dementia, as well as unexpected off-target side effects undetected during conventional drug development programs. This would extend assessment of safety beyond CV safety and include long-term durability of effects. Sample sizes could be much larger using the embedded trial approach because of lower costs per patient, enabling more powerful assessments of the heterogeneity of treatment effects. Trials of this nature also could be crucial in realizing the goal of precision pharmacotherapy in type 2 diabetes, should different classes of drugs or specific drugs within classes be found to be better for patients with different clinical characteristics (31). However, defining the randomization process to avoid confounders and any imbalances between the groups studied will be crucial.
Although registries or EMRs may afford the opportunity for large, pragmatic, and relatively inexpensive trials, the success of such trials likely will depend on having a simple intervention and an appropriate health system with an adequate EMR, as is the case with the ongoing ADAPTABLE (Aspirin Dosing: A Patient-Centric Trial Assessing Benefits and Long-term Effectiveness) trial of aspirin therapy for atherosclerotic CVD (90). As mentioned above, irrespective of trial design, treatment randomization will remain crucial to determining a medication’s effect without undue bias.
Standardized Definitions
Safety and efficacy comparisons would be greatly enhanced by standardizing the definitions of important outcomes. The strategies and definitions used to identify events such as pancreatitis, pancreatic malignancy, progression of retinopathy, and worsening of renal function have varied considerably among the CVOTs conducted to date. Collaborative efforts should be made to generate definitions for high-priority safety and microvascular outcomes, akin to those established for CV outcomes.
Modification of End Points and Analyses
In light of the noted differences in effects of drugs on components of MACE composite end points, it may be desirable to reassess the end points and primary analyses to be adopted in future studies to provide a more nuanced look at CV outcomes. Trials likely will still be powered based on the time to the first primary end point, despite disadvantages that include possible heterogeneity of the composite components, results driven by components of lesser importance, and insufficient power to draw definitive conclusions for all components of the composite (91). To address these limitations, alternative analytic strategies such as weighted composite outcomes (92) and the win ratio approach (93) should be considered. To better capture the full impact of treatments, subsequent and recurrent events also need to be evaluated for both benefits and risks using validated statistical approaches (94,95). When testing drugs within the same class, data from previous trials should be used to inform both the design and sample size requirements, with a Bayesian approach considered to potentially limit sample sizes to more manageable and less costly proportions without compromising study power. Important secondary outcomes, especially mortality or serious irreversible morbidity events, should not be dismissed even if the primary composite outcomes are not achieved; they should be independently considered, especially when the statistical findings are robust, biologically plausible, and supportable by external evidence. This way forward would require buy-in from regulatory bodies, as well as mechanisms to ensure equity for developers of first-in-class interventions and/or those who willingly adopt more complex trial designs.
Another challenge is how best to incorporate predefined safety concerns into the primary analysis. One possibility is to add safety issues as components of the primary composite outcome if the included safety and efficacy components can be weighted appropriately to reflect their clinical importance. Such a combined end point would then reflect the intervention’s overall net clinical benefit to the patient. If there is adequate power, it may be more desirable to evaluate key efficacy and key safety outcomes separately as predefined coprimary end points, with superiority and noninferiority analyses respectively, as was done in EXSCEL (12). In general, however, the FDA typically prefers trials to establish efficacy and safety separately, with approval based on whether efficacy (benefit) outweighs safety (harm).
Establishment of Biorepositories
Many different mechanisms contribute to the occurrence of CV events, including atherosclerosis, hemodynamic changes, arrhythmias, and, for strokes, hemorrhagic mechanisms. To gain additional insight into both mechanisms and subgroups, it would be desirable for future trials to have informed consent to store biological (i.e., serum, urine, fecal, and DNA) samples in case unexpected results warrant further investigation or for additional hypothesis-generating studies. Greater use of patient phenotyping, genotyping, and metabolomic data, similar to what is now being done in the field of oncology, may help to better identify predictors of benefit or harm that so far have escaped detection. Biorepositories will be of key importance in this regard, as well, by allowing for subsequent biomarker, gut microbiota, and genomic analyses to facilitate future precision medicine opportunities.
Enhanced Efficiency and Cost-Sharing Options
The current system for evaluating the CV safety of new diabetes drugs, in which each is assessed individually in a large, long-term CVOT funded by a pharmaceutical sponsor, is cumbersome and expensive. Strategies to enhance the efficiency of trials, such as collecting CV outcomes data from trials designed for other purposes, should be strongly considered. Although evidence that the expense of such studies is suppressing innovation in new diabetes drug development is lacking, it is conceivable that, if the cost of CVOTs were reduced, more innovative research could be supported. Thus, models for cost-sharing among pharmaceutical, governmental, and other organizations should be explored.
Involvement of Patients and Advocacy Organizations
When designing future trials, all stakeholders should be involved. The direct involvement of patients and their advocates will be vitally important to ensure that patients’ views and wishes are taken into account and that patient-related outcome measures are fully integrated. There is some concern, however, that patient-reported outcome measures are not standardized, limiting comparisons between studies. Efforts to improve patients’ understanding of clinical trials and to enlist the help of patients in developing trial information literature and consent processes might improve patients’ willingness to participate as subjects. The importance of minimizing participant discontinuation, improving study drug adherence and treatment persistence, and avoiding missing data cannot be overstated when seeking to obtain definitive trial outcomes. The rapid growth of digital health and monitoring devices with Internet connectivity may offer further opportunities to access patient-specific outcomes or provide mechanistic insights (29,96).
Conclusions
The 2008 FDA guidance for diabetes drug development greatly increased the collection of data on conventional CV outcomes of treatment. Studies completed to date and those now under way are providing strong evidence for CV benefit for several drugs and reassurance about the lack of CV risk for many others. In addition, they have yielded further information on non-CV benefits, such as reduction of renal disease, and potential harms, such as HF and lower-limb amputations. This information has built a strong foundation of outcomes evidence that is truly transforming diabetes care. The information on improved CV outcomes for specific antidiabetes drugs should be considered in revised clinical treatment recommendations, given that cardioprotection is an added benefit.
However, after nearly a decade of experience with CVOTs designed on the basis of the 2008 guidance, it is time to reevaluate how best to conduct such trials. Future trials might benefit from focusing on populations more typical of those seen in routine care, with longer duration of follow-up, greater consistency in the ascertainment of outcomes, and improved statistical methods for analysis. In addition, greater efficiency and relevance to clinical practice might be obtained by collaboration of various stakeholders in health care—including pharmaceutical companies, regulatory groups, insurers, health systems, consumer advocates, and people with diabetes themselves—in the conduct of trials, perhaps by embedding clinical protocols within health systems. It is to be expected that future trials, like those already completed, will provide both answers to the primary research questions posed and new insights regarding previously unexpected benefits and risks.
Supplementary Material
Supplementary Data
Article Information
Acknowledgments. Writing and editing support services for this article were provided by Debbie Kendall of Kendall Editorial in Richmond, Virginia. The authors thank Christian S. Kohler, the ADA’s Associate Publisher, Scholarly Journals, and his staff for their assistance, guidance, and expertise in convening the Expert Forum.
Duality of Interest. W.T.C., prior to joining the ADA, received grant support awarded to his institution from Novo Nordisk and Sanofi. He served as a consultant for Adocia, Intarcia Therapeutics, and Sanofi. He has no active disclosures. S.K. is a consultant to and advisor for Boehringer Ingelheim, Eli Lilly, and Novo Nordisk and is a stock shareholder in Johnson & Johnson. H.C.G. holds the McMaster-Sanofi Population Health Institute Chair in Diabetes Research and Care. He has received research grant support from AstraZeneca, Eli Lilly, Merck, and Sanofi; honoraria for speaking engagements from AstraZeneca, Boehringer Ingelheim, Novo Nordisk, and Sanofi; and consulting fees from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck, Novo Nordisk, and Sanofi. R.R.H. reports receiving grants from AstraZeneca; grants and personal fees from Bayer, Boehringer Ingelheim, and Merck; personal fees from Amgen, Novartis, and Servier; and other support from Elcelyx Therapeutics, GlaxoSmithKline, Janssen, and Takeda. B.Z. is a consultant to and has received honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, and Sanofi and has received grant support from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk. J.S.S. has been an advisor to Adocia, AstraZeneca, BD Technologies, Boehringer Ingelheim, Dance Biopharm, Diavacs, Elcelyx Therapeutics, Eli Lilly, Ideal Life, ImmunoMolecular Therapeutics, Intarcia Therapeutics, Intrexon, Merck, Orgenesis, Sanofi, Servier, vTv Therapeutics, Valeritas, and Viacyte. He has received research funding from Mesoblast and Viacyte. He is a member of the board of directors of Dexcom, Intarcia Therapeutics, Moerae Matrix, and VasoPrep Surgical. He is a stock shareholder in Dance Biopharm, Dexcom, Ideal Life, Intarcia Therapeutics, Intrexon, Moerae Matrix, and VasoPrep Surgical. J.B.G. is a consultant to Boehringer Ingelheim and Daiichi Sankyo and has received research funding from AstraZeneca, GlaxoSmithKline, and Merck. J.B.B. has received contracted consulting fees, paid to his institution, and travel support from Adocia, AstraZeneca, Dance Biopharm, Dexcom, Elcelyx Therapeutics, Eli Lilly, Fractyl, GI Dynamics, Intarcia Therapeutics, Lexicon, Merck, Metavention, NovaTarg, Novo Nordisk, Orexigen, PhaseBio, Sanofi, Shenzhen HighTide, and vTv Therapeutics and grant support from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, GI Dynamics, GlaxoSmithKline, Intarcia Therapeutics, Johnson & Johnson, Lexicon, Medtronic, Merck, Novo Nordisk, Orexigen, Sanofi, Scion NeuroStim, Takeda, and Theracos. He has received fees and holds stock options in Insulin Algorithms and PhaseBio and serves on the board of the AstraZeneca HealthCare Foundation. S.E.I. has served as a consultant to Alere, Janssen, and vTv Therapeutics. He has participated on clinical trial steering or executive committees for AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Eisai, Novo Nordisk, and Sanofi/Lexicon. He has served as a member of a data monitoring committee for Intarcia Therapeutics. L.A.L. has received research funding from, has provided continuing medication education on behalf of, and/or has acted as an advisor to AstraZeneca, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Janssen, Merck, Novo Nordisk, Sanofi, and Servier. I.R. has received fees for serving on advisory boards for AstraZeneca, Boehringer Ingelheim, Concenter BioPharma/Silkim, Eli Lilly, LabStyle Innovations, Merck Sharp & Dohme, Novo Nordisk, Orgenesis, Pfizer, Sanofi, and SmartZyme Innovation; fees for consultant services from AstraZeneca/Bristol-Myers Squibb, Diabetes Medical Center, FutuRx, Gili Medical, Insuline Medical, Kamada, and Medial EarlySign; and fees for serving on speakers’ bureaus from AstraZeneca/Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly, Johnson & Johnson, Merck Sharp & Dohme, Novartis Pharma AG, Novo Nordisk, Sanofi, and Teva. He is a stock shareholder in GlucoMe, Insuline Medical, LabStyle Innovations, and Orgenesis. J.R. has served on scientific advisory boards and received honoraria or consulting fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Intarcia Therapeutics, Janssen, Novo Nordisk, and Sanofi. He has received grants/research support from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Genentech, GlaxoSmithKline, Intarcia Therapeutics, Janssen, Lexicon, Merck, Novo Nordisk, Pfizer, and Sanofi. M.C.R. has received honoraria for consulting, speaking, or serving on committees for Adocia, AstraZeneca, Elcelyx Therapeutics, Eli Lilly, GlaxoSmithKline, Sanofi, Theracos, and Valeritas and has received research support through his institution from AstraZeneca, Eli Lilly, and Novo Nordisk; these potential dualities have been reviewed and managed by Oregon Health & Science University. No other potential conflicts of interest relevant to this article were reported.
Footnotes
References
- 1.U.S. Food and Drug Administration. Guidance for industry: diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes [Internet]. Available from www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071627.pdf. Accessed 31 October 2016
- 2.Laakso M. Cardiovascular disease in type 2 diabetes from population to man to mechanisms: the Kelly West Award Lecture 2008. Diabetes Care 2010;33:442–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457–2471 [DOI] [PubMed] [Google Scholar]
- 4.Caveney E, Turner JR. White paper. A review of FDA guidance: understanding the FDA guidance on assessing cardiovascular risks for new antidiabetic therapies [Internet]. Available from http://www.quintiles.com/library/white-papers/new-fda-guidance-on-antidiabetic-therapies. Accessed 31 October 2016
- 5.European Medicines Agency. Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus [Internet], 2012. Available from http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129256.pdf. Accessed 3 November 2016
- 6.Scirica BM, Bhatt DL, Braunwald E, et al.; SAVOR-TIMI 53 Steering Committee and Investigators . Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–1326 [DOI] [PubMed] [Google Scholar]
- 7.White WB, Cannon CP, Heller SR, et al.; EXAMINE Investigators . Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327–1335 [DOI] [PubMed] [Google Scholar]
- 8.Green JB, Bethel MA, Armstrong PW, et al.; TECOS Study Group . Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:232–242 [DOI] [PubMed] [Google Scholar]
- 9.Pfeffer MA, Claggett B, Diaz R, et al.; ELIXA Investigators . Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373:2247–2257 [DOI] [PubMed] [Google Scholar]
- 10.Marso SP, Daniels GH, Brown-Frandsen K, et al.; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marso SP, Bain SC, Consoli A, et al.; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844 [DOI] [PubMed] [Google Scholar]
- 12.Holman RR, Bethel MA, Mentz RJ, et al.; EXSCEL Study Group . Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017;377:1228–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 14.Neal B, Perkovic V, Mahaffey KW, et al.; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657 [DOI] [PubMed] [Google Scholar]
- 15.Rawshani A, Rawshani A, Franzén S, et al. . Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med 2017;376:1407–1418 [DOI] [PubMed] [Google Scholar]
- 16.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–3421 [PubMed] [Google Scholar]
- 17.Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 18.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 19.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 20.Gerstein HC, Miller ME, Byington RP, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel A, MacMahon S, Chalmers J, et al.; ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 22.Duckworth W, Abraira C, Moritz T, et al.; VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 23.Ohkubo Y, Kishikawa H, Araki E, et al. . Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 1995;28:103–117 [DOI] [PubMed] [Google Scholar]
- 24.Ray KK, Seshasai SR, Wijesuriya S, et al. . Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet 2009;373:1765–1772 [DOI] [PubMed] [Google Scholar]
- 25.Turnbull FM, Abraira C, Anderson RJ, et al.; Control Group . Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009;52:2288–2298 [DOI] [PubMed] [Google Scholar]
- 26.Nathan DM, Cleary PA, Backlund JY, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orchard TJ, Nathan DM, Zinman B, et al.; Writing Group for the DCCT/EDIC Research Group . Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA 2015;313:45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 29.Hayward RA, Reaven PD, Wiitala WL, et al.; VADT Investigators . Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;372:2197–2206 [DOI] [PubMed] [Google Scholar]
- 30.ACCORD Study Group Nine-year effects of 3.7 years of intensive glycemic control on cardiovascular outcomes. Diabetes Care 2016;39:701–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holman RR, Sourij H, Califf RM. Cardiovascular outcome trials of glucose-lowering drugs or strategies in type 2 diabetes. Lancet 2014;383:2008–2017 [DOI] [PubMed] [Google Scholar]
- 32.John M, Gopalakrishnan Unnikrishnan A, Kalra S, Nair T. Cardiovascular outcome trials for anti-diabetes medication: a holy grail of drug development? Indian Heart J 2016;68:564–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA 2005;294:2581–2586 [DOI] [PubMed] [Google Scholar]
- 34.Drugs.com. Bristol-Myers Squibb and Merck joint statement on FDA advisory committee vote on Pargluva (muraglitazar), an investigational oral treatment for type 2 diabetes [Internet]. Available from https://www.drugs.com/nda/pargluva_050909.html. Accessed 3 November 2016
- 35.Home PD, Pocock SJ, Beck-Nielsen H, et al.; RECORD Study Team . Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 2009;373:2125–2135 [DOI] [PubMed] [Google Scholar]
- 36.Mahaffey KW, Hafley G, Dickerson S, et al. . Results of a reevaluation of cardiovascular outcomes in the RECORD trial. Am Heart J 2013;166:240–249.e1 [DOI] [PubMed] [Google Scholar]
- 37.U.S. Food and Drug Administration. FDA drug safety communication: FDA requires removal of some prescribing and dispensing restrictions for rosiglitazone-containing diabetes medicines [Internet]. Available from https://www.fda.gov/drugs/drugsafety/ucm376389.htm. Accessed 9 November 2017
- 38.Dormandy JA, Charbonnel B, Eckland DJ, et al.; PROactive Investigators . Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005;366:1279–1289 [DOI] [PubMed] [Google Scholar]
- 39.Kernan WN, Viscoli CM, Furie KL, et al.; IRIS Trial Investigators . Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016;374:1321–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young LH, Viscoli CM, Curtis JP, et al. . IRIS Investigators. Cardiac outcomes after ischemic stroke or TIA: effects of pioglitazone in patients with insulin resistance without diabetes. Circulation 2017;135:1882–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.U.S. Food and Drug Administration Center for Drug Evaluation and Research. Application Number 204042Orig1s000: summary review [Internet]. Available from https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204042Orig1s000SumR.pdf. Accessed 5 May 2017
- 42.Regier EE, Venkat MV, Close KL. More than seven years of hindsight: revisiting the FDA’s 2008 guidance on cardiovascular outcomes trials for type 2 diabetes medications. Clin Diabetes 2016;34:173–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Novo Nordisk. Novo Nordisk receives complete response letter in the US for Tresiba and Ryzodeg [Internet]. Available from https://www.novonordisk.com/bin/getPDF.1676900.pdf. Accessed 4 November 2016
- 44.Boehringer Ingelheim. CAROLINA: cardiovascular outcome study of linagliptin versus glimepiride in patients with type 2 diabetes. In: ClinicalTrials.gov [Internet]. Bethesda, MD, National Library of Medicine. Available from https://clinicaltrials.gov/show/NCT01243424. NLM Identifier: NCT01243424. Accessed 6 April 2017
- 45.Boehringer Ingelheim. Cardiovascular and renal microvascular outcome study with linagliptin in patients with type 2 diabetes mellitus (CARMELINA). In: ClinicalTrials.gov [Internet]. Bethesda, MD, National Library of Medicine. Available from https://clinicaltrials.gov/show/NCT01897532. NLM Identifier: NCT01897532. Accessed 10 February 2017
- 46.Janssen Research & Development. Evaluation of the effects of canagliflozin on renal and cardiovascular outcomes in participants with diabetic nephropathy (CREDENCE). In: ClinicalTrials.gov [Internet]. Bethesda, MD, National Library of Medicine. Available from https://clinicaltrials.gov/show/NCT02065791. NLM Identifier: NCT02065791. Accessed 10 February 2017
- 47.AstraZeneca. Multicenter trial to evaluate the effect of dapagliflozin on the incidence of cardiovascular events (DECLARE-TIMI 58). In: ClinicalTrials.gov [Internet]. Bethesda, MD, National Library of Medicine. Available from https://clinicaltrials.gov/show/NCT01730534. NLM Identifier: NCT01730534. Accessed 10 February 2017
- 48.Merck Sharp & Dohme. Cardiovascular outcomes following ertugliflozin treatment in type 2 diabetes mellitus participants with vascular disease, the VERTIS CV study (MK-8835-004). In: ClinicalTrials.gov [Internet]. Bethesda, MD, National Library of Medicine. Available from https://clinicaltrials.gov/show/NCT01986881. NLM Identifier: NCT01986881. Accessed 14 February 2017
- 49.AstraZeneca. Study to evaluate the effect of dapagliflozin on the incidence of worsening heart failure or cardiovascular death in patients with chronic heart failure (Dapa-HF). In: ClinicalTrials.gov [Internet]. Bethesda, MD, National Library of Medicine. Available from https://clinicaltrials.gov/show/NCT03036124. NLM Identifier: NCT03036124. Accessed 6 April 2017
- 50.AstraZeneca. A study to evaluate the effect of dapagliflozin on renal outcomes and cardiovascular mortality in patients with chronic kidney disease (Dapa-CKD). In: ClinicalTrials.gov [Internet]. Bethesda, MD, National Library of Medicine. Available from https://clinicaltrials.gov/show/NCT03036150. NLM Identifier: NCT03036150. Accessed 4 April 2017
- 51.Boehringer Ingelheim. Empagliflozin outcome trial in patients with chronic heart failure with reduced ejection fraction (EMPEROR-Reduced). In: ClinicalTrials.gov [Internet]. Bethesda, MD, National Library of Medicine. Available from https://clinicaltrials.gov/show/NCT03057977. NLM Identifier: NCT03057977. Accessed 6 April 2017
- 52.Boehringer Ingelheim. Empagliflozin outcome trial in patients with chronic heart failure with preserved ejection fraction (EMPEROR-Preserved). In: ClinicalTrials.gov [Internet]. Bethesda, MD, National Library of Medicine. Available from https://clinicaltrials.gov/show/NCT03057951. NLM Identifier: NCT03057951. Accessed 6 April 2017
- 53.Intarcia Therapeutics. Intarcia announces successful cardiovascular safety results in phase 3 FREEDOM-CVO trial for ITCA 650, an investigational therapy for type 2 diabetes: company also reports new $75 million financing for manufacturing scale-up and inventory build for anticipated global launch of ITCA 650 [Internet]. Available from https://www.intarcia.com/media/press-releases/2016-may-6-cardiovascular-safety.html. Accessed 14 February 2017
- 54.Gerstein HC, Colhoun HM, Dagenais GR, et al.; REWIND Trial Investigators . Design and baseline characteristics of participants in the Researching cardiovascular Events with a Weekly INcretin in Diabetes (REWIND) trial on the cardiovascular effects of dulaglutide. Diabetes Obes Metab 14 July 2017 [Epub ahead of print]. https://doi.org/10.1111/dom.13028 [DOI] [PubMed] [Google Scholar]
- 55.GlaxoSmithKline. Effect of albiglutide, when added to standard blood glucose lowering therapies, on major cardiovascular events in subjects with type 2 diabetes mellitus. In: ClinicalTrials.gov [Internet]. Available from https://clinicaltrials.gov/show/NCT02465515. NLM Identifier: NCT02465515. Accessed 14 February 2017
- 56.Novo Nordisk A/S. A trial investigating the cardiovascular safety of oral semaglutide in subjects with type 2 diabetes (PIONEER 6). In: ClinicalTrials.gov [Internet]. Available from https://clinicaltrials.gov/show/NCT02692716. NLM Identifier: NCT02692716. Accessed 29 June 2017
- 57.Marso SP, McGuire DK, Zinman B, et al.; DEVOTE Study Group . Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med 2017;377:723–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holman RR, Coleman RL, Chan JCN, et al.; ACE Study Group . Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2017;5:877–886 [DOI] [PubMed] [Google Scholar]
- 59.Zannad F, Cannon CP, Cushman WC, et al.; EXAMINE Investigators . Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet 2015;385:2067–2076 [DOI] [PubMed] [Google Scholar]
- 60.Wanner C, Inzucchi SE, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323–334 [DOI] [PubMed] [Google Scholar]
- 61.Theodorakis MJ, Coleman RL, Feng H, et al. . Baseline characteristics and temporal differences in Acarbose Cardiovascular Evaluation (ACE) trial participants. Am Heart J. 1 September 2017 [Epub ahead of print]. https://doi.org/10/1016/j.ahj.2017.09.001 [DOI] [PubMed] [Google Scholar]
- 62.Gantz I, Chen M, Suryawanshi S, et al. . A randomized, placebo-controlled study of the cardiovascular safety of the once-weekly DPP-4 inhibitor omarigliptin in patients with type 2 diabetes mellitus. Cardiovasc Diabetol 2017;16:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Merck. Merck provides update on filing plans for omarigliptin, an investigational DPP-4 inhibitor for type 2 diabetes [Internet]. Available from http://www.mrknewsroom.com/news/company-statements/merck-provides-update-filing-plans-omarigliptin-investigational-dpp-4-inhibi. Accessed 7 April 2017
- 64.U.S. Food and Drug Administration. FDA drug safety communication: FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin [Internet]. Available from http://www.fda.gov/Drugs/DrugSafety/ucm486096.htm. Accessed 1 January 2017
- 65.Rosenstock J, Marx N, Kahn SE, et al. . Cardiovascular outcome trials in type 2 diabetes and the sulphonylurea controversy: rationale for the active-comparator CAROLINA trial. Diab Vasc Dis Res 2013;10:289–301 [DOI] [PubMed] [Google Scholar]
- 66.Neal B, Perkovic V, Matthews DR, et al.; CANVAS-R Trial Collaborative Group . Rationale, design and baseline characteristics of the CANagliflozin cardioVascular Assessment Study-Renal (CANVAS-R): a randomized, placebo-controlled trial. Diabetes Obes Metab 2017;19:387–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eli Lilly. Jardiance (empagliflozin) tablets to be studied in chronic kidney disease [Internet]. Available from https://investor.lilly.com/releasedetail.cfm?releaseid=1029854. Accessed 11 October 2017
- 68.Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care 2016;39:1108–1114 [DOI] [PubMed] [Google Scholar]
- 69.Mudaliar S, Alloju S, Henry RR. Can shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care 2016;39:1115–1122 [DOI] [PubMed] [Google Scholar]
- 70.Abdul-Ghani M, DeFronzo RA, Del Prato S, Chilton R, Singh R, Ryder REJ. Cardiovascular disease and type 2 diabetes: has the dawn of a new era arrived? Diabetes Care 2017;40:813–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.U.S. Food and Drug Administration. FDA approves Jardiance to reduce cardiovascular death in adults with type 2 diabetes [Internet]. Available from http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm531517.htm. Accessed 14 February 2017
- 72.American Diabetes Association Pharmacologic approaches to glycemic control. Sec. 8. In Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(Suppl. 1):S64–S74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neal B, Perkovic V, de Zeeuw D, et al. . Rationale, design, and baseline characteristics of the Canagliflozin Cardiovascular Assessment Study (CANVAS)--a randomized placebo-controlled trial. Am Heart J 2013;166:217–223.e11 [DOI] [PubMed] [Google Scholar]
- 74.Janssen Pharmaceuticals. FDA briefing document: NDA 204042 Invokana (canagliflozin) tablets [Internet]. Endocrinologic and Metabolic Drugs Advisory Committee Meeting. 10 January 2013. Available from https://wayback.archive-it.org/7993/20170405220010/https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM334550.pdf. Accessed 10 October 2017
- 75.Neal B, Perkovic V, Mahaffey KW, et al.; CANVAS Program collaborative group . Optimizing the analysis strategy for the CANVAS Program: a prespecified plan for the integrated analyses of the CANVAS and CANVAS-R trials. Diabetes Obes Metab 2017;19:926–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Victoza liraglutide injection 1.2 mg/1.8 mg [prescribing information]. Plainsboro, NJ, Novo Nordisk, 2017
- 77.Novo Nordisk. Semaglutide receives positive 16-0 vote in favour of approval from FDA Advisory Committee [Internet]. Available from https://www.novonordisk.com/bin/getPDF.2142854.pdf. Accessed 19 October 2017
- 78.Intarcia Therapeutics. Intarcia submits new drug application (NDA) to FDA for U.S. marketing approval of ITCA 650 in type 2 diabetes [Internet]. Available from https://www.intarcia.com/media/press-releases/2016-nov-21-nda-submission.html. Accessed 10 April 2017
- 79.Lincoff AM, Tardif J-C, Schwartz GG, et al.; AleCardio Investigators . Effect of aleglitazar on cardiovascular outcomes after acute coronary syndrome in patients with type 2 diabetes mellitus: the AleCardio randomized clinical trial. JAMA 2014;311:1515–1525 [DOI] [PubMed] [Google Scholar]
- 80.Erdmann E, Califf R, Gerstein HC, et al. . Effects of the dual peroxisome proliferator-activated receptor activator aleglitazar in patients with type 2 diabetes mellitus or prediabetes. Am Heart J 2015;170:117–122 [DOI] [PubMed] [Google Scholar]
- 81.Vaccaro O, Masulli M, Nicolucci A, et al.; Thiazolidinediones Or Sulfonylureas Cardiovascular Accidents Intervention Trial (TOSCA.IT) study group; Italian Diabetes Society. Effects on the incidence of cardiovascular events of the addition of pioglitazone versus sulfonylureas in patients with type 2 diabetes inadequately controlled with metformin (TOSCA.IT): a randomised, multicentre trial. Lancet Diabetes Endocrinol 2017;5:887–897 [DOI] [PubMed] [Google Scholar]
- 82.Gerstein HC, Bosch J, Dagenais GR, et al.; ORIGIN Trial Investigators . Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012;367:319–328 [DOI] [PubMed] [Google Scholar]
- 83.ORIGIN Trial Investigators Cardiovascular and other outcomes postintervention with insulin glargine and omega-3 fatty acids (ORIGINALE). Diabetes Care 2016;39:709–716 [DOI] [PubMed] [Google Scholar]
- 84.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee Pharmacologic management of type 2 diabetes: 2016 interim update. Can J Diabetes 2016;40:484–486 [DOI] [PubMed] [Google Scholar]
- 85.McMurray JJ, Gerstein HC, Holman RR, Pfeffer MA. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol 2014;2:843–851 [DOI] [PubMed] [Google Scholar]
- 86.Fitchett D, Zinman B, Wanner C, et al.; EMPA-REG OUTCOME® trial investigators . Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur Heart J 2016;37:1526–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weidmann P, Ferrari P. Central role of sodium in hypertension in diabetic subjects. Diabetes Care 1991;14:220–232 [DOI] [PubMed] [Google Scholar]
- 88.Gerstein HC, Miller ME, Genuth S, et al.; ACCORD Study Group . Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 2011;364:818–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.The NNT Group. Quick summaries of evidence-based medicine [Internet]. Available from http://www.thennt.com. Accessed 5 February 2017
- 90.Jones WS, Roe MT, Antman EM, et al. . The changing landscape of randomized clinical trials in cardiovascular disease. J Am Coll Cardiol 2016;68:1898–1907 [DOI] [PubMed] [Google Scholar]
- 91.Bethel MA, Holman R, Haffner SM, et al. . Determining the most appropriate components for a composite clinical trial outcome. Am Heart J 2008;156:633–640 [DOI] [PubMed] [Google Scholar]
- 92.Stolker JM, Spertus JA, Cohen DJ, et al. . Rethinking composite end points in clinical trials: insights from patients and trialists. Circulation 2014;130:1254–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pocock SJ, Clayton TC, Stone GW. Design of major randomized trials: part 3 of a 4-part series on statistics for clinical trials. J Am Coll Cardiol 2015;66:2757–2766 [DOI] [PubMed] [Google Scholar]
- 94.Rogers JK, Jhund PS, Perez AC, et al. . Effect of rosuvastatin on repeat heart failure hospitalizations: the CORONA Trial (Controlled Rosuvastatin Multinational Trial in Heart Failure). JACC Heart Fail 2014;2:289–297 [DOI] [PubMed] [Google Scholar]
- 95.Rogers JK, Pocock SJ, McMurray JJ, et al. . Analysing recurrent hospitalizations in heart failure: a review of statistical methodology, with application to CHARM-Preserved. Eur J Heart Fail 2014;16:33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gallin EK, Bond E, Califf RM, et al. . Forging stronger partnerships between academic health centers and patient-driven organizations. Acad Med 2013;88:1220–1224 [DOI] [PubMed] [Google Scholar]
- 97.Bergenstal RM, Bailey CJ, Kendall DM. Type 2 diabetes: assessing the relative risks and benefits of glucose-lowering medications. Am J Med 2010;123:374.e9–e18 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data