Human Bocavirus: A Novel Parvovirus Epidemiologically Associated with Pneumonia Requiring Hospitalization in Thailand (original) (raw)
Abstract
Background. We detected human bocavirus (HBoV) infection in 4.5% of hospitalized patients with pneumonia in rural Thailand. However, the role of HBoV as a pathogen is unclear.
Methods. We compared HBoV infection in patients with pneumonia with that in asymptomatic control patients enrolled between 1 September 2004 and 31 August 2005 in the same hospitals in Thailand.We examined outpatients with influenza-like illness for HBoV infection and tested for 13 additional respiratory viruses. Epidemiologic and clinical characteristics of HBoV infection are described.
Results. HBoV infection was detected in 20 (3.9%) of 512 outpatients and 3 (1%) of 280 control patients. Coinfection with other viruses was detected in 83% of patients with pneumonia and in 90% of outpatients. Compared with control patients, HBoV infection was significantly associated with pneumonia requiring hospitalization (adjusted odds ratio, 3.56 [95% confidence interval, 1.06–11.91]; P = .04). Eighty-three percent of HBoV infections were detected in patients with pneumonia who were <5 years old. More patients with pneumonia associated with HBoV—respiratory syncytial virus (RSV) or human parainfluenza virus (HPIV) coinfections had wheezing than patients with RSV and HPIV infections alone (9 [53%] of 17 vs. 32 [23%] of 138]; P = .01).
Conclusions. HBoV infection was epidemiologically associated with pneumonia among young children in rural Thailand, but infection and illness may be dependent on coinfection with other viruses.
Human bocavirus (HBoV) is a recently identified parvovirus that was detected in respiratory secretions from children with lower respiratory tract infections (LRTIs). First reported in Sweden [1] in September 2005, it is only the second member of the family Parvoviridae to be potentially associated with human disease and is the first to be linked with respiratory illness. Recently, additional studies identified HBoV from banked respiratory specimens from children with LRTI [2–11]. Together, these studies reported a prevalence of HBoV infection of 1.5%–11.3%, with most HBoV detections occurring in young children. Coinfection with other viruses was examined in several studies and ranged from 17% to 67% [1, 5–10] of specimens. However, he viruses tested and viral diagnostic assays varied between studies. Although several studies detected HBoV in respiratory specimens from patients presenting with LRTI, they neither assessed whether HBoV infection was epidemiologically associated with respiratory illness by comparison with control groups that controlled for age and month of infection nor estimated populationbased rates of disease in different age groups.
We sought to determine whether HBoV infection was associated with severe respiratory illness by comparing HBoV infection among hospitalized patients with pneumonia with infection among persons without respiratory symptoms. Using recently developed real-time polymerase chain reaction (PCR) assays for HBoV [12], we examined hospitalized patients of all ages with pneumonia enrolled through active population-based surveillance in rural Thailand between 1 September 2004 and 31 August 2005 for HBoV infection, to generate estimates of incidence and disease burden. We also tested a sample of patients with influenza-like illness and persons without fever or respiratory illness during the preceding 3 days who attended outpatient departments of the same hospitals during the study period. In addition, we used sensitive PCR assays to test for 13 other respiratory viral pathogens. The association of HBoV infection with pneumonia requiring hospitalization and with viral coinfections and the clinical and epidemiologic characteristics of HBoV infections during the study period are described.
Patients and Methods
We enrolled hospitalized patients with pneumonia identified through active population-based surveillance in Sa Kaeo province, Thailand, between 1 September 2004 and 31 August 2005 in a study to determine the etiologies of pneumonia. During 2002, active surveillance for hospitalized pneumonia was established in Sa Kaeo province by the US Centers for Disease Control and Prevention's (CDC) International Emerging Infections Program, the Thailand Ministry of Public Health (MOPH), and the Sa Kaeo Public Health Office; in August 2003, a study to determine the etiology of pneumonia began. All 8 hospitals in the province, accounting for all acute-care hospitalizations, participated [13]. In general, a patient was enrolled in the study if he or she had at least 1 sign of acute infection (fever, chills, temperature <35.5°C, abnormal white blood cell [WBC] count or differential [>11,000 or <3000 cells/μL]) plus signs or symptoms of lower respiratory tract disease (abnormal breath sounds, tachypnea, cough, sputum production, or dyspnea) and the physician ordered a chest radiograph (CXR) within 48 h of admission. Each enrolled patient provided a nasopharyngeal (NP) swab, acute and convalescent serum, urine, and clinical and demographic information. In addition, a sample of outpatients with acute influenza-like illness was recruited from the outpatient departments of 5 hospitals in Sa Kaeo province [14]. Influenza-like illness was defined by the World Health Organization definition (fever within the preceding 3 days and cough or sore throat in the absence of other diagnoses). Each outpatient participant provided a NP swab and demographic information.
The key to the present study was the collection of respiratory samples from control patients (i.e., patients from the same province who did not have a fever and did not report a fever, cough, or sore throat within the preceding 3 days), which began in August 2004. The control patients were collected from the outpatient clinics described above. We sought to enroll equal numbers of control patients from each age group (persons ⩽2, 3–5, 6–15, 16–54, and ⩾55 years old) during each month of the year and an equal number of control patients each month (median number per month, 25; range, 10–30). Each control patients provided a NP swab and demographic information.
Nasopharyngeal swabs were collected and shipped to the Thailand National Institute of Health (Thai NIH) and then to the CDC as described elsewhere [12]. Acute and convalescent serum specimens were also collected. NP specimens were inoculated into MDCK and HEp-2 cells and tested for respiratory syncytial virus (RSV), human parainfluenza viruses (HPIVs), influenza viruses, and adenovirus by immunofluorescent staining at Thai NIH. Respiratory specimens and select serum specimens were tested for HBoV by real-time PCR assays that targeted the HBoV NS1 and NP-1 genes [12].We defined an HBoV infection as a positive PCR test for both the NS1 and NP-1 targets or a positive test for a single target that was confirmed with a second extraction from a new, previously unopened sample aliquot. Semiquantitative estimates of virus load in clinical samples expressed as genome equivalents (GEs) per milliliter of viral transport media were determined by comparison of sample cycle threshold value with those obtained from standard curves prepared from serial 10-fold dilutions of HBoV recombinant plasmids for each real-time PCR target [12].
The respiratory specimens were also tested for RSV; HPIV 1, 2, and 3; adenovirus; influenza viruses A and B; human metapneumovirus (HMPV); and rhinoviruses using PCR methods described elsewhere [15, 16] and for human coronaviruses (HCoV) 229E, OC43, HKU1, and NL63 using in-house realtime PCR assays. A specimen was considered to have a positive result if either culture or PCR testing was positive. Diagnostic testing for bacterial pathogens during the study period was limited to urine pneumococcal surface antigen assay among patients ⩾18 years old who had pneumonia.
We compared HBoV infection in hospitalized patients with pneumonia and control patients using the χ2 test and a multivariable unconditional logistic regression model controlling for age group (⩽2, 3–5, 6–15, 16–54, and ⩾55 years old) and month. The proportion of patients with pneumonia in each age group was 24%, 9%, 9%, 25%, and 34%, respectively; the proportion of control patients was 18%, 11%, 22%, 25%, and 23%, respectively. Our analysis was limited to 1 September 2004–31 August 2005. We assessed risk factors for HBoV infection among hospitalized patients with pneumonia using an unconditional logistic regression model that included all potential confounders and variables in the model that were significant at the univariate level at P < .05. All 2-way interactions were evaluated. To estimate the minimum age group—specific incidence of HBoV-associated pneumonia requiring hospitalization, we divided the number of HBoV infections by the appropriate age-group population estimate from the 2000 national Thai census of Sa Kaeo Province. Each pneumonia admission was counted individually, including readmissions ⩾14 days since a previous discharge. Readmissions within ⩽13 days were considered to be 1 admission. Clinical characteristics that were obtained from patients with pneumonia associated with HBoV infection with RSV, HPIV 1–3, or rhinovirus infection, but no coinfection with any of the other viruses, were compared with patients with HBoV-RSV and HBoV-HPIV coinfection or with HBoV-rhinovirus coinfection. RSV- and HPIV-associated pneumonias were combined to increase the numbers available for comparison. Variables were compared using the χ2 test for dichotomous variables or the Wilcoxon signed-rank test for continuous variables. Two-tailed P < .05 was considered to be statistically significant. Patients with missing values for clinical characteristics were excluded from the comparison of clinical characteristics, and patients with triple virus infections were excluded from clinical comparisons and logistic regression models. All analysis was performed by use of SAS software (version 9.1; SAS Institute).
All participants were informed of the study objectives, and written consent was obtained. The study protocol was reviewed and approved by the ethics review boards of the CDC and Thai MOPH.
Results
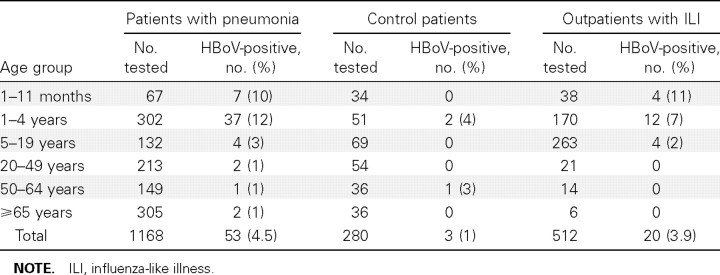
During 1 September 2004–31 August 2005, 3960 patients with signs and symptoms of clinical pneumonia were admitted to a Sa Kaeo province hospital, and 2288 (58%) of these patients had a CXR.We enrolled 1171 (51%) of the hospitalized patients with pneumonia who had a CXR; 3 patients did not have a sufficient amount of specimen remaining to test for HBoV and were excluded from the analysis. Among patients with pneumonia, 51 were readmissions. In addition, we enrolled a sample of 512 outpatients with upper respiratory tract illness and 280 control patients without fever or respiratory illness. One outpatient did not have enough specimen to test for HBoV and was excluded. NP swab specimens from all participants were tested for HBoV (table 1). As reported elsewhere [12], HBoV infection was detected in 4.5% of patients with pneumonia. Most infections were detected among patients with pneumonia who were <5 years old; 13% were among infants <1 year old, and 70% were among children 1–4 years old. Few HBoV infections were found among young or middle-aged adults; 4% were among persons ⩾65 years old. By contrast, only 1% of control patients had HBoV infection. HBoV infection was detected in 20 (3.9%) of 512 outpatients with influenza-like illness; 70% were among children <5 years old. Among HBoVpositive specimens, the estimated virus load ranged from 600 to 4×1010 GE/mL. As noted elsewhere [12], most of the positive hospitalized patients and all of the control patients had moderate to low virus loads (<5×104 GE/mL). Six patients with pneumonia and 3 outpatients had high levels of HBoV in their respiratory samples (⩾107 GE/mL).
Table 1.
Human bocavirus (HBoV) polymerase chain reaction test results from hospitalized patients with pneumonia, asymptomatic control patients, and outpatients with acute respiratory illness from Sa Kaeo province, 1 September 2004–31 August 2005.
Among patients with pneumonia, HBoV was the third most common viral infection detected among children <5 years old, after rhinovirus and RSV, and it accounted for 12% of all pneumonia requiring hospitalization in this age group. The unadjusted estimated annual incidence of HBoV-associated hospitalized pneumonia, including all HBoV infections, in Sa Kaeo province during the study year was 123 cases/100,000 population for infants 0–11 months old, 129 cases/100,000 population for children 1–4 years old, 3 cases/100,000 population for persons 5–19 years old, 1 case/100,000 population for persons 20–49 years old, 2 cases/100,000 population for persons 50–64 years old, and 9 cases/100,000 population for persons >65 years old. Overall, the unadjusted estimated incidence of hospitalized pneumonia in Sa Kaeo province with HBoV detected was 12 cases/100,000 population.
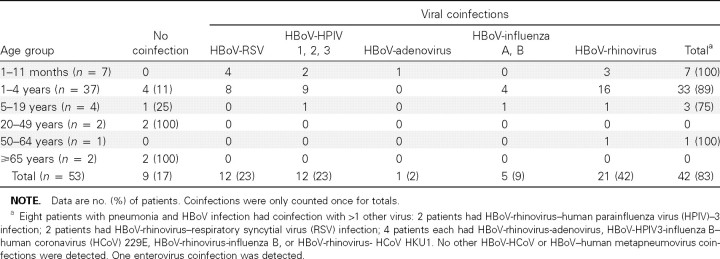
Among the 53 HBoV-infected patients with pneumonia, 44 (83%) had coinfection with other viruses (table 2). Only 9 patients with pneumonia associated with HBoV infection did not have coinfection with 1 of the other 13 viruses tested. Among persons ⩾65 years old, 2 (100%) of the HBoV infections were single virus infections. Among children <5 years old, 40 (91%) of 44 HBoV infections had coinfection with other viruses. HBoV-rhinovirus was the most common viral coinfection, with HBoV-RSV and HBoV-HPIV the next most common. By contrast, viral coinfections were not as common in patients with pneumonia caused by viruses other than HBoV. Coinfections with any viruses were detected among 0%–34% of rhinovirus, RSV, HPIV3, and influenza virus infections. Among the control patients, 1 of 3 with HBoV infections had coinfection with HCoV NL63. Coinfections among outpatients were detected in 18 (90%) of 20 patients and included 4 coinfections with influenza virus A; 3 with rhinovirus; 2 each with adenovirus, influenza virus B, and adenovirus/rhinovirus; and 1 each with HPIV-3, RSV, HPIV3/influenza B, HPIV3/adenovirus, and HMPV/rhinovirus/HCoV NL63.
Table 2.
Viral coinfections among human bocavirus (HBoV)—infected hospitalized patients with pneumonia in Sa Kaeo province, Thailand.
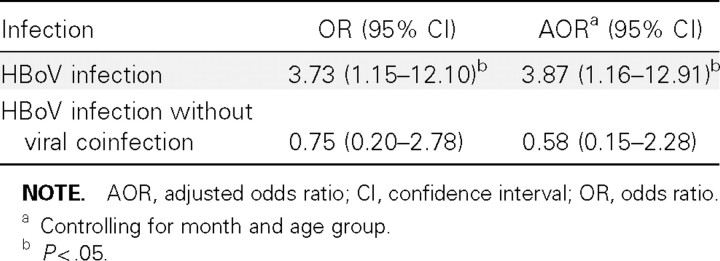
We compared the detection of HBoV in respiratory specimens between hospitalized patients with pneumonia and control patients (table 3). The risk of HBoV infection was 4 times higher among patients with pneumonia than among control patients and was statistically significant even after we controlled for age group and month. However, if we compared HBoV infections in patients with pneumonia who did not have coinfection with other viruses and control patients, the detection of HBoV was not significantly greater in patients with pneumonia, compared with control patients.We also compared outpatients with influenza-like illness with control patients. After controlling for age group and month, the risk of HBoV detection in outpatients with influenza-like illness was slightly higher than that in control patients, but the association was not significant (adjusted odds ratio [AOR], 1.58 [95% confidence interval {CI}, 0.34–7.31]; P = .55).
Table 3.
Comparison of human bocavirus (HBoV) infections among patients hospitalized with pneumonia and asymptomatic control subjects in Sa Kaeo province, Thailand.
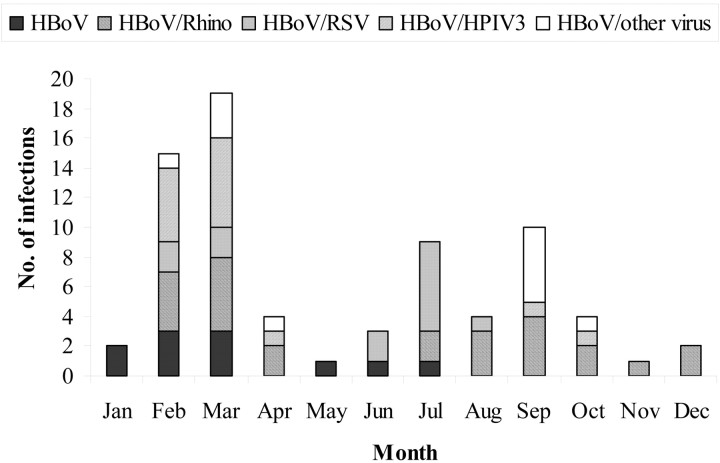
During the study period, HBoV infections in patients with pneumonia and outpatients with influenza-like illness occurred throughout the year. However, 34 (47%) of 73 HBoV infections occurred during February and March (figure 1); 6 (55%) of 11 HBoV infections with no coinfection occurred during February and March. The seasonality of HBoV infections with viral coinfections appeared to coincide with the seasonality of RSV and rhinovirus. HBoV-rhinovirus coinfections occurred throughout the year, similar to rhinovirus infections (data not shown). However, 11 (50%) of HBoV-rhinovirus coinfections occurred during February and March. Also, 8 (62%) of HBoVRSV coinfections occurred in June and July, the months when 55% of RSV infections occurred (data not shown). The remaining HBoV-RSV coinfections occurred during February and March.
Figure 1.
Seasonal occurrence of human bocavirus (HBoV) infection among patients with pneumonia and outpatients with influenza-like illness, 1 September 2004–31 August 2005. HPIV, human parainfluenza virus; Rhino, rhinovirus; RSV, respiratory syncytial virus.
We looked at independent risk factors for HBoV infection among hospitalized patients with pneumonia. Because coinfections with rhinoviruses, HPIV3, and RSV were common with HBoV and coinfection occurred between these viruses, we included these viral infections, as well as age group and month, in our model. Age was the greatest risk factor for HBoV infection, with younger children, <1 year old (AOR, 6.45 [95% CI, 1.08–38.51]) and 2–4 years old (AOR, 15.13 [95% CI, 3.42–66.82]), having the highest risk of infection, compared with patients 5–19 years old (AOR, 4.23 [95% CI, 0.74–23.94]), 20– 49 years old (AOR, 1.32 [95% CI, 0.18–9.56]), 50–64 years old (AOR, 1.06 [95% CI, 0.10–11.91]), and ⩾65 years old (referent). No viral infections were statistically associated with HBoV infection among hospitalized patients with pneumonia, but rhinovirus and HPIV3 infections had elevated point estimates (rhinovirus infection, AOR, 1.76 [95% CI, 0.84–3.74]; and HPIV3 infection, AOR, 2.01 [95% CI, 0.79–5.16]).
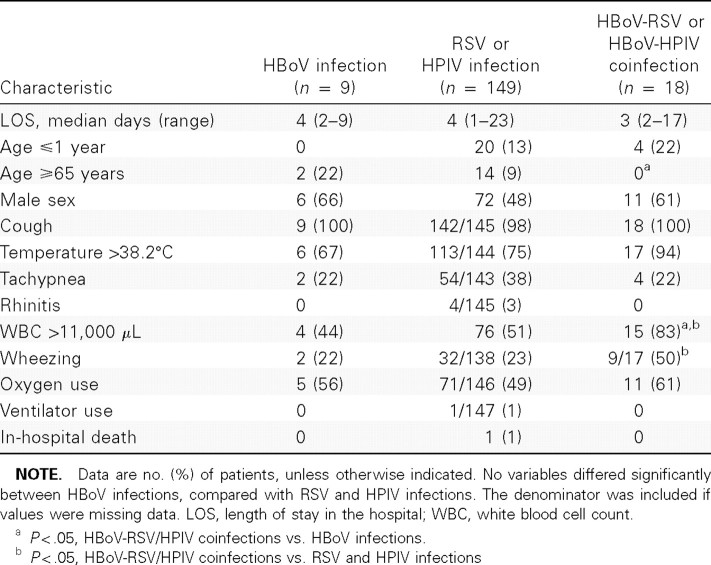
A few clinical characteristics differed when we compared patients with pneumonia associated with HBoV infection with patients with RSV and HPIV infections (combined) and with patients with HBoV-RSV/HPIV coinfection (table 4). More patients with pneumonia associated with HBoV-RSV/HPIV coinfections had wheezing than patients with RSV and HPIV infections. HBoV-RSV/HPIV coinfections also were more likely to cause elevated WBC counts (>11,000 cells/μL) at admission. Individually, results were similar for RSV but not HPIV (6 [60%] of 10 HBoV-RSV coinfections vs. 23 [24%] of 96 RSV infections [P = .02]; and 3 [43%] of 7 HBoV-HPIV coinfections vs. 9 [23%] of 40 HPIV infections [P = .25]).
Table 4.
Comparisons of clinical characteristics among patients with pneumonia and human bocavirus (HBoV) infection, respiratory syncytial virus (RSV), and human parainfluenza virus (HPIV)—3 infection or HBoV-RSV and HBoV-HPIV3 coinfections.
Patients with HBoV-rhinovirus coinfection also had a higher proportion of wheezing than those with rhinovirus infection (7 [58%] of 12 HBoV-rhinovirus coinfections vs. 37 [28%] of 134 rhinovirus infections; P = .03). None of the patients with pneumonia associated with HBoV-rhinovirus infections were <1 year old. Nine (6%) patients with rhinovirus infection were <1 year old. There was no difference in fever or elevated WBC counts between patients with rhinovirus infection and those with HBoV-rhinovirus coinfection.
Finally, we tested serum collected from some hospitalized patients with pneumonia for HBoV DNA by real-time PCR. Initially, we screened serum from 5 patients with the highest levels of HBoV DNA (>107 GE/mL) in their respiratory specimens. HBoV DNA was detected in the acute-phase serum specimen from 4 (80%) of 5 patients and in the convalescentphase serum specimen from 2 (40%) patients. The virus loads were substantially lower than those in the corresponding respiratory specimens. Viral coinfections were detected in 3 (60%) of 5 patients. For comparison, we examined, in a blinded fashion, acute serum from 6 patients with mid- to low levels of HBoV DNA (2×104−4×105 GE/mL) and 5 patients with no detectable HBoV DNA in their respiratory specimens.HBoV DNA was not detected in any of these specimens.
Discussion
Our results suggest that HBoV infection is epidemiologically associated with pneumonia requiring hospitalization in rural Thailand. Because HBoV infection was uncommon among our sample of persons without fever or signs and symptoms of respiratory illness, it seems unlikely that HBoV infection in patients with pneumonia was a coincidental event and not linked to their illness. However, the role that HBoV infection plays in the disease process is confounded by the high proportion of coinfections with other respiratory viruses.We tested for a greater number of viruses than has been reported in other published studies, and we found a much higher proportion of viral coinfections—among children <5 years old, the proportion of HBoV infections with viral coinfections was 91%. In addition, the burden of HBoV-associated pneumonia was substantial. Our population-based estimates of the incidence of HBoV-associated pneumonia place it among such viruses as RSV and influenza, which cause significant morbidity among young children. Finally, our findings suggest that HBoV infection, or coinfection, may influence a patient's clinical presentation.
The apparent dependency of HBoV infection and illness on coinfection with other respiratory viruses is intriguing. Parvoviruses are among the most dependent on host cellular functions for replication, and they only multiply in cells that are in the process of replicating their own DNA [17]. Human parvovirus B19, an Erythrovirus, only replicates during the DNA synthesis phase of the cell cycle. Adeno-associated viruses in the genus Dependovirus depend on coinfection with adenovirus or herpesviruses that induce DNA synthesis in the host cell to facilitate their own replication. However, the other members of the genus _Bocavirus_—bovine bocavirus and canine minute virus—do not require a helper virus, and most of the HBoV coinfecting viruses that we identified do not induce DNA replication in the host cell. One possibility is that coviral-induced cellular damage results in high levels of cellular division and differentiation, creating conditions permissive for HBoV replication. A similar mechanism has been shown for polyomavirus infection in mice [18]. In addition, respiratory viral infections may suppress immune system functions to make the host more susceptible to infection with HBoV. Further studies are needed to understand the interaction between HBoV and other viral infections.
Interestingly, patients with pneumonia associated with HBoV-RSV/HPIV coinfection and HBoV-rhinovirus coinfection appeared to have more wheezing recorded at admission than patients with RSV, HPIV, or rhinovirus infection. Children with viral respiratory infections often present with wheezing [19]. Although we did not have a large sample of patients with only HBoV infection, our results raise the possibility that HBoV coinfection may play an important role in the clinical presentation of wheezing among children. Also, several studies have documented an association with respiratory viral infections and exacerbations of asthma in adults and children [20–22]. Confirmation of our results with other patient cohorts and obtaining additional information on underlying illness, such as a history of asthma, will be important to more fully understand the role of HBoV infection and human disease.
The presence of nucleic acid in acute-phase serumspecimens from patients with pneumonia with high titers of HBoV in their respiratory specimens suggests that HBoV viremia may have occurred in these patients, although virus concentrations were consistently higher in respiratory secretions than in serum collected on the same day. We did not find evidence of viremia in samples from patients with low HBoV respiratory titers or those infected with other respiratory viruses. Therefore, this phenomenon may not be common. Interestingly, HBoV nucleic acid was present in convalescent serum specimens from 2 of the patients with HBoV positive acute serum. Persistent viremia of another parvovirus causing human illness, parvovirus B19, has been described [23]. One study also reported finding HBoV nucleic acid in respiratory tissues and one bowel-tissue specimen from autopsy specimens from 9 children [24]. Therefore, it is possible that this recently described virus does cause viremia and possibly even illness other than that of the respiratory tract. Persistent HBoV viremia could have implications for blood- or organ-donation programs. Tissue-culture techniques for growing HBoV that could establish HBoV viability in serum specimens have not yet been described.
Our study has some strengths and limitations with bearing on its interpretation. In contrast to most studies to date, which reported HBoV in a convenience sample of patients submitting respiratory specimens, the present study was built on an ongoing, prospective, population-based, surveillance system, and it included a control group without respiratory disease, which allowed us to estimate incidence and burden of disease and to determine epidemiologically whether HBoV infection was associated with hospitalized pneumonia. However, the number and rate of cases of HBoV-associated pneumonia were likely underestimated. Only 58% of patients admitted to a Sa Kaeo hospital during the study period with signs and symptoms of clinical pneumonia got a CXR, and only 51% of these enrolled in the etiology study did so. We found a high proportion of HBoV infections that had coinfection with other viruses. However, although we tested for more viruses than other studies, we may have missed additional viral infections not detected by PCR or tissue culture. The PCR primers for viral diagnostics were based on published sequences. Therefore, viral variants that are not recognized by our primers or identified by our culture techniques will have been missed. During the study year, diagnostic testing for bacterial pathogens was limited to urine pneumococcal surface antigen assay among patients with pneumonia who were >18 years old. No adults had HBoV— Streptococcus pneumoniae coinfection. However, we do not know whether HBoV-bacterial coinfections occurred among children or whether HBoV-atypical bacterial coinfections occurred.
To our knowledge, we present the first study that clearly demonstrates an association between HBoV infection and pneumonia requiring hospitalization and likely with less severe respiratory illness that is managed in outpatient clinics. However, understanding the interaction between HBoV and other coinfecting viruses will be crucial for establishing the role of this newly identified virus in human disease. In addition, HBoV coinfection may influence the clinical symptoms that the patient displays. Additional studies to better define HBoV infection, including interactions with other viruses, determining whether HBoV causes illness other than respiratory illness, and whether HBoV infection can persist after acute illness will be critical to determine the burden of HBoV infection.
Acknowledgments
We thank Brian Holloway and Karen McCaustland (Centers for Disease Control and Prevention Biotechnology Core Facility Branch), for oligonucleotide synthesis and expert technical advice; and George Gallucci and Pongpun Sawatwong, for technical assistance.
Footnotes
Potential conflicts of interest: none reported.
Presented in part: International Conference on Emerging Infectious Diseases, Atlanta, 19–22 March 2006.
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the funding agency.
References
- 1.Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA. 2005;102:12891–6. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastien N, Brandt K, Dust K, Ward D, Li Y. Human bocavirus infection, Canada. Emerg Infect Dis. 2006;12:848–50. doi: 10.3201/eid1205.051424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sloots TP, McErlean P, Speicher DJ, Arden KE, Nissen MD, Mackay IM. Evidence of human coronavirus HKU1 and human bocavirus in Australian children. J Clin Virol. 2006;35:99–102. doi: 10.1016/j.jcv.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma X, Endo R, Ishiguro N, et al. Detection of human bocavirus in Japanese children with lower respiratory tract infections. J Clin Microbiol. 2006;44:1132–4. doi: 10.1128/JCM.44.3.1132-1134.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foulongne V, Rodiere M, Segondy M. Human bocavirus in children. Emerg Infect Dis. 2006;12:862–3. doi: 10.3201/eid1205.051523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arden KE, McErlean P, Nissen MD, Sloots TP, Mackay IM. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J Med Virol. 2006;78:1232–40. doi: 10.1002/jmv.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weissbrich B, Neske F, Schubert J, et al. Frequent detection of bocavirus DNA in German children with respiratory tract infections. BMC Infect Dis. 2006;11:109. doi: 10.1186/1471-2334-6-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold JC, Singh KK, Spector SA, Sawyer MH. Human bocavirus: prevalence and clinical spectrum at a children's hospital. Clin Infect Dis. 2006;43:283–8. doi: 10.1086/505399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foulongne V, Oejnik Y, Perez V, Elaerts S, Rodiere M, Segondy M. Human bocavirus in French children. Emerg Infect Dis. 2006;12:1251–3. doi: 10.3201/eid1208.060213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi EH, Lee HJ, Kim SJ, et al. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000–2005. Clin Infect Dis. 2006;43:585–92. doi: 10.1086/506350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kesebir D VM, Weibel C, Shapiro ED, Ferguson D, Landry ML, Kahn JS. Human bocavirus infection in young children in the United States: molecular epidemiological profile and clinical characteristics of a newly emerging respiratory virus. J Infect Dis. 2006;194:1276–82. doi: 10.1086/508213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu X, Chittaganpitch M, Olsen SJ, et al. Real-time PCR assays for detection of bocavirus in human specimens. J Clin Microbiol. 2006;44:3231–5. doi: 10.1128/JCM.00889-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsen SJ, Laosiritaworn Y, Siasiriwattana S, Chunsuttiwat S, Dowell SF. The incidence of pneumonia in rural Thailand. Int J Infect Dis. 2006;10:439–45. doi: 10.1016/j.ijid.2006.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simmerman JM, Lertiendumrong J, Dowell SF, et al. The cost of influenza in Thailand. Vaccine. 2006;24:4417–26. doi: 10.1016/j.vaccine.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 15.Falsey AR, Erdman D, Anderson LJ, Walsh EE. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003;187:785–90. doi: 10.1086/367901. [DOI] [PubMed] [Google Scholar]
- 16.Erdman DD, Weinberg GA, Edwards KM, et al. GeneScan reverse transcription-PCR assay for detection of six common respiratory viruses in young children hospitalized with acute respiratory illness. J Clin Microbiol. 2003;41:4298–303. doi: 10.1128/JCM.41.9.4298-4303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muzyczka N, Berns KI. Parvoviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields virology. 4th ed. Vol 2. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 2327–59. [Google Scholar]
- 18.Atencio IA SF, Zhou XJ, Vaziri ND, Villarreal LP. Adult mouse kidneys become permissive to acute polyomavirus infection and reactivate persistent infections in response to cellular damage and regeneration. J Virol. 1993;67:1424–32. doi: 10.1128/jvi.67.3.1424-1432.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright AL, Taussig LM, Ray CG, Harrison HR, Holberg CJ. The Tucson children's respiratory study: 2. Lower respiratory tract illness in the first year of life. Am J Epidemiol. 1989;129:1232–46. doi: 10.1093/oxfordjournals.aje.a115243. [DOI] [PubMed] [Google Scholar]
- 20.Teichtahl H, Buckmaster N, Pertnikovs E. The incidence of respiratory tract infection in adults requiring hospitalization for asthma. Chest. 1997;112:591–6. doi: 10.1378/chest.112.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–6. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnston SL, Pattemore PK, Sanderson G, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310:1225–9. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaMonte AC, Paul ME, Read JS, et al. Persistent parvovirus B19 infection without the development of chronic anemia in HIV-infected and -uninfected children: the Women and Infants Transmission Study. J Infect Dis. 2004;189:847–51. doi: 10.1086/381899. [DOI] [PubMed] [Google Scholar]
- 24.Battaglioli G, Clement N, Linden M, St George K. High prevalence of human bocavirus in pediatric autopsy respiratory specimens [abstract T-PM17] Abstract and proceedings of the 22nd Annual Clinical Virology Symposium (Clearwater Beach, FL) 2006 [Google Scholar]