An Antibody to the Tetraspan Membrane Protein CD9 Promotes Neurite Formation in a Partially α3β1 Integrin-Dependent Manner (original) (raw)
Abstract
The tetraspan cell surface glycoprotein, CD9, has been implicated in cellular signaling during growth and differentiation in the hematopoietic and nervous systems. Because CD9 expression is induced early in development in sensory and sympathetic neuroblasts, we investigated the role of CD9 in neurite outgrowth. We plated dissociated cells from neonatal sympathetic ganglia on immobilized anti-CD9 antibodies or antibodies against other cell surface molecules. We show here that B2C11, an anti-CD9 antibody that has been shown previously to activate Schwann cells in vitro, promotes robust neurite outgrowth from sympathetic neurons that is greater than that on other antibody surfaces and is comparable to neurite outgrowth on a collagen substratum. In addition, B2C11 causes dramatic morphological changes in neurons and glia from dissociated ganglia, including a flattening of these cells.
Because CD9 interacts with integrins in many cell types including Schwann cells, and specifically with the α3β1 integrin in some cells, we tested whether the effect of B2C11 on neurite outgrowth is mediated by this integrin. An anti-α3β1 antibody, Ralph 3–1, attenuates the extent of neurite outgrowth on B2C11 and collagen, but not on laminin. Because the α3β1 integrin has been shown to mediate neurite outgrowth on different substrates, these results provide a functional significance for the CD9-α3β1 interaction; downstream signaling may be activated by this cis interaction on the cell surface in response to external cues that promote neurite outgrowth.
Keywords: CD9, tetraspan proteins, antibody perturbation, neurite outgrowth, sympathetic neurons, α3β1 integrin
During the course of nervous system development, neurons extend neurites that traverse long distances to establish highly selective connections. This process involves diffusible chemoattractants and chemorepellents such as the recently described netrins and semaphorins (Keynes and Cook, 1995) as well as ECM and cell surface molecules (Venstrom and Reichardt, 1993; Letourneau et al., 1994). The ECM components involved in neurite outgrowth include collagen; laminin; fibronectin; thrombospondin and vitronectin, which interact with heterodimeric cell surface receptor proteins known as the integrins (Rathjen, 1991; Reichardt and Tomaselli, 1991; Hynes and Lander, 1992; Letourneau et al., 1994). Other cell surface adhesion molecules involved include the cadherins and immunoglobulin superfamily members (Rathjen, 1991; Hynes and Lander, 1992). The cellular signaling cascades that lie downstream of these first-order interactions and lead to cytoskeletal rearrangements and process extension are also being elucidated (Doherty and Walsh, 1994; Tanaka and Sabry, 1995).
The tetraspan integral membrane proteins constitute another family of cell surface molecules that is involved in intercellular signaling (Wright and Tomlinson, 1994). One of these proteins, CD9, has been recently described in the rat nervous system (Tole and Patterson, 1993; Kaprielian et al., 1995). CD9 interacts in cis (within the membrane of the same cell) with members of the integrin protein family in many cell types, and many of the functions ascribed to CD9 may depend on CD9–integrin interactions (Higashihara et al., 1985;Slupsky et al., 1989; Letarte et al., 1992; Rubinstein et al., 1994;Nakamura et al., 1995; Shaw et al., 1995).
CD9 is expressed in Schwann cells during development (Kaprielian et al., 1995) and after injury (Banerjee and Patterson, 1995) in a pattern that mimics the myelin genes, suggesting the possibility that CD9 may play a signaling role in vivo. Moreover, experiments using mAbs against CD9 have implicated this protein in Schwann cell adhesion, proliferation, and migration (Anton et al., 1995; Hadjiargyrou and Patterson, 1995).
CD9 is also expressed by neurons (Tole and Patterson, 1993; Kaprielian et al., 1995). Although CD9 is not detectable on migrating neural crest cells, expression is upregulated very soon after these cells coalesce into various tissue derivatives (E11-E12 in the rat), and CD9 is found on the surface of neuroblasts in the SCG at E12 and in DRG at E15. This time of onset of CD9 expression is correlated with the early stages of neuronal differentiation (Tole and Patterson, 1993), and prompted us to investigate the role of CD9 in neurite outgrowth. We find that one anti-CD9 mAb specifically stimulates neurite extension and induces cytoskeletal rearrangement and dramatic morphological changes in the somas and processes of cultured sympathetic neurons and glia. We further demonstrate that an Ab against the α3β1 integrin attenuates the extent of neurite outgrowth induced by the anti-CD9 mAb.
MATERIALS AND METHODS
Antibodies. mAbs utilized in these experiments were described previously (DeFreitas et al., 1995; Hadjiargyrou et al., 1996). Briefly, mAbs to rat surface proteins, CD9 (ROCA1, ROCA2 and B2C11), p75LNGFR (192-IgG), Thy-1 (OX-7), a heparan sulfate proteoglycan (pg22), and the α3β1 integrin (Ralph-1) are all mouse IgGs. They were purified from hybridoma supernatants using the mAb Trap-G kit (Pharmacia) and stored at −80°C in a solution containing 1 m glycine-HCl, pH 2.7, and 60 mm Tris-HCl, pH 9 (final, pH 7.6). The Ralph-1 mAb was a generous gift of Dr. Louis Reichardt (University of California, San Francisco, CA), and purified IgGs and F(ab) fragments of an NCAM mAb were a generous gift of Dr. Urs Rutishauser (Case Western Reserve University, Cleveland, OH).
Sympathetic neuronal cultures. Superior cervical ganglia were dissected from neonatal rats and enzymatically dissociated as described previously (Banerjee and Patterson, 1995). Dissociated cells were plated on various surfaces and grown in complete medium: L15-CO2 containing fresh vitamin mix (Hawrot and Patterson, 1979), 5 μg/ml bovine insulin (Sigma, St. Louis, MO), 100 μg/ml transferrin (Sigma), and 100 ng/ml NGF (Boehringer– Mannheim, Indianapolis, IN).
Neurite outgrowth assays. For quantitation of neurite outgrowth, 8-well glass slides (Roboz Surgical Instrument Co. Inc., Rockville, MD) were sterilized by first being immersed in 95% ethanol, followed by flaming. Each well of the 8-well slide was coated with 5 μl of a solution of 5 cm2 of type BA85 nitrocellulose (Schleicher & Schuell, Keene, NH) dissolved in 6 ml methanol (Lagenaur and Lemmon, 1987), and allowed to dry in a tissue culture hood. Each purified mAb (5 μg/ml), which was diluted in 100 mm carbonate buffer, pH 9.6, and rat tail collagen (Hawrot and Patterson, 1979), were added to the wells (50 μl total volume). The mAbs were allowed to bind to the nitrocellulose for 2–4 hours at room temperature (RT), followed by 2 washes with 1× PBS. To prevent nonspecific cell binding, the wells were then blocked for 1 hr at 37°C with a 5% BSA solution (in PBS), and washed twice with PBS.
Dissociated SCG cells were either directly added to each well at a density of 5 × 103 cells/well in 50 μl L15-CO2 medium, or preincubated with mAbs before plating. In the latter case, 5–6 × 103 cells were incubated for 30 min in 50 μl complete medium containing 100 μg/ml of either Ralph 3-1 anti-N-CAM IgG, anti-NCAM F(ab), or no Ab as controls. In one experiment, cells were incubated for 1 hr in 50 μl complete medium containing 20 μg/ml of either Ralph 3-1 or pg22 (data not shown). Cells were then added in this medium to the wells prepared as described above. The cultures were incubated at 37°C for 16 hr, washed well in L15-CO2 medium by flooding the well twice with medium and removing it, and fixed in 4% paraformaldehyde for 15 min at RT. They were then stained with anti-peripherin or anti-S100 Abs.
Cells were viewed on an inverted Nikon fluorescence microscope (Diaphot 300) and quantitated by scoring neuron numbers in the same standard area (approximately 3.5 mm2) on all surfaces. The length of neurite outgrowth was measured using a eyepiece micrometer. Adhesion and neurite outgrowth was measured in quadruplicate wells in individual experiments for each condition used, and the data are expressed as mean ± SEM of four determinations from a single experiment. Each experiment was repeated 4–5 times, but the data were not combined because the total number of neurons varied somewhat between experiments. The percentage of neurons with neurites was consistent for the different experiments, however, as were the fold differences between the different conditions used.
For the blocking experiments with integrin Abs, results from six wells from two different experiments were combined, and the results presented are mean ± SEM of these six determinations.
Immunocytochemistry. To test the ability of various mAbs to bind to sympathetic neurons, dissociated SCG cells were plated on 8-well sterile glass slides prepared as described above on rat tail collagen at a density of 1.5 × 104/well. The cultures were grown for 16 hr, and the cells were stained for surface antigens with purified mAbs (5 μg/ml) for 60 min at RT. Cells were then washed with culture medium and fixed in 4% paraformaldehyde for 15 min at RT. After fixation, cells were washed twice with PBS and incubated for 45–60 min at RT with FITC-conjugated, goat anti-mouse IgG secondary Ab (Hi-F, Antibodies, Inc.) diluted 1:200 in medium. Cells were then washed three times with PBS, and the slide was mounted in glycerol containing 8 mg/ml _n_-propyl gallate (dissolved in 100 mmTris-HCl, pH 9). Cells were viewed and photographed using an inverted Nikon fluorescence microscope (Diaphot 300).
S100 immunohistochemistry was performed as described previously (Banerjee and Patterson, 1995), except that the secondary Ab was an anti-rabbit Ab conjugated with FITC (Vector Laboratories, Burlingame, CA) and the stained material was mounted in glycerol containing_n_-propyl gallate, as described above.
Peripherin immunohistochemistry was performed by incubating the fixed cultures with anti-peripherin polyclonal Ab (Chemicon International, Temecula, CA) diluted 1:1000 in PBS containing 2% goat serum and 0.1% NP-40. After washes in PBS, cells were incubated with an FITC-conjugated anti-rabbit secondary Ab (Vector Laboratories), washed, and mounted in glycerol containing _n_-propyl gallate.
RESULTS
B2C11 and control Abs recognize cultured sympathetic neurons and glia
All of the purified mAbs used in these experiments bind well to living, dissociated cells from neonatal rat sympathetic ganglia. In addition to the CD9-activating mAb (B2C11; Hadjiargyrou and Patterson, 1995; Kaprielian et al., 1995), other mAbs used recognize CD9 (ROCA1 and ROCA2), the low affinity NGF receptor (192-IgG), Thy-1 (OX-7), and a heparan sulfate proteoglycan (pg22), and were chosen because of their reported ability to recognize sympathetic neurons (Mason and Williams, 1980; Chand-ler et al., 1984; Matthew et al., 1985; Mahanthappa and Patterson, 1992). Also, all the antigens are cell surface molecules, and the corresponding mAbs are of the IgG isotype. As shown in Figure1, pg22, OX-7, 192-IgG, and ROCA2 recognize and strongly immunostain the surface of neurons, as defined by morphology shown in the respective phase contrast micrographs. In addition, 192-IgG and ROCA2 immunostain the surface of Schwann cells (Fig. 1,E–H, arrows). The anti-CD9 mAb ROCA1 is an exception; it does not label sympathetic neurons (data not shown), consistent with the report by Kaprielian et al. (1995) that although ROCA1 immunoprecipates CD9 and recognizes CD9 on Western blots, it does not stain the surface of living cells. B2C11 recognizes both Schwann cells and neurons in dissociated SCG cultures (Fig. 1). The mAb concentration used for histological staining, 5 μg/ml, is the same as that used to coat surfaces in the experiments described below.
Fig. 1.
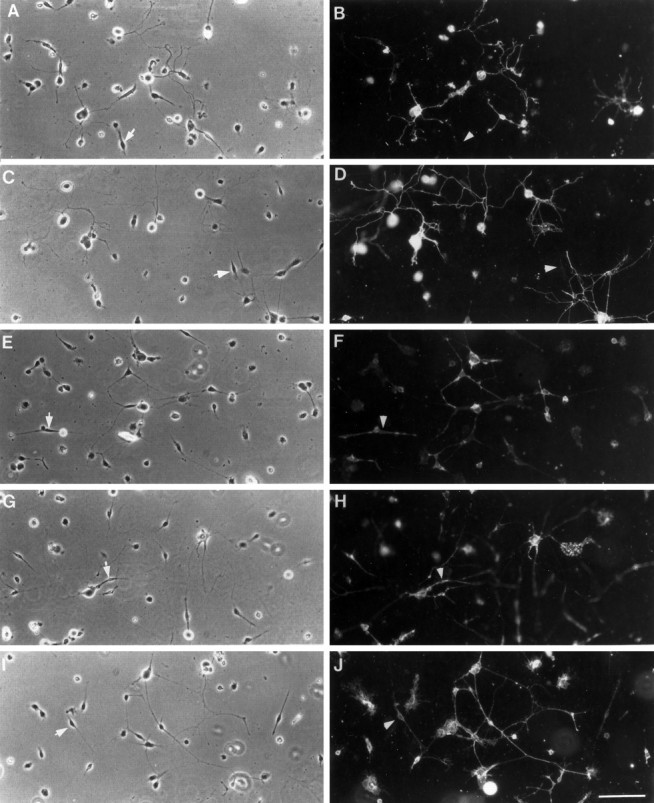
Immonostaining of SCG cultures with various mAbs. All mAbs used here stain sympathetic neurons, and most also stain glial cells. Dissociated SCGs were plated on collagen, cultured for 16 hr, and immunostained with pg22 (B), OX-7 (D), 192-IgG (F), ROCA2 (H), or B2C11 (J). The corresponding phase contrast micrographs are shown in A,C, E, G, and_I_, respectively. The arrows and_arrowheads_ point to glial cells and demonstrate that whereas 192-IgG, ROCA2, and B2C11 recognize Schwann cells, pg22 and OX-7 do not seem to do so. Bar, 100 μm.
Neurons adhere to immobilized B2C11
Using the experimental paradigm used to show that immobilized B2C11 activates Schwann cells (Hadjiargyrou and Patterson, 1995), sympathetic ganglia were dissociated and plated either on rat tail collagen type I or on various mAbs that had been immobilized on a nitrocellulose surface. Cells that had been cultured for 16 hr on the various surfaces were washed gently and fixed. Neurons were identified by immunostaining for peripherin, a neuron-specific intermediate filament protein (Portier et al., 1984). The number of peripherin-immunoreactive cells that remain on each surface after washing was determined. As shown in Figure 2, neurons adhere well on a variety of surfaces; numbers of neurons on B2C11, OX-7, and 192-IgG are comparable to those on the adhesive ECM protein, rat tail collagen type I. Adhesion is somewhat less on immobilized pg22 and ROCA2. In two other experiments, cells adhered well on collagen, B2C11, 192-IgG, and OX-7, and less well on pg22 and ROCA2 (approximately three- to fivefold more cells adhered to B2C11 than to pg22 and ROCA2), although the total number of cells on each surface varied somewhat from experiment to experiment. In experiments where the cultures were not washed before fixation, the numbers of cells on all surfaces were comparable (data not shown).
Fig. 2.
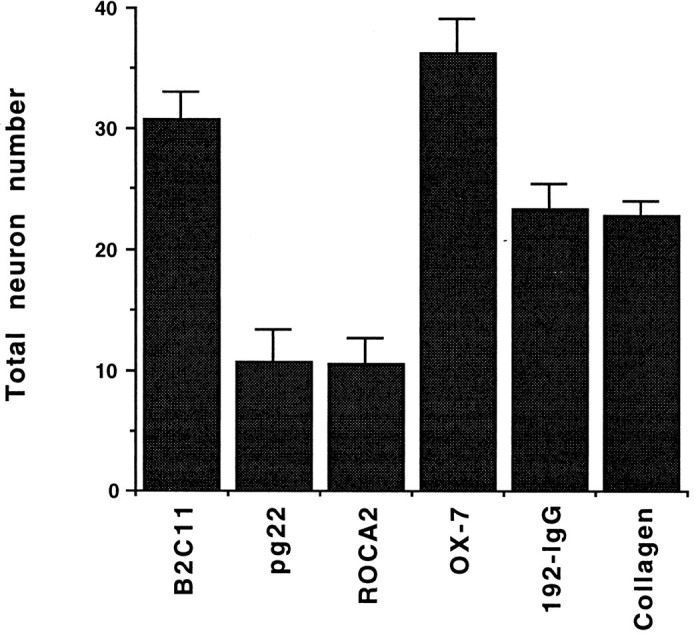
mAbs against NGFR, CD9, and Thy-1 promote neuronal adhesion. Dissociated SCG cells that had been cultured for 16 hr were immunostained for peripherin after careful washing. The number of neurons, identified by peripherin immunoreactivity, which adhered on each surface, was determined by microscopy. For each condition, four separate wells were established, and the data shown are mean ± SEM from quadruplicate determinations.
Neurons extend processes on B2C11
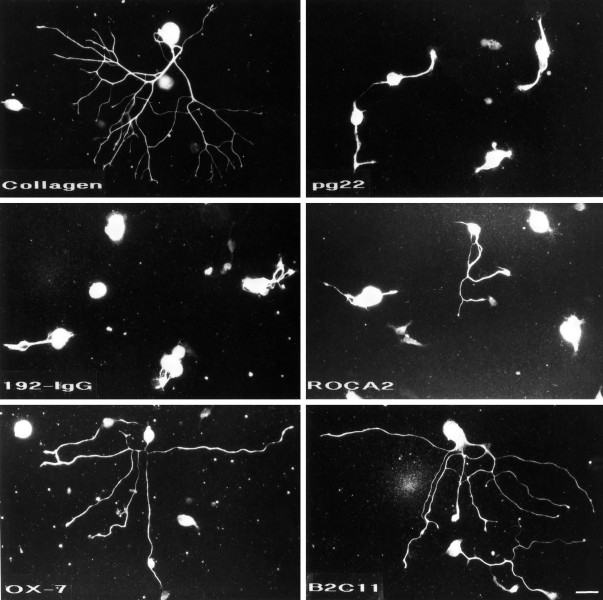
After 16 hr in culture, sympathetic neurons display robust neurite outgrowth on certain surfaces. Shown in Figure 3 are representative examples of neurite outgrowth on each surface tested. As would be expected, extensive neurite outgrowth is observed on rat tail collagen, a surface well characterized as favorable to neurite outgrowth. In contrast, neurons on pg22, 192-IgG, and ROCA2 display limited neurite outgrowth. Consistent with a previously demonstrated role for Thy-1 in neurite outgrowth (Leifer et al., 1984, 1991;Mahanthappa and Patterson, 1992), many neurons put out neurites on immobilized OX-7. Extensive neurite outgrowth that is more robust than that on OX-7, and comparable to that observed on collagen, is observed on B2C11.
Fig. 3.
B2C11 and OX-7 promote neurite outgrowth. Dissociated sympathetic neurons that had been cultured for 16 hr and stained for peripherin display robust neurite outgrowth on collagen, OX-7, and B2C11. On pg22, ROCA2, and 192-IgG, however, there is little or no neurite outgrowth. Bar, 25 μm.
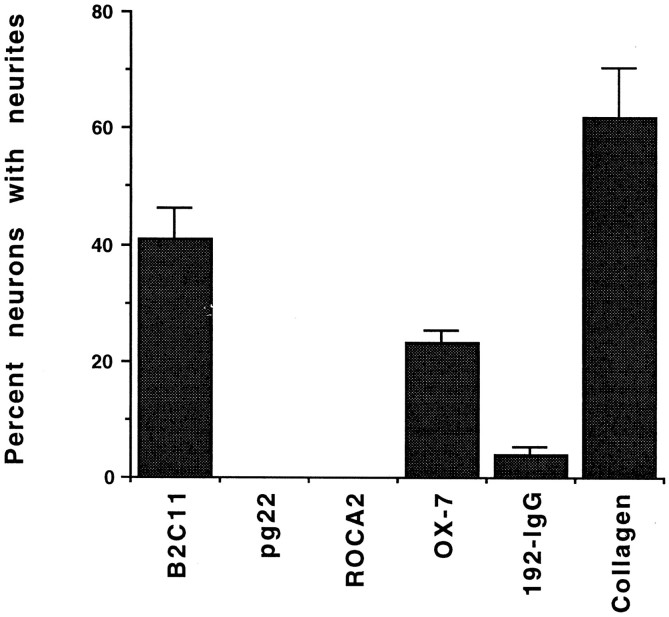
To quantitate the extent of neurite outgrowth, the percentage of neurons bearing neurites, as well as the length of neurites, was determined on each surface. The number of neurons with processes more than 100 μm in length were categorized as neurite-bearing cells. The percentages of neurite-bearing cells on each surface were consistent in four separate experiments. As shown in Figure 4, the percentage of neurons that extend neurites on immobilized B2C11 is similar to that on rat tail collagen type I. A smaller percentage of neurons extend neurites on OX-7. Neurons did not extend neurites on immobilized pg22 or ROCA2 in this experiment, and only a small percentage of cells had neurites on 192-IgG. In three other experiments, the mean percentages of neurite-bearing neurons were 40–62% on collagen, 41–54% on B2C11, 0–15% on pg22, 0–10% on ROCA2, 23–32% on OX-7, and 3–20% on 192-IgG. In experiments where the cultures were not washed before they were fixed and immunostained, numbers of neurons on pg22 and ROCA2 were comparable to the other surfaces. In these cases as well, the percentage of neurons with neurites were 7–17%, suggesting that fewer cells adhering to pg22 and ROCA2 after washing does not result in an underestimation of the percent outgrowth on these surfaces.
Fig. 4.
B2C11 and OX-7 promote neurite outgrowth. Sympathetic neurons were identified by peripherin immunoreactivity after culturing for 16 hr. Neurons with neurite lengths greater than 100 μm were scored as neurite-bearing cells, and the percentage of neurons that were neurite bearing was quantitated. For each condition, four separate wells were established, and the data shown are mean ± SEM from these quadruplicate determinations.
The length of the neurites on each of the surfaces was also quantitated. The representative data shown in Figure 5were obtained from the same experiment shown in Figures 2 and 4, and the data were pooled from quadruplicate cultures in a single experiment. These experiments were repeated three times, with the same overall trends shown in Figure 5. The neurite lengths were pooled in bins, and the number of neurons with neurite lengths in each bin in one experiment are shown in Figure 5, where each bin is represented as a bar. In three experiments, 38–62% of neurons on B2C11 have neurites in the smallest length bin, and 2–15% of neurons have neurites in the longest length bin. On rat tail collagen, the corresponding values are 23–40% (smallest length bin) and 3–23% (longest length bin). On OX-7, the corresponding values are 50–55% and 0–13%. These profiles indicate that neurons on B2C11 have neurite lengths shorter than those on collagen type I and longer than those on OX-7. In all experiments, the majority of neurite lengths of neurons on pg22, 192-IgG, and ROCA2 were confined to the bin with shortest length neurites (Fig. 5; data not shown).
Fig. 5.
Neurite length on B2C11 is equivalent to that on collagen. Neurite length was measured using an eyepiece reticule and was divided into classes, and the number of neurons with neurite lengths in each class was plotted for each condition. Neurite length on B2C11 is almost as long as that on collagen and much longer than that on OX-7. In this experiment, no neurites were observed from neurons on pg22 or ROCA2.
A mAb against the α3β1 integrin attenuates neurite outgrowth on B2C11
To determine whether the effects of B2C11 depend on a previously demonstrated association of CD9 with the α3β1 integrin, we tested the effect of a function-blocking mAb to α3β1, Ralph 3–1 (DeFreitas et al., 1995), on the extent of sympathetic neurite outgrowth on various surfaces. To do so, we preincubated dissociated SCG cells with either Ralph 3–1 or a variety of control mAbs, then plated them on either rat tail collagen, B2C11 or OX-7, as described above. In addition, we also plated these cells on 1 μg/ml laminin, which results in neurite outgrowth that is comparable to that on B2C11 and collagen, at the concentrations used in this study. As shown in Figure 6, Ralph 3–1 decreases the extent of neurite outgrowth by 38% on B2C11, 45% on OX-7, and 36% on collagen type I. Ralph 3–1 does not significantly modify the extent of neurite outgrowth on laminin, consistent with a previous report (DeFreitas et al., 1995). F(ab) fragments of an anti-NCAM mAb were used as one control and do not show any effect on outgrowth on on any surface. In addition, the intact anti-NCAM mAb and an anti-pg22 mAb also do not show any effect on neurite outgrowth (data not shown).
Fig. 6.
Anti-α3β1 mAb attenuates neurite outgrowth on B2C11 and OX-7. Dissociated SCG cells were preincubated with either Ralph 3–1 (anti-α3β1, hatched bar), anti-NCAM F(ab) (gray bar), or no Ab (black bar) and plated on collagen, B2C11, OX-7, or laminin. Percentages of peripherin-immunoreactive cells with neurites on various surfaces were quantitated. Data shown are mean ± SEM from six wells from two different experiments.
Morphology of neurons and Schwann cells is altered on B2C11
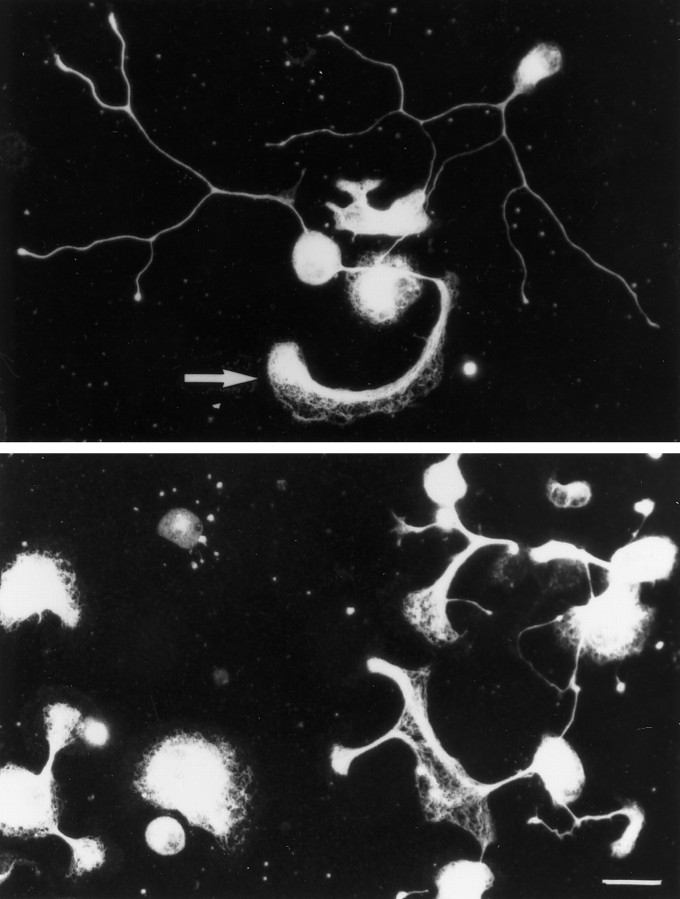
In addition to the robust neurite outgrowth observed on B2C11, this mAb induces dramatic morphological changes in the neurons. Unlike the round, phase-bright morphology seen on all other surfaces (Fig. 3), a large percentage of neurons flatten out on B2C11, and a peripherin-positive cytoskeleton in the flattened soma is easily seen (Fig. 7). In addition, neurites and growth cones also assume a flattened morphology on B2C11 (Fig. 7, arrow). A small percentage of neurons and neurites do, however, retain their usual three-dimensional morphology. Because peripherin is an intermediate filament, these results suggest a dramatic rearrangement of the cytoskeleton induced by neurons binding B2C11.
Fig. 7.
B2C11 induces altered neuronal morphology. Two examples of peripherin-immunoreactive cells on B2C11 are illustrated. Many of the cell bodies flatten out, and a peripherin-positive network is evident in somas, as well as in some neurites and growth cones (arrow). Bar, 25 μm.
To determine whether this altered morphological phenotype on B2C11 is restricted to neurons, we also tested B2C11 on ganglionic glial cells. S-100-positive glia have the expected bipolar morphology on collagen, as well as on 192-IgG, ROCA2, pg22, and OX-7 (Fig. 8). In contrast, on B2C11 (Fig. 8F), Schwann cells lose the bipolar shape and flatten out. A thinning of the cytoplasm, probably because of excessive spreading of the cell membrane, is observed, causing gaps in the cytoplasm in some cases; these areas are apparently devoid of cell membrane (Fig. 8F, arrow). These results are consistent with previously observed spreading of the S-16 Schwann cells on B2C11, observed starting at 2 hr after plating and extending up to 72 hr (Hadjiargyrou and Patterson, 1995).
Fig. 8.
B2C11 induces altered glial morphology. Dissociated SCG cells that had been cultured for 16 hr were immunostained for S100, a glial cell marker. Glia grown on collagen (A), 192-IgG (B), ROCA2 (C), pg22 (D), and OX-7 (E) have the normal bipolar morphology. On B2C11, in contrast, glial cells flatten out, with a thinning of the cytoplasm (F). Bar, 25 μm.
DISCUSSION
CD9, a member of the tetraspan family of cell surface molecules, has been implicated as a signaling molecule in various cellular processes such as adhesion, growth, motility, and differentiation in the hematopoietic and nervous systems (Wright and Tomlinson, 1994;Anton et al., 1995; Banerjee and Patterson, 1995; Hadjiargyrou and Patterson, 1995). The ability of activating anti-CD9 mAbs to cause cellular changes was utilized to elucidate many of these functions. Here we show that B2C11, an anti-CD9 mAb that has previously been demonstrated to stimulate adhesion, migration, and proliferation of Schwann cells (Anton et al., 1995; Hadjiargyrou and Patterson, 1995), induces neurite outgrowth in sympathetic neurons and morphological changes in neurons and glial cells from dissociated sympathetic ganglia.
Neurite outgrowth on B2C11 is comparable to that on rat tail collagen type I and enhanced compared to other mAb surfaces used in our assays, in both the percentage of neurons with neurites as well as in the length of neurites (Figs. 3, 4, 5). Moreover, striking morphological changes in neurons and glial cells are observed only when these cells are plated on B2C11, indicating a reorganization of cytoskeletal components induced by B2C11 activation (Figs. 7, 8).
The neurite outgrowth and morphological changes are not a general consequence of the presence of IgG molecules immobilized on the culture surface because ROCA1 (data not shown), which does not recognize the neuronal surface under these conditions, or pg22, ROCA2, and 192-IgG, which do bind the neurons, cause any significant changes. The observation that ROCA2 does not activate the cells, even though it recognizes CD9 with an affinity similar to B2C11 (Hadjiargyrou and Patterson, 1995), suggests that the effects of B2C11 are a result of an epitope-specific perturbation of CD9. In addition to collagen type I and B2C11, another mAb used as a control in our experiments, OX-7, which recognizes Thy-1, also induces neurite outgrowth. This result is consistent with previous studies showing that OX-7 enhances sympathetic neurite outgrowth when added in solution (Mahanthappa and Patterson, 1992; Doherty et al., 1993). Furthermore, OX-7 and a different anti-Thy-1 mAb, 2G12, enhance neurite outgrowth from retinal ganglion cells when immobilized on glass (Leifer et al., 1984, 1991). The spreading phenotype of both Schwann cells and neurons observed on B2C11 is, however, unique to this surface and is not seen on OX-7, rat tail collagen, or on any other surface tested. Spreading of S-16 Schwann cells has also been observed on B2C11 but not on 192-IgG (Hadjiargyrou and Patterson, 1995).
CD9 perturbation with anti-CD9 mAbs has been used extensively in both the hematopoietic and nervous systems to explore possible functions of CD9. For example, anti-CD9 mAbs cause platelet aggregation (Griffith et al., 1991 and references therein), homotypic adhesion of pre-B cell lines (Masellis-Smith et al., 1990), adhesion of pre-B cells to bone marrow fibroblasts (Masellis-Smith and Shaw, 1994), neutrophil adhesion to endothelium (Forsyth, 1991), increased adhesion of primary Schwann cells, increased adhesion and proliferation of a Schwann cell line (Hadjiargyrou and Patterson, 1995), and enhanced Schwann cell migration (Anton et al., 1995). The ability of anti-CD9 mAbs to activate cells may be a result of the ability of the mAb to mimic a putative ligand for CD9. In this model, the mAb replicates the action of a CD9 ligand. A similar mode of activation has been proposed for neurite outgrowth triggered by the OX-7 mAb, where it may mimic the natural ligand for endogenous Thy-1 (Doherty et al., 1993). An alternative model in which the anti-CD9 mAb works by causing the aggregation and/or internalization of CD9 seems unlikely in view of the fact that in our experiments the ROCA2 mAb, which binds CD9 very well, does not activate the cells.
The dramatic effects of B2C11 on neurite outgrowth and cell morphology support a previously suggested developmental role for CD9 (Tole and Patterson, 1993). During embryogenesis, CD9 is expressed on several neuronal populations (Tole and Patterson, 1993; Kaprielian et al., 1995). In sympathetic neurons, CD9 is expressed at E12, very soon after neural crest cells have formed ganglia. Although sympathetic neuroblasts continue to divide at this stage, neuronal differentiation is concurrent with mitosis (Rohrer and Thoenen, 1987; DiCicco-Bloom et al., 1990). Thus, CD9 expression is correlated with very early neuronal differentiation (Tole and Patterson, 1993). Sensory ganglia express CD9 at E15, which also corresponds to very early neurite outgrowth, which is a postmitotic event in these cells. (Rohrer and Thoenen, 1987; Tole and Patterson, 1993). This correlation is particularly striking in the ventral horn of the spinal cord, where CD9 expression parallels the transient expression of other surface proteins involved in neurite outgrowth such as TAG-1 (Dodd et al., 1988) and DM-GRASP/SC1 (Burns et al., 1991; Tanaka et al., 1991; Tole and Patterson, 1993). CD9 expression in myelinating Schwann cells during development and after injury in vivo also suggests that immature Schwann cells do not express CD9, whereas differentiating Schwann cells do so (Banerjee and Patterson, 1995; Kaprielian et al., 1995).
Our results provide further experimental evidence that CD9 may play an early role in neurite outgrowth. It is therefore interesting that in many cell types, CD9 interacts with various integrins, receptors that are critical for the neuronal response to outgrowth-promoting molecules and that are associated with downstream signaling events leading to cytoskeletal rearrangements and process extension (Reichardt and Tomaselli, 1991). Induction of platelet aggregation by an anti-CD9 mAb causes a specific association of CD9 with the GPIIb-IIIa integrin, and this association is necessary for cell activation (Higashihara et al., 1985; Slupsky et al., 1989). CD9 associates with the α3, α6, and β1 integrins in Schwann (Hadjiargyrou et al., 1996) and neuroblastoma cells (Schmidt et al., 1996). In pre-B and megakaryocytic cell lines, CD9 associates with the β1 chain-containing VLA-4 and VLA-5 integrins, which leads to cell aggregation (Letarte et al., 1992;Rubinstein et al., 1994). The β1 chain specifically interacts in_cis_ with CD9 when transfected into L cells (Rubinstein et al., 1994), and the enhanced motility of a B cell line as a result of CD9 transfection is dependent on β1 integrins (Shaw et al., 1995). Monkey and human CD9 associate with α3β1; in Vero cells, monkey CD9 colocalizes with the juxtacrine growth factor HB-EGF and α3β1 at sites of cell–cell contact (Nakamura et al., 1995). CD9 also associates with α3β1 and α6β1 in various tumor cell lines (Berditchevski et al., 1996).
It is of interest that the β1 integrins have been implicated in neurite outgrowth from a variety of neurons on various substrates (for example, Cohen and Johnson, 1991; Engvall et al., 1992; Tomaselli et al., 1993). Specifically, retinal cells and ciliary neurons use α6β1 as a laminin receptor (DeCurtis et al., 1991; Weaver et al., 1995), and α8β1 promotes neurite outgrowth of sensory neurons on fibronectin (Muller et al., 1995) and sensory and motor neurons on tenascin-C (Varnum-Finney et al., 1995).
In addition to interacting with CD9, the α3β1 integrin has been implicated as a receptor for epiligrin, laminin-5, α2β1, collagen, and fibronectin (Wayner and Carter, 1987; Elices et al., 1991;Symington et al., 1993; Tomaselli et al., 1993; Weitzman et al., 1993). In addition, α3β1 is the neuronal receptor mediating sympathetic neurite outgrowth in response to thrombospondin, and direct binding between α3β1 and thrombospondin has been shown (DeFreitas et al., 1995). α3β1 also mediates sensory neurite outgrowth on laminin-1 and laminin-2 (Tomaselli et al., 1993), neurite outgrowth from ciliary ganglion neurons on laminin (Weaver et al., 1995), and neurite outgrowth from PC12 cells on laminin-1, but only indirectly (Tomaselli et al., 1990). Because most of these interactions were defined by functional perturbation by anti-integrin antibodies, it is possible that in some cases α3β1 is an accessory to ECM ligand interactions with their receptors, rather than itself acting as an ECM receptor. Our results suggest that the α3β1 integrin may be involved in the interaction of CD9 with a putative ECM ligand (mimicked here by B2C11).
The neurite outgrowth-promoting activity of both B2C11 and OX-7 were significantly attenuated by the presence of an anti-α3β1 integrin mAb, suggesting an involvement of this integrin in neurite outgrowth mediated by CD9 and Thy-1. In this context, it is important to note that in addition to expressing CD9, neonatal sympathetic neurons also express α3β1, both in vitro and in vivo(DeFreitas et al., 1995). Because the inhibition caused by the anti-α3β1 mAb is not complete, it is possible that other, nonintegrin, signaling pathways may also be involved. Indeed, it has been proposed that a calcium-dependent mode of signaling is utilized in Thy-1-mediated neurite outgrowth (Doherty et al., 1993). Similarly, some anti-CD9 mAbs are thought to act via calcium- and G-protein-mediated signaling (Seehafer and Shaw, 1991; Kroll et al., 1992). Although there is ample evidence that CD9 interacts with the α3β1 integrin, Thy-1 has not been shown to interact with integrins. However, the specificity of the α3β1 integrin perturbation is illustrated by lack of an effect on laminin, consistent with a previous report (DeFreitas et al., 1995). In addition, none of the control mAbs used shows any effect on neurite outgrowth on any surface. These include anti-NCAM monoclonal IgGs and F(ab) fragments; NCAM was chosen because it has been shown previously not to interact with CD9 in neuronal cells (Schmidt et al., 1996). An anti-pg22 mAb also had no effect, although it binds to these cells.
Thus, integrin α3β1 is involved with CD9-mediated neurite outgrowth. It also mediates sympathetic neurite outgrowth on thrombospondin (DeFreitas et al., 1995) and type I rat tail collagen (this report), but not on collagen IV (DeFreitas et al., 1995) or laminin (DeFreitas et al., 1995, this report).
Integrins α8β1 (Muller et al., 1995) and GPIIb-IIIa (Pelletier et al., 1995) have also been implicated in cell spreading. In our experiments, the cell spreading phenotype was not significantly modified by the presence of the Ralph 3–1 mAb. Thus, the interaction of B2C11 with CD9 leading to cytoskeletal rearrangement and flattening could act by mimicking a putative CD9 ligand that directly causes downstream signaling events, or it may involve a different integrin. In addition, the B2C11-CD9 interaction may induce a _cis_interaction with the α3β1 integrin, triggering an integrin-associated signaling cascade leading to other cellular changes. The possibility that CD9 signaling may involve interactions with another set of cell surface receptors, the integrins, suggests that CD9 may be part of a larger cell surface complex that mediates interactions with the extracellular environment. Such multicomponent complexes have been reported in various cell lines (Berditchevski et al., 1996), including S-16 Schwann (Hadjiargyrou et al., 1996) and N2A neuroblastoma cells (Schmidt et al., 1996). In addition, the ability of CD9 to interact with different integrins in distinct systems raises the possibility that this may be a mechanism that underlies the specificity of CD9 functions.
Footnotes
This work was supported by an NINDS grant to P.H.P., a Muscular Dystrophy Association Research Fellowship to S.A.B., and an National Institutes of Health training grant to M.H. We are grateful to Louis Reichardt (University of California, San Fransisco) for the gift of the Ralph 3–1 mAb and to Urs Rutishauser (Case Western Reserve University, Cleveland) for the NCAM mAbs. We thank Doreen McDowell for media preparation and Karen Allendoerfer, Lisa Banner, Flora De Pablo, and Reto Gadient for providing comments on this manuscript.
Correspondence should be addressed to Dr. Paul H. Patterson, Division of Biology 216-76, California Institute of Technology, Pasadena, CA 91125.
Dr. Banerjee’s present address: Department of Neurosciences, Case Western Reserve University, 10900 Euclid Avenue, Cleveland, OH 44106.
Dr. Hadjiargyrou’s present address: Department of Orthopedics, Musculoskeletal Research Laboratory, State University of New York, Stony Brook, NY 11794.
REFERENCES
- 1.Anton ES, Hadjiargyrou M, Patterson PH, Matthew WD. CD9 plays a role in Schwann cell migration in vitro. J Neurosci. 1995;15:584–595. doi: 10.1523/JNEUROSCI.15-01-00584.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee SA, Patterson PH. Schwann cell CD9 expression is regulated by axons. Mol Cell Neurosci. 1995;6:462–473. doi: 10.1006/mcne.1995.1034. [DOI] [PubMed] [Google Scholar]
- 3.Berditchevski F, Zutter MM, Hemler ME. Characterization of novel complexes on the cell surface between integrins and proteins with 4 transmembrane domains (TM4 proteins). Mol Biol Cell. 1996;7:193–207. doi: 10.1091/mbc.7.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns FR, Von-Kannen S, Guy L, Raper JA, Kamholz J, Chang S. DM-GRASP, a novel immunoglobulin superfamily axonal surface protein that supports neurite extension. Neuron. 1991;7:209–220. doi: 10.1016/0896-6273(91)90259-3. [DOI] [PubMed] [Google Scholar]
- 5.Chandler CE, Parsons LM, Hosang M, Shooter EM. A monoclonal antibody modulates the interaction of nerve growth factor with PC12 cells. J Biol Chem. 1984;259:6882–6889. [PubMed] [Google Scholar]
- 6.Cohen J, Johnson AR. Differential effects of laminin and merosin on neurite outgrowth by developing retinal ganglion cells. J Cell Sci. 1991;15:1–7. doi: 10.1242/jcs.1991.supplement_15.1. [DOI] [PubMed] [Google Scholar]
- 7.DeCurtis I, Quaranta V, Tamura RN, Reichardt LF. Laminin receptors in the retina: sequence analysis of the α6 subunit. Evidence for transcriptional and post-translational regulation. J Cell Biol. 1991;113:405–416. doi: 10.1083/jcb.113.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeFreitas MF, Yoshida CK, Frazier WA, Mendrick DL, Kypta RM, Reichardt LF. Identification of integrin α3β1 as a neuronal thrombospondin receptor mediating neurite outgrowth. Neuron. 1995;15:333–343. doi: 10.1016/0896-6273(95)90038-1. [DOI] [PubMed] [Google Scholar]
- 9.DiCicco-Bloom E, Townes-Anderson E, Black IB. Neuroblast mitosis in dissociated culture: regulation and relationship to differentiation. J Cell Biol. 1990;110:2073–2086. doi: 10.1083/jcb.110.6.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodd J, Morton SB, Karagogeos D, Yamamoto M, Jessell TM. Spatial regulation of axonal glycoprotein expression on subsets of embryonic spinal neurons. Neuron. 1988;1:105–116. doi: 10.1016/0896-6273(88)90194-8. [DOI] [PubMed] [Google Scholar]
- 11.Doherty P, Singh A, Rimon G, Bolsover SR, Walsh FS. Thy-1 antibody-triggered neurite outgrowth requires an influx of calcium into neurons via N- and L-type calcium channels. J Cell Biol. 1993;122:181–189. doi: 10.1083/jcb.122.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doherty P, Walsh FS. Signal transduction events underlying neurite outgrowth stimulated by cell adhesion molecules. Curr Opin Neurobiol. 1994;4:49–55. doi: 10.1016/0959-4388(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 13.Elices MJ, Urry LA, Hemler ME. Receptor functions for the integrin VLA-3: fibronectin collagen and laminin binding are differentially influenced by ARG-GLY-ASP peptide and by divalent cations. J Cell Biol. 1991;112:169–181. doi: 10.1083/jcb.112.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engvall E, Earwicker D, Day A, Muir D, Manthorpe M, Paulsson M. Merosin promotes cell attachment and neurite outgrowth and is a component of the neurite-promoting factor of RN22 Schwannoma cells. Exp Cell Res. 1992;198:115–123. doi: 10.1016/0014-4827(92)90156-3. [DOI] [PubMed] [Google Scholar]
- 15.Forsyth KD. Anti-CD9 antibodies augment neutrophil adherence to endothelium. Immunology. 1991;72:292–296. [PMC free article] [PubMed] [Google Scholar]
- 16.Griffith L, Slupsky J, Seehafer J, Boshkov L, Shaw ARE. Platelet activation by immobilized monoclonal antibody: evidence for a CD9 proximal signal. Blood. 1991;78:1753–1759. [PubMed] [Google Scholar]
- 17.Hadjiargyrou M, Kaprielian Z, Kato N, Patterson PH. Association of the tetraspan protein CD9 with integrins on the surface of S-16 Schwann cells. J Neurochem. 1996;67:2505–2513. doi: 10.1046/j.1471-4159.1996.67062505.x. [DOI] [PubMed] [Google Scholar]
- 18.Hadjiargyrou MH, Patterson PH. An anti-CD9 mAb promotes adhesion and induces proliferation of Schwann cells in vitro. J Neurosci. 1995;15:574–583. doi: 10.1523/JNEUROSCI.15-01-00574.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higashihara M, Maeda H, Shibata Y, Kume S, Ohashi T. A monoclonal anti-human platelet antibody: a new platelet aggregating substance. Blood. 1985;65:382–391. [PubMed] [Google Scholar]
- 20.Hynes RO, Lander AD. Contact and adhesive specificities in the associations, migrations, and targeting of cells and axons. Cell. 1992;68:303–322. doi: 10.1016/0092-8674(92)90472-o. [DOI] [PubMed] [Google Scholar]
- 21.Kaprielian Z, Cho KO, Hadjiargyrou M, Patterson PH. CD9, a major platelet cell surface glycoprotein, is a ROCA antigen and is expressed in the nervous system. J Neurosci. 1995;15:562–573. doi: 10.1523/JNEUROSCI.15-01-00562.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keynes R, Cook GMW. Axon guidance molecules. Cell. 1995;83:161–169. doi: 10.1016/0092-8674(95)90157-4. [DOI] [PubMed] [Google Scholar]
- 23.Kroll MH, Mendelsohn ME, Miller JL, Ballen KK, Hrbolich JK, Schafer AI. Monoclonal antibody AG-1 initiates platelet activation by a pathway dependent on glycoprotein IIb-IIIa and extracellular calcium. Biochim Biophys Acta. 1992;1137:248–256. doi: 10.1016/0167-4889(92)90144-z. [DOI] [PubMed] [Google Scholar]
- 24.Lagenaur C, Lemmon V. An L1-like molecule, the 8D9 antigen is a potent substrate for neurite extension. Proc Natl Acad Sci USA. 1987;84:7753–7757. doi: 10.1073/pnas.84.21.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leifer D, Dreyer EB, Lipton SA. Immunofluorescent characterization of retinal ganglion cell neurites cultured on substrates coated with antibodies against Thy-1. Exp Neurol. 1991;113:386–390. doi: 10.1016/0014-4886(91)90030-g. [DOI] [PubMed] [Google Scholar]
- 26.Leifer D, Lipton SA, Barnstable CJ, Masland RH. Monoclonal antibody to Thy-1 enhances regeneration of processes by rat retinal ganglion cells in culture. Science. 1984;224:303–306. doi: 10.1126/science.6143400. [DOI] [PubMed] [Google Scholar]
- 27.Letarte M, Seehafer JG, Greaves A, Masellis-Smith A, Shaw ARE. Homotypic aggregation of pre-B leukemic cell-lines by antibodies to VLA integrins correlates with their expression of CD9. Leukemia. 1992;7:93–103. [PubMed] [Google Scholar]
- 28.Letourneau PC, Condic ML, Snow DM. Interactions of developing neurons with the extracellular matrix. J Neurosci. 1994;14:915–928. doi: 10.1523/JNEUROSCI.14-03-00915.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahanthappa NK, Patterson PH. Thy-1 involvement in neurite outgrowth: perturbation by antibodies, phospholipase C, and mutation. Dev Biol. 1992;150:47–59. doi: 10.1016/0012-1606(92)90006-3. [DOI] [PubMed] [Google Scholar]
- 30.Masellis-Smith A, Jensen GS, Seehater JG, Slupsky JR, Shaw ARE. Anti-CD9 monoclonal antibodies induce homotypic adhesion of pre-B cell lines by a novel mechanism. J Immunol. 1990;144:1607–1613. [PubMed] [Google Scholar]
- 31.Masellis-Smith A, Shaw ARE. Anti-CD9 monoclonal antibody induce pre-B cell adhesion to bone marrow fibroblasts through de novo recognition of fibronectin. J Immunol. 1994;152:2758–2777. [PubMed] [Google Scholar]
- 32.Mason DW, Williams AF. The kinetics of antibody binding to membrane antigens in solution and at the cell surface. Biochem J. 1980;187:1–20. doi: 10.1042/bj1870001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matthew WD, Greenspan RJ, Lander AD, Reichardt LF. Immunopurification and characterization of a neuronal heparan sulfate proteoglycan. J Neurosci. 1985;5:1842–1850. doi: 10.1523/JNEUROSCI.05-07-01842.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muller U, Bossy B, Venstrom K, Reichardt LF. Integrin α8β1 promotes cell attachment, cell spreading, and neurite outgrowth on fibronectin. Mol Biol Cell. 1995;6:433–448. doi: 10.1091/mbc.6.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura K, Iwamoto R, Mekada E. Membrane-anchored heparin-binding EGF-like growth factor (HB-EGF) and diphtheria toxin receptor-associated protein (DRAP27)/CD9 form a complex with integrin α3β1 at cell–cell contact sites. J Cell Biol. 1995;129:1691–1705. doi: 10.1083/jcb.129.6.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pelletier AJ, Kunicki T, Ruggeri ZM, Quaranta V. The activation state of the integrin αIIbβ3 affects outside-in signals leading to cell spreading and focal adhesion kinase phosphorylation. J Biol Chem. 1995;270:18133–18140. doi: 10.1074/jbc.270.30.18133. [DOI] [PubMed] [Google Scholar]
- 37.Portier M-M, Nechaud Bd, Gros F. Peripherin, a new member of the intermediate filament protein family. Dev Neurosci. 1984;6:335–344. doi: 10.1159/000112360. [DOI] [PubMed] [Google Scholar]
- 38.Rathjen FG. Neural cell contact and axonal growth. Curr Opin Cell Biol. 1991;3:992–1000. doi: 10.1016/0955-0674(91)90119-j. [DOI] [PubMed] [Google Scholar]
- 39.Reichardt LF, Tomaselli KJ. Extracellular matrix molecules and their receptors: functions in neural development. Annu Rev Neurosci. 1991;14:531–570. doi: 10.1146/annurev.ne.14.030191.002531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rohrer H, Thoenen H. Relationship between differentiation and terminal mitosis: chick sensory and ciliary neurons differentiate after terminal mitosis of precursor cells, whereas sympathetic neurons continue to divide after differentiation. J Neurosci. 1987;7:3739–3748. doi: 10.1523/JNEUROSCI.07-11-03739.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubinstein E, Lenaour F, Billard M, Prenant M, Boucheix C. CD9 antigen is an accessory subunit of the VLA integrin complexes. Eur J Immunol. 1994;24:3005–3013. doi: 10.1002/eji.1830241213. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt C, Kunemund V, Wintergerst ES, Schmitz B, Schachner M. CD9 of mouse brain is implicated in neurite outgrowth and cell migration in vitro and is associated with the α6/β1 integrin and the neural adhesion molecule L1. J Neurosci Res. 1996;43:12–31. doi: 10.1002/jnr.490430103. [DOI] [PubMed] [Google Scholar]
- 43.Seehafer JG, Shaw ARE. Evidence that the signal-initiating membrane protein CD9 is associated with small GTP-binding proteins. Biochem Biophys Res Commun. 1991;179:401–406. doi: 10.1016/0006-291x(91)91384-o. [DOI] [PubMed] [Google Scholar]
- 44.Shaw ARE, Domanska A, Mak A, Gilchrist A, Dobler K, Visser L, Poppema S, Fliegel L, Letarte M, Willett BJ. Ectopic expression of human and feline CD9 in a human B cell line confers β1 integrin-dependent motility on fibronectin and laminin substrates and enhanced tyrosine phosphorylation. J Biol Chem. 1995;270:24092–24099. doi: 10.1074/jbc.270.41.24092. [DOI] [PubMed] [Google Scholar]
- 45.Slupsky JR, Seehafer JG, Tang S-C, Masselis-Smith A, Shaw ARE. Evidence that monoclonal antibodies against CD9 antigen induce specific association between CD9 and the platelet glycoprotein IIb–IIIa complex. J Biol Chem. 1989;264:12289–12293. [PubMed] [Google Scholar]
- 46.Symington BE, Takada Y, Carter WG. Interaction of integrins α3β1 and α2β1: potential role in keratinocyte intercellular adhesion. J Cell Biol. 1993;120:523–535. doi: 10.1083/jcb.120.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka E, Sabry J. Making the connection: cytoskeletal rearrangements during growth cone guidance. Cell. 1995;83:177–186. doi: 10.1016/0092-8674(95)90158-2. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka H, Matsui T, Agata A, Tomura M, Kubota I, McFarland KC, Kohr B, Lee A, Phillips HS, Shelton DL. Molecular cloning and expression of a novel adhesion molecule, SC1. Neuron. 1991;7:535–545. doi: 10.1016/0896-6273(91)90366-8. [DOI] [PubMed] [Google Scholar]
- 49.Tole S, Patterson PH. Distribution of CD9 in the developing and mature rat nervous system. Dev Dynamics. 1993;197:94–106. doi: 10.1002/aja.1001970203. [DOI] [PubMed] [Google Scholar]
- 50.Tomaselli KJ, Hall DE, Flier LA, Gehlsen KR, Turner DC, Carbonetto S, Reichardt LF. A neuronal cell line (PC12) expresses two β1 integrins—α1β1 and α3β1—that recognize different neurite promoting domains in laminin. Neuron. 1990;5:651–662. doi: 10.1016/0896-6273(90)90219-6. [DOI] [PubMed] [Google Scholar]
- 51.Tomaselli KJ, Doherty P, Emmett CJ, Damsky CH, Walsh FS, Reichardt LF. Expression of β1 integrins in sensory neurons of the dorsal root ganglion and their functions in neurite outgrowth on two laminin isoforms. J Neurosci. 1993;13:4880–4888. doi: 10.1523/JNEUROSCI.13-11-04880.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Varnum-Finney B, Venstrom K, Muller U, Kypta R, Backus C, Chiquet M, Reichardt LF. The integrin receptor α8β1 mediates interactions of embryonic chick motor and sensory neurons with Tenascin-C. Neuron. 1995;14:1213–1222. doi: 10.1016/0896-6273(95)90268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Venstrom KA, Reichardt LF. Extracellular matrix 2: role of extracellular matrix molecules and their receptors in the nervous system. FASEB J. 1993;7:996–1003. doi: 10.1096/fasebj.7.11.8370483. [DOI] [PubMed] [Google Scholar]
- 54.Wayner EA, Carter WG. Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique α and common β subunits. J Cell Biol. 1987;105:1873–1884. doi: 10.1083/jcb.105.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weaver C, Yoshida CK, Curtis ID, Reichardt LF. Expression and in vitro function of β1-integrin laminin receptors in the developing avian ciliary ganglion. J Neurosci. 1995;15:5275–5285. doi: 10.1523/JNEUROSCI.15-07-05275.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weitzman JB, Pasqualini R, Takada Y, Hemler ME. The function and distinctive regulation of the integrin VLA-3 in cell adhesion, spreading, and homotypic cell aggregation. J Biol Chem. 1993;268:8651–8657. [PubMed] [Google Scholar]
- 57.Wright MD, Tomlinson MG. The ins and outs of the transmembrane 4 superfamily. Immunol Today. 1994;15:588–594. doi: 10.1016/0167-5699(94)90222-4. [DOI] [PubMed] [Google Scholar]